CHAPTER I
Introduction
Nearly a half a century ago, quantitative organic microanalysis which deals with samples ranging in size from one to 10 mg. (1 mg. = 1 0 -3 gram) was de
veloped as a means of both convenience and necessity by Fritz Pregl, a physi
cian, who was the medical examiner of the city of Graz, Austria. While work
ing on bile acids, he obtained crystalline degradation products in quantities too small to be analyzed by the existing methods. Two alternatives were his, either to work for several years repeating his experiments on a much larger scale or to adapt the existing methods to the milligram quantities he had on hand. He chose the latter. He became so interested in this new field that he never returned to the work on the bile acids, but devoted his entire time to the development and teaching of microanalysis. Advanced students of chem
istry from all parts of the world went to Graz to learn the microtechniques.
For his contribution to science, Pregl received the Nobel Prize in 1 9 2 3 .1 4 Arti
cles relating to Pregl, as well as the roles played by Friedrich Emich and others in the development of this field have been published and to these the reader is r e f e r r e d .2 8'6 5-9 6-1 5 9 Early events of importance in the development of micro
chemistry are shown in Table 1, while Table 2 shows the approximate size of the samples used for various determinations.
T A B L E 1
E A R L Y W O R K O F IMPORTANCE IN T H E D E V E L O P M E N T O F MICROCHEMISTRY
Date Author Subject matter
1867 T. G. W o r m l e y2i 4 "Microchemistry of Poisons"—book 1903 W . Nernst, and
Ε. H. RiesenfeldH7,ii8
Determination of atomic weights
1904 G. B a r g e r1 7 Determination of molecular weights of organic compounds
1909 F. E m i c h ^ "5 2 Determination of halogens and sulfur by the Carius method
1911 F. E m i c h5° , 5 i Determination of nitrogen by the Kjeldahl method 1911 J . D o n a u4 7 Recommended use of the Kuhlmann microchemi
cal balance. Previous to this, the Nernst microbalance was used.
1911 F. Preglise Quantitative organic microanalysis was developed through the use of the Kuhlmann microchemi- cal balance
1
TABLE 2
S A M P L E SIZE FOR VARIOUS D E T E R M I N A T I O N S2 4'2 5-4 4'6 1 , 6 2 , 1 1 9 , 1 2 0 , 1 3 6 , 1 4 4 - 1 4 7 , 1 7 4
Determination Sample size (mg.)
Carbon (manometric) 1- 5
Carbon-hydrogen 4 - 6
Nitrogen (Dumas or Kjeldahl) 1- 6
Halogens 5 - 8
Sulfur 2 - 8
Oxygen 3 -10
Phosphorus 4 - 6
Metals 4 - 6
Groups 5 - 8
Modifications of the original methods have since been named semimicro- analysis4 3 (milligram procedures using sample sizes upwards of 10 mg.) and ultramicroanalysis7 7 (samples sizes down to 1 0 ~6 gram). The present t r e n d ,3 7'3 8'1 0 5'1 7 7 however, is to substitute these terms with more descriptive ones such as, ( a ) gram methods for the so-called macro- or classical; ( b ) centigram for the present semimicro-; ( c ) milligram for micro-; ( d ) microgram for ultramicro-; ( e ) nanogram for submicro- (samples down to 1 0- 9 gram) and; ( f ) picogram for subultramicro- (samples down to 1 0- 1 2 g r a m ) . Like
wise, the terms gamma ( γ ) , and lambda (λ) are to be substituted with micro
gram ^ g . ) and microliter (μΐ.) respectively as follows:
1 microgram = 1 μg. — 0.001 mg. = 1 0- 6 gram 1 microliter = 1 μΐ. = 0.001 ml.
Recommendations, Collaboration, and Standardization With the acceptance of microchemical methods by industry, it became necessary for others than those trained by Pregl, or his students, to perform this type of work and to eventually train assistants and/or students. Due to the very nature of the work, the beginner must have correctly proportioned apparatus and specific directions for performing the work, particularly methods known to be successful in the hands of those with comparatively little experience. At least as far as apparatus is concerned, the above is of equal importance to the experienced microanalyst. Recognizing these needs, several groups have been actively engaged in work along these lines.
The initial sources of supply of equipment were from Europe and the various pieces received were not always interchangeable. The American manu
facturers had no experience along these lines and no specifications to act as a guide. In 1937, the Division of Microchemistry (later known as the Division
3 Recommendations, Collaboration, and Standardization
of Analytical and Micro Chemistry and now the Division of Analytical Chem
istry) of the American Chemical Society, appointed the Committee for the Standardization of Microchemical Apparatus, composed of experienced micro- chemists whose purpose "was to investigate the wide variations in dimensions of various common pieces of apparatus which are employed in organic micro
analysis and to suggest to the Division recommended spécifications for such apparatus in so far as it seems practical to do s o . "1 5 0 This action came at a very opportune time inasmuch as European supplies were cut off soon after
wards due to World W a r II. The committee concentrated on the apparatus for the determinations most widely performed and in 1941 was able to recom
mend specifications for the pieces used in the carbon-hydrogen and Dumas nitrogen set-ups.1 4 8'1 5 0 Two years later, recommendations were published for those pieces used in connection with the determinations of halogens and sulfur by the catalytic combustion method.1 4 9 This committee then ceased to function.
After the end of World W a r II, attention again was called to the need for continued work along these lines, and in 1947, the Division of Analytical and Micro Chemistry (now the Division of Analytical Chemistry) of the American Chemical Society appointed a new Committee for the Standardiza
tion of Microchemical Apparatus (now the Committee on Microchemical Ap
paratus). The new committee declared its intentions to be the following3 6-1 8 2: 1. Where needed, to revise the recommendations of the former com
mittee.
2. To recommend specifications for other items of quantitative micro-, semimicro-, and ultramicroapparatus and finally of the qualitative field.
3. All recommendations were to be made with the understanding that the specifications represented the best thought at that time and that revisions would be made when necessary. Also that primary consid
eration would be given to glass apparatus.
The new committee has been very a c t i v e1 6 3'1 7 5"1 8 4 in successfully carrying out its original intentions as shown in the following respective chapters. All recommendations have been made only after considerable experimental work by members of the committee and cooperating chemists. (Alber5 in an address to the First International Microchemical Congress at Graz, Austria, in 1950, discussed the general subject of standardization in the United States in progress up to that time.) The British Standards Institution's accomplishments3 5 have been along the same lines and have been of great value to the British manu
facturers. The Division of Analytical Chemistry of the American Chemical Society also has functioning the Committee on Balances and Weights which makes recommendations along these l i n e s .1 0 3-1 4 0
The American Chemical Society does not sponsor standardization to the extent that the recommended specifications of the Committee on Microchemical Apparatus could become "standards" instead of recommendations. Therefore, this committee now also functions as the nucleus of Subcommittee No. 29 on Microchemical Apparatus of Committee E-l on Methods of Testing of the American Society for Testing Materials. This subcommittee reviews the recom
mended specifications a year or more after these have been published in Analytical Chemistry. In this interval, if changes in dimensions or design have been found necessary, which is rarely the case, they are made. These, together with the recommendations not needing revision, are incorporated into a report to the American Society for Testing Materials and these specifications are adopted as tentative specifications of this Society.1 3 Where such specifications exist, they are included in the following respective chapters.
The Association of Official Agricultural Chemists has been sponsoring a
program of collaborative s t u d i e s2'4 1-4 2'1 2 4"1 2 9'1 6^ on microchemical methods for a number of years in efforts to develop methods
with which good results can be obtained by relatively inexperienced micro- analysts who carefully adhere to the directions. For the first year of each study, samples are submitted to a number of collaborating chemists who analyze the substances by whatever methods they normally employ and report all values obtained. The chemists also provide detailed descriptions of their procedures.
Statistical comparisons are then made between the values obtained by the various methods to determine which one is the most accurate and precise. Similarly, comparisons are made to determine whether or not variations in procedure pro
duce significant differences in results. For the second year's study, all the collaborators are requested to analyze the same samples by a specific procedure based on that method which gave the best results the first year. I f the returns justify such action, the method is adopted as "First Action," and then eventu
ally as "Official." In the event that the method does not prove satisfactory, additional studies are conducted. Those in charge of these collaborative studies are also members of the Committee on Microchemical Apparatus and of Sub
committee No. 29 so that each group has the advantage of the experiences of the others, and the programs are so arranged. The work accomplished is covered in the following respective chapters.
The Commission on Microchemical Techniques of the Section of Analytical Chemistry of the International Union of Pure and Applied Chemistry is now actively engaged in recommending test substances for determining the applica
bility of apparatus and methods for the various determinations when dealing with different types of structures.4 5 This is covered in detail in the section of this chapter dealing with test substances and is mentioned here only for the sake of complete coverage of the general subject under discussion.
5 Blank Tests and Corrections
A great deal of time, effort, and expense is involved in these programs on the part of all parties involved—those conducting the work and those collaborat
ing, both chemists and manufacturers of laboratory apparatus. In spite of this, the benefit to the beginner, the experienced analyst, and the manufacturers is so great that these programs will be continued indefinitely and the reader is advised to follow the literature dealing with these.
The author is a strong advocate of all collaborative work, as shown by his affiliation for years with each of the above-mentioned programs and many of the methods and pieces of apparatus recommended in the following chapters are based on such collaborative efforts.
Blank Tests and Corrections
Entire determinations carried out in the absence of the sample are called blank tests.* The same term applies if a pure sample is used which does not contain the element(s) or group to be determined; for example, a Dumas determina
tion done on benzoic acid, which more nearly duplicates the conditions of the determination than if no sample is used. Blank tests show the amount of error resulting from contamination of the reagents used or from faulty ap
paratus. The volume of standard solution consumed or amount of end product (gas or precipitate) obtained during a blank test is applied as a correction for actual determinations.
Although blank tests have been recommended in connection with the carbon-hydrogen determination, the author does not agree with this practice since a definite hydrogen value will always be obtained due to dehydration of the lead dioxide portion of the combustion tube filling, as explained in the chapter in question. A test sample provides the necessary information as to whether or not the carbon-hydrogen train gives satisfactory results. Except for the few determinations, such as the manometric carbon, that specificallyf call for blank tests, the author prefers to use these only as a means of determining the cause of poor results and then eliminate this cause, if possible, before pro
ceeding with samples. For example, if high nitrogen results are obtained on a test sample by the Kjeldahl method, blank tests on the various individual reagents will show which o n e ( s ) is at fault. Blank values should be applied only if a number of blank tests show that a constant error is present and all attempts to correct the condition have failed. In the opinion of the author, this is an emergency measure rather than a practice that should be encouraged.
* The number of references to the use is too great to list here so that only a few are c i t e d .4 4'6 1'6 2»1 1 9'1 2 0'1 3 6-1 4 4 - 1 4 7-1 7 4
f The reader is referred to the particular chapters in question.
For a few determinations, standard corrections are applied regardless of the results of blank tests.1 3 6 Two per cent of the volume of gas collected during a micro-Dumas determination is automatically deducted as being essen
tially due to the volume of potassium hydroxide adhering to the walls of the nitrometer and to the alteration in vapor tension. For the gravimetric proce
dures for the determination of alkoxyl and alkimide groups, an empirical cor
rection of 0.06 mg. is added to the weight of silver iodide for each milliliter of silver nitrate used. Empirically, Pregl established the factor used in the determination of phosphorus which differs from that used in the determination on the macroscale.
Test Substances*
Compounds in a state of high purity are used to determine the applicability and reliability of methods and pieces of apparatus to the types of compounds to be analyzed. Such materials are known as test substances or test compounds and should be used at frequent intervals. In the selection of a test substance, consideration should be given to the composition, structure, and percentages of the various elements or/and groups present, and, if possible, a compound should be selected which most closely approximates on all points those of the samples which have been submitted for analysis. This, of course, assumes that the analyst has been supplied with all data concerning these samples, in
cluding structures. Any compromise to this practice places the analyst at a dis
advantage inasmuch as methods are often varied according to structural types.
Obviously, when many compounds representing all types must be analyzed routinely, for practical purposes, a variety of types of test substances can be used only when questions of doubt arise regarding the validity of certain re
sults. Otherwise, for general routine checking, the author recommends the use of test substances which contain relatively high percentages of the element ( s ) or group (s) in question.
The Commission on Microchemical Techniques of the Section of Analytical Chemistry of the International Union of Pure and Applied Chemistry is in the process of recommending test substances for the various determinations. The list for use with the carbon-hydrogen determination has been completed.4 5 Work is in process on similar lists for other microchemical determinations and the reader is advised to watch for all of these which are expected to be pub
lished during the next several years. In the meantime, the author recommends the use of the compounds shown in Table 3, most of which are expected to be included in the above lists, and most have been taken from the list for the carbon-hydrogen determination.4 5 These substances are stable, solid at room
* Also referred to as test compounds or test samples.
7 Critical Determinations
temperature, nonhygroscopic, and nonvolatile. The last three properties have been included as requirements so that differences in techniques of the analysts in handling samples with other properties does not become a factor. All of these compounds are either commercially available in a sufficiently pure state to be used for test purposes, based on the accuracy of present-day methods, or may be purified or prepared from commercially available material by conventional laboratory means to meet these standards.
Critical Determinations
A discussion on the subject of critical determinations is in order at this time.
On too many occasions, meaningless analyses are requested by the research groups and the microanalyst should be able to recognize this condition when confronted with it. Habitually, the research chemist requests carbon, hydrogen, nitrogen, etc., without considering the values of such. In general, since we are dealing with organic compounds, the determination of carbon and hydrogen is always in order but is of no value if one is trying to differentiate between two compounds having these values so close together that they fall within the limits of allowable error. For example, suppose two such compounds have theoretical values of the following:
(a) Compound I Compound II C = 6 6 . 2 3 % C = 6 5 . 7 3 % H = 4 . 7 6 % H = 4 . 3 6 % If a carbon-hydrogen determination gave
C = 6 6 . 0 3 % , H = 4 . 5 6 %
it would be impossible from this to say which of the two compounds, I or II, had been analyzed since the allowable error on both carbon and hydrogen are
zbO.3%. In such a case, other determinations, such as nitrogen, halogen, methoxyl, acetyl, etc., often give more information. In many cases, either carbon or hydrogen, but not both, is critical for differentiating as shown by the following:
(b) Compound III Compound I V C z = 7 9 . 8 6 % C = 7 3 . 2 1 % H = 4 . 2 3 % H = 4 . 6 0 % (c) Compound V Compound V I
C = 7 5 . 0 0 % C = 7 5 . 4 2 % H = 7 . 8 6 % H = 3 . 6 2 % In ( b ) the carbon value would be critical while in ( c ) it would be the hydrogen value.
TABLE 3 LIST OF TEST SUBSTANCES SHOWING PERCENTAGES OF ELEMENTS AND GROUPS PRESENT Element Empirical Molecular C Η Ο Ν Hal S (Me) Group Class and compound formula weight (%) (%) (%) (%) (%) (%) (%) (%) (1) CH Anthracene45 C14H10 178.234 94.34 5.66 — — — — — — Naphthalene*
45 128.174 93.71 6.29 — —
—
— ——
(2) 0 (C,H) Anisic acid C8H8°3 152.152 63.15 5.30 31.55 —— — —
20.40% CH30 29 59% COOH Anthraquinone C14H8°2 208.218 80.76 3.87 15.37 — — — — — Benzoic acid45 CTH602 122.125 68.85 4.95 26.20 — — ——
36.85% COOH Cholesterol45 C27H46° 386.665 83.87 11.99 4.14 ——
— ——
D-Glucose (dextrose)45 C6H12Oe 180.162 40.00 6.71 53.29 — — — ——
Phthalic acid anhydride45 C8H4°3 148.120 64.87 2.72 32.41 — — — — — Stearic acid45 C18H36°2 284.486 76.00 12.76 11.25 — — — — 15.82% COOH Vanillin CgHgOg 152.152 63.15 5.30 31.52 — —
— —
20.40% CHsO (3) Ν (C,H, . . . ) Acetanilide45 C8H9ON 135.168 71.09 6.71 11.84 10.36 — — Azobenzene C12H10N2 182.228 79.09 5.53 — 15.37 — — ——
2,4-Dinitrophenylhydrazine45 C6H604N4 198.146 36.37 3.05 32.30 28.28—
- -— — Diphenylamine45 C12HnN 169.228 85.17 6.55 — 8.28 — — — — Hexamethylenetetramine45 C6H12N4 140.194 51.40 8.63 — 39.97 —
—
— — Picric acid45 C6H307N3 229.114 31.45 1.32 48.88 18.34 ——
— Histidine hydrochloride mono-C6H307N3 hydrate C6H1203N3C1 209.643 34.38 5.77 22.90 20.05 16.91% CI— —
6.68% Ν from Benzocaine (ethyl ^-aminoben-6.68% Ν from zoate) C«HN02N 165.195 65.44 6.71 19.37 8.48 — — — 27.28% C2HsO Methyl />-aminobenzoate C8H902N 151.168 63.56 6.00 21.17 9.27 ——
— 20.53% CHsO (4a) F (C, . . . ) ^-Fluorobenzoic acid45 C7H502F 140.117 60.00 3.60 22.84 13.56% F Perfluorodicyclohexylethane45 ^14F26 662.154 25.39 74.6l_% F — Tri fluoroacetani 1 ide45 C8H6ONF3 189.144 50.80 3.20 8.46 7.41 30.14% F_
jw-Trifluoromethyl benzoic acid CRH502F3 190.128 50.54 2.65 16.83 — 29.98% F — — — (4b) CUC,...) Chloroacetamide45 C2H4ONCl 93.519 25.69 4.31 17.11 14.9J 37.91% Cl ^-Chloroacetanilide C8H8ONCl 169.617 56.65 4.75 9.43 8.26 20.90%'Cl 20.90%'ClTABLE 3 (Continued) Element Empirical Molecular C Η Ο Ν Hal S (Me) Group Class and compound formula weight (%) (%) (%) (%) (%) (%) (%) (%) l-Chloro-2,4-dinitrobenzene45 C6H304N2C1 202.563 35.58 1.49 31.60 13.83 17.50% Cl — — — Hexachlorobenzene45 C6Cle 284.808 25.30 — — — 74.70% Cl — — — Hexachlorocyclohexane45 290.856 24.78 2.08
—
— 73.14% Cl — — — Tetrachloro-^-benzoquinone (chloranil, tetrachloro- quinone)45 c6o2ci4 245.894 29.3j_ — 13.01 — 57.68% Cl — ——
(4c) Br (C,H,0, . . .) ^-Bromoacetanilide4.5 C8H8ONBr 214.076 44.88 3.77 7.47 6.54 37.33% Br — ——
2,4,6-Tribromophenol45 C6H3OBr3 330.838 21.78 0.91 4.84 — 72.47% Br — ——
(4d) / (C,H,0, . . .) Erythrosin (iodeosin, 2,4,5,7- tetraiodofluorescein) 45 835.924 28.74 0.96 9.57 — 60.73%) I—
——
o-Iodobenzoic acid45 C7H502I 248.027 33.90^ 2.03 12.90 — 51.17% I—
— — (5) 5 (C,H, . . .) S-Benzylthiuronium chloride45 C8HnN2ClS 202.715 47.40 5.47 — 13.82 17.49% Cl 15.82 ——
L-Methionine C5Hn02NS 149.217 40.25_ 7.43 21.45 9.39 — 21.49 — 9.39% Ν from NH2 30.17% COOH Sulfanilamide45 C6H802N.,S 172.212 41.85 4.68 18.58 16.27 — 18.62 — — Sulfanilic acid (anhydrous)45 C6H-03NS 173.196 41.61_ 4.07 27.71 8.09 — 18.51 ——
Sulfonal [ 2,2'-bis (ethylsulfonyl ) -C6H-03NS propane]45 228.337 36.82 7.06 28.03 — — 28.09 ——
Thiourea45 CH4N2S 76.125 15.7£ 5.30—
36.80 — 42.12— —
(6) P, As (C,H,0} ...) o-Arsanilic acid ( o-Aminophenylarsonic acid )45 C0H8O3NAs 217.048 33.20 3.72 22.11 6.45 ——
34.51% As—
5 -Chloro-4-hydroxy- 3-methoxy-C0H8O3NAs benzyl isothiourea phosphate45-157 C^H^O^CISP 344.725 31.36 4.09 27.82 8. Β 10.29% Cl 9.30 8.99% Ρ—
(7) Me (C, . . .) Calcium oxalate45 CT04Ca 128.102 18.75 — 49.96 — __
31.29% Ca—
Potassium acid phthalate45 C;H.O4K 204.228 47.02 2.47 31.34 — — — 19.12% κ — Sodium oxalate45 Co04Nao 134.004 17.92 — 47.76 — ——
34.31% Na—
Mercuric acetate C;H0O4Hg 318.702 15.07 1.90 20.08 — ——
62.95% Hg—
* May be used for carbon-hydrogen if used IMMEDIATELY after weighing. Notes: The atomic weights proposed by the Commission on Atomic Weights of the IUPAC, 1957™ have been used for the calculation of molecular weights and percentages. The percentages are given to the second decimal. Where the third decimal is less than 0.005, it has been disregarded; where it is greater than 0.005, the second decimal has been increased and underlined, i.e., 0.375 = 0.38.9 Test Substances
In general, low theoretical values are not critical ones unless in one sub
stance the element or group is present while in the other it is absent. Even so, the determination of such elements or groups gives no indication as to the purity.
For example, if a compound theoretically has 4 % of nitrogen, it could be con
taminated with 5 % of some substances containing no nitrogen or with con
siderable amounts of one having a relatively high amount of nitrogen and still give an acceptable analysis (within zbO.2% of the theory).
Laboratory Report Sheets
Good laboratory report sheets are essential in a well-organized laboratory and they are quite important to the analyst, particularly the information contained on them. The sample shown on the next page is that used in the author's laboratory. It is filled out in duplicate so that both research chemist and analyst have a permanent record. It is designed to assist in the bookkeeping of both the research chemist and the analyst. Whenever possible all of the information requested on the sheet should be filled in by the research chemist requesting the analysis. A knowledge of both the physical and chemical properties is helpful to the analyst in handling the sample as discussed in the following chapters. The theoretical percentages of the elements or groups to be determined are valuable inasmuch as a comparison between these and the found values often serve as a guide as to the performance of the apparatus being used. In other words, a pure research compound serves the same purpose as does a test sample for checking.
Laboratory Set-up
PLAN
Plans for microchemical laboratories have been published previously by Niederl and N i e d e r l ,1 1 9^1 2 0 Alber and Harand,7 Kirner,7 8 B o o s ,3 4 and the a u t h o r .1 6 4-1 7 4
Any one of a number may prove satisfactory but in the author's opinion certain features, included in his laboratories, are a necessity and are discussed below.
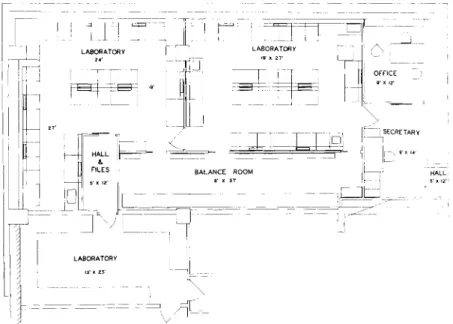
The plan of the author's laboratories which have proven to be most satisfactory is shown in Fig. 1 and serves as an example. The unit consists of an entrance hallway which contains filing space, two offices, a separate filing room, balance room, and three laboratories. At present, these are occupied by nine chemists, a secretary, and a cleaner.
VOLTAGE REGULATION
Modern laboratories should be equipped with electric furnaces for carrying out the combustion procedures described in the following chapters and for most of
11 Laboratory Set-up
these procedures, the operating temperatures are most critical, particularly in regard to constancy. Some pieces have thermostatic control while others do not;
therefore, it is most desirable to have the electricity supplied at a constant voltage to accomplish the above. Either constant voltage transformers or me-
F 2 1 *
HOFFMANN-LA ROCHE INC.
NUTLET, N. J . Microchemical No.
MICROCHEMICAL REPORT Date
Author's Exp. No.
Analysis Requested Formula
Theory m.p.
m-p.
Dry at b . p . _
Submitted by
Code . Sample No.
after c r y s t a l l i z a t i o n from
after times recryetallized from
. ° C , in vacuo hours
°C at
Properties (hygroscopic, etc.) Determination of Carbon and Hydrogen
Weight of sample Weight of H2O Weight of CO2 Ί» Hydrogen 1» Carbon
Structural Formula
Determination of Nitrogen Weight of sample ml.N2 or N/lOO acid Temperature and pressure
<f> of Nitrogen Determination of
Weight of sample
Weight of (or ml. of N/lOO)
Determination of Determination of Weight of sample Weight of sample Weight of Weight of
* * ~
Analyst's Remarks Date Completed
chanical voltage regulators may be used. In the author's laboratories, the line voltage to all table tops is kept constant to within the limits of ± 1 % by means of a 40 K V A regulator of the latter type.
1" 'L
L Π
α
πΠΤΓΤ
LABORATORY
ο
HALL
&
FILES 5' Χ 12'
•
BALANCE ROOM β' Χ 37'
OFFICE )
SECRETARY
FIG. 1. Plan of author's laboratory.
/f
AIR-CONDITIONING
The laboratories (and offices) are air-conditioned to maintain constant tempera
ture and humidity 24 hours per day, 7 days per week, throughout the year, because, by so doing, the balances are not subjected to radical c h a n g e s .5 6'1 1 9-1 2 0'
1 4 4 - 1 4 7 , 1 6 4 , 1 7 4 The temperature is kept constant at approximately 7 5 ° F. (23.9°
C.) and the relative humidity held in the 40 to 5 0 % range,7 0 preferably in the low forties. The exact temperature at which the laboratories are kept is a matter of personal preference, but it is very important that there is no variation either in the rooms or between the different rooms. The relative humidity, however, is closely controlled in the above-mentioned range to reduce weighing errors.
When the percentage is below forty, glassware acquires electrostatic charges while being handled which are not readily lost or dissipated and make weigh
ing either difficult or impossible. The object being weighed appears to rapidly lose weight. I f the relative humidity is above 5 0 % , certain results, particularly carbon and hydrogen have a tendency to be high. The outlets of the air-condi
tioning system are quite large and of the diffusing type so that there is no apparent motion of air. This is of particular importance in the balance room, since balances are seriously affected by air currents.
13 Laboratory Set-up
WEATHER-PROOFING
As an aid to the air-conditioning system and to prevent drafts in winter, all windows in these laboratories are the storm type. The outside frames contain Thermopane glass (double panes with a dehydrated air space between, manu
factured by Libbey-Owens-Ford Glass Co., 570 Lexington Avenue, New York, N . Y . ) . Inside, and approximately one foot away, are plate glass sections extend
ing the entire height of the outside panes. Along the entire base between these two is a vane type of radiator which is used (in winter) solely for the purpose of eliminating drafts from the windows. Experience has shown that these
"extras" are necessary when laboratory tables are situated under or near out
side windows.
BALANCE ROOM
In the author's opinion, a balance room is a "must." Balances should not be placed out in the laboratory. They are the analyst's basic tool and should be so treated and placed accordingly, protected from laboratory conditions. Since a large percentage of the analyst's time is spent at the balance, sufficient space should be provided to prevent crowding. No amount of space should be con
sidered "too much." The author's present balance room is thirty-seven feet long and eight feet wide and contains balance table space for nine balances or approximately four feet per balance which the author considers to be the minimum requirement. The balance room has only inside walls so that no weather-proofing is necessary. However, where outside walls and windows do exist in the balance room, the former should be thoroughly insulated and the latter should be of the heated storm type.1 6 4 Figure 2 shows an electrically heated storm window with thermostatic control, which proved satisfactory for twelve years in a balance room previously occupied by the author. In addition, these balance room windows should be fitted with Venetian blinds and drapes as further protection from the sun's rays. Radiators, if present, should not be in the vicinity of the balances which are very sensitive to temperature changes and for the same reason, fluorescent lighting should be used for illumination.
A temperature change of 1-2° C , no matter what the cause, produces a shift of the balance reading of about 10 μδ.ιΐ9,ΐ20,ΐ44-ΐ47,ΐ74
BALANCE TABLES
The type of tables upon which the balances are mounted is most important.
It was emphasized above that excellent balances will not perform properly in a drafty location subjected to temperature changes. Neither will they give reliable service unless protected from shocks and vibration which affect their sensitivity and precision (see Chapter 3 ) . The cost of a good balance alone should suggest its being properly mounted to increase its lifetime. The location
of the balance room, particularly with reference to machinery in the building as well as disturbances outside the building, such as railroads, trucks, etc., gov
erns the needs for the particular type of balance table which should be used.
Two types are described in detail in this book, both of which the author has used over periods of years. However, in his opinion, the table described in the next section, "Inertia Block-isolator Type Table" is far superior, any loca
tion, to any other which has appeared in the literature. The other, described under "Rigid, Combination Type Table," although satisfactory under certain
FIG. 2. Electrically heated storm window for balance room.
conditions, is a poor, cheaper substitute. Also, it should be borne in mind that future new equipment may create new vibration problems with which the rigid type cannot cope. This was the experience of the author.
Inertia Block-Isolator Type Table187
This type of table consists of three parts, namely, isolators, inertia block, and protective woodwork. The isolators and inertia block form a unit, the size of the former is entirely dependent on the weight of the latter. Each location presents its own vibration problem which should be solved independently, but the same principles are applicable and the selection of the proper combination of springs and inertia block (mass) should make it possible to construct a suitable table. A particularly bad vibration problem existed in the author's laboratory due to the existence of all types of machinery including six 40-inch basket-type centrifuges on the floor beneath the balance room. The principles involved in the construction of the table in the author's laboratory, shown in Figs. 3 - 8 , will serve as an example. Provision is made for nine balances and
15 Laboratory Set-up
for safety, the total weight of the table ( s ) was limited to 9000 pounds, in
cluding isolators, inertia blocks, and woodwork. O f these, the isolators and woodwork approximates 200 pounds per balance leaving 8 0 0 pounds for each block. (Note: It is advantageous to usé the maximum permissible weight for the blocks, but lighter blocks together with lighter isolators would be as efficient as far as vibration absorption is concerned, as shown in the following dis
cussion.)
Vibration isolation depends upon the ratio of the frequency of the disturb
ing vibration, FD, and the natural frequency of the isolation mountings, FN, the efficiency being expressed by the formula,
% efficiency = 1 0 0 ( l _ ^ / ^ ^ ) where
Γ Γ
FN — 188 A/-^ " " cycles per minute and D, the deflection of the loaded isolation mounting, is given by
load
K, the spring constant, is the number of pounds required to make the mounting deflect one inch. I f a force is applied to the loaded mounting to give a slight additional displacement and is then released, as during normal opera
tion of the balance, the mounting will oscillate at a definite frequency (the natural frequency, FN) which depends upon the static deflection of the mount
ing shown in the equation above. From the formula for the efficiency, it can be seen that a mounting having a natural frequency that will isolate the lowest expected disturbing frequency with a particular efficiency will isolate higher frequency disturbances with a higher efficiency.
In these laboratories, the closest source of disturbance is the six centrifuges on the floor beneath the balance room, which operate at 900 r.p.m. I f un
balanced, the centrifuges would generate 900 cycles per minute disturbances;
therefore, it was decided to protect the balances against these. Isolators were selected suitable for a 200-pound load per isolator, with approximately 1.2 inches deflection and a natural frequency o f 172 cycles per minute.9 1 Four of these, uniformly loaded under an 800-pound block, would have, according to the formula above, a theoretical isolation efficiency of 9 6 % for disturbances of 900 cycles per minute. Actually, in practice, an efficiency of 9 3 % was achieved.1 8 7 When properly adjusted, the tables return to their original posi
tion after being displaced. This is an absolute necessity, otherwise, the pre
cision of the balance (Chapter 3 ) would be greatly affected. (In spite of careful handling, a balance, and the table, are displaced every time it is put
into operation.) The springs of the isolators should be completely undamped or the efficiency of vibration isolation is decreased and, what is more important, the table will not return to its original position after being deflected or dis
placed.
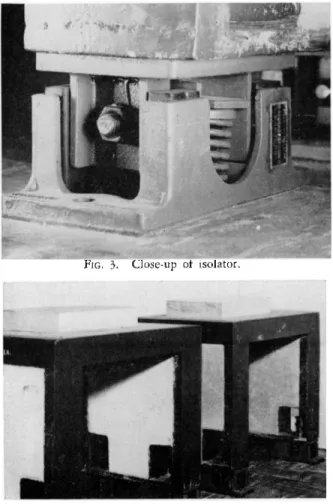
Isolators. The isolators are commercially available. Korfund T y p e9 1 L K / D - 5 2 Vibro-Isolators were recommended by the manufacturer after consideration of the various pieces of equipment causing vibrations in the building. A set of four isolators is required for each reinforced concrete inertia block, one at each of the four corners. Figures 3 and 4 show the isolators in place.
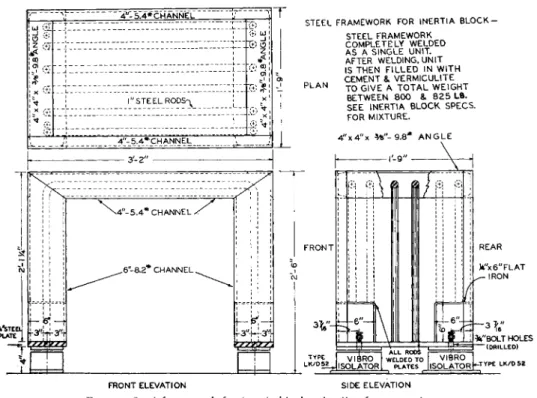
Reinforced concrete inertia blocks. One of these, weighing approximately 800 pounds, is used for each balance. Figure 4 shows the blocks, and Figs. 5 and 6 give the details of construction. Dimensions of the plateau (12 by 20 inches)
FIG. 4. Inertia blocks mounted on isolators.
17 Laboratory Set-up
PLATE
Ψ
4"! 5,4* C H A N N E L
I " S T E E L R O D S - ^
4 " - 5 . 4 * C H A N N E L
\ 4 " - 5 . 4 * C H A N N E L y
^ 6 " - 8 . 2 C H A N N E L ^
STEEL FRAMEWORK FOR I N E R T I A BLOCK - STEEL FRAMEWORK
C O M P L E T E L Y WELDED A S A S I N G L E UNIT.
AFTER WELDING. U N I T IS T H E N F I L L E D I N W I T H o, a m CEMENT & V E R M I C U L I T E
PLAN TO G I V E A T O T A L W E I G H T BETWEEN 8 0 0 & 8 2 5 LB.
SEE I N E R T I A B L O C K SPECS.
FOR M I X T U R E .
4 " χ 4 " χ W- 9 . 8 * A N G L E
-l'-9"
iijULÎ-
J * " x 6" F L A T
^ - I R O N
- 3 V
* " B O L T H O L E S (DRILLED) V I $ R O . I S O L A T O R ( * ~T Y P E L K / D 5 2
F R O N T E L E V A T I O N S I D E E L E V A T I O N
FIG. 5 . Steel framework for inertia block—details of construction.
::-J:-.-.
— τ
I N E R T I A B L O C K S P E C I F I C A T I O N S - S T E E L F R A M E S - A P P R O X . 2 8 0 LB.
C E M E N T - I I O L B .
* J ' B L U E S T O N E - 8 0 L B . S A N D - 2 7 0 LB.
W A T E R —· 7 5 LB.
V E R M I C U L I T E - I O L B . T O T A L W E I G H T W I L L V A R Y
B E T W E E N 8 0 0 AND 8 2 5 LB.
V E R M I C U L I T E V*" B L A C K „ , J , , - *
P R E S T W O O Dν Γ 7 J l 2|
4 - K O R F U N D T Y P E L K / D - 5 2 V I B R O I S O L A T O R S P E R U N I T
F R O N T E L E V A T I O N S E C T I O N T H R U A - A
FIG. 6. Inertia block and surrounding woodwork—details of construction.
(Fig. 6 ) may be varied to accommodate the balance in question. Those shown are suitable for the Ainsworth, Becker, and Bunge balances. A plateau measur
ing 15 by 16 inches is suitable for a Mettler microchemical balance. On top of the concrete plateau is placed an equal size piece of 14 inch plate glass, held in place by resting it on thin strips of plastic tape.
FIG. 7. Inertia block encased in woodwork before table top is put in place.
11 j. 8. Section of finished balance table.
Woodwork. The woodwork construction of the table is designed to protect the inertia block from being touched by the operator. The details of construction are given in Fig. 6. A clearance of one inch exists between the inertia block and the woodwork at all points. This is shown in Fig. 7, a photograph taken during construction before the table top was added. The table top has an open-
19 Laboratory Set-up
ing to allow the small concrete plateau to protrude through it, but not touch it.
(Upon this plateau rest a glass plate 1/4 inch thick and, finally, the balance.) A small molding is attached to the table top to prevent the plateau from coming in contact with notebooks and other objects. Removable panels are used in the
FIG. 9. Rigid type of balance table showing balance mounting.
knee space, so that, if necessary, adjustment of the isolators can always be made with the minimum of difficulty. Figure 8 shows a section of the finished balance table which accommodates nine balances.
Lighting. Since illumination is necessary in the immediate vicinity of the bal
ances, fluorescent fixtures are u s e d .1 6 4-1 7 4-1 8 7 A number of satisfactory fixtures are commercially available. For some balances, such as the Becker and Bunge