CHAPTER T W O
The Essential Nutrient Elements:
Requirements and Interactions in Plants
E . J . H E W I T T
I. Introduction 137 II. Morphological, Anatomical, Chemical, and Physiological Effects of Min-
eral Disorders and Nutrient Interactions 139
A. Macronutrient Elements 140
1. Nitrogen 140 2. Phosphorus 143 3. Sulfur 149 4. Calcium 155 5. Magnesium 172 6. Potassium 176 7. Interrelationships among Nitrogen, Phosphorus, Potassium, Calcium,
and Sodium in Water Content, Dry Matter, Growth, Assimilation
Rates, and Respiration 183 B. Micronutrient or Trace Elements 192
1. Iron 192 2. Relationships between Iron and Other Elements 198
3. Manganese 212 4. Copper 222 5. Zinc . . 229
6. Boron 240 7. Molybdenum 270 8. Chlorine 292 C. Effects of Nutrient Status on Free Amino Acids and Amides . . . . 299
D. Beneficial and Other Elements 318
1. Alkali Metals 318 2. Strontium 322 3. Cobalt 322 4. Selenium 326 5. Aluminum 326 6. Silicon 328 7. Other Elements 329
References 329
I. Introduction
Investigations t h a t lead to t h e recognition of t h e essential m a c r o - a n d m i c r o n u t r i e n t m i n e r a l elements for h i g h e r p l a n t s h a v e been de-
137
scribed in Chapter 1, in w h i c h t h e criteria of their essentiality a n d problems i n t h e determination of essential n u t r i e n t status w e r e dis- cussed. T h e chemical functions of m i n e r a l elements in e n z y m e systems a r e also described i n Chapter 4 b y N a s o n a n d M c E l r o y , a n d the n u t r i - tion of microorganisms is dealt w i t h b y Nicholas, Chapter 3.
T h e action of the essential elements in the growth processes of higher plants has sometimes been revealed b y a study of the visible or an- atomical effects t h a t result from m i n e r a l deficiencies or b y the effects of controlled levels of these elements on t h e chemical composition of the plants affected. Often, however, t h e characteristic symptoms or chemi- cal changes associated w i t h m i n e r a l deficiency or excess in one plant m a y differ, a p p a r e n t l y fundamentally, from those observed in another.
It is necessary to recognize, therefore, t h a t direct manifestations of the specific functions of m i n e r a l elements in different plants m a y be masked or complicated b y differences in t h e indirect or secondary effects of a b n o r m a l m i n e r a l concentrations on metabolism as a whole. This is understandable. Quantitative a n d qualitative differences in the activity a n d distribution of sequentially dependent e n z y m e systems—and pos- sibly quantitative differences in t h e subsequent or prior metabolism of cell constituents t h a t m a y either accumulate or be depleted as a result of such differences in e n z y m e activity, such as occurs w i t h the a m i n o acids (Section II, C ) — w o u l d exert different effects on growth. T h e integrated effects of such variations in metabolic activity m i g h t provide v e r y n u m e r o u s variations in the over-all behavior, w h i c h is revealed in visible, anatomical, or chemical characteristics. T h e s e over-all effects are nevertheless individually recognizable, a n d it m a y be concluded t h a t although distinct in their characteristics t h e y a r e t h e outcome of reproducible a n d p r e s u m a b l y specific metabolic states. T h u s the slight or severe deficiency of a given element might, t h r o u g h a defection in the same basic function, produce a p p a r e n t l y quite different chemical or anatomical responses in different plants w h i c h h a v e different quantita- tive r e q u i r e m e n t s for t h a t element, different quantitative activities in sequential e n z y m e systems, a n d different qualitative capacities to metabolize the intermediate products of t h e n o r m a l or deficient systems (e.g., t h e factors responsible for putrescine formation in relation to potassium deficiency, w h i c h are described l a t e r ) .
Although this view m a y explain t h e diverse characteristics associated w i t h a m i n e r a l deficiency or excess in different plants, it also e m p h a - sizes t h a t caution is needed in d r a w i n g conclusions in regard to func- tion from a specific set of chemical or anatomical symptoms observed in a single plant, even w h e n these symptoms appear to be readily interpreted.
2. E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 139
T h u s , a n d for this reason, confusion m a y exist r e g a r d i n g t h e a p p a r e n t roles of certain m i n e r a l nutrients. T h e diversity of t h e effects of some m i n e r a l deficiencies also justifies the need to specify the relevant p l a n t species a n d even t h e variety concerned i n the observation. T h i s is p a r t i c u l a r l y so w h e n detailed records, such as those r e q u i r e d for diag- nostic purposes, a r e concerned. T h e records of visible s y m p t o m s h a v e often provided t h e first indication of other problems, including such effects as ion antagonism, differential distribution, interaction w i t h en- vironment, metabolic interrelationships w i t h other elements, a n d chemi- cal or physiological action, as, for example, t h e role of m o l y b d e n u m in n i t r a t e assimilation.
T h e roles of m i n e r a l elements, especially t h e m i c r o n u t r i e n t s , in specific e n z y m e activities necessarily comprise t h e m a i n basis for their effects on growth, morphology, cell composition, a n d physiological activities. Discussion of such specific functions in e n z y m e systems is presented separately b y N a s o n a n d M c E l r o y (Chapter 4 ) ; t h e y a r e therefore excluded from this chapter. T h e r e are, however, m a n y aspects of n u t r i t i o n — s u c h as t h e over-all effects of m i n e r a l deficiencies a n d ex- cesses on changes i n morphology or composition, e.g., in carbohydrate or a m i n o acid concentrations, or on such activities as photosynthesis, respiration, a n d protein synthesis—which probably reflect t h e interac- tion of several factors, a n d these can be described only as physiological responses u n t i l m o r e is k n o w n r e g a r d i n g their exact derivation. Inter- relationships between different elements, between e n v i r o n m e n t a n d n u t r i e n t status, a n d between development a n d n u t r i e n t r e q u i r e m e n t also occur a n d modify the effects of deficiency, or excess, as described later.
II. Morphological, Anatomical, Chemical, and Physiological Effects of Mineral Disorders and Nutrient Interactions
T h e visible features described h e r e a r e not intended for diagnostic purposes; for such purposes t h e reader is referred to books b y W a l l a c e (553) a n d H a m b i d g e ( 1 7 7 ) , a n d to comprehensive papers b y M c M u r t r e y (337a) a n d P i p e r (407, 4 0 8 ) , w h e r e extensive references to the literature on this subject a r e also given. Effects of m i c r o n u t r i e n t s on p l a n t growth a n d function a r e also dealt w i t h b y Stiles ( 5 1 2 ) , w h o lists in comprehensive m a n n e r t h e initial records of the evidence for essential micronutrients in m a n y higher plants. This is also given in t h e historical sections of Chapter 1. I n d e p e n d e n t reviews of micro- n u t r i e n t problems h a v e also been given b y H e w i t t (198, 206, 207, 2 0 9 ) , M u l d e r ( 3 6 5 ) , Stiles (513, 5 1 3 a ) , a n d Pirson ( 4 1 1 , 4 1 2 ) , a n d of m a c r o n u t r i e n t s by Baumeister ( 2 8 a ) .
A. M A C R O N uTRiENT E L E M E N T S
1. Nitrogen
Nitrogen is included h e r e as it m a y be classed as a m i n e r a l element w i t h r e g a r d to its availability to m a n y plants a n d to t h e effects of deficiency levels on t h e visible s y m p t o m s a n d n u t r i e n t interrelation- ships to be described below. Nitrogen metabolism is excluded from this account (see V o l u m e I V ) .
a. Deficiency symptoms. A characteristic feature in m a n y plants is the decrease in angle between stem a n d leaf. Leaves become m o r e erect t h a n n o r m a l , especially in cereals, grasses, potato (Solanum tuberosum), tomato (Lycopersicon esculentum), a n d flax (Linum usitatissimum).
L a t e r a l buds often r e m a i n d o r m a n t . T h i s results in the absence of tiller- ing in cereals w h i c h m a y h a v e single shoots; m a n y plants, e.g., flax, potato, tomato, clovers (Trifolium spp.), h a v e a t h i n or spindly habit d u e to t h e reduced growth of lateral buds. T u b e r formation in potatoes is decreased in n u m b e r as well as size of the tubers.
D o r m a n c y is often prolonged a n d n o r m a l swelling a n d opening of buds is delayed, especially in m a n y fruit crops ( 5 5 0 a ) . Delayed d o r m a n c y is often accompanied b y e a r l y senescence. T h i s is seen generally in p r e m a t u r e leaf fall, a n d also in p r e m a t u r e m a t u r a t i o n of stem tissues i n flax. Both these responses would result from early differentiation of meristematic tissues—the first response as a result of differentiation of abscission layers; a n d t h e second, of x y l e m a n d p a r e n c h y m a tissues.
Nitrogen deficiency causes m a r k e d decreases in chlorophyll content a n d leaves a r e pale green. Older leaves become yellow-green a n d often completely yellow, a condition associated w i t h severe proteolysis with- out concurrent synthesis. M a n y plants, however, produce other pig- m e n t s w h e n t h e y lack nitrogen. I n Brassica plants the pigments occur first in t h e mesophyll areas of t h e u p p e r surface w h e r e a s in tomato the dark purple tint is present particularly i n petioles a n d veins. T h i s p i g m e n t appears first in veins on t h e underside of t h e leaf a n d later occurs between veins on both surfaces, i n addition to yellowing follow- ing the loss of chlorophyll. Stems a n d leaf bases of cereals a n d stems of flax a n d fruit trees produce bright red or p u r p l e tints. Apples be- come h i g h l y colored especially u n d e r conditions of high light intensity.
T h e a n t h o c y a n i n formation m a y be suppressed u n d e r greenhouse condi- tions ( 5 5 0 a ) . Slight restriction of nitrogen supply is sometimes used to produce fruit of attractive appearance.
b. Effects on growth. Bosemark (41) described effects of nitrogen
2. E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 141
supplied as n i t r a t e on t h e g r o w t h of w h e a t (Triticum aestivum) roots in a controlled e n v i r o n m e n t . Nitrogen deficiency, produced b y de- creasing the nitrogen supply to ΙΟ"4 M sodium nitrate, resulted in elongated roots w h i c h extended 137 m m i n 10 days compared w i t h those g r o w n at 10~3 to Ι Ο- 2 M concentration, w h i c h extended 105 m m . T h e r e w a s a progressive increase in cell length from 188 to 324 μ w i t h decreasing nitrogen, a condition w h i c h accounted m a i n l y for the effects on root length. Effects of nitrogen level on cell n u m b e r s , calculated as
"relative cell n u m b e r , " w e r e not consistent. I n t h e first e x p e r i m e n t there was initially a decrease followed b y a m a r k e d increase in cell n u m b e r s w i t h increasing nitrogen supply u p to t h e highest nitrogen level. I n a second experiment increasing nitrogen from 10~4 to Ι Ο- 2 M nitrate s h a r p l y decreased cell n u m b e r s . I n a third test, in w h i c h seeds w e r e removed at 4 days, there was a large decrease in cell n u m b e r s w h e n nitrate was increased from 10~4 to Ι Ο- 3 M a n d a further decrease at
Ι Ο- 2 M . Bosemark concluded t h a t high nitrogen supplies inhibited cell multiplication as well as cell elongation. T h e r e appeared to be about three times as m u c h total a n d insoluble nitrogen in t h e inhibited cells as in those of the low nitrogen treatments. Interpretation of these results is complicated, however, b y two factors. T h e basal n u t r i e n t solutions w e r e v e r y dilute b y n o r m a l standards a n d provided only 1 0- 3 M M g+ +, Ι Ο- 4 M C a+ +, 10~3 M P 04- - - , a n d no specified potassium compounds. T h e increases in sodium u p to 1 0- 2 M (i.e., at 100:1 sodium : calcium ratio, w h i c h m i g h t well h a v e been toxic to cell growth) a n d great increases in sodium:po- tassium ratios m i g h t also depress cell expansion. It would a p p e a r de- sirable to extend these observations to account for the possible effects of t h e other ions a n d to use higher levels of the other essential n u t r i e n t s . T o r e n (524a) also observed h y p e r t r o p h y of cells in excised carrot tissue w h e n deficient in nitrogen.
L u t m a n (309) observed t h a t nitrogen deficiency w a s associated w i t h small nuclei in normal-sized cells in the undifferentiated regions of J a p a n e s e millet (Setaria italica) root tips b u t caused small cells in b u c k w h e a t (Fagopyrum esculentum) root tip a n d p r e m a t u r e v a r i o l a - tion i n r a p e (Brassica napus), w h e r e this change could be interpreted as early senescence. Leaf epidermal cells w e r e notably smaller in millet a n d buckwheat, b u t not in rape. T h e outer walls a n d cuticle w e r e obviously t h i n in millet. Leaf palisade cells in potato w e r e relatively slender a n d separated b y large intercellular spaces w h e n nitrogen deficient. Chloroplasts w e r e decreased both in size a n d n u m b e r in millet, b u t in potato, higher nitrogen produced small chloroplasts w i t h a b u n d a n t starch in contrast to large chloroplasts in millet.
Njoku (386) observed t h a t high, as compared w i t h low, nitrogen
supply increased both the m e a n size a n d the total n u m b e r of leaf epidermal cells in Ipomoea caerulea. I n older leaves, w h i c h would h a v e been formed w h e n nitrogen supplies w e r e less limiting on growth, total cell n u m b e r s w e r e about 5 0 % greater a n d t h e size (as reciprocal of n u m b e r s per square millimeter) w a s about 3 0 % greater w i t h high, t h a n w i t h low, nitrogen. T h e s e differences together accounted for double t h e leaf area. I n the youngest leaf, developed after a longer period of low or high nitrogen supply, t h e high nitrogen t r e a t m e n t pro- duced two a n d a half times as m a n y cells, w h i c h w e r e 2 0 % greater in surface area, t h a n in t h e low nitrogen t r e a t m e n t ; together these differences accounted for a threefold r a n g e in leaf area. Related ob- servations of M o r t o n a n d W a t s o n (361) for effects of nitrogen on beet
{Beta vulgaris) are discussed in Section II, A, 7, b.
Cytological changes in cauliflower chloroplasts a n d leaf cells h a v e been studied b y D . P . Hucklesby a n d E. J. H e w i t t (unpublished w o r k ) .
T h e u n i f o r m chlorosis commencing in t h e oldest leaves of cauliflower {Brassica oleracea var. Botrytis) resulted from a progressive enlarge- m e n t of t h e chloroplast starch grains a n d the concomitant reduction of t h e lipoprotein structure of t h e plastid, w h i c h r e m a i n e d a r o u n d the mass of starch grains only as a t h i n skin. T h e ribonucleic acid ( R N A ) staining was similarly reduced. Breakdown followed; it was char- acterized b y t h e gathering of t h e lipoprotein portion into intensely golden yellow globules w h i c h l a y at r a n d o m on the surface of the starch grains or extended in strings along t h e starch grain interfaces.
These globules eventually separated from the starch grains, w h e r e t h e y tended to fuse, becoming progressively larger a n d fewer. Rapid lysis of starch grains followed, accompanied b y the accumulation of a n t h o c y a n i n pigments in t h e vacuoles of t h e epidermal a n d first sub- epidermal layers of cells. After the completion of starch lysis, the a n t h o c y a n i n disappeared, a n d t h e mesophyll cells contained, a p a r t from t h e cytoplasm a n d nuclei, one or two large globules formed from the chloroplast structure, a n d m a n y small colorless cytoplasmic lipid droplets.
T h e over-all effects of nitrogen deficiency are first to decrease the rate a n d extent of protein synthesis b y direct limitation of nitrogen supply. This n a t u r a l l y limits cell expansion a n d still more, cell division.
Similarly t h e lack of tillering probably reflects the decreased production of n e w meristems r a t h e r t h a n excessive apical dominance. T h e second effect of nitrogen deficiency is indirect a n d is due to t h e corresponding tendency for carbohydrates to accumulate d u r i n g the early stages of the deficiency. Chloroplasts are, therefore, initially heavily packed w i t h
2 . E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 1 4 3
starch grains. Cell sap osmotic pressures are also often high owing to t h e h i g h sugar contents.
2. Phosphorus
a. Deficiency symptoms. W a l l a c e ( 5 5 3 ) has pointed out t h a t m a n y of t h e visible effects of phosphorus deficiency resemble those w h i c h are caused b y lack of nitrogen. T h e similarities a p p l y to such effects as acute leaf angles a n d lack of tillering, as shown in flax (Fig. 1 ) , pro-
FIG. 1. Phosphorus deficiency in flax (Linum usitatissimum) ; single stems, small leaves with narrow leaf angles.
longed d o r m a n c y of lateral buds, p r e m a t u r e leaf fall, decreased size and n u m b e r of flower primordia, delay or suppression of flowering, a n d few, small fruits or seeds. Exceptions occur, however, since leaf angles in tomato become v e r y wide a n d leaves curve d o w n w a r d ( 1 9 0 ) . Leaf proportions m a y be altered a n d tobacco leaves (Nicotiana taba- cum) a r e n a r r o w e r in relation to length w h e n t h e y a r e phosphorus deficient ( 3 3 7 ) .
Foliage often lacks the n o r m a l luster a n d the leaf surfaces a p p e a r dull b y reflected light. Leaf color is often a b n o r m a l : it m a y be initially dark green or olive green as in F r e n c h beans (Phaseolus vulgaris), blue green as in tomato a n d some brassicas a n d clovers a n d cereals, or m e r e l y dull pale green as in lettuce (Lactuca sativa) a n d pea (Pisum
sativum). I n t h e oldest leaves, faded yellow-green or b r o w n colors appear. Additional pigments are often produced. These a r e u s u a l l y deep p u r p l e as in tomato, apple (Malus sylvestris), a n d m a i z e (Zea mays), or red a n d p u r p l e as in oat (Avena sativa), swede (Brassica rapa), r a p e , i n brassicas generally, a n d i n oat a n d b a r l e y (Hordeum vulgare). P i g m e n t s are sometimes practically absent as in wheat, sugar beet (Beta vulgaris), potato, celery (Apium graveolens var. dulce).
Bronzing d u e to a b r o w n coloration, w h i c h develops in v e r y small necrotic areas or groups of cells, is also common in m a n y plants, e.g., celery, hop (Humulus lupulus), F r e n c h beans. L a r g e r areas of necrosis, leading to m a r g i n a l scorch, also occur in potato a n d hop. Red clover
(Trifolium pratense) leaflets develop purple-brown tinted necrotic spots. Sugar beet shows dark b r o w n m a r g i n a l scorch a n d potato leaflets develop upcurled scorched margins. Stem bases of cereals a n d leaf petioles of m a n y plants, e.g., carrot (Daucus carota var. sativa), tomato, clovers, a r e also strongly pigmented. Leaf s y m p t o m s u s u a l l y appear first in t h e older leaves a n d progress to y o u n g e r leaves owing to the translocation of phosphorus from t h e older to the y o u n g e r parts (189, 190, 192). I n most plants t h e fading a n d tinting effects are distributed fairly evenly on t h e leaf surface between, or including, the major veins, a n d pronounced interveinal patterns m a y not appear. I n cocoa (Theo- broma cacao) (324) symptoms of chlorosis i m m e d i a t e l y adjacent to major veins, as observed in some virus diseases, m a y occur together w i t h leaf a s y m m e t r y , sometimes leading to mild "sickle leaf" effects similar to those t h a t are associated with zinc deficiency (164, 1 6 5 ) ; stipules r e m a i n green a n d attached to stems from w h i c h the leaves have fallen. Z i n c a n d phosphorus deficiencies a r e also similar in some re- spects for maize, peas, a n d some other plants (218, 221 ).
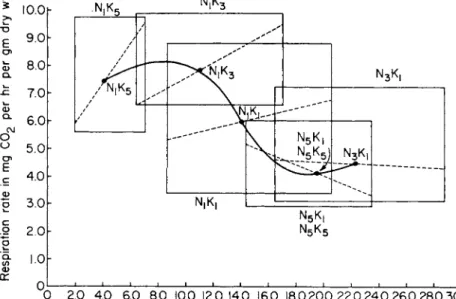
T o p : r o o t ratios m a y be decreased as in nitrogen deficiency; this was observed in s u b t e r r a n e a n clover (Trifolium subterraneum) ( 3 5 6 ) .
Phosphorus interacts with calcium, a n d a m a r k e d example of this effect was observed in s u b t e r r a n e a n clover b y Greenwood a n d Halls- w o r t h ( 1 6 3 ) . T h e phosphorus level of 40 p p m , w h i c h gives good growth w i t h m a n y plants ( 2 0 0 ) , w a s found to b e toxic at a low calcium level of 8 p p m , b u t it was beneficial, or not depressive, at a high calcium level of 64 p p m if t h e nitrogen supply was also high. W i t h low nitrogen supply, w h e n plants w e r e dependent on nitrogen fixation b y root nodules, a n increase in phosphorus level from 8 to 40 p p m caused a m a r k e d depression w i t h 64 p p m calcium (Table X I I I ) . H i g h phosphorus supply also greatly depressed nodulation a n d the total nitrogen content of t h e plants. T h i s effect m i g h t reflect a n induced calcium deficiency since the calcium r e q u i r e m e n t for nodule formation exceeds con-
2 . E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 1 4 5
siderably t h a t r e q u i r e d for t h e growth of t h e host p l a n t w h e n fixed nitrogen is provided ( 3 0 2 , 3 0 3 ) . T h e data are given in T a b l e X I I I in relation to copper r e q u i r e m e n t s .
b. Anatomical and histological effects. L y o n a n d Garcia ( 3 1 0 ) observed t h a t phosphorus deficiency i n tomato resulted in necrosis of thin- walled central pith cells of the stem a n d production of a b n o r m a l l y large intercellular spaces. T h e pericycle cells a n d x y l e m elements w e r e also t h i n walled. P h l o e m was greatly decreased in a m o u n t a n d appeared m a i n l y as undifferentiated p a r e n c h y m a . T h e chlorenchyma tissues w e r e reduced to a few layers a n d w e r e m a i n l y disintegrated.
c. Effects of phosphorus supply on composition. E a t o n ( 1 2 5 , 1 2 6 , 1 2 8 ) studied the effects of phosphorus supply on t h e carbohydrate a n d nitrogen fractions i n t h r e e plants. Phosphorus deficiency i n sunflower
(Helianthus annuus) ( T a b l e I ) caused increased concentrations of total sugars, reducing sugars, sucrose, a n d starch in y o u n g plants, b u t in old plants t h e r e w e r e either no effects or effects t h e reverse of those stated above. I n soybean {Glycine max) ( T a b l e I I ) phosphorus deficiency in- creased the concentrations of all t h e carbohydrate fractions, a n d in black m u s t a r d {Brassica nigra) t h e r e w e r e increases i n total a n d reducing sugars a n d sucrose, b u t not in starch. H a a s ( 1 7 1 ) observed, however, t h a t increasing phosphorus in lemon cuttings {Citrus limonia) caused increases i n total a n d reducing sugars. T h e latter w e r e m a x i m a l at 1 - 1 0 p p m whereas n o n r e d u c i n g sugars increased u p to 1 0 0 p p m phosphorus.
A l t h o u g h the effects of phosphorus deficiency on the carbohydrates observed b y E a t o n w e r e fairly similar for different crops, t h e y w e r e variable for t h e nitrogen fractions in t h e three plants. I n sunflower, phosphorus deficiency resulted i n decreased concentrations of soluble a n d insoluble nitrogen fractions ( T a b l e I ) . I n soybean however there w e r e increases in concentrations of total soluble, insoluble, amide, a n d a m m o n i a nitrogen fractions ( T a b l e I I I ) . Increases i n a m i n o nitrogen could therefore be expected, although this was not estimated separately T h e r e w a s a gradient of increasing a m i d e concentration in leaves from t h e apex to mid-stem w i t h low phosphorus a n d from basal to apical leaves w i t h adequate phosphorus. E a t o n suggested t h a t phosphorus deficiency resulted i n proteolysis in t h e lower leaves a n d impaired resynthesis. I n black m u s t a r d there w e r e only slight increases in amide concentrations.
Ergle a n d E a t o n ( 1 3 5 ) found t h a t the total phosphorus content of cotton {Gossypium sp.) w a s decreased, b u t t h e proportion of organic phosphorus w a s increased, b y phosphorus deficiency. Phosphorylated sugars w e r e decreased, a n d starch a n d reducing sugars w e r e greatly in-
(AS % D R Y W E I G H T ) I N SUNFLOWER (Helianthus annuus)a
70% Alcohol- 70% Alcohol- Total sugars Reducing sugars Sucrose Starch soluble Ν insoluble Ν Stem
level Minus Ρ Plus Ρ Minus Ρ Plus Ρ Minus Ρ Plus Ρ Minus Ρ Plus Ρ Minus Ρ Plus Ρ Minus Ρ Plus Ρ First harvest
Upper 7.74 0.83 5.83 0.82 1.90 0.01 0.86 0.61 2.05 3.79 1.22 1.82 Middle 7.78 0.41 4.41 0.25 3.39 0.16 0.99 0.50 2.35 4.04 0.74 1.03 Lower 3.94 0.29 2.77 0.21 1.17 0.08 0.81 0.34 2.35 3.60 0.71 0.97
Second harvest
Upper 18.97 11.03 16.72 8.67 2.26 2.36 1.04 1.20 1.34 2.77 0.81 1.48 Middle 15.98 5.20 12.45 4.50 3.53 0.71 1.02 0.87 1.31 3.31 0.45 0.86 Lower 10.50 2.96 7.96 2.34 2.55 0.62 1.00 0.80 1.34 2.76 0.42 0.88
Third harvest
Upper 11.91 21.53 10.37 20.05 1.54 1.48 0.87 1.04 1.35 1.88 0.68 1.05 Middle 13.15 13.60 11.19 12.86 1.96 0.75 0.58 0.64 1.08 1.96 0.36 0.61 Lower 9.39 6.80 7.84 5.90 1.55 0.90 0.68 0.43 0.98 1.72 0.32 0.57
aF r o m Eaton (125).
146
2. E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 147 TABLE II
EFFECTS OF PHOSPHORUS SUPPLY ON SOME CARBOHYDRATE FRACTIONS (AS % DRY WEIGHT ) IN SOYBEAN (Glycine max)*
Total sugars Reducing sugars Sucrose Starch Stem
level Minus Ρ Plus Ρ Minus Ρ Plus Ρ Minus Ρ Plus Ρ Minus Ρ Plus Ρ First harvest
Upper 4.41 2.09 3.52 1.84 0.89 0.25 2.25 1.44 Middle 1.34 0.65 0.43 0.32 0.91 0.33 3.34 0.47 Lower 1.59 0.47 0.48 0.24 1.11 0.23 5.52 0.41
Second harvest
Upper 2.65 3.36 1.36 2.51 1.29 0.85 4.64 0.94 Middle 1.63 0.93 0.37 0.32 1.26 0.61 7.29 0.59 Lower 1.69 0.83 0.53 0.34 1.16 0.49 8.19 0.52
Third harvest
Upper 2.13 3.34 1.11 2.54 1.02 0.80 6.11 1.19 Middle 1.01 1.28 0.29 0.42 0.72 0.86 9.27 0.95 Lower 1.14 1.36 0.24 0.38 0.90 0.98 7.96 0.90
aF r o m Eaton (126).
T A B L E III
EFFECTS OF PHOSPHORUS SUPPLY ON SOME NITROGEN FRACTIONS (AS % DRY WEIGHT) IN SOYBEAN (Glycine max)0.
70% Alcohol- 70% Alcohol- 70% Alcohol- soluble solids Total Ν soluble Ν insoluble Ν Stem
level Minus Ρ Plus Ρ Minus Ρ Plus Ρ Minus Ρ Plus Ρ Minus Ρ Plus Ρ Upper 24.6 29.9 2.28 2.34 1.41 1.16 0.87 1.18 Middle 19.3 19.2 3.12 1.73 2.08 0.91 1.04 0.82 Lower 19.5 15.4 3.20 1.16 2.15 0.34 1.05 0.82
Ammonia Ν Amide Ν Nitrate Ν Minus Ρ Plus Ρ Minus Ρ Plus Ρ Minus Ρ Plus Ρ Upper 0.030 0.005 0.372 0.298 0.176 0.363 Middle 0.028 0.000 0.516 0.179 0.182 0.434 Lower 0.051 0.000 0.496 0.059 0.262 0.172
aF r o m Eaton (126).
creased, in deficient plants, b u t sucrose a n d hemicellulose showed little change. Detailed studies b y Gregory a n d Richards a n d others on effects due to interactions of phosphorus w i t h other major elements in barley (Hordeum vulgare) are described later (Section II, A, 7 ) , a n d inter- relationships a m o n g potassium, iron, a n d phosphorus are described in Section II, B, 2, b.
T h e subject of phosphorus metabolism is beyond the scope of this chapter. It m a y be noted, however, t h a t phosphorus as adenosine triphosphate ( A T P ) a n d n u m e r o u s phosphorylated products is involved i n practically every synthetic reaction of t h e cell. T h e p r i m a r y forma- tion of adenosine triphosphate a n d of other phosphorylated compounds w h e t h e r derived from photosynthetic or oxidative phosphorylation will n a t u r a l l y be depressed w i t h inadequate phosphorus supply. Decreased phosphorus also results often in increased nitrogen uptake. U n d e r these circumstances increased accumulation of free a m i n o acids as described in Section II, C would be expected, because a m i n o acid activation as adenosyl derivatives, w h i c h are essential to protein synthesis and to ribonucleic acid production a n d u p o n w h i c h this process is also dependent, would most probably be depressed. Changes in concentra- tions of amides a n d peptides, t h e synthesis of w h i c h is also dependent on A T P , would be decreased for similar reasons, b u t failure to convert a m i n o acids to protein would h a v e a compensating effect in increased a m i d e formation. For these reasons changes in amides a n d peptides m i g h t occur in either direction depending on several factors, such as relative effects on nitrogen uptake a n d on a m i n o acid activation, etc.
T h u s in Eaton's comparative studies amide accumulation was con- siderable in soybean (Table I I I ) b u t it was insignificant in sunflower
(Table I) a n d black mustard.
H e w i t t a n d T a t h a m (225) found t h a t acid phosphatase activity in leaves of tomato plants was increased over tenfold b y phosphorus deficiency ( T a b l e I V ) . This m a y be a response to a decreased in- organic phosphate level, w h i c h inhibits phosphatase activity; if so, the effect m u s t be on the synthesis of the e n z y m e d u r i n g growth, b u t not b y direct inhibition in vitro ( 2 2 5 ) . It cannot be said at present w h e t h e r such increased activity is significant in vivo nor whether, if it is, its effects are useful or harmful, since remobilization of inorganic phosphorus for use in y o u n g e r tissues a n d destruction of phosphate ester bonds m i g h t be either desirable or unfavorable activities according to the circumstances a n d the location.
It would be expected t h a t phosphorus deficiency would decrease deoxyribonucleic acid synthesis as well as t h a t of ribonucleic acid. Such a n effect would chiefly restrict nuclear division or m i g h t cause defects
2. E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 149 T A B L E I V
EFFECTS OF MACRONUTRIENT DEFICIENCIES ON ACID PHOSPHATASE ACTIVITY IN TOMATO (Lycopersicon esculentum) LEAF TISSUES"
Nitrogen Minus Minus Minus
source Control Mg Κ Ρ Means
A. On fresh weight basis*
N 03 8.80 8.38 13.25 71.30 22.93
N 02 8.93 8.13 13.43 83.38 28.46
N H4 9.33 7.25 12.95 105.28 33.70
Means 9.02 7.92 13.21 83.32 —
LSD Separate treatments 5%, 16.15 Nitrogen sources (means) 5%, 8.08 LSD Separate treatments 0.1%, 28.71 Nitrogen sources (means) 1%, 10.85
B. On protein basis0
N 03 0.234 0.293 0.452 2.177 0.789
N 02 0.221 0.221 0.404 2.688 0.884
N H4 0.230 0.256 0.367 4.198 1.263
Means 0.228 0.257 0.408 3.021 —
LSD Separate treatments 5%, 0.666 Nitrogen sources (means) 5%, 0.333 LSD Separate treatments 0 . 1 %r 1.222 Nitrogen sources (means) 1%, 0.453
β From Hewitt and Tatham (225).
6 μΜ Phenolphthalein phosphate hydrolyzed per 100 mg. tissue in 20 minutes.
c μΜ Phenolphthalein phosphate hydrolyzed per 100 mg. tissue per minute.
in parts of chromosomes or gene suppression. These possibilities m e r i t further investigation (see Chapter 1, p. 119, a n d ref. 6 9 ) .
3. Sulfur
a. Morphological and visible effects. P l a n t s deprived of sulfur show some visible effects w h i c h resemble those caused b y nitrogen deficiency.
T h e s e effects m a y include m a r k e d decrease in leaf size, w i t h general paling a n d development of orange, red, or p u r p l e pigments, for example, in gooseberry (Ribes grossularia), s t r a w b e r r y (Fragaria chiloensis var.
ananassa), a n d apple ( 5 5 0 a ) .
A point of difference, however, is t h a t y o u n g leaves w h i c h are sulfur deficient a r e u s u a l l y affected m o r e t h a n , or before, old ones. T h i s happens in tobacco ( 3 3 7 ) , tea {Camellia sinensis), a n d tomato, in w h i c h foliage is m a r k e d l y pale green a n d tips of leaves are char- acteristically t u r n e d d o w n w a r d . I n cocoa (324) t h e y o u n g leaves of each flush of growth a r e chlorotic a n d chlorophyll production is de-
layed. Other plants i n w h i c h y o u n g leaves a r e affected first or m o r e severely include Calceolaria rugosa ( 3 3 7 a ) , soybean ( 1 2 1 ) , pecan (Carya illinoinensis [C. pecan]) ( 3 3 7 a ) , Antirrhinum ( 3 3 7 a ) , sugar cane (Saccharum officinarum) ( 3 3 7 a ) , cotton (Gossypium sp.) ( 1 3 4 ) , a n d citrus species ( 7 2 ) .
Sulfur deficiency in tea was identified b y Storey a n d Leach (515) as t h e cause of "tea yellows." Plants h a v e v e r y chlorotic, u l t i m a t e l y yellow, y o u n g leaves w h i c h are drastically decreased i n size w h e n severely affected. M a r g i n s a n d tips of y o u n g leaves become necrotic a n d rolled. Defoliation is severe a n d rapid, a n d t h e stem apex is killed.
Internodes are m u c h shortened, lateral buds develop p r e m a t u r e l y w i t h small yellow leaves, a n d shoots die back, producing multiple branches w i t h dead tips. T h e symptoms in citrus (72) are similar in several respects. Stems m a y be a b n o r m a l l y stiff a n d woody as in tomato ( 3 8 5 ) , a n d lateral b u d development m a y be suppressed in contrast to tea. Stem a n d root diameter m a y be greatly decreased, as in soybean a n d tomato plants. Internode distances in y o u n g stem regions of tomato m a y be increased in contrast w i t h tea. Flowering is reduced, b u t fruit formation t h a t occurs is p r e m a t u r e . G e n e r a l aspects of sulfur nutrition are reviewed b y Gilbert ( 1 5 4 ) .
b. Anatomical and cytological effects. Anatomical changes reported (310) i n sulfur-deficient tomato plants included a m a r k e d increase in t h e thickness of cell walls of stems, especially in fiber, xylem, a n d collenchyma cells, w h i c h w e r e all increased greatly in proportion to the other tissues. P h l o e m area decreased m o r e t h a n other regions a n d pith increased. W e d i n a n d Struckmeyer (576) found t h a t tobacco stem diameter was decreased from 20 to 12 m m . T h i s was associated w i t h decreased cell sizes in transverse section. Cell walls especially in x y l e m w e r e m u c h thinner. E p i d e r m a l , cortical, a n d pith p a r e n c h y m a cells w e r e rounded. Cambial tissues w e r e decreased from six to three layers of cells. P h l o e m cells w e r e also smaller, a n d p h l o e m fiber cells w e r e lacking. T h e r e w e r e no m a r k e d necrotic lesions, b u t phloem cells collapsed. I n leaves t h e cells w e r e less compactly a r r a n g e d a n d leaves w e r e thicker t h a n n o r m a l . W i t h high sulfate levels leaf cell walls w e r e t h i n n e r t h a n usual, b u t increased p a r e n c h y m a produced thicker leaves w i t h enlarged a n d r o u n d e d cells.
Cell division m a y become a b n o r m a l in sulfur-deficient plants. Steffen- sen (499) observed t h a t sulfur deficiency in Tradescantia paludosa resulted in t h e failure of bivalent chromosomes to separate at t h e first a n a p h a s e of meiosis, a n d t h e y formed micronuclei. I n some instances micronuclei comprised single pairs of bivalent chomosomes. A b n o r m a l formation of tripolar spindles also occurred. I n less acute deficiency
2 . E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 1 5 1
there w a s u n e q u a l , or delayed, separation of t h e chromosomes. Root tips of sulfur-deficient plants also contained micronuclei, owing to the failure of chromosome separation, a n d delayed mitosis occurred. Steffen- sen referred to examples of a b n o r m a l meiosis in other plants caused b y sulfur deficiency. As deoxyribonucleic acid does not contain sulfur, it m a y be supposed t h a t protein synthesis is h e r e a b n o r m a l owing to the insufficient supply of t h e sulfur-containing a m i n o acids. It is also pos- sible t h a t spindle m o v e m e n t s a n d m e c h a n i s m depend on the presence of thiol groupings w h i c h form cross linkages b y oxidation a n d disulfide formation. T h e a p p e a r a n c e of micronuclei, w i t h chromosomes w h i c h a r e defective i n deoxyribonucleic acid, implies t h a t their production m a y be dependent on specific sulfur-containing proteins at some stage in the cell division process.
c. Effects on composition. E a t o n ( 1 2 1 , 1 2 3 , 1 2 4 , 1 2 7 ) described effects of sulfur on t h e composition of tomato, soybean, sunflower, a n d black m u s t a r d (Brassica nigra) plants. Sulfur deficiency in soybean decreased t h e content of total a n d reducing sugars b u t increased those of starch a n d crude hemicellulose fractions ( T a b l e V ) . Effects on total carbohydrates a n d sucrose w e r e not consistent. T h e m a x i m u m effects of sulfur deficiency on sugars occurred in stems; effects w e r e large also in roots compared w i t h leaves. Sulfur deficiency increased sucrose a n d starch b u t decreased reducing sugars in tomato a n d black m u s t a r d . T h e ratio reducing sugar : sucrose was decreased from about 6 to 1.5 b y prolonged sulfur deficiency in tomato ( T a b l e V I ) a n d from 1 0 to about 0 . 6 i n black m u s t a r d ( T a b l e V I I ) . Starch was also increased in sunflower, a n d total sugars w e r e decreased (Table V I I I ) . I n general there w e r e decreases in reducing sugars a n d increases in total carbo- hydrates.
Sulfur deficiency h a d fairly consistant effects on t h e major nitrogen fractions. Insoluble nitrogen, w h i c h probably reflected protein nitrogen, w a s decreased in all species w h e r e a s soluble organic nitrogen, amides, a m i n o a n d a m m o n i a nitrogen, a n d n i t r a t e w e r e increased b y sulfur deficiency. It w a s concluded t h a t protein synthesis was m a r k e d l y im- paired, a n d rapid proteolysis w a s inferred.
E a t o n ( 1 2 4 ) found t h a t sulfur deficiency led to high concentrations of soluble organic sulfur compounds in m u s t a r d . C o n t r a r y to Eaton's findings, earlier w o r k b y Nightingale, S c h e r m e r h o r n , a n d Robbins
( 3 8 5 ) indicated increases in concentrations of reducing sugars a n d sucrose as well as of polysaccharides in sulfur-deficient tomato plants.
T h e r e w e r e also deposits a p p a r e n t l y of calcium oxalate. Nightingale et al. ( 3 8 5 ) examined also the effects of sulfur deficiency on sulfur a n d nitrogen fractions in tomato plants, previously raised in a potting
T A B L E V
EFFECTS OF SULFUR DEFICIENCY ON CONCENTRATIONS OF CARBOHYDRATE FRACTIONS (AS % DRY WEIGHT) IN SOYBEAN (Glycine max)*
Samples taken
Carbohydrate April 22, 1933 May 5, 1933 April 21, 1934 fraction and
plant part Plus S Minus S Plus S Minus S Plus S Minus S Total sugars
Leaves 3.792 4.479 3.091 2.676 1.688 1.544 Stems 3.218 1.580 2.692 1.070 2.3084 0.862 Roots 3.084 2.588 3.412 2.461 1.822 1.315 Reducing sugars
Leaves 1.245 1.174 1.109 0.930 0.682 0.481 Stems 1.411 0.411 1.653 0.300 1.704 0.404 Roots 1.461 0.872 1.562 1.048 1.263 0.712 Sucrose
Leaves 2.419 3.139 1.883 1.659 0.955 1.012 Stems 1.717 1.110 0.986 0.732 0.487 0.440 Roots 1.343 1.630 1.757 1.341 0.530 0.572 Starch
Leaves 10.564 14.140 7.560 7.067 2.324 2.338 Stems 8.586 10.677 3.894 8.386 1.896 2.318 Roots 2.740 2.614 2.071 3.334 1.835 1.811 Hemicelluloses
Leaves 4.784 5.227 4.706 7.047 4.790 5.046 Stems 9.686 10.173 11.878 11.767 12.073 11.612 Roots 9.480 9.228 8.644 8.888 7.578 8.718 Total carbohydrates
Leaves 19.140 23.846 15.357 16.890 8.802 8.928 Stems 21.490 22.430 18.464 21.224 16.277 14.792 Roots 15.304 14.430 14.127 15.474 11.235 11.844 Total carbohydrates 18.651 21.431 16.236 18.000 12.354 11.869
aF r o m Eaton (121).
compost, after 8 weeks w i t h or w i t h o u t sulfur. As c o m p a r e d w i t h t h e initial composition, sulfur deficiency m a r k e d l y decreased free sulfate, sulfur i n proteins, a n d total s u l f h y d r y l groups. T h e r e w a s b y contrast a g r e a t increase i n t h e unidentified n o n p r o t e i n sulfur, o t h e r t h a n sulfate. Sulfur deficiency g r e a t l y increased total n i t r o g e n , n i t r a t e nitrogen, free a m i n o nitrogen, a n d soluble n i t r o g e n o t h e r t h a n n i t r a t e ,
T A B L E V I
EFFECTS OF S U L F U R N U T R I T I O N O N CARBOHYDRATE A N D NITROGEN FRACTIONS (AS % D R Y W E I G H T ) I N TOMATO
(Lycopersicon esculentum)*
Total Reducing 70% Alcohol- 70% Alcohol-
Solution sugars sugars Sucrose Starch Total Ν soluble Ν insoluble Ν Ammonia Amides Nitrates First harvest
Minus S 1.74 1.12 0.62 0.79 3.72 2.44 1.28 0.056 0.099 1.904 Plus S 2.03 1.44 0.59 0.60 3.70 2.49 1.21 0.028 0.081 1.874
Second harvest
Minus S 6.17 3.67 2.50 2.56 3.17 2.44 0.73 0.361 0.180 0.945 Plus S 13.27 11.33 1.94 0.99 2.22 1.29 0.93 0.049 0.066 0.716
"From Eaton (127).
153
T A B L E VII
EFFECTS OF SULFUR DEFICIENCY ON CARBOHYDRATE AND NITROGEN FRACTIONS (AS % FRESH AND DRY WEIGHTS) IN BLACK MUSTARD (Brassica nigra)*
Solution
Dry Green
matter matter Dry Green matter matter
Dry Green matter matter
Dry Green matter matter
Reducing sugars Sucrose Starch Acid-hydrolyzable carbohydrates Minus S
Plus S
0.318 0.023 2.303 0.114
0.549 0.040 0.230 0.011
2.404 0.175 0.608 0.030
10.495 0.764 9.657 0.478
Total Ν Total organic Ν
70% Alcohol- soluble Ν
70% Alcohol- insoluble Ν Minus S
Plus S
6.112 0.445 6.143 0.304
2.996 0.218 2.285 0.113
4.520 0.329 4.391 0.217
1.592 0.116 1.752 0.087 Ammonia Ν Amino Ν Amide Ν Nitrate Ν Minus S
Plus S
0.237 0.0172 0.077 0.004
0.452 0.034 0.114 0.006
0.219 0.016
0.106 0.005 3.116 0.227 3.858 0.191
aF r o m Eaton (124).
EFFECTS TABLE VIII
OF SULFUR DEFICIENCY ON CARBOHYDRATE AND NITROGEN FRACTIONS (AS % DRY WEIGHT) IN SUNFLOWER (Helianthus annuus)a
Stem level Minus S Plus S Minus S Plus S Minus S Plus S Minus S Plus S
Reducing sugars Sucrose Starch Acid-hydrolyzable carbohydrates Upper
Middle Lower
5.005 19.592 3.660 16.767 2.246 10.730
0.365 2.472 0.900 2.480 0.747 1.738
1.296 0.725 0.735 0.537 0.315 0.098
0.516 0.323 0.972 0.512 1.305 1.095
Total Ν Total organic Ν 70% Alcohol-
soluble Ν 70% Alcohol- insoluble Ν Upper
Middle Lower
5.526 3.824 4.673 2.968 2.397 1.801
3.188 1.717 2.431 1.112 1.231 0.766
4.537 2.656 4.145 2.221 1.971 1.249
0.989 1.168 0.528 0.747 9.426 0.552 Ammonia Ν Amino Ν Amide Ν Nitrate Ν Upper
Middle Lower
0.163 0.022 0.092 0.011 0.035 0.002
0.798 0.185 0.625 0.115 0.233 0.085
0.192 0.004 0.156 0.004 0.071 0.000
2.339 2.107 2.243 1.856 1.166 1.035 From Eaton (123).
2 . E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 1 5 5
a n d also increased sucrose a n d reducing sugar levels, compared w i t h levels i n plants given sulfur.
Ergle ( 1 3 3 , 1 3 4 ) observed t h a t sulfur deficiency i n cotton decreased protein a n d protein-bound sulfur a n d soluble sulfur compounds. T h e r e w e r e increased concentrations of amides a n d a m i n o nitrogen com- pounds. Sulfur of chloroplast protein w a s also decreased b u t to a lesser degree t h a n in leaves as a whole. Sulfur deficiency caused n i t r a t e accumulation. It was concluded t h a t sulfur released d u r i n g proteolysis in apical tissues w h e n sulfur deficient, w a s n o t reutilized in protein synthesis.
Biddulph ( 3 4 ) found t h a t trifoliate leaves a n d roots of kidney bean plants (Phaseolus vulgaris) contained m o r e sulfur t h a n stems a n d prophylls. P a r t only of the total sulfur was freely mobile in the plant.
T h e rate of m o v e m e n t of sulfur in t h e p h l o e m was similar to t h a t of sucrose or phosphorus, i n contrast to t h a t of calcium, w h i c h was a p p a r e n t l y nil. P a r t of the sulfur in seeds was translocated to roots, w h e r e it r e m a i n e d immobile u n t i l sulfur was absorbed b y roots from t h e external m e d i u m .
Sulfur is essential for t h e conversion of nitrogen fixed from atmospheric nitrogen b y l e g u m e root nodules into protein nitrogen.
Anderson a n d Spencer ( 1 1 ) concluded that, although sulfur deficiency limited t h e nodulation of s u b t e r r a n e a n clover, this effect was indirect a n d w a s due to a limiting effect of sulfur on t h e nutrition of t h e host plant. Sulfur deficiency, u n l i k e m o l y b d e n u m deficiency ( 1 0 ) , did not prevent nitrogen fixation b y nodules, w h e r e present, but prevented the conversion of nitrogen into protein. I n t h e absence of m o l y b d e n u m , however, sulfur h a d n o effect on t h e efficiency per u n i t weight of the increased nodule mass.
Effects of sulfur deficiency on sulfur-containing a n d other a m i n o acids a r e described i n Section I I , C below. T h e discussion of t h e role of sulfur a n d t h e i n t e r m e d i a r y metabolism of sulfur-containing com- pounds in plants is beyond t h e scope of this chapter.
4. Calcium
a. Visible effects of deficiency, i. Leaves and stems. T h e char- acteristic effects of calcium deficiency m a y occur in m a n y parts of a plant, a n d t h e susceptibility of different tissues differs in various species.
I n m a n y plants w i t h entire broad leaves, t h e first indication of calcium deficiency is a slight paling of a limited region of the leaf m a r g i n , some distance b e h i n d t h e leaf apex. Leaf g r o w t h becomes u n e v e n a n d the leaf b r e a d t h is restricted so t h a t the m a r g i n a l outline is perceptibly concave i n t h e chlorotic regions (Fig. 2 ) . Successively y o u n g e r leaves
FIG. 2. Calcium deficiency in beet (Beta vulgaris); chlorotic and restricted mar- ginal growth about mid-marginal region initially (left hand leaves) and progressing nearer to leaf apex to produce backward hooking of leaf tip (center) and black necrosis of leaf tip (right).
show more pronounced chlorosis a n d m a r g i n a l curvature, w h i c h occurs n e a r e r the leaf tip w i t h decreasing age of the leaf. U l t i m a t e l y the leaf apex is included a n d is killed. On subsequent expansion the leaves develop a sharply pointed, truncated, l a m i n a behind t h e growing tip which is often surrounded b y scorched or blackened tissues. I n the older leaves the areas of restricted m a r g i n a l growth become scorched and t h e y tear, whereas in the youngest leaves only the petiole m a y develop w i t h a blackened tip a n d no l a m i n a at all ( 1 8 9 , 1 9 0 , 192).
2 . E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 1 5 7
M a n y plants, including beet (Beta vulgaris), brassicas, e.g., cauli- flower (Brassica oleracea var. Botrytis), tobacco ( 1 4 7 , 3 3 7 ) , a n d cocoa
( 3 2 4 ) , t h a t produce these symptoms often show a characteristic "hook- i n g " of t h e leaf tip. T h i s arises because t h e differential growth of the m a r g i n a l a n d central regions of the leaf causes strain a n d consequential change in leaf shape. T h e hooking m a y occur in either direction b u t is usually backward.
I n cauliflower a n d i n kale (Brassica oleracea var. acephala), ex- tensive interveinal necrotic spotting m a y a p p e a r in m e d i a n parts of p a r t l y expanded leaves. T h e necrosis m a y be preceded b y g r a y tinting;
it occurs in a well-defined zone w i t h i n t h e m a r g i n s . I n these circum- stances the " t i p hooking" effects occur later in t h e y o u n g e r leaves.
I n tomato, m a r g i n s of t e r m i n a l a n d t h e n lateral leaflets on recently expanded leaves become p u r p l e tinted. Central interveinal areas are chlorotic, develop orange tints, a n d t h e n become necrotic a n d scorched.
T h e s e symptoms precede effects in t h e y o u n g leaves, in w h i c h leaflets r e m a i n folded, become chlorotic at their m a r g i n s , a n d finally necrotic a n d withered. T h e youngest leaves become necrotic a n d die w i t h o u t e x p a n d i n g ; t h e shoot apex withers. Lateral buds tend to r e m a i n d o r m a n t , u n l i k e those in boron-deficient plants, but side shoots t h a t h a v e commenced expansion m a y show similar symptoms after the death of t h e m a i n axis.
I n potato t h e first symptoms on t h e aerial parts of plants t h a t h a v e already m a d e substantial growth occur in t h e y o u n g leaves. T h e leaflets r e m a i n folded, become chlorotic at t h e m a r g i n s , a n d develop small black necrotic spots just w i t h i n the m a r g i n s . Leaflets shrivel a n d the stem apex dies a n d withers. Black streaks a n d lesions occur in t h e stems a little w a y below the apex. W h e n calcium deficiency is v e r y severe, t h e shoots produced from tubers m a y die soon after t h e y begin to extend ( 5 5 5 ) . Stem growth is not injured in this w a y w h e n tubers are m a i n t a i n e d u n d e r " s p r o u t i n g " conditions, i n w h i c h roots are u n a b l e to absorb n u t r i e n t s a n d stems m a y attain 2 0 c m or m o r e without evidence of the i n j u r y produced w h e n plants are rooting in a calcium- deficient n u t r i e n t . Root development is initially vigorous also w h e n tubers a r e set in calcium-deficient sand cultures. It appears, therefore, t h a t stem growth of t h e potato is m o r e sensitive t h a n root growth w h e n stems a r e attached to tubers a n d t h a t stem tissues are injured w h e n their roots develop i n a calcium-deficient m e d i u m , but not w h e n their roots are suppressed b y sprouting tubers in the open air.
U n d e r slight to moderate conditions of calcium deficiency, aerial stem growth m a y a p p e a r n o r m a l or exhibit only a slight m a r g i n a l paling of y o u n g leaflets. T h e tubers from such plants, however, a r e
usually severely malformed (Fig. 3 ) , n u m e r o u s , and m u c h smaller t h a n n o r m a l . T u b e r s are frequently b r a n c h e d or multiple in appearance, and t h e surface m a y be concave. T h e effect on tubers is the most sensitive response a n d m a y be t h e only s y m p t o m to occur w i t h slight deficiency of calcium. I n t e r n a l l y such tubers m a y show a breakdown w h i c h re- sembles the m e d u l l a r y necrosis reported b y V a n Schreven (535) a n d Bolle-Jones (36) or a vascular necrosis w i t h production of excessive corky periderm.
Calcium deficiency is often associated w i t h wilting symptoms, w h i c h appear in two w a y s t h a t m a y be related. I n p a r t l y expanded leaves of
FIG. 3. Calcium deficiency in potato (Solanum tuberosum) tubers; diminutive size, with severe malformation and production of numerous "satellite" tubers.
cauliflower, rape, kale, swede, radish (Raphanus sativus), parsnips (Pastinaca sativa) (190, 192) a n d peach (Prunus persica) wilting oc- curs i n t h e central interveinal areas of t h e leaf ( 9 2 ) . Major veins, mid- ribs, a n d petioles m a y become involved successively, a n d t h e leaf falls over (Fig. 2 2 ) . W i l t i n g also occurs in leaf petioles, pedicels, a n d parts of stems in calcium-deficient plants, often during, or just prior to, flower- ing (192) a n d m a y occur in t h e elongating flower stems of biennials w h i c h h a v e shown only slight leaf symptoms the previous y e a r ; in general it appears to be associated w i t h moderate, r a t h e r t h a n severe, deficiency. It occurs in rape, kale, radish, tulip (Tulipa gesneriana, Rose Copland) ( 2 2 4 ) , pea, clovers ( 1 1 1 , 190), lucerne, flax; in this p l a n t the field condition is called " w i t h e r t i p " ( 3 5 1 , 3 5 5 ) .
T h e leaf symptoms in cereals, such as barley, wheat, oats, include g r a y tinting followed b y total w h i t e chlorosis of leaf tips of y o u n g leaves, w h i c h r e m a i n rolled. A constriction occurs i n the rolled leaf about 3-5 c m behind the tip. T h e leaf wilts at this point a n d the tip portion later withers. T h e s e symptoms m a y closely resemble those caused b y copper deficiency described later (189, 190, 192). M a i z e
2. E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 159
leaves become gelatinous at t h e tips. These d r y out a n d adhere to each other (337a) a n d this effect resembles t h a t of boron deficiency ( 1 3 0 ) .
D e a t h of t h e stem apex is characteristic of calcium deficiency in m a n y plants. I n slight or m o d e r a t e deficiency, t h e n u m b e r of shoots m a y be increased, as in flax or potato, producing a m a r k e d l y b u s h y habit. A possibly related effect is t h e t e n d e n c y for calcium-deficient oat plants to produce lateral shoots from nodes along the prostrate m a i n stem. I n t i m o t h y grass (Phleum pratense), the stem nodes produce adventitious roots. Proteolysis associated w i t h prolonged darkness leads to liberation of calcium from older tissues ( 3 8 3 ) .1
ii. Roots. Calcium deficiency often h a s especially serious growth effects on roots, a n d this is reflected in the fact t h a t the top: root ratio in s u b t e r r a n e a n clover, for example ( 3 5 6 ) , m a y be increased b y calcium deficiency instead of showing the usual decrease caused b y m a n y disorders. Roots of barley, oats, wheat, etc., are particularly sensitive to calcium deficiency. T h e y become stunted or die back from the apex. Adventitious roots from the first node m a y die w h e n only 2.5-5 m m long. M u s t a r d roots become g r a y a n d translucent. Similar translucence a n d gelatinization occurs i n a few days w h e n t h e roots of citrus species a r e i m m e r s e d in calcium-deficient salt solutions ( 4 3 6 a ) . In apple a n d peach (92) a n d tomato (383) the roots become bulbous a n d proliferate just b e h i n d t h e p r i m a r y root tip, w h i c h dies back.
Sorokin a n d S o m m e r ( 4 9 1 , 492) examined t h e effects of calcium defi- ciency over a r a n g e of controlled calcium levels on t h e growth of pea roots. T h e i r methods w e r e similar to those used for t h e study of boron deficiency ( 4 9 0 ) , a n d v e r y low calcium levels w e r e achieved.
T h e roots w e r e clearly far m o r e sensitive t h a n the aerial parts to the first effects of calcium deficiency w h e n t h e plants w e r e transferred to calcium-free solution after a few d a y s ' growth w i t h complete n u t r i e n t . W i t h i n 2 or 3 days t h e apex of t h e m a i n root appeared translucent; t h e root sometimes appeared to be constricted about 2 - 4 m m from the apex. F u r t h e r apical growth t h e n ceased. W i t h 0.06 p p m calcium there w e r e m o r e lateral primordia, most of w h i c h failed to develop; some of these at t h e base of t h e m a i n root, however, elongated slightly. In- creasing calcium u p to 0.25 p p m produced m a i n l y increased lateral root development w i t h restricted m a i n roots. M a i n root elongation was n o r m a l i n the presence of a saturated solution of calcium sulfate.
Histological effects a r e described later.
1 Effects of lack of calcium and other elements are now being observed at the submicroscopic level and in the growing points (cf. Marinos, Amer. J. Bot., 4 9 , No. 8, 1962; and other papers in Proc. 5th Int. Cong. Electron Microscopy, Phila- delphia, 1962). This is a welcome trend. (Ed.)
T h e growth of root hairs as related to calcium supply has been reviewed b y Cormack (84, 8 5 ) . H e concluded from his o w n a n d other work t h a t root h a i r cell walls comprise a n i n n e r elastic l a y e r of cellulose surrounded b y a h a r d e r l a y e r of calcium pectate except at the softer tip area, w h e r e either pectic acid or a modified calcium pectate is said to be present. W h e n adequate calcium is present this softer area is small a n d root hairs are elongated. W i t h low calcium levels the soft area is enlarged a n d t h e root h a i r tip swells to a bulbous state.
T h e formation of root hairs in cabbage roots w a s thought to reflect the extent to w h i c h external calcium would combine w i t h t h e pectic acid of t h e cell wall. Some cells h a d a low i n t e r n a l p H a n d did not readily form root hairs whereas others h a d a higher p H a n d proceeded to form elongated root hairs a n d to produce calcium pectate, the forma- tion of w h i c h Cormack found to be in accordance w i t h t h e effect of p H on t h e ease of combination of calcium a n d pectic acid in vitro. T h e extension of the root h a i r cell from the tip w a s considered to reflect t h e increasing h a r d e n i n g of calcium pectate in t h e basal regions. These views regarding t h e effect of calcium on cell wall plasticity would be in a g r e e m e n t w i t h those of Burström given below. Cormack later con- cluded t h a t a n external supply of calcium w a s required for formation of root hairs initially. T h e m e c h a n i s m was thought to depend on i n t e r n a l w a t e r pressure forcing out t h e cell w a l l a t t h e weakest point left d u r i n g the h a r d e n i n g of the r e m a i n i n g area b y calcium pectate.
Excessive calcium a n d alkaline conditions lead to such rapid calcifica- tion over all the surface t h a t root h a i r formation would t h e n be sup- pressed. W i t h calcium deficiency a n d low p H , lack of h a r d e n i n g of t h e wall results i n gross expansion of t h e w e a k walls w i t h consequent swelling, branching, a n d other abnormalities. Recovery is possible only in regions w h i c h are able to r e s u m e g r o w t h ; a b n o r m a l h a i r cells do not change.
Loneragen a n d Dowling ( 3 0 3 ) , a n d Loneragen ( 3 0 2 ) , h a v e shown t h a t t h e calcium r e q u i r e m e n t for root nodule formation b y s u b t e r r a n e a n clover is about tenfold greater t h a n the a m o u n t needed for t h e growth of t h e host p l a n t w h e n n i t r a t e is t h e nitrogen source. On the other h a n d the calcium r e q u i r e m e n t , if a n y , for the growth of the Rhizobium bacteria, was extremely low, less t h a n 0.1 μΜ. Effects of p H w e r e m o r e critical for t h e rhizobia t h a n for the production of nodules, regardless of calcium supply above the critical values of 0.1 M calcium or p H 4.5 w h e r e increase i n t h e level of t h e one could offset a n unfavorable effect of t h e other.
Burström (59, 60) described effects of calcium a n d hydrogen ion con- centration on the growth of w h e a t roots in w a t e r cultures u s i n g a con- tinuous flow of dilute n u t r i e n t solution. A t concentrations of 10~6 a n d
2 . E S S E N T I A L N U T R I E N T E L E M E N T S F O R P L A N T S 1 6 1
Ι Ο- 8 M calcium t h e r e was a n o p t i m u m effect of p H a r o u n d p H 5 - 6 . 0 . As t h e calcium level w a s progressively increased to 1 0 ~3 M , increasing p H values produced increased g r o w t h in length u p to p H 7 or above.
Analysis of these effects in t e r m s of cell elongation or cell multiplica- tion produced different effects. Cell multiplication w a s slightly de- pressed at 1 0- 3 a n d 1 0- 8 M C a+ + a n d at p H values u p to 6 . 0 , compared w i t h i n t e r m e d i a t e calcium levels of 1 0- 6 to 1 0 ~4 M. T h e r e was little or no effect of p H above p H 5 . 5 , b u t a v e r y great decrease in cell n u m b e r s at p H 4 . Cell elongation, however, w a s m a r k e d l y increased b y in- creasing calcium from 1 0 "8 to ΙΟ"6 M to 1 04 at p H 6 or 7 ; 1 0 ~3 M calcium appeared to be above t h e o p t i m u m for cell multiplication, but not for cell elongation. L o w values (about p H 4 . 4 ) w e r e associated with a b n o r m a l l y elongated cells, especially at t h e higher calcium levels.
Burström advanced t h e suggestion t h a t calcium exerts its effect b y
" h a r d e n i n g " the cell walls a n d t h a t it thus counteracts t h e effect of a u x i n in softening, or increasing t h e elasticity, of cell walls.
I n a later experiment Burström ( 6 0 ) found t h a t cell multiplication was increased b y calcium between 1 0 ~6 a n d 1 0 ~5 M w h e n iron was also given, in accordance with observations of Brown a n d Possingham ( 5 2 ) . T h e low p H o p t i m u m possibly reflects some effect on t h e availability of iron. Calcium r e q u i r e m e n t s for cell division therefore a p p e a r to be about one-tenth or o n e - h u n d r e d t h of those t h a t are optimal for cell ex- pansion. ( T h i s is consistent w i t h t h e v e r y low calcium r e q u i r e m e n t s for growth of bacteria.)
Increasing calcium from 1 0 ~6 to Ι Ο4 M progressively increased protein content a n d fresh weight b u t did not greatly affect d r y weight, a finding from w h i c h Burström concluded t h a t t h e increased cell elongation was d u e to increased w a t e r uptake. T h e increased protein w a s a p p a r e n t l y related to increased n i t r a t e uptake. Calcium appeared to be necessary for the development of the elastic properties of the cell walls b u t did not affect their plasticity. Burström suggested t h a t calcium functioned d u r i n g the growth of t h e cell wall b y intussus- ception b y promoting t h e formation of a n elastic structure a n d also b y increasing t h e u p t a k e of n i t r a t e required for protein synthesis. F r o m t h e results obtained b y Florell ( 1 4 1 , 1 4 2 ) , it would appear t h a t the effect of calcium on n i t r a t e u p t a k e w a s closely related to its effect on mitochondrial formation. Subsequent experiments b y Burström a n d T u l l i n ( 6 1 ) confirmed t h e effect of calcium on cell elongation. This was not inhibited b y ethylenediaminetetraacetic acid ( E D T A ) 10"5 M, but cell multiplication was. T h i s inhibition was reversed b y Ι Ο5 M calcium or m a n g a n e s e , b u t not b y ferrous ions.
C a r r a n d N g ( 6 5 ) suggested t h a t t h e stimulation of coleoptile elongation in the presence of a citrate-containing buffer at p H 5 can
be explained b y t h e sequestration of calcium, w h i c h is present in all walls of w h e a t coleoptiles to the extent of 0 . 1 % of their d r y weight.
E D T A w a s u n a b l e to exert such effects [ N g a n d C a r r ( 3 7 6 ) ] , a n d this result w a s not considered to support t h e view of Bennet-Clark ( 3 2 ) , noted later, that stimulation of cell expansion b y E D T A in Avena coleoptile is d u e to chelation of calcium present as calcium pectate. I n excised carrot root tissues, calcium deficiency resulted in the dis- a p p e a r a n c e of cambial tissues ( 5 2 4 a ) .
Hi. Flowers and fruits. Calcium deficiency severely limits seed pro- duction even w h e n flower formation is otherwise n o r m a l . G r a i n forma- tion is often totally suppressed in cereals; barley a n d w h e a t bear e m p t y glumes though the foliage symptoms m a y h a v e been slight. Flowers fall p r e m a t u r e l y d u r i n g blossoming in broad bean (Vicia faba), F r e n c h beans, clovers, lucerne, a n d m u s t a r d . W h e n organs such as pods are formed, t h e developing ovules abort at a n early stage. Broad b e a n pods m a y show black lesions along their dorsal a n d ventral sutures a n d over t h e sides. T h e spongy u n e v e n surface becomes black a r o u n d the attach- m e n t of the ovule. T h e ovule r e m a i n s small, the integuments t u r n b r o w n irregularly, a n d t h e ovule becomes l i m p a n d finally shrivels.
T h e radicle of t h e e m b r y o a n d the tissue attaching it to the cotyledons m a y also blacken. These symptoms m a y occur even in externally n o r m a l pods. A similar collapse of the ovule, w i t h irregular necrosis of the embryonic cotyledons a n d shrinkage w i t h i n the integuments, which r e m a i n bladder-like, also occurs in pea a n d in dwarf a n d climbing F r e n c h beans (189, 190, 192). This necrosis in embryonic tissues m a y be analogous to the effects of calcium deficiency on t h e gynophore of Arachis, w h i c h is described below.
"Blossom-end r o t " of tomato fruit is readily induced b y calcium deficiency. T h e symptoms commence either as a sunken region a few millimeters in width, n e a r the distal end of the youngest fruit on the truss, or as dark-colored areas beneath t h e surface of a fruit; these are caused b y woody tissue surrounded b y green tissues in t h e otherwise orange-colored flesh of the ovary wall. Mucilage is often lacking and ovules m a y be absent. F r u i t are progressively affected from the apex to t h e base of t h e truss w i t h increasing severity. S p u r r (497) has re- viewed the problem in general a n d has described a detailed anatomical study of the effects of calcium on the incidence of "blossom-end rot."
Proteinaceous inclusions develop in necrotic epidermal cells a n d in deeper layers of the distal region of the pericarp. Calcium deficiency r a t h e r t h a n w a t e r shortage was regarded as the p r i m a r y cause of the disorder. T h e incidence of "blossom-end r o t " u n d e r conditions of high salt nutrition m a y reflect induced calcium deficiency.
T h e role of calcium in the growth of t h e p e a n u t (Arachis hypogaea)