Cyclic Mechanisms of Terminal Oxidation
L . 0 . KRAMPITZ
I. Introduction 209 A. Statement of the Problem 209
B. The Role of Cyclic Mechanisms 210 II. Historical Development of Cyclic Mechanisms 211
A. C4 Dicarboxylic Acid Cycle of Szent-Gyorgyi 211
B. Tricarboxylic Acid Cycle of Krebs 212 III. Criteria for Establishment of Cyclic Mechanisms 213
A. Rates of Reaction 213 B. Quantitative Aspects and Malonate Inhibition 214
IV. Criterion of Rates of Reaction Applied to Microorganisms 218 V. Rate Studies with Bacteria: Micrococcus lysodeikticus and Escherichia
coli 220 VI. Carrier Type of Experiments Employing Isotopes 222
VII. Noncarrier Type of Experiments Employing Isotopes 225
A. Hypothesis of Technique 225 B. The Problem with Yeast 229 VIII. Quantitative Aspects of the TCA Cycle 231
A. Fumarate as Electron Acceptor for Acetate Oxidation 231
B. Evidence against C4 Dicarboxylic Acid Cycle 233
IX. The Criterion of Sequential Induction 235 X . The Criterion of Microbial Mutant Analysis of Metabolic P a t h w a y s . . . . 236
X I . Deviations from the TCA Cycle 238
A. Isocitritase 239 B. Malate Synthetase 239 C. Variation of the TCA Cycle 240
X I I . General Occurrence of the TCA Cycle in Microorganisms: Bacteria, Molds,
and Protozoa 242 X I I I . Alternate Pathways 243
A. Oxidative Pentose Phosphate Cycle 244 B. Occurrence of Cycle in Microorganisms 249
XIV. Summary and Conclusions 252
References 253 Suggested Supplementary Reading 255
I. Introduction
A . STATEMENT OF THE PROBLEM
N a t u r e has endowed cells with unique a n d complicated cyclic mechanisms for t h e oxidation of foodstuffs to carbon dioxide a n d water. Teleologically one would assume t h e more direct these mechanisms, t h e more efficient t h e
209
processes should be for the cell. Were the processes designed solely for ob
taining energy for cellular functions by oxidation, one could question the rationale of the evolutionary processes. On the contrary, as if purposely to confuse researchers, a duality in purpose was conceived b y nature. While the obtainment of energy through electron transfer for cellular functions is most important, the cell requires, in addition, carbon skeletons for the syn
thesis of cellular material. For example, if t h e only mechanism for the oxida
tion of carbohydrate substances were a cyclic pathway, none of the inter
mediates could be removed for biosynthesis of amino acids, fats, or other constituents of the cell, since t h e cyclic process would be interrupted and oxidation would cease. A cyclic mechanism with provisions for replacement of cyclic components appears to be a most efficient mechanism for t h e syn
thesis of these cellular components and for the yield of energy by oxidation.
I t is the intent of this chapter to describe these cyclic mechanisms, particu
larly as they relate to the way by which the various microbial cells obtain energy for their life processes and the synthesis of cellular components.
B . T H E R O L E OF CYCLIC MECHANISMS
After the discovery of the Harden and Young ester (fructose 1,6-diphos- phate) in 1906,1 the elucidation of the mechanism of fermentation of glucose to lactic acid by muscle tissue and to ethyl alcohol and carbon dioxide b y yeast was rapidly achieved (see Chapter 2, Vol. I I ) . T h e two processes were found to be almost identical. T h e main difference concerns t h e fate of py
ruvic acid, which is one of the common intermediates in the sequence of reactions catalyzed by both tissues. I n muscle tissue, the pyruvic acid is reduced to lactic acid by the electrons involved in the oxidative step in t h e conversion of 3-phosphoglyceraldehyde to 1,3-diphosphoglyceric acid. Elec
tron transfer is mediated by diphosphopyridine nucleotide ( D P N+) . I n yeast, on the other hand, pyruvic acid is decarboxylated to acetaldehyde and carbon dioxide, and the electrons involved in the oxidative step re
ferred to above reduce the acetaldehyde to ethyl alcohol. I t was soon estab
lished t h a t m a n y microorganisms metabolized glucose via pyruvic acid in a similar manner. These processes are anaerobic and b y definition are fer
mentative, i.e., the electrons involved in the oxidative step are transferred to an acceptor other t h a n oxygen. I n view of the fact t h a t pyruvic acid a p peared to be a cardinal intermediate of carbohydrate metabolism, m a n y investigators turned their attention toward the elucidation of the mecha
nism of complete oxidation* of this acid. Most of the early information was obtained employing tissues from mammalian origin. T h e reason was two
fold: (1) considerable seemingly related information gained from experi
mental work with mammalian tissue was at hand, and (2) as will be shown subsequently, certain criteria for the ultimate proof of a metabolic series of events were more easily met b y the use of mammalian tissue.
During the last two decades, a great deal of research has been done in a n a t t e m p t to establish these pathways of oxidation in microorganisms, em
ploying the same criteria which were so successful with mammalian tissue.
I n order fully to appreciate the problems concerned with microbial experi
mentation in this area, a brief historical account of the early events and a description of these criteria with mammalian tissue is pertinent. I t is not the purpose of this chapter to describe the detailed enzymic mechanisms of the pathways of oxidative metabolism, which can be found in several excellent textbooks of biochemistry.
II. Historical Development of Cyclic Mechanisms
Szent-Gyorgyi2 found t h a t the oxidation of carbohydrate by minced mus
cle tissue was greatly stimulated b y the addition of very small a m o u n t s of fumaric, malic, oxalacetic, and succinic acids. H e recognized the importance of these C4 dicarboxylic acids for the oxidative metabolism of carbohy
drates, a n d proposed t h a t they were acting catalytically as electron carriers to oxygen. Figure 1 illustrates his concept. I t is important to observe t h a t the C4 dicarboxylic acids are not intermediates in the oxidation of the carbon skeleton of the carbohydrate. T h e y serve only as electron carriers. Thus, while this so-called C4 dicarboxylic acid cycle can explain the transfer of electrons to oxygen, t h e ultimate electron acceptor, with t h e formation of water, it provides no information as to the p a t h w a y of carbon dioxide forma
tion. I n the process, oxalacetic acid accepts two electrons from a n oxidative step in carbohydrate oxidation, forming malic acid. T h e elements of water are removed from malic acid t o form fumaric acid. T h e latter acid is reduced b y another oxidative step in carbohydrate oxidation to succinic acid. T h e
A . C4 DICARBOXYLIC ACID CYCLE OF SZENT-GYORGYI
HOOC CH2-CO-COOH -> Oxalacetic acid
Fumaric acid + 2Η| | - 2Η
HOOC · CH2 · CH2 · COOH - 2 H
O2 via cytochrome system —* H20 Succinic acid
FIG. 1. Szent-Gyorgyi cycle of respiration.
Oxalosuccinic
acid a-Ketoglutaric acid
OOH
Succinic acid FIG. 2. Pathway of oxidation of citric acid.
succinic acid is reoxidized to oxalacetic acid with the electrons passing to oxygen via the cytochrome system with the formation of water. After twelve such oxidation steps the carbohydrate is oxidized to carbon dioxide.
As mentioned previously, the proposed mechanism does not provide in
formation concerning the intermediates of carbohydrate oxidation.
A series of reactions involving tricarboxylic acids which was apparently related to the C4 dicarboxylic acid cycle was studied by Martius and Κ η ο ο ρ .3·4 These investigators found t h a t liver tissue rapidly oxidized citric acid to succinic acid. T h e y presented evidence to indicate t h a t the series of reactions depicted in Fig. 2 were involved. While not all of the intermediate steps in the oxidation of citric acid to succinic acid were rigorously proven, the scheme indicated a possible relationship between tricarboxylic and di
carboxylic acids which were rapidly metabolized b y m a n y types of tissue.
B . TRICARBOXYLIC ACID CYCLE OF K R E B S
Krebs and Johnson in 19376 b y brilliantly conceived experiments were able to establish t h e relationship and to demonstrate the p a t h w a y of carbo
h y d r a t e oxidation b y mammalian tissue. T h e y showed t h a t catalytic amounts of citric acid greatly stimulated the respiration of minced pigeon breast muscle, particularly if carbohydrate was present. Instead of assum
ing t h a t citric acid was serving as a n electron carrier, as had Szent-Gyorgyi for the dicarboxylic acids, Krebs believed t h a t citric acid resulted from the condensation of some derivative of carbohydrate cleavage with oxalacetic acid. T h e oxidation of citric acid to succinate b y reactions shown in Fig. 2, and of succinate to oxalacetate b y the reactions shown in Fig. 1, completed
MAUC ACIO
+HtO ^ < 3 > C O O H
fr
FROM CARBOHYDRATE FATS AMINO ACIDS ETC.
φ COOH
<2>HCH QHCOH
I
CITRIC ACID
FUMARIC ACID
φ COOH (J)CH
®CH φ COOH
-2H
V
Φ COOH®HCH SUCCINIC ®HCH
ACID I
®COOH
<8>C02
CIS-ACONITIC ACID
-KETOGLUTARIC
ACID OXALOSUCCINIC
ACID (» CO,
FIG. 3. Tricarboxylic acid cycle,
a cycle with t h e elimination of two carbon atoms (originally from carbo
hydrate) as carbon dioxide. After completion of t h e cycle, oxalacetate was condensed again with a carbohydrate derivative t o form citric acid a n d t h e cycle initiated again. This cycle is referred to as t h e Krebs cycle or t h e tri
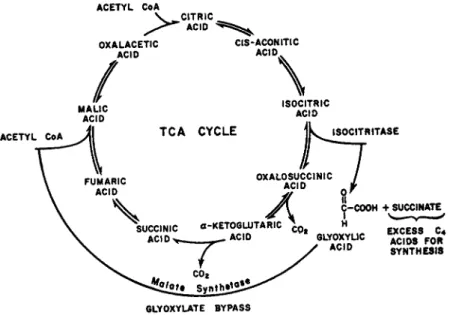
carboxylic acid cycle (TCA cycle) a n d is shown in Fig. 3.
III. Criteria for Establishment of Cyclic Mechanisms
A. R A T E S OF REACTION
Krebs a n d Johnson established t h a t t h e rates of all t h e individual oxida
tive reactions of t h e cycle they were able t o test were commensurate with the total rate of oxidation of citrate. One of t h e premises held a t t h a t time was t h a t for a proposed compound t o be a n intermediate in a sequence of metabolic reactions, it must be converted t o t h e same end products as t h e
parent compound, a n d a t a n equal or greater rate. While this assumption is reasonable, subsequent work, as will be discussed later, has shown t h a t the rate of reactions, particularly with whole cell preparations, is not a re
liable criterion for the establishment of intermediary sequences. Neverthe
less, in these investigations by Krebs and co-workers, rate studies were of incalculable value. T h e y were also able to confirm the observations made by Martius and Κ η ο ο ρ3»4 who had shown the conversion of citric acid to α-ketoglutaric acid. Arsenite, a specific inhibitor of α-keto acid oxidation, was used to inhibit the further oxidation of α-ketoglutaric acid. T h e con
version of citric acid to succinic acid was also demonstrated b y the use of malonic acid as an inhibitor of succinic acid oxidation.
I t has been emphasized t h a t the cycle provides a mechanism for the oxida
tion of carbohydrates to carbon dioxide. Because of the major position held by pyruvic acid in intermediary carbohydrate metabolism, it was thought t h a t this acid was involved in the primary condensation reaction with ox
alacetic acid. As a result of the excellent work b y L i p m a n n6 and his co
workers, Lynen7 and co-workers, Ochoa8 and co-workers, Gunsalus9 and co
workers (see Chapter 1, Vol. I I ) , and Green1 0 and co-workers, it has been established t h a t the acetyl derivative of coenzyme A (acetyl CoA) is t h e moiety derived from the oxidative decarboxylation of pyruvic acid, which condenses with oxalacetic acid to form citric acid.
Perhaps the most important contribution of Krebs and Johnson in 1937 was the demonstration of the synthesis of citric acid from oxalacetic acid.
Prior to this time, considerable work had been done with various microor
ganisms which formed large quantities of citric acid, and it had been sug
gested t h a t a C4 dicarboxylic acid and a C2 fragment were involved in t h e synthesis.1 1*1 2 Detailed evidence, however, was not available. T h e experi
ments by Krebs and Johnson employing minced pigeon breast muscle showed t h a t large amounts of citric acid were synthesized anaerobically from oxalacetic acid. These investigators were not a t t h a t time able to as
certain t h e nature of the moiety which condensed with oxalacetic acid to form citric acid, but as stated above, we now know it to be acetyl CoA. Perhaps in these experiments acetyl CoA was endogenously present and pyruvic acid, which arose from the decarboxylation of a portion of the oxalacetic acid, was oxidized to acetyl CoA by an anaerobic dismutation reaction.
Nevertheless, the synthesis of citric acid, a very important reaction for the concept of the T C A cycle, was demonstrated.
B . QUANTITATIVE ASPECTS AND MALONATE INHIBITION
Krebs and Eggleston1 3 later made the following significant observation which indicated the quantitative importance of the cycle in pigeon breast muscle. T h e y demonstrated t h a t malonate completely inhibited t h e oxida-
tion of pyruvate b y this tissue. This result was compatible with either t h e Szent-Gyorgyi C4 dicarboxylic acid cycle or t h e T C A cycle, since succinic dehydrogenase is required b y both mechanisms. I n t h e former mechanism the flow of electrons would be interrupted inasmuch as t h e oxidation a n d reduction of succinic acid were inhibited. I n t h e T C A cycle mechanism, the inhibitor prevented t h e formation of oxalacetate b y inhibiting t h e oxida
tion of succinic acid, thereby stopping t h e cyclic mechanism. If t h e mecha
nism of t h e T C A cycle were valid, pyruvic acid should be aerobically oxi
dized b y malonate poisoned muscle if t h e oxidation product of succinic acid, i.e., fumaric acid, were also added. I n this manner oxalacetate could be derived from t h e oxidation of fumarate a n d t h e cycle could be initiated in spite of t h e inhibition of t h e oxidation of succinate. Such a n experiment was performed a n d t h e following stoichiometry obtained:
Pyruvate + fumarate + 2 02 j^ t e d> succinate + 3C0* + H* ° C3H4O3 + C4H4O4 + 2 02 > C4He04 + 3 C 02 + H20
Four oxidative steps occur which account for t h e four atoms of oxygen con
sumed. T h e y are: (1) oxidation of fumarate via malate to oxalacetate; (2) oxidation of pyruvate to acetyl CoA a n d carbon dioxide; (3) oxidation of isocitrate, formed via citrate from acetyl CoA a n d oxalacetate, to oxalo- succinate; a n d (4) oxidation of α-ketoglutarate, formed b y decarboxylation of oxalosuccinate, t o succinate a n d carbon dioxide.
This experiment demonstrated clearly t h a t succinate could arise oxida- tively from pyruvate under conditions where t h e reductive p a t h w a y of succinate formation was inhibited b y malonate. T h e oxidation of pyruvate by t h e inhibited muscle tissue could only occur when a C4 dicarboxylic acid (a source of oxalacetate) other t h a n succinic acid was simultaneously added.
While Krebs a n d co-workers were developing t h e important concept of the tricarboxylic acid cycle, Wood a n d W e r k m a n1 4 were developing t h e equally important concept of heterotrophic carbon dioxide fixation (see Chapter 2, Vol. I I I ) . I t h a s been previously stated t h a t in t h e T C A cycle, as it occurs in muscle tissue, if a n y of t h e C4 dicarboxylic acids were removed for a n y other purpose, t h e cycle would be interrupted unless these acids were resynthesized. Working with t h e propionic acid bacteria, Wood a n d W e r k m a n1 6 showed t h a t these organisms formed considerable quantities of succinic acid when glycerol was fermented in t h e presence of sodium bi
carbonate. These results suggested t h a t succinic acid was formed from oxalacetate which h a d been synthesized b y carbon dioxide fixation with pyruvate. This reaction proved to be very important for t h e elucidation of t h e tricarboxylic acid cycle in liver tissue. I n a n a t t e m p t to demonstrate the T C A cycle in pigeon liver, E v a n s1 6 demonstrated t h a t t h e oxidative re
moval of pyruvic acid was not inhibited b y malonate a n d t h a t a-ketogluta-
r a t e and succinate accumulated. I t appeared t h a t a n unknown mechanism for t h e synthesis of C4 dicarboxylic acids existed in liver. These results were in direct contradiction with t h e results of similar experiments employing muscle tissue. Other experiments established t h a t t h e T C A cycle was opera
tive in liver tissue, t h u s posing t h e question of t h e mechanism b y which liver tissue brought about a synthesis of C4 dicarboxylic acids under condi
tions of malonate poisoning. Wood a n d W e r k m a n1 7 and Krebs a n d Eggles- t o n1 8 suggested t h a t t h e carbon dioxide fixation reaction discovered b y Wood and Werkman, i.e., p y r u v a t e + carbon dioxide —> oxalacetate, was t h e mechanism b y which liver tissue synthesized the C4 dicarboxylic acid required for the oxidation of p y r u v a t e under conditions of malonate poison
ing. E v a n s and Slotin1 9 employed the isotope of carbon C1 1 to demonstrate t h a t carbon dioxide was fixed in α-ketoglutarate, which indicated t h a t t h e Wood and W e r k m a n reaction was involved. Wood a n d collaborators2 0 es
tablished t h a t carbon dioxide fixation occurred, a n d determined some quan
titative aspects of the T C A cycle in pigeon liver. I n order to understand more easily the importance of this work, t h e reader is referred to Fig. 3.
These investigators used pyruvic acid a n d N a H C1 303 as substrates under aerobic conditions, in t h e presence of malonate as an inhibitor of succinic dehydrogenase. If it is assumed t h a t pigeon liver can catalyze t h e fixation of carbon dioxide with pyruvic acid to form oxalacetic acid a n d t h a t t h e oxidation of pyruvic acid can occur with t h e formation of acetyl CoA, t h e latter will condense with the oxalacetic acid to form citric acid. T h e malonic acid will inhibit succinic dehydrogenase a n d therefore succinic acid cannot be formed by a reductive reaction, nor can it be oxidized after formation from t h e oxidation of α-ketoglutaric acid. Malic a n d fumaric acids will be formed b y t h e reduction of the oxalacetate formed b y carbon dioxide fixa
tion and will consequently contain C1 3 in their carboxyl groups. T h e tracing of the isotope in t h e various compounds as illustrated in Fig. 3 should be done literally. As depicted two-dimensionally on paper, citric acid appears to be a symmetrical compound and as such one would expect aconitase to remove t h e elements of water in a symmetrical manner. T h a t is, one-half of the molecules of aconitic acid would h a v e t h e double bond above t h e center carbon atom, number 3 as written, a n d t h e remaining molecules would have t h e double bond below t h e center atom. T h e formation of iso- citric acid by t h e action of aconitase would place t h e hydroxyl group alpha to carbon a t o m number one in half of t h e molecules, a n d g a m m a to carbon a t o m one in t h e other half of t h e molecules. After oxidation of t h e isocitric acid to succinic acid, isotope would appear in a carboxyl group of half of t h e succinic acid molecules. T h e experimental results (Table I) showed t h a t t h e succinate did not contain isotope. Wood a n d co-workers interpreted these results to indicate t h a t isocitric acid is t h e product of condensation of
T A B L E I
DISTRIBUTION OP C1 8 IN TRICARBOXYLIC ACIDS BY PIGEON LIVER*
Compound Excess C1 8
(%) α-Ketoglutaric acid
α-Carboxyl group carbon atom 1.1 0.03 0.08 0.8 Remaining 4 carbon atoms
Succinic acid
Citric, malic, and fumaric acid car
boxyl group carbon atoms
° For particulars of experiments see ref. 20.
the moiety from pyruvic acid and oxalacetic acid and t h a t the equilibrium catalyzed by aconitase (i.e., formation of citric acid) did not occur or was extremely sluggish. Subsequent work has shown t h a t citric acid is the pri
m a r y product but t h a t enzymically it exhibits asymmetrical properties which account for the observed results. Under these circumstances it will be observed t h a t the carboxyl group adjacent to t h e keto group of a-ketoglu- taric acid does contain isotope which is evolved as carbon dioxide during t h e oxidative step to succinic acid. Under conditions of the experiment, i.e., malonic acid present as an inhibitor of succinic dehydrogenase, a-keto- glutarate accumulated sufficiently to permit its isolation and analysis for isotopic content. I t was not understood why α-ketoglutarate accumu
lated; but for t h e establishment of the occurrence and quantitative importance of the T C A cycle under these conditions, this accumulation was indeed fortunate. I t must be recalled t h a t while Krebs and co-workers h a d established the framework of the T C A cycle, practically no information was available concerning the intimate mechanisms. H a d α-ketoglutaric acid not accumulated, the employment of C1 302 as the tracer isotope would not have yielded a n y definitive results, since the succinate contained no isotope.
B y determining quantitatively t h e α-ketoglutaric acid and finding the C1 3 exclusively in t h e carboxyl group adjacent to the keto group, Wood and co-workers were able t o confirm t h e existence of t h e T C A cycle and to es
tablish the synthesis of oxalacetic acid from pyruvic acid and C 02 as a mechanism for t h e synthesis of the C4 decarboxylic acid so essential for maintenance of the T C A cycle. Within a short time all of the individual steps of t h e T C A cycle were clearly established.
F r o m all of these results there could be no doubt as to the importance of t h e T C A cycle in mammalian tissue. Nevertheless very little d a t a were a t h a n d which permitted a rigorous evaluation as t o its quantitative impor
tance. T h e possibility remained t h a t some other mechanism which included some of t h e same intermediates as t h e T C A cycle, b u t not in the same se-
quence, was equally as important. Until such d a t a are a t hand it is always dangerous to eliminate other mechanisms. T h e latter warning is exemplified particularly in the area of carbohydrate metabolism (see Chapter 2). A few years ago, the only mechanism which was well established for the break
down of glucose was t h a t referred to as the Embden-Meyerhof scheme. We now know there are several other mechanisms and t h a t these mechanisms exist in organisms and tissues formerly believed to contain exclusively the Embden-Meyerhof pathway. This point will be referred to again.
IV. Criterion of Rates of Reaction Applied to Microorganisms I n their original work, Krebs and Johnson found t h a t m a n y mammalian tissues oxidized citric acid in addition to synthesizing it from oxalacetate.
They concluded t h a t these tissues possessed the T C A cycle. On the other hand, they inferred t h a t yeast and Escherichia coli did not possess t h e cycle, since these organisms could not oxidize citric acid at an appreciable r a t e ; furthermore, some of the other criteria applicable to mammalian tissue were not satisfied with these microbial systems. This immediately posed the problem of the mechanism employed by microorganisms to oxidize carbo
hydrates or their breakdown products. Furthermore, it is well known t h a t m a n y microorganisms can grow with acetic acid as the sole source of carbon.
Since the T C A cycle required a source of dicarboxylic acid for continuance of the cycle, it was reasoned t h a t these organisms must have an unknown mechanism for the synthesis of the C4 dicarboxylic acid from acetic acid, or t h a t a n unknown pathway existed for the oxidation of acetic acid.
I t can be readily shown t h a t m a n y microorganisms either will not oxidize citric acid, or oxidize it a t a rate not commensurate with the rate of oxida
tion of glucose, pyruvate, or acetate. On the other hand, m a n y of these same organisms will oxidize the C4 dicarboxylic acids a t a very rapid rate.
This observation made it attractive to propose t h a t microorganisms pos
sessed a pathway of oxidation comparable to a scheme suggested b y T h u n - berg m a n y years ago.2 1 Figure 4 outlines the essential features of this pathway. The important, yet not conclusively demonstrated, step is the oxi
dative condensation of two molecules of acetic acid to succinic acid. T h e remaining reactions have m a n y features in common with the reactions in
volving C4 dicarboxylic acid components of the T C A cycle. T h e oxidation process has essentially resulted in the oxidation of one mole of acetic acid to carbon dioxide and water. All of the steps, with the exception of t h e oxidative condensation of two molecules of acetic acid to succinic acid, can be demonstrated in m a n y microorganisms. M a n y a t t e m p t s have been m a d e to demonstrate the condensation step, but no conclusive evidence exists t h a t it occurs. I n those cases where claims for its demonstration in bacteria have been made, the reaction appears to be so sluggish t h a t it is of little
FUMARIC ACID
SUCCINIC ACID
COOH HCH
COOH HCH I
I HCH 4 COOH
COOH HCH I
THUNBERGS OXIDATIVE CONDENSATION OF ACETATE
COOH HCH I HCOH I
COOH I MALIC
ACID
DICARBOXYLIC ACID CYCLE + 2 H - 2 H
OXALACETIC ACID
COOH PYRUVIC ACID
FIG. 4. Dicarboxylic acid cycle for oxidation of acetic acid. Net result: The oxida- tion of one molecule of acetic acid to carbon dioxide and water. Cycle repeats.
consequence.2 2 I n keeping with other warnings stated above it would be folly to deny the existence of the reaction; however, more definitive evidence is required. Evidence against this cycle in some microorganisms will be presented later. More important at this stage of the discussion is the fact t h a t if the criterion of rates of reaction is taken as evidence for the opera- tion of a cyclic mechanism in microorganisms, the occurrence of the T C A cycle would appear to be excluded. M u c h of the early work with microor- ganisms was done with E. coli, and additional evidence against the existence of the T C A cycle in this organism was the well-known fact t h a t citric acid will not serve as the sole source of carbon for its growth. On the other hand, it is also known t h a t if some readily metabolized nutrients, such as peptone, glucose, or acetate, are added to the growth medium, citrate will be readily metabolized.2 3 Apparently the cell wall is impermeable to citrate a n d an in- ducible mechanism for its transfer is present which is facilitated by the presence of nutrients t h a t are utilized in a noninducible manner. Unless all t h e intimate details (on a n enzymic level) concerning rate studies of a series of reactions are known, caution must be exercised in interpreting t h e data
obtained from such studies. This caution is well exemplified in the case of acetate oxidation b y muscle tissue. I t has been stated previously t h a t Krebs and his co-workers were confident t h a t some derivative from carbohydrate metabolism condensed with oxalacetate to form citric acid, i.e., t h e first reaction in the cycle. I n an a t t e m p t to elucidate this step they came to t h e conclusion t h a t pyruvic acid condensed with oxalacetate to form a seven- carbon compound which was oxidatively decarboxylated to citric acid. T h e main reason for this proposal was t h a t acetate was not oxidized by muscle a t a rate commensurate with the rate of oxidation of pyruvate. When pyru
v a t e is oxidatively decarboxylated, acetyl CoA is a n early product of the reaction, and it is this derivative and not the free acetic acid which con
denses with oxalacetate to form citrate. When free acetate is added it must first be converted to acetyl CoA, and the preparations used by the early in
vestigators were incapable of performing this metabolic act. Therefore in drawing conclusions from data obtained from rate studies, one must be aware of (1) the permeability problems, and (2) the possibility t h a t the sub
strate one is dealing with is not the one actually employed b y the cell.
V. Rate Studies with Bacteria: Micrococcus lysodeikticus and Escheri
chia coli
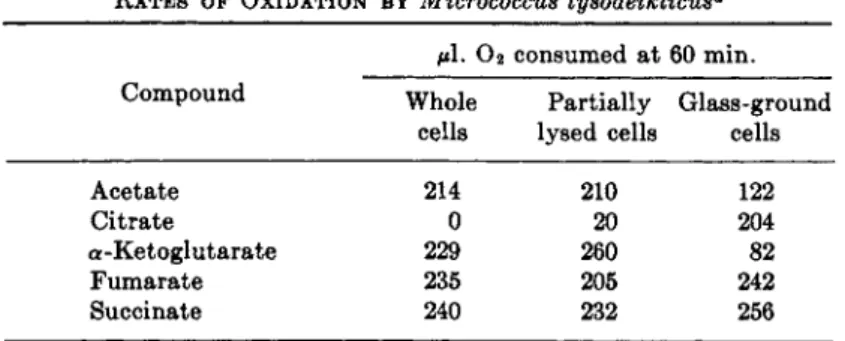
R a t e studies have been extensively performed with two microorganisms, an aerobe, Micrococcus lysodeikticus, and a facultative anaerobe, E. coli.
Typical results obtained with Μ. lysodeikticus are given in Table I I .2 4 W i t h whole cells the rates of oxidation of acetate, α-ketoglutarate, fumarate, and succinate are all comparable. Citrate is not oxidized. Cells t h a t have been partially lysed with lysozyme exhibit similar rates. Cells t h a t have been disrupted by glass-grinding oxidize citrate rapidly but such preparations, in contrast to the whole cells or to cells which have been partially lysed by lysozyme, oxidize α-ketoglutarate very slowly. Neither of these preparations
TABLE II
RATES OF OXIDATION BY Micrococcus lysodeikticus0
μ\. Ο 2 consumed at 60 min.
Compound Whole Partially Glass-ground cells lysed cells cells
Acetate 214 210 122
Citrate 0 20 204
a-Ketoglutarate 229 260 82
Fumarate 235 205 242
Succinate 240 232 256
a For particulars of experiments see ref. 24.
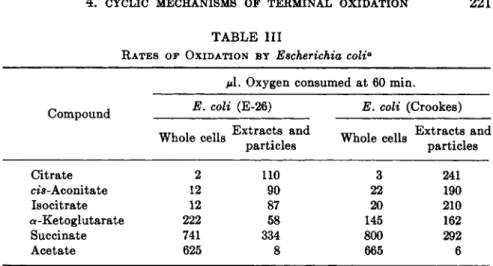
TABLE III
RATES OF OXIDATION BY Escherichia coli*
μ\. Oxygen consumed at 60 min.
Compound E' C°U ^E'2 6^ E' c o l i (C r o o k e s)
Whole cells E x t r*c t sl and Whole cells and
particles particles Citrate 2
m-Aconitate 12 Isocitrate 12 a-Ketoglutarate 222 Succinate 741 Acetate 625
110 3 241 90 22 190 87 20 210 58 145 162 334 800 292 8 665 6
β For particulars of experiments see refs. 25, 26.
satisfies the rate criterion and on this basis if each preparation is taken individually the T C A cycle could be excluded. However, the combined properties of the lysed preparation and the glass-ground preparation are such t h a t the rate criterion is satisfied.
Table I I I illustrates rates of oxidation of some of the tricarboxylic a n d dicarboxylic acids b y whole cells and disrupted cells of two strains of E.
coli?6*26 Whole cells of both strains are practically incapable of oxidizing citrate, cis-aconitate, or isocitrate. Their ability to oxidize a-ketoglutarate is greater, b u t cannot compare with the ability of these cells to oxidize ace
t a t e or succinate. Clearly on t h e basis of r a t e of oxidation t h e Ce tricarbox
ylic acids or α-ketoglutaric acid are not intermediates in t h e oxidation of acetate. On the other hand, succinate could be a n intermediate on this basis.
Malic a n d fumaric acids, d a t a for which are not shown in t h e table, are ox
idized as rapidly as is succinate. An entirely different result is obtained with disrupted cells of these two strains. During t h e preparation of disrupted cells the enzymes responsible for the activation of acetic acid (formation of acetyl CoA) are damaged; therefore the rate of oxidation of p y r u v a t e is more indicative of the true rate of oxidation of acetate. Of particular sig
nificance is t h a t with both preparations and particularly with the prepara
tion obtained from the Crookes strain, the rate of oxidation of citrate, cis- aconitate, a n d isocitrate has been considerably increased. I t might be con
cluded t h a t the whole cells do not oxidize acetate b y way of the T C A cycle, a n d t h a t t h e dicarboxylic acid cycle is of importance. On t h e other hand, t h e criterion of rate would indicate t h a t the T C A cycle is in operation when the cells are disrupted.
These d a t a obtained from experiments with M. lysodeikticus and E. coli illustrate vividly the dangers of depending exclusively on rate studies. I t
will be shown t h a t the T C A cycle is the major p a t h w a y of oxidation of ace
tate by both of these organisms. I n all probability, disrupted preparations are capable of oxidizing the tricarboxylic acids more rapidly t h a n whole cells because a permeability barrier has been destroyed, although this has not been proved. The data on pyruvate and acetate oxidation b y disrupted cells of both strains of E. coli also illustrate the difficulties sometimes en
countered when a substrate which is only related to the actual substrate is added. Acetate is not the actual substrate, and cannot be converted to acetyl CoA, which is the substrate. I t is obvious, therefore, t h a t caution should be exercised when determining whether a compound is an interme
diate. Even if the" criterion of rates of reaction were satisfied, such d a t a do not reveal any information concerning the sequence in which the reac
tions are occurring. I n order to obtain evidence for the oxidation by the T C A cycle it is necessary to establish t h a t the various reactions occur in the sequence dictated by the cycle. Sometimes this important fact is neg
lected. T h e mere demonstration of the presence of all the enzymes required for the T C A cycle does not give any indication of the quantitative impor
tance of the entire cycle in the cell. I t is obvious t h a t even if the cycle ac
counted only for a small percentage of the total oxidation of acetate, the various enzymes could be readily demonstrated. If t h e remaining p a t h w a y of oxidation was entirely unknown, the experiments might not detect it.
Furthermore, the demonstration of the existence of the enzymes of the T C A cycle does not reveal the sequence in which the reactions occur.
VI. Carrier Type of Experiments Employing Isotopes
An ideal approach to the problem of establishing the existence of a cyclic process such as the T C A cycle is the use of specific inhibitors, as done so successfully by Krebs and Wood and their co-workers. I t will be recalled t h a t Krebs and Eggleston added fumarate and pyruvate to the malonate- inhibited pigeon breast muscle preparation in order to initiate the cyclic events. I t was demonstrated t h a t under these conditions the cycle proceeded quantitatively with the accumulation of succinate. Unfortunately, these techniques have not been too successful in causing the accumulation of in
termediates of the cycle with the various microorganisms t h u s far investi
gated. Therefore, the expedient of oxidizing an isotopically labeled sub
strate in the presence of a pool of nonlabeled compounds suspected of being intermediates has been employed. These types of experiments have been termed carrier-type experiments. T h e theory is t h a t if the isotopic substrate were oxidized through an intermediate which is common to the nonisotopic pool, the two should mix and the compound of the pool should contain iso
tope, the pool being sufficiently large to permit isolation of the compound.
B y isolation and determination of the position of the isotope in t h e com-
pound a n d its specific activity, one m a y draw conclusions concerning t h e possible mechanism of oxidation. Unfortunately, this rather simple tech
nique has not been as fruitful as one might predict. T h e hypothesis upon which this technique rests assumes mixing or equilibration of t h e metaboli- cally produced intermediate and t h e added carrier. There is considerable evidence t h a t this is not always t h e case—both with t h e whole cell prepara
tions a n d with soluble preparations. Results obtained from experiments of this type will be discussed, inasmuch as they have been frequently cited either to refute or substantiate t h e T C A cycle in microorganisms.
Saz a n d K r a m p i t z2 7 a n d Ajl a n d co-workers2 8 conducted experiments of this t y p e with Μ. lysodeikticus. Acetate-2-C1 4 was oxidized in t h e presence of nonisotopic α-ketoglutarate a n d succinate. At t h e end of t h e experiment, the residual acetate, α-ketoglutarate, a n d succinate were isolated a n d de
graded, a n d t h e specific radioactivity of t h e various carbon atoms deter
mined (see Table IV). Examination of t h e specific radioactivities indicates serious inadequacies in t h e experimental approach. I t will be observed t h a t t h e specific activities of t h e carbon atoms of α-ketoglutarate are lower t h a n those of t h e corresponding carbon atoms of succinate. If a-ketoglu- t a r a t e is t h e precursor of succinate as it is in t h e T C A cycle, t h e specific radioactivity of t h e corresponding carbon atoms of t h e former cannot be lower, b u t must be equal to or greater t h a n those of succinate. If t h e specific activity in α-ketoglutarate is greater, one can explain t h e results b y postu
lating a source of succinate from some other nonisotopic source within t h e cell which will dilute t h e radioactivity of t h e succinate. Of particular sig-
T A B L E IV
OXIDATION OF ACETATE-2-CU BY M. lysodeikticus IN THE PRESENCE OF NONISOTOPIC ck-KETOGLUTARATE AND SUCCINATE"
Succinate c.p.m./mmole a. -Ketoglut arate c.p.m./mmole
COOH 2,943 COOH
1 661
H2Cl H2C I,
I f 5,217 1 2,241
H2C
1 H2Cj
1
COOH 2,943 c = o 1
1 661
1
COOH 1,409
Initial acetate 300,000 Final acetate 98,500 Respiratory CO 2 50,620
α For particulars of experiments see ref. 27.
nificance is the fact t h a t the specific activity of the respiratory CO2 is many times greater t h a n t h a t of the carboxyl carbon atoms of either a-keto- glutarate or succinate. Since the carboxyl carbon atoms of the various acids in t h e T C A cycle are the precursors of t h e respiratory carbon dioxide, it is impossible, when the cycle operates, for the radioactivity of the respiratory carbon dioxide to be greater t h a n t h a t of the precursor carboxyl carbon atoms. T h e specific activity m a y be the same as or less t h a n t h a t of the cor
responding carboxyl carbon atoms, b u t never greater. If it is less, carbon dioxide has been derived from other nonisotopic sources which will dilute the radioactive carbon dioxide. I t would thus appear t h a t if the T C A cycle is involved in the oxidation of acetate by Μ. lysodeikticus, it accounts for only a fraction of the total process, and some other unknown mechanism is more important. An alternative explanation is t h a t t h e added succinate and α-ketoglutarate, i.e., the carrier compounds, m a y not completely equilibrate with the succinate and α-ketoglutarate which arise from the acetate via t h e oxidative cycle. I n other words, there m a y have been substrate-enzyme or substrate-coenzyme complexes of succinate and α-ketoglutarate which arose as integral components of the cycle and did not mix thoroughly with t h e succinate and α-ketoglutarate which were added as carriers. T h e latter would obviously not contain the full complement of radioactivity present in the acetate.
Similar experiments performed with E. coli?9 produced results which can be interpreted in an entirely different manner (see Table V). T h e relative incorporation of acetate-2-C1 4 into α-ketoglutarate added as a carrier when compared to the succinate is much less t h a n in Μ. lysodeikticus. T h e degree of incorporation of C1 4 into the succinate was indeed relatively great. T h e specific activities of t h e respiratory carbon dioxide and t h e carboxyl carbon
T A B L E V
OXIDATION OF ACETATE-2-C1 4 BY E. coli IN THE PRESENCE OF NONISOTOPIC
«-KETOGLUTARATE AND SUCCINATE0
Compound
(c.p.m./mmole) Distribution of isotope in succinate (c.p.m./mmole)
Initial acetate 386,000 COOH
1 5,060
Final acetate 346,000 1
HCH 39,800
a-Ketoglutarate 1,500 J
HCH 1
39,800 Succinate
Respiratory CO 2 89,500
3,600 COOH 5,060
a For particulars of experiments see ref. 29.
atoms of succinate are of t h e same order of magnitude, indicating t h a t t h e latter m a y be precursors of the respiratory carbon dioxide. These results with E. coli would appear to exclude a mechanism involving both a-keto- glutarate and succinate (as in t h e T C A cycle), b u t would be in conformity with a cycle involving succinate. Such a cycle is t h e Thunberg dicarboxylic acid cycle. Supporting evidence for this concept, not shown in t h e table, is t h a t only small amounts of radioactivity from acetate-2-C1 4 are incorporated into t h e tricarboxylic acids. T a k e n a t face value, these d a t a obtained b y carrier experiments with M. lysodeikticus a n d E. coli suggested t h a t t h e former oxidized acetate via the T C A cycle a n d t h e latter via the dicarb
oxylic acid cycle. Reasons for skepticism about such conclusions have already been presented.
VII. Noncarrier Type of Experiments Employing Isotopes
A. HYPOTHESIS OF TECHNIQUE
I t is possible to circumvent t h e objection t h a t the components of t h e T C A cycle are not in isotopic equilibrium with t h e carrier substances in t h e following manner. If it is assumed t h a t a suspension of cells or a n enzyme preparation which is oxidizing acetate contains small quantities of all t h e intermediates, it should be possible with proper micromethods to isolate the intermediates, if a sufficiently large q u a n t i t y of cells or preparation is employed. I n addition, if isotopic acetate is added in a n a m o u n t which establishes t h e half maximal rate of oxidation of acetate b y t h e enzyme preparation used, t h e size of t h e free pool of isotopic acetate is minimized, and conditions more or less ideal for equilibration of substrate and intermedi
ates are established. Secondly, if the time chosen for t h e experiment is sufficiently short, the possibility of extensive recycling is minimized. I t can be readily shown (Fig. 3) t h a t continuous recycling will randomly dis
tribute the isotope from acetate-2-C1 4 into all t h e intermediates. Should t h e experimental conditions be such to permit extensive recycling, it would be impossible to interpret t h e results in terms of a specific metabolic mechanism.
Results from this t y p e of experiment employing Μ. lysodeikticus and E.
coli will be discussed, in order t h a t t h e reader m a y m a k e a direct compari
son with t h e carrier t y p e of experiment previously described.
Table VI lists the specific activities of t h e various acids isolated from a mixture obtained b y permitting a partially lysed preparation of Μ. ly
sodeikticus to oxidize acetate-2-C1 4 2 7 T h e specific activities of t h e acetate and succinate indicate t h a t t h e two are in isotopic equilibrium. Further
more, t h e α-ketoglutarate a n d citrate are also equilibrated. I n fact, the specific activity of each of t h e latter two acids is greater t h a n t h e specific
TABLE V I
SPECIFIC ACTIVITIES OF TCA ACIDS ISOLATED FROM Μ. lysodeikticus AFTER OXIDATION OF ACETATE-2-C1 4 < 1
Compound c.p.m./mmole /tmole isolated Acetate (final) 2 , 6 0 8 , 2 0 0 4 , 1 9 0 Pyruvate 8 8 0 , 0 0 0 4 8 Succinate 2 , 7 3 4 , 1 5 0 1 7 5 a-Ketoglutarate 3 , 1 7 8 , 2 0 0 2 0 Citrate 3 , 9 5 7 , 9 5 0 4 Respiratory CO 2 2 2 1 , 3 9 0 1 , 3 2 0
0 For particulars of experiments see ref. 2 7 .
activity of the acetate or succinate, which indicates t h a t there are probably nonisotopic precursors of acetate a n d succinate in t h e enzyme preparation which dilute t h e isotopic material. T h e complete equilibration of t h e various acids which occurs in this t y p e of experiment contrasts strikingly with t h e lack of equilibration which occurs in t h e carrier t y p e of experi
ments. These results offer supporting evidence for t h e T C A cycle inilf.
lysodeikticus) however, t h e distribution pattern of t h e isotope in these acids is even more informative, being fully in accord with t h a t which could be expected when acetate-2-C1 4 is oxidized b y w a y of t h e T C A cycle.
T h e distribution d a t a are given in Table V I I a n d t h e reader is again re
ferred to Fig. 3 to facilitate a visualization of t h e mechanism. I n Table V I I the carbon atoms of citric acid have been numbered arbitrarily t o denote their origin in t h e T C A cycle from acetic a n d oxalacetic acids. N u m b e r s 1 and 2 designate t h e carboxyl a n d methyl group of acetic acid a n d numbers 3, 4, 5, a n d 6 correspond to t h e appropriate carbon atoms in oxalacetic acid—carbon 6 (tertiary carboxyl) designating the carboxyl group originally adjacent t o t h e carbonyl group of oxalacetic acid. T h e numbers assigned t o t h e carbon atoms of α-ketoglutaric a n d succinic acids correspond t o their origin from citric acid b y t h e T C A cycle. Since t h e respiratory carbon di
oxide contains radioactivity it is obvious t h a t some recycling of t h e com
ponents of t h e cycle h a s occurred. During t h e first revolution of t h e cycle the acetate-2-C1 4 will condense with nonisotopic oxalacetate which is en- dogenously present, a n d t h e respiratory carbon dioxide will n o t contain isotope. However, t h e succinate formed will literally contain C1 4 in carbon a t o m 2 as numbered in Table V I I . Since succinic acid is a symmetrical molecule, C1 4 will be statistically present in both carbon atoms number 2 and 3, i.e., one-half of t h e molecules will contain C1 4 in position 2 a n d one- half in position 3. During t h e next t u r n of t h e cycle, acetate-2-C1 4 will con
dense with oxalacetate containing isotope in either position 2 or 3, since the latter will be derived from t h e isotopic succinate (see Fig. 3). If t h e
T A B L E V I I
RECOVERY OF AND ISOTOPE DISTRIBUTION IN INTERMEDIATES OF ACETATE-2-C1 4 OXIDATION BY Μ. lysodeikticus11
Compound Structural formula Specific activity (c.p.m./mmole)
Amount isolated (μπιοΐβ)
Initial acetate COOH
1 0
CH1 3 3,620,500
Final acetate 1 COOH
1
2 CH3
33,800 2,608,200
4,190
Citrate 1 COOH
1
2 C H2
439,630 1,278,000
4
6 COOH 3 C
OH 4 C H2
5 COOH
522,700 1,122,000
1,278,000 439,630
a-Ketoglutarate 1 COOH
| 450,300 20
2 CH21
1 1,945,400
3 CH.I 1 1 4 C = 0
1 450,300
1
5 COOH 332,200
Succinate 1 COOH 448,300 175
| 2 CH21
1 1,837,550
3 CH2I 1 1
4 COOH 448,300
Respiratory C 02 — 221,390 1,320
β For particulars of experiments see ref. 27.
original numbering system is maintained t h e citrate will statistically h a v e t h e following isotopic content a t the beginning of the second t u r n of the cycle:
Since none of the carboxyl groups contain isotope, the respiratory carbon dioxide will not contain C1 4 during t h e second t u r n of t h e cycle. I t will be observed, however, t h a t a t t h e conclusion of the second t u r n the carboxyl groups of succinic and oxalacetic acids contain C1 4; therefore, during the third revolution of the cycle, the respiratory carbon dioxide will contain C1 4. Carbon dioxide fixation into oxalacetate has not been considered in the above explanation. T h e a m o u n t of C1 4 found in t h e respiratory carbon dioxide from acetate-2-C1 4 is indicative of the degree of recycling which has occurred. I t can also be seen t h a t after a n appropriate number of cycles a general distribution of C1 4 in the carbon atoms of the various acids will have occurred. T h e d a t a given in Table V I I show t h a t limited recycling h a d occurred under the conditions chosen for the experiment. I n the carrier t y p e of experiments described previously the specific activity of the respira
tory carbon dioxide was much greater t h a n the specific activity of the car
boxyl groups of the various acids. I n the noncarrier t y p e of experiments the specific activity of the respiratory carbon dioxide was somewhat lower t h a n the specific activity of the carboxyl groups. I n all probability, oxida
tions of endogenous substances to carbon dioxide diluted the radioactivity of the respiratory carbon dioxide evolved from the T C A cycle.
T h e specific activity of the individual carbon atoms of each of the acids agrees with t h e mechanism of oxidation of acetate-2-C1 4 b y t h e T C A cycle.
Since limited recycling had occurred, those carbon atoms which have as their precursor the methyl group of acetate-2-C1 4 have t h e highest specific activity. N o t e carbon atoms number 2, 3, and 4 of citric acid, 2 a n d 3 of α-ketoglutaric acid, 2 and 3 of succinic acid, and carbon number 2 of t h e residual acetate. Another important aspect of this noncarrier t y p e of ex
periment is t h a t over 90 % of the total radioactivity added as acetate-2- C1 4 can be found in the recovered acids and respiratory carbon dioxide.
If some other mechanism of oxidation is important for the oxidation of acetate by M. lysodeikticus, the concentration of the intermediates in t h e unknown mechanism is extremely low a n d the specific activity very high.
C O O H 1 C O O H H ^ H 2 H(!>14H C O O H
Η I / 3 C"
+ - 1 \ 4 H C1 4H OH 0 = 0 " —C O O H I I 5 C O O H HC" H
i o O H
T A B L E V I I I
SPECIFIC ACTIVITIES OF T C A ACIDS ISOLATED FROM E. coli AFTER OXIDATION OF ACETATE-2-CU Α
Compound c.p.m./pmole Mmoles isolated Citrate 1 1 , 8 0 0 6 . 3 a-Ketoglutarate 9 , 7 0 0 3 . 9 Succinate 8 , 5 0 0 1 3 6 . 0 Fumarate 7 , 9 2 0 1 0 . 2 Malate 9 , 2 1 5 9 . 9 Respiratory CO2 8 6 0 1 , 7 6 5 Residual acetate 4 , 4 0 0 3 , 2 9 0 Isotope recovery > 9 0 % —
β For particulars of experiments see ref. 2 9 .
Inasmuch as t h e results from carrier t y p e experiments with E. coli were interpreted as showing t h a t t h e oxidation of acetate occurred b y means of t h e C4 dicarboxylic acid cycle, it is interesting to analyze t h e results ob
tained with this organism b y use of the noncarrier technique.2 9 I n contrast to carrier t y p e experiments where isotopic acetate was incorporated into carrier succinate to a high degree, b u t into α-ketoglutarate or citrate in insig
nificant amounts, acetate-2-C1 4 has completely equilibrated with citrate and α-ketoglutarate as well as the C4 dicarboxylic acids (see Table V I I I ) . Actually t h e residual acetate has a lower specific activity t h a n t h e other acids, indicating sources of nonisotopic acetate from endogenous substances which are oxidized to acetate. T h e d a t a depicting t h e distribution p a t t e r n of t h e isotope in the carbon atoms of the various acids are not shown, b u t follow precisely t h e p a t t e r n expected from t h e oxidation of acetate-2-C1 4 by t h e T C A cycle. Furthermore the total recovery of C1 4 from all of t h e isolated compounds is greater t h a n 9 0 % , indicating the quantitative im
portance of the cycle. I t can be concluded t h a t Μ. lysodeikticus and E.
coli, t h e former a strict aerobe and t h e latter a facultative anaerobe, both oxidize acetate by t h e T C A cycle. T h e reader is also referred to t h e work b y Ajl and W o n g3 0 for an appraisal of t h e T C A cycle in E. coli.
B . T H E PROBLEM WITH Y E A S T
One of t h e first observations of t h e biosynthesis of citric acid from acetic acid was m a d e by Wieland and Sonderhoff.1 1 T h e y observed t h a t brewer's yeast converted approximately 1 0 % of total barium acetate present to citric acid. T h e concept of t h e T C A cycle h a d not been established, and these authors believed t h a t t h e synthesis of citric acid which t h e y observed was a cellular side reaction, t h e p a t h w a y of oxidation of acetate b y yeast being via t h e Thunberg dicarboxylic acid cycle. On t h e other hand, the results obtained b y Sonderhoff a n d T h o m a s8 1 with deuterium-labeled ace-
t a t e were in accordance with the T C A cycle. One of t h e objections to the occurrence of the T C A cycle in yeast was the inability to demonstrate m a n y of the enzymes with whole cells. Lynen and Neciullah3 2 demonstrated t h a t such failures were due to the impermeability of the cell membrane of the yeast cell to these substrates. With cells whose structure was destroyed b y freezing in liquid air, the various substrates of the T C A cycle were readily oxidized. Later, Lynen8 3 showed t h a t the oxidation of acetate b y yeast was inhibited by malonate and concluded t h e T C A cycle was operative. Wein- house and Millington3 4 found t h a t the isotope distribution in citrate pro
duced by yeast in t h e presence of magnesium and barium acetate-C1 3 was in agreement with t h e T C A cycle. However, in order to account for the distribution of the amount of isotope in the carboxyl groups of the C4 dicarboxylic acids, these authors suggested t h a t in addition to the T C A cycle, a supplementary mechanism for the synthesis of a dicarboxylic acid from acetic acid was present. T h e mechanism proposed for t h e latter was the Thunberg condensation reaction.
I n 1952, Krebs et al.u employed yeast preparations which h a d been made permeable to di- and tricarboxylic acids by t r e a t m e n t with dry ice. T h e results obtained from studies on the rate of substrate utilization, from car
rier t y p e of experiments and from additional criteria of the t y p e frequently applied to mammalian systems, were interpreted as evidence t h a t t h e tri
carboxylic acid cycle was not the main oxidative p a t h w a y in yeast. T h e y believed t h a t the p a t h w a y existed in yeast, but only for the synthesis of carbon skeletons of compounds required for cellular components. Some un
known mechanism was apparently involved for t h e oxidative energy- yielding process. DeMoss and S w i m3 6 conducted experiments with baker's yeast employing acetate-2-C1 4 without the use of carriers, i.e., experiments similar to those previously described with Μ. lysodeikticus a n d E. coli.
T h e results (Table I X ) of these experiments were very similar to those ob- T A B L E I X
SPECIFIC ACTIVITIES OF T C A ACIDS ISOLATED FROM BAKER'S YEAST AFTER OXIDATION OF A c E T A T E - 2 - C1 4 A
Compound c.p.m./j*mole Mmoles isolated Acetate 5 5 8 0 3 6 Citrate 4 5 5 0 159
a-Ketoglutarate 4 7 0 0 0 . 5
Succinate 4 2 6 0 2 0 8 Fumarate 4 1 5 0 5 6 Malate 4 6 0 0 2 1 7 Respiratory CO 2 9 7 6 5 9 0
α For particulars of experiments see ref. 3 6 .
tained with t h e two bacterial species. One major difference was observed which is not indicated in t h e table. T h e degree of equilibration between t h e residual isotopic acetate a n d all of t h e di- a n d tricarboxylic acids was prac
tically complete; however, while with t h e bacterial species a recovery of greater t h a n 90 % of t h e total C1 4 was obtained in t h e components of t h e T C A cycle, t h e isotope recovery in these components isolated from yeast was lower. Therefore, t h e possibility existed t h a t t h e methods employed did not detect intermediates not related to t h e cycle which were of impor
tance for a n alternate pathway. Yeast cells have a n extremely large intra
cellular pool of amino acids, particularly glutamic acid a n d alanine. I t will readily be recognized t h a t t h e carbon skeletons of these acids have their source in α-ketoglutaric a n d pyruvic acids. If t h e size of t h e intracellular amino acid pool a n d t h e isotopic content of this pool is taken into considera
tion, together with t h e components of t h e T C A cycle, t h e total recovery of isotope is sufficiently large t o indicate a quantitative importance of t h e cycle for oxidative purposes in yeast. These data do not exclude t h e exist
ence of a related mechanism.
VIII. Quantitative Aspects of the TCA Cycle
A. FUMARATE AS ELECTRON ACCEPTOR FOR ACETATE OXIDATION Reference h a s been made to t h e quantitative accumulation of interme
diates of t h e T C A cycle resulting from t h e oxidation of p y r u v a t e b y muscle a n d liver tissue in t h e presence of inhibitors such as malonate. Individual reactions of t h e T C A cycle catalyzed b y enzyme preparations from micro
organisms can be inhibited b y t h e various inhibitors used with mammalian tissue, b u t such preparations usually do not catalyze all of t h e reactions of t h e cycle. With intact microbial cells, inhibitors such as malonate have not been effective. Permeability barriers appear to be involved. For this reason, difficulty has been encountered in t h e design of experiments which would demonstrate t h e quantitative accumulation of intermediates. Krebs,3 7 how
ever, reported t h a t acetate a n d a number of other substrates were oxidized under anaerobic conditions b y nonproliferating suspensions of E. coli when fumarate was added as t h e oxidant. T h e observations resulted from the study of t h e role of fumarate in respiration a n d no consideration was given to t h e mechanism of acetate oxidation. T h e over-all reaction for t h e oxidation of acetate is illustrated in t h e following equation:
Acetate + 4 fumarate + 2 H20 —> 2 C 02 + 4 succinate C2H4O2 + 4C4H4O4 + 2 H20 — 2 C 02 + 4 04Η β 04
Since acetate was found to be practically inert oxidatively in muscle tissue, pyruvate was postulated as t h e substance which condensed with oxalacetate
OXIDATION OP ACETATE-1-C1 4
TABLE X
BY E. coli IN PRESENCE OF FUMARATE0
Compound Quantity
Oumoles)
Specific activity (c.p.m./Mmole)
Added C "
(%)
Acetate Succinate Fumarate Malate
Respiratory C O 2
2 4 . 3 3 7 0
3 1 . 8 5 7 . 6 1 6 2
8 6 6 3 6 2 4 1 4 6 2 3
1 2 . 7 8 1 . 2 0 . 8 1 . 6 2 . 2
β For particulars of experiments see ref. 3 8 .
to initiate the T C A cycle. Therefore, it was difficult a t t h a t time to con
ceive how E. coli could oxidize acetate by a T C A cycle, particularily since such components as citrate, isocitrate, m - a c o n i t a t e , and a-ketoglutarate were not oxidized b y the organism a t a sufficiently high rate when compared to acetate. T h e equation shows t h a t 2 moles of carbon dioxide are pro
duced which are equivalent to the carbon atoms of acetate; since the condi
tions are anaerobic, fumarate is the oxidant, i.e., electron acceptor. Oxi- datively and reductively the equation is satisfied by reduction of 4 moles of fumarate to 4 moles of succinate. Krebs therefore concluded t h a t acetate was oxidized t o carbon dioxide b y some unknown mechanism, and t h a t fumarate simply acted as the oxidant in place of oxygen. Swim a n d K r a m p i t z3 8 repeated the experiment performed by Krebs, substituting 1-C1 4 acetic acid ( C H3- C1 4O O H ) for nonisotopic acetate. Their results are given in Table X .
If the carbon dioxide arose from the carbon atoms of acetate, the carbon dioxide would contain all of t h e radioactivity of t h e initial acetate. I n fact, the specific activity of the carbon dioxide was very low, indicating t h a t only a small fraction of the initial acetate was oxidized to CO2. Upon closer ex
amination these results can be interpreted to be in agreement with the T C A cycle (refer to Fig. 3). T o initiate the T C A cycle the following steps would occur. (1) Two molecules of fumarate would undergo a dismutation forming one molecule of oxalacetate a n d one molecule of succinate. (2) T h e acetate (acetyl CoA) would condense with the oxalacetate to form citrate, with carbons 1 and 2 of the citric acid molecule containing carbon atoms orig
inally present in the acetate. Carbon number 1 (carboxyl group) would contain the C1 4 from C H3- C1 4O O H ) . (3) Transformation to a-ketoglutarate would then occur as outlined in Fig. 3, and during the process nonisotopic carbon number 6 of citric acid would be evolved as carbon dioxide. F u m a rate would act as the electron acceptor for the oxidation of isocitrate t o oxalosuccinate, forming another molecule of succinate. (4) T h e a-ketogluta
rate would be oxidatively decarboxylated to succinate, evolving nonisotopic