Halide Chemistry of Chromium, Molybdenum and Tungsten
J . E . F E R G U S S O N
University of Canterbury, Christchurch, New Zealand 1. General Introduction
2 . Chromium
A . Chromium(I) (d^) B . Chromium(II) (d^) C. Chromium(III) {d^
D . Chromium(IV) {d^
E . Chromium(V) {d^
F . Chromium(VI) {d^) 3 . M o l y b d e n u m a n d T u n g s t e n
A . M o l y b d e n u m a n d T u n g s t e n(0, I) B . M o l y b d e n u m a n d Tungsten(II) ( # ) . . C. M o l y b d e n u m a n d T u n g s t e n ( I I I ) {d^ . . D . M o l y b d e n u m a n d T u n g s t e n ( I V ) {d^) . . E . M o l y b d e n u m and Tungsten(V) ( # ) . . F . M o l y b d e n u m a n d Tungsten(VI) (d^) . . R e c e n t D e v e l o p m e n t s . .
References
2 2 7 2 2 8 2 2 8 2 2 8 2 3 3 2 4 2 2 4 3 2 4 5 2 4 9 2 4 9 2 4 9 2 5 4 2 5 9 2 6 5 2 7 6 2 8 5 2 8 9
1. General Introduction
I n t e r e s t in t h e halogen c h e m i s t r y of elements h a s always existed for chemists, a n d certainly does n o t a p p e a r t o b e diminishing. T h e c h e m i s t r y of chlorides is b y far t h e m o s t fully s t u d i e d a n d r e c e n t researches h a v e p u t fluorides in second place. H o w e v e r , t h e r e is still considerable w o r k t o be done on t h e b r o m i d e a n d iodide c o m p o u n d s of t h e elements. These generalizations will, it is h o p e d , b e exemplified in t h e following pages.
I n view of t h e v a s t a m o u n t of m a t e r i a l for review it h a s been neces
s a r y for t h e a u t h o r t o be selective a n d t o consider only w o r k since a b o u t 1930. T h e p r e s e n t review is therefore confined t o halides, complex halogeno-anions a n d oxyhalides of t h e t h r e e m e t a l s . Detailed discussion of coordination complexes of t h e halides h a s b e e n o m i t t e d a n d t h e w o r k is largely confined t o t h e c h e m i s t r y of t h e a n h y d r o u s m a t e r i a l s . This is especially so for t e r v a l e n t c h r o m i u m . T h e r e c e n t edition of Gmelin
(1962) gives a n excellent coverage of c h r o m i u m chemistry.
2 2 7
228 J. Ε. FERGUSSON
Recent reviews b y Peacock (1960), Figgis and Lewis (1964) and Fowles (1964) touch upon some parts of the present review and can be consulted to see these aspects of the halogen chemistry of chromium, molybdenum and tungsten in the wider field of the transition metals.
2 . C h r o m i u m A. Chromium(I) {d^)
There appears to be no reason to believe that monovalent chromium halides exist, except in the presence of ligands such as carbonyl (in Cr(C0)5X) which stabilize low valency states (Abel et al., 1963).
Various workers have proposed the simple halides as reaction inter
mediates and as species contributing to the band spectra of chromium halides (Hein et al., 1927b; Hein and Bâhr, 1943a, 1953; Hein and Pauling, 1953; Rao, 1949; R a o and Rao, 1949).
Calculated enthalpies of formation of the halides suggest that CrF (—52-7 kcal molc"^) m a y exist. However, if dissociation and dispro
portionation reactions are taken into consideration there is no reason to think that it will (Petrakis, 1962). A derived Cr—CI bond energy of 7 kcal m o l e- i indicates the weakness of any such bond (Sano, 1938b).
On the basis of the greater covalent bonding tendency, ability to TT-bond, and the reducing power of the iodide ion one would expect, contrary to the above, that Cr(I) could be the most stable of the mono halides.
B. Chromium(II) (ι/') (i) Halides
The preparation of a pure sample of anhydrous chromium(II) fluoride has proved to be difficult. Earlier work using the action of hydrogen fluoride on chromium metal, chromium(II) chloride or chromium(II) sulphide as well as the reduction of chromium(III) fluoride with hydro
gen have been shown to give a product contaminated with either un
reacted starting materials or oxidation products (Lux and Illmann, 1958; Muetterties and Castle, 1961; Gmelin, 1962; Sturm, 1962). A recent study has led to the development of new preparations, some of which involve high temperatures. The pyrolysis of (NH4)3CrF6 at 1100°
for 5 h gives chromium (II) fluoride together with a little of the tri
fluoride which itself disproportionates at 1100° according to the reaction:
3 C r F 3 2CrF2 + CrF5
B o t h chromium metal and stannous fluoride satisfactorily reduce the trifluoride at 1100°. The stannous fluoride itself and fluorides of Bi(III), Pb(II), Cu(II), and Cd(II) act as fluorinating agents (Sturm, 1962).
HALIDE CHEMISTRY OF CHROMIUM, MOLYBDENUM AND TUNGSTEN 229 A p a r t i a l r e d u c t i o n of c h r o m i u m ( I I I ) fluoride h a s led t o a n e w p h a s e CrF2.4, also o b t a i n e d b y h e a t i n g a m i x t u r e of t h e di- a n d trifluorides t o 800-900° ( S t u r m , 1962).
T h e t h r e e general m e t h o d s for o b t a i n i n g a n h y d r o u s c h r o m i u m (II) chloride involve r e d u c t i o n of c h r o m i u m ( I I I ) , oxidation of t h e m e t a l a n d t r e a t m e n t of a c h r o m i u m ( I I ) c o m p o u n d w i t h h y d r o g e n chloride. T h e first m e t h o d h a s been a t t e m p t e d using c h r o m i u m m e t a l as t h e r e d u c i n g a g e n t in fused salt b a t h s (Campbell et al,, 1949), or in a sealed silica vessel a t 890° (Corbett et al, 1963; Doerner, 1937). R e d u c t i o n b y h y d r o gen (Hein et al, 1927a; Schlafer a n d Skoludek, 1957) is claimed t o give a p r o d u c t c o n t a m i n a t e d w i t h s t a r t i n g m a t e r i a l . H o w e v e r , a m i x t u r e of h y d r o g e n chloride a n d h y d r o g e n u n d e r controlled conditions p r e v e n t s t h e formation of c h r o m i u m m e t a l (Schlesinger a n d H a m m o n d , 1933;
Doerner, 1937; B u r g , 1950). Oxidation of t h e m e t a l w i t h chlorine ( 9 5 0 - 1000°) gives b o t h t h e di- a n d trichlorides ( D e v y a t o v s k a y a a n d Vil'nyanskii, 1961) while t h e addition of h y d r o g e n chloride (860-885°) p r o d u c e s j u s t t h e dichloride (Kiihnl a n d E r n s t , 1962). T h e blue t e t r a - h y d r a t e CrCl2.4H20 is readily o b t a i n e d b y t r e a t m e n t of c h r o m i u m ( I I ) a c e t a t e w i t h hydrochloric acid.
P r e p a r a t i v e m e t h o d s similar t o t h o s e for t h e dichloride a r e used for a n h y d r o u s chromium(IT) b r o m i d e . H y d r o g e n h a s been used as t h e re
ducing a g e n t (Biltz a n d Birk, 1924; H e i n a n d W i n t e r - H o l d e r , 1931), a n d conversion of c h r o m i u m ( I I ) a c e t a t e h a s b e e n achieved w i t h h y d r o gen b r o m i d e in e t h e r (Brauer, 1962) a n d w i t h acetyl b r o m i d e (Issleib a n d F r o h l i c h , 1959). T h e p u r e s t s a m p l e of t h e d i b r o m i d e is m o s t p r o b a b l y o b t a i n e d b y o x i d a t i o n of t h e m e t a l i n a b r o m i n e - h y d r o g e n s t r e a m a t 800-900° (Fischer a n d Gewehr, 1935; K i i h n l a n d E r n s t , 1962). Oxida
t i o n of t h e m e t a l w i t h 4 0 % h y d r o b r o m i c acid readily p r o d u c e s t h e d a r k blue h e x a h y d r a t e .
T h e p r e p a r a t i o n of c h r o m i u m (II) iodide h a s been largely confined t o t h e reaction b e t w e e n t h e elements. T h e different t e c h n i q u e s of working in vacuo, or in a n inert a t m o s p h e r e a n d a t t e m p e r a t u r e s v a r y i n g from 500-800° h a v e been used (Hein a n d W i n t e r - H o l d e r , 1931b; H e i n a n d B à h r , 1943a; H a n d y a n d Gregory, 1950; T u m a r e v a n d P a n y u s k i n , 1962;
C o r b e t t et al, 1963). I t is a n a d v a n t a g e t o k e e p t h e s u p p l y of iodine a t 175°. Some tri-iodide is usually formed a n d careful s u b l i m a t i o n is re
quired t o o b t a i n p u r e c h r o m i u m ( I I ) iodide. T h e tri-iodide itself disso
ciates t o t h e di-iodide a r o u n d 500° (Gregory a n d H a n d y , 1957; C o r b e t t et al, 1963). C h r o m i u m m e t a l dissolves in a q u e o u s hydroiodic acid t o give a blue-violet solution from w h i c h t h e h e x a h y d r a t e can b e isolated.
T h e reaction
CrBr2(s) -f 2HCl(g) -> CrCl2(s) + 2 H B r ( g )
TABLE I. Chromium(II) halides* ComFormation data^ Colour Melting Specific Cr—X Magnetic Cr—Χ pound -àG° point (°C) heat bond moment bond (kcal mole~^) (kcal mole~^) (e.u.) (cal mole~^ energy (B) at length deg-i) (kcal room temp (A) mole-i) CrF^ 181, 182c,d 170, 172c»d 34d green 894e 4-3f 2 at 2-43f 2 at 2-Olf 2 at l-98f CrCla 92-97c,ci,g,ii,i,t 84, 8oC'g'ii 31-32d,ii white 820-824J 16-76^^ 91-31 5-13°^ 2 at 2-91^ {Θ = -149° 4 at 2-40^ CrBrg 81, 86<i.e 34d white 844 θ = -166° 2 at 3-OOP 4 at 2-54P Crl2 38^1 37^ red-brown 868 55-71 θ = -770 2 at 3-24S 4 at 2-74IÛ a This and following tables of thermodynamic data do not pretend to be exhaustive in their coverage of values in the literatin-e. A selection of what appear to be the best values is given. ^ The formation data is for the formation of the crystalline compound from its elements at 298°K unless otherwise stated. c Rossini et al. (1952). d Brewer et al. (1950). e Sturm (1962). f Jack and Maitland (1957). g Doerner (1937). h Sano (1938b).
I Stout and Chisholm (1962). i Fischer and Gewehr (1935). k Anderson (1937). 1 AUen (1957). m Starr et al. (1940). η Tracy et al. (1961).
ο Klemm and Grimm (1942). Ρ Tracy et al. (1962a). <i. Gregory and Burton (1953). r Allen (1956). s Tracy et al. (1962b). t Shchukarev et al. (1956a).
230 J. Ε. FERGXJSSON
HALIDE CHEMISTRY OF CHROMIUM, MOLYBDENUM AND TUNGSTEN 231
h a s been s t u d i e d a n d evidence for t w o distinct solid solutions CrClg-ajBra., w i t h a l m o s t zero h e a t of mixing, h a s been o b t a i n e d . One solution is b a s e d o n t h e dichloride s t r u c t u r e a n d t h e o t h e r o n t h e d i b r o m i d e (Gregory a n d T r a c y , 1963).
T a b l e I lists some of t h e physical d a t a on t h e dihalides of c h r o m i u m . H i g h e r values for t h e e n t h a l p y of formation of Grig h a v e been recorded b u t t h e v a l u e in t h e t a b l e a p p e a r s t o be t h e m o s t reasonable.
T h e dihalides of c h r o m i u m ( I I ) are v e r y susceptible t o oxidation, especially w h e n wet, a n d t h e y can be used t o r e m o v e t r a c e s of o x y g e n from gas s t r e a m s (Biltz a n d Birk, 1924; A n d e r s o n , 1937; B a l t h i s a n d Bailar, 1939; H e i n a n d B â h r , 1943a). T h e halides b u r n in air t o give t h e c h r o m i u m ( I I I ) oxide. E x c e p t for t h e fluoride t h e y are soluble in w a t e r a n d from t h e solutions, w h i c h are stabilized b y acid, v a r i o u s h y d r a t e s c a n b e isolated. I n t h e case of c h r o m i u m ( I I ) chloride 6, 4, 3, a n d 2 h y d r a t e s can b e isolated a n d readily i n t e r con v e r t e d . T h e b r o m i d e a n d iodide are crystallized as t h e h e x a h y d r a t e s ( L u x a n d I l l m a n n , 1958;
Gmelin, 1962). T h e halides are readily r e d u c e d w i t h h y d r o g e n t o t h e free elements. T h e difluoride is c o n v e r t e d t o t h e trifluoride w i t h fluorine w h e r e a s t h e iodide even a t 300° is only p a r t i a l l y oxidised b y iodine (Jellinek a n d R u d a t , 1928; H e i n a n d B a h r , 1943a). T h e chloride c a n b e oxidised in acid solution w i t h ultraviolet r a d i a t i o n (Collinson et al.,
1959). A c o m p a r i s o n of t h e calculated a n d e x p e r i m e n t a l v a l u e s of t h e e n t h a l p y of f o r m a t i o n indicates a high degree of ionic c h a r a c t e r in t h e chromium-fluorine b o n d s . H o w e v e r , in t h e case of t h e b r o m i d e a n d iodide t h e c o m p o u n d s a r e m o r e s t a b l e t h a n e x p e c t e d , w h i c h m a y b e d u e t o g r e a t e r covalent b o n d i n g (Barber et al., 1961).
T h e dichloride a b s o r b s gaseous a m m o n i a t o give CrClg.^NHg, w h e r e 7^ = 6, 5, 3, 2, a n d 1, a n d t h e dichloride, b r o m i d e , a n d iodide r e a c t w i t h h y d r a z i n e t o give 1:6, 1:4, 1:3, a n d 1:2 a d d i t i o n p r o d u c t s . T h e 1:2 p r o d u c t s a r e p r o b a b l y polymeric (Hein a n d B â h r , 1943b; E a r n s h a w et al., 1964).
B e c a u s e c h r o m i u m ( I I ) iodide readily dissociates i n t o its elements a t 1100-1400° i t h a s been u s e d t o p r e p a r e p u r e c h r o m i u m m e t a l . T h e dissociation is said t o involve t h e C r I radical (Hein a n d W i n t e r - H o l d e r , 1931b; Ageev a n d T r a p e z n i k o v , 1956; E m e l ' y a n o et al.,
1960).
T h e dichloride a n d d i b r o m i d e are dimeric in t h e v a p o u r s t a t e ( 4 4 0 - 700°) a n d h a v e h e a t s of d i m e r i z a t i o n —47·9 kcal dimer"^ a n d —47*0 kcal dimer-^ respectively ( S c h o o n m a k e r et al., 1959).
The m a g n e t i c properties of the c h r o m i u m ( I I ) halides are interesting, especially a t low t e m p e r a t u r e s , w h e r e t h e y b e c o m e a n t i f e r r o m a g n e t i c in b e h a v i o u r . B o t h c h r o m i u m ( I I ) fluoride and chloride h a v e been
232 J. Ε. FERGUSSON
studied a t low t e m p e r a t u r e s a n d n e u t r o n diffraction studies i n d i c a t e some a d d i t i o n a l s t r u c t u r e in t h e diffraction p a t t e r n s a t 4·2°Κ n o t observed a t r o o m t e m p e r a t u r e . T h e p o i n t of a p p e a r a n c e of t h e a d d i tional s t r u c t u r e a p p e a r s t o coincide w i t h a m a x i m u m in t h e specific h e a t curve (at 16·06°Κ for CrClg). T h e effect is p r o b a b l y d u e t o long r a n g e antiferromagnetic i n t e r a c t i o n s via σ-bonding for t h e fluoride, a n d
hg'-ViT overlap for t h e dichloride. T h e Neel t e m p e r a t u r e s for t h e t w o halides a r e 53°K a n d 20°K respectively (Cable et at., 1960). A b o v e t h e t e m p e r a t u r e of t h e specific h e a t anomalies only t h e s h o r t r a n g e effects occur, a n d a t r o o m t e m p e r a t u r e t h e m a g n e t i c m o m e n t s are as e x p e c t e d for a d^-spin-free configuration (Starr et al., 1940; J a c k a n d M a i t l a n d , 1957; S t o u t a n d Chisholm, 1962). T h e d i b r o m i d e a n d di-iodide h a v e h a r d l y been studied, b u t p r o b a b l y show t h e s a m e antiferromagnetic b e h a v i o u r , especially as t h e s t r u c t u r e s of t h e four halides a r e so similar
( K l e m m a n d G r i m m , 1942). T h e h y d r a t e s a r e m a g n e t i c a l l y n o r m a l ( E a r n s h a w et al., 1963, 1965). T h e a n h y d r o u s halides h a v e a d i s t o r t e d o c t a h e d r a l s t e r e o c h e m i s t r y (see below) as a result of a d o u b l y d e g e n e r a t e orbital g r o u n d s t a t e (Jahn-Teller distortion). T h e electronic a b s o r p t i o n spectra can be i n t e r p r e t e d on t h i s basis. T h e a n h y d r o u s chloride h a s a b a n d a t 11,300 c m- i assigned t o ^Eg -> ^T^g, while for t h e solid t e t r a - h y d r a t e CrCl2.4H20 t h e s a m e b a n d h a s been resolved i n t o t h r e e (13,100,
15,200, 18,800 cm"^). T h e difluoride shows a similar splitting of t h e
^Eg -> ^T^g b a n d 11,400 a n d 14,700 cm-^ ( R u n c i m a n a n d S y m e , 1963;
Clark, 1964).
T a b l e I (p. 230) fists t h e C r — X b o n d l e n g t h s of four ' ' s h o r t " b o n d s a n d t w o " l o n g " b o n d s . T h e ' ' l o n g " b o n d s are g r e a t e r ; h a n t h e conven
tional covalent single b o n d length. T h e difluoride is isostructural w i t h copper(II) fluoride a n d s t r u c t u r a l d a t a from n e u t r o n diffraction (at r o o m t e m p e r a t u r e ) a n d single crystal X - r a y analysis are in full accord ( J a c k a n d M a i t l a n d , 1957; Cable et al., 1960).
T h e fluoride CrF2.4 ( S t u r m , 1962) h a s been shown t o b e Cr2F5 (Cr^Tg-Cr^^Ta). T h e s t r u c t u r e of each u n i t is t h e s a m e as t h e individual fluorides. T h e o c t a h e d r a are joined t h r o u g h corners a n d edges (Steinfink a n d B u r n s , 1964). I n t h e dichloride each o c t a h e d r a l u n i t is u n i t e d t o t w o others b y m e a n s of t h e s h o r t Cr—CI b o n d s giving a chain s t r u c t u r e a n d t h e chains are i n t e r l i n k e d b y m e a n s of t h e long Cr—CI b o n d s . These t w o t y p e s of interlinking are p r o b a b l y r e l a t e d t o t h e s h o r t r a n g e a n d long r a n g e antiferromagnetic i n t e r a c t i o n s ( H a n d y a n d Gregory, 1951;
Oswald, 1961; T r a c y et al., 1961). T h e d i b r o m i d e a n d di-iodide are also long ribbon-like m a t e r i a l s ( K l e m m a n d G r i m m , 1942; T r a c y et al., 1962a,b).
HALIDE CHEMISTRY OF CHROMIUM, MOLYBDENUM AND TUNGSTEN 233 (ii) Halide Complexes
T h e complex h a l o g e n o - c h e m i s t r y of c h r o m i u m ( I I ) h a s n o t b e e n s t u d i e d t o a n y g r e a t e x t e n t , a n d a t p r e s e n t is l i m i t e d t o a few fluoro a n d chloro complexes.
T h e complexes Na2CrF4, ( N H 4) C r F 3. 2 H 2 0 , a n d K C r F a h a v e b e e n iso
l a t e d . T h e l a t t e r c o m p o u n d is o b t a i n e d b y t r e a t i n g c h r o m o n s a c e t a t e w i t h p o t a s s i u m h y d r o g e n fluoride. W h e r e a s Na2CrF4 is d e s c r i b e d as being s t a b l e in air, KCrFg is v e r y r e a d i l y oxidized, especially w h e n w e t
( T r a u b e et al, 1925; D e y r u p , 1959, 1964; E d w a r d s a n d P e a c o c k , 1959;
S c a t t u r i n etal, 1961).
B o t h X - r a y p o w d e r p h o t o g r a p h i c a n d n e u t r o n diffraction studies h a v e b e e n carried o u t on t h e p o t a s s i u m salt. T h e former indicates t h a t t h e c h r o m i u m is o c t a h e d r a l l y c o o r d i n a t e d w i t h four C r — F b o n d s a t 2-14 A a n d t w o a t 2-00 Â. T h e result is in accord w i t h simple ligand field t h e o r y , b u t is u n u s u a l i n t h a t t h e distortions a r e t h e opposite t o t h o s e m o s t c o m m o n l y found ( E d w a r d s a n d P e a c o c k , 1959). T h e n e u t r o n diffraction studies i n d i c a t e t h a t t h e crystal consists of ferromagnetic sheets coupled antiferromagnetically. T h i s t y p e of lattice a c c o u n t s for a m a g n e t i c m o m e n t of 4-2 B. T h e s a l t Na2CrF4 h a s a m o m e n t of 5-2 Β ( S c a t t u r i n et al, 1961 ; D e y r u p , 1964). T h e p o t a s s i u m salt also e x h i b i t s a r a t h e r high C r — F s t r e t c h i n g frequency in t h e infrared a t 481 cm*^
(Peacock a n d S h a r p , 1959). T h i s h a s b e e n r e l a t e d t o t h e p a r t i c u l a r d i s t o r t i o n s h o w n b y t h e c o m p o u n d . H o w e v e r , in view of t h e four long b o n d s in t h e s t r u c t u r e of KCrFg it w o u l d a p p e a r t h a t t h e s i t u a t i o n is m o r e complex t h a n indicated.
P h a s e d i a g r a m studies of t h e s y s t e m s CrCl2-NaCl, CrCl2—^KCl, CrCla-CsCl, a n d CrCl2-R'bCl i n d i c a t e t h a t t h e complexes NagCrClg (m.p.
458°, i n c o n g r u e n t ) , KCrClg (495°, 501°), K2CrCl4 (481°, 490°), CsCrClg (718°), Cs2CrCl4 (565°), RbCrClg, a n d Rb2CrCl4 exist (Shiloff, 1960;
G u t a n d G n e h m , 1962; Seifert a n d K l a t y k , 1962, 1964; S h k o F n i k o v a n d Volkov, 1964). A m m o n i u m c o m p o u n d s h a v e also b e e n r e p o r t e d (Gmelin, 1962). T h e crystals of CsCrClg a n d RbCrClg a r e h e x a g o n a l w i t h t h e CsNiClg t y p e s t r u c t u r e (Seifert a n d K l a t y k , 1962, 1964). T h e e u t e c t i c m i x t u r e Cr2+-LiCl-KCl (400-1000°) h a s a b r o a d electronic a b s o r p t i o n b a n d a t 9800 cm~^, consistent w i t h a CrCl4^~ ion in a dis
t o r t e d o c t a h e d r a l e n v i r o n m e n t . T h e a s s i g n m e n t is given as (Gruen a n d M c B e t h , 1962a,b).
C. C h r o m i u m ( i n ) {d^) (i) Halides
A n h y d r o u s conditions a r e necessary t o o b t a i n c h r o m i u m ( I I I ) fluoride, either b y t r e a t i n g c h r o m i u m ( I I I ) c o m p o u n d s w i t h h y d r o g e n fluoride a t
234 J. Ε. FERGUSSON
[CrCl24(OH2)JC1.2H20 [CrCl2(OH2)4]Cl 85«
CrCl3.3H20 ^ ' Q - ^ ' ^ : CrCla Acetylchloride w i l l convert c h r o m i u m ( I I I ) acetate t o t h e trichloride ( W a t t et al., 1955).
C o m m o n impurities i n t h e chloride, such as oxychlorides a n d moisture, can be r e m o v e d b y distillation a t 500° i n a n atmosphere of chlorine a n d carbon tetrachloride (Doerner, 1937).
Similar methods have also been used for anhydrous c h r o m i u m ( I I I ) bromide. B r o m i n a t i o n of t h e m e t a l (800-1000°) gives a product con
t a m i n a t e d w i t h the dibromide ( H e i n et al., 1927a; H a n s e n a n d Griffel, elevated t e m p e r a t u r e s ( W a r t e n b e r g , 1942a; Gmelin, 1962; S t u r m , 1962), or b y prolonged t r e a t m e n t of t h e m e t a l w i t h h y d r o g e n fluoride. E v e n so, t h e l a t t e r reaction does n o t go t o completion (Muetterties a n d Castle, 1961). T h e h e a t i n g of (NH4)3CrP6 vacuo is a successful m e t h o d of p r e p a r a t i o n (Hein et al., 1927a; S t u r m , 1962). T h e h y d r a t e d fluoride is o b t a i n e d readily in a q u e o u s conditions. F o r a n excellent review on t h e h y d r a t e d c h r o m i u m( I I I ) halides see Gmelin (1962). T h e species CrF^^
a n d CrF2+ p r o b a b l y exist in solution (Scheffer a n d H a m m a k e r , 1950;
Wilson a n d T a u b e , 1952).
P r i o r t o 1930 a considerable n u m b e r of p r e p a r a t i o n s of c h r o m i u m (III) chloride h a d been evolved a n d these are reviewed in Mellor (1931).
More recent m e t h o d s are, in general, b a s e d on t h e earlier work. T h e y consist of chlorinating c h r o m i u m( I I I ) oxide w i t h chlorine ( R y a b c h i k o v a n d S h u l ' m a n , 1934; A n n i s et al., 1959; U h l e m a n n a n d F i s c h b a c k , 1963), s u l p h u r monochloride ( F u n k a n d Miiller, 1940; F u n k et al., 1957;
U h l e m a n n a n d F i s c h b a c k , 1963), t h i o n y l chloride, c a r b o n tetrachloride, a n d w i t h a chlorine-carbon t e t r a c h l o r i d e m i x t u r e (Doerner, 1937;
Heisig et al., 1946; B a s m a n o v a , 1959; Vavoulis et al., 1960; U h l e m a n n a n d F i s c h b a c k , 1963). T h e m e t h o d s v a r y from sealed t u b e s t o flow techniques, a t high t e m p e r a t u r e s . T h e reaction b e t w e e n chlorine a n d c h r o m i u m h e x a c a r b o n y l h a s been used (Hieber a n d R o m b e r g , 1935).
Chlorination of t h e m e t a l is n o t a satisfactory m e t h o d (Osthoff' a n d W e s t , 1954; Gmelin, 1962) for t h e p u r e a n h y d r o u s m a t e r i a l .
A useful p r e p a r a t i v e t e c h n i q u e is t o d e h y d r a t e t h e m o r e readily o b t a i n e d h y d r a t e d chloride. T h e r e a g e n t s t h a t h a v e been used a r e 2 , 2 - d i m e t h o x y p r o p a n e (Starke, 1959), t h i o n y l chloride (Hecht, 1947;
Grdenic a n d Gjorgjevic, 1958), carbonyl chloride (Hecht, 1947), a n d d r y h y d r o g e n chloride (Fernandez-Masaguer, 1957) w i t h difi"erent degrees of success. T h e d e h y d r a t i o n w i t h h y d r o g e n chloride can be s u m m a r i z e d b y t h e e q u a t i o n s
HALIDE CHEMISTRY OF CHROMIUM, MOLYBDENUM AND TUNGSTEN 235
1959; Brauer, 1962). The reaction between acetylbromide and chro
mium (III) acetate is a good preparative route as all the products except CrBrg are volatile and can be removed under vacuum (Watt et al., 1955).
Thermal decomposition of the tribromide hexammine complex is said t o give an '^active" form of the anhydrous compound (Hein and Kraft, 1940a). In aqueous H B r the green-violet hydrate [CrBr2(OH2)4]Br.2H20 is formed from chromic oxide.
The tri-iodide has been found difficult to prepare free of the di-iodide.
However, b y careful fractional sublimation of the t w o products obtained b y reacting iodine with chromium metal or with the di-iodide in an atmosphere of iodine a pure product has been obtained. I t is an advan
tage to keep the source of iodine at 225° while the reaction vessel is at 475-500° (Hein and Winter-Holder, 1931b; H a n d y and Gregory, 1950;
Gregory and H a n d y , 1957). Aluminium iodide will react w i t h chro- mium(III) sulphide, but not the oxide, at 350° to give the tri-iodide (Chaigneau and Chastagnier, 1958).
The mixed halides CrICl2, CrIBr2 and CrBrCl2 are prepared from the reaction between CrXg and Y2 at 500°, where Yg is the less reactive of the two halogens. Their structures are based on that of chromium (III) chloride. As y e t thermodynamic and structural data do not rule out the possibility of mixed solutions (Handy and Gregory, 1952a,b).
Table I I lists some of the physical properties of the trihalides. The chemistry of these compounds is vast and only a few aspects bearing directly on the anhydrous halides will be touched on here. Calculated heats of formation indicate that, particularly in the chloride, bromide and iodide, the bonds are predominantly covalent (Barber et al., 1961).
This is confirmed b y the absence of a precipitate of silver chloride from a solution of the chloride and from nuclear quadrupole coupling measurements and studies of the X-ray iT-absorption edge (Stelling,
1940; Barnes and Segel, 1959; Barnes et al., 1962).
The compounds are relatively stable t o heat, the iodide least so.
Chromium(III) chloride has a vapour pressure of 278-2 m m at 1000°
(Jellinek and K o o p , 1929; Wagner and Stein, 1943). I t is monomeric in the gaseous state and at 1300° dissociates to the dichloride and chlorine.
The bromide begins to dissociate at 700° (Hein and Winter-Holder, 1931a; Wagner and Stein, 1943), and the iodide at 500° t o give first the di-iodide and finally the free metal (Basmanova, 1959). The entropy difference between the tervalent and divalent iodides is 10-4 e.u. The heat of formation of chromium(III) fluoride from the difluoride is 157-9 kcal mole-^. The trichloride and tribromide are readily reduced with hydrogen according to the equilibrium reaction 2 CrXg -f Hg
^ 2 C r X 2 + 2 H X (Hein and Winter-Holder, 1931a; Doerner, 1937;
TABLE I I . C h r o m i u m ( I I I ) h a l i d e s C o m - F o r m a t i o n d a t a
p o u n d - Δ ί Γ ° -AG° -AS°
( k c a l m o l e - ^ ) ( k c a l m o l e - ^ ) (e.u.)
>^ 298 (e.u.)
M e l t i n g C o l o u r M a g n e t i c C r — X p o i n t (°C) m o m e n t (B) a t b o n d
r o o m t e m p e r a t u r e l e n g t h (A) CrF3
C r C l g C r B r g C r I ,
130-134a»b,c,h 94, 102i>.i^
48k
250to 1 1 3 - 3 , l l S a . t i
54i>
28-2h 50^
22-4, 25-0b,d 29-4, 28-2rt,i
1100i>
1152i>
g r e e n r e d - v i o l e t 1130^ g r e e n ( e s t i m a t e d )
b l a c k
4-1, 3-85e.f θ = - 1 2 4 133
3 - 6 9 ' Θ = - 1 3 1
3 · 9 4 ί θ - 5 1
4 - 0 3 ' θ = - 7 0
1·90«
2 - 3 8 i
2-57 cl
03
Α
a R o s s i n i et al. ( 1 9 5 2 ) . to B r e w e r et al. ( 1 9 5 0 ) . β v o n W a r t e n b e r g ( 1 9 4 2 a ) . d H a n s e n a n d G r i f f e l ( 1 9 5 8 ) .
e B o z o r t h a n d K r a m m e r ( 1 9 5 9 ) . f H a n s e n a n d G r i f f e l ( 1 9 5 9 ) . δ Κ η ο Ε ( 1 9 6 0 a ) .
^ D o e m e r ( 1 9 3 7 ) .
i A n d e r s o n ( 1 9 3 7 ) . 3 W o o s t e r ( 1 9 3 0 ) .
k G r e g o r y a n d B u r t o n ( 1 9 5 3 ) .
236 J. Ε. FERGUSSON
HALIDE CHEMISTRY OF CHROMIUM, MOLYBDENUM AND TUNGSTEN 237
Sano, 1938a), a n d t h e chloride b u r n s in air t o give CrgOg a n d chlorine (Pechkovskii et al., 1964). T h e calculated b o n d energy of t h e chloride, 84-5 kcal mole-^, is slightly less t h a n t h a t of t h e dichloride (Allen, 1956, 1957). T h e halides are stable in w a t e r b u t will h y d r o l y s e a t h i g h t e m p e r a t u r e s (Domange, 1937). T h e y are all insoluble in w a t e r b u t will dissolve in t h e presence of a little c h r o m o n s ion, or in t h e presence of a reducing a g e n t w h i c h p r e s u m a b l y p r o d u c e s some chro- m i u m ( I I ) . T h e dissolution process m a y involve a charge transfer com
plex of t h e t y p e [Cr2+ · · · X · · · Cr^+ · · · X ] ( H a n d y a n d Gregory, 1950; S t r a a t e n a n d A t e n , 1954; T a u b e a n d K i n g , 1954; Chia a n d K i n g , 1960). Zinc m e t a l also assists t h e dissolving of t h e trichlo
ride in d i m e t h y l s u l p h o x i d e (Schlafer a n d Schafiernicht, 1960).
F r o m a q u e o u s acids t h e halides can b e isolated as h y d r a t e s , cf.
[Cr(H20)6_«,ClJCl3_a. (x = 1, 2, 3). A n i n t e r e s t i n g h y d r a t e of t h e fluoride is [Cr(H20)6]CrF6 w h i c h is p r e p a r e d b y m i x i n g Cr(H20)6Cl3 a n d (NH4)3CrF6 (Birk, 1927). T h e trifluoride will fluorinate c e r t a i n m e t a l oxides a n d chlorides, such as t h o s e of m a g n e s i u m a n d calcium (Mergault a n d D a r m o i s , 1954; M e r g a u l t a n d Laizeau, 1962; M e r g a u l t a n d P i n c e t , 1963; M e r g a u l t a n d T a n n e , 1963). T h e r e a c t i o n b e t w e e n c h r o m y l chloride a n d p h o s p h o r u s p e n t a c h l o r i d e gives a n a d d u c t CrCl3.PCl5, which m a y b e a complex halogeno species (Groeneveld, 1952).
T h e trihalides h a v e b e e n used b y H e i n et al. (1927a, 1959) a n d H e i n a n d K r a f t (1940b) as s t a r t i n g m a t e r i a l s for reactions w i t h organic r a d i cals. H e n c e a considerable knowledge of t h e halides h a s come from t h i s source.
T h e m a g n e t i c p r o p e r t i e s of t h e trihalides are c o m p l e x . R o o m t e m p e r a t u r e m a g n e t i c m o m e n t s a r e given in T a b l e I I . C h r o m i u m t r i fluoride shows a specific h e a t a n o m a l y a t 69·8°Κ a n d t h i s a p p e a r s t o b e associated w i t h a m a g n e t i c t r a n s i t i o n ( H a n s e n a n d Griffel, 1958). Simi
lar t r a n s i t i o n t e m p e r a t u r e s observed for t h e chloride a n d b r o m i d e a r e 16·8°Κ a n d 37·7°Κ respectively ( T r a p e z n i k o v a et al., 1936; A n d e r s o n , 1937). A b o v e t h e s e t e m p e r a t u r e s t h e c o m p o u n d s o b e y t h e Curie-Weiss law. Below t h e m t h e m a g n e t i c b e h a v i o u r h a s b e e n described as ferro
m a g n e t i c b y some (Leech a n d Manuel, 1956b), a n d a n t i f e r r o m a g n e t i c b y o t h e r s (Bizette a n d Terrier, 1962; Cable et al, 1961). I n t h e t r i fluoride, n e u t r o n diffraction studies i n d i c a t e t h a t t h e s t r u c t u r e is basic
ally a n t i f e r r o m a g n e t i c in w h i c h t h e t w o sublattices a r e n o t e x a c t l y aligned, giving rise t o a small ferrimagnetic effect (Wollan et al, 1958).
Also, n o hysteresis w a s observed for t h e fluoride a t 4·2°Κ. I n t h e case of t h e o t h e r halides w h e r e hysteresis for t h e chloride a n d b r o m i d e a r e observed it would a p p e a r , from n e u t r o n diffraction work, t h a t t h e a t o m s w i t h i n t h e layers a r e aligned ferromagnetically a n d t h a t t h e layers are
238 J. Ε. FERGUSSON
coupled antiferromagnetically (Cable et al., 1961). Further discussion of the subject would be inappropriate in this review. Interested readers m a y consult the following references, and references therein, in addition to those given above: Schultz (1940), Leech and Manuel (1956a), Hansen (1959), Tsubokawa (1960), Narath (1961).
The electronic absorption spectra of the fluoride, chloride and bro
mide have been recorded: CrFg, 1460 cm-^ (Ferguson et al., 1961, 1962; Wood et al, 1963), 1610 c m - i (Clark, 1964); CrClg, 1350 c m - i (Clark, 1964), 1370 c m - i (Wood et al, 1963); CrBrg, 1340 c m - i (Wood et al, 1963). The rather different results reported for for the fluoride need resolving. A big difference between the Dq values for the fluoride and chloride does not carry on to the bromide. However, the fit between experiment and theory for the bromide is not good (Wood et al, 1963), probably because of an intense absorption edge at 22,000 c m - i . This is described as being due to the magnetic domain structure of the bromide which diffracts visible radiation so that only on satura
tion does it disappear (Dillon and Remeika, 1963). The detailed struc
ture of a band at 19,000 cm-^ (^^2 ^^2) is said to be related to the magnetic ordering in the compound (Dillon et al, 1962, 1963).
The trichlorides all have a layer structure in which the chromium has octahedral stereochemistry. The chromium-halogen bond lengths are given in Table I I (p. 236). The short chromium-fluorine bond length (0-1 A less than the short bonds of the difluoride) suggests considerable covalent character (Jack and Maitland, 1957; K n o x , 1960). Early work on the trichloride appears to be in error due to difficulties in handling the compound. The Cr—Cr distance between layers is 5·76 A and it is 3-46 A within layers in the chloride (Leech and Manuel, 1956b; Cable et al, 1961). This type of structure will account for the ribbon-like dislocations found in the tervalent chloride (Amelinckx and De- lavignette, 1962). The tri-iodide is isomorphous with the chloride while the bromide is not. However, the bromide has a very similar structure to the chloride.
(ii) Halide Complexes
Of the complex halogeno-anions the fluorides are the most common and fully investigated. Table I I I lists the various halide complexes known.
The usual methods of preparation of the mononuclear species are to fuse the alkali metal halide and chromium(III) halide together, or pre
cipitate the complex from an aqueous acid solution of the chromium halide. The latter method works especially well for large cations where solubilities are favourable. Reduction of chromium(IV) has been used for the fluorides and pyrolysis of CrCl3.3NH3 at 175° gives (NH4)3CrCl6.
TABLE III. Halogeno anions of chromium(III) Anion Cations f Remarks t References [CrFe]3- [CrF^H^OJ^- [CrClel^- [CrClsH^O]^- [CrBr^l^- [CrBr^H^Ol^- [Crl,]2- [CrsFi,]^-
Li, Na, K, Rb, Cs, NH4, Mn, (K^Na) Na, K, Rb, Cs, Tl K, Na, Li, NH4, Rb, Cs, Co(ppd)3, Rh(ppd)3 Li, K, Rb, Cs, NH4 CgHgN Rb Κ j Na NH4, Cs, K, (CA)4N (K) tetragonal /A = 3-79 Β (NH4) cubic (KaNa) cubic = 1610 cm-i Cr—F bond length 1·84-2·01 Â (Rb, Tl) cubic (K) AH° = -466-1 kcal mole-i (Na) AH°f = -415-7 kcal mole-i Co(ppd)3 /X = 3-87 Β Rh(ppd)3 = 1318 cm-i with [ACle]^- (A = Mn, Fe, In) (K) also reported as KaCrClg isomorphous Have not been studied in recent years (Cs) μ = 3-82 Β (Κ) ΑΗ° = -568 kcal mole-i (Κ and Cs) isomorphous
1-10, 28 2, 4, 8, 11-13 14-20 21-23 21 24 16, 17a, 18, 25-27 t (ppd) = l,2-propanediamine. 1. Insley et al. (1956). 2. Talipov and Antipov (1952). 3. Peacock (1957a). 4. Talipov and Krukovskaya (1958). 5. Ryss and Vitukhnovskaya (1958). 6. Knox and Mitchell (1961). 7. Sturm (1962). 8. Passerini and Pirani (1932). 9. Nyholm and Sharpe (1952). 10. Wood et al. (1963).
11. Talipov and Fedorova (1953). 12. Sandermann et al. (1954). 13. Pirani (1932). 14. Shkol'nikov and Valkov (1962). 15. Korshunov and Raskin (1962). 16. Cook (1963). 17. Vasil'kova et al. (1964a, b). 18. Schlesinger and Worner (1929). 19. Efimov and Pitirimov (1963). 20. Hatfield et al. (1963).
21. Gmelin (1962). 22. Krauss et al. (1963). 23. Dubsky and Wagenhofer (1936). 24. Knox and Gellier (1958). 25. Wessel and Ijdo (1957). 26. Earnshaw and Lewis (1961). 27. Adams et al. (1963). 28. Bode and Voss (1957).
HALIDE CHEMISTRY OF CHROMIUM, MOLYBDENUM AND TUNGSTEN 239
240 J. Ε. FERGUSSON
Further pyrolysis at 270° gives the dinuclear species
(NH4)3Cr2Cl9
(Schlesinger and Worner, 1929), which can also be obtained from melts and by using non aqueous solvents (Wessel and Ijdo, 1957; Earnshaw and Lewis, 1961; Adams et al, 1963a; Cook, 1963).
The mononuclear fluoro complexes are green in colour while the chloro complexes are either green or red-violet. They are soluble in water and aqueous acids—^the chloro more so than the fluoro complexes.
The monohydrated fluoro species retain their molecule of water up to 140-150° (Talipov and Krukovskaya, 1958). The stability of the CrClg'- anion appears t o decrease with increasing cation size and the reverse appears to be true for the dinuclear chloro species (Vasil'kova et al, 1964b).
The magnetic moments (Table III) of the mononuclear anion CrXg^- and the electronic absorption spectra are consistent with a spin-free cZ^-conflguration in an octahedral field (Harrington and Sundheim, 1960; Gruen and McBeth, 1962a). The greater value for KaNaCrFg compared with CrFg has been related to the influence of next nearest neighbours (Wood et al, 1963). The crystal field energy is also less in the chloro anion than the fiuoro anion, and the difference m a y be due to increased repulsive effects between the chlorine atoms (Hatfield et al, 1963). A reduction in the Β parameter from 920 to 760-740 cm-^ for the fiuoro anion indicates orbital expansion and possible covalent bond
ing (Wood et al, 1963).
Hardly any structural data are available on the mononuclear species (Table III). Cr—F stretching at 535 and 522 cm*^ has been reported in the infrared (Peacock and Sharp, 1959).
The polynuclear species
Na5Cr3Fi4
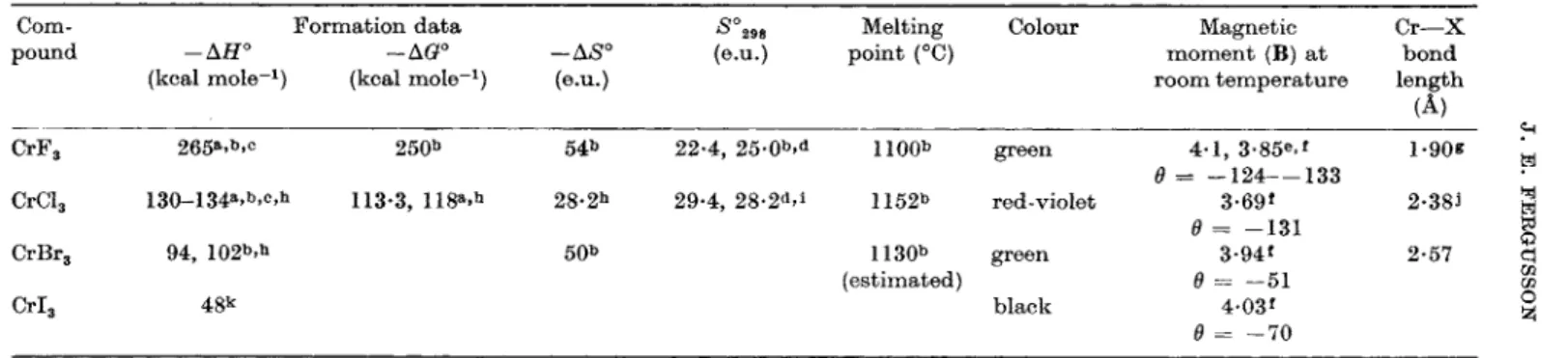
is unexpected with the high charge associated with the anion. Its magnetic moment is reported as low at 20°K (Knox and Gellier, 1958). However, in the anion CrgClg^- the magnetism is normal, indicating no metal-metal interaction (Wessel and Ijdo, 1957; Earnshaw and Lewis, 1961), which is in agreement with a Cr—Cr internuclear distance of 3-12 A (Cr—Cr in metal is 2-5 A) (Wessel and Ijdo, 1957). The stereochemistry of each chromium is octahedral, each octahedron sharing a face, as shown in Fig. 1. The chro
mium atoms are not quite at the centres of the octahedra as there are t w o Cr—CI bond lengths, viz. 2-52 and 2-34 Â. The similar complex anion of tungsten (see below) is, however, diamagnetic, with a short metal-metal bond. The infrared spectrum of CrgClg^- has two bands which have been assigned as follows: 341 cm"^ (Cr—CI stretch), 322 c m - i (Cr—CI—Cr bridge) (Adams et al, 1963a). A band at 316 cm-^
for chromium(III) chloride is also attributed to ν (Cr—CI) bridge (Clark, 1965).
HALIDE CHEMISTRY OF CHROMIUM, MOLYBDENUM AND TUNGSTEN 241
FIG. 1. T h e configuration of t h e anion AgCl^^- (Cr, W ) .
(iii) Oxyhalides
T h e r e a p p e a r s t o b e only one definitely c h a r a c t e r i z e d o x y h a l i d e of c h r o m i u m ( I I I ) CrOCl, t h o u g h o t h e r s h a v e b e e n r e p o r t e d .
D a r k olive green C r O F a n d NagCrOFg h a v e b e e n r e p o r t e d b u t further investigations a r e n e e d e d t o confirm t h e i r existence ( W a r t e n b e r g ,
1942a; Welch, 1944).
T h e green CrOCl h a s b e e n r e p o r t e d as t h e p r o d u c t of t h e r e a c t i o n b e t w e e n CrClg a n d CrgOg w i t h i n t h e t e m p e r a t u r e g r a d i e n t 840-1040°.
O t h e r oxides t h a t h a v e b e e n used are BigOa, TiOa, SiOg a n d HgO. T h e c o m p o u n d is r e m a r k a b l y stable, r e s i s t a n t t o w a t e r , acids, alkalis, a n d air a t r o o m t e m p e r a t u r e . F o r m a t i o n d a t a a r e AH^ = —139 kcal mole-^
a n d a s ; = 16-3 e.u. a t 298°K (Schafer a n d W a r t e n p f u h l , 1961).
T h e c o m p o u n d h a s a layer s t r u c t u r e w i t h t h e - C r — 0 — C r — 0 - layers s e p a r a t e d b y d o u b l e sheets of chlorine a t o m s . T h e Cr—Ο b o n d l e n g t h is 1-97-2-01 Â a n d Cr—CI is 2-32 A (Forsberg, 1962). T h e Cr—Cr d i s t a n c e is 3-04 A, i n d i c a t i n g n o m e t a l - m e t a l i n t e r a c t i o n , as confirmed b y a m a g n e t i c m o m e n t of 3-9 Β (^ = 20°) a t 295°. T h e Curie-Weiss law is o b e y e d (Schafer a n d W a r t e n p f u h l , 1961).
T h e r m a l d e c o m p o s i t i o n of t h e h y d r a t e s of c h r o m i u m ( I I I ) chloride also gives t h e oxychloride according t o t h e e q u a t i o n s :
[ C r ( H 2 0) 6 ] C l 3 C r ( H 2 0) C l 3 C r ( 0 H ) C l 2
100-250°
2 5 0 - 3 3 0 ° 3 4 0 - 4 0 0 °
C r ( H 2 0) C l 3 -f 5 H 2 O C r ( 0 H ) C l 2 + H C l
CrOCl + H C l (Cueilleron a n d H a r t m a n s h e n n , 1959; K i r a l y et al,, 1959).
T h e c o m p o u n d Cr20Cl4.5H20 h a s b e e n r e p o r t e d b u t n o t confirmed (Chatelet a n d Chatelet, 1934; Cueilleron a n d H a r t m a n s h e n n , 1959).
242 J. Ε. FERGUSSON
D. Chromium(IV) (</') (i) Halides
T h e t e t r a v a l e n t s t a t e of c h r o m i u m is poorly characterized as r e g a r d s its halogen chemistry. Only t h e tetrafluoride a n d tetrachloride defi
nitely exist.
T h e fiuoride is p r e p a r e d w h e n t h e u n u s u a l m i x t u r e of c h r o m i u m m e t a l , c h r o m i u m ( I I I ) chloride a n d fluoride are t r e a t e d w i t h fluorine.
Some pentafluoride is also formed in t h i s process ( W a r t e n b e r g , 1941).
T h e reaction b e t w e e n c h r o m i u m ( I I I ) chloride a n d chlorine gas a t 600°
gives t h e t e t r a c h l o r i d e (Doerner, 1937; W a r t e n b e r g , 1942b). I t also m a y form a t 900° in t h e s y s t e m NaCl-CrClg (Cook, 1963).
T h e r m o d y n a m i c d a t a on t h e halides are given in T a b l e I V . T h e com
p o u n d s are readily h y d r o l y s e d b y w a t e r . W h e r e a s t h e fluoride is stable t o h e a t e v e n a b o v e its melting p o i n t (Brewer et al., 1950), t h e t e t r a chloride decomposes t o t h e trichloride a n d chlorine on w a r m i n g from
—78°. H o w e v e r , it is stable as a gas in t h e presence of chlorine (Doerner, 1937; W a r t e n b e r g , 1942b). T h e c h r o m i u m ( I V ) fluoride is also r e m a r k a b l y stable t o chemical reagents. BrPg a n d S e P 4 will reduce it t o lower fluorides a n d a m i x t u r e of BrPg a n d BrFg gives a n oxyfluoride CrOFg.
0-25 BrFg (Clark a n d S a d a n a , 1963).
TABLE I V . C h r o m i u m ( I V ) h a l i d e s C o m p o u n d F o r m a t i o n d a t a
~ A H - A G
(kcal mole-^) (kcal mole"^)
- A S
(e.u.)
Melting p o i n t
(°C)
Colour
CrF^ 286-5^ 65a 277a b r o w n
(estimated)
CrCl4 98-8 (g)b 91-6 ( g p - 2 8 a b r o w n gas
104 (g)e
110 {ψ
a Brewer et al. (1950). ^ Doerner (1937). c Rossini et al. (1952).
I n view of t h e low t h e r m a l stability of t h e t r i b r o m i d e a n d tri-iodide of c h r o m i u m , t h e formation of t h e t e t r a v a l e n t c o m p o u n d s seems u n likely (Brewer et dl., 1950). H o w e v e r , evidence h a s been given for t h e m b o t h as gases w h e n t h e conditions are such t h a t t h e d i v a l e n t halides can
n o t decompose t o t h e m e t a l a n d a high pressure of t h e free halogen is used (Sime a n d Gregory, 1960; T u m a r e v a n d P a n y u s h i n , 1962).
(ii) Complex halides
Complex halogeno anions occur only for fluorine as CrFg^" (K, R b , Cs) a n d CrFg- (K, R b ) (Clark a n d S a d a n a , 1963). T h e former are t h e b e t t e r characterized. F l u o r i n a t i o n of a m i x t u r e of p o t a s s i u m chloride a n d
HALIDE CHEMISTRY OF CHROMIUM, MOLYBDENUM AND TUNGSTEN 243
c h r o m i u m ( I I I ) chloride gives KgCrPe w h i c h dissociates a t 300° t o give KgCrPe a n d c h r o m i u m ( V ) fluoride (Huss a n d K l e m m , 1950). T h e c h r o m i u m ( I V ) c o m p o u n d s a r e flesh-coloured a n d h y d r o l y s e readily in w a t e r . KgCrFg h a s a m a g n e t i c m o m e n t of 2-8 B, i n d i c a t i n g a spin-free
^^-configuration ( K l e m m , 1954, 1956).
T h e p o t a s s i u m salt is i s o m o r p h o u s w i t h KgMnFg, a n d t h e h e x a g o n a l Κ a n d R b salts c o n v e r t t o t h e cubic s t r u c t u r e of CsgCrPg on h e a t i n g . I t a p p e a r s t h a t t h e larger t h e cation t h e higher t h e crystal s y m m e t r y (Huss a n d K l e m m , 1950; B o d e a n d Voss, 1956).
T h e r e d o n o t a p p e a r t o be a n y oxyhalides of c h r o m i u m ( I V ) k n o w n . E. Chromium(V) (d')
(i) Halides
C h r o m i u m ( V ) fiuoride is t h e only p e n t a h a l i d e k n o w n . I t h a s b e e n o b t a i n e d b y fluorination of (a) c h r o m i u m m e t a l , c h r o m i u m ( I I I ) chloride a n d fluoride m i x t u r e ( W a r t e n b e r g , 1941), (b) c h r o m i u m m e t a l in a nickel a u t o c l a v e a t 400° a n d 200 a t m ( E d w a r d s , A. J . , 1963; Glemser et al., 1963), (c) a m i x t u r e of p o t a s s i u m a n d c h r o m i u m ( I I I ) chlorides, a n d (d) b y fluorination of c h r o m i u m ( V I ) oxide ( W a r t e n b e r g , 1941).
D i s p r o p o r t i o n a t i o n of t h e complex KgCrFg also gives t h e pentafluoride (Huss a n d K l e m m , 1950). T h e c o m p o u n d c a n b e p r e p a r e d o n l y using d r y m e t h o d s , as it is r e a d i l y h y d r o l y s e d a n d it is h a r d t o purify from last traces of m o i s t u r e . T h e crimson r e d solid m e l t s a t 30° a n d boils a b o u t 117° w i t h considerable d i s p r o p o r t i o n a t i o n . A n e s t i m a t e d e n t h a l p y of f o r m a t i o n is —290 kcal m o l e - ^ (Brewer et al., 1950).
T h e r e d o n o t a p p e a r t o b e a n y c h r o m i u m ( V ) halogeno-anions. Cer
t a i n l y t h e i r f o r m a t i o n would r e q u i r e rigorous exclusion of m o i s t u r e . (ii) Oxyhalides
T h e oxyhalides of c h r o m i u m ( V ) are CrOFg a n d CrOClg. T h e former is p r e p a r e d b y t r e a t i n g c h r o m i u m ( V I ) oxide w i t h either BrFg or CIF3 a t r o o m t e m p e r a t u r e . I t c a n n o t be entirely purified from t h e r e a c t i n g fluoride, which p r o b a b l y is a t t a c h e d as a solvate molecule, CrOF3.0-25 BrFa (Clark a n d S a d a n a , 1963, 1964). T h e t r e a t m e n t of either chro- m i u m ( V I ) oxide or c h r o m y l chloride w i t h t h i o n y l chloride, s u l p h u r y l chloride or b o r o n ( I I I ) chloride a t low t e m p e r a t u r e s gives t h e o x y chloride CrOClg ( K r a u s s a n d Muenster, 1962; K r a u s s et al., 1963;
J o h a n n e s e n a n d K r a u s s , 1964). B o t h c o m p o u n d s h y d r o l y s e readily a n d t h e chloride, which is a m o n o m e r in n i t r o b e n z e n e , is s t a b l e only a t low t e m p e r a t u r e s in t h e absence of light. A b o v e 0° it d i s p r o p o r t i o n a t e s t o Cr(VI) a n d C r ( I I I ) . T h e m a g n e t i c m o m e n t s of t h e t w o c o m p o u n d s lie in t h e r a n g e 1-80-2-02 B, consistent w i t h a (i^-conflguration ( K r a u s s et al., 1963; Clark a n d S a d a n a , 1964).
244 J. Ε. FERGUSSON
C o m p o u n d μ{Β) θ R e f e r e n c e s
K[ C r O F 4 ] 1-76 ( 2 9 3 ° K ) + 4 1
p y H[ C r O C l 4 ] 1-77 ( 2 8 9 · 5 ° Κ ) 2
[ C, H 3 N] [ C r O C l J 1-82 - 7 3
K^ECrOCl^] 1-92 - 1 4 3
[•NJÎMCTOOk] 1-82 4
R b a C C r O C l s ] 1-83 ( 2 9 0 ° K ) 0 2, 5
1-69 ( 2 9 5 ° K ) t
C s ^ L C r O C l s ] 1-80 - 1 4 6
t L o w v a l u e d u e t o c h r o m i u m ( I I I ) i m p u r i t y ?
1. N y h o l m a n d S h a r p e ( 1 9 5 2 ) . 4 . B a r r a c l o u g h et al. ( 1 9 5 9 ) . 2. T j a b b e s ( 1 9 3 2 ) . 5. H a r e et al. ( 1 9 6 2 ) . 3. K r a u s s et al, ( 1 9 6 3 ) . 6. B r o w n (1964).
T h e complex oxyhalogeno anions of chromium(V) a r e of t w o t y p e s , C r O X ^ - a n d CiOCh^- ( X - F a n d CI). T h e Κ a n d Ag salts of C r O F ^ - are k n o w n a n d a r e o b t a i n e d b y reducing t h e a p p r o p r i a t e d i c h r o m a t e salt w i t h either b r o m i n e trifluoride or chlorine trifluoride (Sharpe a n d Woolf, 1951; Clark a n d S a d a n a , 1964). A c o m p o u n d K C r O g F g . H a O w a s r e p o r t e d a n u m b e r of y e a r s ago (Gmelin, 1962).
T h e t w o series of oxychloride complexes MgiCrOClg] (M = K , R b , Cs, NH4 a n d organic bases, e.g. dipyridyl) a n d M[ C r O C l 4 ] (M = p y r i d i n i u m , quinolinium, etc.) a r e p r e p a r e d b y reducing c h r o m i u m ( V I ) w i t h h y d r o g e n chloride i n acetic acid ( H a r e et al,, 1962; K r a u s s et al,, 1963).
T h e p r o b a b l e r e d u c t i o n steps a r e :
CrOg + 2HC1 ~ ^ ° > CrO^Cla + H^O
ΟνΟΆ + 3HC1 -^J;^,^ > H( C r O C l 4 ) + H^O + iCl^
H( C r O C l 4 ) + H C l ^ H 2 ( C r O C l 5 ) H 2 ( C r O C l 5 ) + 2HC1 H 2( C r( H 2 0 ) C l 5 ) + Cl^
A d d i t i o n of t h e cation a t t h e a p p r o p r i a t e t i m e gives t h e c o m p o u n d required. H o w e v e r , t h e CrOClg^" anion is best p r e p a r e d using acetyl chloride in chloroform ( K r a u s s et al., 1963).
T h e oxyfluoro a n d chloro c o m p o u n d s a r e readily h y d r o l y s e d in moist air. T h e m a g n e t i c properties of t h e complexes a r e in accord w i t h a cZ^-configuration (Table V ) . Because of t h e strong C r= 0 b o n d t h e complex ion CrOClg^" should show a m a r k e d t e t r a g o n a l distortion. T h e electronic a b s o r p t i o n s p e c t r u m of (NH4)2CrOCl5 h a s been recorded a n d t h e limited d a t a is consistent w i t h a m o l e c u l a r orbital description of t h e
TABLE V . M a g n e t i c p r o p e r t i e s o f C r ( V ) o x y h a l i d e c o m p l e x e s
HALIDE CHEMISTRY OF CHROMIUM, MOLYBDENUM AND TUNGSTEN 245
s t r u c t u r e . Discussion of t h i s will b e delayed u n t i l t h e corresponding m o l y b d e n u m c o m p o u n d is described (Gray a n d H a r e , 1962). A C r — 0 stretching frequency for t h e ion CrOClg^- is r e p o r t e d a t 952 cm"^ in t h e infrared (Barraclough et al., 1959; B r o w n , 1964).
T h e r e a p p e a r t o be n o o x y b r o m o or oxyiodo c o m p o u n d s of p e n t a - v a l e n t c h r o m i u m .
F . Chromium(VI) (rf") (i) Halides
C h r o m i u m ( V I ) fluoride is described as a n u n s t a b l e lemon-yellow c o m p o u n d o b t a i n e d d u r i n g t h e fluorination of c h r o m i u m m e t a l in a nickel a u t o c l a v e a t 400° a n d 200 a t m o s p h e r e s . A higher pressure a n d t h e a d d i t i o n of a little m a n g a n e s e increases t h e yield. T h e c o m p o u n d decomposes b e t w e e n —100° a n d —80°, according t o t h e e q u a t i o n CrFe -> CrFs + (Glemser et al., 1963).
(ii) Oxyhalides
P u r e chromyl fluoride (m.p. 31*6°) h a s only b e e n o b t a i n e d i n r e c e n t y e a r s (Engelbrecht a n d Grosse, 1952). I t h a s been r e p o r t e d m a n y t i m e s , b u t all pre-1950 r e p o r t s were of p r o d u c t s c o n t a m i n a t e d w i t h t h e fluori
n a t i n g a g e n t , either e l e m e n t a r y fluorine or h y d r o g e n fluoride. T h e i m p u r i t i e s g a v e rise t o t h e observations t h a t t h e c o m p o u n d w a s u n s t a b l e , undergoing polymerization, a n d t h a t i t w a s a gas a t n o r m a l t e m p e r a t u r e s ( W a r t e n b e r g , 1941). I t can be p r e p a r e d p u r e b y t r e a t i n g c h r o m i u m ( V I ) oxide w i t h excess h y d r o g e n fluoride from which it can b e recrystallized as violet-red crystals (Engelbrecht a n d Grosse, 1952).
O t h e r fluorinating a g e n t s t h a t h a v e been used a r e iodine pentafluoride (Aynsley et al., 1953), c o b a l t ( I I I ) fluoride (Flesch a n d Svec, 1958), selenium tetrafluoride ( B a r t l e t t a n d R o b i n s o n , 1961), a n d fluoro- sulphonic acid. O t h e r m e t h o d s are t o r e a c t p o t a s s i u m d i c h r o m a t e w i t h h y d r o g e n fluoride a n d fluorinate c h r o m i u m m e t a l w i t h a m i x t u r e of p o t a s s i u m n i t r a t e a n d h y d r o g e n fluoride (Wiechert, 1950), or w i t h n i t r y l fluoride (Aynsley et al., 1954). F l u o r i n a t i o n of t h e m e t a l is also said t o give CrOF4, a d a r k r e d solid melting a t 55° ( E d w a r d s , A. J . , 1963).
T h e p r e p a r a t i o n of chromyl chloride h a s been extensively studied.
T h e usual q u a l i t a t i v e t e s t for chloride ions involving t h e reaction be
t w e e n a c h r o m a t e , c o n c e n t r a t e d sulphuric acid a n d chloride ions can b e employed on a p r e p a r a t i v e scale (Sisler, 1946a; V a k h r u s h e v , 1957).
T h e various chlorinating a g e n t s acting on c h r o m i u m ( V I ) oxide t h a t h a v e b e e n used a r e : p h o s p h o r u s p e n t a c h l o r i d e , chlorosulphonic acid, t h i o n y l a n d s u l p h u r y l chloride, a n d a l u m i n i u m trichloride ( F r e e m a n a n d R i c h a r d s , 1958; K r a u s s a n d Muenster, 1962). Puriflcation from
![TABLE III. Halogeno anions of chromium(III) Anion Cations f Remarks t References [CrFe]3- [CrF^H^OJ^- [CrClel^- [CrClsH^O]^- [CrBr^l^- [CrBr^H^Ol^- [Crl,]2- [CrsFi,]^-](https://thumb-eu.123doks.com/thumbv2/9dokorg/1130783.80156/13.658.97.564.93.903/table-halogeno-chromium-cations-remarks-references-crclel-crclsh.webp)

![TABLE XXVH. Oxyfluoride complexes of molybdenum and tungsten(VI) Anion Metal Cations [A04F]«-Mo\ W Na, K, Rb, Cs [AOaFa]^-Mo W K, Rb, Cs, NH4 [AOaF^]^-Mo W K, NH4, Ni, H Κ [AO3F]-W NH^ [AO^F,]^- [AO^FJ^-Mo Mo\ W ) NH4 wide variety [AO2F3]- [AOF,](https://thumb-eu.123doks.com/thumbv2/9dokorg/1130783.80156/58.658.135.546.87.880/table-oxyfluoride-complexes-molybdenum-tungsten-anion-cations-variety.webp)