CHAPTER 2
Fermentation of Carbohydrates and Related Compounds
W . A . W O O D
I. Introduction 59 A. Pathways of Carbon 60
B. Hydrogen Acceptor 60 C. Effect of Chain Length and Oxidation-Reduction State of the Sub-
strate 61 D . Energy Yield 61 E. Substrates Fermented 62 F. Relationship of Fermentation Mechanism to Type of Organism 62
II. Methodology 63 A. Fermentation Balances 63
B. Use of Radioisotopes 65 C. Biochemical Studies 67 III. Carbohydrate Fermentation Types 67
A. Hexose Diphosphate Pathway (Embden-Meyerhof Glycolytic System).. 67
B. Hexose Monophosphate Pathways 93
C. Multiple Pathways 101 D . Influence of Chain Length upon Fermentation 110
E. Effect of Oxidation-Reduction State 122
IV. Organic Acid Fermentations 129
A. Citrate 129 B. The Ethanol-Acetate Fermentation of Clostridium kluyveri 131
C. Methane Fermentations 134
References 138
I. Introduction
Fermentation was one of the first biological phenomena to stimulate the curiosity of natural philosophers and inquisitive observers and t h u s t o become the object of intensive investigations. I n the past half-century, studies have been made on nearly every conceivable phase of Pasteur's
"La vie sans air." These explorations, aided b y a wide spectrum of organ- isms, substrates, culture conditions, and recently b y advanced analytical methods, have revealed an equally broad spectrum of products formed in greatly varying yields. T h e results, in most cases, can now be interpreted in terms of a unified concept of reaction patterns providing energy and material for vital processes of living cells. Although t h e information avail- able is comprehensive and detailed, it will become evident as this presen- tation develops t h a t m a n y details, and probably new principles, remain to be elucidated.
59
60 W. A. WOOD
T h e fundamental characteristics of the fermentation processes which will be discussed in detail are: (a) the pathways of carbon, (b) the deriva- tion and role of hydrogen acceptors in permitting fermentation to proceed and in determining its products, (c) the influence of substrate (oxidation- reduction state, chain length, etc.) on process and products, and (d) the site and yield of energy in the several patterns. Exposition and under- standing of present knowledge requires t h a t the details of the organisms, substrate(s) conditions, products, and yields be given attention. Often the reasons will be given why a unique metabolic p a t t e r n — a genetic heritage—
particularly fits a selected organism for the elucidation of a general princi- ple.
T h e magnitude of the material presented on microbial fermentation products, pathways, and mechanisms does not reflect a universal inherent merit b u t the fact t h a t a sourcebook m a y well have reference value which permits contemporary investigators to review, refine, and explain phe- nomena passed over briefly in the course of the growth of microbiology as a science. As opposed to this, the outline is meant to reflect what appears at present to be the general principles and the primary types of microbial fermentations in terms of products and the effects of substrates and con- ditions on their yield as well as the best explanation which this author can deduce as to their mechanism and biological meaning in terms of energy metabolism. An a t t e m p t is made, in addition, to indicate those areas in which information is fragmentary and the conclusions are based on un- tested inference.
A. PATHWAYS OF CARBON
T o evaluate the type of energy generating system in a given fermenta- tion, one must consider the routes for carbon atoms during the process.
As recently discussed by Elsden,1 the several primary pathways of fermen- tation deliver characteristic amounts of energy utilizable for biological work including biosynthesis. T h e energy-mobilizing steps and the products m a y or m a y not vary with the process. Therefore, energy yield alone, where measured or measurable, m a y not reveal carbon flow patterns. The most useful tool for formulation of pathways is the accurate quantitative evalua- tion of isotope distribution in the products from a uniquely labeled sub- strate. I n most cases, this alone is insufficient and must, as illustrated b y examples in the text, be followed b y evidence of enzyme type and abun- dance.
B . HYDROGEN ACCEPTOR
I n fermentation, as in aerobic metabolism, energy mobilization derives ultimately from oxidation (dehydrogenation). Hence for fermentation pro-
2. FERMENTATION OF CARBOHYDRATES 61 ceeding in the absence of oxygen, the generation of the hydrogen acceptors is of primary consequence. A wide variety of hydrogen acceptors, including pyruvate, acetaldehyde, dihydroxyacetone phosphate, fructose, acetoin, and carbon dioxide, serve in this capacity. T h e corresponding reduced products, lactate, ethanol, glycerol (α-glycerophosphate), mannitol, 2 , 3 - butanediol and methane, accumulate. T h e most common biological hydro
gen acceptors are aldehydes and carboxylic acids, which yield primary alcohols upon reduction. T h e reduction of ketones and ethylenic double bonds to secondary alcohols and saturated carbon chains and the forma
tion of molecular hydrogen also serve. However, organisms devoid of or limiting in these processes comprise the greater portion of known species.
C. E F F E C T OF C H A I N LENGTH AND OXIDATION-REDUCTION STATE OF THE SUBSTRATE
F u n d a m e n t a l b u t predictable changes in the fermentation mechanism occur with substrates of different carbon chain lengths and degrees of oxidation with a resultant alteration of product distribution and energy yield. For C3 substrates and its multiples (including hexose polymers) a common p a t h w a y exists, b u t for C4, C6, and C7 sugars and polyols differ
ent sequences come into play. W i t h polyols, two hydrogen atoms more reduced t h a n the corresponding carbohydrates, there appears to be little change from the hexose fermentation route, the main difference being an increased yield of reduced products. I n contrast, with substrates more oxi
dized t h a n carbohydrates, i.e., hexonic acids, the carbon flow and fermen
tation mechanisms in some instances change drastically.
D . E N E R G Y Y I E L D
T h e d a t a of several physiologists indicate for growing cultures under conditions of energy source limitation a constant relationship between t h e amount of energy source and cell yield.1* Such a relationship would imply a relatively tight, or at least a constant coupling between substrate turn
over, energy yield, and biosynthesis (see Volume I I , Chapter 1). Until recently, the generation and transfer of biochemically useful energy in fer
mentation processes was considered to be substrate coupled, i.e., trans
ferred between chemical groups one of which was a substrate molecule.
These groups, in the terminology of L i p m a n n ,l b appear first as high energy phosphate or thiol ester bonds on intermediates derived from the substrate (substrate phosphorylation). Energy generation as phosphate anhydrides by electron transport (oxidative phosphorylation), as characteristically seen in aerobic metabolism, was considered to be absent from anaerobic metabolic processes. Although not demonstrated in all anaerobic processes, a form of oxidative phosphorylation appears t o be present in certain in-
62 W. A. WOOD
s t a n c e s .l c-l d Whether or not electron transport energy is. coupled to phos- phate anhydrides, the level of energy available will depend on the availa- bility of electron acceptors to permit further substrate oxidation, the amount of phosphate anhydride formed depending upon the number of energy-trapping events per electron pair transferred. T h e formation of re- duced products is a requisite of enhanced energy yield. I n the propionic and butyric acid fermentations, electron transport coupled to oxidative phosphorylation appears to account in part for the large growth yield re- ported in these fermentations (see Volume I I , Chapter 1).
E . SUBSTRATES FERMENTED
A wide variety of substrates is fermented by relatively few distinct path- ways. T h e ability to ferment sugars and related compounds of different configuration from glucose results from the cell's ability to convert sub- strates to intermediates common to the pathways for glucose fermentation, often a m a t t e r of induced formation of enzymes for a few additional reac- tions rather t h a n the creation of new mechanisms. W i t h changes in chain length or oxidation state an altered carbon flow p a t t e r n develops in which a pentose rather t h a n a hexose pathway becomes the common sequence.
T h u s far three general patterns have been observed in microorganisms.
Presumably, the genetic potential to shift adaptively from one pattern to another with changes in available substrate bestows in natural habitats survival value upon an organism.
F . RELATIONSHIP OF FERMENTATION MECHANISM TO T Y P E OF ORGANISM
T h e energy-generating mechanism and the available systems for dis- posing of hydrogen atoms are fundamental characteristics of an organism and express themselves in the type of products formed, i.e., the fermenta- tion characteristic of the organism. This relationship presumably underlies the importance of fermentation products and substrates in systematic bacteriology.
T h e fundamental importance of glucose metabolism requires detailed discussion of the major fermentation patterns. T h u s the homolactic, hetero- lactic, ethanol, propionic, formic, acetone-butyl, and other well-known fermentations of glucose will be considered. Fermentations involving (a) other carbohydrates, although convertible to intermediates of known pathways, and (6) substrates of differing chain lengths and oxidation-re- duction states will be treated in a second section. T h e fermentation of organic acids and the reduction of carbon dioxide t h a t are related only to the final reactions of the carbohydrate fermentations are discussed in a third section.
2. FERMENTATION OF CARBOHYDRATES 63 II. Methodology
T h e classic procedures of microbiology provide rudimentary information on t h e fermentation pattern. Observations of acid production, (indicator added, change in p H ) , gas formation and rate and amount of growth fur
nish (a) a means of surveying the substrates fermented and comparing the range of substrate availability among strains, species, and genera, and (b) a guide to products formed.
A . FERMENTATION BALANCES
T h e stoichiometry of substrate conversion t o products in a fermentation can be derived only from accurate quantitative determination of substrate used and products formed. Fermentations involve as over-all reactants only the substrate and water. I t is therefore convenient t o construct a balance to account for all of the carbon atoms in the substrate distributed among the products. T h e Η and Ο atoms can be balanced only b y com
paring the ratio in the substrate and products with H20 since a net u p t a k e or loss of water m a y occur. Needless t o say, useful information can be ob
tained only when a large proportion of the carbon, hydrogen, and oxygen can be accounted for, and very little is converted to cells.
M a n y of the classic fermentation balances were prepared two or more decades ago when accurate and specific methods for m a n y products were lacking, or when not all products were quantitatively known or measured.
For instance, C 02 (frequently added as carbonate buffer) was considered to be metabolically inert and was not considered in balances prepared before the pioneering work of Wood and W e r k m a n1 6 in 1936. I n this in
stance and in numerous others,- i.e., glycerol fermentation, balances con
structed before this concept and observation require reconsideration. On the other hand, balances of Lavoisierl f as early as 1784 to 1789 were suffi
ciently accurate to formulate the stoichiometry of the alcoholic fermentation a n d are valid today.
I n the absence of specific methods, reasonably specific procedures for separating related compounds were employed and resulted in m a n y of the successful balances. Titration was a means of determining acid concen
tration. For instance, volatile and nonvolatile fractions were separated, followed by identification and quantitation of the volatile acids b y such determinations as Ducleaux distillation constants, ether-water partition co
efficients, or azeotropic distillations. There were, however, no rapid proce
dures for estimating ethanol and mixtures of glycerol and mannitol. I t is not surprising, therefore, t h a t some balances do not show a full carbon re
covery or oxidation-reduction balances corresponding to the substrate.
T h e recent development of partition and ion exchange chromatography
64 W. A . WOOD
T A B L E I
CALCULATION OF FERMENTATION BALANCE OF Lactobacillus lycopersici'' 8* 3 1
Moles/ liter
\ φ β
l i d
Moles ci bon (2) O/R
value (4)
Oxidized (5)
Reduced (5)
Calc.
Ci Β (7)
a
O/R value
(4)
Calc.
Ci (7)
Glucose fermented 112.2 100.0 300.0 0 — — — Products formed:
Lactic acid 89.7 40.0 120.0 0 — — 0
Acetic acid 19.7 8.7 17.4 0 — — 8.7
Ethanol 78.9 35.2 70.4 - 2 — - 7 0 . 4 35.2 Carbon dioxide 95.1 42.4 42.4 + 2 + 8 4 . 8 — — Glycerol 43.5 19.4 58.2 - 1 — - 1 9 . 4 0
308.4 + 8 4 . 8 - 8 9 . 8 43.9
α Numbers (1) to (7) refer to text.
NOTE: (3) Carbon recovered = 308.4/300 - 102.7%; (6) O/R balance = 84.8/
89.8 = 0.943; (8) Ci recovery = Ci observed/Ci calculated X 100 - 96.5%.
has made t h e identification and quantitative determination of fermenta
tion acids simple and accurate. Similar techniques applied t o glycerol, mannitol, and mixtures of carbohydrates allow separation and determina
tion b y nonspecific methods. Specific enzymic methods for succinate, ace
tate, ethanol, and glucose, and relatively specific colorimetric methods for lactate, pyruvate, and carbohydrates have greatly simplified t h e prepara
tion of fermentation balances. A series of methods developed b y Neish2 and associates a n d published in monograph form is t h e best source of modern methods available.
As an aid t o understanding t h e balances presented in subsequent sec
tions, t h e fermentation balance and its preparation will be described briefly. T h e steps below, which have evolved from t h e procedures of John
son et aZ.,3 E r b et aZ.,4 and Barker,5 are generally applicable and m a y be followed in connection with Table I .
(1) Express t h e amount of substrate fermented and products formed in mmoles per 100 mmoles of substrate (Ce) utilized (mmoles product formed/
mmoles substrate used X 100).
(2) Calculate t h e mmoles of carbon in t h e substrate and in each product by multiplying t h e mmoles per 100 mmoles of Ce b y t h e number of carbon atoms in each substrate and product molecule.
(3) Determine t h e percent of carbon recovered (mmoles of C in prod- ucts/mmoles of C in substrate X 100).
(4) Determine t h e oxidation-reduction state of t h e substrate a n d prod-
2. FERMENTATION OF CARBOHYDRATES 65 ucts. One system is based upon a comparison of t h e ratio of hydrogen and oxygen atoms in t h e products with t h a t in water. When this ratio is 2 or C H20 , the O/ R state is zero. Each 2 H in excess of t h e above ratio is ex- pressed as — 1 , whereas a decrease of each 2 H is expressed as + 1 . T h u s glucose, lactate, and acetate have a n oxidation-reduction number of zero, whereas carbon dioxide is + 2 and ethanol is —2.
(5) Multiply t h e "mmoles of product per 100 mmoles Ce fermented"
by t h e oxidation-reduction number. Place t h e plus values in one column, the minus values in another.
(6) Calculate t h e O/ R balance (mmoles oxidized/mmoles reduced).
(7) Estimate the amount of Ci expected from the number of mmoles of C2 compound and substances derived from C2 compounds.
(8) Express as the ratio (Ci observed/ C i calculated). These m a y be C 02, formate, etc.
T h e above steps were performed in t h e balance illustrated in Table I . T h e number in parentheses above each column indicates t h e step involved.
Errors result if (a) substrate carbon provides a major source of cell car- bon; recoveries in t h e fermentation balance m a y be low t o a n extent of 2 0 % ; (b) other ingredients of the medium also yield the same fermentation products; and (c) carbon dioxide fixation occurs.
Aside from allowing a formulation of the fermentation equation, balances also m a y yield information as t o mechanisms involved. I n such studies t h e effect of changes in p H (see Tables I I and V I I I ) , oxidation-reduction level of the substrate, and in time are observed. Most of the information so de- rived, however, relates t o t h e terminal reactions of fermentation rather t h a n t o the major pathways involved.
B . U S E OF RADIOISOTOPES
Whereas fermentation balances reflect the over-all result of a fermenta- tion and allow certain inferences as t o t h e mechanism, fermentation of substrates containing C1 4 in specific positions gives information of which atoms of the substrate appear in particular positions of the products. Since the mechanisms of intermediary metabolism of carbohydrates in plant, animal, and mammalian cells have become well-known, interpretation of the labeling patterns is facilitated. T h u s , the yield of information concern- ing a fermentation mechanism is far greater t h a n is inherent in t h e method.
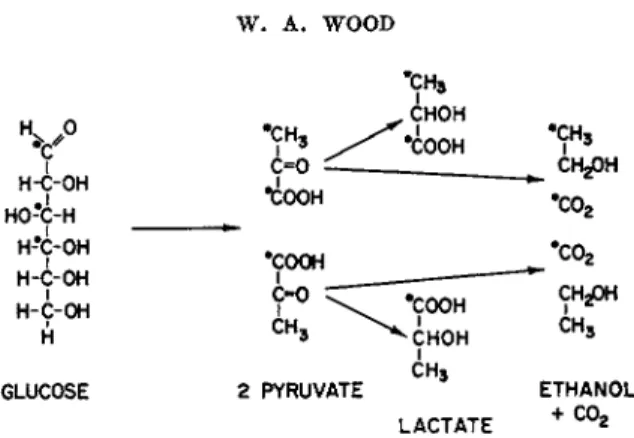
Gibbs and associates,6 for instance, have found in the homolactic fermenta- tion of glucose-3,4-C1 4 b y Lactobacillus casei t h a t only the carboxyl group of lactate was labeled with C1 4 and t h a t t h e specific activity corresponded to t h a t of carbon atoms 3 or 4 of the substrate. Similarly, with glucose-1- C1 4 the radioactivity was present in the methyl group of lactate a t one- half the specific activity of carbon 1. Therefore, lactate must have been derived from 2 molecules of pyruvate whose carbon atoms arose from glu-
66 W. A . WOOD
H-C-OH
Υ
ΗΟ-C-H > - H-C-OH
H-C-OH H-C-OH
GLUCOSE 2 PYRUVATE Η
LACTATE
FIG. 1. Labeling pattern of Embden-Meyerhof pathway.
cose, as shown in Fig. 1. Similar tests with t h e ethanolic fermentation b y yeasts have been performed b y Koshland and Westheimer.7 Since this pattern is in agreement with t h e w a y pyruvate is derived from glucose in the Embden-Meyerhof pathway of glycolysis and from no other known route, labeling d a t a of this sort are taken as evidence t h a t t h e E m b d e n - Meyerhof pathway functions in t h a t fermentation.
Other fermentative pathways give different labeling in t h e products.
For instance, in t h e hexose monophosphate route, carbon a t o m 1 of hexose yields carbon dioxide or t h e carboxyl rather t h a n t h e methyl group of p y ruvate. Hence, in simple cases, t h e amount of radioactive carbon dioxide released in t h e fermentation of glucose-l-C1 4 is considered t o indicate t h e amount of hexose monophosphate pathway which is functioning. A varia
tion of this route, exemplified b y t h e ethanolic fermentation of Pseudomonas lindneri,* yields 2 moles of pyruvate, as shown in Fig. 2. I n this case car- boxyl-labeled pyruvate is formed. If t h e pyruvate is then decarboxylated, labeled carbon dioxide is released as in t h e hexose monophosphate pathway.
However, when substrates labeled in other positions, particularly glucose- 3,4-C1 4, are metabolized, it is possible to distinguish between these varia
tions of t h e hexose monophosphate pathways (see Section I I I , B ) .
I n simpler cases t h e use of isotopically labeled substrates allows verifica
tion of t h e postulated metabolic pathways under physiological conditions.
H^/P *COOH *C° 2
4 « t ~* 4 Γ
HO--C-HH-*C-OH C H* C H 3
ι *C00H *C02
H-C-OH ..Q _
Τ
Έ" 3 *GLUCOSE 2 PYRUVATE ETHANOL + C02
FIG. 2. Labeling pattern of Entner-Doudoroff pathway.
2. FERMENTATION OF CARBOHYDRATES 67 Conclusions derived from this t y p e of experiment are not subject to t h e criticism leveled against biochemical studies t h a t ascertain the presence of enzymes and intermediates of a given p a t h w a y b y the use of broken cell preparations or inhibitors. I n addition, unexpected labeling patterns are effective indicators of anomolous reactions or new pathways. A striking example is presented by the fermentation of glucose b y Leuconostoc mesen- teroides.9'10 I n this case, instead of glucose-l-C1 4 yielding methyl-labeled ethanol or lactate as expected from an Embden-Meyerhof mechanism, the carbon dioxide was labeled. F u r t h e r investigations of this anomolous result in conjunction with enzyme studies established a new p a t h w a y for ethanol formation. When detailed biochemical information is lacking, however, as in the Propionibacterium arabinosum fermentation,1 1 ·1 2 studies with radio- isotopes give clues as to which pathways would be possible, b u t this tech- nique alone is not effective in establishing the details of the new process.
C. BIOCHEMICAL STUDIES
T h e following types of biochemical evidence have been sought in estab- lishing pathways, particularly the Embden-Meyerhof glycolytic system- (a) isolation of intermediates which accumulated during glucose fermenta- tion, (b) fermentation of intermediates to typical end products or to other intermediates of the postulated pathway, (c) demonstration of individual reactions and enzymes. W i t h t h e exception of the phosphorus balance studies of O'Kane and Umbreit,1 3 artificial and often drastic conditions were imposed. For example, sodium fluoride (0.2M to 0.5 M) has been widely used to cause the accumulation of phosphoglycerate during the fer- mentation of glucose.1 4 Since phosphoglycerate is more oxidized t h a n glu- cose, an external hydrogen acceptor such as acetaldehyde was supplied.
F r o m a historical point of view, experiments utilizing these methods played an important role in establishing the Embden-Meyerhof system as the primary mechanism of fermentation. I n the light of newer knowl- edge indicating t h a t several other pathways also utilize the initial and final steps of t h e glycolytic system, it has become evident t h a t only t h e central portion of the Embden-Meyerhof pathway, i.e., reactions between fructose-
1,6-phosphate and glyceraldehyde-3-phosphate, is unique to t h a t p a t h - w a y .1 5 All of the other intermediates are common to several routes. I n spite of this, it is desirable in t h e appropriate sections to present salient informa- tion obtained with the above procedures.
III. Carbohydrate Fermentation Types
A. H E X O S E DIPHOSPHATE PATHWAY ( E M B D E N - M E Y E R H O F GLYCOLYTIC SYSTEM)
T h e first fermentation p a t h w a y visualized in detailed steps, and perhaps t h e best documented today, is the Embden-Meyerhof scheme for fermenta-
68 W . A . WOOD
tion of glucose. T h e initial d a t a were well known a n d assembled principally from investigations of glucose conversion t o lactate in muscle a n d to alco
hol in yeast. Although its adequacy t o explain t h e energetic and mechanis
tic requirements for muscle and yeast created t h e impression of its being the only pathway for glucose utilization, general acceptance of its function as a n important, b u t not sole, route in microbial fermentations was long delayed. A clear statement a n d evaluation of t h e evidence u p t o 1950 h a s been presented b y Elsden.1 6
Since 1950, two important developments in understanding glucose fer
mentation have occurred: (a) enzymic a n d isotopic studies have estab
lished t h e occurrence in microorganisms and in plant and animal tissue of patterns not explainable as p a r t of t h e Embden-Meyerhof p a t h w a y a n d (6) a realization t h a t t h e reactions of t h e Embden-Meyerhof (fructose-1,6- diphosphate) system serve also in other glycolytic a n d oxidative patterns.
All of t h e " a l t e r n a t e " routes of hexose utilization, in fact, show t h e reac
tions from glyceraldehyde-3-phosphate t o pyruvate.
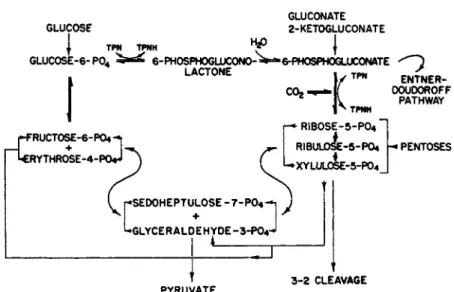
T h e intermediates of t h e Embden-Meyerhof p a t h w a y are indicated in Fig. 3. M a n y detailed accounts of these reactions are available in t e x t s1 7'1 8 and reviews.1 5 ·1 6 All of t h e known intermediates and dissociable coenzymes
0-GLUCOSE GLUCOSE-6-PO4 •I
I
FRUCTOSE -6-ΡΟ4 FRUCT0SE-l,6-di-P04 •1
I
di-HYDROXYACETONE-PQ^— ο -GLYCER ALDEH YDE-3-P0J * - DPNH IP » - J DPNH * 4
0C-GLYCEROL-PO4 1,3-di-PHOSPHOGLYCERIC ACID
V ~Α τ ρ , p / - I
GLYCEROL / 3-PH0SPH0GLYCERIC ACID
\
2-PH0SPH0GLYCERIC ACID
\
2-PHOSPHOENOL PYRUVATE
•ι PYRUVATE
-DPNH-
C 02~~ f ^ LACTATE ACETALDEHYDE
| - « DPNH
ETHANOL FIG. 3. Embden-Meyerhof-Parnas glycolytic scheme.
2. FERMENTATION OF CARBOHYDRATES 69 have been isolated and carefully characterized and most of the enzymes prepared in crystalline form from several cell types.
Concomitant with the understanding of the steps in carbohydrate break- down has come an understanding of the mechanism of substrate-coupled energy generation involving the u p t a k e of inorganic phosphate and the eventual formation of "high energy" ( L i p m a n n ,l b) mixed phosphoric-acyl anhydride and thiol ester bonds transferable to the nucleotide carrier sys- tems for maintenance and growth (see Chapter 1).
1. Y E A S T ETHANOLIC FERMENTATION
T h e study of ethanol production in relation to wine and beer manufac- ture supplied in the late eighteenth and throughout the nineteenth century our earliest understanding of microorganisms as agents of fermentation.
While this treatise is concerned with the bacteria, the background of in- vestigation is so coupled with the yeasts t h a t it is considered desirable to document the classical studies with yeast which have initiated the current concepts of fermentation. Lavoisierl f and Black1 9 in the late eighteenth century, followed by Gay-Lussac2 0 in 1815, made accurate determinations of fermentation products which led to the classical Gay-Lussac equation:
glucose —• 2 ethanol + 2 CO 2
Pasteur, in 1857,1 9 a showed b y more detailed measurements the forma- tion of small amounts of glycerol and succinate and perhaps more impor- t a n t to the development of concepts of t h a t day, showed t h a t some of the sugar was incorporated into cellular material. (See Harden2 0* for discussion of early concepts of fermentation.) More recent investigations2 1 with mod- ern methods and automatic p H control have established the p a t t e r n of minor products (Table I I ) , b u t have not altered the basic concept (see Section I I I , A, lc).
T h e availability of large quantities of yeast as a by-product of beer manufacture was a major factor in the early development of knowledge of fermentation mechanisms. A major contribution b y Buchner2 2 in 1897 was the demonstration t h a t a cell-free yeast juice converted carbohydrate to ethanol. This fundamental observation initiated research which devel- oped steadily until, b y the mid-1940V, the nature of the individual reac- tions, enzymes, metabolic intermediates, coenzymes, and energy relation- ships of the alcoholic fermentation had been established. Parallel studies on the mechanism of muscle glycolysis demonstrated a fact of greatest in- terest t o comparative biochemistry, i.e., t h a t most of the reactions of mus- cle glycolysis are identical to those of the yeast ethanolic fermentation.
This unifying concept emerged in detailed form in the early 1940*8 as the Embden-Meyerhof glycolytic system. F u r t h e r details of yeast glycolysis as
70 W. A. WOOD
T A B L E I I
ETHANOL FERMENTATION BY YEAST"
mMoles/100 mmoles of glucose fermented r r o a u c i
pH 3.0 pH 6.0 pH 6.06 pH 7.6 pH 7.6*
2,3-Butanediol 0.75 0.53 0.39 0.68 0.33
Acetoin Nil Nil Nil 0.19 0.01
Ethanol 171.5 160.5 165.9 129.9 148.0
Glycerol 6.16 16.2 10.4 32.3 25.1
Butyric acid 0.13 0.36 0.39 0.21 0.35
Acetic acid 0.52 4.03 4.27 15.1 9.16
Formic acid 0.36 0.82 0.46 0.49 0.43
Succinic acid 0.53 0.49 1.14 0.68 0.43
Lactic acid 0.82 1.63 1.73 1.37 0.87
Carbon dioxide 180.8 177.0 178.0 148.5 167.8
Glucose carbon assimilated 12.4 12.4 — — —
Fermentation time, hr. 29.0 15.5 16.0 25.0 32.0 Glucose fermented, % 98.5 98.0 98.5 60.3 98.1 Carbon recovered, % 93.8 96.4 94.0 91.3 94.1
O/R balance 1.03 1.05 1.03 1.01 1.04
β Neish and Blackwood.2 1
6 Automatic pH control using ammonium hydroxide or sodium hydroxide.
deduced from studies with enzyme preparations are contained in the chap
ter by Nord and Weiss in Cook's recent volume on the biology of the yeasts.1 8 Tracer studies of flow patterns of glucose-C1 4 carbon in intact fungal cells have been published for Saccharomyces cerevisiae,7 and Fu- sarium UniP From the energetic viewpoint, the phosphate balance in both the ethanolic (yeast) and the lactic (muscle) glycolysis is 2 moles of in
organic phosphate consumed and two moles of adenosine triphosphate (ATP) formed:
glucose + 2 iP + 2 ADP 2 ethanol + 2 C 02 + 2 ATP + 2 H20 T h e free energy of hydrolysis of the terminal pyrophosphate bond on adenosine triphosphate is currently estimated at 7.7 kilocalories per mole,2 4 thus approximately 15.4 kilocalories of biologically useful energy are mo
bilized during the fermentation of 1 mole of glucose via either the ethanolic or the lactic (homolactic) versions of glycolysis.
a. First Form of Fermentation. T h e ethanol fermentation in yeast can be altered to yield glycerol as a major product. Carl Neuberg and his asso
ciates2 6"2 8 investigated the yeast fermentation in detail just prior to 1920 and described three kinds on the basis of products formed. A fourth kind was added in the 1930's on the basis of products formed by dried cells.3 7"4 1
2. FERMENTATION OF CARBOHYDRATES 71 T h e " n o r m a l " alcoholic fermentation—glucose —> 2 ethanol + 2 C 02— i s considered as the first form.
b. Second Form of Fermentation. This form occurs during glucose fer
mentation in the presence of sodium sulfite. T h e acetaldehyde formed from pyruvate by carboxylase is trapped as a bisulfite addition compound, and is thus unavailable to serve as a hydrogen a c c e p t o r .2 5 - 3 0 Under these condi
tions, dihydroxyacetone phosphate replaces acetaldehyde as the oxidant for the reduced diphosphopyridine nucleotide, forming α-glycerol phosphate which is dephosphorylated to glycerol. T h u s both glycerol and acetalde
hyde accumulate (Table I I ) . The triose phosphates and pyruvate do not form stable bisulfite addition compounds; other acetaldehyde fixatives, i.e., dimedon, thiosemicarbazide, and the hydrazines, have the same effect. T h e fermentation t h u s approaches:
glucose + H S 03" —> glycerol + acetaldehyde · HSO3 + CO2
E t h a n o l formation is not completely suppressed because the presence of sufficient trapping agent to bind all of the acetaldehyde becomes toxic.
Based upon the reduction of one mole of triose phosphate to glycerol per mole of glucose, there is no net energy yield in t h a t portion of the fermen
tation shifted b y sulfite. Evidently the unaffected portion of t h e fermenta
tion supplies sufficient energy.
Neuberg's bisulfite fermentation was employed during World W a r I for the production of glycerol (the Constein-Ludecke and the Cocking-Lilly industrial processes in Germany and England, respectively).1 9
c. Third Form of Fermentation. As indicated earlier, yeast fermentation in alkaline medium forms glycerol at the expense of ethanol. T h e reduction of dihydroxyacetone phosphate to glycerol leaves a deficiency in reducing power which results in acetaldehyde accumulation. T h e acetaldehyde undergoes dismutation to equal amounts of acetate and ethanol; thus ace
t a t e becomes a quantitatively significant p r o d u c t2 1 ·3 1'3 5 as follows:
2 glucose —» 2 glycerol + acetic acid + ethanol -f- 2 CO 2
This represents a balance among the following oxidations and reductions:
glyceraldehyde-3-phosphate + iP + D P N+ —>
1,3-diphosphoglycerate + D P N H + H+
acetaldehyde + DPN+ -+ acetic acid + D P N H + H+
dihydroxyacetone phosphate + D P N H + H+ —> α-glycerophosphate -f- DPN+
acetaldehyde + D P N H + H+ -> ethanol -> DPN+
Since acetaldehyde oxidation in yeast produces acetate rather t h a n a c e t y l ^ SCoA, there is no net energy yield in this form of the yeast fermentation.
72 W. A. WOOD
Neish and Blackwood,2 1 in recent experiments with automatic p H con- trol, observed rapid fermentation between p H 2.4 and 7.4; the rate was very slow at p H 2.0 and a t 8.0. Glycerol yields as high as 29 % were ob- tained in t h e more alkaline region. Nickerson and Carroll3 6 reported for one organism (Zygosaccharomyces acidifaciens) the third form of fermenta- tion as the normal process, i.e., without p H control.
d. Fourth Form of Fermentation. T h e fourth fermentation type, reported by Neuberg and Kobel around 19303 7 - 4 1 appears t o result from t h e use of dried preparations or cell-free extracts. I n this case pyruvate is not m e - tabolized rapidly and ethanol and carbon dioxide are not found. T h u s pyruvate and glycerol accumulate:
glucose —• pyruvate + glycerol 2. HOMOLACTIC FERMENTATION
Scheele (1780) isolated and identified the acid of sour milk as lactic acid.
The identification and association of lactic acid-producing organisms with fermentation was established by Bondeau, b y Pasteur and Schultze, and by Lister (see reference 19). Since the 1880's when t h e first commerical fermentations were initiated, t h e production of lactic acid b y fermentation has become an important industry. All members of t h e genera Strepto- coccus, Pediococcus, Microbacterium, a large number of lactobacilli, certain bacilli, and Rhizopus species ferment glucose predominantly to lactic acid with formation of trace amounts of volatile acids, ethanol, fumarate a n d carbon dioxide (Table I I I ) . Kluyver and Donker4 2 have applied t h e term
"homofermentative" as contrasted with t h e "heterofermentative" t y p e in which other products occur in major amount. Orla-Jensen4 3 recognized the heterofermentative organisms among t h e lactic acid bacteria a n d applied the terms "Betacoccus" and "Betabacterium" to denote t h e hetero- fermentative cocci and rods respectively. Although t h e homofermentative streptococci yield from glucose only traces of products other t h a n lactate in t h e usual growing or cell suspension fermentation, Gunsalus and N i v e n4 4 showed t h a t a t alkaline p H t h e production of formate, acetate, and ethanol was increased a t t h e expense of lactate t o t h e extent of 25 t o 40 % of t h e sugar utilized. T h e latter products appeared in t h e ratio of 2 : 1 : 1 . I n con- trast t o earlier studies, P i a t t and F o s t e r4 5 with more modern methods found small amounts of carbon dioxide, glycerol, diacetyl, acetoin, a n d 2,3-butanediol which in aggregate accounted for as much as 1 8 % of t h e glucose carbon (Table I I I ) . I n addition, C1 402 was incorporated into lac- tate, acetate, and cell material. C1 402 incorporation was not obtained with Lactobacillus casei,6 Lactobacillus plantarum, and Streptococcus lactis™ in earlier studies. I t would be interesting t o investigate t h e route of C1 402 incorporation into acetate and lactate.
2. FERMENTATION OF CARBOHYDRATES 73 TABLE III
GLUCOSE FERMENTATION BY Streptococcus faecalis AND Streptococcus liquefaciens' jtiMoles/100 mmoles of glucose fermented
Product S. faecalis S. liquefaciens
pH 5.0e pH 7.0« pH 9.0» N o pH
control pH 7.0 p H 7 * pH 7C
Lactic acid 174 146 122 175 173 183 185
Acetic acid 12.2 18.8 31.2 18.0 23.3 14.3 10.4 Formic acid 15.4 33.6 52.8 0.52 6.55 0.91 0.10
Carbon dioxide — — — 6.00 6.61 6.00 5.44
Ethanol 7.0 14.6 22.4 4.45 2.00 4.00 5.53
Glycerol — — — 2.67 14.1 0 0.07
Bi acetyl — — — 0 0 0.16 0.09
Acetoin — — — 0.10 0.9 0.12 0.01
2,3-Butanediol — — — 0 0 1.24 0
Carbon recovery, % 95.0 90.0 88.0 97.5 105.0 99.8 98.8 O/R Index 1.02 1.18 1.18 1.06 0.99 1.07 0.98
β Initial pH.
6 Sparged with N2 .
e Deficient medium.
T h e mechanism of microbial lactic acid fermentation has, in anaology to muscle glycolysis, been considered as an Embden-Meyerhof glycolysis wherein pyruvic acid is reduced t o lactic acid rather t h a n being decar- boxylated and reduced to ethanol as in the yeast fermentation. T h e earliest evidence of Embden-Meyerhof intermediates in lactic bacteria was t h a t of Stone and W e r k m a n ,1 4 who reported t h a t Lactobacillus plantarum accu
mulates phosphoglyceric acid when glucose is fermented in the presence of fluoride and acetaldehyde or other hydrogen acceptors. Evidence for other Embden-Meyerhof intermediates was reported b y O'Kane and Umbreit,1 3 who observed t h a t during glucose fermentation Streptococcus faecalis re
moved inorganic phosphate from the medium and accumulated organic phosphate compounds with the solubility characteristics of the hexose mono and diphosphates and the glyceric acids. A complete enzymic anal
ysis of the lactic bacteria has not been made, b u t some scattered reports in connection with other studies do exist.4 6 a
Evidence from biochemical studies supports the view t h a t t h e homo- lactic fermentation utilizes the glycolytic system, whereas the heterolactic fermentation follows a hexose monophosphate p a t h w a y (Section I I I , B , 2).
Since homolactic organisms form other products in major amounts under
74 W. A. WOOD
certain conditions and m a y contain enzymes of the hexose monophosphate pathways a more precise definition of "homolactic" has been sought. As a result of assays for aldolase and enzymes of the hexose monophosphate pathways, it has been proposed t h a t "homolactic" refer to those lactic acid bacteria containing aldolase, b u t not phosphoketolase.4 6 b»c
Gibbs and associates6 have provided the most convincing evidence for Embden-Meyerhof glycolysis b y showing t h a t L. casei, L. pentosus (plan- tarum), and S. faecalis ferment glucose-l-C1 4 to methyl-labeled lactate with a 50 % dilution of the specific activity in the methyl group over t h a t of carbon atom 1 of glucose. Similarly, the fermentation of glucose-3,4-C1 4 yielded carboxy-labeled lactate without dilution of the specific activity.
Since these labeling patterns exclusively fit the distribution of carbon atoms expected from the Embden-Meyerhof p a t h w a y (Fig. 1), there is no doubt t h a t the glycolytic pathway is the quantitatively significant mechanism functioning in the homolactic fermentation. T h e following equation is as- sumed to describe the chemical and energetic transformations involved.
glucose + 2 iP + 2 ADP -» 2 lactate + 2 ATP + 2 H20
Based upon a net formation of 2 moles of A T P , an energy yield of 15.4 kilocalories2 4 per mole of glucose fermented is realized. Therefore, the lactic and similar fermentations are distinctly inferior to other (aerobic) methods of energy generation. Nevertheless, this energy transfer allows rapid de- velopment of cultures in an otherwise complete medium to a point where growth in 16 hours or less is limited by the amount of acid accumulated.
Since Streptococcus faecalis can grow anaerobically on p y r u v a t e4 7 or cit- rate (which produces pyruvate, Section IV, A), and achieves greater growth on glucose under aerobic conditions,4 8 it is likely t h a t substrate level energy generation results from pyruvate dismutation or oxidation with accom- panying formation of a c e t y l ^ S C o A . (See Gunsalus4 8 a for review of reac- tions.)
T h e stereoconfiguration of the lactic acid produced varies with the genus or species involved. T h e literature on this point is confusing and citations as to the configuration of the lactate produced m a y be erroneous for the following reasons: (a) " d e x t r o " and "dextrorotatory" are used in- terchangeably, b u t now have different meanings; (6) the relationship be- tween configuration (D , L) and optical rotation (d,l) was established after some of the rotations were published; (c) previous to the publication of Bancroft and Davis4 8 1* the effect of ionization of the carboxyl group and of salt formation upon rotation was not appreciated; (d) the same culture m a y produce different forms of lactic acid depending upon conditions or the phase (rough or smooth) of the culture.4 9 T h e available d a t a as to t h e configuration produced is summarized in Table IV.
2. FERMENTATION OF CARBOHYDRATES 75
T A B L E I V
CONFIGURATION OF LACTIC ACID PRODUCED BY VARIOUS MICROORGANISMS
Organism Configuration I. Homofermentative type
Streptococcus sp. L ( + ) °5 0
Lactobacillus caucasicus, lactis, i > ( - )6 Lactobacillus leichmanii D( _ ) 5 0 - W
Lactobacillus helveticus, bifidis, D Lc 2 4 3 Lactobacillus plantarum DL2 4 8 Lactobacillus thermophilis, delbrueckii L( + ) 51-68
Lactobacillus bulgaricus DL or D ( — )5 2
Lactobacillus casei L ( + ) , L ( + ) , and D ( - ) »
Pediococcus sp. D LM
Bacillus sp. L ( + )5 5
Clostridium sp. D L1 0 0 , 106
Butyribacterium rettgeri DL8 9 II. Heterfermentative type
Lactobacillus brevis, buchneri pasteuria- DL5 0 nusy fermenti
Leuconostoc sp. D ( - )6 0
Microbacterium sp. L ( + )4 8
Rhizopus sp. L ( + )6 2
III. Mixed fermentations
Serratia sp. D ( - )1 9«
• L ( + ) Zinc salt-2 H20 , [a]D - - 8 . 2 5 ° (4%, 25°C); H20 = 12.89%."
* D ( - ) Zinc salt-2 H20 , [«]D = +8.25° (4%, 25°C); H20 = 12.89%.
C DL Zinc salt-3 H20 , [a]D = 0, H20 - 18.18%.
Lactic acid of L-configuration (dextrorotatory-sarcolactate) is produced b y streptococci, whereas the opposite configuration is produced b y mem- bers of the genus Leuconostoc. T h e lactate-producing Bacillus calidolactis, Bacillus coagulans, and Microbacterium and Rhizopus species also form L ( + ) - l a c t a t e , whereas pediococci and heterolactobacilli produce mixtures of isomers. Among the homofermentative lactobacilli, great variation exists in the t y p e of lactate produced.5 0
One determinent of lactic acid configuration is the stereospecificity of the lactic dehydrogenase involved. I n Lactobacillus plantarum (arabinosus), which produces DL-lactate, two lactic dehydrogenases are present, each specific for a different isomer of l a c t a t e .5 7 T h e combined action of these dehydrogenases can racemize either isomer of lactate with pyruvate being the intermediate. Katagiri, K i t a h a r a , and their associates claim t h a t the combined action of a stereospecific lactic dehydrogenase forming D ( - ) lactate and a lactic acid racemase is more important in their strains of L. plantarum. I n this system D-lactate is formed first, then racemized b y a
76 W. A. WOOD
mechanism which is distinct from the combined function of the two stereo- specific dehydrogenases.6 8 - 6 9 A similar racemase is secreted by Clostridium acetobutylicum™-™ which racemizes α-acetolactate stereoisomers as well as those of lactate. Racemizing enzymes are not unknown in nature. An α-hydroxy acid racemase has also been found in animal tissue.7 4 I n addi
tion, there is evidence for an interconversion of phosphoglycerate isomers in L. plantarum.*2 Although these structural differences are of considerable usefulness in classification or identification, they do not reflect fundamental differences in fermentation mechanism or in energy yield.
I n addition to glucose, other hexoses such as fructose, mannose, galac
tose, disaccharides including lactose, maltose, and sucrose, and starch and dextrin among the polysaccharides serve as substrates for the homolactic fermentation. I t is presumed t h a t these sugars are converted to intermedi
ates of the glycolytic system by inducible enzymes. Galactose is conspicu
ous for a variation in its fermentation b y hyaluronic acid-producing strains of Streptococcus pyogenes. Whereas glucose yields predominantly lactate in the usual manner, about 50 % of the galactose carbon appears as acetic and formic acids and ethanol in the ratio 2 : 1: 1 .7 5 - 7 8 T h e ratio is strikingly similar to t h a t obtained by Gunsalus and N i v e n4 4 for the fermentation of glucose b y S. liquefaciens at a high p H . Although the fermentation balances are imperfect, p H does not appear to be the sole factor responsible for di
version of the homolactic fermentation of galactose. T h e results m a y be explained by differences in t h e r a t e of pyruvate formation from the two hexoses, b u t the possibility of an independent p a t h w a y for galactose fer
mentation should serve to motivate further consideration of these observa
tions. Use has been made of the inducible nature of the galactose fermenta
tion system in S. agalactae (mastiditis) for the quantitative estimation of glucose and galactose in mixtures.7 9
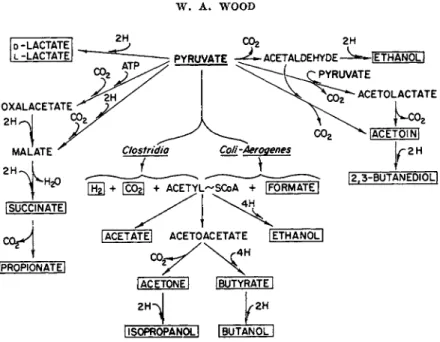
3. BUTYRIC ACID AND SOLVENT-PRODUCING FERMENTATIONS
Among the Clostridia and certain bacilli, butyric acid and solvents such as n-butanol, acetone, and isopropanol are characteristic products of carbo
hydrate fermentation.
a. Fermentation Types. Three closely related types are recognized. T h e differences reside in the presence of additional terminal reactions super
imposed upon the basic scheme.
T h e butyric type exemplified by Clostridium butyricum*1 and also dis
played by C. tyrobutyricumf2 and C. lacto-acetophilum*z produces butyric and acetic acids, carbon dioxide, and hydrogen. C. perfringens (welchii)*4'85 and C. tetania6 produce, in addition, lactate, ethanol, and sometimes for
mate. According to the fermentation balances in Table V, the following equation approximately fits the d a t a for C. butyricum:
2. FERMENTATION OF CARBOHYDRATES 77
T A B L E V
FERMENTATION BALANCES FOR CLOSTRIDIA
Products
mMoles/100 mmoles glucose fermented
Butyric acid 76 73 9β 3 46 4.3 17.2 29
Acetic acid 42 28 15 60 14.2 17.2 88 Lactic acid — — 160 33 — — 107 Carbon dioxide 188 190 24 176 221 203.5 48 Hydrogen 235 182 21 214 135 77.6 74 Ethanol — — 10 26 7.2 —
Butanol — — — — 56 58.6 - Acetone — — — — 22.4 — — Acetoin — — — — 6.4 — — Isopropanol — — — — — 12.1 — Carbon recovered, 96.0 91.0 98.3 97.1 99.6 96.2 110.0e
%
O/R balance 0.97 1.16 0.81 1.05 1.01 1.06 0.74
β Iron deficient.
6 Iron sufficient.
e Ci/Ct ratio - 0.33.
4 glucose —• 2 acetate + 3 butyrate + 8 CO2 + 8 H2
If it is assumed t h a t b u t y r a t e arises b y condensation of 2 acetate units, the a m o u n t of carbon dioxide observed agrees with t h e a m o u n t expected.
Further, a 1:1 ratio between hydrogen a n d carbon dioxide was observed.
Examination of fermentation balances in t h e literature reveals consider
able fluctuation in t h e quantity of products. I t is n o t possible t o rationalize all of these reports with current concepts, however, often because of ir
regularities in t h e experimental procedures employed. F o r instance, high ethanol and low butyric acid yields have been observed with a m u t a n t of C. tetani** and b y resting cells of C. botulinum*1 Also formate, n o t normally an end product in clostridial fermentations, h a s been reported as a major product for several species.8 4»8 5 T h e c. tetani balance, the p H of fermentation, and t h e amount of carbohydrate fermented in t h e formate-producing exper
iments should b e checked.
Although lactate is n o t fermented b y C. bvtyricum, under ordinary con-
Clostridium butyricum*1 Clostridium lacto acetophilum62 Clostridium perfringens8* ·81 Clostridium acetobutylicum9* Clostridium butylicum100 Butyribacterium rettgeri*9
78 W . A . WOOD
ditions, B h a t a n d Barker found t h a t a closely related organism, C. lacto- acetophilum, fermented lactate when acetate was added as t h e hydrogen acceptor.8 3 More recently B r y a n t et al. have reported t h a t C. tyrobutyricum, isolated from grass silages, and C. butyricum also ferment lactate under these conditions.8 2 Hence, from t h e standpoint of fermentation mechanisms, the similarities among C. butyricum, C. tyrobutyricum, a n d C. lacto-aceto- philum appear t o be greater t h a n t h e differences.
Another t y p e of butyric acid fermentation is displayed b y Butyribac- terium rettgeri. T h e products formed from glucose, pyruvate, or lactate are DL-lactate (glucose-substrate), carbon dioxide, hydrogen, acetic, and butyric acids. Caproic acid also is formed in lesser a m o u n t s8 8 (Table V ) . As observed with t h e Clostridia,85 t h e yield of lactate is increased with iron deficiency.8 9 I n contrast t o C. butylicum, which produces more t h a n one mole of carbon dioxide and not more t h a n one mole of C2 (acetic and b u - tyric acid X 2) per mole of triose, Butyribacterium produces 0.4 mole of car- bon dioxide and 1.2 moles of C2 per triose. Butyribacterium resembles several of t h e Clostridia in t h a t C14C>2 is fixed into b o t h t h e carboxyl and methyl groups of acetate during fermentation.9 0 Pine and B a r k e r9 1 have shown t h a t this process does not involve carbon dioxide fixation into di- carboxylic acids or t h e glycine-serine interconversion, and is stimulated rather t h a n inhibited b y the lack of iron.8 9 I n addition, acetate was shown to be t h e precursor of b u t y r a t e .9 0 During growth in a defined medium, with lactate as an energy source, there is an absolute requirement for lipoic acid. With glucose or pyruvate, however, lipoic acid is not required. Sev- eral lines of evidence indicate t h a t lipoic acid does not function in pyru- v a t e metabolism b y this organism. Hence, a role in lactate metabolism is indicated.9 2
T h e fermentation of glucose-l-C1 4 b y Butyribacterium rettgeri produced predominantly methyl-labeled products.8 9 Although t h e specific activity of t h e methyl group was less t h a n 50 %, it appears t h a t t h e Embden-Meyer- hof p a t h w a y is t h e main fermentation system involved.
The acetone-butyl fermentation b y C. acetobutylicum results from addi- tional terminal reactions which utilize t h e butyric acid and t h e precursors between acetyl a n d b u t y r a t e acetic acid t o produce butanol a n d acetone, re- spectively9 8 (Table V ) . M a n y studies have established t h e time course of product development a n d t h e effect of conditions a n d nutrition upon t h e outcome of t h e fermentation.9 4 -"9 6 Stiles et al., for instance, observed t h a t t h e acids appeared early in t h e fermentation a n d t h a t t h e solvents were pro- duced later. Again formic acid was encountered a n d was metabolized when added. Acetoacetate was implicated as a n important precursor of both b u t y r a t e a n d acetone.9 6 ·9 7 T h e decarboxylase responsible for conversion of acetoacetate t o acetone h a s been purified from extracts.9 6 - 9 8 ·9 9
2. FERMENTATION OF CARBOHYDRATES 79 T h e isopropyl fermentation b y C. butylicum closely resembles t h a t of C.
acetobutylicum except t h a t isopropanol is produced a t t h e expense of ace
t o n e .1 0 0 Again alkali suppresses alcohol formation a n d increases t h e acid p r o d u c t i o n .1 0 1*1 0 2 There can be little doubt t h a t acetone is t h e precursor of isopropanol, because its addition enhances t h e production of isopropanol.
Moreover, t h e addition of hydrogen acceptors increases t h e a m o u n t of isopropanol a n d acetone, a n d decreases t h e alcohols, presumably b y com
peting for t h e hydrogen utilized in alcohol formation.1 0 2 Added butyric acid is reduced t o butanol a n d increases t h e yield of isopropanol b y elimi
nating t h e demand for hydrogen atoms in butyric acid synthesis.1 0 1 b. General Characteristics of Clostridial Fermentations. I t is of consid
erable interest t o find t h a t Clostridia, which normally do not produce lac
tate, can carry out a homolactic fermentation under abnormal conditions.
Kempner and K u b o w i t z1 0 3*1 0 4 showed with C. butyricum t h a t carbon mono
xide a n d cyanide diverted t h e fermentation t o t h e homolactic type in a manner which could be reversed b y light or b y removal of t h e inhibitor.
103, io5 τη β effective level of carbon monoxide or cyanide was of t h e same order of magnitude required t o inhibit respiration of aerobic cells. A similar sensitivity h a s not been found in other lactic a n d ethanolic fermentations.
T h e lactate formed is DL with a slight excess of t h e L-component.1 0 8 Pappenheimer and S h a s k a n8 6 produced a homolactic fermentation in C.
perfringens b y decreasing t h e iron content of t h e medium (Table V ) . W i t h iron-deficient medium (0.04 Mg. of iron per ml.) growth was decreased about 50 % a n d 1.7 moles of lactate per mole of glucose were produced. Ad
dition of iron u p t o 0.64 μg. per ml. increased growth a n d decreased lactate production t o typical levels. A t suboptimal iron concentrations t h e iron was completely taken u p b y t h e cells. Similarly, t h e addition of iron t o whey fer
mentation b y C. acetobutylicum decreased t h e lactate, formate, a n d ethanol content, a n d increased t h e a m o u n t of hydrogen, carbon dioxide, acetone, acetoin, b u t y r a t e , a n d acetate. Also, cultures obtained b y serial transfer in low iron medium produced a homolactic fermentation.1 0 7
T h e d a t a on (a) shift t o lactic fermentation b y carbon monoxide, cyanide, and iron deficiency, (b) t h e reversal of t h e monoxide a n d cyanide effect b y light, and (c) inhibition b y t h e same concentrations needed t o inhibit res
piration parallel t h e behavior expected from iron porphyrin function in p y r u v a t e dissimilation. However, no spectral evidence has been obtained for these respiratory catalysts in Clostridia.107* Further, Lerner a n d Mueller
1 0 8 found t h a t cells from a n iron-deficient medium, which do not ferment glu
cose, could be activated b y glutamine, thereby suggesting a n indirect effect of iron.
T h e early fragmentary biochemical evidence on t h e mechanism of fermen
tation suggested a non-Embden-Meyerhof process (lack of fluoride inhi-
80 W. A. WOOD TABLE VI
DISTRIBUTION OF C1 4 IN THE PRODUCTS OF GLUCOSB-C1 4
FERMENTATION BY Clostridium perfringens Relative specific activity0
Product
Glucose- 3,4-C1 4
Glucose- 1-C1 4
Glucose-2-C1 4
Glucose- 6-C1 4
Carbon dioxide 9 5 . 0b 0b 0* 0C 0b
Ethanol
CH3— 4.2 25.2 0.3 0 22.7
— C H 2 O H 2.1 0.4 21.3 48.2 0.2
Acetic acid
CH8— 1.1 41.5 0.3 0 35.2
—COOH — 0.2 46.8 47.0 0
α Specific activity of each labeled carbon atom of glucose = 100.
6 From Paege and co-workers.1 1 6 c From Cynkin and Gibbs.1 1 7
bition,1 4 methylglyoxal formation1 0 0' 1 0 9) . More recent enzymic and iso- topic evidence, although incomplete, clearly indicates the existence of the classical fermentation route. An iron-requiring aldolase, triose phosphate isomerase and glyceraldehyde-3-phosphate dehydrogenase, were reported by Bard and Gunsalus.1 1 4 Also, the reduction of diphosphopyridine nucleo
tide ( D P N ) b y glucose, glucose-6-phosphate, fructose-6-phosphate, and fructose diphosphate is interpreted as evidence for t h e hexose diphosphate p a t h w a y .1 1 5 F u r t h e r evidence for operation of t h e hexose diphosphate p a t h w a y was obtained from t h e fermentation of glucose-l-C1 4, -2-C1 4, -3,4-C1 4, and -6-C1 4 b y C. perfringens.11* T h e labeling of products (Table VI) was qualitatively t h a t expected of the glycolytic system. However, ethanol and acetic acid formed from glucose labeled in carbon atoms 2, 6, or 3 and 4 were not similarly labeled as would be expected if these products were derived from a common precursor and produced a greater t h a n 50 % decrease in specific activity. M o r e recently Cynkin and Gibbs1 1 7 found equal labeling in ethanol and acetate with the expected 5 0 % decrease in specific activity.
During glucose fermentation, a large number of added compounds are converted to normal products. P y r u v a t e and acetate contribute to all of the products1 1 8 and butyrate, acetoacetate, acetone, acetaldehyde, and acet- oin are precursors of butanol, acetone, isopropanol, ethanol, and 2,3-bu- tanediol, r e s p e c t i v e l y .9 9 , 1 0 1 ·1 1 3 , 1 1 8 A comprehensive survey of compounds utilized in this manner has been reported b y D a v i e s9 6 (Table V I I ) .
P y r u v a t e fermentations v a r y from t h a t of C. acetobutylicum110 and C.