ContentslistsavailableatSciVerseScienceDirect
Applied Catalysis B: Environmental
j ourna l h o me pa g e :w w w . e l s e v i e r . c o m / l o c a t e / a p c a t b
Comparison of the photocatalytic efficiencies of bare and doped rutile and
anatase TiO 2 photocatalysts under visible light for phenol degradation and E. coli inactivation
G. Veréb
a, L. Manczinger
b, G. Bozsó
c, A. Sienkiewicz
d, L. Forró
d, K. Mogyorósi
a,∗, K. Hernádi
a, A. Dombi
aaResearchGroupofEnvironmentalChemistry,InstituteofChemistry,FacultyofSciencesandInformatics,UniversityofSzeged,H-6720,Szeged,TiszaLajoskrt.103,Hungary
bDepartmentofMicrobiology,FacultyofSciencesandInformatics,UniversityofSzeged,H-6701,P.O.Box533,Szeged,Hungary
cDepartmentofMineralogy,Geochemistry,andPetrology,FacultyofSciencesandInformatics,UniversityofSzeged,H-6701,P.O.Box651,Szeged,Hungary
dFSB,IPMC,LPMC,Station3,EcolePolytechniqueFédéraledeLausanne,CH-1015Lausanne,Switzerland
a r t i c l e i n f o
Articlehistory:
Received15June2012 Receivedinrevisedform 22September2012 Accepted26September2012 Available online 8 October 2012
Keywords:
Photocatalysis Visiblelight Disinfection Hydroxylradical Dopedtitania ESR Singletoxygen
a b s t r a c t
ThisstudyaimedatcomparingthephotocatalyticefficienciesofvariousTiO2basedphotocatalystsfor phenoldegradationandbacteriainactivationunderilluminationwithvisiblelight.Commercialundoped anataseandrutile(bothfromAldrich),AeroxideP25(EvonikIndustries),nitrogen-dopedanatase(Sumit- omoTP-S201,SumitomoChemicalInc.),nitrogenandsulphurco-dopedanatase(KronosVLP7000,Kronos TitanGmbH),andourcustom-synthesizednitrogen-andiron-dopedTiO2,aswellasnitrogenandsulphur co-dopedAeroxideP25andsilver-andgold-depositedAeroxideP25werestudied.Thephotocatalytic efficiencyofdifferenttypesoftitaniumdioxidebasedphotocatalystswasdeterminedbyinactivationof EscherichiacoliK12bacteriaandbyphenoldecomposition.Electronspinresonance(ESR)incombination withspintrappingwasusedtogetinsightintothereactiveoxygenspecies(ROS)-mediatedphotocatalytic processesinthepresenceofTiO2-basedphotocatalysts.ESRresultsconfirmedthattitaniaswhichgener- atedOH•radicalswereefficientinE.colidisinfection,whereastitaniasthatwereunabletoproduceOH• radicalsdidnotrevealsignificantbactericidalaction.Threeofourhome-madetitanias(iron-,nitrogen-, nitrogen/sulphur)aswellasthecommercialnitrogen/sulphurcodopedKronosVLP7000TiO2showed higherefficiencyofphenoldegradationthanthewell-establishedreferencephotocatalyst,AeroxideP25, butshowedmuchlower(ifany)activityforbacteriainactivation,includingKronosVLP7000,which revealedextremelyhighefficiencyforphenoldecomposition.InterestinglyundopedAldrichrutile(with largeparticles-100–700nm)hadthehighestefficiencyforinactivationofE.coliandalsohadfairlyhigh activityofphenoldegradation.
© 2012 Elsevier B.V. All rights reserved.
1. Introduction
Photocatalysisisanintensivelyinvestigatedalternativewater treatmentmethodnowadayswhich isapromisingtechniqueto decomposeorganicpollutantsinwater.Averyimportantadvan- tageofphotocatalyticpurificationproceduresisthatitisapplicable forawiderangeoforganicpollutants.Themethodissuitablefor deactivationofvariousmicroorganismsaswell.Duetoitshigh efficiency,lowtoxicity,excellentphysico-chemicalstability,and lowrelativecosts,titaniumdioxide(titania,TiO2)isconsideredas themostpromisingphotocatalystforenvironmentalpurification.
ThefirstreportonTiO2basedwaterdisinfectionwaspublishedin 1985byMatsunagaetal.[1].Sincethiswork,inactivationstudies
∗Correspondingauthor.Tel.:+3662544334;fax:+3662420505.
E-mailaddress:k.mogyorosi@chem.u-szeged.hu(K.Mogyorósi).
ofmanymodelmicroorganismswerecarriedoutinthepresence ofTiO2-basedphotocatalysts.
Inthemajorityofthesestudies,UVirradiationwasappliedto purifywaterandinactivatemicroorganismsinthepresenceofvar- iousformsoftitania[1–14].Howeveritiswellknownthatinthe solarspectrumthereisamuchhigherintensityinthevisiblelight range(∼43%oftotalsolarenergy)thanintheUVrange(∼3%)[8,15], andinanenvironmentallyfriendlyandeconomicalprocess,solar irradiationcouldbeappliedtoactivatethephotocatalyst[16–21].
Thereforeitisimportanttodevelopvisiblelightactivephotocata- lystswhichareabletodecomposeorganicpollutantsandtokill bacteria.Visiblelightactivephotocatalystsarealsoimportantfor indoorapplications,e.g.inair andsurface purificationatwhich naturallyUVlightispracticallyabsent.Inparticular,visiblelight activateddetoxification and/ordisinfectionprocesseshavebeen reportedforTiO2 dopedwithnitrogen[13,22–25],iron[26–28], iodine[8,29–33],sulphur[13,22,34],aswellasformetal-modified TiO2,e.g.withsilver[8,23,35]orgold[36].
0926-3373/$–seefrontmatter© 2012 Elsevier B.V. All rights reserved.
http://dx.doi.org/10.1016/j.apcatb.2012.09.045
Themaingoalofthisstudywastogiveanoverallpictureof theperformanceof differenttypeof bareand modifiedtitanias usingvisiblelightforthedecompositionoforganicpollutantsand killingbacteria.Theefficiencyofseveralbare,doped(N,Fe,S)and noblemetaldeposited(Ag,Au)titaniumdioxideswerecompared byphenoldegradationandbydeactivationofEscherichiacoli.
We also aimed to investigate the correlation between the efficiencyoforganicpollutiondegradationand disinfectionper- formance, as well as to get an insight into the underpinning photocatalyticalmechanisms.Itiswellknownthatexcitationof TiO2 byphotonswithenergiesgreaterthanthebandgapyields formation of electron–hole (e−cb)/(h+vb) pairs, which can either recombine or take part in redox reactions. As a result, differ- enttypes of reactiveoxygenspecies (ROS) can beformed that arecapableofdegradingorganicpollutantsor damagingbacte- rialcells[2,8,24].Irelandetal.[2]emphasizedtheimportanceof OH• radicalsbecauseincontrastwithothertypeofwatertreat- menttechnologies(e.g.:ozonation,directphotolysisofhydrogen peroxide,andradiolysis)whichinherentlyproduceOH•radicalin verysmallquantities(<10−12M)[37],inaphotocatalyticsystem 10−9MOH•concentrationwasmeasured[38].Authorspublished thattherewasnoinactivationofE.coliinthepresenceoftheOH• radicalscavenger[2].Manyotherauthorsrecognizedtheimpor- tanceofOH• generationinphotocatalyticdisinfectionprocesses [2,3,5,6,8,24,34,39–43].Kikuchietal.[40]publishedthatthepho- tocatalyticbactericidal effectof E.colionilluminated TiO2 was confirmedonboth oxidationandreduction sites,corresponding to OH• and O2•− production, respectively. However theactual lethalagentisH2O2,subsequentlyproducedfromOH•andO2•−, particularlyinthelong-rangebactericidaleffect[40].Inaddition Rengifo-Herreraetal.[13]reportedthatsuperoxideradical(O2•−) anditsoxidationproduct:1O2–singletoxygenwereresponsible forE.coliinactivationbyN,Sco-dopedTiO2nanoparticlesunder visiblelightillumination.Inthisstudy,weusedelectronspinres- onance(ESR)spectroscopytoexplorethepossibledifferencesin efficienciesandthegeneratedROSbyselecteddopedandundoped titania-basedphotocatalysts.
2. Experimental
2.1. Commercialphotocatalysts
Aeroxide P25 (TiO2-P25, Evonik Industries), Aldrich anatase (TiO2-AA),andAldrichrutile(TiO2-AR)wereselectedascommer- ciallyavailableundopedphotocatalysts.
TiO2-TP-S201(SumitomoChemicalInc.)andTiO2-VLP700(Kro- nos Titan GmbH) doped with nitrogen and nitrogen/sulphur, respectively,werechosenascommerciallyavailabledopedTiO2
basedphotocatalysts.
2.2. HomemadeTiO2basedphotocatalysts
Thehome-madetitaniaswerenitrogen(TiO2-N),iron(TiO2-Fe), sulphur and nitrogen (TiO2-P25-NS) doped and silver (TiO2- P25-Ag) and gold (TiO2-P25-Au) deposited titanium dioxide photocatalysts.
Nitrogendopedtitania(TiO2-N)waspreparedbythehydroly- sisoftitanium(IV)chlorideinnitricacidsolutionfollowedbythe additionofaqueoussolutionofammonia.Theprecipitatewasthen driedandcalcinatedinournewlydevelopedcalcinationprocess usingrapidheating(∼60◦C/min)andshortexposuretothehot environmentat400◦Cfor10min[25].
Irondopedsample (TiO2-Fe)wasprepared by theoxidative hydrolysisoftitanium(III)chlorideinthepresenceofiron(III)chlo- rideunderair[27,44].Thissamplewasalsocalcinatedapplying
rapidheatingandshortexposuretothehotenvironment(at600◦C for10min).
SilverandgoldnanoparticleswerephotodepositedunderUV irradiation fromsilver acetate and HAuCl4 solutions on Aerox- ideP25toproduceTiO2-P25-AgandTiO2-P25-Auphotocatalysts [44,45].Thesetwosamplescontained1wt%ofthenoblemetals.
AeroxideP25wasalsoco-dopedwithsulphurandnitrogenby calcinationwiththioureafor1h,at400◦Cinstaticair(TiO2-P25- NS)similarlylikeinthemethodappliedbyRengifo-Herreraetal.
[22].
Allofthetitanias(includingthecommercialtitaniumdioxides) werewashedthreetimesbycentrifugationin0.1mMNaCl(Spek- trum3D,99.0%)aqueoussolution,resuspendedinMilliQwater, thendriedat80◦Cfor24handregroundinagatemortarbefore thephotocatalyticexperiments.
2.3. Methodsandinstrumentation 2.3.1. XRD
ARigakudiffractometerwasappliedforX-raydiffraction(XRD) measurements (Cu K␣=0.15406nm, 30kV, and 15mA,in the 20–40◦(2)regime).Theaveragediametersoftheparticleswere derivedusingtheScherrerequation.Theweightfractionofanatase andrutilewascalculatedfromthepeakareasoftheanataseand rutilepeaksat25.3◦(2)and27.5◦(2),respectively.
2.3.2. DRS
TheDRspectraofthesamples(=220–800nm)weremeasured byaJASCO-V650diodearraycomputercontrolled(SpectraManager Software)spectrophotometerwithanintegrationsphere(ILV-724).
2.3.3. TEM
TEMmicrographswererecordedonaPhilipsCM10instrument operatingat100kVusingFormvarcoatedcoppergrids.
2.3.4. BET
Thespecificsurface areaof thecatalystswasdeterminedby nitrogenadsorptionat77KusingaMicromeriticsgasadsorption analyser(GeminiType2375).Thespecificsurfaceareawascalcu- latedusingtheBETmethod.
2.3.5. X-rayphotoelectronspectroscopy(XPS)
X-rayphotoelectronspectraweretakenwithaSPECSinstru- mentequippedwithaPHOIBOS150MCD9hemisphericalelectron energyanalyseroperatedintheFATmode.Furtherdetailsofthe measurementsaredescribedelsewhere[46].
2.3.6. X-rayfluorescencespectroscopy(XRF)
AHoribaJobinYvonXGT-5000X-rayfluorescentspectrometer, equippedwithRhX-raysourcewasusedtomeasuretheelement contentofthesamples.Therecordsweremadeat30kVexcitation voltage,0.5mAanodecurrentand1000smeasuringtime.
2.3.7. ESRspintrappingmeasurements
The ESR measurements were performed at room tempera- ture by using a Bruker ESP300E spectrometer (Bruker BioSpin, Germany),operatingattheX-bandfrequencyandequippedwith astandardrectangularTE102cavity.Aftereachilluminationstep, small aliquots of ∼20L were transferred into 0.7mm ID and 0.87mmODglasscapillarytubes(VitroCom,NJ,USA).Tomaximize thesamplevolumeintheactivezone oftheESR cavity,assem- bliesofseventightlypackedcapillarieswerebundledtogetherand insertedintoa wide-bore quartzcapillary (standardESRquartz tubewith2.9mmIDand 4mmOD,Model707-SQ-250M,from Wilmad-LabGlassInc.,Vineland,NJ,USA).Suchsetupresultedin ca.140LsamplevolumeintheactivezoneoftheTE102cavity,
Fig.1. Photographofusedphotoreactorsystemequippedwithconventional24W energysavingcompactfluorescencelamps.
which,togetherwiththedivisionoftheaqueoussampleintoseven physically-separatedvolumes,markedlyimprovedtheoverallsen- sitivityofmeasurements[47,48].Thetypicalinstrumentalsettings were:microwavefrequency9.77GHz,microwavepower10.1mW, sweepwidth100G,modulationfrequency 100kHz,modulation amplitude0.5G,receivergain2×104,timeconstant81.92ms,con- versiontime40.96msandtotalscantime41.9s.
2.4. Measurementsofphotocatalyticactivity 2.4.1. Experimentalconditionsforphenoldegradation
Theexperimentsofphenol(Spektrum3D,99.0%)degradation werecarried out in a special photoreactorwhich was anopen glass vesselwithdouble walls, surroundedby a thermostating jacketat 25.0◦C. Aroundthereactor fourcompactfluorescence lamps(DÜWI25920/R7S-24Wtype–conventional 24Wenergy savingcompactfluorescencelamps)weremounted(Fig.1).The spectrum of thelamp wasslightly modified bycirculating 1M NaNO2 (MolarChemicals, min.99.13%)aqueoussolution inthe thermostatingjacket. This cut-off solution absorbs UV photons below400nm,providingvisiblelightirradiationforthesamples (Fig. 2).Theradiation intensitywas determinedby ferrioxalate actinometryfortheVISlampswithNaNO2cut-offfiltersolution asIVIS,1=1.07±0.03×10−5einstein/dm3/sinthephotoreactor.(It shouldbenotedthattheaveragequantumyieldofferrioxalateacti- nometryisabout0.9inthewavelengthrangebetween400and 540nm,anditisnearlyzeroabove540nm[49].)
Theefficiency ofdifferent type ofphotocatalysts was deter- minedbydecomposingphenol(0.1mM)inNaClsolution(0.9wt%) whichcontainedthetitaniapowdersin1.0g/Lconcentration.The suspension(100mL)wassonicatedbeforethephotocatalytictests (for5min) then it was stirredby magnetic stirrerand air was bubbledduringtheexperiments.Changesinphenolconcentration werefollowedusinganAgilent1100seriesHPLCsystemequipped withLichrospherRP18columnapplyingmethanol/watermixture aseluent(thedetectionwascarriedoutat210nm).
2.4.2. ExperimentalconditionsforinactivationofE.coli
Thesamephotoreactorandsimilarconditionswereappliedlike forthephenoldecompositionmeasurements.Anothertypeoflight filtrationwasalsocarriedoutinsomeexperiments.Applying5mM
0 5000 10000 15000 20000 25000 30000
300 400 500 600 700 800
Wavelength (nm)
Intensity (a.u.)
1M NaNO2 filtration
5mM K2Cr2O7 filtration
0 1000 2000 3000 4000 5000
400 402404 406408 410
Fig.2.Spectraoftheappliedconventionalenergysavingcompactfluorescence lamps(24W)with1MNaNO2and5mMK2Cr2O7lightcut-offfiltrationsbythe recirculationofthesesolutionsinthethermostatingjacket.
K2Cr2O7(Reanal,analyticalgrade)aqueoussolutioninthethermo- statingjacket,thelightintensitywasreducedto4%below420nm Fig.2.Theradiationintensitywasdeterminedbyferrioxalateacti- nometryfortheVISlampswiththeK2Cr2O7cut-offfiltersolution asIVIS,2=1.75±0.01×10−6einstein/dm3/sinthephotoreactor.
TheE.colisuspensionswerepreparedusingthefollowingpro- cedure.Firstly,E.coliculturesweregrownfor24hin0.9%NaCl solutionsupplementedwithnutrients:1%Tripton (Reanal,ana- lyticalgrade)and0.5%yeastextract(Scharlau,analyticalgrade).
Thenthecultureswerewashedtwotimeswitha0.9%salinesolu- tionbycentrifugationat4000rpmfor2minandthesedimentwas re-suspendedin0.9%NaClsolution.Priortothedisinfectionexper- iments,theTiO2-basedphotocatalystswereaddedtoa0.9%NaCl solutionsandsonicatedtoformhomogeneoussuspensions,which werethensupplementedwiththepreviouslypreparedE.colisus- pensions.
Alowvalueofinitialcolonyformingunit(104CFU/mL)wasset inthetitaniumdioxidesuspensionsinordertodetermineverylow disinfectionefficienciesaswell.Sampleswereplatedonagargels duringtheexperimentsandthecolonieswerecountedafter24hof incubationat37◦Cindark.Allofthesampleswereplatedon2–3 agargelsforgettingreliabledata.Allofthepresentedresultswere takenfromtheaverageoftwoparallelexperiments(resultswere fairlyreproducible).
2.4.3. ExperimentalconditionsforESRmeasurements
IntheESRmeasurementsweusedtwo(ROS) scavengers,i.e.
10mMconcentrationof2,2,6,6-tetramethyl-4-piperidinol(TMP- OH)and50mMconcentrationof5,5-dimethyl-1-pyrrolineN-oxide (DMPO).ThesolutionsofROSscavengerswerepreparedeitherin H2Oordeuteratedwater(D2O).TMP-OH,andD2O(isotopicpurity of99.9at%D)werepurchasedfromSigma–Aldrich(Switzerland) andusedasreceived.DMPOwasalsoobtainedfromSigma–Aldrich (Switzerland).BeforetheESRmeasurementsthesuspensionswere sonicatedfor5minandthen,duringthesubsequentphotocatalytic experimentsunderexposuretovisiblelight,theywerevigorously stirredbymagneticstirrerandairwasbubbledthrough.Theaque- oussuspensionscontainingthetitaniumdioxidenanoparticlesand ROS scavengers were exposed to the visible light in the same photoreactor in which phenol decomposition and disinfection experimentswerecarried out.Similarly,thesame conventional (24W)energysavingcompactfluorescencelampsand1MNaNO2 solutioninthethermostatingjacketforlightcut-offfiltrationwere
0.0 0.2 0.4 0.6 0.8 1.0 1.2
200 300 400 500 600 700 800
Absorbance
TiO2-N TiO2-TP-S201
TiO2-Fe
TiO2-VLP7000 0.0
0.2 0.4 0.6 0.8 1.0 1.2
200 300 400 600 700 800
(nm)
(nm)
Absorbance
TiO2-P25-Au TiO2-P25-Ag TiO2-P25-NS TiO2-P25
0.0 0.2 0.4 0.6 0.8 1.0
300 350 400 450 500
TiO2-AA TiO2-AR TiO2-P25
TiO2-P25
TiO2-P25-NS TiO2-P25-Ag
TiO2-P25-Au
a
b
Fig.3.Diffusereflectancespectraofinvestigatedphotocatalysts:(a)non-doped commercialandP25basedmodifiedhome-madetitaniumdioxides;(b)dopedcom- mercialandhome-madetitanias.
used.Theconcentrationofthetitaniumdioxidesuspensionswas of1.0g/L,andthetotalvolumewasof20mL.
3. Resultsanddiscussion
3.1. Characterizationofthephotocatalysts
The photocatalysts were characterized by several methods, including: XRD,transmission electron microscopy (TEM),X-ray fluorescencespectroscopy(XRF),diffusereflectancespectroscopy (DRS),X-rayphotoelectronspectroscopy(XPS)and nitrogengas adsorptionanalysis(BET)method.Themostimportantphysico- chemical parameters of the studied photocatalysts, i.e. anatase andrutilecontent,theparticlesize,thedopantcontent(derived fromXRF,and/orXPS)aswellastheBETspecificsurfacearea,are showninTable1.Thereareinvestigatedtitaniaswhichcontain onlyanatasephase(suchasTiO2-AAand TiO2-VLP7000),inthe P25basedtitaniasmorethan90%oftheparticlesareinanatase phase,inthenitrogendopedtitania5%brookitecontentwasdeter- minedbyXRD,whileAldrichrutilecontains96%rutile.Theparticle sizesareverydifferent:TiO2-VLP7000hasthesmallestparticles (DXRD=7.8nm)withthelargestspecificsurfacearea(297m2/g), whileAldrichrutilecontainslargeparticles(100–700nm),which resultsinverylowspecificsurfacearea(2.7m2/g).Thelightabsorp- tionwasmeasuredbydiffusereflectancespectroscopyforallof thephotocatalysts(Fig.3aandb).Thesefiguresshowthatdoped
Fig.4. TEMimagesof(a)Aldrichrutile,(b)SumitomoTP-S201and(c)Kronos VLP7000titanias.TEMimagesofotherinvestigatedphotocatalystsarepublished elsewhere[44].
titaniasabsorbvisiblelight.Aldrichanatasedoesnotabsorbany photonsbeyond400nm,howeverAldrichrutilehasabandgap at about420nm. These titaniaswere allexamined withtrans- missionelectronmicroscopytoobservethesizedistributionand themorphologyoftheparticles.TheTEMimagesofTiO2-TP-S201, TiO2-VLP7000and TiO2-ARareillustratedin Fig.4,while other imagesofthephotocatalystsarepublishedelsewhere[44].Aldrich rutilesamplecontainsrelativelylargeparticleswhicharelarger than100nm(DTEM≈315nm)whichresultsinverylowspecific surfacearea.Theparticlediametervaluescalculatedfromtheline
Table1
Structuralparametersoftheinvestigatedphotocatalysts(phasecontent,particlesize,theconcentrationofdopantsandspecificsurfacearea),theinitialdegradationratesof phenol,irradiationtimefortotalsterilizationandtheresultsofESRmeasurementsareshown.Thesamplesarelistedintheorderofdecreasingactivityforphenoldegradation formthetoptothebottom.Greyrowsindicatethehome-madetitaniumdioxides,thewhiterowsareforcommercialtitanias.
Sample Anatase
(wt%) Rutile (wt%)
DA(nm) DR(nm) Dopantcontent (at%)f
aSBET(m2/g) r0,phenol
(×10−8M/s)
tsterilization
(min)g
ESRMeasurements
WithTMP-OH scavenger
WithDMPO scavenger
WithDMPO scavenger
1O2 O2•- OH•
TiO2-VLP7000 100 – 7.8 – S:0.33c/N:1.21d 297 29.9 – High No No
TiO2-AR 4 96 ∼315b – 2.7 4.2 20 No No High
TiO2-P25-NS 94 6 25.4 ∼40 S:0.13c/N:n.d. 55 3.7 – Notmeasured
TiO2-N 95a – 6.5 – 1.32d 139 2.4 – No No No
TiO2-Fe 29 71 ∼35 ∼31 0.37c 28 1.7 – Notmeasured
TiO2-TP-S201 100 – 17.3 – N:0.82d 80 1.5 60 Notmeasured
TiO2-P25 90 10 25.4 ∼40 – 49 1.4 60 No No Yes
TiO2-P25-Ag 90 10 24.5 ∼42 0.24c,e 51 1.3 60 Notmeasured
TiO2-P25-Au 90 10 24.1 ∼37 0.13c,e 51 0.4 – Notmeasured
TiO2-AA 100 – >85 – – 9.9 0.4 – Notmeasured
aCalculatedcontentofbrookiteis5wt%,DB=14.4nm.
b AverageparticlediameterwascalculatedfromTEMpictures.
c MeasuredbyXRF.
d EstimatedbyXPS.
eNominal(added)metalcontentis1.0wt%asmD/mcatalyst×100;measuredvaluesforAgandAucontentsare0.94wt%and0.96wt%,respectively.
f ExpressedasnD/ntotal×100.
gCFU=104;1MNaNO2lightcut-offfiltration(>400nm).
broadeningoftheanatasepeakforTiO2-TP-S201(DXRD=17.3nm) andTiO2-VLP7000(DXRD=7.8nm)samplesareingoodagreement withthenanoparticlesizesobservedontheirTEMimages(Table1, Fig.4bandc).
3.2. Phenoldegradation
ThedecompositionofphenolwasfollowedbyHPLC.Thedecay curvesarepresentedinFig.5aandb.Therewasnotanydecrease intheconcentrationunderirradiationwithoutphotocatalyst,and only very slow degradations were observed applying Aldrich anataseorTiO2-P25-Au.Itmeansthatinthelatercasedepositing Aunanoparticlesontitaniumdioxidereducesthephenoldegrada- tionefficiencyinthevisiblelightrange.TheTiO2-TP-S201andthe TiO2-P25-Agsamplesshowedsimilarperformancethanthewell knownreferenceAeroxideP25(TiO2-P25),whichdecomposed17%
ofthephenolfromthe0.1mMsolutionafter4hofirradiation.Three home-madetitaniasshowedhigherefficiencythanTiO2-P25.Only commerciallyavailableKronosVLP7000(co-dopedbynitrogenand sulphur)hadbetterperformancethanthenon-dopedAldrichrutile (TiO2-AR).Itshouldbenotedthatrutileabsorbslightbetween400 and420nm(incontrastwiththeanatase),inwhichwavelength rangetherearetwopeaksinthespectrumofthelamp(Fig.2).
Nevertheless,themostactivephotocatalyst(TiO2-VLP7000)con- tains100% anatasephase. The highperformance of this titania wasresultedbytheefficientdopingprovidingtheabilitytoacti- vatetheparticlesbyvisiblelight.Thedecaycurves,andalsothe initialdegradationratesofphenoldemonstrateaswell,thatTiO2- VLP7000hasextremelyhighefficiencyforphenoldegradation,94%
ofphenolwasdecomposedin4h.KRONOSVLP7000isanitrogen andsulphurco-dopedtitaniumdioxidelikeourhome-madeTiO2- N,S,but thecommercialtitaniaownsvery highspecificsurface areawhichcanresultinthishighperformancebesidestheefficient doping(KimandChoi[50]publishedthathighspecificsurfacearea isbeneficialforthedegradationofphenol).
3.3. Disinfectionperformance
Investigating Aldrich anatase, TiO2-P25-Au, TiO2-Fe, TiO2-N;
TiO2-P25-NSandTiO2-VLP7000titaniumdioxides,therewasnot anydisinfectioneffectobservedafter2hofirradiation.Theresults
0.0 0.2 0.4 0.6 0.8 1.0
0 60 120 180 240
Irradiation time (min) cphenol (10-4 M)
Light - without TiO2 TiO2-P25 TiO2-Fe TiO2-P25-NS TiO2-N TiO2-AR TiO2-VLP7000 0.80
0.85 0.90 0.95 1.00
0 60 120 180 240
Irradiation time (min) cphenol (10-4 M)
Light - without TiO2 TiO2-AA TiO2-P25-Au TiO2-TP-S201 TiO2-P25-Ag TiO2-P25
a
b
Fig.5.(aandb)DecaycurvesofphenolunderVISirradiation(conventional24W energysavingcompactfluorescencelampswith1MNaNO2lightcut-offfiltration (>400nm).
1.E+00 1.E+01 1.E+02 1.E+03 1.E+04 1.E+05
0 20 40 60 80 100 120
Irradiation time (min)
CFU (1/mL)
Light without photocatalyst TiO2-P25 in dark TiO2-TP-S201 in dark TiO2-AR in dark TiO2-P25-Ag in dark TiO2-TP-S201 + light TiO2-P25 + light TiO2-P25-Ag + light TiO2-AR + light
> 400 nm λ
Fig.6. Disinfectionexperimentswith1MNaNO2filteredvisiblelightirradiation (initialcolonyformingunitwas104).
ofthefiveotherphotocatalystsactivitiesarepresentedinFig.6.All oftheexperimentswererepeatedtwotimes,andtheaveragesare showed.ApplyingTiO2-TP-S201,TiO2-P25andTiO2-P25-Agtita- nias,thecolonyformingunitwasreducedtozeroin1h.Fromthe shapeofthemortalitycurve,itseemsthatthesilvercontaining samplehasalittlebithigherdisinfectionabilitythanthesilverfree P25.Itismostlikelyduetothewellknowndisinfectioneffectof silverions[51–57]whichcanbereleasedfromthesilvernanopar- ticlestothesolutionin lowquantity(inthedarkexperiment a slowdisinfectioneffectwasalsodeterminedonthisphotocatalyst).
TiO2-ARsamplehadmuchhigheractivityforinactivationofE.coli.
Thewaterwassterilizedtotallybythistitaniain20min(Fig.6).
A seriesof experimentswasalso carried outtoexcludethe possibilitythatthedetermineddisinfectioneffectswereresulted bytoxiccompoundsdissolvedfromtheirradiatedphotocatalysts.
Suspensionsofthetitanias(withoutbacteriaaddition)wereirra- diatedforthesametimeatwhichtheydisinfectedpreviouslythe solution,andthenthenanoparticleswereseparatedbycentrifuga- tionandfiltration.Aftertheseparation,104CFU/mLwasadjusted tothissolution,anditwaspouredbackintothephotoreactorand thechangesincolonyformingunitwasfollowedfor2hofirradia- tion.InthecaseofTiO2-P25,TiO2-TP-S201,TiO2-ARtitanias,there wasnotseenanynotableCFUreduction.Onlyinthecaseofsilver containingTiO2wasnoticedasimilarCFUreductionlikeinTiO2-Ag suspensionindark.
Investigationofdisinfectionperformancewascarriedoutalso at higher initial CFU (105CFU/mL) for the most active titania (TiO2-AR).Thistitaniatotallysterilizedthewaterafter30minof irradiationinthiscase.
Disinfectionexperimentswerealsocarriedout withanother type oflight cut-off filtration.Applying5mMK2Cr2O7 aqueous solutioninthethermostatingjacketthelightintensitywasreduced to4%below420nm(Fig.2).Resultsofdisinfectionexperimentsare presentedinFig.7.Fig.8representsaphotographwhichdemon- stratestheE.colicoloniesofthephotocatalytictestwithAldrich rutile(after24hofthermostatingin37◦C,indark).ApplyingAerox- ideP25orTiO2-TP-S201therewasnotseenanydisinfectioneffect after2h.ThesilvercontainingTiO2 sampleshowedalowactiv- ityforkillingbacterialikeindarkcondition(duetothepresence ofAg+ ions).Non-dopedAldrichrutileshowedanotableactivity consideringtheverylowlightintensitybelow420nm(onlylight <420nmcanexcitethisphaseoftitaniumdioxide).WithK2Cr2O7 filtrationthreetitaniumdioxides(TiO2-P25,TiO2-P25-Ag,TiO2-TP- S201)losttheirphotocatalyticdisinfectionproperty,howeverwith
1.E+00 1.E+01 1.E+02 1.E+03 1.E+04 1.E+05
0 20 40 60 80 100 120
Irradiation time (min)
CFU (1/mL)
TiO2-TP-S201 + light TiO2-P25 + light TiO2-P25-Ag + light TiO2-AR + light
λ > 420 nm
Fig.7.Disinfectionexperimentswith5mMK2Cr2O7filteredvisiblelightirradiation (initialcolonyformingunitwas104).
NaNO2cut-offfiltration,thesetitaniasdisinfectedcompletelythe water in1h. Thismeansthat theintensityof thecommercially availablelightsources inthewavelengthrangefrom400nmto 420nmiscrucialforefficientindoorair/surfacecleaning.
3.4. Comparisonofphenoldegradationandthedisinfection performance
Theresultsofphenoldecompositionanddisinfectionexperi- mentsaresummarizedinTable1.Thevaluesofinitialdegradation ratesofphenolforthedifferenttitaniasaredecreasingfromthetop tothebottom.TiO2-VLP7000showedextremelyhighefficiencyfor phenoldecomposition,butithadnotanynotabledisinfectionprop- erty.Aldrichrutilehadhighefficiencytokillbacteriaandalsohad highefficiencyforphenoldegradation.Therewerethreetitanias whichdegradedphenolwithhighefficiencybutdidnotshowany antibacterialproperty.Moreover,therewerethreeothertitanias whichkilledE.coliafter1hofirradiation,howeverthesetitanium dioxideshadrelativelylowperformanceforphenoldegradation.It seemsfromTable1,thereisnocorrelationbetweenthebacteria killingabilityofcatalystandtheircrystallinestructure.Interpre- tationfortheseinterestingobservationsbasedontheparticlesize oronthespecificsurfaceareacannotbeprovided.Togetsome
Fig.8. PhotographofE.colicoloniesofthephotocatalytictest(>420nminitial CFU=105)withAldrichrutile(after24hthermostatingin37◦C,indark).
-1.0E-03 -5.0E-04 0.0E+00 5.0E-04 1.0E-03
3440 3450 3460 3470 3480 3490 3500 3510 3520 3530 Magnetic Field (G)
ESR signal (a.u.)
TiO2-VLP7000 - TMP-OH - D2O - 30 min Irradiation TiO2-VLP7000 - TMP-OH - D2O - NaN3 - 0 min Irradiation TiO2-VLP7000 - TMP-OH - D2O - NaN3 - 30 min Irradiation
-1.0E-03 -5.0E-04 0.0E+00 5.0E-04 1.0E-03
3440 3450 3460 3470 3480 3490 3500 3510 3520 3530 Magnetic Field (G)
ESR signal (a.u.)
TiO2-VLP7000 - TMP-OH - H2O - 60 min Irradiation TiO2-VLP7000 - TMP-OH - D2O - 60 min Irradiation
-1.5E-04 -5.0E-05 5.0E-05 1.5E-04
3440 3450 3460 3470 3480 3490 3500 3510 3520 3530
Magnetic Field (G)
ESR signal (a.u.)
TiO2-AR - DMPO - H2O - 3 min Irradiation TiO2-VLP7000 - DMPO - H2O - 0 min Irradiation TiO2-VLP7000 - DMPO - H2O - 3 min Irradiation
-1.0E-03 -5.0E-04 0.0E+00 5.0E-04 1.0E-03
3440 3450 3460 3470 3480 3490 3500 3510 3520 3530
Magnetic Field (G)
ESR signal (a.u.)
TiO2-AR - TMP-OH - H2O - 60 min Irradiation TiO2-AR - TMP-OH - D2O - 60 min Irradiation
a b
d c
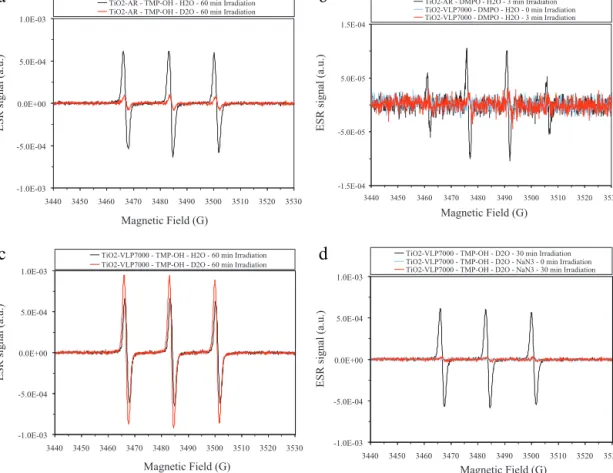
Fig.9. ResultsofESRmeasurements:(a)visiblelightilluminatedaqueous(H2OandD2O)suspensionsofTiO2-ARcontaining10mMconcentrationofTMP-OH;(b)visiblelight illuminatedaqueoussuspensionsofTiO2-ARandTiO2-VLP7000containing50mMconcentrationofDMPO;(c)visiblelightilluminatedaqueous(H2OandD2O)TiO2-VLP7000 suspensionscontaining10mMconcentrationofTMP-OH;(d)visiblelightilluminatedD2OsuspensionofTiO2-VLP7000containing10mMTMP-OH(inthepresenceand absenceofNaN3singletoxygenquencher).
insightintoROS-mediatedbacteriainactivationmechanisms,ESR spin-trappingmeasurementswerecarriedoutforselectedphoto- catalysts.
3.4.1. ESRmeasurements
Four selected titanium dioxides were investigated. TiO2- VLP7000and TiO2-N showed rapidphenol degradation,but no E.colideactivation.TiO2-ARhadhighefficiencyforphenoldegrada- tionandfordisinfectionaswell,whileTiO2-P25hadantibacterial propertybutithadrelativelylowperformanceforphenoldegra- dation.TheresultsoftheESRmeasurementsaresummarizedin Table1.
3.4.1.1. TiO2-AR. InthepresenceofTMP-OHscavengerintheVIS irradiatedTiO2-ARsuspensionastrongESRsignalofTEMPOLwas observedafter1hofillumination(Fig.9a).TEMPOLmightbepro- ducedbyanattackofsingletoxygen(1O2)orhydroxylradical(OH•) onTMP-OH[58].
TheESRsignalofTEMPOLisslightlydistorted.Asitisseenin Fig.9athecentralESRfeatureismarkedlyhigherthanthelow-and high-fieldfeatures.Thisdistortionoriginatesfromtheformationof anothernitroxideradical,TEMPONE.OH•radicalscanreactwith TEMPOLbyattackingontheOH-groupswhichyieldthegeneration ofTEMPONE[59].
InD2O,asitcanbeseeninFig.9a,thesignalamplitudeofthe ESRsignalofTEMPOLwasreducedca.7.3timesascomparedtoH2O (forthesameilluminationtime).ItisknownthatD2Osuppresses theformationofOD•radicalsattheTiO2/D2Ointerface[60,61].
InthepresenceofDMPOanintenseDMPO-OH•signal(inH2O) wasobservedundervisiblelightirradiationwhichprovedtheOH• radicalgenerationinthecaseofAR(Fig.9b).Thisresultcorrobo- rateswiththemarkeddiminishmentoftheTEMPOLsignalinD2O, andsuggeststhatthephotocatalyticactivityofthissamplemightbe relatedtothephotocatalyticgenerationofhydroxylradicals(OH•).
ItshouldbenotedthatTiO2-ARprobablyproducessome1O2via theclassicalmechanismsuggestedbyNosakaetal.[62]butsince applying DMPO scavenger no DMPO-OOHsignal was detected, TiO2-ARdonotproduceO2•−radicalinmeasurablequantity.
3.4.1.2. TiO2-VLP7000.Under VISlight illumination in H2O,the TiO2-VLP7000samplegaveastrongTEMPOLsignal.Thissignalis evenstronger(byca.10%)thanthesignaloftheTiO2-AR(Fig.9c).
InD2O,theESRsignalamplitudeofTEMPOLincreasedca.1.4 times as compared to H2O (Fig. 9c) which suggested that the detectedsignalcanbeduetothegenerationofsingletoxygen(1O2) onthesurfaceofTiO2-VLP7000.Togetmoreevidenceofthegen- erationof1O2 anexperimentwithsingletoxygenquencherwas alsocarriedout.InD2OaverystrongsuppressionoftheTEMPOL signalwasobserved(itwasreducedca.19times)inthepresence of100mMNaN3(whichisa1O2quencher)(seeFig.9d).
SoboththeESRsignalenhancementinD2Oandsignalquench- ingbyNaN3suggestedthatthestrongsignalofTEMPOLisdueto theformationof1O2.Howevertheseresultsdonotexcludethepos- sibilitythatthescavengerwasdirectlyoxidizedonthesurfaceof thephotocatalyst,assuggestedbyNosaka(in2006)forothertita- niaphotocatalysts[63],consideringthatthistitaniahasextremely highspecificsurfacearea.
InthepresenceofDMPO scavengernoDMPO-OHor DMPO- OOHsignalwasdeterminedforVISlightirradiatedTiO2-VLP7000 (Fig.9b)whichprovedthatthereisnoOH•radicalgenerationon thistitania.Thisobservationcorroborateswiththeresultsofthe experimentswithTMP-OHscavenger.
3.4.1.3. TiO2-P25. AtthecaseofTMP-OHscavengerinthevisible lightilluminatedTiO2-P25suspensionawellmeasurablesignalof TEMPOLwasobserved,whichwasabout30%ofthesignalofTiO2- AR.ThissignalwasstronglydecreasedinD2Olikeinthecaseof TiO2-AR,andtheexperimentswithDMPOscavengeralsoproved againthegenerationofOH•radical(butinsmalleramountsthan inthecaseofTiO2-AR)andtheabsenceofO2•−.
3.4.1.4. TiO2-N.NomarkedformationofOH•,O2•−orsingletoxy- gen(1O2)wasobservedforthenitrogendopedhome-madetitania (TiO2-N).
4. Conclusions
Undervisiblelightillumination,threeofourhome-madetita- nias(TiO2-Fe,TiO2-N,S,TiO2-N,)andcommercialnitrogen/sulphur- dopedKronosVLP7000titaniumdioxideshowedhigherefficiency forphenoldegradationthanthewellknownreferenceAeroxide P25, however onlyVLP7000 had betterperformance than non- dopedAldrichrutile.
AldrichrutilehadhighefficiencyofE.coliinactivationandalso hadsignificantactivityforphenoldegradation;howeverfourdoped titaniashadhigherefficiencyforphenoldegradationthanTiO2-P25 buthadnotanyactivityforinactivationofbacteria.
ESR measurements pointed out that titanias that generated OH•radicalswereactiveforkillingE.coli,andthose,whichwere unabletoproduceOH•radicalsdidnotshowanydisinfectionprop- erty.Thisisconsistentwiththestatementsofmanyauthorswho described that OH• radicals play a majorrole in photocatalytic disinfectionexperiments[2,5,6,24,34,39,42,43],andrecentstudy provedthatthispersistinthecaseofvisiblelightirradiationas well.ThisisingoodagreementwiththeresultsofthestudyofYu etal.[34]whodemonstratedthedeactivationofMicrococcuslylae byvisiblelightirradiatedTiO2duetothegenerationofhydroxyl radical.
KronosVLP7000showedextremelyhighefficiencyforphenol decompositionduetoitshighspecificsurfacearea,butthistitania didnotshowanydisinfectionpropertyunderourcircumstances whichmightbeduetoabsenceofOH•radicalgeneration.
TiO2-N titania showedhighperformance for phenol decom- position,howeverneitherOH•radicalnorsuperoxideradicalion (O2•−)orsingletoxygen(1O2)wasdetected.Thismeansthatphe- noldegradationoccurredviathereactionbytheholeonthesurface, andthehighefficiencyismostlikelyduetothehighspecificsurface area(thistitaniumdioxidehasthesecondhighestspecificsurface areaamongtheinvestigatedsamples).
The experiments with K2Cr2O7 cut-off filtered light source pointed outthat therutile couldhave high-performanceappli- cability forutilizing visible light in self-cleaningor disinfecting processes.Rutilehadanotableefficiencyalsowiththisverylow intensitybelow420nm.Thisstudyfirstlyinvestigatedthedisin- fectionperformanceofrutileparticleswithrelativelylargeparticle size(d∼315nm)usingsolelyvisiblelightforactivatingthephoto- catalyst.
PureandsilverdopedP25andSumitomotitaniasterilizedwater in1hwhen>400nmconditionwasused,butthesephotocata- lystslosttheirdisinfectionpropertywhenK2Cr2O7 light cut-off filtrationwasapplied(>420nm).Theseresultshighlightedthat theintensityofthecommerciallyavailablelampsinthewavelength
range from400nmto420nm iscrucialtoapplyeffectively the photocatalystsforindoorair/surfacecleaning.
Acknowledgements
This work was partially financed by the European Union throughtheHungary-SerbiaIPACross-borderCo-operationPro- gram,HU-SRB/0901/121/116.Itwasalsoco-financedbythegrant from the Hungarian National Office of Research and Technol- ogy (OTKACK 80193), by theEuropean Regional Development Fund(TÁMOP-4.2.1/B-09/1/KONV-2010-0005andTÁMOP-4.2.2/B- 10/1-2010-0012)andtheSwissContribution(SH/7/2/20).
KM thanksthe financial supportof the Hungarian Research Foundation(OTKAPD78378)andtheJánosBolyaiResearchSchol- arshipoftheHungarianAcademyofSciences.
A.Sand L.F. acknowledgethe financialsupport of theSwiss NationalScienceFoundationthroughtheNano-TeraNTFproject
“Core-shellsuperparamagneticandupconvertingnano-engineered materialsforbiomedicalapplications–NanoUp”.
TheauthorsareindebtedtoEvonikIndustries,toKronosGmbh., toSumitomoChemicalsInc.forsupportingourworkbysupplying freeTiO2samplesforthesestudies.
References
[1] T.Matsunaga,R.Tomoda,T.Nakajima,H.Wake,FEMSMicrobiologyLetters29 (1985)211–214.
[2]J.C.Ireland,P.Klostermann,E.W.Rive,R.M.Clark,AppliedandEnvironmental Microbiology59(1993)1668–1670.
[3] J.A.Herrera-Melian,J.M.D.Rodriguez,A.V.Suarez,E.T.Rendon,C.V.D.Campo,J.
Arana,J.P.Pena,Chemosphere41(2000)323–327.
[4]K.Kühn,Chemosphere53(2003)71–77.
[5]M.Cho,H.Chung,W.Choi,J.Yoon,WaterResearch38(2004)1069–1077.
[6] A.Vohra,D.Y.Goswami,D.A.Deshpande,S.S.Block,AppliedCatalysisB64 (2006)57–65.
[7]A.K.Benabbou,Z.Derriche,C.Felix,P.Lejeune,C.Guillard,AppliedCatalysisB 76(2007)257–263.
[8] C.Hu,J.Guo,J.Qu,X.Hu,Langmuir23(2007)4982–4987.
[9] C.Guillard,T.Bui,C.Felix,V.Moules,B.Lina,P.Lejeune,ComptesRendusChimie 11(2008)107–113.
[10]D.M.A.Alrousan,P.S.M.Dunlop,T.A.McMurray,J.A.Byrne,WaterResearch43 (2009)47–54.
[11] L.Caballero,K.A.Whitehead,N.S.Allen,J.Verran,JournalofPhotochemistry andPhotobiologyA:Chemistry202(2009)92–98.
[12]F.Chen,X.Yang,Q. Wu,EnvironmentalScience &Technology43(2009) 4606–4611.
[13]J.A.Rengifo-Herrera,K.Pierzchała,A.Sienkiewicz,L.Forró,J.Kiwi,C.Pulgarin, AppliedCatalysisB88(2009)398–406.
[14]E.A.Kozlova,A.S. Safatov,S.A.Kiselev,V.Y.Marchenko,A.A.Sergeev,M.O.
Skarnovich,E.K.Emelyanova,M.A.Smetannikova,G.A.Buryak,A.V.Vorontsov, EnvironmentalScience&Technology44(2010)5121–5126.
[15]S.Malato,P.Fernández-Ibá ˜nez,M.I.Maldonado,J.Blanco,W.Gernjak,Catalysis Today147(2009)1–59.
[16]A.Vidal,A.I.Dıaz,A.E.Hraiki,M.Romero,I.Muguruza,F.Senhaji,J.González, CatalysisToday54(1999)283–290.
[17]E.Duffy,SolarEnergy77(2004)649–655.
[18]C.Sichel,J.Blanco,S.Malato,P.Fernández-Ibá ˜nez,JournalofPhotochemistry andPhotobiologyA:Chemistry189(2007)239–246.
[19]C.Sichel,J.Tello,M.deCara,P.Fernández-Ibá ˜nez,CatalysisToday129(2007) 152–160.
[20]A.I.Gomes,J.C.Santos,V.J.P.Vilar,R.A.R.Boaventura,AppliedCatalysisB88 (2009)283–291.
[21]C.Karunakaran, G.Abiramasundari,P.Gomathisankar, G. Manikandan,V.
Anandi,JournalofColloidandInterfaceScience352(2010)68–74.
[22]J.A.Rengifo-Herrera,J.Kiwi,C.Pulgarin,JournalofPhotochemistryandPhoto- biologyA:Chemistry205(2009)109–115.
[23] P.Wu,J.A.Imlay,J.K.Shang,Biomaterials31(2010)7526–7533.
[24]P.Wu,R.Xie,J.A.Imlay,J.K.Shang,AppliedCatalysisB88(2009)576–581.
[25]Z.Pap,L.Baia,K.Mogyorosi,A.Dombi,A.Oszko,V.Danciu,CatalysisCommu- nications17(2011)1–7.
[26]K.Nagaveni,G.Sivalingam,M.S.Hegde,G.Madras,EnvironmentalScience&
Technology38(2004)1600–1604.
[27]Z.Ambrus,N.Balazs,T.Alapi,G.Wittmann,P.Sipos,A.Dombi,K.Mogyorosi, AppliedCatalysisB81(2008)27–37.
[28] E.G.Bajnoczi,N.Balazs,K.Mogyorosi,D.F.Sranko,Z.Pap,Z.Ambrus,S.E.Canton, K.Noren,E.Kuzmann,A.Vertes,Z.Homonnay,A.Oszko,I.Palinko,P.Sipos, AppliedCatalysisB103(2011)232–239.