CHAPTER FIVE
Biological Nitrogen Fixation
A R T T U R I I . V I R T A N E N A N D J O R M A K . M I E T T I N E N
I. Introduction 539 A. The Significance of the Fixation of Molecular Nitrogen in Nature. . 539
B. The History of Biological Nitrogen Fixation . 542 II. Nitrogen Fixation by Free-Living Microorganisms 544
A. Detection of Nitrogen Fixation 544 B. The Species That Fix Nitrogen 546 C. Metabolism of Nitrogen-Fixing Microorganisms and Factors That In-
fluence Fixation 552 III. Symbiotic Nitrogen Fixation in Leguminous Plants 565
A. Characteristics of the Nodule Bacteria 565
B. Formation of the Nodules 572 C. Effectiveness of the Nodules as Nitrogen Fixers 579
D. Competition between Rhizobial Strains 587 E. Microbial Antagonism and Phage Action in the Soil 591
F. Transfer of the Fixed Nitrogen from the Nodules to the Host Plant. . 593 G. Excretion of Nitrogenous Compounds from the Nodules into the Rooting K. Nitrogen Fixation with Excised Root Nodules and Cell-Free Preparations 598 H. Carbohydrate-Nitrogen Relationships in Symbiotic Nitrogen Fixation,
and the Influence of Combined Nitrogen and Other Nutrients on
L. Symbiotic Nitrogen Fixation in Nonleguminous Plants 605 M. The Chemical Mechanism of Nitrogen Fixation 609
References 621 Medium 625 Nodulation and Nitrogen Fixation 628
I. The Red Pigment, Its Significance in Root Nodules 635 J. The Importance of Bacteroids in Root Nodules 645
I. Introduction
A . T H E S I G N I F I C A N C E O F T H E F I X A T I O N O F M O L E C U L A R N I T R O G E N I N N A T U R E
Biological fixation of m o l e c u l a r n i t r o g e n is considered a f u n d a m e n t a l p h e n o m e n o n i n t h e m a i n t e n a n c e of life. H o w e v e r , n o t long ago it w a s found b y Stevenson (299) t h a t rocks contain v a r i a b l e a m o u n t s of a m - m o n i u m n i t r o g e n a n d t h a t t h e total n i t r o g e n i n rocks exceeds t h e m o l e c u l a r n i t r o g e n i n t h e a t m o s p h e r e . T h e combined n i t r o g e n i n rocks is, however, so f i r m l y fixed i n t h e crystal lattice of t h e m i n e r a l t h a t
539
540 A . I. V I R T A N E N A N D J. K . M I E T T I N E N
only w h e n rocks w e a t h e r m a y it be utilized b y plants. Because this process is v e r y slow, the a n n u a l l y liberated a m m o n i u m nitrogen is insignificant for t h e growth of higher plants. H e n c e life on earth practically depends on the fixation of atmospheric nitrogen. A p p a r e n t l y only a v e r y small portion of t h e fixed nitrogen results from the non- biological nitrogen fixation brought about b y photochemical reactions or electrical discharges in t h e atmosphere. I n t h e literature widely v a r y i n g values are found for the a m o u n t of combined nitrogen w h i c h is brought to the surface of t h e earth b y precipitation. U n t i l 1950 in seventy investigations from different parts of the world t h e a n n u a l rain- fall w a s found to contain combined nitrogen a m o u n t i n g to from 1 to n e a r l y 30 kg per hectare. I n this discussion these results cannot be treated in detail.
I n t h e n o r t h e r n E u r o p e a n countries, w h e r e settlement is relatively sparse a n d i n d u s t r y per square kilometer small, the y e a r l y rainfall per hectare contains only small a m o u n t s of nitrogen compounds ( 3 4 1 ) . Recent investigations in different parts of Scandinavia h a v e given v e r y variable values for the a m m o n i u m a n d n i t r a t e nitrogen in t h e a n n u a l precipitation. Eriksson (118) reports v e r y low values for both in n o r t h e r n Scandinavia (the lowest values for n i t r a t e nitrogen being 0.23 kg a n d for a m m o n i u m nitrogen 0.15 kg per hectare per y e a r ) . I n southern Scandinavia, w h e r e the population density a n d agricultural a n d industrial activities a r e relatively high, m u c h higher values a r e to be found: in D e n m a r k the highest value for n i t r a t e nitrogen is 2.21 kg, a n d for a m m o n i u m nitrogen 4.94 kg, p e r h e c t a r e per y e a r a n d the lowest values a r e 1.74 a n d 2.50 kg, respectively ( 1 1 8 ) . Since it is probable t h a t t h e a m m o n i u m nitrogen found i n r a i n w a t e r origi- nates m a i n l y from t h e a m m o n i a evaporated from the soil a n d oceans, a n d since n i t r a t e nitrogen according to recent investigations is absent from snow collected from regions in N e w Z e a l a n d w h e r e no plants or animals exist [Wilson ( 4 0 3 ) ] , the nitrogen fixation in the atmosphere seems to be v e r y low—far lower even t h a n was earlier thought. T h e large a m o u n t s of a m m o n i u m a n d n i t r a t e nitrogen (28.2 kg total nitro- gen per hectare per y e a r ) observed in Central E u r o p e b y Scharrer a n d Fast (287) in the r a i n w a t e r are a p p a r e n t l y due mostly to industry, agriculture, a n d other activities in densely populated areas. Earlier results even from relatively n e a r b y regions often differ greatly [ M a n -
shard (220) ] , in p a r t perhaps owing to t h e methods used.
T h e industrial production of nitrogen compounds from atmospheric nitrogen has opened u p n e w possibilities in this c e n t u r y for nitrogen fertilization a n d thus for improving agricultural production. T h e fact is, however, t h a t perhaps only 2 or 3 % of the nitrogen contained in
5. B I O L O G I C A L N I T R O G E N F I X A T I O N 541 the a n n u a l harvests of the world originates, at present, from nitrogen- ous fertilizers produced b y industry. Most of t h e nitrogen of plants is still a product of biological nitrogen fixation or is derived from the nitrogen reserves of t h e soil. I n t h e latter case t h e soil is depleted of its fertility.
Effective against this depletion is t h e cultivation of legumes. T h e r e is n o m e t h o d m o r e effective in preserving the h u m u s a n d nitrogenous contents of t h e soil t h a n t h e cultivation of legume-rich leys,* especially leys of clover (Trifolium species) a n d alfalfa (Medicago sativa).
N e i t h e r is t h e r e a m o r e effective or economical w a y of producing pro- teins for animals. Therefore, t h e significance of leguminous plants has not yet been diminished b y t h e t r e m e n d o u s development of t h e m a n u - facture of nitrogenous fertilizers. F r o m the standpoint of world economy of energy, a n effective cultivation of legumes, a n d t h u s t h e utilization of biological nitrogen fixation, is of the greatest importance [ V i r t a n e n
( 3 4 2 ) ] . . . . .
T h e nitrogen fixation w h i c h takes place i n t h e root nodules of legumes is, u n d e r favorable conditions, sufficient for t h e m a x i m a l growth of m a n y legumes. I n this laboratory greenhouse experiments w i t h red clover (Trifolium pratense), g r o w n in pots w i t h q u a r t z sand in t h e absence of combined nitrogen, h a v e at best given fixation w h i c h can be calculated to about 1000 kg of nitrogen p e r h e c t a r e per year. I n these experiments clover w a s cut t h r e e times i n 6 m o n t h s [ V i r t a n e n
( 3 3 2 ) ] . U n d e r field conditions such high nitrogen fixation cannot of course be obtained since t e m p e r a t u r e , moisture, nutrients, a n d other factors w h i c h influence growth can be most favorably adjusted d u r i n g the whole period of growth only i n a greenhouse. However, even in a country as far n o r t h as F i n l a n d , a r e a l l y good red clover sward (giving yields of from 6000 to 9000 kg of d r y m a t t e r per hectare in three cuttings per s u m m e r ) fixes 2 0 0 - 3 0 0 kg of nitrogen p e r hectare in one growing season [ V i r t a n e n ( 3 3 3 ) ] , a n d a pea (Pisum sativum) crop fixes about 100 kg. I n countries w h e r e t h e s u m m e r is longer a n d t h e w e a t h e r m o r e favorable, u p to 400 kg of nitrogen m a y be fixed in a v e r y good red clover sward, a n d i n a n alfalfa sward even more. T h e s e amounts exceed b y m a n y times t h e a m o u n t s of nitrogen in t h e fertiliz- ers used i n effective agricultural production. O n t h e average, however, t h e nitrogen fixed i n clover a n d alfalfa fields is n a t u r a l l y m u c h lower t h a n t h e m a x i m a l values given above [cf. ( 1 3 4 ) , page 2 1 8 ] .
I n n a t u r e m a n y wild leguminous herbs a n d trees a r e i m p o r t a n t as fixers of atmospheric nitrogen a n d i n increasing t h e nitrogen content of the soil. Also some nonlegumes, such as several species of Alnus (on
* The term "leys" relates to cultivated grass for cutting.
542 A. I. V I R T A N E N A N D J. K . M I E T T I N E N
t h e roots of w h i c h nitrogen-fixing nodules a r e formed b y specific micro- organisms), are valuable contributors to the formation of a h u m u s rich in nitrogen.
Free-living nitrogen-fixing bacteria, such as anaerobic Clostridium a n d aerobic Azotobacter, compete w i t h other microorganisms in the soil for their nutrition, especially for carbon sources. T h e nitrogen fixed per hectare b y these nitrogen fixers is, therefore, m u c h smaller t h a n in symbiotic nitrogen fixation. Accurate information about the a m o u n t s of nitrogen thus fixed is lacking, b u t it is obvious t h a t t h e y v a r y greatly in different soils, possibly from a few kilograms to 20 kg per hectare per year. Allison ( 1 3 ) , on t h e basis of t h e h i g h l y controversial literature on this point, stated t h a t although as m u c h as 20 pounds per acre probably m a y be fixed a n n u a l l y on a n occasional, well-limed grassland, even the old estimate of 6 pounds per acre p e r y e a r in the U n i t e d States [ L i p m a n a n d Conybeare ( 2 1 1 ) ] m a y be too high for a n average rate. I n n a t u r a l l y acid soils Clostridium is evidently the most i m p o r t a n t free- living nitrogen fixer both in forest a n d cultivated soils. I n n e u t r a l a n d slightly alkaline soils Azotobacter m a y be important, b u t its role is still u n d e r debate. Since in t h e recent years n u m e r o u s microorganisms h a v e been found to be able to fix nitrogen, our concepts of the role of free- living nitrogen-fixing organisms in n a t u r e m a y in t i m e change.
T h e most autotrophic microorganisms, w h i c h assimilate both carbon dioxide a n d nitrogen, are t h e nitrogen-fixing blue-green algae. I n m a r i n e waters their occurrence is scanty, b u t from tropical to polar regions t h e y a r e common in fresh water. T h e i r nitrogen-fixing capacity becomes m o r e clearly distinguishable in nitrogen-deficient surround- ings. Information about their importance in n a t u r e a n d agriculture is still v e r y deficient, b u t their role m a y be greater t h a n has been sup- posed hitherto [Calder ( 8 9 ) ] . I n rice (Oryza sativa) fields t h e y even m a y be of great importance (100, 3 9 7 ) , b u t their role relative to nitro- gen-fixing bacteria is still u n d e r dispute ( 9 5 ) .
B. T H E H I S T O R Y O F B I O L O G I C A L N I T R O G E N F I X A T I O N
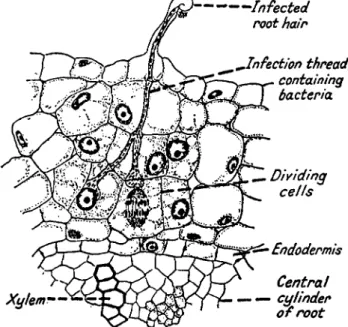
F r o m the beginnings of agriculture symbiotic nitrogen fixation has been u n w i t t i n g l y utilized in t h e cultivation of legumes. After m a n y contradictory a n d negative results, in the latter p a r t of the n i n e t e e n t h c e n t u r y t h e ability of legumes to grow on poor soils a n d to improve their fertility was finally explained w h e n , in 1886, Hellriegel a n d W i l f a r t h (153) showed experimentally t h a t legumes actually utilize atmospheric nitrogen if their roots bear nodules formed b y bacteria.
T w o years later Beijerinck (35) isolated a b a c t e r i u m in p u r e c u l t u r e w h i c h formed nodules on the roots of the host plant. [Beijerinck gave
5. B I O L O G I C A L N I T R O G E N F I X A T I O N 543 this bacterium t h e n a m e Bacillus radicicola, w h i c h P r a z m o w s k i (267) changed to Bacterium radicicola because it did not form spores. F r a n k (133) began to use t h e n a m e Rhizobium leguminosarum. I n this article Rhizobium is used as the n a m e for the genus a n d rhizobia as the general n a m e for the nodule bacteria of leguminous plants.] T h e formation of root nodules b y specific bacteria t h u s was conclusively proved.
Of special historical interest is t h e peculiar fact t h a t t h e earlier nega- tive results regarding the ability of legumes to utilize atmospheric nitrogen (especially the experiments performed in Rothamsted in 1 8 5 7 -
1859) w e r e due to a "too careful" test methodology, in other words the destroying of bacteria indispensable for t h e fixation. I n t h e excellent m o n o g r a p h of F r e d et al. (134) a n d in t h a t of Wilson (407) the dra- m a t i c history of t h e studies on symbiotic nitrogen fixation is thoroughly treated. Especially interesting is a recently published article b y Wilson
( 4 1 0 ) .
Beijerinck's original discovery about the inability of rhizobia to fix gaseous nitrogen w h e n growing free on a m e d i u m was challenged b y m a n y c o n t r a r y findings. T h e n u m e r o u s positive claims w e r e probably influenced b y t h e a p p a r e n t increase of t h e Rhizobium mass in a nitro- gen-free or exceedingly nitrogen-deficient m e d i u m . T h e inoculated bacteria can continue to divide to some extent u n t i l their nitrogen con- tent is severely lowered, a n d t h e y form a voluminous nitrogen-free gum. Cells of Rhizobium from a m e d i u m containing a n excess of a suit- able nitrogen source, e.g., asparagine, h a v e a nitrogen content of 8 - 9 % of their d r y weight, whereas cells g r o w n on a m e d i u m deficient in nitrogen contain only 3 . 5 % of nitrogen [Burris a n d Wilson ( 8 1 ) ] . T h e dependence of free-living rhizobia on combined nitrogen, a n d their inability to fix nitrogen, was unequivocally shown in critical experi- m e n t s in different laboratories d u r i n g the 1920's a n d 1930's (cf. 4 1 7 ) . M o r e recently this result has been confirmed b y Wilson (411) b y the sensitive isotope method w i t h N2 1 5.
T h e first observations on fixation of atmospheric nitrogen b y micro- organisms g r o w n in n u t r i e n t solution w e r e m a d e in 1862 b y Jodin
( 1 7 7 ) , w h o , soon after Pasteur's discovery of t h e microbial cause of fermentation, demonstrated t h a t a n u t r i e n t solution containing phos- p h a t e a n d a source of carbon such as sugar, glycerol, or tartaric acid, b u t no combined nitrogen, supports the growth of " m y c o d e r m s . " By sealing t h e growing culture into a closed vessel h e could prove t h a t both oxygen a n d nitrogen w e r e utilized, t h e latter to the extent of about
6 - 7 % of t h e former.
Berthelot ( 4 3 ) , about a q u a r t e r of a c e n t u r y later, showed b y quanti- tative determinations a n increase of combined nitrogen in soils left in
544 A . I. V I R T A N E N A N D J. K . M I E T T I N E N
open jars d u r i n g a s u m m e r , w h e r e a s no increase took place if the soil was previously sterilized b y heating. This pointed to a microbial cause of t h e fixation. T h e first to obtain a nitrogen-fixing organism in p u r e culture was W i n o g r a d s k y ( 4 2 3 ) . H e isolated from soil a n anaerobic organism, Clostridium pasteurianum, which, growing i n a m e d i u m con- taining glucose a n d phosphate b u t no combined nitrogen, fixed about 2 m g of gaseous nitrogen p e r g r a m of glucose fermented.
A few years later Beijerinck (37) isolated from soil a n d canal w a t e r two aerobic nitrogen-fixing organisms, Azotobacter chroococcum a n d A.
agilis, respectively. T h e s e soon t u r n e d out to be h i g h l y efficient in fixing nitrogen a n d became t h e most c o m m o n l y used microorganisms in studies of nonsymbiotic nitrogen fixation.
Observations on t h e nitrogen-fixing capacity of photosynthetic blue- green algae w e r e m a d e as e a r l y as in 1889, a t w h i c h t i m e t h e r e was, however, no certainty y e t about t h e p u r i t y of algal cultures regarding bacterial contamination. T h e first observation on nitrogen fixation b y a p u r e culture of a blue-green alga (Nostoc punctiforme) was m a d e b y Drewes in 1928 ( 1 0 7 ) . I n addition to free-living forms, t h e r e a r e m a n y blue-green algae t h a t live i n association w i t h other plants. T h e w a t e r fern Azolla is, i n fact, able to grow w i t h o u t combined nitrogen b y m e a n s of its symbiotic alga [Bortels ( 6 0 ) ] . Bond a n d Scott (54) recently found t h a t some lichens fix nitrogen w i t h t h e help of blue- green algae as their algal component.
After N2 1 5 c a m e into use in t h e studies on nitrogen fixation, a n u m - ber of n e w microorganisms h a v e been found to possess some capacity to fix molecular nitrogen.
T h u s d u r i n g t h e last seventy y e a r s t h e gaseous nitrogen of air has been found to be a suitable nitrogen source for n u m e r o u s micro- organisms to a greater or lesser degree. On t h e other h a n d , it has never been possible to prove t h a t multicellular green plants fix nitrogen except in symbiosis w i t h specific microorganisms.
IL Nitrogen Fixation by Free-Living Microorganisms
A. D E T E C T I O N O F N I T R O G E N F I X A T I O N
D u r i n g a half c e n t u r y of research since t h e earliest investigations, the ability to fix nitrogen has been attributed to n u m e r o u s species of organisms, m a i n l y bacteria, molds, a n d yeasts. M a n y of these claims w e r e evidently at fault owing to uncritical methods. W e a k nitrogen fixation is often v e r y difficult to prove w i t h certainty a n d requires t h a t special attention be paid to t h e methods used.
5. B I O L O G I C A L N I T R O G E N F I X A T I O N 545 Growth without combined nitrogen, so useful i n the isolation of ni- trogen-fixing organisms a n d i m p o r t a n t as evidence for nitrogen fixation, is not a n unequivocal proof of it p e r se. T i n y a m o u n t s of combined nitrogen in t h e inoculum, in t h e m e d i u m , a n d in t h e laboratory air m a y be able to sustain some growth d u r i n g weeks or m o n t h s , as already mentioned. Lack of g r o w t h of identical cultures incubated in the absence of nitrogen is a valuable negative control.
Increase of total nitrogen in t h e culture, u s u a l l y determined b y the Kjeldahl procedure, is the most widely used a n d i m p o r t a n t proof of fixa- tion. If nitrogen fixation is strong or moderate, this m e t h o d is quite satisfactory, b u t if t h e fixation is v e r y small a n d the organism re- quires t h e addition of p l a n t extracts or nitrogenous growth factors to the m e d i u m , reliable demonstration of nitrogen fixation b y the classic procedures of nitrogen determination becomes difficult. I n such cases, w h e n t h e absolute a m o u n t of nitrogen m u s t be determined w i t h high accuracy, it is often advantageous to use reductive substances, like zinc or iron, in the Kjeldahl procedure to ensure the complete reduction of all nitrogenous compounds to a m m o n i a .
Gasometric determination of t h e consumption of nitrogen ( a n d oxy- gen) d u r i n g growth in a closed vessel is t h e oldest technique; it was used b y Jodin (177) as early as 1862, b u t it is somewhat tedious a n d it also h a s its pitfalls. T h e Warburg manometrical technique, though not practical i n t h e detection of n e w nitrogen-fixing species, is invaluable in chemical a n d physiological studies of the nitrogen fixation.
I n nonsymbiotic organisms like Azotobacter, in w h i c h it is b o u n d to the g r o w t h of t h e organism, nitrogen fixation as well as growth in physiologically adequate conditions follow t h e kinetics of a first-order reaction. Therefore, nitrogen fixation can be m e a s u r e d b y the velocity constant g of a first-order reaction
g = 2.30J log (a + y)/dt = dy/(a + y) dt (1) w h e r e a = initial cell concentration, γ = increase in t hours.
I n Eq. 1, g can be determined b y plotting against t i m e the logarithm of t h e experimental values of a n y q u a n t i t y w h i c h is proportional to growth, such as t h e total n u m b e r Ν or the d r y m a t e r i a l of the cells, or t h e respiration r a t e (cubic millimeters of oxygen consumed per h o u r per milliliter of c u l t u r e ) . Of these t h e r a t e of respiration (oxygen consump- tion) is generally used because it can be easily determined b y the m a n o - metric m e t h o d of W a r b u r g . Especially Burk [cf., e.g. ( 7 2 ) ] has used this technique for studies of the dependence of nitrogen fixation on
variable factors like t e m p e r a t u r e , p H , p N2, inhibitors, etc.
546 A . I. V I R T A N E N A N D J. K . M I E T T I N E N
T h e isotope method w i t h N2 1 5 as a tracer, first introduced to research on nitrogen fixation b y Burris a n d Miller [78, cf. also ( 7 6 ) ] at the beginning of t h e 1940's, is t h e most sensitive a n d critical t e c h n i q u e so far available. N a t u r a l nitrogen contains 0 . 3 7 % of the h e a v y isotope N1 5 a n d a n increase of 0.02% above the n a t u r a l level can be accurately determined b y the mass spectrograph. Since N2 1 5 is n o w a d a y s available enriched to 6 0 % excess (over the n a t u r a l concentration), it m a y be diluted about 3000-fold in the experiment a n d still be demonstrated w i t h certainty. T h i s m e a n s t h a t for 1 m g nitrogen i n t h e organism, fraction, or isolated compound, only 0.35 fig need be derived from fixa- tion. T h e isotope m e t h o d has t h e additional advantage t h a t it is not sensitive to the q u a n t i t y of nitrogen in the sample, since the size of sample does not appreciably influence t h e mass spectrographic deter- m i n a t i o n of its isotopic composition.
However, in t h e studies on nitrogen fixation b y t h e symbiotic system the most critical item of technique is often not the determination of nitrogen, b u t t h e use of aseptic culture methods. As will be seen later, the use of open pots in experiments has probably led to m o r e erroneous results t h a n a n y other technical deficiency.
B. T H E S P E C I E S T H A T F I X N I T R O G E N
A brief s u m m a r y of t h e free-living nitrogen-fixing organisms is presented in Table I. O n l y one m e m b e r of each of the most typical genera or species is mentioned w i t h a few of its characteristics, the n a m e of t h e investigator w h o detected its nitrogen-fixing property, a n d t h e y e a r of its detection.
Î. Azotobacter and Beijerinckia
As mentioned earlier, Beijerinck in 1901 isolated from soil t h e type species, A. chroococcum, w h i c h is ubiquitous in n e u t r a l soils, a n d also t h e motile species A. agilis, w h i c h is m a i n l y a w a t e r bacterium. Some authorities separate as a third species A. vinelandii, w h i c h in Bergey's m a n u a l is classified as a subspecies of A. agilis. Azotobacter vinelandii was first isolated b y L i p m a n in 1903 at Vineland, N e w Jersey. Azoto- bacter species a r e strongly aerobic, fast growing, a n d active in nitrogen fixing; t h e y h a v e a p H o p t i m u m at about 7 a n d do not grow w i t h nitro- gen gas below p H 6. T h e y get e n e r g y for their physiological processes b y oxidizing carbohydrates (e.g., mono- a n d disaccharides a n d s t a r c h ) , organic acids, * a n d other substances to carbon dioxide. T h e a m o u n t of nitrogen fixed is usually 10-20 m g nitrogen per g r a m of sugar utilized
— a t the very highest, 2 5 - 3 0 m g per g r a m . Nitrogen fixation is most efficient at the time of fastest growth, w h e n a n Azotobacter culture m a y
T A B L E I
REPRESENTATIVE F R E E - L I V I N G N I T R O G E N - F I X I N G MICROORGANISMS
Efficiency of fixation Organism Growth characteristics Investigator0 Year6 (mg Ν / g m carbohydrate) Bacteria
Azotobacter Heterotrophic, aerobic Beijerinck 1901 10-30
Beijerinckia Heterotrophic, aerobic Starkey and De 1939 10-20
Clostridium Heterotrophic, anaerobic Winogradsky 1893 2-12
Aerobacter Heterotrophic, facultative anaerobic Hamilton et al. 1953 4
Methanobacterium Chemoautotrophic, anaerobic Pine and Barker 1954 —
Pseudomonas Heterotrophic, facultative anaerobic Anderson 1955 1-15
Achromobacter Heterotrophic, aerobic Jensen 1958 1
Bacillus polymyxa Heterotrophic, aerobic Hino and Wilson 1958 —
Rhodospirillum Photoautotrophic, aerobic Kamen and Gest 1949 —
Chlorobium Photoautotrophic, aerobic Lindstrφm et al. 1949 —
Chromatium Photoautotrophic, aerobic Lindstrφm et al. 1950 —
Rhodomicrobium Photoautotrophic, aerobic Lindstrφm et al. 1950 —
Yeasts
Saccharomyces, Rhodotorula Heterotrophic, facultative anaerobic Metcalfe and Chayen 1954 4 Pullularia Heteroautotrophic, facultative
anaerobic
Brown and Metcalfe 1957 5 Algae
NostoCj Anabaena Photoautotrophic, aerobic Drewes 1928 10 (in dar
Cylindrospermum, Aulosira, Photoautotrophic, aerobic For references see Fogg —
—
Calothrix, Tolypothrix, (126, 128, 129)
Anabaenopsis, Mastigocladus
a Investigator who detected nitrogen-fixing property.
6 Year when nitrogen-fixing property was detected.
547
548 Α . Ι. V I R T A N E N A N D J. Κ . M I E T T I N E N
fix 10 m g nitrogen per 100 m l per day. W i t h this r a t e fixation can be determined b y t h e Kjeldahl procedure after only 1-2 hours.
Azotobacter indicus, first isolated b y Starkey a n d D e (297) in 1939 a n d earlier classified as t h e t h i r d species of Azotobacter, is currently, according to D e r x ( 1 0 6 ) , classified as t h e separate genus Beijerinckia (173).* I n addition to the t y p e species B. indica, several subspecies are recognized in this n e w genus [Kluyver a n d Becking ( 1 9 4 ) ] , w h i c h is found only in the calcium-deficient tropical laterite soils of Asia, Africa, America, a n d Australia. T h e m a i n characteristics of Beijerinckia, w h i c h distinguish it from Azotobacter, are its broader p H spectrum (3 to 7, with o p t i m u m a r o u n d 4 ) a n d its low calcium ("calcifrige") r e q u i r e m e n t .
2. Clostridium
T h i s is a large genus of strictly anaerobic, u s u a l l y motile, g r a m - positive rods, w h i c h typically do not ferment cellulose. Clostridium butyricum, isolated in 1880 b y Prazmowski, is t h e t y p e species; it fixes atmospheric nitrogen moderately. Of the n u m e r o u s species of Clost- ridium, t h e most active in t h e assimilation of nitrogen is C. pasteuri- anum, first isolated b y W i n o g r a d s k y (422) in 1893. N u m e r o u s other species of t h e genus also fix nitrogen, though less efficiently. F o r a list of the nitrogen-fixing species see Bergey's M a n u a l . I n a study of 15 species of Clostridium, Rosenblum a n d Wilson (278) found 12 capable of fixing nitrogen. T h e nitrogen-fixing efficiency of Clostridia is usually 2 - 3 m g nitrogen p e r g r a m of s u g a r — a t t h e highest 1 0 - 1 2 m g nitrogen per g r a m of sugar. W h e n serially cultivated i n synthetic m e d i u m , Clostridia g r a d u a l l y lose their ability to grow, b u t t h e y r e g a i n it after one "soil passage," i.e., w h e n cultivated once again i n soil. However, w h e n a synthetic n u t r i e n t solution contains folic acid, the ability to grow is not lost, according to V i r t a n e n a n d L u n d b o m ( 3 7 9 ) .
I n n a t u r e , Clostridia thrive best in association w i t h cellulose-degrad- ing fungi a n d bacteria, utilize the mono- a n d disaccharides produced b y t h e m [cf. Vartiovaara ( 3 2 9 ) ] . Clostridia cannot u s e polysaccharides other t h a n starch (cf. Section II,C,3).
E v e n a small a m o u n t of a m m o n i u m or n i t r a t e nitrogen inhibits nitro- gen-fixation, since t h e combined forms of nitrogen are used prefer- entially b y Clostridium as well as b y Azotobacter.
3 . Aerobacter
It was shown b y Skinner (294) t h a t Aerobacter aerogenes is able to fix nitrogen, a n d this observation has been recently confirmed, w i t h the
* Bergey's Manual (7th Edition, 1957) still classifies it as a third species of Azotobacter "until further comparative studies are made."
5. B I O L O G I C A L N I T R O G E N F I X A T I O N 549 isotopic N1 5- t e c h n i q u e , b y H a m i l t o n et al. ( 1 4 8 ) , H a m i l t o n a n d W i l s o n
( 1 5 0 ) , a n d J e n s e n ( 1 7 5 ) . N i t r o g e n fixation b y this organism is most intensive if t h e m e d i u m contains traces of n i t r a t e nitrogen ( 1 0 - 2 0 pg/
m l ) a n d a relatively h i g h concentration of glucose or saccharose ( 2 - 4 % ) . T h e strains of H a m i l t o n a n d co-workers required strictly anaerobic conditions for nitrogen fixation, b u t t h e supply of oxygen h a d no appreciable influence on t h e growth a n d nitrogen fixation of t h e two strains studied b y Jensen. Similar results u n d e r anaerobic condi- tions w e r e later reported b y P e n g r a a n d W i l s o n (262a) w h e r e a s Johnstone a n d Pfeffer (177a) report results on fermentation of w h e y b y a nitrogen-fixing strain of Aerobacter aerogenes i n aerobic condi- tions. I n t h e isotopic e x p e r i m e n t of H a m i l t o n et al. a n excess of 17.4 atom p e r cent w a s reached i n 3 days, w h i c h corresponds to a doubling of t h e original nitrogen or a n increase of 30 μg nitrogen p e r milliliter, a n a m o u n t w h i c h is certainly easily demonstrable b y t h e Kjeldahl method.
P i n e a n d Barker (266) found t h a t t h e chemautotrophic, anaerobic Methanobacterium omelianskii fixed nitrogen, a n d t h e y demonstrated this also b y t h e N1 5- t e c h n i q u e . A small a m o u n t (5 /Ag/ml) of am- m o n i u m i n t h e m e d i u m increased t h e nitrogen fixation, b u t a greater a m o u n t (30 /xg/ml) inhibited it.
A n d e r s o n (22) showed t h a t a facultatively anaerobic soil b a c t e r i u m of t h e Pseudomonas type, for w h i c h h e proposed t h e n a m e P. azotocol- ligans, fixed atmospheric nitrogen; h e demonstrated this both b y t h e N1 5- t e c h n i q u e a n d b y t h e Kjeldahl method.
Roy a n d M u k h e r j e e (281) described a nitrogen-fixing microorganism w h i c h resembles Anderson's t y p e i n some respects b u t also has distinct differences. Recently Proctor a n d W i l s o n (268) h a v e isolated six dif- ferent species of Pseudomonas, w h i c h all possess t h e ability to fix nitro- gen. T h e nitrogen-fixing e n z y m e system seemed to be adaptive in these microorganisms.
A n u m b e r of other genera, mostly anaerobic bacteria, h a v e been shown to possess t h e p o w e r of nitrogen fixation, although t h e evidence is not a l w a y s indisputable. Of these m a y be m e n t i o n e d Bacillus hydro- genes [Belayaeva ( 3 8 ) ] , Achromobacter sp. [Jensen ( 1 7 6 ) ] , a n d Bacil- lus polymyxa [ H i n o a n d W i l s o n ( 1 6 3 ) ] . B. hydrogènes fixes nitrogen both on totally inorganic, nitrogen-free m e d i u m i n a n atmosphere of hydrogen, carbon dioxide, a n d nitrogen a n d on glucose-contain- ing m e d i u m i n t h e absence of h y d r o g e n ; i n t h e latter case t h e yield is 0.85-2 m g nitrogen per g r a m of sugar. Quite recently Jensen
(176) i n D e n m a r k isolated from w a t e r a nitrogen-fixing organism which h e identified as a n Achromobacter species. I n a later survey
550 A. I. V I R T A N E N A N D J. K. M I E T T I N E N
Proctor a n d Wilson (268) demonstrated aerobic nitrogen fixation in eight strains of Achromobacter; of these only Jensen's strains fixed nitrogen also anaerobically. As i n Pseudomonas species, the nitrogen- fixing e n z y m e system seemed to be adaptive. Bacillus polymyxa is another species w h i c h only quite recently has been shown b y H i n o a n d Wilson (163) to be able to fix nitrogen. It contains a n active hydrogenase a n d its nitrogen fixation is sensitive to molecular oxygen.
As little as 1 % of oxygen significantly reduced nitrogen fixation, com- peting w i t h nitrogen for the final electrons produced b y the cytochrome system. On the contrary, growth of t h e organism on n i t r a t e nitrogen requires oxygen. U n c e r t a i n t y still prevails r e g a r d i n g the reported
[Sisler a n d ZoBell (293) ] observation t h a t t h e anaerobic chemautotroph Desulfovibrio desulfuricans, w h i c h stores e n e r g y for its life processes from t h e reduction of sulfates b y hydrogen, is able to fix atmospheric nitrogen. Bach (30) reports t h a t a study, b y m e a n s of the N1 5- t e c h n i q u e , on the nitrogen-fixing ability of this organism has given a negative re- sult. Bisset (44a) has discussed n a t u r a l relationships of t h e nitrogen- fixing bacteria.
4. Soil Yeasts
Metcalfe a n d C h a y e n (223) isolated from u n d e r n e a t h Calluna vul- garis, growing on a n acid h e a t h soil at p H 4.5, two yeastlike organisms, one resembling Saccharomyces, the other Rhodotorula. W h e n cultivated 14 days on nitrogen-free m e d i u m , t h e organisms fixed 1-4 m g nitrogen per g r a m glucose, t h a t is, h a v i n g about a t e n t h of t h e efficiency of Azotobacter. T h e s e results also h a v e been confirmed b y t h e N1 5- t e c h - n i q u e [Roberts a n d W i l s o n ( 2 7 6 ) ] . S o m e w h a t m o r e active is t h e Pullularia-Xy^e soil yeast recently isolated b y Brown a n d Metcalfe ( 6 5 ) , w h i c h is able to fix 4 - 5 m g nitrogen p e r g r a m glucose. On a m e d i u m containing combined nitrogen it r a p i d l y loses its nitrogen- fixing power. N e m e t h (239, 240) reports t h e isolation in p u r e culture, from the root nodules of Lupinus luteus, of a yeastlike microorganism w h i c h is able to grow r a p i d l y on nitrogen-deficient " r h i z o b i u m a g a r "
a n d to fix nitrogen a n d w h i c h should differ from all previously k n o w n free-living nitrogen fixers b y containing a red pigment, at first sup- posed to be hemoglobin b u t later on (239a) found not to be so.
5. Photosynthetic Bacteria
W h e n studying the photoevolution of h y d r o g e n b y Rhodospirillum rubrum, K a m e n a n d Gest (184) noticed t h a t nitrogen a n d a m m o n i a competitively inhibit this process. W i l s o n a n d U m b r e i t (417) h a d shown in 1937 t h a t h y d r o g e n competitively inhibits nitrogen fixation in
5. B I O L O G I C A L N I T R O G E N F I X A T I O N 551 the symbiotic system a n d later in other organisms (see page 5 6 0 ) . K a m e n a n d Gest studied w h e t h e r Rhodospirillum m i g h t also fix gaseous nitrogen, a n d w i t h N1 5 found this to be so. Since t h e n , nitrogen-fixing genera h a v e been found in all t h r e e families of t h e suborder Rhodo- bacteriineae:
Thiorhodaceae: Chromatium [ L i n d s t r ö m et al. ( 2 0 7 , 2 0 9 ) ]
Athiorhodaceae: Rhodospirillum rubrum [ K a m e n a n d Gest ( 1 8 4 ) ] ; Rhodopseudomonas sp. [ L i n d s t r ö m et al. ( 2 0 8 ) ] , Rhodomicrobium vannielii [ L i n d s t r ö m et al. (208, 2 0 9 ) ]
Chlorobacteriaceae: Chlorobium thiosulfatophilum [ L i n d s t r ö m et al.
(207, 2 0 9 ) ]
L i n d s t r ö m et al. (208) tested 20 species of Rhodopseudomonas for nitrogen fixation, 19 of w h i c h gave a positive result; it seems to be t h e general concensus t h a t this ability is probably possessed b y all photo- synthetic bacteria. T h e s e bacteria u s u a l l y r e q u i r e organic growth factors ( t h i a m i n e , biotin, a n d nicotinic a c i d ) , a n d m a n y of t h e m are able to fix nitrogen also in the dark w h e n growing heterotrophically, though the efficiency of fixation is t h e n low.
6. Blue-Green Algae
T w o of the f u n d a m e n t a l processes of organic nature—photosynthesis a n d nitrogen fixation—are u n i t e d i n most species of t h e widely dis- tributed blue-green algae (Schizophyceae or M y x o p h y c e a e ) .
T h e first evidence of t h e nitrogen-fixing ability of these simple organisms, w h i c h in their mode of reproduction resemble bacteria, was provided b y F r a n k (132) in 1889. However, since his algal cultures w e r e n o t free of bacteria a n d t h e first p u r e cultures of microscopic algae
(cultures of Chlorophyceae) could not fix nitrogen, the reported nitro- gen fixation of earlier algal cultures w a s suspected to be due to nitrogen- fixing bacteria, w h i c h thrive well in t h e slimy layer covering the algae.
D r e w e s (107) finally showed conclusively, b y u s i n g p u r e cultures of Nostoc punctiforme a n d Anabaena variabilis, t h a t t h e y are able to fix nitrogen; this result was confirmed b y Allison a n d M o r r i s (15) a n d others. Since t h e n this ability has been proved in m o r e t h a n 20 species distributed i n t h e genera Nostoc, Anabaena, Cylindrospermum, Calo- thrix, Anabaenopsis, a n d Mastigocladus [for references see Fogg's monographs (126, 128, 1 2 9 ) ] . It has been established t h a t t h e ability to fix nitrogen is common a m o n g t h e blue-greens although some species lack this p r o p e r t y [cf. Fogg ( 1 2 8 ) ] . All t h e blue-green algae that can fix nitrogen seem to h a v e a c o m m o n peculiarity, n a m e l y , t h e y form thick-walled cells, called heterocysts, w h i c h appear at r e g u l a r intervals
552 A . I. V I R T A N E N A N D J. K . M I E T T I N E N
in the chains of algal cells. T h e role of heterocysts is not fully u n d e r - stood, but it is generally believed t h a t t h e y are resting cells or organs of perennation. Fogg a n d Wolfe (130) suspect t h a t the heterocysts and t h e nitrogen fixation of blue-green algae m a y h a v e h a d a common phylogenetic origin.
Nitrogen fixation of t h e blue-green algae has been confirmed b y W i l l i a m s a n d Burris (402) b y the isotopic tracer technique ( N1 5) . T h e blue-green algae h a v e a special role in t h e economy of n a t u r e , because they, being autotrophic w i t h respect to carbon as well as nitrogen, are able to thrive in places w h e r e no other organisms a r e able to grow, e.g.
on h u m i d rocks. T h e y , as well as the photosynthetic bacteria, are be- lieved to belong a m o n g the earliest forms of life.* Most of the blue- green algae can also grow heterotrophically in a nitrogen-free m e d i u m , but nitrogen fixation is t h e n slower. One strain of Nostoc muscorum has been reported to fix 10 m g nitrogen per g r a m of glucose in darkness.
C . M E T A B O L I S M O F N I T R O G E N - F I X I N G M I C R O O R G A N I S M S A N D F A C T O R S T H A T I N F L U E N C E F I X A T I O N
1. General Remarks
Nitrogen fixation in free-living microorganisms is n o r m a l l y insepa- r a b l y b o u n d w i t h growth. It is not achieved in n o n g r o w i n g cultures and w a s not, at first, consistently obtained w i t h cell-free e n z y m e prepara- tions, although M a g e e a n d Burris (218) reported erratic positive results w i t h cell-free Azotobacter vinelandii preparations. However, positive results obtained b y a dependable method, even occasional ones, m e a n t h a t the reaction does take place. A l t h o u g h all t h e factors in- volved a r e not yet mastered, cell-free nitrogen fixation is expected to be consistently obtained sooner or later.f
* By some—e.g., van Niel—the blue-green algae are regarded as relatively advanced forms among the microorganisms because of their special pigments. Also there is a view that nitrogen fixation came late in the evolution of bacteria, after all the ammonia of the original atmosphere had been used. [Ed.]
f After this manuscript was completed, fixation of nitrogen by cell-free extracts of Clostridium was reported by Carnahan et al. (90). They obtained from cells of Clostridium pasteurianum, dried at 50 °C under vacuum and autolyzed at 30 °C in anaerobic conditions, soluble preparations capable of reproducible and readily meas- urable nitrogen fixation in the presence of pyruvate. The techniques applicable to Clostridium were not successful for Azotobacter and Nostoc. However, soon after the above report Wilson and Burris (412a) applied the same method successfully to an- other blue-green alga, Mastigocladus laminosus, and also to a photosynthetic bac- terium, Rhodospirillum rubrum. At about the same time, Nicholas and Fisher (241b) reported that cell-free extracts from Azotobacter vinelandii could fix nitrogen when the cells were disrupted during the early log phase of growth in the culture medium
5. B I O L O G I C A L N I T R O G E N F I X A T I O N 553 A t this point, however, w e m u s t e x a m i n e t h e results obtained d u r i n g the last two decades w i t h growing cultures, even t h o u g h it is often extremely difficult to decide w h e t h e r a n observed effect was specific to the process of nitrogen fixation itself or to t h e metabolic processes in the organism in general.
U n t i l quite recently it h a s been considered t h a t nitrogen-fixing organisms use gaseous nitrogen as a source only of nitrogen, b u t h a r d l y of energy. T h e latter possibility has also to b e t a k e n into con- sideration, however. Some years ago, P a r k e r (261a) suggested t h a t nitrogen fixation m i g h t well be a form of respiration w i t h nitrogen act- ing as a n alternative to oxygen as a t e r m i n a l h y d r o g e n acceptor. I n a recent paper, P a r k e r a n d Scutt (261b) emphasize this possibility, based on t h e competitive inhibition of nitrogen fixation b y oxygen in Azotobacter, a n d on t h e calculations of Bayliss (33a) on the e n e r g y changes associated w i t h t h e reduction of nitrogen to a m m o n i a . Dil- w o r t h a n d P a r k e r (106a) h a v e just found t h a t t h e inhibition of respira- tion b y oxygen is m o r e severe w i t h the organic acids t h a n w i t h t h e sugars. W h e n glycerine is t h e o n l y carbon source, no inhibitory effects of oxygen w e r e observed. N o explanation could be offered for t h e lack of inhibition. H a m i l t o n a n d W i l s o n (150) w e r e the first w h o reported oxygen-inhibited nitrogen fixation b y t h e faculative aerobe Aero- bacter aerogenes. T h e specific n a t u r e of t h e inhibition w a s shown b y P e n g r a a n d W i l s o n (262a) for this organism a n d b y H i n o a n d W i l - son (163) for another facultative b a c t e r i u m of t h e Bacillus polymyxa type. T h e value obtained b y P a r k e r a n d Scutt (261b) for t h e Michaelis constant was 0.0229 at 2 0 % oxygen concentration, a n d was in agree- m e n t w i t h t h a t obtained b y W i l s o n et al ( 4 1 3 ) .
2. Humidity, Temperature, and pH
Regarding t h e general conditions of growth, t h e Clostridium species favor a relatively high, Azotobacter a moderate, h u m i d i t y in the soil.
T h e o p t i m u m t e m p e r a t u r e for growth as well as for nitrogen fixation is for C. pasteurianum 25°C, for A. chroococcum a n d A. agilis 2 5 - 2 8 ° C , for Beijerinckia indica (formerly A. indicus) 30°C and, for some tropical strains of t h e latter, even 33°C.
Both Clostridium a n d Azotobacter h a v e t h e p H o p t i m u m of nitrogen fixation slightly on the alkaline side, b u t t h e p H evidently does not h a v e
in which they were grown. The fixation was shown by them to occur in particle- free extracts provided the "media factors" were present. Thus new possibilities have been opened up for the elucidation of factors necessary for nitrogen fixation and the mechanism of this process.
554 A . I. V I R T A N E N A N D J. K . M I E T T I N E N
a direct effect on fixation, since t h e same o p t i m u m ( p H 7.6-7.8) is obtained for Azotobacter w h e n growing on a m m o n i u m nitrogen, as Burk et al. (72) showed in 1934. W i t h t h e exception of A. indicus—
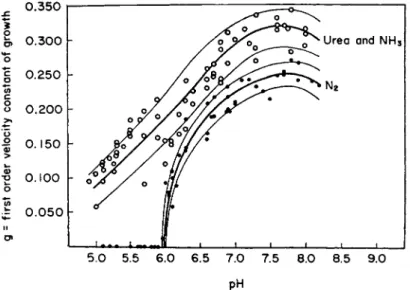
w h i c h c u r r e n t l y is classified as a separate genus, Beijerinckia—Azoto- bacter does not grow on gaseous nitrogen or fix it below p H 6.0. T h e cessation of g r o w t h at this p H w i t h nitrogen is v e r y abrupt, although growth w i t h a m m o n i u m nitrogen diminishes o n l y slowly a n d ceases somewhere below p H 5 (Fig. 1 ) . I n blue-green algae assimilation of nitrogen does not take place below p H 5.7 [Allison et al. ( 1 4 ) ] . On the
5.0 5.5 6.0 6.5 7.0 7.5 8.0 8.5 9.0 pH
FIG. 1. Growth of Azotobacter with urea and ammonia or with nitrogen as a function of pH. From Burk et al. (72).
alkaline side t h e p H dependence of nitrogen fixation for bacteria is similar to t h a t of respiration, being evidently d u e to t h e inhibition of t h e latter.
3. Carbon and Energy Metabolism
It is neither possible n o r useful to discuss the carbon metabolism of nitrogen-fixing organisms in great detail h e r e because it does not differ m a r k e d l y from t h a t of the nonnitrogen-fixing organisms. But brief m e n t i o n m a y show, first, w h i c h carbon compounds a r e most readily utilized b y a given organism and, second, t h e efficiency of fixation w i t h a given carbohydrate.
T h e anaerobic nitrogen-fixing bacteria obtain e n e r g y for g r o w t h as well as for nitrogen fixation from fermentation of carbohydrates (espe-
5. B I O L O G I C A L N I T R O G E N F I X A T I O N 555 cially glucose, saccharose, a n d / o r soluble starch) m a i n l y to fatty acids.
T h e n a t u r e of t h e carbohydrates w h i c h t h e organism is able to use has even been adopted as a m e a n s of classification [ B r e d e m a n n (63) ; W a k s - m a n ( 3 9 3 ) ] . Carbohydrates utilized a r e degraded b y t h e glycolytic m e c h a n i s m , or b y t h e so-called pentose cycle, to the level of three-car- bon compounds, w h i c h a r e t h e n used for building u p cell constituents or a r e further degraded, m a i n l y to acetate. T h e efficiency of nitrogen fixation b y p u r e cultures of anaerobic nitrogen-fixing bacteria u s u a l l y has been reported to be about 2 - 3 m g nitrogen per g r a m of glucose used.
Rosenblum a n d Wilson ( 2 7 9 ) , however, report a n efficiency as high as 1 0 - 1 2 m g nitrogen p e r g r a m of sugar used for Clostridium pasteurianum.
T h i s is n e a r l y t h e s a m e as t h e average efficiency of Azotobacter. I n anaerobic fermentation per mole of glucose only a small a m o u n t of en- ergy is released compared w i t h aerobic respiration (total combustion to carbon dioxide a n d w a t e r ) . Since t h e low e n e r g y production in anaerobic organisms is sufficient for nitrogen fixation, it seems t h a t t h e e x t r e m e l y h i g h respiration of Azotobacter is not directly needed for nitrogen fixation.
Aerobic nitrogen-fixing agents can use a m u c h greater variety of carbon sources t h a n t h e anaerobic ones. Azotobacter, for example, utilizes fatty a n d h y d r o x y acids, lower a n d higher alcohols, a n d mono-, di-, a n d polysaccharides. It can also use a m i n o acids, b u t n o t peptones, as a source of carbon a n d energy. Several a m i n o acids are also used as a source of nitrogen (see page 5 5 9 ) .
Azotobacter is t h e fastest-growing organism a m o n g t h e nitrogen- fixing bacteria. I n n a t u r a l conditions its rates of growth a n d of nitrogen fixation a r e u s u a l l y directly dependent on the supply of carbohydrate a n d oxygen. I n a special fermentor w i t h h i g h l y efficient aeration a n d w i t h a high carbohydrate concentration, A l e x a n d e r a n d W i l s o n (4) reported fixation of 0.175 m g nitrogen per milliliter per h o u r d u r i n g the exponential period of growth. Such a n e t increase can be m a i n - tained, of course, o n l y for a short time.
Carbon compounds a r e partially converted to cellular substances, partially oxidized to carbon dioxide a n d water. W h e n n o combined nitrogen is present, Azotobacter m a y fix even 2 0 - 3 0 m g nitrogen per g r a m of glucose, although a n average efficiency is o n l y about 12 m g . Meyerhof a n d B u r k (225) h a v e a l r e a d y shown t h a t a v e r y h i g h respira- tion r a t e is typical of t h e Azotobacter: Q0 j- v a l u e s obtained v a r y between 2000 a n d 4000 μΐ 02 per h o u r p e r m i l l i g r a m d r y weight of bacteria. T h e highest r a t e is found at a n oxygen pressure of 0.10-0.15 a t m — s o m e - w h a t below t h e atmospheric. I n this respect Azotobacter differs from the excised, sliced soybean nodules, w h e r e fixation increases even at
5 5 6 A . I. V I R T A N E N A N D J. K . M I E T T I N E N
oxygen pressures higher t h a n atmospheric, t h e m a x i m u m lying at about p02 0 . 5 a t m (see Section I I I , K ) . T h e high respiration r a t e seems to h a v e no direct significance for t h e fixation process. E n z y m e s for the glycolytic as well as t h e "direct oxidation" p a t h w a y (see Chapter 3 , Vol.
I A ) seem to b e present in Azotobacter as well as in all t h e other nitro- gen-fixing agents studied. Intermediates of t h e above " p a t h w a y s " — pentose, hexose, a n d sedoheptulose phosphates—as well as t h e e n z y m e s of the tricarboxylic acid cycle h a v e been shown in Azotobacter b y Wilson's group [for references see W i l s o n ( 4 1 1 ) ] . P y r u v a t e produced b y t h e glycolytic m e c h a n i s m or "direct oxidation" is further oxidized b y t h e tricarboxylic acid cycle, w h i c h is probably t h e m a i n source of e n e r g y for t h e process of fixation as well as t h e m a i n source of carbon acceptors for t h e reduced nitrogen. T h e nitrogen-fixing blue-green algae n o r m a l l y use atmospheric carbon dioxide as the source of carbon and light as t h e source of energy, b u t m a n y of t h e m a r e also able to fix nitrogen heterotrophically in darkness, u s i n g glucose as a source of carbon a n d energy. Allison et al. ( 1 4 ) h a v e reported r a t h e r high efficiencies of heterotrophic fixation for Nostoc muscorum, 1 0 - 1 2 m g nitrogen per g r a m of glucose, w h i c h a r e quite comparable to t h e aver- age efficiency of Azotobacter a n d equal to the highest value reported for Clostridium (see above). On the other h a n d , a n o t h e r strain of N.
muscorum was a n obligate phototroph.
4. Utilization of Molecular and Combined Nitrogen Burk ( 6 8 ) was t h e first to study the dependence of nitrogen fixation on t h e partial pressure of nitrogen ( p N2) . H e showed t h a t t h e relation- ship follows t h e Michaelis-Menten equation for a n e n z y m a t i c reaction, b u t his value for Km, t h e p N2, w h e r e t h e reaction r a t e of the fixation is half m a x i m a l , was too high, because h e used molecular h y d r o g e n as a diluent gas. Later, w h e n h y d r o g e n w a s found b y W i l s o n a n d U m b r e i t ( 4 1 7 ) to be a specific inhibitor of nitrogen fixation, Km w a s redeter- m i n e d in a n inert atmosphere; these determinations gave for Azoto- bacter Km = 0 . 0 2 a t m [ W y s s et al. ( 4 3 1 ) ; Wilson et al. ( 4 1 3 ) ] , a n d also for Nostoc Km = 0 . 0 2 atm, values comparable w i t h those obtained for detached soybean root nodules (Km = 0 . 0 2 5 ) [Burris et al. ( 7 7 ) ] . These results show t h a t already half of t h e m a x i m a l r a t e of fixation is reached b y each of these systems at a nitrogen pressure w h i c h is only about one-fortieth t h a t of t h e atmospheric pressure. Gaseous nitrogen, therefore, seldom, if ever, becomes a limiting factor of nitrogen fixation u n d e r n a t u r a l conditions.
I n most nitrogen-fixing organisms even low concentrations of N H4 +
diminish nitrogen fixation m a r k e d l y , a n d 5 - 1 0 m g a m m o n i u m nitrogen
5. B I O L O G I C A L N I T R O G E N F I X A T I O N 557 per liter suffices to arrest it completely [Burk ( 6 8 ) ] . T h i s is about the same concentration, in g r a m atoms of nitrogen, at w h i c h utilization of molecular nitrogen reaches its m a x i m u m . Below this concentration both forms of nitrogen are utilized simultaneously, t h e inhibition b y a m m o n i a being "competitive." Above this concentration a m m o n i u m nitrogen is u s u a l l y utilized preferentially. T h e gram-negative aerobic bacterium recently isolated b y Roy a n d M u k h e r j e e (281) from the soil of J a v a n e s e j u t e (Corchorus sp.) fields is the only exception so far re- ported. This b a c t e r i u m seems to be a n obligate nitrogen fixer.
T h e e n z y m e system responsible for a m m o n i u m ion assimilation seems to be constitutive, not adaptive, in most nitrogen-fixing organisms because no lag period is u s u a l l y found i n the utilization of a m m o n i u m nitrogen w h e n it is added into a nitrogen-fixing culture, but fixation of gaseous nitrogen ceases a n d utilization of a m m o n i u m nitrogen starts immediately. A lag period has been observed in Azotobacter o n l y if a relatively high a m m o n i u m - i o n concentration was used (above 10"4 M;
0 . 1 % w / v of nitrogen as a m m o n i u m acetate stops respiration for a period of several hours) [Wilson a n d Roberts (421 ) ; A z i m a n d Roberts
( 2 8 ) ] , b u t this m a y be d u e to secondary effects, e.g., disturbance of t h e p H inside the cells because of a n excessive assimilation of the a m - m o n i u m ion.
N i t r a t e nitrogen is utilized b y most nitrogen-fixing organisms also, but, e.g., i n a n Azotobacter c u l t u r e growing on molecular nitrogen, utilization of n i t r a t e nitrogen begins only after a lag period of about 1 h o u r [Burk ( 6 8 ) ] . T h i s length of t i m e is required for adaptation, i.e., for t h e formation of a nitrate-reducing e n z y m e system in the cells. I n recent years this e n z y m e system has been extensively investigated [see Chapter 3 of this volume on inorganic nutrition of microorganisms, b y Nicholas; cf. also N a s o n a n d T a k a h a s h i ( 2 3 8 ) ] . N i t r a t e is reduced to a m m o n i a i n four steps probably b y four enzymes, t h r e e of w h i c h — n i t r a t e reductase, nitrite reductase, a n d h y d r o x y l a m i n e reductase—
h a v e been shown experimentally to exist [ E g a m i et al. ( 1 1 0 ) ] . T h e identity of t h e t h i r d e n z y m e w h i c h leads to t h e formation of h y d r o x y l - a m i n e is still not clear [ Y a m a d a a n d V i r t a n e n ( 4 3 2 ) ] . M e d i n a a n d Nicholas ( 2 2 1 , 222) h a v e shown, i n Neurospora g r o w n on nitrate ni- trogen, a n e n z y m e called h y p o n i t r i t e reductase w h i c h m a y be t h e miss- i n g e n z y m e . About the same concentration of n i t r a t e nitrogen (5 m g nitrogen p e r l i t e r ) , at w h i c h nitrogen fixation reaches its m a x i m u m is usually sufficient to p r e v e n t nitrogen fixation. T h e blue-green alga Anabaena cylindrica is a n exception as it utilizes molecular nitrogen also in t h e presence of high concentrations of n i t r a t e [Allen ( 7 ) ] . A m - monia, however, stops nitrogen fixation in this case also.
558 A . I. V I R T A N E N A N D J. K . M I E T T I N E N
It has been k n o w n for some years t h a t nitrous oxide ( N20 ) competi- tively a n d specifically inhibits nitrogen fixation [ M o l n a r et al. ( 2 3 2 ) ] . M o z e n a n d Burris (234) found N20 to be slowly assimilated—with a r a t e of about 5 % t h a t of N2 assimilation. According to Burris (75) utilization of N20 is competitively inhibited b y N2 a n d H2, a fact w h i c h suggests t h a t N20 , N2, a n d H2 a r e all adsorbed to t h e same active site of t h e e n z y m e surface. According to Roberts ( 2 7 5 ) , however, t h e ob- served utilization of N20 m a y be due to its decomposition, w h i c h leads to h i g h l y labeled N2, N20 itself being a nonutilizable competitive in- hibitor of N2 fixation.
H y d r o x y l a m i n e is not utilized b y a n y N2-fixing organism. After Blom (47) a n d E n d r e s (115) h a d presented the idea t h a t it m i g h t be a n intermediate in nitrogen fixation, it was tested b y Burk a n d H o r n e r ( 7 1 ) , w h o found it nonutilizable a n d h i g h l y toxic to Azotobacter. E v e n in nontoxic concentrations (below 3 m g p e r liter) it was not utilized, a n d 5 m g per liter w a s sufficient to d a m a g e t h e cells. T h i s w a s con- firmed b y Novak a n d Wilson (249) a n d b y Pethica et al. ( 2 6 3 ) , w h o found even 1.5-2 m g p e r liter toxic to Azotobacter. Clostridium is some- w h a t less sensitive, 5 - 1 0 m g per liter being t h e lower limit of toxicity
[Rosenblum a n d W i l s o n ( 2 8 0 ) ] .
Nonutilization a n d toxicity of exogenous h y d r o x y l a m i n e h a v e often been cited against its possible role as a n i n t e r m e d i a t e in nitrogen fixa- tion, b u t these facts are not proof of this. However, there a r e other findings w h i c h suggest t h a t h y d r o x y l a m i n e m a y not be a probable in- termediate i n nitrogen fixation. Spencer et al. (296) recently studied soluble n i t r a t e a n d h y d r o x y l a m i n e reductases of Azotobacter a n d found t h a t these e n z y m e s are adaptive to n i t r a t e b u t not to molecular nitrogen or to a m m o n i u m sulfate. This finding suggests t h a t t h e enzymes func- tion i n n i t r a t e a n d nitrite assimilation, b u t not in nitrogen fixation.
Derivatives of h y d r o x y l a m i n e w i t h keto acids, oximes, are also non- utilizable [Novak a n d W i l s o n ( 2 4 9 ) , Burris ( 7 5 ) ] .
H y d r a z i n e has been found to be nonutilizable a n d extremely toxic for Azotobacter [Burk a n d H o r n e r ( 7 1 ) ] a n d for Clostridium [Rosen- b l u m a n d Wilson ( 2 8 0 ) ] . Suzuki a n d Suzuki (301) noticed t h a t in a heavy, well-aerated suspension of Azotobacter h y d r a z i n e disappears.
A z i m a n d Roberts (29) found t h a t nitrogen fixation is completely inhibited in Azotobacter b y h y d r a z i n e in a concentration of 2 χ 10"4
M. Below 2 X 1 0- 5 M, nitrogen fixation is stimulated. Respiration is completely inhibited o n l y in concentrations above 10~2 M. According to Roberts (275) h y d r a z i n e is, however, nonutilizable a n d is only
"sequestrated" b y t h e cells. Bach (31) showed t h a t w i t h keto acids it forms cyclic derivatives t h r o u g h a chemical reaction. T h e possible role of such derivatives is discussed below (page 6 4 3 ) .
5. B I O L O G I C A L N I T R O G E N F I X A T I O N 559 N 02N H2 ( n i t r a m i d e ) a n d ( N O H )2 (hyponitrite) a r e two other simple inorganic nitrogenous compounds t h a t h a v e been tested because of their possible role as intermediates i n nitrogen fixation. Both are labile compounds, r a p i d l y decomposed i n w a t e r solution. K l u y v e r a n d Verhoeven (195) h a v e shown t h a t t h e compound used b y t h e earlier workers [Allen a n d v a n N i e l ( 9 ) ] r e a l l y w a s imidonitric acid
( N H N 02H ) a n d n o t n i t r a m i d e . Tests b y M o z e n a n d Burris [cf. Burris ( 7 5 ) ] m a d e i n a n atmosphere of 2 0 % 02 + 8 0 % H2 w i t h N1 5- l a b e l e d n i t r a m i d e added i n successive small aliquots to keep t h e concentration nontoxic, gave no evidence t h a t it was utilized b y Azotobacter.
H y p o n i t r i t e is still m o r e labile t h a n n i t r a m i d e . I t h a s been tested b y Rosenblum a n d W i l s o n (280) using Clostridium pasteurianum as test organism a n d b y C h a n d h a r y et al. (94) using Azotobacter vinelandii.
These authors h a v e found it to inhibit nitrogen fixation without being utilized itself.
A m i n o acids a n d purines in general seem not to be as readily utilized b y nitrogen-fixing organisms as b y m a n y other microorganisms. Burk a n d H o r n e r (71) a n d H o r n e r a n d Allison (168) h a v e studied t h e utili- zation of n u m e r o u s organic nitrogen compounds b y Azotobacter, showing t h a t it can utilize u r e a a n d several a m i n o acids a n d p u r i n e s as sources of nitrogen, although only urea, a m i n o dicarboxylic acids, a n d a d e n i n e are readily utilizable. T h e s e compounds do not, however, block nitrogen fixation as a m m o n i u m a n d n i t r a t e nitrogen according to Wilson et al. ( 4 1 6 ) . T h e blue-green alga Anabaena cylindrica is also able to utilize several a m i n o acids a n d other nitrogenous com- pounds [Fogg a n d Wolfe ( 1 3 0 ) ] although blue-green algae in general prefer inorganic forms of nitrogen.
5. Excretion of Fixed Nitrogen
Excretion of fixed nitrogen m a y take place in the case of free-living nitrogen fixers as it does i n t h e case of t h e symbiotic system (page 5 9 8 ) , although it usually is v e r y low. H i g h excretion from Clostridium pasteurianum, about 6 0 % of t h e nitrogen fixed, w a s reported b y V i r t a n e n a n d H a k a l a ( 3 5 1 ) . Zelitch et al. (435) somewhat later showed b y N1 5 t h a t t h e excreted nitrogen, u p to 5 0 % of the fixed nitrogen m a i n l y in t h e form of asparagine, g l u t a m i n e , a n d a m m o n i a , w a s
" j u v e n i l e " nitrogen, since its labeling w a s m a n y times h i g h e r t h a n i n t h e same compounds inside t h e cells. W a t a n a b e (396) studied t h e ex- cretion of nitrogen b y blue-green algae; h e found t h a t of several species tested only Calothrix brevissima showed a noticeable e x c r e t i o n — 4 0 % of fixed n i t r o g e n — i n the form of aspartic acid, glutamic acid, a n d alanine. Fogg (127) m a d e a detailed study of excretion from blue- green algae. H e found liberal excretion only from A. cylindrica; t h e
560 A . I. V I R T A N E N A N D J. K . M I E T T I N E N
excreted nitrogen n o t being i n t h e form of free a m i n o acids as from Calothrix brevissima ( 3 9 6 ) , b u t i n t h e form of polypeptides containing principally serine a n d threonine, small a m o u n t s of glutamic acid, gly- cine, a n d tyrosine, a n d traces of alanine, valine, a n d leucine as well.
I r o n deficiency increased excretion.
I t seems t h a t excretion of nitrogenous compounds takes place w h e n t h e conditions for nitrogen fixation are good b u t t h e r e is a shortage of some other element, e.g., carbon or iron. T h e significance of the ex- cretion p h e n o m e n o n from t h e standpoint of nitrogen fixation is dis- cussed further i n connection w i t h t h e symbiotic system (Section I I I , G ) . 6. Inhibition of Nitrogen Fixation by Hydrogen and Carbon Monoxide
It w a s shown b y W i l s o n a n d U m b r e i t (417) t h a t h y d r o g e n com- petitively inhibits symbiotic nitrogen fixation, b u t not growth on a m - m o n i u m or n i t r a t e nitrogen. L a t e r it was shown t h a t the same is t r u e for Azotobacter [ W y s s et al. ( 4 3 1 ) ] , Nostoc [Wilson ( 4 0 9 ) ] , a n d Rhodospirillum rubrum [ K a m e n a n d Gest ( 1 8 4 ) ] . I n Clostridium pasteurianum, in w h i c h h y d r o g e n is a n o r m a l product of metabolism, it does not inhibit the r a t e of fixation but o n l y decreases t h e efficiency of t h e reaction [Rosenblum a n d W i l s o n ( 2 7 9 ) ] . Also in Rhodospirillum rubrum h y d r o g e n does n o t inhibit nitrogen fixation [Gest et al. ( 1 4 5 ) ] .
It has been established b y W i l s o n a n d his associates [for references see, e.g., Shug et al. (290) ] t h a t all free-living nitrogen-fixing microbes possess t h e e n z y m e hydrogenase, w h i c h activates molecular h y d r o g e n
H2 ^ 2H+ + 2e~
Quite recently t h e presence of hydrogenase in t h e symbiotic system, too, w a s confirmed [ H o c h et al. ( 1 6 5 ) ] .
All nitrogen-fixing systems hitherto examined thus contain h y d r o - genase. T h i s e n z y m e is nonspecific for t h e oxidant; e.g., m e t h y l e n e blue, cytochrome c, nitrate, p y r i d i n e nucleotides, or oxygen m a y act as electron acceptors [ L e e et al. ( 2 0 0 ) ; S h u g et al. ( 2 8 9 ) ] . A n o t h e r inter- esting feature of hydrogenase is t h a t it is adaptive to nitrogen fixation r a t h e r t h a n to t h e presence of h y d r o g e n ; if t h e nitrogen source of a n actively nitrogen-fixing Azotobacter culture is changed to a m m o n i u m ion, h y d r o g e n a s e activity is i m m e d i a t e l y greatly diminished [ L e e et al.
( 2 0 0 ) ; Lee a n d Wilson ( 2 0 1 ) ] . All t h e above facts corroborate the hypothesis t h a t t h e e n z y m e is connected w i t h nitrogen fixation [Wilson (408, 4 0 9 ) ] . T h e first supposition t h a t t h e " h y d r o g e n a s e " a n d "nitro- genase" w e r e identical, i.e., t h a t each organism possessing hydrogenase w a s also able to fix nitrogen, w a s invalidated w h e n it was shown t h a t