ContentslistsavailableatScienceDirect
Data in Brief
journalhomepage:www.elsevier.com/locate/dib
Data Article
Monthly data of stable isotopic composition ( δ 18 O, δ 2 H) and tritium activity in
precipitation from 2004 to 2017 in the Mecsek Hills, Hungary
István Fórizs
a,∗, Zoltán Kern
a,∗, József Csicsák
b, Gergely Csurgó
b, Gábor Földing
b, Zoltán Máthé
b, János Ország
c, Géza Szreda
b, Roland Vendégh
baInstitute for Geological and Geochemical Research, Research Centre for Astronomy and Earth Sciences
bMECSEKÉRC Zrt. Esztergár Lajos u. 19. H-7633 Pécs, Hungary
cPublic Limited Company for Radioactive Waste Management (PURAM), H-7031 Pf. 12. Paks, Hungary
a rt i c l e i n f o
Article history:
Received 5 June 2020 Revised 14 August 2020 Accepted 18 August 2020 Available online 21 August 2020 Keywords:
Precipitation isotope monitoring
3H
Oxygen isotopes Hydrogen isotopes D-excess Isotope hydrology Hungary
a b s t r a c t
The stableisotopiccomposition(δ18O, δ²H)and tritium ac- tivityofmonthlyaggregatedprecipitationsampleswerecol- lectedbetween April 2004and December2017 atsix sites representingthefirstpublishedprecipitationisotopedataset fromthe MecsekHills (Hungary). Thedataset includes 697 stableisotopicand653tritiumactivityconcentrationdataof monthly precipitationsamples collectedacross the Mecsek Hills.Atthebeginningofthemonitoringperiod,theisotopic compositionvaluessuggestaninsufficientprotectionagainst evaporationandthisissuehasoccasionallyreappearedlater onlyinlimitedperiods.Thesedataarepresentedinbrackets intheSupplementaryTableandshouldbedisregardedfrom furtheranalysisuntiladditionalverification.Thisdatasetpro- vides isotope hydrological benchmark in comparison with otherlocaland regionaldatasetsofstableisotopesand tri- tiumactivitiesinsurfacewaterandgroundwaternotonlyin the Mecsek Hills butalso inthe surroundings. It can sup- portwaterresourcemanagement,andpaleoclimatologicalre- search.Isotopehydrologicalevaluationandfurtherdiscussion
∗ Corresponding author.
E-mail addresses: forizs@geochem.hu (I. Fórizs), zoltan.kern@gmail.com (Z. Kern).
https://doi.org/10.1016/j.dib.2020.106206
2352-3409/© 2020 The Author(s). Published by Elsevier Inc. This is an open access article under the CC BY-NC-ND license ( http://creativecommons.org/licenses/by-nc-nd/4.0/ )
onthe seasonaltrendsinthe precipitationisotopiccharac- teristics areinprogress and thetritium datawereused in the derivationofagridded database(1×1km)ofamount- weighted annualmeanprecipitationtritiumactivityforthe Adriatic-PannonianRegion(AP3H_v1,[1]).
© 2020TheAuthor(s).PublishedbyElsevierInc.
ThisisanopenaccessarticleundertheCCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/)
SpecificationsTable
Subject Earth and Planetary Sciences
Specific subject area
isotope hydrology
Type of data Table
Figure How data were
acquired
Aggregated monthly precipitation samples were collected. Stable oxygen and hydrogen isotope composition of monthly precipitation samples were measured by isotope ratio mass spectrometry from 2004 to 2010, and by laser spectrometry (with cavity enhanced absorption technique) from 2011 to 2017.
Tritium activities have been analyzed by low-level liquid scintillation counting following electrolytic enrichment.
Data format Raw data are provided in Excel files as supplementary material.
Parameters for data collection
No pretreatment was required before the stable isotope analysis. Electrolytic enrichment was applied before the analysis of the tritium activities.
Description of data collection
Stable oxygen and hydrogen isotopic compositions were measured by isotope ratio mass spectrometry using a dual inlet Finnigan MAT Delta S, and Finnigan Delta plusXP mass spectrometer in continuous-flow mode until the end of 2010, and by laser spectrometry using a Liquid Water Isotope Analyser (LWIA-24d, Los Gatos Research) thereafter. Tritium activities have been analyzed by low-level liquid scintillation counting following electrolytic enrichment.
Data source location
Six sites in the Mecsek Hills, Hungary Z 46.03744 °N 18.12443 °E 117 m a.s.l.
B 46.06854 °N 18.11269 °E 172 m a.s.l.
V 46.12153 °N 18.09230 °E 330 m a.s.l.
II 46.09927 °N 18.09149 °E 332 m a.s.l.
Boda 46.08668 °N 18.04671 °E 233 m a.s.l.
Het 46.12524 °N 18.04776 °E 165 m a.s.l.
Data accessibility With the article
ValueoftheData
• Thedatasetprovidesbenchmarkdataforisotopehydrologicalevaluationofthesurfacewater andgroundwaterintheMecsekregion(e.g.estimatingthemeanresidencetimeofstreamwa- terandkarsticreservoirs).
• Thedataareespeciallyimportantbecausethey providenecessarybasicisotopehydrological background datafortheenvironmentalmonitoring oftheplanned highactivityradioactive wasterepository.
• Regionalpaleoclimatologicalresearchcouldalsobenefitfromthesedatabecausetheisotopic
‘temperature’effectinmodernprecipitationintheMecsekregioncanbeinferred,whichcan beacrucialparameterinpaleoclimatologicalapplications.
• Thedatasetprovidesreferencedatafor(i)authenticityandtraceability ofagriculturalprod- ucts from this region e.g. forlocal wines, and (ii) archaeological research of migration of peoples,animalsandobjectsmadeoforganicmaterial.
1. DataDescription
Thestudyoftheisotopiccompositionofsurfaceandgroundwaterstartedin2004(Fig.1)in theMecsek region(South Hungary)inordertoprovidefield datatohydrogeological modeling forthegeologicalresearchprogrammeofBodaClaystoneFormation(BCF)withinthesiteselec- tion ofa planned highactivityradioactivewaste repository.The research programmeis man- agedbythePublicLimitedCompanyforRadioactive WasteManagement.Precipitationsamples werecollectedbytheMECSEKÉRCZrt,stableisotopeanalysiswasperformedbytheInstitutefor
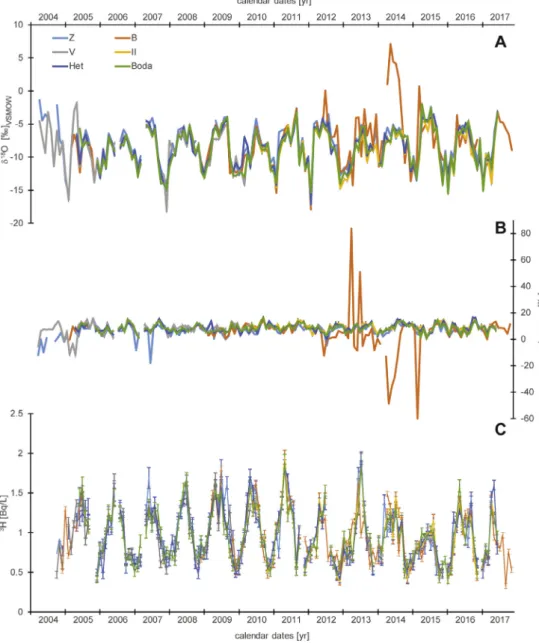
Fig. 1. Isotopic characteristics of monthly precipitation collected in the Mecsek Hills (Hungary) between April 2004 and November 2017. Stable oxygen isotopic composition (A), d-excess (B), and tritium activity concentration (C).
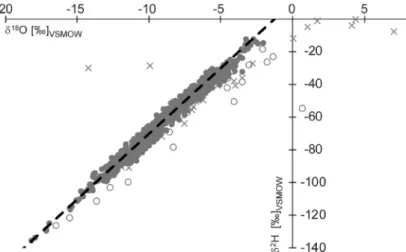
Fig. 2. Distribution of monthly averaged δ2H–δ18O for precipitation in the Mecsek Hills between 2004 and 2017. The dashed black line shows the Global Meteoric Waterline [3] . Data obtained from samples before May 2005 are marked by open circles and the extreme outliers in the dataset of station B (from July 2012 to August 2014 and also in March 2015) are marked by crosses.
Geological andGeochemical Research,Research Centre for Astronomy andEarth Sciences and itspredecessor(Institute forGeological andGeochemicalResearch,HungarianAcademyofSci- ences),andtritiumactivityofthewatersampleswereanalyzedbytheHYDROSYSLaborLtd.
AuniquemonthinthedatasetisApril2007whentherewasnoprecipitationatanystation ofthenetwork,howeverNovember2011,December2013,andDecember2016werealsorather drymonthswhensomeofthestationsdidnotrecordprecipitation.Inaddition,inmonthswith lowprecipitationamountitwasaquite frequentsituationthatthecollectedprecipitationwas sufficientonlyforstableisotopeanalysisandtherewasnotenoughmaterialfortritiumactivity measurements.Inonecase, October2010thesamplesubmittedforstableisotopeanalysishas beenlost.Altogether,stableoxygen andhydrogenisotopiccompositionsare presentedfor697 samplesandTritiumactivityisavailablefor653samples(SupplementaryTable).
The time series of the primary isotopic parameters of monthly precipitation data show a highdegreeofcoherency(Fig.1).Therearetworemarkableperiodswhenthecoherencyinthe stableisotope data is weaker. Especially d-excess [2] indicates the problematic data in these periods becaused-excess values obviouslystand out fromthe typical rangeof distribution of thisparameter.ThefirstperiodisfromthebeginningoftherecordtoMay2005andthesecond oneisbetweenJuly2012andMarch2015.Inthefirstperiodunusuallylowd-excessvalueswere observedatstationsZandVsuggestingevaporationeffectuntilthecollectorvesselwasburied.
FailureinevaporationprotectioncanbeassumedforsamplescollectedinFebruaryandJulyof 2007atstationZ.There isno deviationinthemetadata ofstationB whichcould explain the extremelyanomalousvaluesseenintherecordfromJuly2012toAugust2014andalsoinMarch 2015.
For the sake of completeness these potentially problematic data are also reported in the dataset,howeverpresentedinbracketsanditisrecommendeddisregardingtheseisotopicdata inisotopehydrologicalcharacterizationofthemodernprecipitationintheMecsekHills.Tritium activitydata donot seem to reflectthesedisturbances (Fig.1c). The point cloud inthe dual- isotopespacedefinedbythevastmajorityofthedata(Fig.2)fitsnicelytotheGlobalMeteoric WaterLine,describingtheglobalrelationoftheseparameters[3].Hencethesedatawillbesuit- ableinputdataforfurtherisotopehydrologicalmodellingandprovidearobustmodernreference forpaleoclimatestudies.Wepointoutagainthatproblematicdata,presentedinbracketsinthe SupplementaryTable,shouldnotbeconsidereduntiladditionalverification.
2. ExperimentalDesign,MaterialsandMethods 2.1. Samplecollection
The collectionof precipitationsamples forisotope hydrological monitoringstartedin April 2004attwostations(Z andV)andfora shortperiod(fromNov2004toNov2005) atstation B. Dailyprecipitation sampleswere collectedineach morning andaggregatedina 5Lplastic (HDPE) container on a monthlybasis. Winter precipitationwas collectedby the same instru- mentation as(sono heatedfunnelwasused) ifsnow hadaccumulatedin thecollectorwhen thesamplewasharvested,itwasaddedtothecollectionvesselbymelting.Specialprecautions forevaporationpreventionwasnotappliedwhenmeltingthesnow.Thenetworkwasexpanded withtwoadditionalstationsfromMay2005.Fromthenon,thesamplecollectingvessels were placed ingroundcavity,primarily toprevent summerevaporationandwinterfreezing. Thisis an approvedoptionforcollectorconfigurationforprecipitationisotopeanalysis(‘Buriedcollec- tor’:[4]).MonitoringceasedatstationVbyMayof2010andthecollectionplacewasmovedto
∼4kmsouthward(stationII)by June2011.Thesamplecollectorswere installedintherecom- mendedsafetydistancefrompotentialphysicalbarriers[4].Thewatersamplesweretransferred toleak-proofplasticbottleandshippedtolaboratories. Sampleswere keptinglassvialsclosed withplasticcapswithconicalinteriorinrefrigerator.
2.2. Analyticalmethods
Stableisotoperatiosinwaterwereanalyzedinthedifferentperiodsoftimebythefollowing methodsandanalyticalinstruments:
2.2.1. From2004to2005
- the
δ
18Ovalueofwaterwasdeterminedby FinniganMAT deltaS massspectrometerusing CO2 gasequilibratedwithH2Oat20°Ctemperatureduringovernight,wheresamplecontain- ingvesselswereshaken.ThedetailsofthesamplepreparationsetupisdescribedbyRoether [5],theoriginalprincipleistheonedescribedbyEpstein&Mayeda[6].- the
δ
2Hvalue ofwater wasdetermined by Finnigan MAT deltaSmass spectrometerusing H2 gasliberatedfrom3μ
Lwaterbyreactingwith100mgspecialzincalloy[7]inasealedvacuumglasstubeat500°Cduring30min[8,9].
The applied laboratory standard was the BTW (Budapest Tap Water) calibrated against SMOW andSLAP. The normalizationfactors [10] for both H and O, which were found to be rather stable, were determined circa monthly by applying reference materials W-63333 as- signed
δ
2HVSMOW-SLAPandδ
18OVSMOW-SLAPvaluesof-399.8and–52.28‰,andW-39500assignedδ
2HVSMOW-SLAP andδ
18OVSMOW-SLAP valuesof-1.5and-1.52‰,respectively[11,12,13].Theseref- erencematerialswerekindlyprovidedbyTylerB.Coplen,theRestonStable IsotopeLaboratory (RSIL),ofU.S.GeologicalSurvey(USGS).Thereproducibilitywasaround±0.06–0.07‰foroxygen.Whenthedifferencebetweenthe duplicates exceeded 0.2‰(foroxygen)then we dida thirdanalysis(or a fourthoneifit was recommended)forthesakeofkeepingthereproducibilitybelow±0.1‰.Occasionallywerean- alyzedsomesamplesafterseveralmonthsandtheremeasureddeltavalueswerealwayswithin the±0.1‰rangetotheoriginallydeterminedone.Thereforewestatethattheoverallestimated (sample storage+sample preparation+analysis) uncertainty was±0.1‰ foroxygen and ±1‰ forhydrogen.
2.2.2. From2006to2010
- the
δ
18O value of water was determined by Finnigan DeltaplusXP mass spectrometer in continuous-flowmode. The sample preparingprocess before themass spectrometric mea- surementwas the following: 1 mL waterfrom each sample (unknown andstandard) waspipetted intoa 10mLvialclosedbyseptum+cap.EachvialwasflushedbyHegascontain- ing 0.3v/v%CO2 during6minutes,andthewhole setwaskeptina heatingblock at32°C forminimum18hoursforgettingtheisotopicequilibriumintheCO2-H2Osystem.
- the
δ
2H value of water was determined by Finnigan DeltaplusXP mass spectrometer in continuous-flow mode. The samplepreparing process before the mass spectrometric mea- surement wasthe following: platinized catalyzer wasinserted and 1mL water fromeach sample(unknownandstandard)waspipettedintoa10mLvialclosedbyseptum+cap.Each vialwasflushedbyHegascontaining2.1v/v%H2 during6min,andthewholesetwaskept ina heatingblock at32°C forminimum40minforgettingtheisotopic equilibriuminthe H2-H2Osystem[14].The applied laboratory standard was the BTW (Budapest Tap Water) calibrated against SMOW and SLAP. The normalization factors [10] for both H andO, which were found to be veryclose to1.0, were determined circa weekly by applyingreferencematerials W-63333 as- signed
δ
2HVSMOW-SLAPandδ
18OVSMOW-SLAPvaluesof-399.8and–52.28‰,andW-39500assignedδ
2HVSMOW-SLAP andδ
18OVSMOW-SLAP valuesof-1.5and-1.52‰,respectively[11,12,13].Theoverall estimated(sample storage+sample preparation+analysis) uncertainty was±0.2‰ foroxygen and±2‰forhydrogen.2.2.3. Since2011
Both
δ
2H andδ
18O values of water were determined by a Liquid Water Isotope Analyser(LWIA-24d,Los GatosResearch). Thesamplepreparing andmeasurementprocess wasthefol- lowing:1mLwaterwaspipettedintoeach 2mLvialclosedwithseptum+cap.Thevialswere arrangedonthetrayinthefollowingway:3labstandards– 5unknownsamples– 3labstan- dards,etc.inthesaketopreventtheeffectofambienttemperaturevariation[15].Anautosam- pler(CTCAnalyticsGCPAL)usingaHamiltonsyringetook 1
μ
Lwaterfromthe2mLvialandinjecteditthroughapreformedseptumintothevaporizerofthelaseranalyzerwherethewater wasevaporated at80°Cinlow vacuuminto thecavityoftheanalyzer.From eachvial6injec- tions were carried out,but becauseof memoryeffect only thedata of thelatest 4 injections weretakenintoaccount.Theoverallestimateduncertaintywas±0.2‰foroxygenand±1‰for hydrogen.Theappliedlaboratorystandardswerethefollowingoneswiththeassigned
δ
2Handδ
18Ovaluesinbrackets:BWS1(-9.0;-0.53),BWS2(-74.9;-10.41),BWS3(-147.7;-19.95)[16].Eachsampleduringboth themassspectrometric andlaser instrumentalanalysiswasmea- suredin duplicate and the meanvalues are here presented. All stableisotope measurements werecarriedout intheInstituteforGeologicalandGeochemicalResearch,ResearchCentrefor AstronomyandEarthSciences(Budapest,Hungary)anditspredecessors.
Theresultsare expressedinthestandard
δ
-notation[17] relativeto ViennaStandard MeanOceanWater:
δ
(‰)=(R−Rstd)/Rstd∗1000[‰]VSMOW,whereRandRstdare18O/16Oor2H/1Hratiosinthesampleandinthestandard(ViennaStandard MeanOceanWater-VSMOW),respectively[18].
Duringthetransitionfrommassspectrometrictolaserspectroscopicanalysesseveralsetsof sampleswere measuredby both techniques,anditwasfound that themeasured deltavalues werethesamewithintheanalyticaluncertainties.Thelaboratory– usingbothmassspectrom- eters(FinniganMATDeltaS,andFinniganDeltaplusXP)andtheLiquidWaterIsotopeAnalyser– hassuccessfullyparticipatedinthewaterisotopelaboratoryproficiencytests[19].
A secondary isotopic parameter of natural waters, d-excess [2], has been calculated as d=
δ
2H-8×δ
18O.Tritiumactivitieshavebeenanalyzedbylowlevelliquidscintillationcountingfollowingelec- trolyticenrichmentatHYDROSYS LaborLtd, Budapest.Tritiated water,SRM4361C H-3 (NIST), wasused asstandard referencematerial.Activityconcentration ofthesamplesis expressedin Bq/L.
DeclarationofCompetingInterest
Theauthorsdeclarethattheyhavenoknowncompetingfinancialinterestsorpersonalrela- tionshipswhichhave,orcouldbeperceivedtohave,influencedtheworkreportedinthisarticle.
Acknowledgments
TheauthorsacknowledgetheconsentofPublicLimitedCompanyforRadioactiveWasteMan- agement andMECSEKÉRCZrt.to publishtheir data. Thiswork wassupported by the National Research,DevelopmentandInnovationOffice,Hungary[SNN118205].
Supplementarymaterials
Supplementary material associatedwiththisarticle canbe found, inthe onlineversion, at doi:10.1016/j.dib.2020.106206.
References
[1] Z. Kern, D. Erdélyi, P. Vre ˇca, I. Krajcar Broni ´c, T. Kandu ˇc, M. Štrok, I. Fórizs, L. Palcsu, M. Süveges, G. Czup- pon, B. Kohán, I.G. Hatvani, Isoscape of amount-weighted annual mean precipitation tritium ( 3H) activity from 1976 to 2017 for the Adriatic-Pannonian region - AP 3H_v1 database, Earth Syst, Sci. Data 12 (2020), doi: 10.5194/
essd- 12- 2061- 2020 .
[2] W. Dansgaard , Stable isotopes in precipitation, Tellus 16 (1964) 436–468 . [3] H. Craig , Isotopic variation in meteoric waters, Science 133 (1961) 1702–1703 .
[4] IAEA GNIP Precipitation sampling guide, http://www-naweb.iaea.org/napc/ih/documents/other/gnip _ manual _ v2.02 _ en _ hq.pdf , 2020 (accessed 10 May 2020).
[5] W Roether , Water-CO 2Exchange Set-up for the Routine 18Oxygen Assay of Natural Water, Int. J. Appl. Radiat. Isot.
21 (1970) 379–387 .
[6] S. Epstein , T. Mayeda , Variation of O 18 content of waters from natural sources, Geochim. et Cosmochim. Acta 4 (1953) 213–224 .
[7] J.M. Hayes , M.W. Johnson , Reagent and procedure for preparation of H 2for hydrogen-isotopic analysis of water, Indiana University, Bloomington (Indiana, USA) (1988) .
[8] M.L. Coleman , T.J. Shepherd , J.J. Durham , J.E. Rouse , G.R. Moore , Reduction of Water with Zinc for Hydrogen Isotope Analysis, Anal. Chem. 54 (1982) 993–995 .
[9] A. Demény , H-isotope fractionation due to hydrogen-zinc reactions and its implications on D/H analysis of water samples, Chem. Geol. 121 (1995) 19–25 .
[10] D. Paul , G. Skrzypek , I. Fórizs , Normalization of measured stable isotopic compositions to isotope reference scales – a review, Rapid Commun, Mass Spectrom. 21 (20 07) 30 06–3014 .
[11] A. Demény , S. Kele , Z. Siklósy , Empirical equations for the temperature dependence of calcite-water oxygen isotope fractionation from 10 to 70 °C, Rapid Commun, Mass Spectrom. 24 (2010) 3521–3526 .
[12] H. Qi , T.B. Coplen , L. Tarbox , J.M. Lorenz , M. Scholl , USGS48 Puerto Rico precipitation – a new isotopic reference material for δ2H and δ18O measurements of water, Isot. Environ. Health Stud. 50 (2014) 4 42–4 47 .
[13] T.B. Coplen , J. Hopple , in: Audit of VSMOW distributed by the United States National Institute of Standards and Technology, IAEA-TECDOC-825 Reference and intercomparison materials for stable isotopes of light elements, IAEA, Vienna, 1995, pp. 35–38 .
[14] S.J Prosser , C.M. Scrimgeour , High-precision determination of 2H/ 1H in H 2and H 2O by continuous-flow isotope ratio mass spectrometry, Anal. Chem. 67 (1995) 1992–1997 .
[15] G. Lis , L.I. Wassenaar , M.J. Hendry , High-Precision Laser Spectroscopy D/H and 18O/ 16O Measurements of Microliter Natural Water Samples, Anal. Chem. 80 (2008) 287–293 .
[16] Gy. Czuppon , A. Demény , Sz. Leél- ˝Ossy , M. Óvari , M. Molnár , J. Stieber , K. Kiss , K. Kármán , G. Surányi , L. Haszpra , Cave monitoring in the Béke and Baradla Caves (Northeastern Hungary): implications for the conditions for the formation cave carbonates, Int. J. Speleol. 47 (2018) 13–28 .
[17] C.R. McKinney , J.M. McCrea , S. Epstein , H.A. Allen , H.C. Urey Improvements in mass spectrometers for the measure- ment of small differences in isotope abundance ratios, Rev. Sci. Instrum. 21 (1950) 724–730 .
[18] T.B. Coplen , Reporting of stable hydrogen, carbon and oxygen isotopic abundances, Pure App. Chem 66 (1994) 273–276 .
[19] L.I. Wassenaar, S. Terzer-Wassmuth, C. Douence, L. Araguas-Araguas, P.K. Aggarwal, T.B. Coplen, Seeking excel- lence: an evaluation of 235 international laboratories conducting water isotope analyses by isotope-ratio and laser- absorption spectrometry, Rapid Commun, Mass Spectrom. 32 (2018) 393–406, doi: 10.1002/rcm.8052 .