General Chemistry of Fluorine-Containing Compounds
B Y J. H . S I M O N S
The University of Florida, Gainesville, Florida
I. Introduction 1 II. H y d r o g e n Fluoride 2 III. T h e Alkali and A m m o n i u m Fluorides 15
IV. Copper, Silver, and G o l d Fluorides. 18
V. Alkaline Earth Fluorides I9
V I . Zinc, C a d m i u m , and Mercury Fluorides 22 V I I . Boron, A l u m i n u m , Scandium, Yttrium, L a n t h a n u m , and Actinium Fluorides 23
V I I I . Galium, I n d i u m , and T h a l l i u m Fluorides • 28
I X . Carbon, Silicon, T i t a n i u m , Zirconium, and H a f n i u m Fluorides 29
X . Germanium, T i n , and Lead Fluorides 46 X I . Vanadium, N i o b i u m , and T a n t a l u m Fluorides 47 X I I . Nitrogen, Phosphorous, Arsenic, Antimony, and Bismuth Fluorides 49
X I I I . C h r o m i u m , M o l y b d e n u m , and T u n g s t e n Fluorides 60 X I V . Oxygen, Sulfur, S e l e n i u m , and Tellurium Fluorides 63
X V . Manganese and Rhenium Fluorides 75 X V I . Fluorine and the Halogen Fluorides 78 X V I I . Iron, Cobalt, and Nickel Fluorides 91 X V I I I . R u t h e n i u m , R h o d i u m , and Palladium Fluorides 93
X I X . O s m i u m , Iridium, and Platinum Fluorides 95
X X . Lanthanide Metal Fluorides 9 9
X X I . Actinide Metal Fluorides 100
References 109
I. Introduction
Since the publication of Volumes I and II of "Fluorine Chemistry", much new knowledge has accumulated relative to the general chemistry of fluorine-containing compounds. This chapter is an attempt to bring this subject up to date as of the time of the writing of this book in the year
1962. In the previous volumes divisions of the subject were made such as between organic and inorganic, volatile or nonvolatile, and simple and complex compounds, for convenience of organization. These divisions are of course, purely arbitrary; and the enormous number of fluorine-contain
ing compounds with such wide ranges of variations of properties shows how diffuse are the boundaries between these divisions. A distinction between volatile and nonvolatile must depend upon a choice of the measured
1
2 J. H . SIMONS
volatility at some selected temperature. Likewise, the properties of fluorine- containing compounds show the inadequacy of dividing descriptive chemistry into organic and inorganic, probably more forcibly than the modern chemistry of the compounds of other elements. It seems to be highly inappropriate to classify together the chemistries of many large fields of compounds under a term which is the negative of another field, regardless of the recognized importance of that field.
Much of the new information presented in this chapter is improved or more detailed knowledge in areas already broadly treated in Volumes I and II or is filling in the gaps in these areas. For this material there is made no attempt to complete the subject. New types of compounds have been discovered, and for these the subject is treated more completely. As is traditional in organizing chemical information, the subject is divided into groups of the periodic table and proceeds across the table and down it.
A compound is listed under its most significant element other than hydro
gen, oxygen, or fluorine, if this can be recognized, otherwise, it may be referred to in several places. If two elements seem equally significant, the compound will most likely be found under the element occurring first as the table is traversed. As the lowest molecular weight compound is hydrogen fluoride, the subject begins with it.
A collected summary table of the thermodynamic properties of the fluorides of all the elements as taken from the literature is presented by Glassner<2 6 9). Sharpe( 6 7 1) has published an excellent review of some of the general aspects of the chemistry of fluorine, and Domange( 1 7 9) has reviewed the inorganic chemistry of fluorine.
II. Hydrogen Fluoride
During the past ten years hydrogen fluoride has been extensively studied spectroscopically. Attention has also been directed toward its thermodynamics, its polymerization in the vapor phase, and its properties as a liquid solvent. Other properties such as ionic equilibrium and ioniza
tion potentials have had some attention. Publications dealing with its catalytic properties for organic chemical reactions have been chiefly in patent literature, and these contain chiefly minor details of reactions already well known.
It is difficult to separate the thermodynamics, the association, and the experimentally determined physical properties such as spectra, because the first must take into consideration the second and must employ the third. Also the properties of both the atom and molecule of the element are involved. For example, the thermodynamic functions Cp°, 5°,
-CFO - F0°)IT, and ( # ° - Fo0)/^, for F, F2, and HF were calculated from 1000°K to 5000°K from spectral data by Cole, Farber, and
Elverum*1 3 9) and the heats, Ai/°/T, free energies, AF0/T, and the equili
brium constants in terms of pressure for the reactions HF ^ \H% + \F2, H F + H, and F2 ^ 2F from 298.16 to 5000°K. Potter*5 5 6) also used spectroscopic data to calculate — (AFo — ^Ho)jTy Cp°> 5°, and (H° - Ho°)T for F, F2, HF, DF, and the naturally occurring isotopic mixture of H F and DF. In a report by Smith*6 9 9) on the molecular pro
perties of hydrogen fluoride which included the spectroscopic constants, the experimental measurements used are chiefly from infrared spectra with the addition of some dielectric constant measurements. In addition to the properties of the monomer, the polymers of H F were shown in the infrared bands. The gas was shown to be chiefly the monomer at high temperatures and the hexamer at low ones. The tetramer was shown to exist, but it is always in small concentration relative to either H F or HeF6. Spectral evidence also showed the presence of a dimer, but its absorption is so small that it can be concluded to exist in very small concentrations. It was also concluded that the original postulation of a monomer-hexamer equilibrium for the vapor by Simons and Hilde- brand*6 8 9) is essentially correct. The dielectric constant measurements indicated that the hexamer has a low dipole moment and probably has a ring structure, which is also in agreement with crystal structure data from X-rays. This author also showed that H F in dilute solutions in MOF6, UF6, and WF6 is also polymerized. He also found that in the vapor H F forms complexes with other molecules of two types. The first class are complexes with inorganic compounds such as CIF3, SO2, and CO2.
They are 1 to 1 complexes with a small heat of formation. Aii/for C I F 3 H F and S O 2 H F is about — 4 kcal per mole and for C O 2 H F it is about —2.
In the other class are organic compounds such as diethyl ether, acetone, methanol, and dioxane. One to one complexes are also formed which are only slightly dissociated at room temperatures and 100 mm Hg. The heats of formation are about — 11 kcal per mole.
Smith*6 9 9) found the heat of polymerization for the tetramer to be
— 19 kcal per mole and for the hexamer —40 kcal per mole.
The determination of the infrared spectra has been reported by a number of authors; Safary, Vodar, and Coulon*6 1 2) reported Raman spectra in addition to the infrared for both gaseous and liquid HF.
Coulon*1 4 6) reported the spectra of the gas from 1400 c m-1 to 700 c m -1 and Safary*610) reported the absorption curve of H F gas 1500 to 1650 A and the Raman spectra of the liquid and the infrared spectra of both gas and liquid from 1 to 5 JLI. The infrared spectra of both H F and D F were reported by Adams and Katz*1). The ultraviolet spectra of H F and D F were reported by John and Barrow*3 7 1), and the dissociation energy of H F was found to be 5.86 ± 0.01 ev. The spectra of monomeric H F
4 J. H. SIMONS
was given by Kuipers*4 1 8*4 1 9). The infrared spectrum of H F was also given by Kuipers, Smith, and Nielsen*4 2 0) and dissolved in CCI4 and some aromatic solvents by Josien, Grange, and Lascombe*3 8 2). The absorption spectra in the ultraviolet 1480-1850 A for temperatures 16.5, 53, 100, and 165° were given by Romand and Safary*5 9 6).
The controversies relative to the molecular species present in hydrogen fluoride gas have apparently been satisfactorily resolved by Smith.*6 9 9) The original quantitative interpretation of the hydrogen fluoride vapor system by Simons and Hildebrand*6 8 9) of an equilibrium between a monomer and a hexamer with the latter assumed to have a ring structure has been seriously questioned by numerous authors, but Smith modified it only to provide for a small fraction of a tetramer and a much smaller fraction of a dimer. He showed that the vapor density measurements of Fredenhagen*2 4 5) which were interpreted by the assumption of polymers higher than six can be explained by gas law deviations without the higher polymers. Doubt was cast upon the existence of a ring hexamer by the failure of Bauer, Beach, and Simons*54) to find evidence for such a structure in their electron diffraction studies, by the ultraviolet absorption spectra reported by Safary, Romand, and Vodar*6 1 1) and by the fact that X-ray crystal data seemed in agreement with the electron diffraction data.
However, Atoji and Lipscomb*22) re-examined the X-ray diffraction of crystalline HF and concluded that the interpretations of previous workers were incorrect. In the solid, infinite zig-zag coplaner chains of hydrogen bands exist. The hydrogen band angle is 120.1° and the F H - F distance is 2.49 A. This would be the correct angle for a benzene like ring structure.
The measurements of Safary, Romand, and Vodar*6 1 1) extend down to 1500 A at temperatures of 16.5, 53, 100, and 165°. They found continuous absorption below 1650 A. Oriani and Smyth*5 2 3) measured the di
electric constant of the vapor and found the dielectric polarization to appear to increase with pressure in the pressure ranges where polymeriza
tion was appreciable. From this they concluded that the monomer-ring hexamer model cannot be nearly the complete story. However, Mag- nuson*4 4 8) made dielectric constant measurements using microwave techniques and found the dielectric polarization to decrease with pressure.
This indicates that the dominant polymer is probably cyclic. Infrared studies of polymer bands in H F vapor such as those reported by Shelton, and Nielsen*474) in which the results were interpreted on the basis of chain polymers have been superseded by more recent studies. The vapor density measurements of Strohmeier and Briegleb*723) and the interpretation by Briegleb and Strohmeier*88) that the simple monomer-hexamer system could be excluded and that a hexamer is in no way preferred has been satisfactorily answered by Smith by showing that their results can be
brought into agreement with the monomer, tetramer, hexamer system by considering gas law imperfections.
Other workers have recently presented measurements and interpreta
tions in agreement with the Simons andHildebrand assumptions as modified by Smith. Jarry and Davis*3 6 4) have determined the vapor pressure, vapor density, and heat of vaporization of HF. The vapor pressure curves
logio Pmm = 8.38036 - (1952.55)/(335.52 + t)
logioPmm = -1.91175 - (918.27jT) + 3.21542 logioT are in agreement with those of Simons*6 8 3) and the vapor density measure
ments agree with those of Simons and Hildebrand*6 8 9). The boiling point is given as 19.51° and for 20 grams H F the heats of vaporization are
13.42 kcal at 4.40°, 15.75 at 17.56, and 16.08 at 19.54°. In a series of three papers, the first by Frank and Spalthoff*240) reported the specific heat, vapor pressure, and vapor density of hydrogen fluoride up to 300°C and 300 atm. The critical temperature is 188 ± 3°C, critical pressure 66.2 ± 3.5 kgm per cm3, and critical density 0.29 ± 0.03 gm per cm3. The heat of vaporization is 89.5 cal per gm. at 19.4°C, rises to a maximum of 146 cal per gm at 130°, declines to 101 cal per gm at 170°, and then de
creases rapidly. In a second paper Spalthoff and Franck*7 0 5) considered the association of hydrogen fluoride in the gas at high pressure using the data reported in the previous paper. They concluded there is association in both chains and HQFQ rings. They calculated the AH of association per H F monomer to be — 7000 ± 500 cal per mol with a AS of reaction of
— 25.0 ± 1 e.u. and a AS of ring closure of —9.2 e.u. In the third paper Franck and Meyer*2 3 8) reported the specific heat and association of H F gas at pressures between 100 and 700 mm Hg and temperatures between - 2 0 and + 100°C. They found A H for the reaction 6HF = (HF)6 to be
— 33.5 kcal per 120 gm, AS = - 195 e.u. for 120 gm, for chain formation, and A H = 40.2 and AS = - 1 9 9 for ring formation. Tsitsishvil*7 4 1) discussed the theoretical aspects of the association of hydrogen fluoride.
Tsitsishvil also calculated the properties of the H F molecule. He gives the energy of dissociation as 6.68 ev = 153.97 kcal, the H F distance as 0.92 A, the vibration frequency 4141 c m- 1, the dipole moment as 1.91 D, and the polarizability as about 0.8 x 1 0- 24 cm3. The ionization potentials of the H F molecule (15.77 ev and 16.97 ev) and the ground state of the HF+ ion were given by Coope, Frost, and McDowell*1 4 1). They reported the first ionization to be 15.77 ev and the second 16.97 ev. Johns and Barrow*3 7 0) reported the ultraviolet band systems of HF+ and DF+.
The heat and entropy of ionization of hydrofluoric acid in aqueous solu
tion was given by Hepler, Jolly, and Latimer*3 1 6). They found
6 J . H . SIMONS
AH0 = - 3 1 8 0 cal per mole, A S0 = - 2 5 . 2 e.u., and the partial molal entropy of the bifluoride ion to be 26 e.u. Jones and Penneman*3 8 1) have determined the infrared absorption spectra of aqueous H F 2- and D F 2- ions and have indicated polymeric species such as H2F3~. The infrared spectra of crystalline HF at — 180°C between 300 and 5000 c m-1 was given by Giguere and Zengin*2 6 7). Waddington*7 5 5) has calculated the lattice energies of potassium, rubidium, and cesium bifluorides and the strength of the bonds in the bifluoride ion, HF2"". He found for the reaction HF2~(g) = HF(g) + F(g)~~, AH = 58 ± 5 kcal per mole. Adams and Katz*1) have measured the infrared spectra of both H F and DF. The thermodynamic properties of D F were given by Potter*5 5 6), and Olah and Kuhn*5 1 7) prepared D F by the deuterolysis of various acyl fluorides and from HSO3F and D20 in 90% yield.
Kastler*3 8 6) discussed the quantum theory of the H F molecule and concluded that the band is 64% of ionic character. Butler and Brokow*102) provided a theoretical calculation of the thermal conductivity of H F gas based upon the 6 H F ^ (HF)6 equilibrium and obtained agreement with the experimentally determined values of Franck and Spalthoff*239). Talley, Kaylor, and Nielsen*7 2 7) studied the infrared spectra of H F and D F and evaluated the molecular constants. Mann et #/.*4 5 4 a) determined the vibrational-rotational emission spectra of HF.
The physical properties of solutions of inorganic fluorides in liquid hydrogen fluoride have been studied by various investigators. Nikolaev and Tananaev*5 0 9) and Nikolaev*506) have determined the activities of the alkali fluorides KF, NaF, and LiF in liquid hydrogen fluoride. Koerber and DeVries*4 0 8) have measured cells of the type M, MF2(S) || HF(NaF)
II Hg2F( S) || Hg where M represents cadmium, copper, or lead at 10 and
0°C and evaluated the standard free energies of formation of mercurous, cupric, and lead fluorides. By using the AF°273 = - 154.8 kcal calculated from Jahn-Held and Jellinek*3 6 2) for C d( s) + F2(g) ->• CdF2(S) they
Vapor pressure measurements were made for solution of the fluorides in liquid H F at 0°. It is concluded that PbF2 forms the compound P b F2 • 2.5HF and that for P b F2 ( s) + 2.5HF -> P b F2 • 2. 5 H F( S) AF°273 = —4.5 kcal. The activity coefficients of silver fluoride in liquid hydrogen fluoride at 0° were calculated from the potential of cells.
F o r A g( s) + |-F2(g) + H F ( 1) -> AgF • HF( S), AF2730 = - 4 9 . 0 kcal.
obtained
2 H g(i ) + F2(g) -> H g2F2( s) A i™ 2 7 3 = - 104.5 kcal P b( S) + F2(g) -> P b F2(S) A F°27 3 = - 149.3 kcal Cu(s) + F2(g) -> C u F2(S) A F°27 3 = — 117.3 kcal
By the use of great care in the preparation and purification of hydrogen fluoride, Runner, Balog, and Kilpatrick*6 0 3) were able to reduce the specific conductivity at 0° to 2.6 x 10~6 o h m -1 c m - 1. Kilpatrick and Lewis*3 9 7) measured the conductance and transference numbers of NaF, KF, SbFs, and NaSbFe in liquid hydrogen fluoride and determined ionic mobilities.
They reported ionic mobilities at infinite dilution at 20°, 0°, and —15° of Na+as 150, 117, and 99 respectively; K+as 150, 117, and 99; H+ as 102, 79 and 67; SbF6~ as 251, 196, and 167; F~ as 350, 273, and 231; and BF4_
as 234, 183, and 140. Hyman et alS™*>) studied the H F - S b F5 system.
They found the substances miscible in all proportions. These mixtures are very corrosive even attacking nickel and other base metals satisfactory for use with liquid hydrogen fluoride. Platinum and Kel-F were satis
factory materials of construction. From a variety of measurements, in
cluding optical, they conclude the following equilibria to be established:
2 H F + S b F5 ^ H2F+S b F6- ^ H2F + + S b F6~
At 20 mole % of SbF5, KX - 4.4 x 10"4 and K2 z 1.7 x 102. The SbFs increases the acidity of the liquid. At 3M SbFs the HO value is estimated to be —15.2. The infrared spectra of liquid hydrogen fluoride and its mixtures with liquid SO2 were reported by Maybury, Gordon, and Katz*4 6 4) from 1 to 25 JU. Polymer peaks were found and the spectra of HF2~ solvated in liquid H F determined. Seel and Sauer*6 5 7) found a violet color caused by N2C>2+ for liquid hydrogen fluoride into which is passed NO2 plus an excess of NO. Solutions of NOF, N2O3, and KNO3 also gave the violet color.
The very strongly acidic properties of hydrogen fluoride have been employed to determine the base strength of aromatic hydrocarbons.
Kilpatrick and Luborsky*3 9 8) accomplished this by means of conductance measurements of solutions of the hydrocarbons in liquid hydrogen fluoride.
They showed that the same order of basicity is obtained when BF3 is added to the HF. McCaulay and Lien*4 6 7) had previously determined the relative basicities of methyl benzenes by extracting the compound from its solu
tion in heptane with liquid H F in which was dissolved BF3. The aromatic compounds although slightly soluble in liquid hydrogen fluoride are more soluble in the presence of BF3. This was explained by the basicity of the aromatic hydrocarbon which causes it to react with hydrogen fluoride
Ar + H F ^ ArH+ + F".
The BF3 reacts with the fluoride ion
B F3 + F - ^ B F4-
to drive the former reaction to the right and increase the solubility of the hydrocarbon in the polar solvent. Kilpatrick and Luborsky*3 9 9) have
8 J. H . SIMONS
determined the equilibrium constant at 20° for the above reaction by means of both conductance and vapor pressures. McCaulay, Higley, and Lien*466* showed that T1F4, TaFs, and CbFs also undergo reactions similar to BF3 and enhance the acidity of liquid HF by the reactions
F " + 2 T i F4 ^ T i2F9- F - + T a F5 ^ T a F6~ F - + C b F5 ^ C b F6~
and that PF5 showed the same property but less strongly. SiF4, BaF2, PbF2, B1F3, SbF3, CrF3, ZrF4, WF6, and ZnF2 did not show this property.
Mackor, Hofstra, and van der Waals*442> also determined the basicity of aromatic compounds by their distribution between n-heptane and liquid HF containing dissolved BF3. An explanation of the weakness of aqueous hydrogen fluoride was given by Pauling*531) and by McCorbrey*469* by means of thermodynamic reasoning. The acidity of aqueous solutions of hydrogen fluoride have been investigated by Hyman, Kilpatrick, and Katz*353) and the Hammett acidity function Ho determined. The driest HF had a Ho value of —10.2 which compares with a value of —11.0 for 100% sulfuric acid. It was suggested that the addition of BF3 or SbFs would increase this acidity. In a more recent paper*3 5 3 a) they estimate the Ho value for 3M SbF5 in HF to be - 1 5 . 2 . Hyman and Garber<352>
studied the Ho function for solutions of hydrogen fluoride in solution in trifluoroacetic acid.
The catalytic properties of hydrogen fluoride for organic chemical reactions which have been treated in Volume I are of course related to the high acidity of the substance. This is enhanced by the presence of BF3 or other substance that act similarly. This increases the catalytic activity and many patents have been issued in which various reactions are reported using this catalytic system. Such reactions are condensations, alkylations, isomerizations, polymerizations, and oxidations.
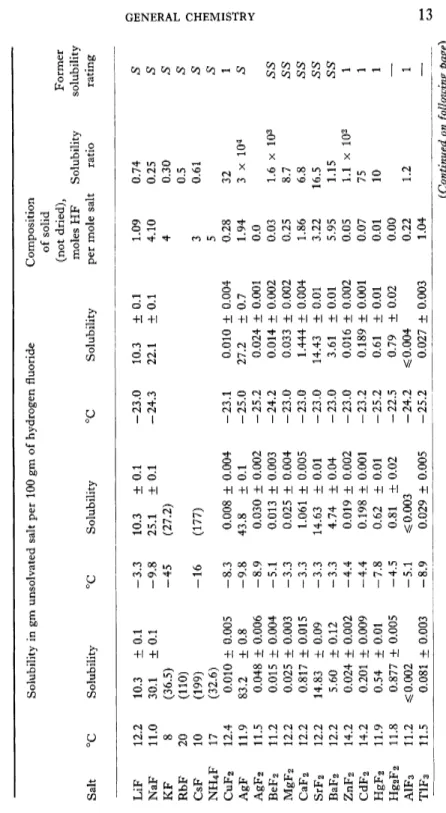
Liquid hydrogen fluoride is an extremely good solvent for many substances. The solubility of aromatic hydrocarbons has been noted above especially when the acidity of the liquid is enhanced by BF3, SbFs, TaFs, CbFs, or T1F4. Katz*387> has reported its use as a solvent for proteins and Rutter*605> has found it a good solvent for purines and pyrimidines which dissolve at 0° to about 12.5 gm per 100 ml and can be recovered unchanged. Jache and Cady*358) have determined quantitatively the solubility of many fluorides in liquid hydrogen fluoride at three tempera
tures. Their results are given in Table V. In this table, the solubility ratio is the concentration of the metal in gram-atoms per lOOgmof hydrogen fluoride in a solution saturated with the fluoride of the metal at 10° divided
Fluoride forming
acid in Soluble Sparingly Soluble Insoluble liquid H F
B e F2 N H4+ , Na+
B F3 Na+, K + , M g +2, M n +2 C 0 +2, C r +3 Ag+, C u +2, A g+ 2, C e +3, C e +4
AIF3 N a +
C r F3 Na+, K+ (solvolyse)
S i F4 N a+K+ (decompose) Ag+
G e F4 Na+ Ag+
S n F4 C u +2 Ag+
T i F4 Na+ C u +2 Ag+
ReF4(?) C a +2 A g+( ? )
S e F4 A g+, H g2+ 2( ? ) C u +2, H g +2
P F5 Ag+ C u +2, A g+ 2( ? )
A s F s M g +2, M n +2, C r +3 Ag+, A g +2, P b +2, H g +2
S b F s C e +3, C o +3, C e +4 Na+ Ag+, Cu+2
B i F5 K+(?)
V F5 Ag+, C u+ 2( ? ) P R+ 3( ? ) H g +2
N b F5 Ag+
T a F5 K+ Ag+
I F s Ag+ H g +2
T e F e A g+
M o F6 Na+ Ag+, C u +2
W F6 Ag+ C u +2
R e F6 C a +2 A g+( ? )
by the concentration of the metal in gram-atoms per 100 gm of water in a solution saturated with the hydroxide of the metal at 25°. The former solubility rating is from Table III, Volume I, p. 234. Clifford and co
workers have studied acid-base reactions in liquid hydrogen fluoride.
Clifford, Beachell, and Jack*1 3 2) studied the solubilities of metallic fluorides in hydrogen fluoride containing fluorides such as BF3 which could pro
duce fluoroacids by reaction with the solvent. The free acids were not isolated but some of the salts were such as AgSbFe, NaSbF6, AgAsF6, Mg(AsF6)2, Mn(AsF6)2, Cr(AsF6)3, Pb(AsF6)2, Hg(AsF6)2, AgPF6, AgBF4, and NabF4. In Table I are found these reported solubilities. These acid solutions were also tested by reaction with the metallic elements and the reaction results are found in Table II. From this information these authors then classified the relative acidity of the fluorides of the elements
T A B L E I
SO L U B I L I T Y O F FL U O R I D E I N LI Q U I D H F A N D AC I D FO R M I N G FL U O R I D E S
10 J . H . SIMONS
Acid
forming Reaction Slow reaction N o reaction fluoride
B F3 M g , M n Cr Be, Al, N b , S n , P b , H g , A g
G e F4 M g , M n , A g
S n F4 M g Ca(?), B e , M n
T 1 F4 H g , A g
S e F4 Ca, M g , M n , Z n , Cr, P b , C u , A g
PF5 A g Ca, M g , M n , Z n , Cr, S n A s F5 M g , M n , Cr, N b , P b , H g , A g C u , Be
V F5 H g , A g M g , N b
N b F5 A g M g
I F5 H g , A g M g , M n , S n , C u
T e F6 S n , A g M g , M n , Cr
M o F6 A g M g , M n
W F6 A g M g , Z n
R e F6 S n M g , M n
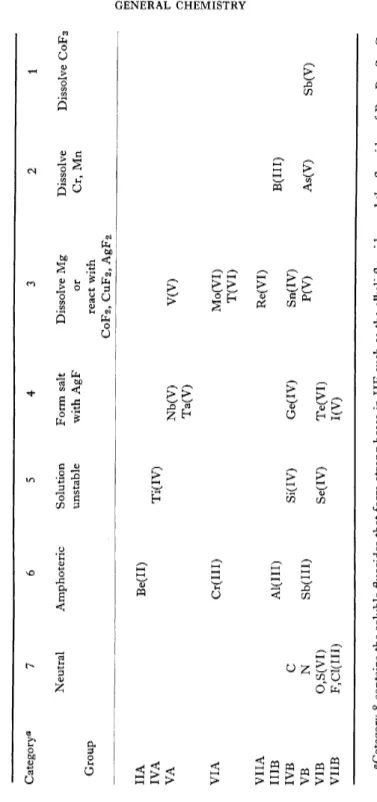
in hydrogen fluoride as is seen in Table III. Clifford and Morris*1 3 4) identified a number of salts of fluoroacids. Among these are AgPF6, Ba(PF6)2, NaPF6, Ba(AsF6)2, NaAsF6, AgTeF7, NaGeF5, and BaHF3.
They also concluded that the acid strengths in liquid H F is in the follow
ing order: (BF3, SbF5, AsF5, PF5) > GeF4 > TeF6 > 1F5 > SeF4.
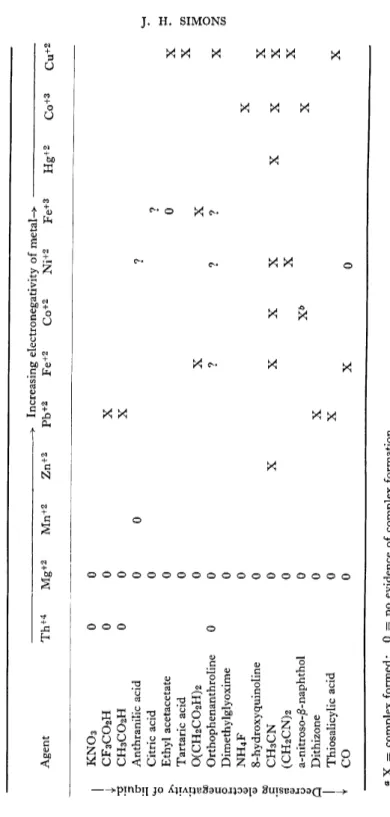
Clifford and Kongpricha*1 3 3) prepared pure AgBF4 by precipitation of solutions of A g N 03 in liquid H F by BF3. Clifford and Sargent*1 3 5) have studied the formation of complexes of metallic fluorides dissolved in liquid hydrogen fluoride and the results are reproduced in Table IV.
The possibility of complex formation between hydrogen fluoride and the halogen fluorides has been indicated, as for example, by an infrared band as reported by Pemsler and Smith*5 4 3) with an indicated heat of reaction, H F + CIF3 ^ H F • CIF3, of 3.9 kcal per mole. They also found a band for D F • CIF3. However, Rogers, Speirs, and Panish*5 9 1) found a positive deviation from Raoult's law for a solution of the liquids and a small conductivity indicating a low degree of ionization of H F in CIF3.
Rogers, Speirs, Panish, and Thompson*5 9 0) made conductance and vapor pressure measurements on the system HF-IF5 and found no evidence of complex formation. Positive deviations from Raoult's law were found and indications of very small ionization of H F in solution. The same
T A B L E I I
RE A C T I O N O F ME T A L S W I T H LI Q U I D H F A N D AC I D FO R M I N G FL U O R I D E S
TABLE III RELATIVE STRENGTHS OF FLUOROACIDS IN LIQUID HF Category" 7 6 5 4 3 2 1 Neutral Amphoteric Solution Form salt Dissolve Mg Dissolve Dissolve C0F3 unstable with AgF or Cr, Mn Group react with C0F2, CuF 2, AgF 2 IIA Be(II) IVA Ti(IV) VA Nb(V) V(V) Ta(V) VIA Cr(III) Mo(VI) T(VI) VIIA Re(VI) IIIB Al(III) B(III) IVB C Si(IV) Ge(IV) Sn(IV) VB N Sb(III) P(V) As(V) Sb(V) VIB 0,S(VI) Se(IV) Te(VI) VIIB F,C1(III) I(V) "Category 8 contains the soluble fluorides that form strong bases in HF such as the alkali fluorides and the fluorides of Ra, Ba, Sr, Ca Ag+andTl+.
TABLE IV METALLIC COMPLEXES IN LIQUID HFa > Increasing electronegativity of metal-> — Agent Th+4 Mg+2 Mn+2 Zn+2 Pb+2 Fe+2 Co+2 Ni+2 Fe+3 Hg+2 Co+3 Cu+2 | KNOs 0 0 { CF3CO2H 0 0 X 3 CH3CO2H 0 0 X cr Anthranilic acid 0 0 ? ^ Citric acid 0 ? ^ Ethyl acetacetate 0 0 X •£ Tartaric acid 0 x '§ 0(CH 2C0 2H) 2 0 XX g Orthophenanthroline 0 0 ? ? ? X g Dimethylglyoxime 0 J NH4F 0 x 0 8-hydroxyquinoline 0 ^ .1? CH3CN 0 X XXX XXX g (CH 2CN) 2 0 X X 0 a-nitroso-j5-naphthol 0 X& X Q Dithizone 0 X 1 Thiosalicylic acid 0 X X 1 CO 0 X 0 0 X = complex formed; 0 = no evidence of complex formation. 6 Gives Co(III) complex.
12 J. H. SIMONS
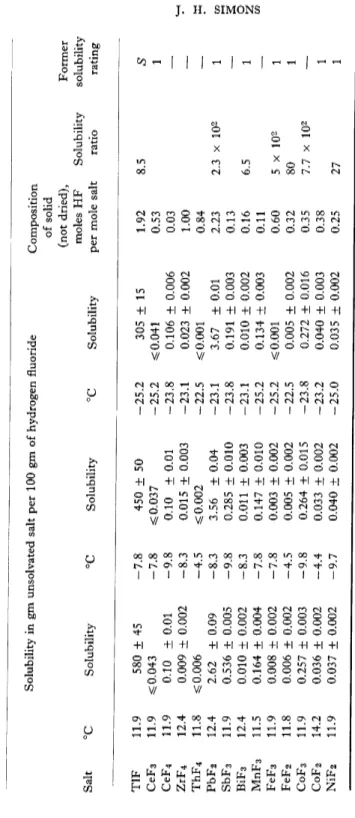
TABLE V SOLUBILITY OF FLUORIDES IN HYDROGEN FLUORIDE Solubility in gm unsolvated salt per 100 gm of hydrogen fluoride Composition of solid (not dried), Former moles HF Solubility solubility Salt °C Solubility °C Solubility °C Solubility per mole salt ratio rating LiF 12.2 10.3 ± 0.1 -3.3 10.3 ± 0.1 -23.0 10.3 ± 0.1 1.09 0.74 S NaF 11.0 30.1 ± 0.1 -9.8 25.1 ± 0.1 -24.3 22.1 + 0.1 4.10 0.25 S KF 8 (36.5) -45 (27.2) 4 0.30 S RbF 20 (110) 0.5 S CsF 10 (199) -16 (177) 3 0.61 S NH 4F 17 (32.6) 5 S CuF 2 12.4 0.010 ± 0.005 -8.3 0.008 ± 0.004 -23.1 0.010 ± 0.004 0.28 32 1 AgF 11.9 83.2 ± 0.8 -9.8 43.8 ± 0.1 -25.0 27.2 ± 0.7 1.94 3 x 104 S AgF 2 11.5 0.048 ± 0.006 -8.9 0.030 ± 0.002 -25.2 0.024 ± 0.001 0.0 BeF 2 11.2 0.015 ± 0.004 -5.1 0.013 ± 0.003 -24.2 0.014 ± 0.002 0.03 1.6 x 103 SS MgF 2 12.2 0.025 ± 0.003 -3.3 0.025 ± 0.004 -23.0 0.033 ± 0.002 0.25 8.7 SS CaF 2 12.2 0.817 ± 0.015 -3.3 1.061 ± 0.005 -23.0 1.444 ± 0.004 1.86 6.8 SS SrF 2 12.2 14.83 ± 0.09 -3.3 14.63 ± 0.01 -23.0 14.43 ± 0.01 3.22 16.5 SS BaF 2 12.2 5.60 ± 0.12 -3.3 4.74 ± 0.04 -23.0 3.61 ± 0.01 5.95 1.15 SS ZnF 2 14.2 0.024 ± 0.002 -4.4 0.019 ± 0.002 -23.0 0.016 ± 0.002 0.05 1.1 x 102 1 CdF 2 14.2 0.201 ± 0.009 -4.4 0.198 ± 0.001 -23.2 0.189 ± 0.001 0.07 75 1 HgF 2 11.9 0.54 ± 0.01 -7.8 0.62 ± 0.01 -25.2 0.61 ± 0.01 0.01 10 1 Hg 2F 2 11.8 0.877 ± 0.005 -4.5 0.81 ± 0.02 -22.5 0.79 ± 0.02 0.00 — A1F 3 11.2 ^0.002 -5.1 ^0.003 -24.2 ^0.004 0.22 1.2 1 TIF3 11.5 0.081 ± 0.003 -8.9 0.029 ± 0.005 -25.2 0.027 ± 0.003 1.04 — (Continued on following page)
TABLE V (Continued) Solubility in gm unsolvated salt per 100 gm of hydrogen fluoride Composition of solid (not dried), Former Salt °C Solubility °C Solubility °C Solubility moles HF per mole salt Solubility ratio solubil ratin TIF 11.9 580 ± 45 -7.8 450 ± 50 -25.2 305 ± 15 1.92 8.5 S CeF 3 11.9 s$ 0.043 -7.8 ^0.037 -25.2 ^0.041 0.53 1 CeF 4 11.9 0.10 ± 0.01 -9.8 0.10 ± 0.01 -23.8 0.106 ± 0.006 0.03 ZrF 4 12.4 0.009 ± 0.002 -8.3 0.015 ± 0.003 -23.1 0.023 ± 0.002 1.00 ThF 4 11.8 ^0.006 -4.5 ^0.002 -22.5 ^0.001 0.84 PbF 2 12.4 2.62 ± 0.09 -8.3 3.56 ± 0.04 -23.1 3.67 ± 0.01 2.23 2.3 x 102 1 SbF 3 11.9 0.536 ± 0.005 -9.8 0.285 ± 0.010 -23.8 0.191 ± 0.003 0.13 BiF 3 12.4 0.010 ± 0.002 -8.3 0.011 ± 0.003 -23.1 0.010 ± 0.002 0.16 6.5 1 MnF 3 11.5 0.164 ± 0.004 -7.8 0.147 ± 0.010 -25.2 0.134 ± 0.003 0.11 FeF 3 11.9 0.008 ± 0.002 -7.8 0.003 ± 0.002 -25.2 ^0.001 0.60 5 x 102 1 FeF 2 11.8 0.006 ± 0.002 -4.5 0.005 ± 0.002 -22.5 0.005 ± 0.002 0.32 80 1 CoF 3 11.9 0.257 ± 0.003 -9.8 0.264 ± 0.015 -23.8 0.272 ± 0.016 0.35 7.7 x 102 CoF 2 14.2 0.036 ± 0.002 -4.4 0.033 ± 0.002 -23.2 0.040 ± 0.003 0.38 1 NiF 2 11.9 0.037 ± 0.002 -9.7 0.040 ± 0.002 -25.0 0.035 ± 0.002 0.25 27 1 14 J. H. SIMONS
conclusions result from the measurements of the system HF-BrFs by Rogers, Speirs, and Panish*590).
The production of radioactive fluorine F18 has made possible studies of the exchange of fluorine atoms between various fluorides. Dodgen and Libby<176) have shown that HF does not exchange with F 2 at room temp
erature but will exchange in a brass vessel slowly at 200°. Evidence indi
cated that this exchange is heterogeneous and is catalyzed by metal fluorides on the walls of the container. Rogers and Katz<585) studied the exchange between HF and the halogen fluorides, C C I 2 F 2 and SF6. They found that exchange of fluorine atoms between HF and liquid BrF3, C I F 3 , BrFs, and IF5 to be essentially complete at room temperature in ten minutes, with HF and gaseous C I F 3 , BrFs, and I F 7 at atmospheric pressure and room temperature, the exchange was essentially complete in 3 min, with SF6, and C C I 2 F 2 , HF did not exchange readily; F 2 did not readily exchange with C I F 3 .
III. The Alkali and Ammonium Fluorides
There is not much new information concerning the hydrogen fluoride complexes with the alkali fluorides. Schutza, Euchen, and Namsch^642) reported the heat of formation of N H 4 F • HF from NH3 and HF to be
A # 2 5 = — 5 1 . 4 ± 0 . 2 kcal per mole and from N2, H 2 , and F 2 A / / 2 5 =
— 1 9 0 . 8 . Higgins and Westrum^3 2 8 a) studied the solution of sodium and ammonium fluorides in liquid hydrogen fluoride by thermochemical methods. At 2 9 8 . 1 5 ° K they found — AH (in kcal per mole of solute in
2 3 8 moles of liquid hydrogen fluoride) to be 4 2 . 4 5 for N H 3 (g) , 1 4 . 3 1 for NH4F, 5 . 7 6 for N H 4 H F 2 , 3 . 3 4 for N H 4 H 3 F 4 , and 9 . 5 4 for K H F2. They calculate the enthalpy of formation of H F(D at 2 9 8 . 1 5 ° K to be — 7 1 . 8 kcal per mole. The AH0 and (AjF°) of formation from the elements in standard states are calculated as follows: for NH4F, - 1 1 1 . 0 ( - 84.0); for N H4H F2,
- 1 9 1 . 4 ( - 1 5 4 . 9 ) ; for N H 4 H 3 F 4 , - 3 3 7 . 4 ( - 2 9 7 . 2 ) ; for NaHF2, - 2 1 8 . 0 ( — 2 0 7 . 2 ) ; and for NaH2F3, —292.5 ( — 2 6 8 . 3 ) . The dissociation pressure of NaF • HF(S) has been measured by Fisher^231) from 157 to 2 6 9 ° .
3.521
logPmm = 9.475 X 103 ± 2.l<>/0. T
AH0 is calculated to be 16.1 kcal per mole in this temperature range, and AF° = 1 6 . 1 1 x 1 03 - 3 0 . 1 7 71. This AH0 value should be compared with the value of Ai/o at 5 0 0 ° K for KF • H F( S) = K F( S) + H F( g) of 1 8 . 4 kcal given by Westrum and Pitzer^776). The Raman spectra of KF • HF in single crystal form was given by Mathieu and Couture-Mathieu<461> and the infrared spectra of this compound was reported by various
16 J. H. SIMONS
authors*1 4 3*3 9 3'5 0 3*. Kruh, Fuwa, and McEver*4 1 7) determined the crystal structures of lithium and sodium bifluorides; and both a and j8 bifluorides of potassium, rubidium, and cesium were determined. The lattice energies of potassium, rubidium, and cesium bifluorides were calculated by Waddington*755>.
The alkali fluorides have many common or similar properties, and these properties are frequently studied for the series of compounds.
The heats of fusion of LiF was found to be 6.2 kcal per mole and that of KF, 6.7 by Petit and Cremiev*5 4 8). The ultraviolet absorption spectra of gaseous NaF, KF, RbF, and CsF were reported by Barrow and Count*4 3).
From this they calculated the dissociation energy of the fluorine molecule at 298°K to be 37.6 ± 3.5 kcal. Yim and Feinleib*810> determined the electrical conductivity of LiF, NaF, and KF. The solubilities of He, Ne, and A at pressures of 1 to 2 atm and at temperatures of 600, 700, and 800°C, were determined in the eutectic mixture of 46.5% mole, LiF, 11.5%.
NaF, and 42% K F by Blander, Grimes, Smith, and Watson*63). By means of measurements of the velocity distribution of molecules in a beam pro
duced as the vapor effused through a small slit, Miller and Kusch*4 7 5) found both dimers and trimers in the vapors of NaF. This is a common property of alkali halides. By use of the same method Eisenstadt, Rothberg, and Kusch*1 9 9) found the dissociation energies of the dimers of LiF, NaF, KF, RbF, and CsF to be 58.9, 54.3, 47.6, and 37.8 kcal per mole respectively.
LiF trimer was found to have a heat of dissociation of 38.3 ± 2.3 kcal per mole. The heats of sublimation of the monomers were given as 61.6 ± 0.8, 63.6 ± 0.4, 55.9 ± 0.9, 50.8 ± 0.5, and 44.6 ± 0.8 for the salts in the order Li, Na, K, Rb, and Cs fluorides. For the dimers the values are 62.2 ± 1.1, 71.1 ± 0.6, 62.3 ± 0.7, 57.6 ± 0.7, and 49.7 ± 1.0. For the LiF trimer the value is 83.6 ± 2.8. From the electrical conductance of aqueous solutions of NaF and LiF at 25°. Kahlweit*3 8 4) obtained the limit
ing conductance of the F~ ion as Ao = 53.8 ± 0.2.
Schmitz-Dumont and Heckman*6 2 7) studied thermally the systems of the alkali fluorides with the alkali carbonates and sulfates. They found that LiF, NaF, and CsF formed no mixed carbonates but K F and RbF formed M3CO3F compounds. LiF formed no mixed sulfate but KF, RbF, and CsF formed M3SO4F compounds. Alkali perfluorides have been reported by Bode*65) by treating an alkali metal or halide with elementary fluorine at 170 to 180°. He reports K F2, RbF2, and CsF3.
Ammonium fluoride has been found not to form double salts in the N H 4 F - K F - H 2 O system by Haendler and Jache*2 8 8), but Haendler, Johnson and Crocket*2 8 9) prepared the following ammonium fluoro- metallates by a reaction of anhydrous metal bromides in methanol with ammonium fluoride. NH4BiF4, N H 4C d F 3, NH4C0F3, N H 4C u F 3,
N H 4 M 1 1 F 3 , N H4Z n F 3, N H 4M g F 3, N H 4F e F 3, (NH4)2NiF4, ( N H 4) 2G e F 6 (NH4)2, SnF6, ( N H 4) 2T h F 6, (NH4)2TiF6, (NH4)3A1F6, (NH4)3FeF6, (NH4)3InF6, and (NH4)3ZrF7.
The elastic constants of LiF have been determined by acoustic velocities by Roa*5 6 3). Douglas and Dever*1 8 1) determined the melting point to be 848.1 ± 1°C and the heat of fusion to be 1043.6 absolute Joules per gram. The vapor pressure was given by Evseev, Pozharskaya, Nes- meyanov, and Gerasimov*2 1 3) as l o g p = 10.366 — 12.733/JT from 926 to 1052.5°K with the heat of sublimation at 989°K = 58.27 kcal per mole.
The solubility of LiF in water was determined by Booth and Bidwell*79) from 198 to 375°C.
Sodium fluoride has been studied thermodynamically by Kind*4 0 0) and the heat capacities and entropies at 298.15 obtained. S029 8 . i 5 of NaF = 12.26 ± 0.07. At higher temperatures, 1285 to 1800°K, the heat of fusion was found to be 8030 cal per mole and the entropy of fusion 6.25 cal per deg per mole by O'Brien and Kelley*5 1 2) and HT — # 2 9 8 . 1 5
forNaF(i)isl6.40r + 170 ± 0.1%,from 1285to 1 8 0 0° K ;HT- H 29 8 . nf o r NaF(c) is 10.40T + 1.98 x 1 0 "3r 2 + 0.33 x l O5^ 1 ± 0.3% from 298 to 1285°K. Novoselova et #/.*511) found that the vapor pressure of sodium fluoride from 1071 to 1193° fitted the equation, logio P(mm) = 8.2263
-(11,029.9/T) with A H evaporation = 50,463 cal per mole and the boiling point 1785°. Coughlin*1 4 5) found the heat of formation of NaF, from the elements at 298.15°K, to be — 136.3 kcal per mole.
Kusch*4 2 3) found that sodium fluoride vapor in a beam is about 10%
dimers or higher polymers. Morrison and Jache*4 9 1) studied the ternary system N a F - H F - H20 at 0 and - 15° and found in addition to NaF • H F the solid phases NaF • 2HF, NaF • 3HF, and NaF • 4HF at both tempera
tures.
The crystal structure of K F • H20 has been determined by Anderson and Lingafelter*13).
The high temperature heat content of RbF have been determined by Kaylor, Walden, and Smith*3 9 0). Its melting point was found to be 1048°K and heat of fusion 5490 cal per mole.
Zhuravler*8 1 9) prepared cesium from its fluoride by reaction with magnesium at 580 to 600°.
2 C s F 4- M g -> M g F2 + 2 C s
The system CsF-BeF2 has been studied by Breusov, Novoselova, and Simonov*85) and the following complex found, Cs3BeF5, Cs2BeF4, CsBeF3, and CsBe2Fs. CsBeF3 and Cs2BeF4 are crystallized from aqueous solutions.
Ammonium fluorometallates were prepared by Haendler, Johnson,
18 J. H. SIMONS
and Crocket*2 8 9) by treating a methanol solution of the metal bromide with ammonium fluoride. The following compounds were reported:
NH4BiF4, N H4C d F3, N H4C o F3, N H4C u F3, N H4M n F3, N H4Z n F3, N H4H g F3, N H4F e F3, (NH4)2NiF4, (NH4)2GeF6, (NH4)2SnF6, (NH4)2ThF6, (NH4)2TiF6, (NH4)3A1F6, (NH4)3FeF6, (NH4)3InF6, and (NH4)3ZrF7. Crocket and Haendler*1 5 2) prepared potassium, rubidium, and cesium fluorometallates by the same method. The following compounds are listed: KBiF4, KCdF3, KCoF3, KCuF3, K M n F3, KNiF3, KZnF3, K3F e F6, K2Z r F6, RbBF4, RbCdF3, RbCoF3, RbCuF3, R b M n F3, RbNiF3, RbZnF3, Rb3FeF6, Rb3ZrF7, CsBi2F7, CsCo2F5, Cs2FeF5, and Cs2ZrF6.
IV. Copper, Silver, and Gold Fluorides
Electron microscope and X-ray studies were made of CuF2 • H20 , Cu(OH)F • CuF2, and CuF2 by Crabtree, Lees, and Little*1 5 0). Wadding- ton*7 5 6) calculated the lattice energy and thermodynamic properties of CuF and found it unstable to CuF2 + Cu.
Geller and Bond*2 5 8) studied the crystal structure of CuF • 2 H20 , and Billy and Haendler*6 2) determined the structure of a single crystal of CuF2. The structure of K2CuF4was determined by Knox*4 0 6) and N H4C u F3 prepared by Haendler, Johnson, and Crocket*2 8 9). Crocket and Haendler*1 5 2) prepared K C u F3 and RbCuF3. Sharp and Sharpe*6 6 8) obtained solutions in toluene of cuprous fluoroborate, fluorosulfonate, and hexafluoro- phosphate, arsenate, niobate, and tantalate by displacing silver from the corresponding silver salts by copper in the solution in the aromatic hydro
carbon. The solid cuprous salts could not be obtained by evaporation of these solutions. Copper (II) fluoride nitrosyl, CuF2NO, was prepared by Fraser*2 4 1 a). Okazaki and Suemune*5 1 5 a) report the crystal structure of K C u F3.
Methforyl thiosilver, AgSCF3 was prepared by Emeleus and Mac- Duffie*2 0 2 a) by the reaction at 140° of silver fluoride and carbon bisulfide.
It decomposes above 80° in vacuo.
Thomas and Jache*7 3 3 a) studied the A g F - H F - H20 system at 0°
and 15°. They found the solid phases at 0° to be AgF, AgF • Z H20 , AgF • 4 H20 , 3AgF • 2HF, AgF • 2HF, AgF • 3HF, AgF • 5HF, and 6AgF • 7HF • H20 . At 15° the solid phases were AgF, AgF • 2 H20 , AgF • 4 H20 , AgF • H F3, AgF • 3HF, and AgF • 5HF.
Olah and Quinn*5 1 8 a) prepared AgBF4 by the reaction in C H3N 02 of either AgF and B F3 or Ag, HF, and BF3.
Ag2F crystals were prepared by the electrolysis of a saturated solution of AgF with a silver block anode by Hilsch, Minnigerrode, and Warten- berg*3 2 9). The preparation of the compound by the reaction of AgF with