CHAPTER 3
Radiochemistry and Radiation Chemistry of Fluorine*
B Y J O H N A. W E T H I N G T O N , Jr.
The University of Florida, Gainesville, Florida
I. Introduction 237 II. Isotopes of Fluorine 238 I I I . Preparation of the K n o w n Radioactive Isotopes 238
IV. Preparation of Other Isotopes F r o m F19 247
V. T h e Isotope F16 247
V I . U s e f u l M e t h o d s for Producing F18 and F20 251
V I I . Detailed Nuclear Properties of the Fluorine Isotopes 257
V I I I . Radiochemical Properties of F18 and Counting M e t h o d s 257
I X . Radiochemical Properties and Counting of F20 261
X . Correction of Activity to a Standard T i m e 262
X I . Exchange Reactions 265 X I I . Preparation of Labeled C o m p o u n d s 266
X I I I . Chemical Studies with F18 268
X I V . Chemical Studies with F2<> 275
X V . Analytical Determinations 277 X V I . Fluorine Activities in Nuclear Reactors 278
X V I I . Biological Investigations—Fluorine Isotopes 278
X V I I I . Radiation Chemistry 282
X I X . Stars 287 References 287
I. Introduction
Fluorine chemistry represents a paradox in modern science. The chemistry of this element has received increasing attention since about 1940 when organic and inorganic compounds began to play an important role in nuclear energy work. Despite the wide use of the element in this field, there have been few papers exploiting the use of fluorine isotopes in studying chemical reactions and physical changes. More recent literature has shown little change in this tendency despite the fact that fluorine compounds cover the spectrum of chemical reactivity from rocket fuels to dielectrics. People in physics, on the other hand, have used fluorine nuclei as tools for studying nuclear structure, and hundreds of papers
* T h e literature surveyed in this chapter includes papers abstracted by Nuclear Science Abstracts up to the early part of 1961 and by Chemical Abstracts through the 1959 subject index.
237
238 JOHN A. WETHINGTON, JR.
have been published in this scientific area. Chemists and biologists have not kept pace with this rate of publication.
II. Isotopes of Fluorine
Fluorine, as found in nature, is monoisotopic. A number of other ele
ments including sodium, aluminum, phosphorus, etc., also share this distinction. If fluorine had been used as a basis for the atomic weight scale, the difference between the physical and chemical scale of atomic weights would have been avoided.
The known radioactive isotopes of fluorine, all man-made, are F1 7, F1 8, F2 0, and F2 1. All are of short half-life as shown in Table I, and this property probably accounts for the lack of work in the field.
T A B L E I Fluorine Isotopes
Nuclide Particle emitted Product nuclide Half-life
- pi? P+ O17 6 0 - 7 2 sec
F18 P+ 9 7 % , Orbital Elec Q 1 8 1 0 7 - 1 1 2 m i n
tron Capture 3 %
Stable — —
P- N e20 1 0 - 1 2 sec
J T 2 1 P~ N e21 5 sec
The isotopes with fewer neutrons undergo beta decay by positron emission designated as /3+. The two isotopes F18 and F20 are of most inter
est to the chemist. The isotope F16 has been predicted, but it has not been identified.
III. Preparation of the Known Radioactive Isotopes In this section, an attempt has been made to list all methods for pre
paring F1 7, F1 8, F2 0, and F21 that have been described in the literature.
Representative references have been given with each method. This list of references is not intended to be exhaustive or complete; however, an attempt was made to present a few representative papers with each method.
These were selected both from the newer and older literature. Many of the methods listed are of limited use to the chemist. The tables contain information about target material, cross section, particle energy used, and other information.
Table II summarizes the preparation of F1 7, Table III, the preparation of F1 8, Table IV, the preparation of F2 0, and Table V, the preparation of F2 1.
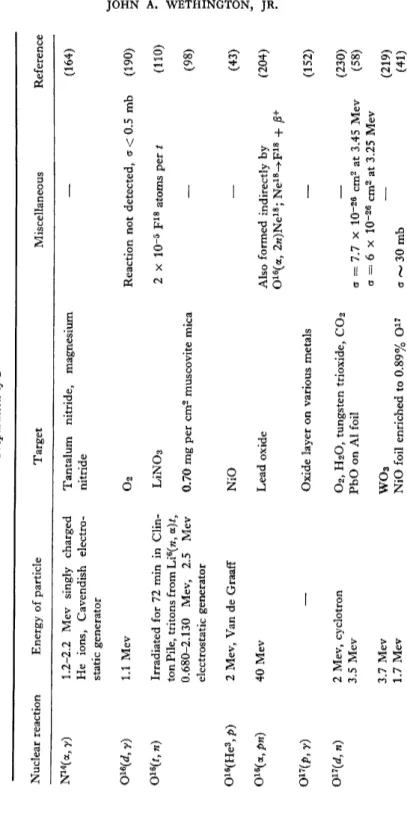
TABLE II Preparation of'F17 Nuclear reaction Energy of particle Target Miscellaneous Reference N14 (a, n) 0™(p, y) Q™(d, n) F19 (y, In)
15 mC Rn Ra or Rn 9 Mev, 0.04/xa, cyclotron 5.5-7.5 Mev, cyclotron 4 Mev, cyclotron, can be as low as 1.6 Mev 0.8-2.1 Mev, electrostatic generator, 6-8/na 3 Mev 3.2 Mev 2.1 Mev, 3 Mev electrostatic generator 1.8-4.3 Mev 100 Mev, betatron 50 Mev, 100 Mev, betatron 12-28 Mev, betatron N2, air, NaN3 Adsorbed air Air NH4NO3 on Pb Any oxygen containing target Ice on Au backing and WO2 Quartz, Fe 20 3, H2O 0 2 PbO on Al foil WO3 PbQ 2 NaF, LiF LiF LiF
a~0.15 x 10-28 cm2 CT = 6 ± 3 x IO"30 cm2 for radia tion of 1.38 Mev and E p = 1.35 Mev Neutron yield = 82 mb per stera- dian at 3.5 Mev d energy y activity of 0.51 Mev noticed in reactors at Savannah River, experi ment showed this caused by F17 0.22 at 100 Mev, 0.15 at 50 Mev relative to N14 (y, ra)N13 Integrated cross section, up to 26 Mev = 0.0024 Mev-barns (220) (67) (173) (61) (63) (217) (151) (150) (228) (133) (83) (68) (17) (159) (95)
FLUORINE RADIOCHEMISTRY AND RADIATION CHEMISTRY 239
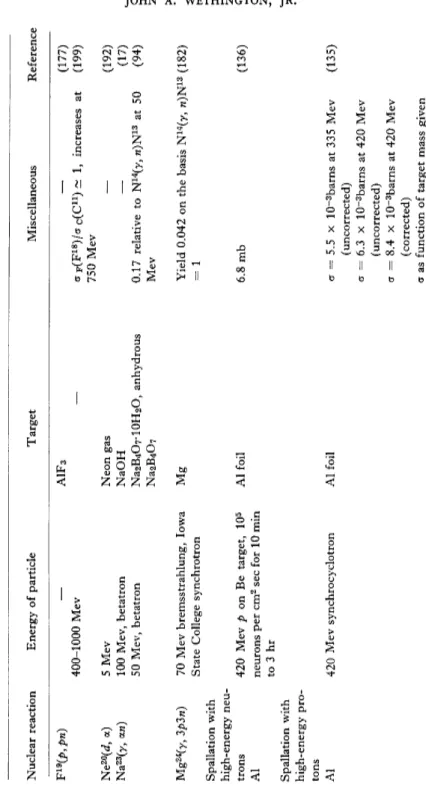
TABLE III Preparation of F18 Nuclear reaction Energy of particle Target Miscellaneous Reference N14 (a, y) 016 (J, y) D1G (t, n) Oie (He3 ,/>) 016 (a,^> w) 017 (/>, y) Oi7(rf, n)
1.2-2.2 Mev singly charged He ions, Cavendish electro static generator 1.1 Mev Irradiated for 72 min in Clin ton Pile, tritons from Li6 (w, a)/, 0.680-2.130 Mev, 2.5 Mev electrostatic generator 2 Mev, Van de GraafT 40 Mev 2 Mev, cyclotron 3.5 Mev 3.7 Mev 1.7 Mev Tantalum nitride, magnesium nitride o 2 LiN0 3 0.70 mg per cm2 muscovite mica NiO Lead oxide Oxide layer on various metals O2, H2O, tungsten trioxide, CO2 PbO on Al foil WO3 NiO foil enriched to 0.89% O17
(164) Reaction not detected, cr < 0.5 mb (190) 2xl0-5 F18 atoms per t Also formed indirectly by Oie (a, 2«)Ne18 ; Ne18 ->F18 4- JS+ cr = 7.7 X 10-26 cm2 at 3 45 Mev a = 6 x IO"26 cm2 at 3.25 Mev '30 mb (110) (98) (43) (204) (152) (230) (58) (219) (41) 240 JOHN A. WETHINGTON, JR.
01S (P, n) 018 (He3, t) F19 (w, In) F19 (/>, d) F19 (J, t) F19 (y, n)
~ 4 Mev, cyclotron (sets in sharply at 2.6 Mev) 0.8-3.5 Mev 3.0-3.5 Mev 2-6-4.3 Mev 21 Mev, cyclotron Cyclotron, neutrons up to 20 Mev from d, n reaction on Li target ~ 10 Mev 9 Mev, cyclotron 8.2 Mev 8.9 Mev, Liverpool cyclotron 17 Mev, Van de GraafT, and Li7 (/>, y)Be8 100 Mev, betatron 100 Mev, betatron 70 Mev, Queen's College synchrotron 20 Mev Water with high concentration of O18 Ni foil oxidized with O18 Ta 2C>3 enriched in O18 H2O, 33% O18 o 2 Teflon foil, 11.9 mg per cm2 NaF Teflon, 1 mg per cm2 LiF, CaF 2 LiF, NaF LiF LiF At 3.55 Mev one F18 per 109 pro- (63) tons, a = 0.75 x IO-25 cm2 — (92) — (134) — (200) F18 due to 016 (He3 ,/>)F18 and/or (115) Oie (He3 , w)Ne18 ->F18 + jS+ 6.3 Mev deuterons used (163) Differential cross section in center (171) of mass system at 11° = 8.5 + 1.5 mb per sterad a = 3.9 ± 0.4 x IO"27 cm2 at 8.8 (114) Mev Cross section for t plotted 0.75% relative to Cu63 (y, «)Cu62 2.7 relative to N14 (y, «)N13 Yield relative to C12 (y, n)C11 = 0.382, J or dE = 0.11 Mev-barns Peak cross section = 3.48 mb, integrated cross section = 0.039 Mev-barns
(34) (66) (96) (17) (159) (64) (95) (Continued on following page).
FLUORINE RADIOCHEMISTRY AND RADIATION CHEMISTRY 241
TABLE III (Continued) Nuclear reaction Energy of particle Target Miscellaneous Reference F19 (P,pn) Ne20 (J> a) Na23 (y, aw)
400-1000 Mev 5 Mev 100 Mev, betatron 50 Mev, betatron A1F 3 Neon gas NaOH Na2B4(V10H2O, anhydrous Na 2B 40 7
- (177) a F(F18 )/CT c(Cn ) ~ 1, increases at (199) 750 Mev - (192) - (17) 0.17 relative to N14 (y, «)N13 at 50 (94) Mev Mg24 (y, 3p3n) Spallation with high-energy neu trons Al Spallation with high-energy pro tons Al
70 Mev bremsstrahlung, Iowa Mg State College synchrotron 420 Mev p on Be target, 105 Al foil neurons per cm2 sec for 10 min to 3 hr 420 Mev synchrocyclotron Al foil
Yield 0.042 on the basis N14 (y, w)N13 (182) = 1 6.8 mb (136) a = 5.5 x 10-3 barns at 335 Mev (135) (uncorrected) a = 6.3 x 10-3 barns at 420 Mev (uncorrected) a = 8.4 x 10"3 barns at 420 Mev (corrected) a as function of target mass given 4^ N> O a >
it
H o H O200-980 Mev, Birmingham Al foil, 19 mg per cm2 and a Al (F18 ) proton synchrotron polyethylene catcher foils = 0.75 (400-980 Mev) (47) a Al (Na24 ) 0.4-3.0 Bev, 1010 p per pulse, 0.00025 in or 0.003 in. Yield relative to Na24 as unity (74) 12 pulses per min, Cosmotron Al foils, purity > 99.9% E, Bev F18 , yield " 0.41 065 0.60 0.64 1.00 0.70 1.40 0.62 2.20 0.66 3.00 0.65 CI 420 Mev, synchrocyclotron NH 4C1 2.2 x 10~3 barns (135) 160 Mev Fused KC1 F18 unwanted product, cross section (139) - 1 mb at 160 Mev, 0.2 mb at 80 Mev Co 370 Mev, Nevis Cyclotron Co sponge ^1 gm per cm2 0.07 ± 0.03 mb for F18 formation (24) Cu 370 Mev, Nevis Cyclotron Cu metal, impurities < 0.01% 3 x 10~5 barns for F18 formation (82) 2.2 Bev, Brookhaven Cos- Cu foil, 40-1200 mg per cm2 1 mb + 0.2 mb for F18 formation (75) motron 420 Mev, synchrocyclotron Cu foil, 99.9% pure 8 x K)~5 barns (135) Ag 420 Mev, synchrocyclotron Ag foil, 99.9% pure 1.6 x 10"5 barns (135) Au 420 Mev, synchrocyclotron Au foil, 99.9% pure 4.4 x 10"6 barns (135) (Continued on following page).
FLUORINE RADIOCHEMISTRY AND RADIATION CHEMISTRY 243
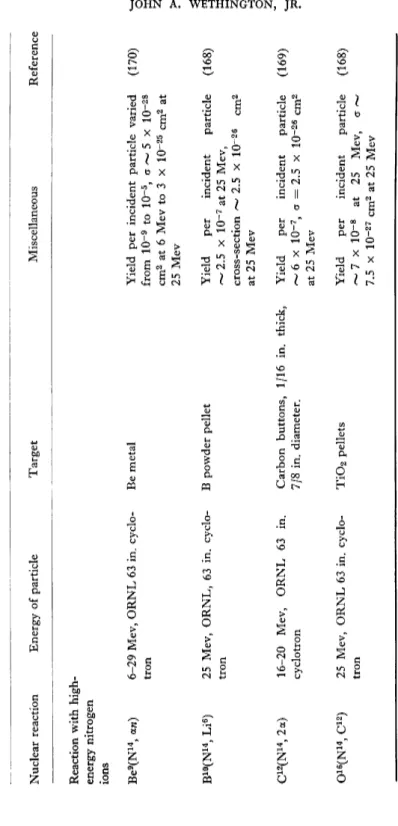
TABLE III (continued) Nuclear reaction Energy of particle Target Miscellaneous Reference Reaction with high- energy nitrogen ions Be9 (N14 , aw) 6-29 Mev, ORNL 63 in. cyclo- Be metal tron Yield per incident particle varied (170) from IO"9 to 10~5 , o ~ 5 x IO-28 cm2 at 6 Mev to 3 x IO-25 cm2 at 25 Mev B10 (N14 , Li6 ) 25 Mev, ORNL, 63 in. cyclo- B powder pellet tron Yield per incident particle (168) ~ 2.5 x 10-7 at 25 Mev, cross-section ^ 2.5 x 10~26 cm2 at 25 Mev C12 (N14 ,2a) 16-20 Mev, ORNL 63 in. Carbon buttons, 1/16 in. thick, Yield per incident particle (169) cyclotron 7/8 in. diameter. / 6 x IO"7 , a = 2.5 x 10~26 at 25 Mev 016 (N14 , C12 ) 25 Mev, ORNL 63 in. cyclo- Ti0 2 pellets tron Yield per incident particle (168) — 7 x IO"8 at 25 Mev, cr ~ 7.5 x IO"27 cm2 at 25 Mev 244 JOHN A. WETHINGTON, JR.
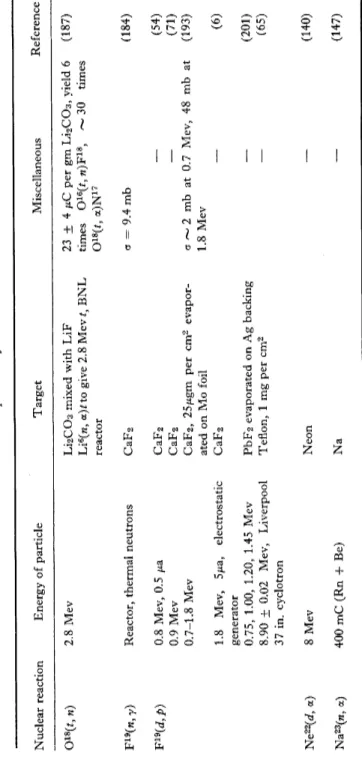
TABLE IV Preparation of F20 Nuclear reaction Energy of particle Target Miscellaneous Reference 018 (*, n) 2.8 Mev Li2C03 mixed with LiF 23 ± 4 /LiC per gm Li 2C0 3, yield 6 (187) 018 (*, n) Li6 («, a)*to give 2.8 Mev/, BNL reactor times 016 (*,w)F18 , —30 times 018 (t, a)N17 F19 (rc, y) Reactor, thermal neutrons CaF 2 a - 9.4 mb (184) F19 (J,£) 0.8 Mev, 0.5 /*a CaF 2 — (54) 0.9 Mev CaF 2 — (71) 0.7-1.8 Mev CaF 2, 25/xgm per cm2 evapor ated on Mo foil a — 2 mb at 0.7 Mev, 48 mb at 1.8 Mev (193) 1.8 Mev, 5/xa, electrostatic CaF 2 — (6) generator (201) 0.75, 1.00, 1.20, 1.45 Mev PbF 2 evaporated on Ag backing — (201) 8.90 ± 0.02 Mev, Liverpool Teflon, 1 mg per cm2 — (65) 37 in. cyclotron Ne22 (d, a) 8 Mev Neon — (140) Na23 («, a) 400 mC (Rn + Be) Na — (147)
FLUORINE RADIOCHEMISTRY AND RADIATION CHEMISTRY 245
TABLE V Preparation of F21 Nuclear reaction Energy of particle Target Miscellaneous Reference F19 (t,p) 0.9 Mev 1.82 Mev thorn a 2.5 Mev Van de Graaff accelerator 1.8 Mev from a 2.5 Mev electrostatic generator An attempt to Neutrons from reactor LiF irradiated in the Low Inten- No fluoride activity observed other (32) produce F21 by sity Test Reactor than F18 the sequence Li6 («, a)t, F19 a,£)F21
~ - (28) CaF 2, PbF 2 — (99) CaF 2, PbF 2 evaporated on Au — (100) disks
246 JOHN A. WETHINGTON, JR.
FLUORINE RADIOCHEMISTRY AND RADIATION CHEMISTRY 247
IV. Preparation of Other Isotopes from F19
Table VI summarizes the preparation of other radioactive isotopes from F1 9. Some duplication of reactions occurs between Tables II through VI. Table VI is intended mainly to serve as a guide for the chemist who has to conduct irradiation studies of fluorine-containing materials. With this table, plus a knowledge of the incident particle and its energy, a pre
diction can be made of the activities which might be encountered in the experimental work. Nuclear reactions which lead only to stable isotopes have not been included in Table VI.
V. The Isotope F1*
This isotope has not been isolated at this time. Possible methods for making F16 have been discussed by Barkas( 2 0>2 1 ). He postulated that this nuclide might be produced by the reaction N1 4( H e3, w)F16 which has a theoretical Q value of — 1.9 M e v( 2 0) and by the reaction C1 2( a ) F16 which would have a threshold of 207 Mev( 2 1 ).
The quantity Q> commonly called the "Q value/' is determined by energy or mass differences for the particular nuclear reaction involved.
The analysis* is similar to that used for chemical reactions except that the relativistic relation between mass and energy must be taken into account. Consider the reaction X(x, y) Y. Let the target nucleus X be at rest, the incident particle x have kinetic energy Ex, the product nucleus Y have kinetic energy EYi and the outgoing particle y have kinetic energy Ey. Then for total energy to be conserved, it is necessary that
Mxc2 + Ex + mxc* = EY + MYc2 + Ey + myc*
where mXy Mx, and My are masses of the incident particle, target nucleus, product particle, and product nucleus. The difference in kinetic energy Q of the products and the incident particle is expressed as
Q = EY + Ey - Ex, and conversion into masses shows that
Q = (Mx + mx- My - my)c*.
Thus Q can be expressed in terms of either energy difference or mass difference. If Q is positive, the kinetic energy of the products is greater than the reactants, and the reaction is said to be exothermic or exoergic.
A reaction with a negative O value is endothermic or endoergic.
* T h i s material is abstracted from I. Kaplin, "Nuclear Physics", pp. 2 1 7 - 2 2 0 . A d d i s o n - Wesley, Reading, Massachusetts, 1955.
TABLE VI Preparation of Radioisotopes from F19 Nuclear reaction Energy of particle Target Miscellaneous Reference F19 (y, «)F18 F19 (y, 2«)F17 F19 (y, 2/>)N17 F19 («, y)F2 ° F19 (w,/>)019
17 Mev Van de Graaff and re action Li7 (/>, y)Be8 100 Mev 100 Mev 70 Mev 12-28 Mev 100 Mev 100 Mev 12-28 Mev 50 Mev betatron spectrum 400 mC (Rn + Be) Thermal neutrons, reactor 800 mC (Rn 4- Be), E n = 8 Mev 400 mC (Rn + Be) Fast neutrons indirectly from cyclotron Fast neutrons indirectly from cyclotron LiF, CaF 2 LiF, NaF LiF LiF LiF, NaF LiF LiF LiF, NaF LiF CaF 2 LiF Liquid HF, Teflon Teflon 0.75% relative to Cu63 (y, «)Cu62 where a = 5 x IO-26 cm2 Yield = 2.7 at 100 Mev, 2.8 at 50 Mev, relative to N14 (y, «)N13 S a dE = 0.11 Mev-barns Peak position = 20 Mev, peak c = 3.48 mb, J a dE= 0.039 Mev- barns to 26 Mev Cloud chamber studies Produced 70-sec F17 Peak position = 26 Mev, CT = 0.66 mb peak Produced N17 , 4 sec emitter a = 9.4 mb
(96) (17) (159) (64) (95) (17) (159) (95) (186) (147) (184) (10) (147) (161) (102) 248 JOHN A. WETHINGTON, JR.
800 mC (Rn + Be) Neutrons from Be target bom barded by 2.6 Mev d at l/*a 400 mC (Rn + Be) 100 mC (Rn 4- Be) Neutrons up to 4.9 Mev from p bombardment tritium target, also d, d reactions gave neu trons of 4.6 to 8 Mev
CaF 2 LiF CaF 2 target and detector 6 Mev 1-6.48 Mev, cyclotron PbF 2 on Ta foil LiF Cyclotron 400-980 Mev
BaF 2, CsF A1F 3 10 Mev Teflon, 11.9 mg per cm2 0.8 Mev, 0.5/ua 0.9 Mev 0.7-1.8 Mev
CaF 2 CaF 2 25 /xgm per cm2 CaF 2 evaporated Mo foil 3.9 Mev CaF 2 on Pt foil - (69) Activation a = 3.5 x IO"26 cm2 (128) - (147) - (148) a as function of E n shown (132) - (224) Threshold = 4.3 Mev, a « 25 mb (30) at 5 Mev - (181) - (177) a F(F18 )/ac(Cn ) -1; increase at (199) 750 Mev Differential cross section in center (171) of mass system at 11° = 8.5 ±1.5 mb per steradian - (54) - (71) CT = 10 mb at 1 Mev, 32 mb at 1.5 (193) Mev - (149) (Continued on following page).
FLUORINE RADIOCHEMISTRY AND RADIATION CHEMISTRY 249
F19 («, <x)N16 F1Q (p, «)Ne19 F19 (£,/m)F18 F19 (£, d)F18 F19 (d,/>)F20