Electrochemical and Surface analysis techniques applied to the investigation of MIC.
László Trif1, Abdul Shaban1, Judit Telegdi1, 2
1Institute of Materials and Environmental Chemistry, Research Centre for Natural Sciences, Hungarian Academy of Sciences (RCNS HAS, Magyar tudósok körútja 2., H-1117 Budapest, Hungary
2Óbuda University, Faculty of Light Industry and Environmental Engineering, Doberdó u. 6., 1034 Budapest, Hungary
Contents
Techniques used for MIC monitoring and evaluation 1 Electrochemical methods in the study of MIC
1.1 Methods requiring no external signal 1.1.1 Redox potential
1.1.2 Corrosion potential (Ecorr)
1.1.3 Electrochemical Noise Analysis (ENA) 1.1.4 Microsensors
1.1.5 Dual-Cell Technique 1.1.6 Capacitance
1.2 Methods requiring a small external signal
1.2.1 Electrochemical Impedance Spectroscopy (EIS) 1.2.2 Polarization Resistance Method
1.3 Methods that apply large signal polarization 2 Surface analytical methods
2.1 Microscopic techniques
2.2 Scanning electron microscopy (SEM) and environmental scanning electron microscopy 2.3 Atomic force microscopy (AFM)
2.4 Confocal laser microscopy (CLM)
2.5 Confocal laser scanning microscopy (CLSM) 2.6 Confocal Raman microscopy (CRM)
3. Piezoelectrical methods: quartz crystal microbalance (QCM) 4. Spectroscopic analytical methods
4.1 Fourier transforms infrared spectroscopy (FTIR) 4.2 X-ray photoelectron spectroscopy (XPS)
Abstract
To follow and measure the microbially influenced corrosion acknowledged by academics and engineers needs analytical technique of broad spectrum. The presence of microbes changes not only the aqueous environment, but has a decisive impact on the solid surface whereto they adhere and form biofilm with heterogenic composition. This layer with very high water content, ions and microorganisms involved from the bulk solution modify the solid surfaces and deteriorate them. This explains the need for instrumentation of wide spectrum in order the get the measure of the dissolution of metals, deterioration of passive film. In this chapter, the mostly used techniques are summarized. Some of them (electrochemical noise measurement, electrochemical impedance spectroscopy, quartz crystal microbalance) are apt to follow electrochemical reactions, deposition, and the change in the layer permeability induced by the presence of microorganisms, by their metabolites or by the biofilm they formed. Other techniques (scanning electron microscopy, environmental scanning electron microscopy, confocal laser microscopy, confocal scanning microscopy, atomic force microscopy) can visualize the microorganisms, their distribution in the gelatinous biofilm and give information about the surface deterioration in 3D and in section. Spectroscopic techniques (FTIR, X-ray photoelectron spectroscopy) inform us about the material composition, on the change of the surface. The techniques are not only introduces and shows how is possible to use in case of MIC, but their advantages and disadvantages are also mentioned. It is important to open the eyes on the fact that, because of the complex phenomenon the microorganisms generate, parallel application of different techniques is important. These techniques are reviewed regarding the heterogeneous characteristics of microbial consortia and their possible influences on metal substrata. Our intention is that this chapter will motivate application and combination of new and previously used techniques for practical, industrial, detection and on-line monitoring to determine the impact of biofilms on structural deterioration in different systems in the oil and gas industry.
Key words:
Electrochemical techniques, microbiologically influenced corrosion in oil and gas industry, electrochemical noise analysis, microsensors, electrochemical impedance spectroscopy, quartz crystal microbalance, confocal laser microscopy, scanning electron microscope, atomic force microscopy, confocal Raman microscopy, FTIR, XPS.
Techniques used for MIC monitoring and evaluation
Any investigation of MIC needs input from scientists and engineers from interdisciplinary fields, and it is especially important to combine various areas of expertise to tackle the phenomenon. In particular, the diagnosis of MIC cannot be based only on microbiological data. Techniques utilized for detection and monitoring of MIC include conventional electrochemical direct current (DC) methods, alternating current (AC) electrochemical impedance spectroscopy (EIS) , optical and electron microscopy, atomic force microscopy (AFM) , Fourier transform infrared spectrometry (FTIR), scanning vibrating electrode mapping (SVEM), concentric electrode technique, x-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES) [1- 3]. The best approach to determine the presence of MIC in pipelines, apart from microbial test results, is the integration of data from those different types of corrosion detection and monitoring methods and system operating parameters. These data include main operating parameters such as flow rate, temperature, pressure, pigging frequency, chemical treatment history, and fluid physico-chemistry (chemical composition of gases, liquids and solids) which affect MIC directly or indirectly.
1. Electrochemical methods in the study of MIC
Electrochemical methods applied to study MIC include those in which no external signal is applied (e.g., measurement of redox potential (Er-o) or corrosion potential (Ecorr), and electrochemical noise analysis (ENA)), those in which only a small potential or current perturbation is applied (e.g., polarization resistance (Rp) and electrochemical impedance spectroscopy (EIS)), and those in which the potential is scanned over a wide range (e.g., anodic and cathodic polarization curves, cyclic voltammetry) [1].
It is important to note that electrodes used in all electrochemical techniques measure the current and/or voltage in order to estimate a corrosion rate. The surface of the electrode alters during measurements as electric double layers form. However, in the presence of microorganisms, biofilm will build up during the measurement, and thus the thickness and character of the film formed on the electrode changes continuously. This explains why it is necessary to use, in parallel, several different electrochemical techniques in order to obtain more precise results on the electrode surface reactions, which can then be used to help explain MIC mechanisms [1–4].
1.1 Methods requiring no external signal 1.1.1 Redox potential
Redox potential in general is a measure of the oxidizing power of the environment. A prepassivated platinum (Pt) electrode and an electrode of the metal of interest follow biofilm development and its effects on the corrosion behavior of structural materials [5]. Since the potential of Pt changes in the positive direction when either the oxygen concentration is increased or pH is decreased, an indicator is added to the solution to determine pH changes independently. Time dependence of the open-circuit potential (OCP or Ecorr) of several steels was compared to that of the prepassivated platinum electrode. Micro-organisms, by their presence or their metabolic activity, during their growth and multiplication, can drastically alter the local physical chemistry at the interface material environment, resulting in the initiation and acceleration of localized corrosion [5, 6].
This technique has a great advantage as it could be used both in laboratory and field tests. In the case of MIC, this method is not useful for determination of the corrosion rates and this is a disadvantage. Such techniques also require simultaneous measurement of the pH. It is important to choose the immersion time carefully, and so when the microbial colonization on the electrode starts, the measured value will correspond to the chemistry at the electrode under the biofilm rather than to that of the bulk environment. The redox potential measurements of electrochemical reactions should be conducted under equilibrium conditions.
1.1.2 Corrosion potential (Ecorr)
Corrosion potential, also known as open circuit potential, measurements require a stable reference electrode (RE), usually assumed to be unaffected by biofilm formation, and a high- impedance voltmeter. Ecorr values are difficult to interpret, especially when related to MIC [6].
Dickinson et al [7] demonstrated that microbiologically deposited manganese oxide on 316L stainless steel coupons caused an increase in Ecorr and increased cathodic current density at more anodic potentials.
Because of its simplicity, Ecorr has been applied in MIC studies both in the laboratory and in the field for many years. It measures both the anodic and cathodic processes simultaneously but only the trends are assessed [8].
1.1.3 Electrochemical Noise Analysis (ENA)
Electrochemical noise (EN) data can be obtained either as fluctuations of Ecorr, (fluctuations of potential (E)) at an applied current (I), or as fluctuations of I at an applied E [6]. No external
signals are applied that may influence biofilm properties. It measures the potential and current fluctuations simultaneously.
In this approach, two electrodes of the same material are coupled through a zero resistance ammeter (ZRA). Simultaneous collection of potential and current electrochemical noise data allows analysis in time and frequency domains. Analysis of EN data in the time domain results in values of the mean potential (Ecoup) and mean current (Icoup) of the coupled electrodes. This technique gives the most plausible result on the electrochemical consequence of MIC [9].
Padilla-Viveros et al.[10] have investigated the electrochemical behavior of SAE1010 carbon steel, which was exposed to a Desulfovibrio alaskensis (strain IMP-7760). With the mathematical analysis of the electrochemical noise, and by using the localization index (LI), it was possible to differentiate among corrosion processes, and the factors influencing each other were determined. By determining LI (which can be defined as the standard deviation of the current noise divided by the root mean square current), information about the proceeding corrosion process can be obtained, as follows: if the value of LI is lower than 0.05, the corrosion process is considered to be uniform, while an LI value between 0.05 and 0.1 corresponds to a mixed corrosion type. A value of LI higher than 0.1 is typical to a localized corrosion process [10].
1.1.4 Microsensors
Microsensors, usually in the form of microelectrodes and microoptodes (fiber optic micro sensors), are crucial tools in biofilm research because they permit for the probing of local environments and the quantification of local interactions at the microscale with high spatial resolution, obtaining information that is problematic to get otherwise.
In a biofilm, as a result of the metabolic activity of the microorganisms, some materials are consumed and some are produced. The concentration profiles of these substances convey useful information about microbial activity and about mass transport.
Most of microsensors used in biofilm research are electrochemical sensors, where the most useful are amperometric microsensors, which can be used to measure the concentrations of dissolved gases, ions, and organic and inorganic molecules.
Ultimate microsensors have the following characteristics: small tip diameters to prevent distortion of the local environment, small sensor surfaces for optimal spatial resolution, low noise levels, stable signal, high selectivity, and strength to resist breakage [11].
Electrochemical microsensors of interest for microbial ecology are of H2S, methane etc. are available commercially, thus it has become a relatively simple task to analyze the microenvironment in stratified microbial communities for several chemical species.
The microsensors for measuring H2S in biofilm or in sediments have (measuring, counter and guard) platinum electrodes (a modified oxygen microelectrode). The method is based on amperometric technique when potassium ferricyanid is the redox mediator. This method could be useful in neutral and moderate alkaline biofilm [12]. Microsensors have been used to establish profiles of mixed species biofilms [13].
1.1.5 Dual-Cell Technique
The dual cell, split cell or biological battery allows continuous monitoring of changes in corrosion rates due to the presence of a biofilm [14]. A semi-permeable membrane separates two identical electrochemical cells biologically. The two working electrodes are connected to a potentiostat set at null (0 mV) potential. Bacteria are present in one of the cells, and the sign and magnitude of the resulting galvanic current are monitored to show the bacterial corrosivity. The dual-cell technique does not provide the determination of corrosion rates, but monitor changes in corrosion rate due to the biofilm presence.
1.1.6 Capacitance
Capacitance can be determined using the galvanostatic transient method. A constant-current pulse is applied to the sample under investigation to produce an overvoltage–time response. The applied current (Iapp) generates cathodic overvoltage (η). After amplification through a high- impedance differential amplifier, signals are recorded by a computer. The capacitance (C) is determined by nonlinear fitting as:
(1 exp[ / ])
app p p
I R t R C
, (1)
wheretis the time, assuming a simple parallel combination of polarization resistance (Rp) andC as the model for the electrode/solution interface. In most cases, this produces an acceptable fit.
Close proximity of the working and reference electrodes and the small Iappmakes corrections for uncompensated resistance unnecessary [6, 7].
1.2 Methods requiring a small external signal 1.2.1 Electrochemical Impedance Spectroscopy (EIS)
EIS technique records impedance data as a function of the frequency of an applied signal at a fixed electrode potential. To obtain a complete impedance spectrum a large frequency range
must be investigated. Small signals required for EIS do not adversely affect the numbers, viability, and activity of microorganisms within a biofilm [15]. EIS data determine the polarization resistance (Rp) values that allow the calculation of the corrosion rate. EIS is commonly used for steady-state conditions (at OCP, and uniform corrosion); however, sophisticated models have been developed for localized pitting corrosion [16]. This technique provides information about the properties of the formed layer on the electrode surface, such as information about the compactness or porosity by analyzing the Rp values, which allows its use in the MIC evaluation investigations.
1.2.2 Polarization Resistance Method
By measuring Rp, the corrosion rate of any metal can be continuously monitored [17]. Rp is defined as:
(d / d ) 0
Rp E I i (2)
Rp is the slope of a potential (E) versus current density (i) curve at Ecorr, where i= 0. Corrosion current density (icorr) is calculated fromRpas:
corr / p
i B R (3)
where B b b a c/ 2.303(b ba c) (4)
The exact calculation of icorr for a given time requires simultaneous measurements of Rp and anodic and cathodic Tafel slopes (ba and bc). Modern instruments are able to determine the precise values of icorr. A simplification of the polarization resistance technique is the linear polarization technique when it is assumed that the relationship between E and i is linear in a narrow range aroundEcorr[18]. Usually, only two points (E,i) are measured, andBis assumed to have a constant value of about 20 mV. This approach is applicable to field tests and forms the basis of commercial corrosion rate monitors. Rp can also be determined as the dc limit of electrochemical impedance (i.e. at the low frequency limit). For localized corrosion, experimental Rp data should be used as a qualitative indication for rapid corrosion. Large fluctuations of Rp with time are often observed for systems undergoing pitting or crevice corrosion as Rp data are meaningful for general or uniform corrosion but less so for localized corrosion, including MIC. Additionally, the use of Stern–Geary theory (where corrosion rate is inversely proportional to Rp at potentials close to Ecorr) is valid for conditions controlled by electron transfer, but not for diffusion-controlled systems [19], as it is frequently the case in
MIC. The advantage of the polarization resistance method is the rapid and easy interpretation of the results, and it shows good correlation with the gravimetrical method, which represents the measured difference in weight before and after suffering corrosion. Its disadvantage is that it is not applicable in localized corrosion cases. The presence of biofilms introduces additional electrochemical reactions which complicates the linear polarization interpretation thus can lead to nonlinear polarization behavior. Those uncertainty is the reason behind coupling this method with other complementary techniques.
1.3 Methods that apply large signal polarization
Large signal polarization techniques require potential scans ranging from several hundred millivolts to several volts. Potentiostatic or potentiodynamic polarization curves as well as pitting scans use large signal polarization [20]. Polarization curves can be used to determine corrosion currents (icorr) by Tafel extrapolation. Mechanistic information can be obtained from experimental values of the anodic and cathodic Tafel slopes (baandbc). Pitting scans are used to determineEpitand the protection potential (Eprot).
In numerous cases, polarization curves have been used to determine the effects of microorganisms on the electrochemical properties of metal surfaces and on the corrosion behavior [1, 19]. The advantage of this technique is that it can be used under laboratory or field conditions because of the easy interpretation of data. The major disadvantage of the large signal polarizations is their destructive nature, that is, the irreversible changes of surface properties due to application of large anodic or cathodic potentials which influence the microbial behavior, the growth and multiplication and biofilm formation.
Choice of potential scan rate is important in MIC studies to reduce the effects on biofilm structure and character. The faster the scan rate, the less the impact on microbial activities. The advantage of potentiodynamic sweep techniques is their usefulness to predict the corrosion behavior of passive metals in biotic media in the presence of biofilms. Disadvantages are that the results depend on the sweep rate and experimental conditions. Slow potential sweep rates can affect localized conditions at the metal-solution interface [20, 21].
To provide complex information about a surface covered by biofilm and data on MIC, some of the techniques presented above should be used in parallel as a complementary method. The reason is that the biofilm changes the electrode surface dramatically: the metal dissolution is enhanced by its presence and with increasing film thickness (together with the continuous
internal reaction of microbes and production of aggressive metabolites) the surface properties changes continuously.
2 Surface analytical methods 2.1 Microscopic techniques
Microscopy includes three well-known branches: optical, electron, and scanning probe. Each branch has advantages and disadvantages. During the last decades the microscopic methods have significantly improved, enhanced the understanding of microbial growth, biofilm formation, the microorganism’s interaction with surfaces (i.e. study of MIC) [22]. Visualization of microorganisms has always been critical because of its soft consistency, always changing in size and surface character, and due to the EPS content that surround them. Some of the earliest microscopic observations were in situ microscopy of algae and bacteria. In this chapter, three microscopic techniques are highlighted. Confocal laser microscopy (CLM), scanning electron microscopy (SEM) together with the environmental scanning electron microscopy (ESEM) and atomic force microscopy (AFM) allow visualization of microorganisms and observation of biofilm in real time. There is increasing number of reports of these innovative technologies in the recent MIC literature [23]. The complementary nature of the microscopy methods, scanning electron microscopy and atomic force microscopy make it possible to visualize and assess surfaces modified by the single cells and by presence of different biofilms formed from microorganisms.
The evaluation of the shape and organelles of cells and their visibility could be enhanced by special techniques like staining and the evaluation by fluorescence microscope.
2.2 Scanning electron microscopy (SEM) and environmental scanning electron microscopy (ESEM)
SEM produces images of a sample by scanning it with a focused beam of electrons. The electrons generated in a cathode, amplified by anode, focused by a magnetic lens, interact with atoms of the sample, producing various signals (backscattered and secondary electrons, X-ray).
The backscattered and secondary electrons give morphologic information in 3D images, the X- ray determines the composition of the solid surface. The most common mode of detection is by secondary electrons emitted by atoms excited by the electron beam. The best scanning electron microscopes can achieve a resolution better than 1 nanometer [24].
Even though SEM produces photographic information on the sample, but is unable to produce quantitative data on the sample surface. SEM operates under high vacuum, which is a significant drawback of this technique specially when applied in investigation of MIC. That’s because the biofilm, with its gelatinous consistence and cells of microorganism embedded into the extracellular matrix of the biofilm suffer shrinkage when the water content evaporates.
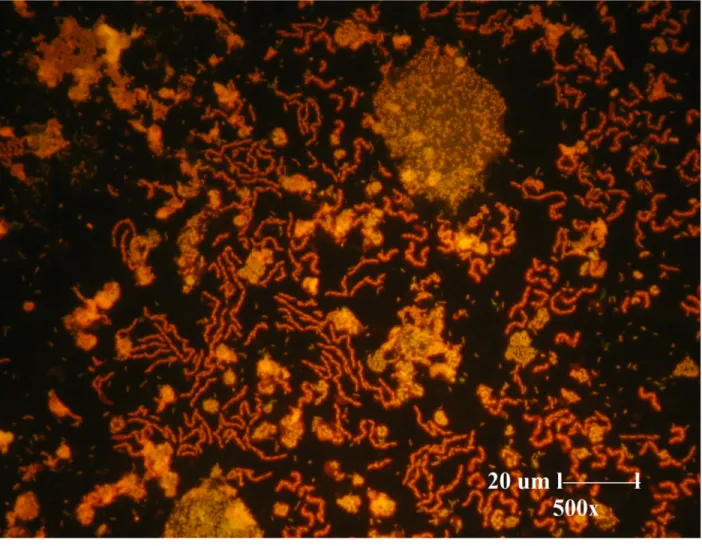
Figure 1 Fluorescence microscopic image of mixed culture isolated from oil well.
The ESEM was developed by substantial alteration of SEM. The ESEM instrument deviates substantially from the SEM as in the case of the ESEM only a part of the electrons can scan the spot as the other electrons are scattered on the gas particles (mainly water molecules or other inert gases), which allow the measurement at high pressure. The vacuum regions are separated from the high pressure and the low pressure instrumental area. The differential pumping enables
that the electron beam, because of scattering on different gas molecules, progressively loses electrons. The measure of the lost electrons depends on the gas molecules, pressure and the accelerating voltage. But at the end, the quantity of electrons that reach the surface is enough for scanning and imaging the solid surface. All these adjustments make the ESEM proper for imaging specimens in their natural state, i.e. the samples keep their water content and their structure is visualized in the original form, which helps the visualization of biological samples.
ESEM is useful tool to demonstrate the real structure of biofilms, cells embedded in the extracellular matrices on solid surfaces [25-27].
Specimens are placed in high vacuum chamber of SEM or low vacuum in case of environmental scanning electron microscope (ESEM), which helps in visualization of biological samples.
ESEM is useful tool to demonstrate the real structure of biofilms, cells embedded in the extracellular matrices on solid surfaces [25, 26].
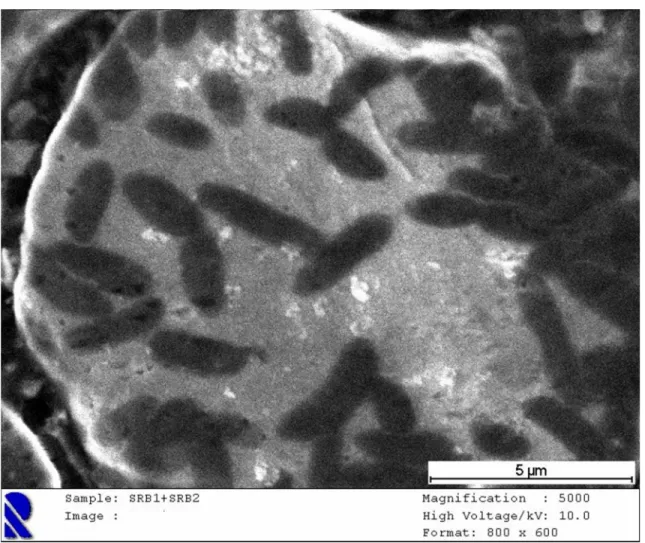
Figure 2 Backscattered SEM image of SRB on mild steel.
2.3 Atomic force microscopy (AFM)
Atomic force microscopy, which has very high resolution, was developed to extend the application field for visualization of not only conductive but also of non-conductive materials. It enables the study of surfaces with and without layers (passive oxide layer, molecular or thicker coatings etc.). The technique is based on the measurement of forces between the tip and the sample surface under investigation.
The AFM can be used for imaging, force measurement and manipulation experiments. From the MIC point of view, the imaging and the force measurements are mostly used.
When the imaging function is applied, 3D images provide important information on the form of the microbial cells, on the outer part of the cell surface, and via analyzing the images; the section mode furnishes information to the length and width of microorganisms. Additionally, the surface coverage by exopolymeric substances as well as the biofilm formation can be followed in time.
The other operational option is the measurement of the force formed between the tip and the surface. It could be followed in time during scanning or at focusing onto one spot on the surface.
In MIC studies, this operational option is importance to visualize not only the formation of scattered patchy EPS layer but also the continuous biofilm with various components as well.
AFM could visualize the topography of surface covered by different, corrosion relevant bacteria which produces iron sulfide and initializes localized accumulation of sulfide, regenerating anodic sites and, in the case of iron, the cathodic sites are also activated in the vicinity of the anodes [28, 29]. In contrast to the SEM the advantage of AFM is that it can provide higher resolution than SEM, and it does not require high vacuum. AFM is a versatile technique that allows visualization under atmospheric conditions as well as in liquid; additionally, it is possible to combine the AFM method with electrochemical measurement when the change in the electrochemical parameters (Ecorr, icorr, etc.) are followed parallel with the morphological consequence of the electric field [30].
The AFM operates by an ultra-fine needle (so-called tip) affixed to a cantilever. The extra sharp top of the needle (where only some atoms are present) runs over the surface. As the tip moves up and down due to the surface irregularity, the laser beam, which illuminates the cantilever, reflects and allows the direct measurement of the deflection by simply changing the angle of incidence for the laser beam. In this way, an electronic image is the result. There are different operating modes of the AFM. One is the "contact mode", when between the tip and the sample
repulsive force acts. In case of attracting force between the tip and the sample, the so-called
“non-contact mode” is the operating manner. The third action form is the “tapping-mode” when the tip moves constantly between the “contact” and “non-contact” force ranges. The measurement in tapping mode is very beneficial in case of soft samples; this mode is useful for biological samples, because the tip will not damage the soft surface (and the soft material will not contaminate the tip) [31]. This technique allows measuring the section of special objects and gives numerical values on the surface objects and its roughness. AFM is able for topographical imaging of bacteria, biofilm, and corroded steel surfaces in high-resolution, and for quantification of localized corrosion [32]. By the atomic force microscope, it is possible to study the surface topology of various materials in the presence of bacteria [33]. It provides not only the observation of specimens at molecular resolution, but also the quantification of surface feature.
The AFM has been useful in elucidating corrosion phenomena related to biofilms on metal surfaces. Bacterial colonization on a copper surface was observed by AFM and the pits were found to be associated with microbial activity [34].
AFM successfully visualized the microorganisms as single cells as well as embedded in biofilms, the formation of biopolymer network, and the deteriorating effect of corrosion relevant microbes in the microbiologically influenced corrosion, the slime with bacteria and the etched metal surface caused by the present of acid producer microorganisms [35-37].
2.4 Confocal laser microscopy (CLM)
CLM is widely used in the biological sciences. The instrument forms a physical barrier system (confocal apertures) to create a thin plane-of-focus in which out-of-focus light has been eliminated. A laser light source provides the intense, coherent, collimated light necessary to penetrate deep into a thick specimen. The laser light excites fluorophores, (intrinsic or added to the sample). Photomultiplier tubes detect the resulting fluorescence, and the results are digital images. Images of the x-y plane (parallel to the surface) are collected automatically as a computer-controlled stepping motor alters the z dimension (depth). The ability of CLM to resolve three-dimensional structures in the micrometer range allows the exploration of biofilm architecture in the native state: hydrated, living cells within an exopolymer matrix. CLM facilitates the visualization of biofilm structures by eliminating the interference arising from out- of-focus objects [37].
Figure 3 Pseudomonascells on mild steel (a,b) and their colony (c)
2.5 Confocal laser scanning microscopy (CLSM)
In this instrument a laser beam, which passes the surface and excites fluorophores, is focused into an area of the sample under investigation by an objective lens, and the collected emitted photons from the sample will realize images of the structure in 3D on the pre-selected depth level and time. The CLSM is suitable for in situ studying biofilms, and monitoring their formation.
The importance of this method is that the microorganisms are visualized in viable form; the distribution of the microbes within the biofilm is also monitored. Additionally, the visualizes interaction of bacteria (or other, corrosion relevant microorganisms) with the solid surface could help in understanding the MIC, as well as enables the kinetic study of MIC. The results got by this technique can be compared by results of other methods (e.g. EIS), it helps the more precise understanding of chemical processes caused by microbes, the determination of diffusion processes in the biofilm, and enables in situ registration of microbial responses to anodic and cathodic interactions.
2.6 Confocal Raman microscopy (CRM)
The spectral information in confocal microscopy can be obtained through different techniques such as absorption, reflection, transmission, emission, photoluminescence, fluorescence or Raman spectroscopy. In the 1990s an optical microscope was coupled with a Raman spectrometer, and the so-called micro-Raman spectroscopy was established. In this case, the microscope was used to focus the excitation light to a small spot of a few micrometers in diameter to obtain a Raman spectrum from a microscopic area. The advantage of using a microscope objective instead of a simple lens is the high collection efficiency for the Raman signal due to the high numerical aperture of the microscope objective. This technique, which was called Raman mapping, could be used to extract the relevant chemical information from each spectrum and to create a map of the distribution of the chemical components in a sample with a lateral resolution of a few micrometers [38].
It is important to reduce the fluorescence background as much as possible. Unfortunately, it is not always possible to have not any fluorescence; so in this case the confocal detection setup limits the collection of fluorescence to photons emitted from the focal plane. This reduces the fluorescence background signal, so that in many cases Raman images can be obtained. In biological samples, such as microbial cells and tissues, infrared spectra often show broad spectral features, which can give information regarding cellular components. The advantage of confocal
Raman microscopy is that it allows its use in industry and in academic as well as in bioscience research. The unique advantage of the confocal Raman microscopy is that it does not need any sample preparation, it gives improved axial and spatial resolution; and it furnishes detailed analysis of cells in their natural state. The images can contain full spectral information at each pixel so that the distribution of components within the cell can be visualized based upon their Raman signature [39]. Using confocal Raman method, the changes in a variety of cells, including bacteria can be monitored over time [40] and comparison between vial and dead cells can be easily analyzed. Sandt et al. [41] have used confocal Raman microspectroscopy successfully in the study of chemical heterogeneities of Pseudomonas aeruginosa biofilms in situ. They monitored the formation of extracellular polymeric substances (EPS). The spectroscopic signature of the cells in the biofilm and of the EPS were differentiated and their distribution in biofilm colonies and within water channels was mapped in-plane and in-depth.
Beier et al. [42, 43] have used confocal Raman microspectroscopy to differentiate species of bacteria grown in biofilms.
3. Piezoelectrical methods: quartz crystal microbalance (QCM)
QCM uses acoustic waves generated by oscillating a piezoelectric, single crystal quartz plate to measure mass variations. QCM is a sensitive gravimetric tool that has been used to a narrow extent in biofilm and MIC related studies. The advantages of the QCM include high sensitivity (ng.cm−2), continuous data in situ, the nondestructive of monitoring the processes on surfaces, besides being able to combine with other applied techniques such as voltammetry, EIS, optical spectroscopy, etc. QCM is a gravimetric tool that can measure mass changes on the crystal surface at nanogram levels, which makes it a useful tool for monitoring the kinetics of bacterial mass accumulation during the process of biofilm formation [44-46]. Originally developed for applications in the field of metal corrosion and its inhibition, QCM was first used to monitor biofilm formation inPseudomonas cepacia[44]. Several research works demonstrated that QCM is a useful technique for monitoring anchorage-dependent cell attachment and detachment on metal surfaces [47]. QCM long-term monitoring of biofilm formation of P. aeruginosa on gold combined with optical reflectance allowed studying the viscoelastic biofilm properties [48].
However, general limitations due to the viscoelastic nature of biofilms, modified systems were developed namely, the quartz crystal microbalance with dissipation mode (QCM-D). Changes in dissipation energy (ΔD) and crystal frequency (Δƒ) over time, predict the adhesion behavior of
bacteria, which enables the possibility to follow the bacteria interaction and adhesion process with the surface [49]. Resolution of frequency and dissipation in liquids is on the order of ± 0.1 Hz and 1 × 10−7, respectively [49]. The change in dissipation provides knowledge to how readily an adsorbed layer elastically deforms when the crystal shears (its elastic modulus) and how much the film repels deformation (its viscosity).
Incorporated simulation software, usually included in the instrument package with commercial instruments, permits modeling the experimental data with theory to extract meaningful parameters such as mass, thickness, density, viscosity, or storage modulus in order to characterize the biofilms [50].
The role of microbes in MIC processes is mainly due to their metabolisms associated with microbial growth and reproduction (by-products). Several papers appeared on bacterial adhesion but little attention was given to the use of QCM in monitoring biocidal activity on biofilm formation [47-53]. Even though QCM is an easy-to-use, low-cost device, but it has not been applied extensively in research laboratory studying biofilms and MIC related mechanisms.
4. Spectroscopic analytical methods
Spectroscopic analytical methods are based on measuring the amount of radiation produced or absorbed by molecular or atomic species of interest. Spectroscopical methods have been applied in microbiology related fields in different ways for quantitative and qualitative analysis. Two methods will be discussed are Fourier transform infrared spectroscopy (FTIR-spectroscopy) and X-ray photoelectron spectroscopy (XPS).
4.1 Fourier transforms infrared spectroscopy (FTIR)
In the last decades there was a significant development in the FTIR-spectroscopic technique (wavelength accuracy, spectral reproducibility). The wavelength interesting in the case of MIC study ranges between 2.5 and 20 μm i.e. in the medium infrared. The molecules are vibrationally excited, the remaining radiation results in adsorption bands at different frequencies, as well as vibrational and rotational bands. The adsorption bands expressed in wavenumbers has advantage as it is proportional to the absorbed energy. In the spectrum of functional groups present in molecules are represented in the 4000 and 1500 cm-1 range and the deformation, bending and ring vibrations are below 1500 cm-1. This range could denominate to the “fingerprint” of molecules.
Within the FTIR techniques there are special others like the transmission mode, diffuse reflectance and attenuated total reflectance spectroscopy (J. Schmitt et al [54].)
The advantage of FTIR in studying of biofilm was recognized more than 20 years ago as this technique is suitable for identification of microorganisms in a non-invasive way. Spectra got in non-destructive mode are fingerprints of microorganisms. By the FTIR-attenuated total reflection technique thein situ observation of biofilm formation is possible on a ATR crystal (germanium) as well as it is proper for differentiation between inorganic and organic materials and microbes.
The application of a flow cell allowed the investigation of binding mechanisms between the biofilm and the solid surface and it was proved that this binding process was caused by the EPS [55]. The identification of microbial samples by FTIR spectra was demonstrated by Helm et al.
[56]. The FTIR spectroscopy was successfully used by Nicholas et al. [57] to analyze bacteria alone, bacteria embedded in EPS matrix as well as in gelatinous, thick microbial biofilms. The disadvantage of the FTIR technique used to characterize MIC is that it cannot make and differences between vial and dead cells, it is useable only under laboratory conditions and the high water in the biofilm disturbs the spectrum. This is the reason that though the FTIR is a very important measuring technique, it should be combined together with other, electrochemical methods and the results should be compared.
4.2 X-ray photoelectron spectroscopy (XPS)
In this technique the sample under investigation, which is placed into ultra-high vacuum chamber, is irradiated with X-rays that leads to formation of photoelectrons. These photoelectrons (originate from some micrometer to some nm of the solid surface) with their characteristic energies represents the atoms they are originate from. The intensities of their spectra are proportional with the concentration of atoms and indicate their oxidation states. With other words, the XPS spectrum gives information about the surface composition and the chemical state. Another possibility to determine the depth profile by use of Ar+ bombardment is by changing the take-off angles of photoelectrons [58, 59]. XPS makes possible the determination of the cell surface composition and its modeling mainly by three molecular classes like polysaccharides, peptides and hydrocarbon-like compounds. There were experiments when the microbial cell surface structure of a group of special bacilli were determined [60] and they have found that the spectral carbon components varied significantly among strains but not the nitrogen and oxygen peaks. The individual cell components were also determined.
The disadvantage of the XPS technique is that it works under ultra-high vacuum. This is the reason that cooling devices should prevent the biological samples from dehydration and decomposition. Results got in chemical states should also be compared with similar results derives from other technique [62, 63]. Another problem is that this technique is not applicable under industrial conditions.
5. Summary.
As the MIC is recognized better and better by specialists, the analytical methods used for identify and characterize the MIC came into the focus in the last decades and the importance of these techniques is re-evaluated and further developed. One should keep in mind that several scientists of different fields (e.g. corrosion engineers, microbiologists) have been using as well as developing new techniques which are better capable to follow the damages caused by corrosion relevant microorganisms and give numerical data on its measure and on the kinetic of these undesired processes. The understanding of the MIC needs continuous collaboration among scientists and engineers as well as microbiologists.
This chapter gives a brief review on the techniques used for investigation of biocorrosion on solid surfaces in different oil and gas industrial environments.
Electrochemical methods reviewed in detail demonstrated the wide variety of techniques to measure the rate of microbial deterioration, to follow the kinetics of corrosion processes, and, at the same time, the advantages and disadvantages of each techniques used in MIC evaluation was pointed out. The problem is that, in the course of measurements, the electrode surface changes continuously because of electrode surface alteration caused by microbial adhesion and biofilm formation. Among the electrochemical techniques the most proper method for evaluation of microbial corrosion is the noise analysis but its disadvantage is the need of complex mathematical models to get the real current and voltage values. It is recommended to use, in parallel the other electrochemical methods in order to clearly see corrosive deterioration due to the presence of microbes and their metabolic activity. It is necessary to evaluate the biocorrosion by different (not only by) electrochemical methods and, after comparing the results, more precise evaluation of biocorrosion will take shape.
Biofilms formed in different environments under either field or laboratory conditions on naturally occurring or man-made surfaces have been extensively studied in various stages of bioorganic film development using a wide range of microscopy techniques. These investigations
provide mainly qualitative assessments, while surface chemical techniques can provide quantitatively estimation of the changes in surface composition caused by the biofilm formation and electrochemical corrosion processes.
Fluorescence microscopy allows visualization of microbial cells scattered on solid surfaces. This is a useful technique as, after staining with different dyes, not only the microorganisms are visible but we get information about their status (the cell are vial or dead, the different constituents of a cell are “illuminated” etc.). Scanning electron microscope provides quasi-3D images on single cells and colonies as well as patchy or continuous EPS layers (but mainly in dehydrated state). Additionally, the opportunity of EDX it informs us about the change in the surface composition caused by the presence of microorganism. The environmental scanning electron microscope works under less high vacuum, and this microscopy is more useful for investigation of microbes under “quasi normal” environment and visualize the biofilm in 3D form. Among the microscopic methods the CLM, CLSM, CRM and the AFM were detailed, in all cases both the advantages and the disadvantages were summarized in studying the biocorrosion. The advantage of the confocal Raman microscopy is that it needs no sample preparation and it is able to improved axial and spatial resolution over conventional microscopy, and it makes possible to perform extremely detailed analysis of cells in their natural state.
Atomic force microscope, which works under atmospheric conditions and also in liquid, visualizes the microbial cells, the mats and the biofilms under natural conditions in 3D and the analytical software enables to measure the dimension of microbes (characteristic for each species) as well as the pits they generate on the metal surface, and to calculate the roughening of surface caused by the presence of microorganisms and their biofilm. The introduction of spectroscopic techniques (FTIR, XPS) explains why and how these methods support the cognition and understanding of MIC.
The review of the various techniques explains the importance of parallel application of several methods in order to get more precise information about the corrosion initialized by microorganisms and to be sure that the microorganisms are responsible for the undesired deterioration.
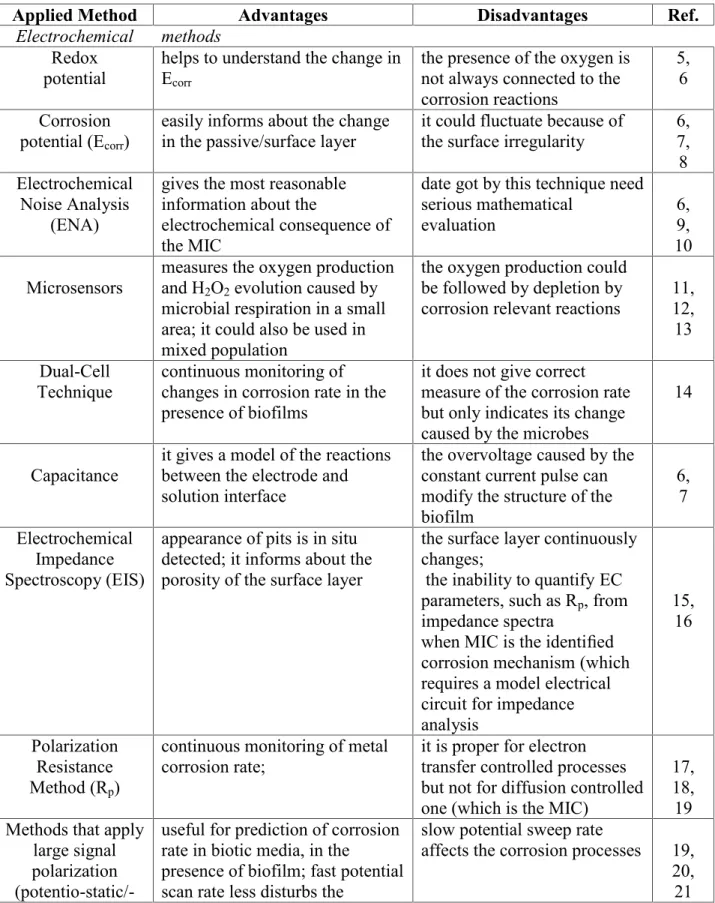
Table 1. A summary of advantages and disadvantages of methods applied in MIC related research investigations.
Applied Method Advantages Disadvantages Ref.
Electrochemical methods Redox
potential helps to understand the change in Ecorr
the presence of the oxygen is not always connected to the corrosion reactions
5,6
Corrosion
potential (Ecorr) easily informs about the change
in the passive/surface layer it could fluctuate because of
the surface irregularity 6, 7,8 Electrochemical
Noise Analysis (ENA)
gives the most reasonable information about the
electrochemical consequence of the MIC
date got by this technique need serious mathematical
evaluation 6,
109,
Microsensors measures the oxygen production and H2O2evolution caused by microbial respiration in a small area; it could also be used in mixed population
the oxygen production could be followed by depletion by
corrosion relevant reactions 11, 12,13
Dual-Cell
Technique continuous monitoring of changes in corrosion rate in the presence of biofilms
it does not give correct measure of the corrosion rate but only indicates its change caused by the microbes
14
Capacitance it gives a model of the reactions between the electrode and solution interface
the overvoltage caused by the constant current pulse can modify the structure of the biofilm
6,7
Electrochemical Impedance Spectroscopy (EIS)
appearance of pits is in situ detected; it informs about the porosity of the surface layer
the surface layer continuously changes;
the inability to quantify EC parameters, such as Rp, from impedance spectra
when MIC is the identified corrosion mechanism (which requires a model electrical circuit for impedance analysis
15,16
Polarization Resistance Method (Rp)
continuous monitoring of metal
corrosion rate; it is proper for electron transfer controlled processes but not for diffusion controlled one (which is the MIC)
17,18, Methods that apply 19
large signal polarization (potentio-static/-
useful for prediction of corrosion rate in biotic media, in the
presence of biofilm; fast potential scan rate less disturbs the
slow potential sweep rate
affects the corrosion processes 19, 20,21
dynamic methods) microbial activity Surface analytical methods
Scanning electron microscope (SEM), EDX Environmental scanning electron
microscopy (ESEM)
easy to use for monitor microbial adhesion and biofilm formation as well as the visualization of deteriorated metal surface; when combined together with EDX:
quantitative data on the surface composition
at the SEM: dehydration of the microorganisms/biofilm
destroys the original structure 24, 25,26, 27
Atomic force microscopy
(AFM)
visualization of vial and dead cells as well as microbial adhesion, biofilm formation under atmospheric condition;
numerical evaluation of microbes
it does not give any
information about the surface composition
28,29, 30,31, 32,33, 34,35, Confocal laser 36
microscopy (CLM);
Confocal laser scanning microscopy
(CLSM)
visualization of hydrated living cells and the exopolymeric matrix
use of stain; focal length can cause difficulties in
visualization of microbial
community 37
Confocal Raman microscopy
(CRM)
useful in studying chemical heterogeneity; distribution of microbes in biofilms could be mapped in-plane and in-depth
It cannot make distinction
between living and dead cells 38,39, 40,41, 42,43 Piezoelectrical methods
Piezoelectrical
methods, QCM easy-to-use technique,
cost effective the conductivity of the solution changes during the biofilm formation; difficult to obtain same alloy as applied in industry; unable to
differentiate between processes
44,45, 46,47, 48,49, 50,51, 52,53
Spectroscopical methods Fourier transform
infrared spectroscopy
(FTIR)
it canin-situanalyze the change
in the biofilm composition it can’t differentiate between
vial and dead cells 54,55, 56,57 X-ray
photoelectron spectroscopy (XPS) and Auger
electron spectroscopy
(AES)
Individual cell components and microbial cell surface structure could be measured
dehydration by ultra-high
vacuum 58,
59,60, 61,62, 63
References
[1] B. J. Little, J. S. Lee, Microbiologically influenced corrosion, Wiley Series in Corrosion. Hoboken, New Jersey: John Wiley & Sons, Inc. (2007).
[2] B. J. Little, P. Wagner, F. Mansfeld, An overview of microbiologically influenced corrosion, Electrochim.
Acta 37(12) (1992) 2185-2194.
[3] B. J. Little, F. B. Mansfeld, P. J. Arps, J. C. Earthman, Microbiologically Influenced Corrosion, In:
Encyclopedia of Electrochemistry, Wiley-VCH Verlag GmbH & Co., (2007), doi:10.1002/9783527610426.bard040603.
[4] L. Huabing, Z. Enze, Z. Dawei, X. Dake, X. Xia, Y. Chunguang, F. Hao, J. Zhouhua, I. Xiaogang, G.
Tingyue, Y. Ke, Microbiologically Influenced Corrosion of 2707 Hyper-Duplex Stainless Steel by Marine Pseudomonas aeruginosa Biofilm, Scientific Reports 6 (2016) 20190, doi:10.1038/srep20190.
[5] X. Zhang, R. A. Buchanan, E. E. Stansbury, N. J. E. Dowling, Electrochemical responses of structural materials to microbially influenced corrosion, CORROSION’89, NACE International, Houston, TX. (1989) No. 512.
[6] B. J. Little, P. A. Wagner, Application of electrochemical techniques to the study of MIC, In: Modern Aspects of Electrochemistry, New York: Kluwer Academic, Plenum Publishers (2001).
[7] W. H. Dickinson, F. Caccavo, Z. Lewandowski, The ennoblement of stainless steel by manganic oxide biofouling, Corros. Sci. 38 (8) (1996) 1407-1422.
[8] R. Javaherdashti, Microbiologically Influenced Corrosion, an engineering insight In: Engineering Materials and Processes, Springer-Verlag London Ltd. (2008).
[9] U. Bertocci, C. Gabrielli, F. Huet, M. Keddam. Noise resistance applied to corrosion measurements. I.
Theoretical analysis, J. Electrochem. Soc. 144 (1) (1997) 31-37.
[10]A. Padilla-Viveros, E. Garcia-Ochoa, D. Alazard. Comparative electrochemical noise study of the corrosion process of carbon steel by the sulfate-reducing bacterium Desulfovibrio alaskensis under nutritionally rich and oligotrophic culture conditions. Electrochimica Acta. 51 (2006) 3841-3847.
[11]W. Lee, D. de Beer, Oxygen and pH microprofiles above corroding mild steel covered with a biofilm, Biofouling 8 (4) (1995) 273-280.
[12]M. Kühl, C. Steuckart, G. Eichert, P. Jaroschewski, A H2S microsensor for profiling biofilms and sediments: application in an acidic lake sediment, Aquatic Microbiol. Ecology 15 (1998) 201-209.
[13]Z. Lewandowski, MIC and biofilm heterogeneity, CORROSION/2000, NACE International, Houston, TX, (2000) No. 400.
[14]R. B. Miller, A. Sadek, A. Rodriguez, M. Iannuzzi, C. Giai, J. Senko, C. N. Monty, Use of an Electrochemical Split Cell Technique to Evaluate the Influence of Shewanella oneidensis Activities on Corrosion of Carbon Steel, A. Al-Ahmad (ed), PLoS ONE 11 1 (2016) e0147899, http://doi.org/10.1371/journal.pone.0147899.
[15]P. L. Beese, H. Venzlaff, J. Srinivasan, J. Garrelfs, M. Stratmann, K. J.J. Mayrhofer, Monitoring of anaerobic microbially influenced corrosion via electrochemical frequency modulation, Electrochimica Acta, 105, (2013), 239–247, doi:10.1016/j.electacta.2013.04.144.
[16]M. Kendig, F. Mansfeld, S. Tsai, Determination of the long-term corrosion behavior of coated steel with A.
C. impedance measurements, Corros. Sci. 23 (4) (1983) 317-329.
[17]F. Mansfeld, The polarization resistance technique for measuring corrosion currents, in M.G. Fontana, R.W. Staehle (Eds.), Advances in Corrosion Science and Technology New York, Plenum Press 6 (1976) 163–262.
[18]F. Mansfeld, B. Little, A technical review of electrochemical techniques applied to microbiologically influenced corrosion, Corros. Sci. 32 (3) (1991) 247-272.
[19]Uhlig's Corrosion Handbook, third ed., R. Winston Revie (Ed.), John Wiley & Sons INC, (2011).
[20]G. Schmitt, Sophisticated Electrochemical methods for MIC investigation and monitoring, Materials and Corrosion, 48 (1997) 586–601, doi:10.1002/maco.19970480905.
[21]Zs. Kereszetes, J. Telegdi, J. Beczner, E. Kálmán, The influence of biocides on the microbiologically influenced corrosion of mild steel and brass, Electrochim. Acta 43 1-2 (1998) 77–85.
[22]A. Steele, D. Goddard, I. B. Beech The use of atomic force microscopy in study of the biodeterioration of stainless steel in the presence of bacterial biofilms, Int. Biodeter. Biodegr. 34 (1990) 35-46.
[23]Y. F. Dufrêne, Application of atomic force microscopy to microbial surfaces: from reconstituted cell surface layers to living cells, Micron 32 (2001) 153–165.
[24] R S Khandpur, Handbook of Analytical Instruments, third ed., McGraw Hill Education (India) Private Limited, (2015).
[25]A. B. Ronner, A. C. L. Wong, Biofilm development and sanitizer inactivation of Listeria monocytogenes andSalmonella typhimuriumon stainless steel and buna-n rubber, J. Food Prot. 56 (9) (1993) 750–758.
[26]J. W. Arnold, G. W. Bailey, Surface finishes on stainless steel reduce bacterial attachment and early biofilm formation: scanning electron and atomic force microscopy study, Poult Sci. 79 (12) (2000) 1839- 45.
[27]M. G. Langer, A. Koitschev, Methods in Cell Biology, In: B. P. Jena, J. K. H. Hörber (eds), Atomic Force Microscopy In Cell Biology, San Diego, Academic 68 (2002) 141–169.
[28]S. A. James, L. C. Powell, C. J. Wright, Atomic Force Microscopy of Biofilms-Imaging, Interactions, and Mechanics, (chapter 6), in: Microbial Biofilms - Importance and Applications, D. Dhanasekaran (Ed.), InTech, (2016), doi: 10.5772/63312, Available from: http://www.intechopen.com/books/microbial- biofilms-importance-and-applications/atomic-force-microscopy-of-biofilms-imaging-interactions-and- mechanics.
[29]G. Binning, C. Quate and Ch. Gerber, Atomic Force Microscope, Phys. Rev. Lett. 56 (1986) 930.
[30]U. Hasse, S. Fletcher, F. Scholz, J. Solid State Electrochem. 10 (2006) 833, doi:10.1007/s10008-006-0170- 7.
[31]Y. F. Dufrêne, D. Martínez-Martín, I. Medalsy, D. Alsteens, D. J. Müller, Multiparametric imaging of biological systems by force-distance curve–based AFM, Nature Methods 10 (2013) 847–854, doi:10.1038/nmeth.2602.
[32]L. Ch. Xua , K. -Y. Chanb, H. H. P Fanga, Application of atomic force microscopy in the study of microbiologically influenced corrosion, Materials Characterization 48 (2–3) (2002) 195–203, doi:10.1016/S1044-5803(02)00239-5.
[33]L. C. Xu, H. H. P. Fang, K. Y. Chan, Atomic force microscopy study of microbiologically influenced corrosion of mild steel, J. Electrochem. Soc. 146 (1999) 4455–4460.
[34]I. B. Beech, V. Zinkevich, L. Hanjangsit, R. Gubner, R. Avci, The effect ofPseudomonas NCIMB 2021 biofilm on AISI 316 stainless steel, Biofouling 15 (2000) 3–12.
[35]J. Telegdi, Zs. Keresztes, G. Pálinkás, E. Kálmán, W. Sand, Microbially influenced corrosion as revealed by atomic force microscopy, Appl. Phys. A 66 (1998) S639-S649, doi:10.1007/s003390051215.
[36]Zs. Keresztes, T. Rigó, J. Telegdi, E. Kálmán, Investigation of biopolymer networks by means of AFM, Applied Physics:A 72 (2001) S113-S116, doi:10.1007/s003390100680.
[37]M. Kuehn, M. Hausner, H-J. Bungartz, M. Wagner, P. A. Wilderer, S. Wuertz, Automated Confocal Laser Scanning Microscopy and Semi-automated Image Processing for Analysis of Biofilms, Applied and Environmental Microbiology 64 11 (1998) 4115-4127.
[38]T. Dieing, O. Hollricher, J. Toporski (Eds.). Confocal Raman Microscopy. Springer-Verlag Berlin Heidelberg (2010) ISBN 978-3-642-12521-8.
[39]Princeton Instruments, Confocal Raman Microscopy General Overview.
http://forum.sci.ccny.cuny.edu/core_facilities/microscopy-imaging/raman-confocal/Confocal-raman- note.pdf (Accessed 10thof November 2016).
[40]C. Xie, D. Chen, Y. Q. Li. Raman sorting and identification of single living micro-organisms with optical tweezers. Opt. Lett. 30 (2005) 1800-1802.
[41]C. Sandt, T. Smith-Palmer, J. Pink, L. Brennan, D. Pink. Confocal Raman microspectroscopy as a tool for studying the chemical heterogeneities of biofilms in situ. Journal of Applied Microbiology. 103 (2007) 1808-1820.
[42]B. D. Beier, R. G. Quivey Jr., A. J. Berger. Identification of different bacterial species in biofilms using confocal Raman microscopy. Journal of Biomedical Optics 15(6) (2010) 066001-1 – 066001-5.
[43]B. D. Beier, R. G. Quivey, A. J. Berger. Raman microspectroscopy for species identification and mapping within bacterial biofilms. AMB Express. 2(35) (2012) doi 10.1186/2191-0855-2-35.
[44]D. E. Nivens, J. Q. Chambers, T. R. Anderson, D. C. White, Long-term, on-line monitoring of microbial biofilms using a quartz crystal microbalance, Analytical Chemistry 65 (1993) 65–69.
[45]J. J. T. Babauta, C. A. Beasley, H. Beyenal. Investigation of Electron Transfer by Geobacter sulfurreducens Biofilms by using an Electrochemical Quartz Crystal Microbalance. Chem. Electro. Chem. 1(11) (2014) 2007 – 2016..
[46]J. Wegener, A. Janshoff, C. Steinem. The quartz crystal microbalance as a novel means to study cell- substrate interactions in situ. Cell. Biochem. Biophys. 34 (2001) 121-151.
[47]A.-C. Olofsson, M. Hermansson, H. Elwing. Use of a Quartz Crystal Microbalance To Investigate the Antiadhesive Potential of N-Acetyl-l-Cysteine. Appl. Environ. Microbiol. 71 (5) (2005) 2705-2712.
[48]V. Reipa, J. Almeida, KD Cole, Long-term monitoring of biofilm growth and disinfection using a quartz crystal microbalance and reflectance measurements, J. Microbiol. Methods, 66 (2006) 449–459, http://dx.doi.org/10.1016/j.mimet.2006.01.016.
[49]M. C. Dixon. Quartz Crystal Microbalance with Dissipation Monitoring: Enabling Real-Time Characterization of Biological Materials and Their Interactions. Journal of Biomolecular Techniques. 19(3) (2008) 151-158.
[50]D. Johannsmann. The Quartz Crystal Microbalance in Soft Matter Research. Fundamentals and Modeling.
Springer International Publishing, Switzerland (2015).
[51]A.-C. Olofsson, M. Hermansson, H. Elwing. Use of a Quartz Crystal Microbalance To Investigate the Antiadhesive Potential of N-Acetyl-l-Cysteine. Appl. Environ. Microbiol. 71 (5) (2005) 2705-2712.
[52]V. Reipa, J. Almeida, KD Cole, Long-term monitoring of biofilm growth and disinfection using a quartz crystal microbalance and reflectance measurements, J. Microbiol. Methods, 66 (2006) 449–459, http://dx.doi.org/10.1016/j.mimet.2006.01.016.
[53]J. Telegdi, A. Shaban, E. Kálmán. EQCM study of copper and iron corrosion inhibition in presence of organic inhibitors and biocides. Electrochimica Acta. 45(22-23) (2000) 3639-3647.
[54]J. Schmitt, H.-C. Flemming. FTIR-spectroscopy in microbial and material analysis. International Biodeterioration & Biodegradation 41 (1998) 1-11.
[55]A. Tunlid, D. E. Nivens, H. Jansson, D. C. White. Infrared monitoring of adhesion of Catenaria anguillulaezoospores to solid surfaces. Experimental Mycology 15 (1991) 206-214.
[56]D. Helm, H. Labischinski, G. Schallehn, D. Naumann. Classification and identification of bacteria by Fourier-transform infrared spectroscopy. Journal of general microbiology 137 (1991) 69-79.
[57]P. D. Nichols, J. M. Henson, J. B. Gugkert, D. E. Nivens, D. C. White. Fourier transform-infrared spectroscopic methods for microbial ecology: analysis of bacteria, bacteriapolymer mixtures and biofilms.
Journal of Microbial Methods 4 (1985) 79-94.
[58]S. Hofmann. Depth profiling. In: D. Briggs, M. P. Seah (Eds). Practical Surface Analysis by Auger and X- ray Photoelectron Spectroscopy. John Wiley & Sons Inc., New York (1983) 141–179.
[59]D. Briggs, J. C. Riviere. Spectral interpretation. In: D. Briggs, M. P. Seah (Eds). Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy. John Wiley & Sons Inc., New York (1983) 87–139.
[60]F. Ahimou, C. J. P. Boonaert, Y. Adriaensen, P. Jacques, P. Thonart, M. Paquot, P. G. Rouxhet. XPS analysis of chemical functions at the surface of Bacillus subtilis. Journal of Colloid and Interface Science 309 (2007) 49-55.
[61]S. L. McArthur, G. Mishra, C. D. Easton. Applications of XPS in Biology and Biointerface Analysis.
Chapter 2 in: V. S. Smentkowski (Ed). Surface Analysis and Techniques in Biology. Springer International Publishing Switzerland, ISBN 978-3-319-01359-6 (2014) 9-36.
[62]G. Chen, R. J. Palmer, D. C. White. Instrumental analysis of microbiologically influenced corrosion.
Biodegradation 8 (1997) 189-200.
[63]I. B. Beech, C. C. Gaylarde. Recent advances in the study of biocorrosion - an overview. Revista de Microbiologia 30 (1999) 177-190.