VERA G. COLLINS
Freshwater Biological Association. The Ferry House, Ambleside, Cumbria
1 Introduction . . . . 2 1 9
2 Sampling apparatus . . . 221
3 Monitoring the environment . . . . . . . . 223
4 Methods for handling samples . . . . . . . . 223
4.1 Jenkin surface-mud core samples . . . . . . . 225
4.2 Deep sediment cores . . . . . . . . . 226
4.3 Sediment samples obtained by diving techniques . . . . 228
4.4 Procedures for measuring the redox potential (electrode potential) of sediment samples taken with the Jenkin surface-mud sampler . 229 4.5 Procedures for measuring oxygen uptake in situ in Jenkin surface-mud core samples . . . . 2 3 1 5 Methods and media for enumerating bacteria in sediments . . . 2 3 3 5.1 Counting procedures . . . . . . . . . 233
5.2 Media 240 6 Distribution of bacterial populations . . . . . . . 242
6.1 T h e distribution of heterotrophic bacteria in a stratified lake . . 243
6.2 T h e distribution of heterotrophic bacteria in surface sediments and at the sediment-water interface in different lakes . . . . 248
6.3 T h e vertical distribution of bacteria in Jenkin surface-mud cores . 254 6.4 T h e vertical distribution of heterotrophic bacteria in deep sediment cores . . . . . . . . . . . 262
7 Discussion . . . . . . . . . . . 266
Acknowledgements . . . . . . . . . . 268
References . . . . . . . . . . . 269
1 Introduction
In 1947 the author, at the start of her career in aquatic microbiology, had the privilege of meeting and talking with Claude E. Zobell, when he visited the laboratories of the Freshwater Biological Association
219
in Wray Castle, which is on the western shores of Windermere. An autographed copy of Marine Microbiology by Zobell (1946) and discussions with Dr Zobell could be considered as being responsible for the initiation of experiments designed to explore the microbiology of the sediments of freshwater lakes in the English Lake District.
This paper, therefore, will be concerned primarily with studies on the microbiology of mud with particular emphasis on the bacteria present in the surface sediments of freshwater lakes. Where relevant, attempts will be made to link up parallel studies in the marine aquatic environment by the simple expedient of cross-reference.
The environment of a stratified freshwater lake (Collins, 1969) provides some unique ecological niches for potential exploitation by microorganisms. Current interest in the trophic status of lakes centres around the degree of enrichment of the lake through the main agencies, the use of agricultural fertilizers on the land surrounding the drainage basin of the lake and the input of sewage effluents from domestic and farming sources. The morphometry of the lake basin has a pronounced effect on the capacity of the waters of the lake to continue to receive enrichment from the drainage area and to maintain a biological equi- librium between supply and demand of extraneous organic matter.
The results of the long-term monitoring programme on lakes in the English Lake District, using the criterion of the dissolved oxygen con- centration of the " b o t t o m " waters, i.e. hypolimnetic waters of these stratified lakes, provides comparative data in support of the effect of the morphometry of the lake basin, particularly depth, on the trophic status of the lakes. The results, covering a five-year period, are shown in Table 1 (p. 241). In general it can be stated that lakes with a depth of over 20 m have the capacity under continuous enrichment to main- tain an oxygen balance in excess of biological demand, whereas lakes with a depth of 20 m or less demonstrate an oxygen deficit. These results support the criteria for "grouping" lakes proposed by Thomas
(1965) and they also place the lakes of the English Lake District into two main groups; these are "unproductive" or oligotrophic, " p r o - ductive" or eu trophic. The present-day grouping of these lakes, on the basis of the oxygen deficit of the bottom waters, still provides support for the "lake series" proposed by Pearsall (1921) and the studies of algae by Lund (1957) and the studies of Jones (1972b) on the phospha- tase activity of freshwater bacteria. A great deal of information on the morphometry, physics and chemistry of this "lake series" is available
in Macan (1970) along with dissolved oxygen depth profiles for seven- teen lakes in the series obtained by the present author in 1963 (see Figs 30 and 31, Macan (1970)). The studies mentioned above have clearly demonstrated the differing trophic status of a group of fresh- water lakes. In other sections of the text an attempt will be made to examine the contribution of some of the bacterial groups present in the hypolimnetic zone, at the sediment-water interface and in the sedi- ments, to the microbiology of the "lake series".
2 Sampling apparatus
" T h e r e is no completely sterile sediment sampler." (Sorokin and Jannasch, 1972.) This statement neatly summarizes the existing
situation with regard to the large variety of sampling devices now available for the purpose of sampling sediments, whether they be of marine or freshwater origin. Various samplers are described by Zobell (1946) for obtaining samples and mud cores from marine sedi- ments under varying depths of water. A sampler for collecting un- disturbed cores of marine sediments is described by Craib (1965).
The type of sediment greatly influences the choice of sampling apparatus and the majority of samplers fall into two types: grab samplers and coring devices. One such grab sampler is the Shipek model described by Collins et al. (1973) ; other models of grab samplers are manufactured and supplied by Hydro-Bios Apparatebau GmbH, 23 Kiel-Holtenau, Germany. Another useful reference on manufacturers and suppliers of sediment samplers is the source list of Limnological and Océanographie apparatus and supplies (Special Publication, 1964).
The use of the grab type of sampler means that the mud samples so obtained will be subjected to a great deal of disturbance. For some microbiological studies this will not be important; for example, when the nature of the investigation merits a random mixed sample of the type of sediment that would not easily consolidate into the tube of a coring device.
However, when samples of relatively undisturbed surface mud and its accompanying overlying water are required a core sampling device must be used. For microbiological studies, such samplers provide the means of determining the distribution of the microbial populations with depth in mud cores, with minimal disturbance of the m u d - water interface of the sediments.
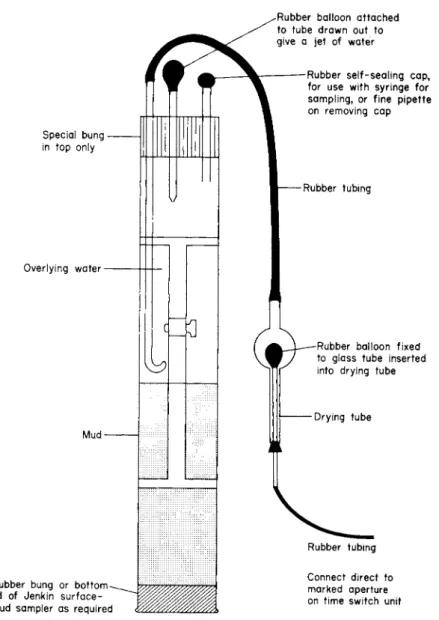
A core sampler designed for use on lakes and known as the Jenkin surface-mud sampler was described and used by Mortimer (1941,
1942). This sampler was subsequently modified (Mortimer, 1971), and is available from The Lakes Instrument Co. Ltd, Oakland, Winder- mere, Cumbria.
The Jenkin surface-mud sampler is not suitable for obtaining cores of surface mud from under shallow depths of water in the littoral regions of lakes. An alternative sampler, designed for sampling sediments in these regions, was designed by Mr H. C. Gilson and up to 1972 was known as the FBA Automatic Mud Sampler. Since some difficulty may be experienced in obtaining this sampler from commercial sup- pliers, the details of its design are as follows. The apparatus is intended for sampling soft, muddy deposits at the bottoms of lakes and rivers. In water not more than 3-4 m deep it can be used on a pole; in deeper water it can be loaded with weights and used on a line. It will not in general retain a sample where the bottom is weedy, sandy or gravelly, nor where the deposit is less than about 15 cm deep.
The gunmetal headpiece contains a valve which is open as the sampler descends and closes when the operator begins to pull the sampler out of the deposit. The top is threaded (19 mm BSW) to fit the sockets used on jointed net poles, several of which may be screwed together to provide the length required; a hole to take a standard 7-9 mm D- shackle is also provided, so that a line (which should be spliced round a thimble) can be attached. Lead weights (one of about 6-0 kg and three of about 2*0 kg each) are provided so that when used in deep water the sampler can be loaded to suit the consistency of the deposit and the depth of sample required.
Sample tubes are normally of perspex 53 mm bore, 50 cm long.
Sample tubes only, at the time of writing this paper, can be obtained from Griffin Biological Laboratories Ltd, 113 Lavender Hill, Ton- bridge, Kent.
The Jenkin surface-mud sampler takes a core of mud ranging in depth from 10-30 cm, a short core sampler designed by Mackereth (1969) takes a mud core 1 m in length and a pneumatic corer (Mackereth, 1958) takes mud cores 6 m in length.
3 Monitoring the environment
The determination of temperature and dissolved oxygen in stratified lakes, at the time of sampling for microbiological analyses, is carried out by means of a Mackereth oxygen electrode (Mackereth, 1964).
This instrument (obtainable from The Lakes Instrument Co. Ltd, Oakland, Windermere, Cumbria) simultaneously records temperature and dissolved oxygen.
Mention has already been made of the specialized ecological niches that can occur in stratified lakes and the oxygen deficit regime that can exist in the hypolimnetic zones of enriched shallow lakes. The position and extent of these particular ecological niches can be determined at the sampling site before taking samples for microbiological analysis.
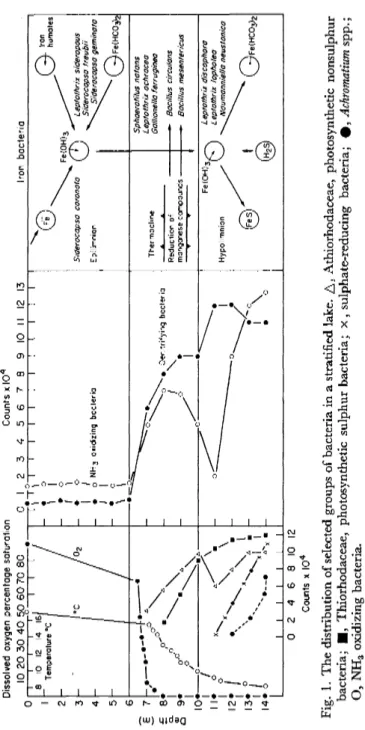
Long-term routine monitoring for temperature and dissolved oxygen provides a background of environmental data that enables the micro- biologist to study a stratified lake in sufficient detail to determine the predominant groups of organisms present in the different zones of the lake. An example of this type of study is shown in Fig. 1 for a eutrophic lake with a depth of 16 m; the results, for a few selected groups of organisms, were obtained using the media and methods described by Collins (1969). This example also shows the depth of the hypolimnetic oxygen deficit zone, starting below the thermocline and extending to the profundal water immediately overlying the sediments.
4 Methods for handling samples
Regardless of the type of sediment sampler used, it is essential to process the samples in the laboratory as soon as possible. This is because of the rapid changes that can take place at the sediment-water inter- face, particularly in the case of mud-core samples contained in narrow tubes. The increased surface area, offered by the walls of the core tube, encourages the rapid multiplication of the bacterial populations of the overlying water on storage. If oxygen uptake measurements of the surface mud are to be made, the results will be affected by the respira- tion rate of the developing bacterial "storage" population. If the mud- core samples are required only for microbiological analysis it is an acceptable procedure to store the samples overnight at a temperature range of between 5 °C and 10 °C. This procedure is not acceptable, for reasons explained above, if the purpose is to determine the numbers and
Dissolved oxygen percentage saturation 10 20 30 40 %0 60 70 80 _ 0 Counts xlO4 4 5 6 7 8 9 10 II 12 13 Iron bacteria Denitrifying bacteria
*® .
& Iron humâte Siderocapsa coronata Epilimnionrevv-ri/· Thermocline Reduction of manganese compounds -
Leptothrix sideropous Siderocapsa treubii ^Siderocapsa geminata
•G
Fe(HCO^ Sphaerotilus notons Leptothrix oc h race a Gollionella ferruginea Bacillus circulons Bacillus mesentericus HypolimnionFe (OH )3 s->v L eptothrix discophora Γ^- J Leptothrix lopholea Noumanniella neustonico t
•Q -
(HC0 0 2 4 6 8 10 12 Counts x I04 Fig. 1. The distribution of selected groups of bacteria in a stratified lake. Δ, Athiorhodaceae, photosynthetic nonsulphu bacteria; ■, Thiorhodaceae, photosynthetic sulphur bacteria; x, sulphate-reducing bacteria; φ, Achromatium spp. O, NH3 oxidizing bacteria.types of bacteria present in the overlying water of the sediment sample.
This sampling zone will be referred to as the "core water" zone of Jenkin surface-mud samples.
4.1 JENKIN SURFACE-MUD GORE SAMPLES
The general procedure for sediment samples obtained by means of the Jenkin surface-mud sampler is to transfer the tube of the sampler from its carrier to a piston-slicing device fixed to a laboratory bench. A re- vised method of fixing the sampling tubes to the apparatus has been devised by I. Haigh, a member of the FBA Workshop staff. The modi- fication and a piston-slicing device designed and made by Haigh are shown in Fig. 2. The basic design of the Jenkin sampler (Mortimer, 1971) allows a complete interchange and transfer of core tubes from the sampler to convenient carrying racks and then to a piston-slicing device in the laboratory.
For bacteriological examination procedures the core water of the sample is carefully siphoned off the mud, using a sterilized siphon tube, into a sterile container. The operation of the piston device is such that the mud core is pushed up the tube 1 cm at a time. As each 1-cm slice of mud is ejected from the tube, a sterile slicer is used to transfer the slices into sterile plastic petri dishes. This is the standard procedure adopted when the purpose of the experiment is to determine the depth distribution patterns of the bacterial populations in the mud.
The core tubes of the Jenkin sampler are manufactured from perspex and this material allows for a series of adaptations to be made to the basic core tubes so that they can be used for a variety of experimental purposes connected with sediment microbiology.
A more discrete method of sampling can be carried out using hypo- dermic syringes with a range of different needle sizes. Micro samples of mud can be obtained from layers of 1 cm or less in depth by the in- sertion of the syringe needles through holes of the appropriate diameter drilled in the walls of the tubes at the depth intervals required. Before being used to take a sediment sample the holes in the core tube are sealed with pliable polythene sealing tape. After the sediment cores have been taken, the procedure is to sample the mud by inserting the syringe needle through the sealing tape ; the sample is then withdrawn into the syringe barrel. The samples of mud obtained in this way can then be processed directly for bacteriological examination, thus élimina-
ting the method involving storage in petri dishes previously described It should be noted that the syringe sampling method is feasible only when the mud is of a soft oozy consistency. When the sediment samples are in a more consolidated state and discrete samples are required from 1-cm depth intervals, a No. 4 cork borer, inserted through holes of the appropriate diameter, makes an excellent sampling device. The micro-mud cores obtained by this method can then be pushed out of the barrel of the cork borer with a close-fitting plunger. Aseptic condi- tions can be maintained throughout this cork borer sampling method by using the technique of alcohol flaming between sampling the different layers of mud.
The method of transferring 1-cm slices of mud from the sediment cores enables all the mud in the layer to be processed, whereas the syringe sampling method removes a sub-sample from each sliced layer in the mud-core profile. There is less disturbance of the mud due to handling procedures when the syringe or cork borer methods are used to remove samples of mud from the core tubes.
An alternative method of sampling all of the mud within each 1-cm layer is by cutting a series of slots at 1-cm depth intervals down one side of the core tubes and inserting a series of close-fitting steel plates or slicers into the slots. As in the methods described earlier, the slots cut in the sides of the core tubes are sealed with polythene tape, before the sediment core samples are obtained. Samples are taken from the mud cores by pushing the sharpened edge of each steel plate through the sealing tape. The forward edge of the steel plate is cut to fit exactly against the inside wall of the core tube. This ensures the complete isolation of each layer within the mud-core profile. The mud from each layer is then scooped out with a long-handled scoop. The forward edge of the scoop is shaped to fit snugly against the internal wall of the core tube and the bottom of the scoop is flattened to ensure complete re- moval of the layer of mud from the surface of the steel plates used to isolate each layer. This sampling method has been successfully used and is illustrated by Goulder (1971) for studies on the protozoan populations of sediments.
4 . 2 DEEP SEDIMENT GORES
For studies on the vertical distribution of bacteria in mud cores ex- ceeding 30 cm in depth the following standard procedure has been
from the sampler tube, (c) Control mechanism for extruding m u d cores at 1-cm depth intervals. (Photographs by A. E. Ramsbottom.)
adopted. The outside surface of the mud core is scraped off, using asep- tic precautions and a sample of mud from the central area of the core is removed aseptically using a spatula. The samples of mud are then placed in sterile screw-capped wide-mouth polystyrene containers of 100 ml capacity for subsequent handling procedures.
This sampling method has been generally adopted for vertical dis- tribution studies on the microbial populations of mud in an attempt to reduce the effect of the "carry d o w n " of surface mud material by the coring devices. It is of interest to note at this stage in the "description of procedures" section of the text that the majority of results, obtained on the vertical distribution of bacteria in sediments, demonstrate a general pattern of decreasing numbers of bacteria with increasing depth. This type of distribution pattern could be considered to be a reflection of the
"carry d o w n " effect of surface material by the coring apparatus from present-day deposits to depths within the sediments and to sediment material laid down some 15000 years ago. This applies to most of the long cores (i.e. 6 m) taken from the sediments of lakes in the English Lake District with the Mackereth pneumatic sampler, the bottom layers of which contain glacial clay laid down during the last ice-age.
A field sampling procedure, designed to overcome the surface carry- down effect of the coring apparatus, involves the enclosure of the end of the corer by means of two large polythene bags each containing industrial alcohol. The corer descends into the sediments and when it reaches a depth below that of the organic sediment zone in the mud, the polythene bags are burst and a core of mud enters the sampler, without the end of the corer tube having been in contact with surface- mud material.
4 . 3 SEDIMENT SAMPLES OBTAINED BY DIVING TECHNIQUES
The statement made by Sorokin and Jannasch (1972) that "there is no completely sterile sediment sampler" certainly applies to the major- ity of coring devices when it is desired to obtain cores from depths of mud greater than 30 cm in the sediments. The development of diving techniques now makes it possible to obtain cores of less than 30 cm in length from the surface sediments, using sterile core tubes that can be hand operated by the diver from above the sediments. Material from other depths within the lake system will therefore be carried down into the hypolimnetic zone, due to disturbance created by the
movement of the diver proceeding from the surface waters to the profundal zone. However, the degree of disturbance and "carry d o w n "
effect will be of the same order, if not less, than that created by coring devices in their progress through the depths of the lake being sampled.
During bacteriological studies on the central basin of Lake Erie, Menon et al. (1971) reported that sediment samples were taken by divers "using sterile rubber bulbs". The availability of divers and their expertise opens up the development of novel methods of sampling and studying sediments for incorporation into experimental design in the future.
It is appropriate here to attempt to summarize the situation with regard to sterile versus nonsterile sediment samplers. The situation is neatly summarized by a quotation from Zobell (1946):
While a sampler which reaches the bottom in a sterile condition may be desirable for the collection of mud samples, for bacteriological analysis, such a sampler is not prerequisite. There is little likelihood that either quantitative or qualitative results will be influenced by contaminating organisms from the overlying water, because whereas samples have been carefully examined, hundreds to thousands of times as many bacteria have been found per unit volume of bottom deposits as in the over- lying water, and any bacterial species found in the water may be carried to the bottom by the process of sedimentation or otherwise. By taking certain precautions to minimize the possibilities of contamination, samples of bottom deposits, which are satisfactory for bacteriological analysis, can be collected with coring tubes which of necessity go down open.
4 . 4 P R O C E D U R E S F O R MEASURING T H E R E D O X P O T E N T I A L ( E L E C T R O D E P O T E N T I A L ) OF SEDIMENT SAMPLES T A K E N W I T H T H E J E N K I N SURFACE-
MUD SAMPLER
The method used for measuring the redox potential of the sediment- water interface of core samples was that described by Mortimer (1971).
This method, shown in Fig. 3, was also used for the determination of the electrode potential at different depths in the core profiles. However, the disturbance of the mud created by the insertion of the " b u n c h "
of electrodes into the mud cores caused a considerable delay in ob- taining "steady" electrode potential readings at any given depth in the sediment sample. Despite the effect of the "disturbance factor", this electrode system, so far, has provided the only reliable method of establishing the "zero oxygen level" (Mortimer, 1971) at the sediment- water interface of Jenkin surface-mud cores. It is important in relation to microbiological studies on sediments that the position of the zero
Fig. 3. Arrangement for measuring the distribution of electrode potential across the sediment-water interface, redrawn from Mortimer (1941, 1942). a, Sampling tube;
b, platinum electrode array; c, calomel electrode; d, leads to potentiometer; e, KG1- agar bridge. (After Mortimer, 1971.)
oxygen level should be determined when the enumeration or culture of selected groups of organisms is being attempted.
Another method used to determine the within-depth electrode potential of sediment samples, taken with the Jenkin surface-mud sampler, involved the use of microelectrodes made out of syringe needles. The microelectrodes were inserted into holes, drilled at 1-cm intervals, in one side of the perspex wall of the sampling tube, as pre- viously described. The microelectrodes were constructed within the syringe needles using a platinum-to-copper wire connection with a mercury contact and sealed into the syringe needle with fused ground glass. The platinum tip of the needle was ground down to a fine point, thus completing an intact microprobe electrode. This system gave electrode potential readings with less initial drift at the various depths in the sediment samples.
4 . 5 PROCEDURES FOR MEASURING OXYGEN UPTAKE IN SITU IN JENKIN SURFACE-MUD GORE SAMPLES
Another facet of the versatility of the use of core samples taken with the Jenkin surface-mud sampler is described and shown in Fig. 4. This is a perfuser technique designed in collaboration with the late F. J. H.
Mackereth of this laboratory. The core tubes can be conveniently fixed to a rack system that allows them to be handled for transport and storage. Experimental work on the sediment samples can then be carried out over a range of temperatures in cold rooms, deep freezers, incubators or water baths without the necessity for removing or dis- turbing the sediment sample from its collecting tube after sampling.
Since the principle of the system is to circulate the overlying water of the sediment cores, without introducing air into the core tubes, it is then possible by means of a syringe sampling technique to perform the Winkler test on a 30-ml volume of sample (Mackereth, 1963). The oxygen uptake value for the surface mud can then be determined. The volume of the overlying water above the mud core is kept at a constant level, i.e. the tube completely full of water, by the simple expedient of replacing the volume of abstracted sample, each time, with deoxygen- ated 022-/£m membrane filtered tap water. (The tap-water supply at Ferry House is lake water pumped from a well, situated in a bed of gravel by the lake shore.)
If it is desired to perform oxygen uptake measurements on the sedi-
Special bung in top only
Overlying water
Mud
Rubber bung or bottom lid of Jenkin surface- mud sampler as required
Rubber balloon attached to tube drawn out to give a jet of water
Rubber self-sealing cap, for use with syringe for sampling, or fine pipette on removing cap
Rubber tubing
Rubber balloon fixed to glass tube inserted into drying tube
Drying tube
Rubber tubing Connect direct to marked aperture on time switch unit
Fig. 4. Perfuser method for Jenkin surface-mud cores. The Jenkin tube must be com- pletely full of water. The two small tubes on the top bung must be filled with water.
Attach the tubing from either a vacuum pump, or water filter pump to the appro- priately marked aperture on the time switch unit. This switch unit is set to "make and break" contact approximately once every minute. The two rubber balloons will depress during this cut-out time for a period of 5 s. When the switch operates the balloons will eject water into the system, then refill as the switch makes contact again, hence the core water can be stirred without the introduction of air. (After Collins et al, 1973.)
ment core sample in a respirometer, then samples of the mud can be removed from the core tubes by any of the methods previously des- cribed, using the modified core tubes of the sampler, with either holes or slots in the side walls of the tubes.
5 Methods and media for enumerating bacteria in sediments
5.1 COUNTING PROCEDURES
The sub-samples of mud obtained by the various procedures described in the previous section of the text were processed for bacteriological analysis using the following procedures. Serial dilutions were made of 1-g portions of mud weighed out under aseptic conditions and tap water, sterilized at 121 °C for 20 min was used as the diluent. The dilution tubes were shaken by hand to disperse the bacteria and mud particles within the diluent. A known volume (1Ό ml) of a suitable dilution was then inoculated into media appropriate to the particular group of organisms being studied.
Sediment sub-samples of a known volume, taken by the syringe method of sampling, were inoculated directly into the dilution tubes.
This method had the additional advantage that it reduced the hazards due to aerial contamination during the handling procedures.
5.1.1 Heterotrophic bacteria
The organisms within the heterotrophic microbial population of sedi- ments can be conveniently placed in groups, based on the criterion of their response to different concentrations of oxygen, both under field conditions and in culture in the laboratory. Therefore the heterotrophic organisms present in sediments will be discussed as aerobes, facultative aerobes, facultative anaerobes, anaerobes and microaerophilic organ- isms.
a. Aerobes Aerobic organisms were counted by a pour plate method in which 1-0 ml of a suitable dilution of the original sub-sample of sediment was pipetted into a sterile petri dish. The agar medium was held in a molten state at 46 °C, cooled to 42 °C, added to the sample in the petri dish and then mixed by rotating six times in a clockwise direction and six times in an anticlockwise direction.
The agar and sample mixture in the petri dishes was then allowed to set and the plates were incubated for 10 days at 20 °C.
The main criticism of this method is that psychrophilic organisms in the sample will be adversely affected by a very short exposure time to a temperature of 46 °C, i.e. the gelling temperature of agar. This seems to be a valid criticism when the pour plate method is used for water samples from both marine and freshwater environments (Collins et al.9 1973). It would also seem to be a valid objection to the pour plate method when consideration is given to the range of environmental temperatures determined for the hypolimnetic waters of the lakes listed in Table 1. The temperature range of the waters immediately overlying the surface sediments, at the depths given in Table 1, lies anywhere between 4 °C and 12 °C. However, exhaustive tests of the temperature tolerance range of over 1000 pure culture isolates of aerobic bacteria from the sediment-water interface of freshwater lakes have shown that the organisms will grow at a temperature range of from 0 to 25 °C. The author has not succeeded in isolating any truly psychrophilic organisms from freshwater sediments.
With regard to aerobic organisms in sea water and marine sediments
TABLE 1
A comparison of dissolved oxygen values in different lakes
Lake Wastwater Ennerdale Water Goniston Water Haweswater
Windermere North Basin Ullswater
Buttermere Thirlmere Derwentwater
Windermere South Basin Loweswater
Bassenthwaite Lake Rydal Water Grasmere Esthwaite Water Blelham T a r n
1969
85 64
—. 71 61 61 62 49 5 39 0
— 0 2 0 0
Lowest oxygen value 1970 1971
Percentage 81
66 65
— 61 48 62 58 81 38 5 75 3 4 0 0
80 70 60
— 56 56 35 48 42 25 0 52 0 1 0 0
1972 1973 saturation
90 85 65 79 62 64 74 78 20 22 0 3 0 0 0 0
90 76 71 64 63 58 50 42 24 22 1 1 0 0 0 0
1974
87 78 68 54 58 54 65 57 67 13 0 24 0 0 0 0
Depth of recording
m 75 40 40 37 60 54 25 40 19 40 14 20 17 20 16 13
and their enumeration by the pour plate method, Zobell (1946), using the data of Zobell and Conn (1940), showed that the temperature of the molten agar at the time of pouring was important in relation to the numbers of colonies developing on pour plates. The standard technique for pour plates used in the author's laboratory is based on the work of Zobell and Conn (1940). Their data, relevant to the period of incubation and temperature of incubation of pour plates and to the
" p o u r i n g " temperature of the agar, is shown in Table 2.
TABLE 2
(a) Relative number of bacterial colonies appearing on nutrient sea-water agar after different periods of incubation at different temperatures, the average plate counts being expressed as percentages of the plate count at 18 days at 18 °G (from data of Zobell, 1946)
Incubation
time Incubation temperature 4°G
0 0 4 9 17 26 33
12 °G 18 28 46 67 90 97 98
18 °G 30 41 67 91 98 100 96
22 °G 36 60 82 96 97 95 87
25 °G 41 65 78 84 85 82 74
30 °G 44 61 69 71 70 63 53
37 °G 8 12 12 13
(b) Relative numbers of colonies developing on nutrient agar inoculated with sea water or marine mud when the agar was poured at different temperatures, the plate counts being expressed as percentages of the average plate count on media poured at 42 °G (from data of Zobell and Gonn, 1940)
Inocula Sea water Marine mud
Number of samples
14 9
42 °G 100 100
Pour temperature 45 °G 50 °G 55 °G
95-8 89-4 34-2 93-4 82-1 26-9
60 °C 17-5 11-4
The spread plate procedure is an alternative method for enumerating bacteria, and the application of the technique for counting bacteria in water is described in Collins et al. (1973). This counting method eliminates the use of molten agar, held at a temperature of 46 °C, by the simple expedient of using pre-poured and dried agar plates. A known volume of sample or diluted sample is spread on the surface
of an agar plate using a sterile glass spreader. The plates are then incubated at a standard temperature for a given time period.
The use of the spread plate method for enumerating bacteria from sediment samples has severe limitations and has not been adopted as a standard procedure. The main objection to the method is concerned with the fact that "spreading" bacterial colonies overgrow the surface of the agar plates and cause aberrant counts of the other colonies that have developed on the plates. These "spreading" colonies have occurred in all sediment samples examined.
b. Facultative aerobes By the author's definition, this group of hetero- trophic organisms prefer aerobic conditions for growth but can tolerate environmental situations where there is a slight reduction in oxygen concentration. They occur in large numbers at the sediment-water interface at the onset of stratification in lakes when the overlying water above the sediments has an oxygen saturation value from 50 per cent to 60 per cent.
These organisms are counted by the M P N method using 1-ml aliquots from serial dilutions of the sediment sub-samples.
c. Facultative anaerobes The organisms within this heterotrophic group are considered to be those organisms that are capable of survival and growth under anaerobic environmental conditions, but they demon- strate a preference for an environment containing dissolved oxygen at a concentration ranging from 0 per cent to 20 per cent saturation. These organisms occur at the sediment-water interface in lakes, when the period of stratification has become well established.
Colony counts of this group of organisms are obtained by the pour plate method with an overlay of plain agar. The layer of uninoculated agar permits the diffusion of some oxygen into the petri dish and the oxygen tension is "poised" by the growth and oxygen uptake of the developing colonies in the inoculated layer of agar underneath.
d. Anaerobes Anaerobic heterotrophic organisms can be counted by the pour plate method and the plates incubated in anaerobic jars (Willis, 1969), or by the Hungate roll-tube technique (Hungate, 1969).
The roll-tube method has been adopted as the standard method for enumerating strictly anaerobic organisms from sediment samples from freshwater lakes.
e. Microaerophilic organisms The heterotrophic organisms within this group are unable to grow under strictly anaerobic conditions ; they prefer an environment containing " m i c r o " amounts of oxygen. These organ- isms are the predominant types present at the sediment-water interface when the dissolved oxygen concentration of the overlying water reaches a saturation value of 1-5 per cent.
These organisms are counted by the M P N method using 1-ml ali- quots from serial dilutions of the sediment sub-samples inoculated by stabbing into tubes of semi-solid agar medium, with the agar concen- tration reduced to 0-3 per cent. Since the agar medium is in a semi- solid state, the inoculum can be dispersed in the tubes by mixing on a Rotamixer. This method of mixing the contents of the tubes has the added advantage that oxygen is introduced into the agar by the action of the mixing process.
The semi-solid state of the agar entraps bubbles of oxygen throughout the depth of the stab. This enables the organisms to grow at all depths within the agar stab. As the oxygen becomes depleted in the agar at the bottom of the stab, the microaerophilic bacteria migrate upwards to form a discrete band of growth at a level in the agar where the oxy- gen concentration will support their growth. The organisms do not grow at the surface of the agar stab. This zone of the agar is usually colonized by a thin layer of growth composed of aerobic organisms.
5.1.2 Other heterotrophic organisms
Other microorganisms found in sediments include yeasts, fungi, algae and protozoans. Methods for the isolation and enumeration of some groups of yeasts from the aquatic environment are given by Aaronson (1970); he also describes some methods for the isolation and enumera- tion of fungi and algae from both marine and freshwater environments.
The work of Zobell et al. (1943) demonstrated the presence of certain species of actinomycetes in marine sediment samples and the studies of Johnston (1972) include detailed descriptions of methods used to
isolate and enumerate species of actinomycetes in the sediments of freshwater lakes. This author also gives a most useful cross-reference review of published work on actinomycetes in marine and freshwater habitats. A brief cross-reference source for yeasts and actinomycetes is given in Collins et al. (1973).
Methods for the enumeration of some protozoan species are de- scribed by Goulder (1971). This author used one of the previously described modifications of the Jenkin surface-mud sampler tubes to study the vertical distribution of protozoans in sediment core samples.
Some general isolation procedures for other groups of protozoans are described by Aaronson (1970). Webb (1961) describes studies on the distribution of benthic protozoa in the sediments of a stratified eu trophic lake and useful résumés of the role of protozoans in the aquatic environ- ment are given by Bick (1973), Corliss (1973) and Legner (1973).
5.1.3 Autotrophic bacteria
The organisms within this major group can be considered as autotrophs and chemoautotrophs in relation to their participation in the carbon cycle, the nitrogen cycle, the sulphur cycle and their chemical activities in relation to the iron and manganese cycle in sediments and at the sediment-water interface of freshwater lakes. Some of the members of this group are present in large numbers, within the hypolimnetic zones of stratified eu trophic lakes. The oxygen concentration ranges from 0 per cent to 3 per cent saturation within a water column extending in some lakes from 6 m to 16 m. The distribution of some selected groups of chemoautotrophic bacteria in a stratified eutrophic lake is shown in Fig. 1 (p. 230) in relation to oxygen concentration with depth.
The isolation, cultivation and methods for the enumeration of twelve of the main groups of organisms has already been described in detail in Collins (1969) and I do not propose to repeat all of the pro- cedures in this text.
Most of the studies, reported by Collins (1969), on autotrophic and chemoautotrophic organisms were carried out on Jenkin surface-mud cores. These core samples covered a depth range of 20-30 cm in the sediments. The vertical distribution of these organisms in deep sedi- ment cores has not been studied. The main emphasis, in relation to the chemical activities of autotrophic and chemoautotrophic organ- isms, has been concentrated on studies involved with the surface sediments, the sediment-water interface and the hypolimnetic waters of lakes.
Enrichment techniques. One of the most useful methods for obtaining crude enrichment cultures of chemoautotrophic, autotrophic and photoautotrophic bacteria from marine and freshwater sediments can
be achieved by creating a " m o d e l " system in the laboratory, by using the Winogradsky cylinder or column (Winogradsky, 1887; Larsen, 1952; Collins, 1963; Collins, 1969, Aaronson, 1970). A novel modifica- tion of the Winogradsky column system was devised by Wynn-Williams and Rhodes for the study of photoautotrophic bacteria from marine sediments and is described in Collins et al. (1973).
The sediment cores, obtained by means of the Jenkin surface-mud sampler, make ideal Winogradsky columns. The sediment sample as collected can be enriched in situ in the core tube by the addition of various chemical compounds and then submitted to a variety of incu- bation conditions. The different modifications of the core tubes, as previously described, enables either intermittent or continuous en- richment to be carried out during the period of incubation.
For the purpose of studying the vertical distribution of selective groups of chemoautotrophic bacteria with depth in Jenkin surface- mud cores, the sub-samples of mud can be taken from the various layers in the sediment cores by any of the previously described methods.
The sub-samples can then be inoculated into suitable liquid media developed for the growth of these organisms derived from either marine or freshwater sediments.
The Winogradsky column can be considered as one of the main methodological links between freshwater and marine sediment micro- biology. The method offers the microbiologist the opportunity to study a small representative model of a cross-section of the natural environment under a great variety of controlled conditions in the laboratory.
5.1.4 Alternative counting procedures
a. Direct counts These were done on sediment sub-samples using the method of Jones and Mollinson (1948) for direct counts of soil micro- organisms. Considerable difficulty is encountered with all " d i r e c t "
counting techniques when they are applied to sediment samples be- cause of the masking effect of the particulate matter in the sediment sample. Microscopic examination reveals aggregates of bacterial cells attached to particulate matter and the observer is faced with the dilemma of whether to count an aggregate of cells as one unit, or to attempt to differentiate and count single cells as one unit. This particular direct counting method does not provide an estimate of the proportion of
dead or " l i v e " cells present in the sample at the time of performing the counting procedures.
The development of fluorescence microscopy has greatly advanced the usefulness of direct counting procedures, both in soil microbiology (Trolldenier, 1973) and in aquatic microbiology (Jones, 1974). The use of fluorescence microscopy has now made it possible to estimate, by direct microscopic counts, the proportion of live (fluorescing cells) and dead (nonfluorescing cells) in samples from the natural environ- ment of the aquatic ecosystem.
b. Counts on membrane filters The methods for obtaining viable counts of bacteria on membrane filters, from both marine and freshwater en- vironments, are described in Collins et aL (1973). The use of membrane filters in sediment microbiology is governed by the selection of the most suitable dilution of the sediment sample, the selectivity of the medium used and the conditions of incubation. The development of "spreading"
colonies under aerobic incubation produces aberrant counts on mem- brane filters and the same effect has been mentioned previously with reference to the use of the spread plate method of counting.
The membrane filter method of counting bacteria has its greatest use in sediment microbiology when it is applied selectively for the pur- pose of enumerating some groups of heterotrophic anaerobic bacteria.
The combined effect of anaerobic conditions of incubation and the selectivity of the medium used tends to inhibit the development of
"spreading" colonies.
5.2 MEDIA
A quotation from Collins et aL (1973) provides an appropriate preface to this section of the text :
T h e choice of medium to be used depends on the nature of the sample and information required from the sample. T h e medium may be a general one which will support the growth of the aerobic heterotrophic bacteria present in either freshwater or marine environments, or it may be selective in that it will enhance the growth of or demonstrate the presence of certain types of organisms, e.g. heterotrophic anaerobes, autotrophic types, fluorescent types, chitinoclastic bacteria, etc. T h e mineral requirements of marine bacteria are reviewed by MacLeod ( 1965) and media were discussed at the Interdisciplinary Conference (Marine Biology, 1968).
5.2.1 Heterotrophic bacteria
a. Aerobes, b. Facultative aerobes, c. Facultative anaerobes and e. Micro- aerophilic organisms The heterotrophic organisms within these four groups were enumerated by the pour plate method, the spread plate method (limited studies), and by the use of semi-solid agar in tubes.
The basic medium used for all of these methods and groups was the standard plate count medium developed for the study of fresh- water bacteria, and designated as Collins casein-peptone-starch (CPS medium (Collins and Willoughby, 1962).
The basic CPS medium can be modified for the purpose of estimating viable heterotrophic bacteria from surface sediments, capable of pro- ducing exo-enzymes. The use of this modified CPS medium, for estimating exo-enzymes producing bacteria in freshwater, has been described by Jones (1971) and Collins et al. (1973).
Pure cultures of the heterotrophic organisms from sediment samples from freshwater lakes can be successfully maintained on Collins' ENA medium (Collins, 1963). The long-term maintenance of pure cultures can be achieved by the use of stab cultures in both CPS and ENA medium. The agar concentration of the medium should be reduced to 0-3 per cent w/v and the medium should be dispensed into screw- capped glass bottles of 7-ml capacity.
Several useful media for marine heterotrophic bacteria are described in Collins et al. (1973) and by Aaronson (1970); most of the media described in these publications were used to enumerate and culture heterotrophic bacteria in both sea water and marine sediment samples.
It is of interest in this context to consider a statement made by Zobell (1946):
It has been our experience that the colonies developing on plates inoculated with marine m u d differ grossly from those inoculated with sea water. While m a n y of the bacteria are common to both habitats, some mud-dwelling bacteria have never been observed in water, and others isolated from water have not been found in m u d . Unfortunately there are not enough data on this point to be statistically significant.
I n general, the colonies developing from marine m u d are smaller, grow more slowly, and are less likely to be pigmented than those developing from sea water.
d. Anaerobes Heterotrophic anaerobic bacteria from the sediments of freshwater lakes were enumerated and isolated using the methods and media as described by Willis (1969) and Hungate (1969).
For the isolation of heterotrophic anaerobes from marine sediments,
the methods quoted from Collins et al. (1973) can be used: " . . . a portion of sample (c. 2 g) was added to Robertson's cooked meat broth and incubated at various temperatures and then plated out on various media recommended for clostridia (Davies, 1969) and incubated anaerobically at the appropriate temperature. Liston et al. (1969) used similar methods."
5.2.2 Autotrophic bacteria
A description of a wide variety of media used for the isolation and enumeration of autotrophic, facultatively autotrophic and chemo- autotrophic bacteria is given by Collins (1969), Aaronson (1970) and Collins et al. (1973). The studies reported by these authors include both the marine and freshwater environment.
Detailed studies on two selective groups of chemoautotrophic bacteria found in freshwater sediments were made by Cappenberg (1974a, b). The two groups of organisms studied by Cappenberg were the sulphate-reducing and methane-producing bacteria. He describes in full the methods and medium used to enumerate the meth- anogenic bacteria and the counting methods for sulphate-reducing bacteria are described by Cappenberg (1972).
For the purpose of illustrating the use of Jenkin surface-mud core samples for studying the vertical distribution of chemoautotrophic bacteria in surface sediments, two groups of organisms have been selected. These are the sulphate-reducing bacteria and the thiosulphate oxidizing bacteria. The sulphate-reducing bacteria were counted using Starkey's (1938) medium and the thiosulphate oxidizers using Starkey's (1934) medium.
6 Distribution of bacterial populations
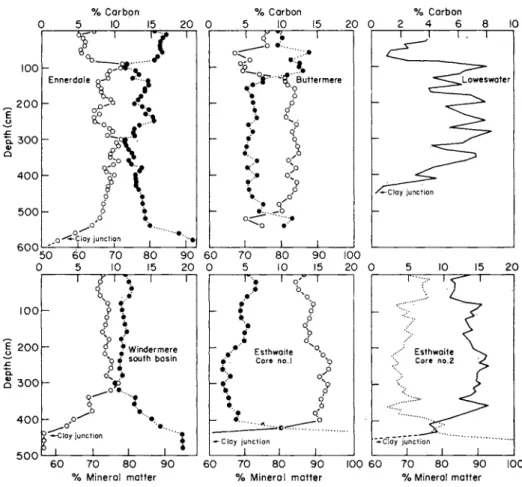
This section of the text will be mainly concerned with the presentation of a few selected examples of the distribution patterns and numbers of bacteria found in the sediments and the sediment-water interface of freshwater lakes.
6.1 THE DISTRIBUTION OF HETEROTROPHIG BACTERIA IN A STRATIFIED LAKE
The results of long-term ecological studies on the bacteriology of strati- fied freshwater lakes have clearly demonstrated that the highest numbers of bacteria occur in the surface muds of sediments. To illus- trate this point, data are presented in Table 3. These data were ob- tained by means of depth contour sampling on a transect line from the shore of a shallow eutrophic lake out to the area of maximum depth of the lake. Each of the depth profiles was sampled at 1-m depth inter- vals; the water samples were taken with a Friedinger water bottle of 1-litre capacity and the mud samples were taken with a Jenkin surface-mud core sampler. The bacterial counts were made on CPS medium under aerobic conditions of incubation, using the pour plate method, and incubated for 10 days at 20 °C. The temperature and dissolved oxygen concentration were measured in situ at the time of sampling with a Mackereth oxygen electrode.
The general trend of the distribution pattern of the bacterial popu- lation in the water is one of decreasing numbers of bacteria with in- creasing distance from the shore of the lake out to the open water. The same trend is shown by the results obtained from the sediment-water interface samples designated in this instance as the "core water".
However, the results for the surface sediments, under the various depth contours sampled, show a different general trend, that is one of in- creasing numbers of bacteria with increasing distance from the shore of the lake. It is of interest to note that the temperature and dissolved oxygen results indicated that the thermocline zone of the lake, on this occasion, was positioned around the 6-m depth contour. Below the thermocline zone, in the hypolimnion of the lake, there is another general trend in the distribution pattern of the bacterial population.
This population trend is one of decreasing numbers of bacteria correlat- ing with a decrease in the dissolved oxygen concentration. This particu- lar trend has been discussed previously by Collins (1970) and is thought to represent the ability of certain components of the heterotrophic bacterial population to survive under conditions of low concentrations of dissolved oxygen and able to demonstrate their viability, by colony growth under aerobic conditions of incubation. The inference is that these organisms either prefer conditions of low oxygen tension or that aerobic plate counts simply record the low numbers of strict
TABLE 3 Distribution of heterotrophic bacteria in a stratified lake Counts in water column in millions per 100 ml, in sediment in millions per g wet mud. Dissolved oxygen (in mg per litre) in parentheses Sampling profile depth m 0 1 2 3 4 5 6 7 8 9 10 11 12
2 28 (8-8
) 0-8 (8-9)
4 24 (8-7)
32 (8-3
) 19 (7-9)
36 (8-3
)
Contou
r m
6 13 (8-7
)
17 (8-8
) 15 (8-8) 19 (8-6)
22 (7-7
)
24 (5-8
)
depth 8 13 (8-7) 16 (8-5) 13 (8-2)
13 (7-8
)
19 (7-8
)
14 (5-9
) 18 (1-3)
14 (0-49
)
10 0-9 (8-3) 0-5 (8-6) 13 (8-4) 0-5 (8-3) 20 (8-3)
17 (7-1
) 0-8 (1-6) 0-6 (0-38) 0-3 (0-29) 0-4 (0-27)
12 0-5 (8-6) 0-6 (8'6) 0-9 (8-6) 0-8 (8'7) 0-8 (8-1) 0-5 (54) 0-5 (1-7) 0-7 (0-50) 0-3 (0-26) 0-2 (0-22) 0-1 (0-18) 0-9 (0-14) 0-3 (0-14)
Water and Surface Temperature sediment-water sedimen (°C) interface (counts x 10 16-6 — — 16-5 — — 16-3 31 0-328 16-0 15-7 14-7 13-0 11-4 10-18 9-75 9-55 9-48
21 0-796 14 2-00 0-4 1-82 0-3 2-21 0-3 2-5
aerobes capable of surviving in an environment with an oxygen con- centration range from 0*14 to 1-7 mg 02 per litre. The numerical range for bacteria per ml in this instance ranged from 1000 to 17000. Whereas aerobic plate counts from surface sediment samples, within this same dissolved oxygen regime, yielded bacterial numbers in the range 1-8
x 106 to 2-4 x 106 g_ 1 wet mud. Above and in the thermocline zone, the dissolved oxygen concentration ranged from 8-9 to 5-4 mg 02 per litre and the aerobic bacterial count range was 5000-36000 m l- 1.
The results discussed above tend to indicate that there is an associa- tion between bacteria and particulate matter and the availability of readily oxidizable organic matter for bacterial utilization (Waksman and Vartiovaara, 1938). At the same time the results also indicate, in this particular lake system, that the hypolimnetic waters represent a zone of low activity for heterotrophic aerobic bacteria, the zone extending in this case from a depth of 6 m on the 8-10 and 12-m depth contours to the sediment-water interface.
The dissolved oxygen data indicated that a considerable biochemical oxygen demand was evident in the hypolimnetic waters, against a trend of decreasing numbers of aerobic bacteria. Part of this oxygen demand can be accounted for by littoral surface-mud material, in an oxidized state, being transported to profundal regions reduced in oxy- gen concentration. This effect represents a chemical oxygen demand rather than a microbiological oxygen demand. However, it is accepted by most workers in this field that oxygen uptake or oxygen removal from sediments can be considered as being due to a combination of factors and microbial respiration is recognized as one of these factors
(Schroepfer, 1931; Wisely and Klassen, 1938).
The distribution pattern, in a stratified lake, of heterotrophic aerobic organisms is represented by the results presented in Table 3 ; and in Table 4 the counts for facultative anaerobes and anaerobes are presented. These results were obtained by using the methods des- cribed in section 5 and the counts were done on a selection of the same water sediment-interface (core waters) and sediment samples of the surface mud as shown in Table 3. The selected samples used were those taken from the hypolimnetic zone of the lake, where the dissolved oxygen concentration ranged from 0-14 to 1-7 mg 02 per litre. I n relation to depth within the lake, this represented a zone extending from a depth of 6 m on the 8-, 10- and 12-m depth contours.
The results shown in Table 4 clearly indicate that the numbers of
TABLE 4 Heterotrophic bacteria in a stratified lake, facultative anaerobes and anaerobes, hypolimnetic zone only. Counts in water column per ml, in sediment per g wet mud Water at Profile Depth contour sediment-water Surface sedimen sampling 8m 10m 12m interface depth Facultative Facultative Facultative Facultative Facultative m anaerobes Anaerobes anaerobes Anaerobes anaerobes Anaerobes anaerobes Anaerobes anaerobes Anaerobe 6 15xl04 3xl04 16xl04 0-2 xlO4 23xl04 0-1 x 104 — — — — (1-3) (1-6) (1-7) 7 12xl04 2-5 xlO4 19xl04 0-35 xlO4 50xl04 0-15 xlO4 — — — — (049) (0-38) (0-5) 8 22xl04 0-15 xlO4 55xl04 0-2 x 104 16xl04 0-1 x 104 14x 106 2xl0 (0-29) (0-26) 9 31xl04 0-2 xlO4 41 x 104 0-1 x 104 _____ (0-27) (0-22) 10 65xl04 0-1 xlO4 60xl04 1 x 104 1·9χ106 6χ10 (0-18) 11 82xl04 0-45 xlO4 _ _ _ _ (0-14) 12 250 xlO4 3xl04 2xl06 8-5 xlO (0-14)