Modes of Action of the Essential Mineral Elements
A L V I N N A S O N A N D W I L L I A M D . M C E L R O Y
I. Introduction 451 II. General Functions of the Essential Nutrient Elements 453
III. Catalytic Properties of Metalloproteins 455 A. Model Systems for Catalytic Activity of Metal Ions 455
B. Metalloproteins 460 IV. Metal Requirements of Enzymes 464
V. Mechanism of Action of the Micronutrient Elements 465
A. Zinc 465 B. Copper 471 C. Iron 478 D. Molybdenum 483
E. Manganese 494 F. Boron 499 G. Vanadium 502 H. Cobalt, Selenium, and Iodine 504
VI. Mechanism of Action of the Macronutrient Elements 508
A. Nitrogen 508 B. Phosphorus 508 C. Calcium 509 D. Magnesium 511
E. Potassium 512 F. Sulfur 515 G. Chlorine* 517 VII. Mineral Nutrients in Metabolic Pathways and Processes 518
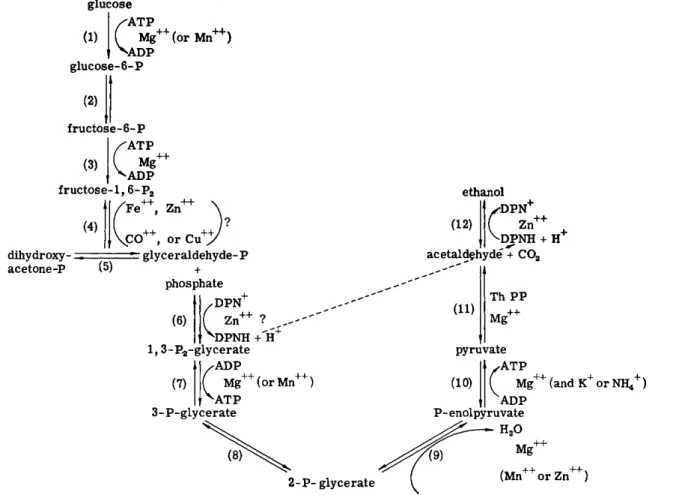
A. Glycolysis 518 B. Hexose Monophosphate Shunt (Pentose Phosphate Pathway) . 518
C. Krebs Citric Acid Cycle 518 D. Terminal Respiration 518 E. Photosynthesis 518 VIII. Concluding Remarks 521
References . 522 I. Introduction
N u t r i e n t s a r e necessary for m a i n t e n a n c e of t h e physical organization a n d activities of living cells b y v i r t u e of t h e i r function i n t h e generation or release of e n e r g y , t h e b u i l d i n g a n d r e p a i r of protoplasm, a n d t h e r e g u l a t i o n of metabolic processes. T h e y a r e u s u a l l y classified into those groups w h i c h c a n be used as ( a ) a n e n e r g y source, (b) a carbon source,
* For the authors' reasons for treating chlorine in this section rather than as a micronutrient see page 517. (Ed.)
451
452 A . N A S O N A N D W . D . M C E L R O Y
(c) a nitrogen source, (d) organic g r o w t h factors, a n d (e) m i n e r a l salts or inorganic n u t r i e n t s . T h e latter group is t h e subject of t h e present chapter.
All cells a n d organisms require t h e presence of certain inorganic ions for g r o w t h a n d reproduction. Salts contribute to t h e regulation of osmotic pressure (see Chapters 2 a n d 4 i n V o l u m e I I ) , t h e m a i n t e n a n c e of cellular m e m b r a n e s a n d to t h e functioning of t h e metabolic m a c h i n e . Some of the elements are required i n large a m o u n t s ( m a c r o n u t r i e n t or major elements) while others are r e q u i r e d i n relatively small a m o u n t s (micronutrient or trace e l e m e n t s ) . O u r most complete knowledge of the m i n e r a l r e q u i r e m e n t s of living forms h a s been attained w i t h h i g h e r plants in contrast to t h e relatively limited information i n this respect w i t h animals a n d microorganisms. T h i s is probably explained b y t h e fact t h a t higher plants lent themselves best to controlled nutritional ex- p e r i m e n t a t i o n i n t h e late n i n e t e e n t h a n d early t w e n t i e t h centuries, a n d t h e r e w a s a n obvious incentive i n t h e economic importance of p l a n t crops. It has been established t h a t for higher plants t h e m a c r o n u t r i e n t elements include nitrogen, phosphorus, sulfur, potassium, m a g n e s i u m , a n d calcium. Recent evidence (cf. Chapters 1 a n d 2) h a s also impli- cated sodium a n d chlorine as essential n u t r i e n t elements. T h e micro- n u t r i e n t elements required b y plants a r e iron, zinc, m a n g a n e s e , copper, m o l y b d e n u m , boron, a n d v a n a d i u m . T h e last t h r e e h a v e not been shown to b e essential for animals. Iodine a n d cobalt a r e essential for animals, b u t a p p a r e n t l y not for plants. Although most of the elements of the periodic table h a v e been found in living cells, this does n o t necessarily m e a n t h a t all are essential to life ( 2 1 8 ) . About 9 5 % of the d r y weight of most plants is composed of four elements—carbon, hydrogen, oxygen, a n d nitrogen. A n o t h e r 4 % is m a d e u p of potassium, phosphorus, cal- cium, m a g n e s i u m , silicon, a l u m i n u m , sulfur, chlorine, a n d sodium. T h e r e m a i n i n g 1 % or less of t h e weight of the p l a n t is accounted for b y t h e m i c r o n u t r i e n t a n d b y certain dispensable elements.
T h e function of t h e essential n u t r i e n t elements, especially t h e micro- n u t r i e n t s , h a s received considerable attention in recent years. P a r t i c u l a r emphasis h a s been placed u p o n their role i n catalytic processes. I n several reviews (40, 128, 136) t h e r e is discussion, in some detail, of t h e general problems of m e t a l chelation a n d metalloprotein complexes, chiefly from t h e standpoint of the physical properties of the ions a n d t h e structural linkages between m e t a l ions a n d organic molecules. Re- views h a v e also appeared on t h e function of m e t a l s in peptidases ( 2 4 2 ) , on t h e problems of phosphorylation a n d t h e influence of m e t a l ions on these processes ( 1 3 4 ) , on t h e general p r o b l e m of multiple m e t a l effects on e n z y m e systems a n d on the alteration of the e n z y m e patterns d u r i n g
g r o w t h ( 1 4 5 ) . D u r i n g t h e last few years t h e r e h a v e also been reviews on t h e m e c h a n i s m of action of m e t a l ions i n e n z y m e systems ( 1 4 6 , 1 7 4 ) including a detailed account of t h e role of metals i n yeast fermentation
( 1 7 3 ) as well as a comprehensive t r e a t m e n t of t h e metabolic role of t h e trace elements i n plants a n d a n i m a l s ( 9 8 , 2 5 7 ) . T h e broad area of m i n e r a l n u t r i t i o n of both plants a n d animals, covering m a n y papers i n this field a n d including the history a n d geographical distribution of in- dividual elemental deficiencies, has been t h e subject of a r e c e n t l y p u b - lished v o l u m e ( 8 3 ) . N o a t t e m p t h a s been m a d e , therefore, to refer to all t h e literature w h i c h is concerned w i t h t h e function of t h e essential elements. T h e r e a d e r is referred to t h e reviews m e n t i o n e d for additional references a n d details.
II. General Functions of the Essential Nutrient Elements
A n u m b e r of attempts h a v e been m a d e to uncover u n d e r l y i n g gen- eralizations w h i c h relate t h e essential elements a n d their chemical properties to function a n d m e c h a n i s m of action. Of some interest is t h e proposal b y T h a t c h e r ( 2 6 2 ) i n 1 9 3 4 suggesting a classification of t h e essential elements based on their role i n p l a n t nutrition. H e indicated t h a t n e a r l y all t h e elements k n o w n or proposed to h a v e a function i n plants occur i n t h e first four periods of t h e periodic table. H e classified ( a ) h y d r o g e n a n d oxygen as energy-exchange elements; (b) carbon, nitrogen, sulfur, a n d phosphorus as e n e r g y storers; (c) sodium, potas- sium, calcium, a n d m a g n e s i u m as translocation regulators; a n d (d) m a n g a n e s e , iron, copper, a n d zinc (as well as cobalt a n d nickel) as oxidation-reduction regulators. T h e r e m a i n i n g elements of t h e scheme w e r e placed into four other groups, b u t their functions w e r e listed as u n k n o w n . Similar relationships between biological essentiality of the elements a n d their atomic structures h a v e been suggested b y other workers ( 7 3 , 2 5 3 ) .
O u r present knowledge of m i n e r a l n u t r i t i o n indicates t h a t one of t h e most i m p o r t a n t functions of t h e essential elements is to act as cofactors or activators i n e n z y m e systems. T h e r e are obvious exceptions. Nitrogen a n d sulfur a p p e a r to serve p r i m a r i l y in a structural capacity, t h e former as a component a t o m of proteins a n d nucleic acids a n d t h e latter of certain a m i n o acids a n d vitamins. Phosphorus, in addition to its struc- t u r a l role i n nucleic acids, nucleotides, phosphagens, a n d phospholipids h a s a n i m p o r t a n t function in i n t e r m e d i a r y metabolism (e.g., glycolysis a n d t h e oxidative p a t h w a y as well as i n t h e utilization, transfer, a n d release of t h e e n e r g y of m e t a b o l i s m ) . All t h e other elements, except boron, h a v e been shown to h a v e a direct catalytic role in some e n z y m e system. I t is h i g h l y likely t h a t a n e n z y m a t i c role for boron will be
454 A . N A S O N A N D W . D . M C E L R O Y
demonstrated i n d u e time. Aside from t h e catalytic role assigned to most of t h e m i n e r a l elements, a n u m b e r of t h e m h a v e additional func- tions. T h e structural organization of protoplasm depends to a great ex- t e n t u p o n t h e binding of metals to proteins a n d other organic molecules.
M u c h r e m a i n s to b e done before w e can clearly state h o w these metals function i n t h e m a i n t e n a n c e of such structures as the chromosomes, mitochondria, microsomes, a n d the various cell m e m b r a n e s .
It is at present widely accepted t h a t t h e p r i m a r y role of the micro- n u t r i e n t elements a n d m a n y of t h e m a c r o n u t r i e n t elements i n cell physiology can be explained i n terms of their function as active groups of various i m p o r t a n t enzymes. T h e fact t h a t t h e micronutrients a r e needed i n only small quantities is taken as a n indication t h a t t h e y act i n some catalytic role, u s u a l l y as p a r t of a n e n z y m e system. I n this respect t h e function of these elements is similar to t h a t of t h e organic m i c r o n u t r i e n t s — t h e vitamins.
I n t e r m s of the m e t a l r e q u i r e m e n t s of e n z y m e s two broad groups can be designated: (a) those enzymes in w h i c h a specific m e t a l h a s been shown to be a n integral component, a n d (b) those e n z y m e s for w h i c h o n e or m o r e metals serve as a n activator. W h i l e t h e physiological signi- ficance of t h e specific m e t a l components of e n z y m e s seems well defined, t h a t of m e t a l activators at times is open to question. A n activating effect b y a m e t a l i n a cell-free system does not necessarily establish a physio- logical function for t h e m e t a l in question. F o r example, arginase can be activated b y N i+ +, C o+ +, F e+ +, a n d M n+ +. Although M n+ + is regarded as t h e n o r m a l l y active ion, the physiological effects of t h e other ions are not k n o w n . This also raises a question of the possible role of metals w h i c h a r e regarded as not being essential although t h e y do exercise a n activating effect in vitro (e.g., N i+ + a n d C o+ + i n t h e above e x a m p l e ) , and, therefore, possibly in vivo. I t m u s t also be considered t h a t physio- logical control of e n z y m e action b y m e t a l ions m i g h t also be governed b y m e t a l ion availability a n d m e t a l ion antagonisms. T h e classical antagonism between K+ a n d C a+ + or t h e competition between tungstate a n d m o l y b d a t e in a process such as nitrogen fixation reflects the need for evaluating these p h e n o m e n a in terms of their role i n the intact organism. F o r example, t h e growth of certain microorganisms has been shown to be dependent on alkali m e t a l ions, a n u m b e r of antagonistic effects h a v i n g been demonstrated a m o n g the metals of this series ( 1 5 0 ) . T h e r e also appears to be a complete functional replacement of K+ b y R b+ i n t h e n u t r i t i o n of some varieties of Chlorella (203) a n d certain bacteria ( 1 5 0 ) . Similarly a r e q u i r e m e n t for C a+ + for growth has been r e p o r t e d to b e m e t equally well b y S r ^ for t h e phycomycete Allomyces
( 1 1 1 ) . I t is difficult to decide w h i c h is the essential ion i n these cases.
It would seem t h a t whichever one is available could be used efficiently, t h u s reflecting the fact t h a t organisms h a v e a w i d e r use for m e t a l ions i n their e n v i r o n m e n t t h a n is indicated b y their m i n i m u m nutritional r e q u i r e m e n t s . I n addition to the complete substitution of one metallic ion for a n o t h e r there is the m o r e common partial r e p l a c e m e n t or sparing action t h a t one ion m a y show for another. As a n example, t h e m a n g a n e s e r e q u i r e m e n t of Lactobacillus arabinosus ( = L. plantarum) is m a r k e d l y lowered b y m a g n e s i u m a n d to a lesser extent b y calcium or strontium.
I n evaluating effects of m e t a l ions as well as r e q u i r e m e n t s , for m e t a l ions, consideration m u s t also be given to the substrates used a n d the metabolic p a t h w a y s involved. M o l y b d e n u m is required in m u c h h i g h e r a m o u n t s b y fungi a n d higher plants w h e n n i t r a t e r a t h e r t h a n a m m o n i a serves as t h e nitrogen source (cf. Section V, D , 2 ) . T h i s has been demonstrated to be due to t h e fact t h a t m o l y b d e n u m is the m e t a l com- ponent of t h e nitrate-reducing e n z y m e , n i t r a t e reductase. It is quite likely t h a t m a n y m o r e of these p h e n o m e n a will be explained eventually w i t h i n t h e scope of metal-enzyme-substrate interactions. H e w i t t (98) has recently reviewed various aspects of this i m p o r t a n t problem of m e t a l interaction.
111. Catalytic Properties of Metalloproteins
T h e basic question, therefore, w i t h r e g a r d to the m o d e of action of almost all the m i n e r a l elements centers about the role of their ions in e n z y m e systems.
A. M O D E L S Y S T E M S F O R C A T A L Y T I C A C T I V I T Y O F M E T A L I O N S
I n a n u m b e r of cases the catalytic activity of metalloenzymes is a l r e a d y present i n a primitive form in t h e free m e t a l ions. Model sys- tems h a v e provided some basis for insight into t h e m e c h a n i s m of action of a limited n u m b e r of metals at t h e e n z y m e level, n o t a b l y in oxidases a n d other oxidation-reduction reactions involving copper a n d iron. T h i s has also been t r u e to a m o r e limited extent in decarboxylation a n d hydrolysis reactions. On t h e other h a n d , t h e r e a r e no model catalyses k n o w n for m a n y other metalloenzymes or metal-activated enzymes.
1. Copper-Catalyzed Oxidations
T h e n o n e n z y m a t i c catalyses of t h e autoxidation of ascorbic acid a n d catechol b y h e a v y metals including copper are well k n o w n . T h e i r copper-enzyme counterparts a r e ascorbic acid oxidase a n d polyphenol oxidase, respectively (cf. V o l u m e I A, C h a p t e r 3, pp. 2 8 7 - 2 9 3 ) . T h e catalysis of ascorbic acid oxidation b y C u+ + is increased a p p r o x i m a t e l y
456 A . N A S O N A N D W . D . M C E L R O Y
1000-fold b y t h e copper e n z y m e , ascorbic acid oxidase. A n o t h e r impor- t a n t difference between t h e inorganic model a n d e n z y m e systems con- cerned with ascorbic acid oxidation is t h a t hydrogen peroxide is not produced b y t h e e n z y m a t i c reaction, a fact indicating a possible dif- ference in m e c h a n i s m s in the two cases. Copper catalyses probably in- volve a reversible C u+ + «-> C u+ reaction in both the models a n d the oxidases. T h i s has been clearly demonstrated for polyphenol oxidase (132, 133) a n d experimentally suggested for ascorbic acid oxidase (114, 115). A study of t h e m e c h a n i s m of copper ion catalyses of autoxidation of ascorbic acid implies (143, 286, 287) t h a t C u+ + is reduced to C u+ b y a n intramolecular electron shift w i t h i n the coordination complex of the monovalent ascorbate ion a n d C u+ + to give a semiquinone free radical of ascorbate. T h e resultant C u+ w h i c h does not readily coordinate w i t h h y d r o x y l compounds is released a n d oxidized b y oxygen while the ascorbate free radical is t h e n pictured as reducing 02 to either H202 or H20 . A similar proposal for the m e c h a n i s m of metal-catalyzed catechol oxidation has been indicated b y Szent-Györgyi a n d his colleagues ( 1 9 ) . A n u m b e r of t h e copper enzymes such as the polyphenol oxidase of potatoes {Solanum tuberosum) a n d of m u s h r o o m s , b u t not laccase, are inhibited b y carbon monoxide, a n d these inhibitions a r e not reversed b y light, in a g r e e m e n t w i t h t h e k n o w n properties of copper carbonyls ( 1 9 7 ) . I n view of the existence of a v e r y active copper ion catalysis of ascorbic acid autoxidation as well as a n e n h a n c e m e n t of catalytic activity w h e n copper is complexed w i t h nonspecific proteins, t h e ex- istence of a t r u e ascorbic acid oxidase has been questioned. T h i s is dis cussed u n d e r Section V, B, 1 on copper enzymes.
2. Iron-Catalyzed Oxidations
I r o n provides a n o t h e r example of t h e e n h a n c e m e n t of t h e catalytic properties of m e t a l ions w h e n combined w i t h a specific protein. T h e various catalytic properties of h e m e proteins are a l r e a d y present in simple iron compounds. I r o n salts are k n o w n to catalyze t h e oxidation, b y molecular oxygen, of different organic compounds such as phenols, thiols, ascorbic acid, etc. T h i s is analogous to cytochrome oxidase ac- tivity, w h i l e t h e ability of ferricyanide ions to accept electrons to form ferrocyanide is suggestive of electron transfer activity (87, 2 5 5 ) . T h e oxidation of ferrous hydroxide b y 02 to t h e ferric state in t h e presence of a reducing substance, such as thioglycolic acid, is a n example of primitive oxidase activity. T h u s t h e ferric ion as a thioglycolic acid complex m a y be reduced to the ferrous state again w i t h the formation of dithioglycolic acid followed b y t h e subsequent oxidation of Fe""" to F e+ ++ b y molecular oxygen. T h i s results in the over-all catalytic oxida-
tion of thioglycolic acid. T h e ferricyanide system cited above represents a model electron transport substance, being reversibly oxidized a n d reduced w i t h t h e exchange of single electrons.
I r o n salts also exhibit catalase a n d peroxidase properties of a low degree. W h e n iron is incorporated into t h e p o r p h y r i n ring, t h e catalase a n d peroxidase activities originally associated w i t h the inorganic iron a r e increased although t h e p o r p h y r i n s b y themselves show n o catalytic action. W h e n t h e iron p o r p h y r i n s i n t u r n become attached to specific proteins, t h e resulting protein complex shows a t r e m e n d o u s increase in catalytic activity a n d specificity i n addition to being stabilized a n d pro- tected. T h e catalase activities of t h e e n z y m e a n d t h e iron p o r p h y r i n is 1 09 a n d 1 03 times greater, respectively, t h a n t h a t of iron salts. T h e function a n d specificity of action of t h e h e m e is dependent on the pro- tein to w h i c h it is attached ( 1 9 7 ) . H e m e w h e n combined w i t h certain proteins can serve as a transporter of molecular oxygen (hemoglobin), a transporter of electrons (cytochrome b or c ) , a n activator of oxygen (cytochrome oxidase), a n activator of h y d r o g e n peroxide (peroxidase), a n d a decomposer of h y d r o g e n peroxide (catalase). I n o r g a n i c iron salts are regarded as evolutionary precursors of the iron p o r p h y r i n s , the properties of t h e h e m e proteins being a l r e a d y present in a primitive form i n ferrous a n d ferric iron itself ( 8 7 ) . I r o n in its ionic state tends to form octahedral complexes w i t h six coordinate bonds, coordinating generally w i t h oxygen or nitrogen groups containing u n s h a r e d electron pairs as in the h e m e compounds.
3 . Metal-Catalyzed Decarboxylations
Other model systems concerned w i t h m e t a l ion catalysis a n d its rela- tion to e n z y m e catalyses h a v e been investigated in decarboxylation re- actions. A l t h o u g h n u m e r o u s studies h a v e been carried out on non- enzymatic, metal-catalyzed, a n d enzyme-catalyzed decarboxylations of certain metabolically significant organic acids, t h e r e is n o general agree- m e n t as to t h e m e c h a n i s m s involved. H e r e model systems h a v e only partially elucidated t h e m o d e of action of a m e t a l component or acti- vator of a catalytic protein. T h i s aspect h a s been treated in a n u m b e r of reviews ( 4 0 , 1 3 6 , 1 4 5 , 1 4 6 , 1 9 3 , 2 9 0 ) .
Catalytic a m o u n t s of some polyvalent cations m a r k e d l y accelerate the decarboxylation of oxalacetic a n d oxalosuccinic acid. T h i s was first observed b y Krebs ( 1 3 1 ) , w h o found t h a t the most active polyvalent cations for t h e n o n e n z y m a t i c decarboxylation of oxalacetic acid w e r e C o+ +, Z n+ +, Cu+% F e+ +, F e ^ , a n d A l ^ . A t similar concentrations Ca+%
B a+ +, Mg*+, a n d M n+ + w e r e m u c h less effective. T h e decarboxylation of oxalosuccinic acid, like t h a t of oxalacetic acid, is accelerated b y aniline
458 A . N A S O N A N D W . D . M C E L R O Y
a n d b y polyvalent cations ( 1 9 2 ) . T h i s catalysis seems to be due to the capacity of the active cations to form labile m e t a l complexes w i t h the keto acids or their enol forms ( 1 2 9 ) , the m e t a l complexes undergoing rapid decarboxylation. Acetoacetic acid is exceptional in t h a t it appears to form stable m e t a l complexes. T h e complex w i t h F e+ ++ forms a n in- tense red color whereas the complex formed w i t h A l+ ++ absorbs light only in the ultraviolet region. T h e absorption bands of the m e t a l com- plexes of both oxalacetic a n d oxalosuccinic acid disappear v e r y r a p i d l y as a result of decarboxylation. M g+ + or M n+ + as relatively ineffective catalysts of the n o n e n z y m a t i c decarboxylation gave only slight changes
+ (JÔCu
FIG. 1. Metal-catalyzed decarboxylation of ketosuccinic acid derivatives. From Calvin (40) after Steinberger and Westheimer (254).
in the absorption spectrum of t h e keto acids indicating t h a t t h e low catalytic activity of these cations is related to their small capacity to form complexes.
Steinberger a n d W e s t h e i m e r (254) h a v e studied the detailed mech- a n i s m of n o n e n z y m a t i c /^-decarboxylation of the dimethyl-substituted acids. T h e y proposed, as the active intermediate, t h e formation of a chelate structure between t h e m e t a l a n d t h e carbonyl a n d t h e a- carboxyl group of the substrate. It was suggested t h a t the formation of t h e chelate structure results in a n electron shift toward the m e t a l ion a n d a w a y from the ß-carboxyl, leading to decarboxylation as shown in Fig. 1. Other evidence has been reviewed ( 1 4 6 ) , however, w h i c h em- phasizes the possibility of formation of several different complexes, de-
p e n d e n t in p a r t on the p H of the m e d i u m . Some of these complexes m a y be inactive. A m o r e serious objection arises, however, w h e n these proposals a r e applied to e n z y m a t i c reactions. W h i l e zinc, copper, iron, a n d other metals a r e effective i n catalyzing t h e n o n e n z y m a t i c decar- boxylation of keto acids, these a r e not the active metals in t h e en- z y m a t i c reactions. M a n g a n e s e , t h e most effective m e t a l in e n z y m a t i c decarboxylation of oxalacetate a n d oxalosuccinate, is v i r t u a l l y ineffec- tive in t h e n o n e n z y m a t i c reaction. It was found t h a t M n+ + catalyzed t h e oxidative n o n e n z y m a t i c decarboxylation of oxaloacetic acid, b u t t h e product w a s malonic acid in contrast to p y r u v i c acid w h i c h is formed enzymatically. A somewhat similar effect of Mn++ on a-keto- glutaric acid oxidation yielded u n k n o w n products. A p p a r e n t l y M n+ + does not catalyze a n oxidative decarboxylation similar to t h a t obtained w h e n t h e e n z y m e is present.
T h e r e is no question t h a t the various metals will form bridges be- tween t h e substrate a n d the protein, b u t w h e t h e r these will lead to t h e formation of a n inhibitory complex or a n active intermediate ap- p a r e n t l y depends on a n u m b e r of factors. Additional studies on the n a t u r e of the metal-substrate-protein complex are necessary before the formation of a chelate structure can be completely accepted as t h e active intermediate in enzyme-catalyzed decarboxylations. Evidence in favor of t h e formation of a complex between t h e protein, keto acid, a n d M n+ + in t h e e n z y m a t i c decarboxylation of oxalacetic a n d oxalosuccinic acid has been provided b y t h e experiments of R o m b e r g , Ochoa, a n d M e h l e r ( 1 2 9 ) . On m i x i n g M n+ +, oxalosuccinate, a n d oxalosuccinic car- boxylase, t h e r e is a v e r y r a p i d a n d pronounced increase in light absorp- tion at 240 m/*, suggesting complex formation, followed b y a decrease due to decarboxylation. It w a s assumed t h a t despite t h e low affinity of M n+ + for the keto acids, the specific binding of both M n+ + a n d keto acid b y t h e carboxylation protein would enable t h e formation of t h e u n - stable complex at low Mn++ concentrations. It is a n open question w h e t h e r the carboxylase accelerates t h e formation of a keto acid-man- ganese complex w h i c h would t h e n decarboxylate spontaneously or w h e t h e r decarboxylation occurs in a t e r n a r y complex of protein, M nH, a n d keto acid.
4. Other Metal-Catalyzed Models
T h e r e are also cases of metal-catalyzed n o n e n z y m a t i c reactions w h i c h resemble certain e n z y m a t i c reactions although t h e latter h a v e n o m e t a l requirement. F o r example, M e t z l e r a n d Snell (160) showed t h a t the t r a n s a m i n a t i o n between glutamic acid a n d pyridoxal is catalyzed b y m e t a l ions i n t h e ascending order of effectivity: M n+ +, C o+ +, N i+ +, C u+ +,
460 A . N A S O N A N D W . D . M C E L R O Y
a n d Z n+ +. T r a n s a m i n a s e , however, h a s n o k n o w n m e t a l r e q u i r e m e n t . A c t u a l l y transaminations w e r e discovered as spontaneous reactions be- fore t h e e n z y m a t i c reactions w e r e k n o w n . I t h a s been suggested t h a t a Schiff's base between t h e aldehyde of pyridoxal condensed w i t h t h e a m i n o group of t h e acid is a n i n t e r m e d i a t e i n t r a n s a m i n a t i o n . T h e model systems implicate t h e formation of a Schiff's base b y pyridoxal w i t h a n a m i n o acid i n t h e presence of a m e t a l ion (Fig. 2 ) , although a
R
Ov Ν—C—H CHII 2OH
W / / % /
/ CH2 > = N - OH HR/ _C_H
HO—C=0 Ο Η3
Pyridoxal Pyridoxamine + + Amino acid Ketoacid Transamination; oxidative deamination and reductive amination.
Cu++ > A l ?+~ F e+ + - F e3 + > N i+ + - C o+ +
(relative activities in nonenzymatic reaction)
FIG. 2. Model system for the formation of a Schiff's base by pyridoxal with an amino acid in the presence of a metal ion (40).
m e t a l r e q u i r e m e n t for t h e e n z y m a t i c system h a s n o t y e t been demon- strated.
B . M E T A L L O P R O T E I N S
T h e increased catalysis, w h i c h arises from t h e combination of a m e t a l ion w i t h a specific protein resides a t least i n p a r t i n t h e n a t u r e of t h e physicochemical bonding b e t w e e n t h e m e t a l ion a n d t h e protein.
T w o types of bonds a r e involved i n m e t a l ion reactions w i t h organic molecules. First, t h e r e is t h e ionic bond w h i c h arises from electrostatic attraction b e t w e e n t w o oppositely charged ions. A n i m p o r t a n t class of electrostatic bonding is t h e ion-dipole bond resulting from electrostatic attraction between a positively-charged m e t a l ion a n d a dipolar mol- ecule. E x a m p l e s of such complexes r a n g e from t h e simple C u i H a O ) ^
to t h e intricate forms such as t h e ionic iron complex hemoglobin. T h e second kind of bond, the covalent linkage, is one i n w h i c h a pair of electrons is shared between t h e m e t a l ion a n d a n a t o m of t h e group bound. T h e transition metals a r e quite active i n participating i n co- ordination complexes, forming essentially covalent linkages between t h e m e t a l a n d t h e coordinated molecule. T h e transition metals m a y also form ionic complexes. A c t u a l l y ionic complexes a n d covalent linkages represent two extremes. M a n y coordination complexes h a v e properties w h i c h a r e intermediate between these two types of bonds.
T h e m e t a l ion m a y coordinate w i t h a n u m b e r of molecules of a sub- stance as indicated b y t h e coordination n u m b e r . G e n e r a l l y t h e r e a r e definite spatial a r r a n g e m e n t s such as p l a n a r a n d t e t r a h e d r a l for the
metals w i t h coordination n u m b e r 4 , a n d often octa- H
h e d r a l for t h e metals w i t h n u m b e r 6 . F r e q u e n t l y co- HoN^ I ordination m a y take place w i t h two groups of t h e same yfe y Λ molecule. I n the formation of chelate complexes t h e % ,Xs ^C=0 most stable a r e those leading to relatively strain-free
rings such as 5 - or 6- m e m b e r e d rings. M a n y a m i n o acids 3 Metal form m e t a l chelate complexes b y coordination t h r o u g h io a r m no a ci d t h e carboxyl a n d a m i n o groups (Fig. 3 ) . Despite t h e chelate struc- preparation a n d study of various coordination com- ture. R is the plexes of metals w i t h organic molecules, o u r knowl- carbon chain edge of t h e n a t u r e a n d stability of complexes of physi- ^a^0™1 S ^ ologically i m p o r t a n t ions w i t h physiologically i m p o r t a n t
substances (e.g., proteins, phosphorylated compounds, etc.) u n d e r physiological conditions is meager. T h e best examples of chelates of high stability u n d e r biological conditions a r e t h e h e m e s a n d chloro- phylls.
T h e reactions of m e t a l ions w i t h protein molecules a r e of great in- terest. O n l y certain specific polar side chains of proteins act as ligands for t h e formation of m e t a l protein complexes. A ligand is u s u a l l y described as a n atom, or a group of atoms, w h i c h is capable of donating electrons to a separate m e t a l atom. T h i s t e n d e n c y to donate electrons will lead to t h e formation of " c o m p l e x " compounds between two or m o r e such ligands. Such compounds a r e chelates ( C l a w ) . Klotz ( 1 2 8 ) reviewed t h e various properties of polar side chains of proteins a n d concluded t h a t t h e following a r e k n o w n to be involved in complex formation w i t h at least some metals: phosphoric acid, carboxyl, imida- zolium, a- a n d c- a m m o n i u m , phenolic, a n d sulfhydryl groups. Most proteins h a v e at least a n u m b e r of these side chains. Therefore, it is expected t h a t t h e y form stable complexes w i t h m a n y metals. M e t a l ions m a y also function b y acting as a bridge, t h u s linking proteins to
462 A . N A S O N A N D W . D . M C E L R O Y
low molecular weight compounds. It has been possible to show t h e bind- ing of u n c h a r g e d organic molecules to s e r u m a l b u m i n provided certain metals a r e p r e s e n t — t h e metal, protein, a n d organic molecule ap- p a r e n t l y acting together to form a t e r n a r y complex. F o r example, in the binding of azopyridine dyes w i t h pepsin those metals w h i c h pro- moted t h e combination of dye w i t h protein w e r e effective in forming chelates w i t h the d y e in the absence of protein. Calcium a n d m a g n e s i u m did not combine w i t h the dye a n d w e r e also inactive in bringing about complex formation. Studies of t h e complex formation of metals w i t h proteins a n d chelate formation of metals w i t h low molecular weight compounds provide useful information in interpreting t h e m e c h a n i s m of action of m e t a l ions in certain enzyme-catalyzed reactions.
Klotz (128) stressed t h r e e general categories in w h i c h metals in com- bination w i t h proteins or prosthetic groups m a y act as catalysts.
Î. Primary Effects of Protein on the Properties of the Metal Examples of this group include the copper a n d h e m e e n z y m e s already indicated above. This frequently involves oxidation-reduction reactions i n w h i c h t h e primitive catalytic properties of t h e metallic ions are con- siderably e n h a n c e d b y combination w i t h specific proteins.
2. Primary Effect of Metal on the Properties of the Enzyme Protein T h e combination of metals w i t h proteins m a y alter a n u m b e r of properties of t h e protein. T h i s could possibly occur b y changing t h e n e t charge of t h e proteins a n d t h u s from p u r e l y electrostatic effects alter t h e combination of substrate w i t h e n z y m e . By changing the ratio of zinc a n d m a g n e s i u m , Sadasivan (222) observed a shift in t h e p H opti- m u m of phosphatase activity to either the acid or alkaline range.
M a s s e y (158) demonstrated t h a t a n u m b e r of anions, such as sulfate, selenate, a n d borate, w e r e effective i n activating salt-free crystalline fumarase as well as shifting t h e optimal p H to t h e alkaline side. Studies b y others h a v e shown a n activating effect of such cations as C a+ +, C o+ +, Cd+% a n d M n+ + on the esterase a n d amidase activities of trypsin although t h e r e is n o absolute r e q u i r e m e n t for a metal. T h e electrostatic effect of metals on proteins m a y be responsible for t h e profound influence of nonessential metals on t h e metabolism of both plants a n d animals. I n addition to this direct electrostatic effect, however, metals m a y also activate b y t h e removal of inhibitory substances. T h e e n z y m e leucine aminopeptidase provides a n o t h e r e x a m p l e of a n indirect effect of a metal. T h e purest e n z y m e preparations a r e strongly activated b y M n+ +, b u t t h e action of Mg++ is quite poor. However, the stability of the crude or purified e n z y m e is greatly increased b y M g+ +. A protecting effect on
yeast a r g i n i n e desiminase (arginine->citrulline + N H3) has been ex- hibited b y cobalt a n d nickel i n crude extracts, b u t not in partially puri- fied fractions.
3 . Cooperative Effects of Metal and Protein
M e t a l s a n d protein m a y act cooperatively to increase catalytic activity. H e l l e r m a n a n d Stock ( 9 5 ) w e r e a m o n g t h e first to suggest
H2^f ^CHCOO"
H2C CH2
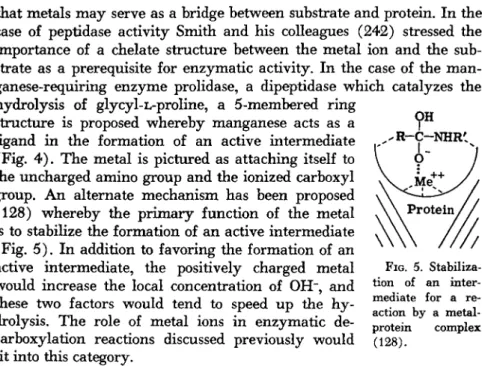
FIG. 4. Postulated coordination of glycyl-L-proline with M n++ and prolidase (242).
t h a t metals m a y serve as a bridge between substrate a n d protein. I n t h e case of peptidase activity S m i t h a n d his colleagues ( 2 4 2 ) stressed t h e i m p o r t a n c e of a chelate structure between t h e m e t a l ion a n d t h e sub- strate as a prerequisite for e n z y m a t i c activity. I n t h e case of t h e m a n - ganese-requiring e n z y m e prolidase, a dipeptidase w h i c h catalyzes t h e hydrolysis of glycyl-L-proline, a 5- m e m b e r e d r i n g
structure is proposed w h e r e b y m a n g a n e s e acts as a ligand i n t h e formation of a n active i n t e r m e d i a t e (Fig. 4 ) . T h e m e t a l is pictured as attaching itself to t h e u n c h a r g e d a m i n o group a n d t h e ionized carboxyl group. A n alternate m e c h a n i s m h a s been proposed
( 1 2 8 ) w h e r e b y t h e p r i m a r y function of t h e m e t a l is to stabilize t h e formation of a n active intermediate (Fig. 5 ) , I n addition to favoring t h e formation of a n active intermediate, t h e positively charged m e t a l w o u l d increase t h e local concentration of OH", a n d these two factors would tend to speed u p t h e h y - drolysis. T h e role of m e t a l ions in e n z y m a t i c de- carboxylation reactions discussed previously w o u l d fit into this category.
U n d e r appropriate conditions or w i t h suitable substrates such as pyrophosphate-containing compounds, M g+ + or M n+ + m a y be expected to form chelate structures m o r e readily. Bauer ( 2 3 ) suggested t h a t t h e action of inorganic pyrophosphatase depended u p o n t h e formation of a n enzyme-metal-substrate chelate structure. Calvin emphasized t h e i m - portance of t h e pyrophosphate structure for t h e linkage of coenzymes
FIG. 5. Stabiliza- tion of an inter- mediate for a re- action by a metal- protein complex (128).
4 6 4 A . N A S O N A N D W . D . M C E L R O Y
or substrates to t h e e n z y m e via a chelate structure w i t h M g+ + or M n+ + (Fig. 6 ) . Other groups on t h e protein m u s t be effective i n bringing about complex formation w i t h pyrophosphate since metals a r e not re- quired in all cases. I n those cases w h e r e metals are required, t h e chelate structure m a y be a n intermediate. T h i s m e c h a n i s m w o u l d h a v e a bear- ing on those M g+ +- or M n+ +- a c t i v a t e d e n z y m e s w h e r e a pyrophosphate- containing compound such as A D P or A T P is involved.
It is i m p o r t a n t to stress t h a t t h e combination of a m e t a l ion w i t h sub- strate a n d e n z y m e is not sufficient to produce biological activity. T h e effect of m e t a l ions i n activating e n z y m e s m u s t h a v e some specificity beyond m e r e combination w i t h substrate a n d protein. Enolase, t h e e n z y m e w h i c h catalyzes t h e conversion of 2-phosphoglycerate to phos- pho-enol p y r u v a t e , was shown b y M a l m s t r ö m (149) to be inhibited b y
"O^l I ^O—Ribose-Adenine
Enzyme
FIG. 6. Chelate structure of the pyrophosphate group of A D P (or A T P ) with M g++ and its chelate binding to the enzyme.
beryllium, calcium, a n d nickel although these ions formed complexes w i t h t h e e n z y m e . M g+ +, w h i c h h a p p e n s to form t h e weakest complex w i t h enolase a n d substrate, gives t h e best e n z y m a t i c activity, w h e r e a s Z n+ +, w h i c h binds strongly w i t h t h e e n z y m e , shows high activity also.
It is a p p a r e n t t h a t t h e effect of m e t a l ions in activating enzymes m u s t h a v e some specificity beyond m e r e combination w i t h the substrate a n d protein.
IV. Metal Requirements of Enzymes
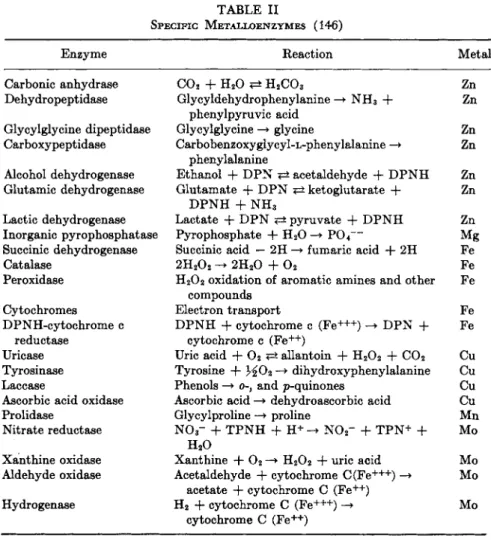
I n t e r m s of t h e m e t a l r e q u i r e m e n t s of enzymes, two broad groups can be designated: (a) those e n z y m e s i n w h i c h a specific m e t a l has been shown to be a n integral component, a n d (b) those e n z y m e s for w h i c h one or m o r e metals serve as a n activator. A t times t h e lines dividing these two groups a r e difficult to distinguish. T h e emerging pat- t e r n shows t h a t such trace elements as zinc, iron, copper, a n d molyb- d e n u m h a v e been clearly established as specific a n d integral com- ponents for a n u m b e r of e n z y m e systems. M a g n e s i u m a n d m a n g a n e s e , on t h e other h a n d , a r e most frequently involved as activators. I n certain enzymes, however, t h e y a r e regarded as specific components.
All these examples will b e discussed u n d e r t h e individual metals i n the sections w h i c h follow.
V. Mechanism of Action of the Micronutrient Elements
N o a t t e m p t will b e m a d e to review historical development, effects of deficiencies, excesses or interrelationships of t h e n u t r i e n t elements in p l a n t metabolism since these aspects h a v e been covered in Chapters 1 a n d 2 of this volume. Special phases of these topics will be included, however, i n those cases w h e r e t h e y m a y h a v e a particular bearing on t h e m o d e of action of t h e m i n e r a l element i n question.
A. Z I N C
A l t h o u g h zinc w a s shown b y earlier workers to stimulate t h e growth of various organisms, p r o b a b l y t h e first definite evidence for zinc as a n essential element was presented i n 1914 b y M a z e ( 1 5 9 ) , w h o demonstrated t h a t w i t h o u t added zinc, n o r m a l g r o w t h of m a i z e
(Zea mays) was not possible. H e considered zinc to be a n essential element for growth, n o t m e r e l y a stimulant. I n 1919 Steinberg (250) provided proof t h a t zinc is also indispensable for t h e n o r m a l growth of fungi. Since t h a t t i m e a host of reports h a v e presented irrefutable data i n support of zinc as a m i c r o n u t r i e n t element [see reviews b y Chesters a n d Rolinson ( 4 2 ) , Gilbert ( 8 3 ) , a n d H o c h a n d Vallee ( 1 0 5 ) ] . M o r e recently t h e w o r k of Vallee a n d his associates (105) has pro- vided n e w evidence for the role of zinc as a component of p y r i d i n e nucleotide dehydrogenases.
1. Relationship with Auxin
T h u s far the most p r o m i n e n t role for zinc in plants appears to be in its interrelationship w i t h a u x i n . Z i n c deficiency i n h i g h e r plants is characterized b y a failure of stem elongation as first described b y Skoog ( 2 4 1 ) . T o m a t o (Lycopersicon esculentum) plants deficient i n zinc w e r e shown to be deficient in auxin, incipient deficiencies result- ing i n a reduction of 5 0 % or m o r e i n a u x i n content before the ap- p e a r a n c e of diminished growth. T h i s effect was n o t produced b y deficiencies of copper or m a n g a n e s e , w h i c h gave m a r k e d growth depressions before affecting a u x i n content. T h e addition of zinc to deficient plants resulted i n a n increase in a u x i n w i t h i n 24 hours.
Skoog considered t h e relation of zinc to a u x i n to be a n indirect one.
H e suggested t h a t t h e low level of a u x i n in zinc-deficient plants was due to destruction of t h e h o r m o n e b y oxidation r a t h e r t h a n to a lack of synthesis resulting from a n altered oxidation-reduction balance in
466 A . N A S O N A N D W . D . M C E L R O Y
deficient plants. T h e disappearance of a u x i n w a s accompanied b y a n increase in t h e oxidizing capacity of zinc-deficient plants, arising in p a r t from a n increased peroxidase activity. Sections from zinc-deficient stems inactivated indole-3-acetic acid m o r e r a p i d l y t h a n sections from h e a l t h y plants. I n contrast, T s u i (267) furnished evidence i n tomato plants implicating zinc in t h e synthesis of a u x i n b y w a y of t r y p t o p h a n . Zinc-deficient plants, w h i c h w e r e also low in auxin, similarly showed a decrease in t r y p t o p h a n content. T h e e n z y m e system allegedly re- sponsible for the formation of indole-3-acetic acid b y oxidative deami- nation of t r y p t o p h a n was found to be t h e same in deficient as in h e a l t h y plants. F r o m these results it would a p p e a r t h a t zinc is required for t h e synthesis of t r y p t o p h a n a n d therefore indirectly for t h e synthesis of auxin. I n keeping w i t h this suggestion is t h e earlier indication b y H o a g l a n d (104) of a direct correlation between t h e distribution of a u x i n a n d t r y p t o p h a n in different parts of t h e leaf as well as t h e fact t h a t t h e apical p a r t of the leaf w h i c h contains m o r e a u x i n a n d t r y p t o p h a n also accumulates zinc. F u r t h e r support for this hypothesis was provided b y the finding t h a t in Neurospora t r y p t o p h a n synthetase, the e n z y m e w h i c h catalyzes t h e formation of t r y p t o p h a n from indole a n d serine, is m a r k e d l y a n d specifically decreased b y a zinc deficiency ( 1 7 8 ) . T h e results i m p l y t h a t zinc is either a component of the e n z y m e system or t h a t a deficiency indirectly leads to a decrease in t h e e n z y m e protein. T h e first possibility has not been eliminated.
2. Zinc Deficiency and Enzyme Systems
T h e necessity of a m e t a l ion for the activity or synthesis of specific enzymes m a y at times be indicated b y growing t h e organism u n d e r conditions of m e t a l deficiency a n d comparing its e n z y m e systems with those of n o r m a l tissues. This approach has been taken in a t t e m p t i n g to uncover the mode of action of zinc. Reed (209) reported t h a t in zinc- deficient tomato plants dehydrogenase activity w a s lowered, w h i l e t h e quinones arising b y action of phenol oxidases w e r e not decreased. T h e accumulation of inorganic phosphate suggested a possible role of zinc in t h e activation of a phosphate-transferring e n z y m e , perhaps hexo- kinase. It was also found t h a t p y r u v i c carboxylase is absent in zinc- deficient Rhizopus nigricans ( 7 2 ) . Z i n c is n o t regarded as a constituent of t h e carboxylase since no correlation was obtained between zinc con- tent a n d e n z y m a t i c activity of mycelial extracts. T h e m e t a l is probably necessary for t h e synthesis of t h e e n z y m e itself.
Z i n c deficiency in Neurospora does not lead simply to t h e production of less m y c e l i u m , b u t it specifically results in t h e production of fungus h a v i n g drastically altered metabolic characteristics as indicated b y t h e
m a r k e d changes in e n z y m a t i c constitution ( 1 7 8 ) . T h e alterations in- volved not o n l y t h e virtual disappearance of certain e n z y m e activities such as t h a t of alcohol dehydrogenase a n d t h e t r y p t o p h a n synthetase, b u t also r e m a r k a b l e increases in t h e activities of other e n z y m e s . T h e concentration of diphosphopyridine nucleotidase ( D P N a s e ) , a n e n z y m e w h i c h splits D P N a t the nicotinamide riboside linkage, increased ten- to twentyfold. O t h e r e n z y m e s including fumarase, hexokinase, aldolase, a n d triosephosphate dehydrogenase w e r e unaffected. T h e basic defect in zinc deficiency seemed to be not in the synthesis of vitamins, a m i n o acids, purines, or pyrimidines, b u t p r e s u m a b l y in their subsequent metabolism.
A w o r k i n g hypothesis to explain the above p h e n o m e n a is t h a t those e n z y m e s w h i c h increase i n n u t r i t i o n a l l y deficient cells are proteins of relatively simple structure. T h e i r synthesis can proceed even in t h e absence of certain k e y reactions w h i c h are necessary for the building of m o r e complex protein molecules. A m e t a l deficiency such as zinc m a y eliminate one of several competing reactions for available a m i n o nitrogen, resulting i n a relative increase i n certain enzymes a n d de- creases i n others. T h e competition m a y be for the polypeptide m a d e at t h e enzyme-forming center. F o r example, in the case of t r y p t o p h a n synthetase, zinc appears to b e essential not only for its function, b u t also for t h e synthesis of the protein p a r t of t h e e n z y m e . T h u s in m a n y w a y s zinc behaves as a specific inducer for t h e formation of certain enzymes. U n d e r limited zinc deficiency, however, protein synthesis a p p a r e n t l y proceeds n o r m a l l y . W i t h t h e loss of specific enzymes, the polypeptide pool can be used for t h e increased synthesis of other enzymes. It is possible, therefore, t h a t a single template can function as a site for t h e formation of a single polypeptide chain, w h i c h in t u r n is t h e p r i m a r y structure for a n u m b e r of enzymes. T h e secondary a n d t e r t i a r y structure, w h i c h in p a r t is u n d e r nutritional control, determines the specificity of catalytic activity.
Protein synthesized in t h e zinc-deficient mycelia is not available for n e w e n z y m e synthesis since nitrogen as well as zinc is essential for t h e restoration of t h e diphosphopyridine nucleotidase a n d alcohol dehydrogenase levels of zinc-deficient m a t s ( 1 7 9 ) . Also a zinc deficiency as well as a n u m b e r of other m e t a l deficiencies i n tomato plants elevated t h e concentration of polyphenol oxidase, peroxidase, ascorbic acid oxidase, a n d glycolic acid oxidase ( 1 8 0 ) . Although it has been shown (180) t h a t such metalloenzymes as polyphenol oxidase, ascorbic acid oxidase, a n d peroxidase a r e decreased in concentration in plants deficient i n the specific m e t a l concerned, it does not neces- sarily follow t h a t other nutritive conditions would fail to decrease these
468 A . N A S O N A N D W . D . M C E L R O Y
enzymes. I t would be fallacious to conclude t h a t a particular e n z y m e contains a specific m e t a l component simply because a deficiency of the latter results in a decreased concentration of t h e e n z y m e . A t most such evidence is suggestive. As pointed out below, t h e decrease i n alcohol dehydrogenase in zinc-deficient Neurospora can n o w be ascribed to its properties as a zinc protein. Q u i n l a n - W a t s o n (208) reported a decrease i n aldolase of higher plants u n d e r conditions of zinc deficiency, w h e r e a s a copper deficiency h a d n o effect. F a r m o r e evidence is necessary to establish this e n z y m e as a zinc protein. T h i s is exemplified b y t h e case of alcohol dehydrogenase given below.
3. Alcohol Dehydrogenase and Other Pyridine Nucleotide Dehydrogenases
T h e above-mentioned decreases i n t h e content of alcohol dehydro- genase as a result of a zinc deficiency can be attributed to t h e fact t h a t t h e e n z y m e is a zinc protein. T h e recent w o r k of Vallee a n d his as- sociates (105) h a s implied t h a t p y r i d i n e nucleotide dehydrogenases contain zinc, w h i c h probably serves to b i n d t h e p y r i d i n e nucleotide to the protein moiety. T h e y first showed t h a t yeast alcohol dehydrogenase ( A D H ) contains u n i f o r m l y large a m o u n t s of zinc firmly b o u n d to the protein a n d suggested t h a t t h e m e t a l is a functional component of the molecule in its e n z y m a t i c activity. T h e i r data demonstrated t h a t four molecules of zinc are b o u n d to one molecule of t h e e n z y m e protein, constituting a n integral p a r t of the protein molecule i n the n a t u r a l state. T h i s complex w h i c h has a zinc content of approximately 0 . 2 % has been assigned t h e empirical formula ( A D H ) Z n4. One molecule of crystalline yeast A D H has also been shown to bind four molecules of D P N (or D P N H ) . I n a m o r e recent report, Kägi a n d Vallee (116) observed t h a t the irreversible, time-dependent inhibition of t h e e n z y m e b y 1,10-phenanthroline a n d 8-hydroxyquinoline-5-sulfonic acid is ac- companied b y t h e dissociation of its a p o e n z y m e of molecular weight 151,000 into four subunits w i t h a molecular weight of 36,000 each.
T h e y envisage t h e empirical structural formula of yeast alcohol de- hydrogenase as [ ( Y A D H )4Z n4] ( D P N )4 w h e r e Y A D H represents a single a p o e n z y m e u n i t of molecular weight 36,000. T h e y h a v e reported t h a t t h e rates of inactivation a n d of t h e loss of zinc from t h e e n z y m e as well as t h e degree of dissociation of t h e a p o e n z y m e are directly correlated. T h e zinc atoms a r e thought to stabilize the q u a t e r n a r y structure of t h e e n z y m e t h r o u g h t h e formation of bridges between the m o n o m e r s to form the enzymatically active tetramer. A three-point a t t a c h m e n t of D P N to two adjacent m o n o m e r i c units of t h e a p o e n z y m e a n d to t h e binding zinc atom is postulated to account for t h e inter-
relationship between t h e functional a n d structural features of t h e e n z y m e a n d its zinc content.
Z i n c has also been reported b y Vallee a n d his colleagues to be a com- ponent of liver alcohol dehydrogenase a n d other p y r i d i n e nucleotide dehydrogenases such as liver g l u t a m i c dehydrogenase ( 1 ) a n d muscle lactic dehydrogenase ( 1 0 5 ) . T h e presence of two to four atoms of zinc i n crystalline beef liver glutamic dehydrogenase, t h e inhibition of t h e system b y a n u m b e r of metal-binding agents, a n d t h e demonstration b y F r i e d e n ( 7 5 ) t h a t t h e e n z y m e can be dispersed into protein subunits b y one of these chelating agents as well as b y t h e addition of D P N H have led to t h e suggestion t h a t four e n z y m e fragments are bound b y zinc atoms to form t h e larger kinetic molecular u n i t ( 1 ) . T h e recent work of Yielding a n d T o m k i n s ( 2 9 6 ) , however, in w h i c h various steroid hormones also promoted disaggregation of t h e glutamic dehydrogenase macromolecule into subunits, has cast some doubts on t h e above in- terpretation. It m a y also well be t h a t these e n z y m e s from the tissues of higher plants will also prove to h a v e a zinc component.
H o c h a n d Vallee ( 1 0 5 ) feel t h a t t h e hypothesis of a m e t a l as a com- ponent of m a n y , if not all, of t h e p y r i d i n e nucleotide-dependent de- hydrogenases is a n a t u r a l extension of their studies on t h e above enzymes. T h e y report zinc, copper, a n d iron to be t h e o n l y metals consistently present i n significant concentrations in glyceraldehyde 3 - phosphate dehydrogenase of yeast a n d rabbit muscle, t h e α-glycero- phosphate dehydrogenase of rabbit muscle, t h e m a l i c dehydrogenase of pig heart, a n d t h e TPN-glucose 6-phosphate dehydrogenase of yeast.
These e n z y m e s a r e all inhibited b y a n u m b e r of chelating agents k n o w n to form strong complexes w i t h these metals.
4. Carbonic Anhydrase
T h i s is t h e first e n z y m e for w h i c h zinc was established as a m e t a l component. Carbonic a n h y d r a s e w a s h i g h l y purified from bovine red blood cells a n d shown to contain a p p r o x i m a t e l y 0 . 3 % zinc in a tightly bound form ( 1 1 9 ) . Based on a molecular weight of 3 0 , 0 0 0 , t h e reported zinc content varies from 0 . 9 to 1.5 g r a m atoms per mole. T h e e n z y m e w h i c h catalyzes t h e reaction, H20 + C 03 H2C 03, is inhibited b y such metal-binding agents as cyanide, azide, a n d sulfide a n d is t h e o n l y zinc-containing substance w i t h carbonic a n h y d r a s e properties. Keilin a n d M a n n reported t h a t inorganic zinc salts, simple organozinc com- pounds, zinc p o r p h y r i n , insulin, a n d Zn++ complexes of various proteins lacked this activity. I n contrast to earlier reports ( 2 2 8 , 2 6 8 ) of t h e inability to dissociate the zinc of carbonic a n h y d r a s e from t h e protein, Lindskog a n d M a l m s t r ö m ( 1 4 0 ) have recently been able to remove
470 A. N A S O N A N D W . D . M C E L R O Y
t h e m e t a l reversibly from t h e e n z y m e at p H 5 in t h e presence of certain chelating agents. T h e dissociation of zinc causes a loss of e n z y m a t i c activity w h i c h can be fully restored b y the addition of zinc. Other types of substances h a v e catalytic activity in the h y d r a t i o n of carbon dioxide or t h e dehydration of carbonic acid, although all b u t one a r e not metals.
T h e s e include h y d r o g e n peroxide, hypochlorite, various organic bases such as histidine, sulfate, selenite, a n d arsenite. T h e i r m e c h a n i s m of action is different from t h a t of the e n z y m e .
Carbonic a n h y d r a s e activity h a s been observed b y a n u m b e r of workers to be present in t h e leaves of several plants [see review b y H o c h a n d Vallee (105) ] . As y e t zinc h a s not been clearly demonstrated to be a component of t h e p l a n t e n z y m e . D a y a n d F r a n k l i n (52) w e r e a m o n g t h e first to establish t h e presence of carbonic a n h y d r a s e in plants. T h e y found t h a t zinc w a s associated in a nondialyzable form w i t h t h e e n z y m e protein b u t did not prove a functional relationship.
Bradfield (34) reported a w i d e distribution of t h e e n z y m e in several plants a n d indicated t h e susceptibility of t h e system to zinc deficiency.
Wood a n d Sibly (295) obtained a decrease in carbonic a n h y d r a s e in zinc-deficient oats. T h e y questioned w h e t h e r such data w e r e indicative of the zinc n a t u r e of t h e e n z y m e a n d decided t h a t t h e observed decrease could best be ascribed to a n indirect effect on protein formation.
W h e t h e r or not zinc is a component of carbonic a n h y d r a s e from plants m u s t await further study. T h e basic m e c h a n i s m of the role of zinc in carbonic a n h y d r a s e from animals has not been investigated, although t h e suggestion has been m a d e t h a t zinc m a y function b y u n i t i n g e n z y m e a n d substrate in a coordination complex.
5. Other Zinc Enzymes
Z i n c has been reported to be a m e t a l component of the a n i m a l peptidases, dehydropeptidase ( 2 9 9 ) , glycylglycine dipeptidase ( 1 3 9 ) , a n d carboxypeptidase ( 2 6 9 ) . Crystalline preparations of t h e latter contain 0.98 g r a m atoms of zinc per mole of e n z y m e . T h e m e t a l can be removed b y dialysis, t h e loss of e n z y m e activity being proportional to the loss of m e t a l ( 2 7 0 ) . E n z y m a t i c activity can be restored to the metal- free e n z y m e b y t h e addition of Z n+ + or b y certain ions of t h e first transition period, such as C r+ + +, N i+ +, C o+ +, F e+ +, a n d M n+ +. Evidence has also been presented w h i c h suggests t h a t t h e phosphatases of Pénicillium chrysogenum (221 ) a n d certain a n i m a l tissues m a y be zinc-containing enzymes. Z i n c h a s been implicated b y W a r b u r g a n d Christian (276) as a possible m e t a l component of yeast zymohexase. T h e e n z y m e w a s characterized as a dissociable metal-protein w h i c h could be inhibited b y t h e formation of complexes w i t h pyrophosphate, cysteine, a n d
glutathione. A l t h o u g h a n earlier investigation found t h a t h i g h l y p u r i - fied preparations of uricase, t h e e n z y m e t h a t catalyzes t h e oxidation of u r i c acid to allantoin, contained 0 . 1 3 % zinc ( 1 0 6 ) , a m o r e recent report h a s implicated the e n z y m e from k i d n e y as a copper protein containing 0 . 0 5 % copper ( 1 5 2 ) . A n u m b e r of isolated e n z y m e systems such as lecithinase, histidine deaminase, a n d oxaloacetic decarboxylase have been shown to be activated b y zinc, a m o n g other m e t a l ions. T h e effect is not specific for zinc a n d raises the question of its significance in the intact cell.
6. Other Effects of Zinc
I n addition to t h e a p p a r e n t relationship between zinc a n d auxin content i n plants as previously discussed, other biological p h e n o m e n a h a v e been associated w i t h the metal. T h e above-described a u x i n effect possibly accounts for i m p a i r e d flower setting a n d seed production, w h i c h is a conspicuous s y m p t o m of zinc deficiency in high plants. T h e r e a r e reports t h a t in fungi zinc increases the u p t a k e of calcium, phosphorus, a n d m a g n e s i u m as well as t h e efficiency of sugar utilization. Some workers h a v e indicated zinc to be unfavorable for citrate production in fungi, b u t others h a v e reported t h e opposite effect [see Chesters a n d Rolinson ( 4 2 ) ] .
B . C O P P E R
S o m m e r ( 2 4 4 ) is credited w i t h the first demonstration t h a t copper is a n essential element for higher plants. T h e m e t a l has been established as a component of a n u m b e r of different p l a n t enzymes—polyphenol oxidase, monophenol oxidase, laccase, a n d ascorbic acid oxidase.
Enzymatic Role
O n e of t h e general properties ascribed to t h e copper e n z y m e s is the catalysis of the direct oxidation of their substrates b y atmospheric oxygen according t h e equation
Copper enzyme
B H2 + y202 > Β + H20
a n d their failure to function anaerobically [see review b y D a w s o n a n d T a r p l e y ( 5 0 ) ] . T h e i n h e r e n t p r o p e r t y of inorganic copper salts in catalyzing t h e oxidation of various organic substrates b y molecular oxygen w a s discussed previously. Polyphenol oxidase, also called catecholase or tyrosinase, catalyzes t h e oxidation of o-diphenols b y molecular oxygen to form the corresponding quinones a n d w a t e r (Fig.
7 ) , as shown b y t h e early work of Raper. m- or p-Diphenols are n o t
472 A . N A S O N A N D W . D. M C E L R O Y
acted u p o n b y this e n z y m e . Monophenolase, as exemplified b y the e n z y m e from m u s h r o o m , catalyzes t h e conversion of a monophenol to t h e o-diphenol b y molecular oxygen. T h i s e n z y m e has also been called cresolase or tyrosinase; it is always accompanied b y polyphenolase activity, although polyphenolase does not a l w a y s h a v e monophenolase activity. Laccase, w h i c h is obtained from t h e latex of certain species of lacquer trees can oxidize p-diphenols a n d o-diphenols to the correspond- ing quinones a n d water. Ascorbic acid oxidase catalyzes t h e oxidation of ascorbic acid to form dehydroascorbic acid a n d w a t e r (see Fig. 7 ) .
HAC-
II IOH
OHHC. ^CH Catechol
H
+ V2
o
9 tyrosinase HC'HC II
+ H20
H
CH
o-Benzoquinone
Ο II C-I
c-
IIc-
I HC--OH
-OH + ^ 02
ascorbic acid oxidase
HOCH
L - Ascorbic acid
Ο II C -
c=o
I C = 0HC I
0 + H20
HOCH I CH2OH
Dehydro-L -ascorbic acid
FIG. 7. Over-all reactions of tryosinase and ascorbic acid oxidase.
T h e activities of these oxidases are dependent on t h e copper content, w h i c h i n purified preparations ranges between 0.1 a n d 0 . 3 5 % . T h e copper is tightly b o u n d a n d cannot be removed b y dialysis against water. However, t r e a t m e n t w i t h acids or cyanide a n d subsequent dialysis result in t h e removal of copper from t h e protein. Reconstitution of these e n z y m e s has been accomplished b y adding back copper. T h e copper oxidases a r e inhibited b y a n u m b e r of m e t a l binding agents, carbon monoxide inhibiting the phenol oxidases of potato a n d m u s h - room b u t not laccase. Inhibition b y carbon monoxide is not reversed by light i n a g r e e m e n t w i t h t h e properties of copper-carbon monoxide models.
T h e best evidence thus far for t h e m e c h a n i s m of action of cop- per w a s presented b y Kubowitz (132, 133). Using potato polyphenol
oxidase, h e obtained data indicating t h a t copper is concerned in electron transport a p p a r e n t l y b y undergoing cyclic oxidation-reduction between C u ^ a n d C u+ d u r i n g t h e e n z y m a t i c transfer of electrons from sub- strate to oxygen. H e showed t h a t 1 mole of o-diphenol reduced t h e C u+ + e n z y m e to t h e cuprous form, yielding 1 mole of o-quinone. I n the re- action of a molecule of o-diphenol, 2 cupric atoms a r e reduced to t h e cuprous ( C u+) form as the carbon monoxide complex, the u p t a k e of car- bon monoxide being determined manometrically. I n the presence of one molecule of o-diphenol, 2 cupric atoms are reduced to the cuprous form, since one molecule of carbon monoxide is b o u n d to two atoms of phenolase copper. T h i s constitutes the best evidence t h u s far for a n oxidation-reduction role of copper at t h e e n z y m a t i c level. T h e copper of laccase, b y analogy to polyphenolase, p r e s u m a b l y acts in the same m a n n e r .
T h e monophenolase activity w h i c h often accompanies polyphenolase activity has been a controversial a n d much-discussed subject. T h e facts t h a t t h e ratio of t h e two activities is easily altered a n d t h a t added copper readily exchanges w i t h the enzyme's copper d u r i n g poly- phenolase action, a n d to a lesser extent d u r i n g monophenolase action, suggest t h a t t h e two activities a r e i n d e p e n d e n t of one another. T h i s does not exclude t h e possibility t h a t these two activities a r e due to two dif- ferent sites on t h e same protein molecule a n d t h a t each m a y r e q u i r e
copper as a prosthetic group. I n support of this idea a r e t h e observations t h a t t h e two activities a r e associated w i t h t h e same electrophoretic a n d ultracentrifugal component a n d t h a t t h e y are similarly inhibited b y t h e same metal-binding reagents a n d competitive substrates [see re- views b y D a w s o n a n d T a r p l e y (50) a n d M a s o n ( 1 5 6 ) ] .
T h e recent experiments of Dressier a n d D a w s o n (57, 58) h a v e shed further light on this question. B y studying the exchange between radioactive C u6 4 cupric ions a n d resting (nonfunctioning) tyrosinase (purified from t h e m u s h r o o m Psalliota campestris) as well as the catalytically functioning e n z y m e t h e y obtained data w h i c h support t h e hypothesis of two distinct activity centers, i.e., monophenolase (cresolase) a n d polyphenolase (catecholase) sites. T h e i r results sug- gested t h a t copper is firmly b o u n d to t h e tyrosinase protein a n d t h a t t h e resting enzyme essentially does not undergo exchange w i t h radio- active cupric ions except w h e n the e n z y m e contains copper bonded at inactive sites, i.e., o n l y w h e n the e n z y m e is i m p u r e or partially in- activated. However, w h e n the e n z y m e w a s actively catalyzing the oxidation of polyphenols a n exchange between t h e copper of the e n z y m e w i t h ionic C u6 4 occurred depending on the a m o u n t of substrate employed (on t h e n u m b e r of o-dihydric phenol molecules oxidized)