Symposia Biologica Hungarica
SYMPOSIUM
ON THE MUSCLE
Akadémiai Kiadó, Budapest
S Y M P O S IU M O N T H E M U S C L E (S y m p o sia B io lo g ica H u n g a r ic a 17)
E d ite d b y
E . N. A. B ÍR Ó a n d N. G ARAM V Ö LG Y I
T h e E u ro p e a n M uscle C lu b h e ld its th i r d m e e tin g in B u d a p e s t in 1974.
T h is b o o k c o n ta in s th e p a p e rs o f o u t s ta n d in g re s e a rc h e rs a n d s c ie n tis ts d is cu ssin g th e m o s t tim e ly p ro b le m s o f m u scle re s e a rc h a n d s u p p ly in g n u m e r o u s v a lu a b le d a t a o n t h e su b je c t.
Som e c o n tr ib u tio n s r e p o r t on c o m p le t
ed e x p e rim e n ts w h ile o th e r s d e a l o n ly w ith th e m o s t sig n ific a n t a sp e c ts o f a re s e a rc h w o rk . T h e m e e tin g o f th e E u ro p e a n M uscle C lu b is o f g r e a t im p o rta n c e a s t h e q u ic k m o to riz a tio n a n d m e c h a n iz a tio n s e rio u sly e n d a n g e r th e h u m a n ity a n d it s e n v iro n m e n t.
T h e v o lu m e is re c o m m e n d e d fo r th o s e w o rk in g in th i s sp ecial field o f science.
A K A D É M IA I K IA D Ó
Publishing H ouse o f th e H u n g arian A cadem y o f Sciences
B U D A P E ST
ISBN 963 05 1006 5
Symposia Biologica Hungarica
17
Symposia Biologica Hungarica
R e d ig it
E . N . A . B Í R Ó e t N . G A R A M V Ö L G Y I
Vol. 17
A K A D É M I A I K I A D Ó , B U D A P E S T 1976
SYMPOSIUM ON THE MUSCLE
E d ite d b y
E. N. A. BIRO
and
N. G A R A M V Ö L G Y I
A K A D É M I A I K I A D Ó , B U D A P E S T 1976
The symposium was held in B udapest, 31 August 1 Septem ber, 1974
ISBN 963 05 1006 5
© A k a d é m ia i K ia d ó , B u d a p e s t 1976 P r i n te d in H u n g a r y
CONTENTS
L is t o f P a r t ic i p a n ts 7
F o re w o rd 11
O p en in g a d d re s s 13
CALCIUM AN D R EG U LA T IO N
Ha m o ir, G.:
E a r li e r a n d r e c e n t w o rk o n p a rv a lb u m in s 17
Pe c h è r e, J . F .:
Som e r e c e n t r e s u lts in th e s t u d y o f m u s c u la r p a rv a lb u m in s 35 C o x , J . , Be n z o n a n a, G. Ko h l e r, L . a n d St e i n, E . A .:
A n e w c a lc iu m -b in d in g p r o te in in c ru s ta c e a n t a i l m u sc le 39 Mu l v a n y, M . J .:
P o ss ib le m e c h a n is m b y w h ich c a lc iu m a ffe c ts th e m e c h a n ic a l p r o p e r tie s o f
rig o r fro g m u sc le s 45
Al b r e c h t, V ., Sc h u l z e, W ., Ga s s m a n n, B . a n d Ra p o p o r t, S. M .:
S tu d ie s o n th e b io c h e m is try o f rig o r m o r tis (A b s tra c t) 51 Rü e g g, J . C., Ku h n, H . J . , He r z ig, J . W . a n d Dic k h a u s, H .:
E f f e c t o f c a lc iu m io n s a n d A T P o n th e s e rie s -e la s tic ity o f g ly c e r o l- e x tra c te d
m u s c le fib re s 63
Ze l c k, TL, Jo n a s, L ., Ka r n s t e d t, U . a n d Po p e s c u, L . M .:
C o rre la te d b io c h e m ic a l a n d u ltra h is to c h e m ic a l s tu d ie s o n C a lo c a lis a tio n
a n d C a m o v e m e n t in s m o o th m u s c le (A b s tra c t) 57
Bo t ta zzo, G. F ., Fl o r in-Ch r is t e n s e n, A . a n d Do n ia c h, D .:
C la ss ific a tio n o f h u m a n s m o o th m u s c le a n tib o d ie s (SM A) b y im m u n o
flu o re sc e n c e 59
Ga r a m v ö l g y i, N .:
S a rc o p la sm ic re tic u lu m a n d tr ia d s in h u m a n s t r ia t e d m u sc le 61
M ECHANICS AND E N E R G E T IC S
Pr i n g l e, J . W . S.:
T h e r e s tin g e la s tic ity o f in s e c t f lig h t m u sc le 67
Op l a t k a, A ., Tir o s h, R ., Lir o n, FT. a n d Bo r e j d o, J . :
M ech a n o c h e m ic a l c o u p lin g in s y s te m s in w h ic h th e m y o s in h e a d s d o n o t fo rm p a r t o f a n a g g re g a te d s t r u c tu r e a n d m a y o r m a y n o t fo r m p a ir s 79 He r z ig, J . W . a n d He r z ig, U . B .:
E f f e c t o f C a-ions o n c o n tr a c tio n sp eed a n d fo rce g e n e ra tio n in g ly c e r in a te d
h e a r t m u s c le 85
5
Tr o m b it á s, К . a n d Ti g y i-Se b e s, A .:
I r r e g u la r c h a n g e s o f th e s t r ia t e d p a t t e r n in f lig h t m u s c le o f th e in s e c t 89 Pa u l, R . J . a n d Ku s h m e r ic k, M . J . :
C o m p a riso n o f in itia l a n d re c o v e r y m e ta b o lic p ro cesses in is o m e tric te t a n i
o f fro g s a r to r iu s m u sc le 93
EX C IT A T IO N
Va r g a, E ., Da n k ó, M ., Do m o n k o s, J . a n d Ge s z t e l y i, I . :
In v e s tig a tio n o f m e ta b o lic d e p e n d e n c y o f m e m b ra n e p o te n t ia l o sc illa tio n
e v o k e d b y v e r a tr in e o n s k e le ta l m u s c le 101
Kó n y a, L . a n d Kö v é r, A .:
S tu d ie s o n th e m e c h a n is m o f th e e le c tro -m e c h a n ic a l c o u p lin g in fro g s k e le ta l
m u s c le 105
Ka t o n a, G . a n d Sz é k e s s y-He r m a n n, V ilm a:
C o m p a ra tiv e s tu d ie s o n so m e m e m b ra n e - b o u n d e n z y m e s in n o rm a l a n d
d y s tro p h ic m u s c le 111
Le r c h, R ., Ra f f l e n b e u l, W ., Kl e b e r, A ., Me i e r, W ., Je n n y, E . a n d Ru t is h a u e r, W .:
E f f e c t o f ra p id d ig ita lis a tio n o n e a r ly isc h a e m ic h e a r t fa ilu re in d o g s 119 (A b s tra c t)
Jó l e s z, F . A ., Il l é s, I . , Ra p c s á k, M. a n d Fr e n k l, R .:
T h e o re tic a l a n a ly s is o f m u s c le fa tig u e a n d th e m u scle a s m a c r o s y s te m 121
M Y O F IB R IL L A R PR O T E IN S
Ga b b ia n i, G .:
A c tin in n o n m u s c u la r cells (A b s tra c t) 127
B . Pá p a i, Má r ia:
A p r o te in f a c to r in h ib itin g th e p o ly m e riz a tio n o f a c tin 129 Br u g g m a n n, St., Bü c h i, K . a n d Je n n y, E .:
Im m u n o c h e m ic a l s tu d ie s o n m y o s in w ith th e a id o f q u a n ti ta t iv e m icro -
c o m p le m e n t-fix a tio n 135
Mü l l e r, G ., Er m in i, M. a n d Je n n y, E .:
T h e d if fe re n tia tio n o f re d a n d w h ite s k e le ta l m u scle in th e r a b b i t 139 De l c a y r e, C. a n d Sw y n g h e d a u w, B .:
H e a r t m y o s in A T P a s e a n d lig h t s u b u n its (A c o m p a r a tiv e s tu d y ) (A b s tra c t) 143 Kl o t z, C., Le g e r, J . J . a n d Sw y n g h e d a u w, B .:
S tu d ie s o n h u m a n h e a r t m y o s in (A b s tra c t) 145
Op l a t k a, A ., Mü h l r a d, A . a n d La m e d, R .:
A ffin ity c h r o m a to g r a p h y o f a c tin , m y o s in a n d m y o s in fr a g m e n ts b y im
m o b iliz e d a c tin , m y o s in a n d n u c le o tid e s 147
Sy r o v y, I .:
L ig h t c h a in s o f m y o s in in r e la tio n to m u sc le fu n c tio n (A b s tra c t) 151 Ta k á c s, Ö ., G. Mé s z á r o s, Ma g d a a n d Gu b a, F .:
T h e in flu e n c e o f m o to r n e rv e o n th e c o m p o s itio n o f m u sc le p r o te in s 153 Me d u g o r a c, I . :
A T P a s e a c ti v it y a n d s u b u n its o f m y o s in in th e m y o c a r d iu m o f r a t s c o n
d itio n e d b y sw im m in g 101
LIST OF PARTICIPANTS
Aj t a i, K. D ep artm en t of Biochemistry, Eötvös L oránd U niversity, B u d a
pest, H ungary
Al b r e c h t, V. Research Center for Molecular Biology and Medicine, Central In stitu te for A lim entation, Academ y of Sciences of the GDR, P o tsd a m — Rehbrücke, GDR
Al l é r a, A. In stitu te for Cytology, Bonn, FR G
Ap o r, P. Research U nit, H ungarian College of Physical E ducation, Budapest, H ungary
Bá l in t, M. D epartm ent of Biochem istry, Eötvös L oránd U niversity, Budapest, H ungary
Ba r y l k o, B . Nencki In stitu te o f Experim ental Biology, Polish Academ y of Sciences, W arsaw, Poland
Be l á g y i, J . Biophysical In stitu te, Medical U niversity o f Pécs, Pécs, H ungary
Be r g s t r ö m, К . K arolinska H ospital, Stockholm, Sweden
Bír ó, E. N. A. D epartm ent of Biochemistry, Eötvös L oránd U niversity, Budapest, H ungary
Bo tta zzo, G. E. D epartm ent of Im m unology, The Middlesex H ospital Medical School, London, E ngland
Br u g g m a n n, St. In stitu te of Pharm acology and Biochem istry, Zürich, Sw itzerland
Ca r a f o l i, E. In stitu te of General Pathology, Modena, Ita ly
Cox, J . A. M. D epartm ent of Biochemistry, U niversity of Geneva, Geneva, Switzerland
Da l la Li b e r a, L. In stitu te of General Pathology, U niversity of Padova, Padova, Ita ly
Da m b r o w s k a, R. Nencki In stitu te of E xperim ental Biology, Polish Academy of Sciences, Warsaw, Poland
D ’Ha e s e, J . In stitu te for Cytology, Bonn, FR G
Dr a b ik o w s k a, G. Nencki In stitu te of E xperim ental Biology, Polish Academy of Sciences, W arsaw, Poland
Dr a b ik o w s k i, W. Nencki In stitu te of E xperim ental Biology, Polish Academy of Sciences, Warsaw, Poland
Dy d y n s k a, M. Nencki In stitu te of E xperim ental Biology, Polish Academ y of Sciences, W arsaw, Poland
Eb a s h i, S. In stitu te of Pharm acology, U niversity of Tokyo, Tokyo, J a p a n Er m i n i, M. In stitu te of Pharm acology an d Biochem istry, Zürich, Sw itzer
land
7
Er n s t, E . Biophysical In stitu te , Medical U niversity of Pécs, Pécs, H ungary Fá b iá n, F. D epartm ent of B iochem istry, E ötvös L oránd U niversity,
B udapest, H ungary
Fa z e k a s, S. 2nd D ep artm en t o f B iochem istry, Semmelweis Medical U niversity, B udapest, H ungary
Fe r e n c z, K. D epartm ent of Biochem istry, Eötvös L oránd U niversity, B udapest, H ungary
Fo c a n t, B. D epartm en t of General Biology, In stitu te Ed. Van Beneden, Liège, Belgium
Ga b b i a n i, G. In stitu te of Pathology, Geneva, Switzerland
Ga r a m v ö l g y i, N. Research U nit, H ungarian College of Physical E d u cation, B udapest, H ungary
Ge r g e l y, J . B oston Biomedical Research In stitu te , Boston, Mass., USA
Gu b a, F. D e p artm en t of Biochem istry, Medical U niversity of Szeged, Szeged, H ungary
Ha m o ir, G. D epartm ent of General Biology, In stitu te Ed. Van Beneden, Liège, Belgium
Ha r d w ic k e, P. MRC Research Unit of Biophysics, K ings College, London, E ngland
He g y i, G. D epartm ent of Biochem istry, E ötvös L oránd U niversity, B udapest, H ungary
Hi n s s e n, H. In stitu te for Cytology, Bonn, FR G
Je n n y, E. In stitu te of Pharm acology and Biochem istry, Zürich, Switzer
land
Jo a s s in, L. D epartm ent of Zoology, In stitu te Ed. Van Beneden, Liège, Belgium
Jó l e s z, F. K álm án K andó College o f Electrical Engineering, B udapest, H ungary
Ka k o l, I. Nencki In stitu te of E xperim ental Biology, Polish Academ y of Sciences, W arsaw, Poland
Ka l a m k a r o v a, M. B. In s titu te of Biophysics, Academ y of Sciences of th e USSR, Pushchino, USSR
Ka t o n a, G. 2nd D ep artm en t of Biochem istry, Semmelweis Medical U niversity, B udapest, H ungary
Ko h l e r, L. D ep artm en t of Biochem istry, U niversity of Geneva, Geneva, Switzerland
Kó n y a, L. C entral R esearch L aboratory, Medical U niversity of D ebre
cen, Debrecen, H ungary
Kö v é r, A. C entral Research L aboratory, Medical U niversity of D ebre
cen, Debrecen, H ungary
Le f v e r t, A. K . K arolinska H ospital, Stockholm, Sweden
Le r c h, R. Medical Polyclinic U niversity of Zürich, Zürich, Switzerland Lo w y, J . Biophysics In stitu te , Aarhus U niversity, A arhus, D enm ark Ma k a r ie w ic z, W. In stitu te of Biochem istry, U niversity of K rakow ,
K rakow , Poland
Ma k i n o s e, M. D ep artm en t of Physiology, M ax-P lanck-Institute for Medical Research, Heidelberg, FR G
Ma r g r e t h, A. In stitu te of General Pathology, U niversity of Padova, Padova, Ita ly
Me d u g o r a c, I. In stitu te of Physiology, U niversity of Tübingen, T übin
gen, FR G
Mü l l e r, G. In stitu te of Pharm acology and Biochemistry, Zürich, Sw itzer
land
Mu l v a n y, M. J . Biophysics In stitu te, Aarhus U niversity, Aarhus, D en
m ark
Na g y, B. F. B oston Biomedical Research In stitu te, Boston, Mass., USA No w a k-Ol s z e w s k a, E. Nencki In stitu te of Experim ental Biology, Polish
Academ y of Sciences, W arsaw, Poland
Op l a t k a, A. The W eizmann In stitu te of Sciences, R ehovot, Israel Pá p a i, M. 2nd D epartm ent of Biochemistry, Semmelweis Medical U niver
sity, B udapest, H ungary
Pa u l, R. J . Physiology D epartm ent, H a rv a rd Medical School, Boston, Mass., USA
Pe c h è r e, J . F. D epartm en t of Biochem istry of Macromol. CNRS, M ont
pellier, France
Pe r r y, S. V. D epartm ent of Biochem istry, U niversity of Birm ingham , Birm ingham , E ngland
Pf i s t e r, M. In stitu te of Pharm acology, U niversity of Zürich, Zürich, Sw itzerland
Pil a r s k a, M. Nencki In stitu te o f Experim ental Biology, Polish Academy of Sciences, W arsaw, Poland
Pi n s e t, I. Centre of Nuclear Studies de Saclay, Gif sur Y vette, France
Pr i n g l e, J . W. S. D epartm ent of Zoology, A gricultural Research Council U nit, Oxford, E ngland
Rie d h a m m e r, H. 2nd In stitu te of Physiology, U niversity of Heidelberg, Heidelberg, FR G
Ri e g l e r, B. Research U nit of th e H ungarian College of Physical Education, B udapest, H ungary
Rü e g g, C. 2nd In stitu te of Physiology, U niversity of Heidelberg, H eidel
berg, FR G
Sa l v a t o r i, S. In stitu te of General Pathology, U niversity of Padova, Padova, Ita ly
Sa l y ia t i, S. In stitu te of General Pathology, U niversity of Padova, Padova, Ita ly
Sc h a u b, M. C. In stitu te of Pharm acology, U niversity of Zürich, Zürich, Switzerland
Sr é t e r, F. A. B oston Biomedical Research In stitu te, Boston, Mass., USA
St a n k ie w ic z, A. D epartm ent of B iochem istry, U niversity of K rakow, K rakow, Poland
St r z e l e c k a-Go l a e w s k a, H. Nencki In stitu te of E xperim ental Biology, Polish Academy of Sciences, W arsaw, Poland
Sv e n d s e n, K . H . Biophysics In stitu te , A arhus U niversity, A arhus, D en
m ark
Sy r o v y, I. In stitu te of Physiology, Czechoslovak Academy of Sciences, Prague, Czechoslovakia
Sw y n g h e d a u w, B. D epartm ent of Biomedical Physiology, INSERM -U 127, Paris, France
9
Sza bó- Ke l e m e n, G. D ep artm en t of Biochemistry, Eötvös Loránd U ni
versity, Budapest, H ungary
Sz é k e s s y-He r m a n n, V. 2nd D epartm ent of Biochem istry, Semmelweis Medical U niversity, B udapest, H ungary
Sz il á g y i, L. D ep artm en t of Biochem istry, Eötvös L oránd U niversity, Budapest, H ungary
Sz v e t k ó, D. D epart ment of Physical E ducation, Memorial U niversity, St. Johns, New Foundland, Canada
Ta k á c s, Ü. D epartm ent o f Biochem istry, Medical U niversity o f Szeged, Szeged, H ungary
Tr o m b it á s, К . Central Research L aboratory, Medical U niversity of Pécs, Pécs, H ungary
Vá c z y, К . Research U nit, H ungarian College of Physical E ducation, B udapest, H ungary
Va r g a, E. D epartm ent of Physiology, Medical U niversity of Debrecen, Debrecen, H ungary
Wa l l im a n n, T. In stitu te for Cellular Biology, Zürich, Sw itzerland Wa t t e r s o n, J . In stitu te of Pharm acology, U niversity of Zürich, Zürich,
Switzerland
We r b e r, H. The W eizmann In stitu te of Sciences, R ehovot, Israel Wi l l, H. Academy of Sciences, Central In stitu te for H e a rt and Circula
to ry Research Division of Cellular and Molecular Cardiology, B erlin- Buch, GDR
Wo l f, I. D epartm ent of Biochem istry, Eötvös L oránd U niversity, B u da
pest, H ungary
Ze l c k, U. In stitu te for Pharm acology an d Toxicology, U niversity of Rostock, Rostock, GDR
Zu b r z y c k a, E. Nencki In stitu te of E xperim ental Biology, Polish Acad
emy of Sciences, W arsaw, Poland
FO REW O R D
This volume contains th e papers presented a t th e T hird Meeting of the E uropean Muscle Club held in B udapest on 31 August and 1 Septem ber, 1974. Some authors described completed experim ents while others outlined only th e most im p o rtan t aspects o f a research work. Finally th e editors included the abstracts of a few contributions supplying valuable inform a
tion on th e subject as a whole. Thus, th e diverging topics give an overall picture o f th e work of the meeting.
The Muscle Club was organized sim ultaneously w ith th e 9th F E B S Meet
ing held in B udapest in 1974 when one of th e symposia dealt w ith the
“Proteins of Contractile System s” . Our aim is to stu d y and discuss th e m ost im portant aspects of th e muscle functions in co-operation with th e researchers working in this special field of science all over th e world.
E. N. A. Bíró
l l
O PEN IN G ADDRESS
Ladies and Gentlemen,
I t is m y pleasant d u ty to welcome our guests on behalf of the Organizing Committee. A fter Belgium and Switzerland it is now H u ng ary ’s tu rn to be th e venue for th e yearly meeting of the European Muscle Club. The very fact, th a t this is already th e th ird m eeting on this subject, clearly shows how useful th e idea of establishing the E uropean Muscle Club was. A club of informal character, w ithout any form al membership or organization which does n o t belong to an y national or international organ seems to be the best m eans for extensive discussions and for creation of personal con
nections.
Though our club is in Europe, we are m ost happy to be able to greet outstanding scientists from overseas countries as well, as such Prof. Ebashi from Ja p an , Prof. Gergely from th e U nited States and Prof. O platka from Israel. W e sincerely regretted th a t our B ritish colleagues were n o t present a t th e first two meetings an d it is now a pleasure to welcome here Prof.
Pringle, Prof. P erry and other B ritish experts. I should like to express my hope th a t th e meeting of th e E uropean Muscle Club will not be restricted to a continental character in th e future.
Following our traditions in muscle research we have organized this m eeting w ith the greatest pleasure, though we have to apologize for some possible organizational failures.
By way of introduction I should like to point out th e special im por
tance of muscle research in our days. In modern tim es, autom atization of labour, increasing m otorization and m echanization endanger th e hum an
ity —sim ultaneously w ith environm ental contam ination—leading to the im poverishm ent of m ovem ent. Muscle represents the basis of all hum an m ovem ents from th e m ost simple ones to physical education and sport by which this danger can be overcome. I t is not too difficult to predict th a t in a few years muscle research will be regarded as im po rtant as are to day to p ro tect th e environm ent an d to p reven t th e hazards of radiation.
W ith these thoughts I open th e T hird E uropean Muscle Meeting.
N. Garamvölgyi
13
, ' /
CALCIUM AND REGULATION
S'утр. Biol. H ung. 17, p p . 17 — 33 (1974)
E A R L IE R AND R E C EN T W ORK ON PARVALBUM INS*
b y
G. H a m o i r
LA B O RATO RY O F G E N E R A I. BIOLOGY, FACULTY O F SCIEN CES, U N IV E R SIT Y O F L IÈ G E , B-4000 L IÈ G E , BELGIUM
The parvalbum ins (PA), initially described 20 years ago (H enrotte, 1955) have received a t first little attentio n . They have been originally considered as strange sarcoplasmic proteins of unknown function occurring only in the white muscles of fish and am phibia. B ut th e recent discovery of th eir phylogenetic relationship to troponin C (TN-C) and to both alkali- and DTNB-light chains (LC) has draw n th e a tte n tio n tow ards them . I shall review here briefly th e earlier work carried out on PA and exam ine th e present developm ent of th e subject. Before th a t, I would like to m ention some of m y collaborators who have contributed largely to th e work done in Liège: H enrotte who isolated and crystallized carp PA I I I in 1955;
Konosu, Peclière, Focant, B hushana Rao, Gerday and Gosselin-Rey.
As soon as a com parative stud y of the muscle proteins of th e carp has sta rte d (Hamoir, 1951), it became clear th a t th e fish sarcoplasmic extracts differ very m uch from the rab b it ones. This is already visible by u ltra ce n tri
fugation (Fig. 1). R ab b it myogen separates into two peaks having sedi
m entation coefficients of about 5 and 7 Svedberg units. B ut in th e case of the carp, three peaks are seen of about 1.5, 5 and 7 Svedberg units. The slow peak which was first observed by D euticke (1934) in frog extracts, corresponds to th e low molecular weight proteins of fish and th a t of the am phibians’ white muscles. I t was soon realized as a result of a com parative
F ig . 1. U ltr a c e n tr if u g a l p a tt e r n s o f r a b b i t (left) a n d c a rp (rig h t) sa rc o p la s m ic e x tr a c ts (w h ite m u s cle) a f t e r ca. 1 h a t 60,000 rp m . T h e s e d im e n ta tio n c o e ffic ie n ts o f t h e p e a k s a r e g iv e n in S v e d b e rg
u n its
F ig . 2. C o m p a riso n o f th e a s c e n d in g m o v in g b o u n d a r y e le c tro p h o r e tic p a tt e r n s o f r a b b i t (b ro k e n line) a n d c a rp (solid line) w h ite m u sc le s a rc o p la sm ic e x tr a c ts a t I 0.075 a n d p H 7.3 a f t e r c a . 5 h
e le c tro p h o re s is a t 5.8 V /c m
* I n v ite d p a p e r
2 17
work on plaice (Jebsen an d Ham oir, 1958) and cod (Connell, 1958) th a t these proteins generally occur in th e muscle low ionic stren gth ex tracts of th e lower vertebrates and correspond to 25 (+ 1 0 ) % of these extracts.
The composition of th e slow u ltracentrifugal peak of th e carp ex tracts has been determ ined first by moving boundary electrophoresis. The dif
ferences between th e rab b it and carp sarcoplasm ic ex tracts are illustrated in Fig. 2. The broken line corresponds to th e ascending p a tte rn of th e rab b it and the solid one to th e ascending p a tte rn of th e carp. All proteins are negatively charged a t n eu tral p H and m igrate from the right to th e left.
In the rab b it ex tract, th e only acidic electrophoretic component detected by this m ethod, (h) corresponds to serum album in, b u t th e carp ex tract contains 5 components m igrating in front of the diagram . The fourth is creatine kinase (EC 2.7.3.2) which has always a higher electrophoretic m obility in fish th a n in warm-blooded vertebrates. The slow ultracen tri
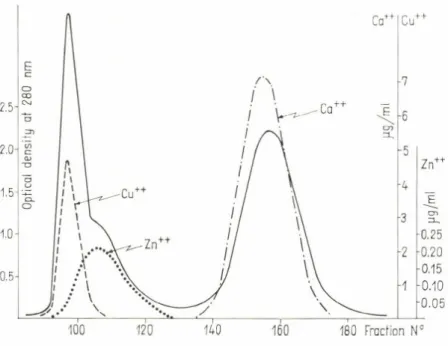
fugal peak is m ade up of th e com ponents 1, 2, 3 and 5, which has been unequivocally detected by filtration of th e sarcoplasmic ex tracts on Sepha- dex G-75 (Pechère and Focant, 1965; Fig. 3). The proteins of low molecular weight sep arate completely from th e other proteins. The retard ed peak m easured by TJV absorption is m uch smaller th a n th a t m easured b y biuret, th is discrepancy, due to th e low absorption of these proteins a t 254 nm , indicate th eir low try p to p h a n and tyrosine content.
The carp components 2, 3 and 5 were isolated by am m onium sulphate fractionation, crystallized a n d described between 1955 and 1965 (Konosu e t ah, 1965). I t is th e carp component 3 crystallized initially b y H enrotte (1955) (Fig. 4) which has been used by K retsinger to determ ine th e te rtia ry stru ctu re of these proteins (Kretsinger and Nockolds, 1973).
The stu dy of these low m olecular weight proteins was extended from 1965 up to now to m any species of fish an d of other lower verteb rates using
F ig . 3. S e p a ra tio n o f th e sa rc o p la s m ic p ro te in s o f c a r p w h it e m u sc le b y f i lt r a ti o n o n a S epha- d e x G-75 c o lu m n . F u ll sy m b o ls: b iu r e t d e te r m in a tio n s ; e m p ty o n es: u ltr a v io le t a b s o r p tio n . T h e m a in fr a c tio n c o rre sp o n d s to t h e u n r e t a r d
e d g ly c o ly tic e n zy m e s
F ig . 4. P h a s e c o n tr a s t p ic tu r e o f c ry s ta ls o f t h e f i r s t is o la te d p a r v a lb u m in (c a rp c o m p o n e n t 3) (H e n ro tte , 1955) X 450
different analytical and separation m ethods: acetone fractionation, filtra tion of Sephadex G-75, D E A E cellulose chrom atography, starch gel and polyacrylam ide gel electrophoresis. In 1968 Pechère proposed to designate these proteins of low molecular weight parvalbum ins, in view of th eir small molecular weight and their great solubility. One year later we proposed also a new nom enclature based on the electrophoretic m obility in starch gels (Bhushana R ao et ah, 1969). At th a t tim e we com pared m any species o f lower vertebrates by starch gel electrophoresis in collaboration w ith R. K . Scopes. This work has m ade clear th a t PA m igrate usually w ith th e largely different mobilities shown by th e carp components. This regularity is illustrated in Fig. 5 which represents starch gel electrophoretic p a tte rn s a t p H 8.5 of muscle extracts of carp, of Tilapia m acrochir, a fresh w ater fish living in African lakes and rivers, of codling and of pike. The rom an capitals I, I I , I I I and IY designate th e different types of PA which can be encountered. R ecently a slower com ponent (V) has been described b y P iro n t and Gerday (1973) an d a still slower one (VI) exists in few spe
cies of fish (Hamoir and Gerday, unpublished).
While this com parative work was in progress, some a tte m p ts were made, of course, to determ ine th e biological function of these proteins. Before exam ining this point, let us first consider some of th eir general properties (isoelectric points, th e sedim entation coefficients, th e diffusion coefficients of 9 PA, etc.) which are sum m arized in th e Tables I and II. These proteins have isoelectric points situated between 4 and 5.5 and their molecular
2* 19
CP TM CL P
О
CK.
ff
ж <zzz>
■CK.
-CK. -CK.
<77T> < Z7/T>
<ZZZ>
! <% £> --- <ZZZ>
I
æV /^ --- --- —
F ig . 5. S c h e m a tic re p r e s e n ta tio n o f s t a r c h gel e le c tro p h o r e tic p a tt e r n s a t p H 8.5 o f s a rc o p la sm ic e x tr a c ts o f w h ite m u sc le s o f c a rp (C P), T ila p ia m a c r o c h ir В (TM ), co d lin g (CL) a n d p ik e (P ). L o c a tio n o f th e in s e rtio n s lo t: 0. T h e b o tt o m c o rre sp o n d s to th e a n o d e . T h e p o s itio n o f c re a tin e k in a s e (CK) a n d o f t h e P A o c c u rrin g in th e s e sp ecies is sh o w n
o n t h e le f t
4.0 -I
1---*--- 1--- г
250 275 300 325 350
Pike com ponent II
250 275 300 325
nm nm
F ig. 6. U ltr a v io le t a b s o r p tio n s p e c tr a o f th e p ik e P A I I a n d I I I in p h o s p h a te -N a C l b u ffe r a t p H 7.3 a n d in 0.1 N X a O H
weights range between 11,000-12,000. Some can be crystallized fairly easily. As H enrotte has observed w ith th e carp component I I I , th eir u ltra violet absorption is quite unusual. They contain approxim ately 10 residues of phenylalanine per molecule, 0 or 1 residue or tyrosine and usually no trypto p h an . The spectra of th e tw o pike PA , shown in Pig. 6, correspond to component I I I devoid of try p to p h a n and tyrosine and to com ponent I I which contains one residue of tyrosine per molecule and gives therefore the usual shift and alkaline p H (Bhushana Kao an d Gerday, 1973).
The amino acid compositions of 10 PA from 5 teleosts and 2 rays (Gerday and Teuwis, 1972) are compared in Table I I. All PA are as a whole made up of a single polypeptide chain of 108 (+ 1 ) amino acids. Several AA are absent or present as one or no more th a n 2 residues per molecule. This is the case of tyrosine and try p to p h an , of th e sulfur-containing amino acids, of proline, of histidine and of arginine. The am ount of this last amino acid is particularly constant : all th e PA exam ined so far contain a single residue of arginine per molecule except th e frog one which contains 3 arginine residues (Pechère et al., 1971). In view of all these analogies, it was obvious th a t th e PA represent a fam ily of homologous proteins (Pechère, 1968;
G erday and B hushana Rao, 1969) which appeared a t first very different irom o th er proteins described a t th a t tim e.
B u t these properties did not indicate w hat function these proteins could play in th e muscle cell. The first interpretatio n was th a t th ey were involved m some way in glycolysis. As Sephadex G-75 allows to fractionate th e sarco
plasmic ex tracts into an unretard ed fraction and a retarded one containing the PA, th e glycolytic powers of both fractions were easily exam ined Pechère and Focant, showed in 1965 th a t glycogenolvsis is carried out only bv the unretarded fraction and is not influenced by th e addition of PA.
The comparison of th e PA content of fish w hite and red muscle afforded also another argum ent against th e contribution of these proteins to an aerobic glycogenolvsis In fish, th e red muscle of th e laleral line (Fig. 7)
21
Some properties of P A of fish white muscle Table I
Component
Carp (1) (2) Tilapia maer. B (3) Cod (Gadus call.) (4) Pike (5)
II I I I IV
a + h I l i a IT Ib II II I II III
i.p . 3.95 4.25 4.37-4.47 4.1 4 .4 4 .4 4.1 4 .2 5.0
S2 0-W (S) 1.7 1.6 1.7 1.6 1.6 1.7 1.7 1.55 1.55
D 2o.wX IO7 — 12.0 15.0 11.0 11.0 10.7 10.7 —
M.W. (UC) — 9.300-12.500 11.100-11.300 13.100 13.100 14.700 14.800 — —
(AA) 12.300 11.500 11.300 9.900 10.600 11.500 11.200 11.700 11.700
Crystallization + + + +
UV. abs. R es. Phe/m ol. 9.0 9.4 10.9 9. s 10.7 13.0 13.0 12.6 9.9
Res. Tyr/m ol. 1.0 0 0 0 0 0 1.3 1.25 0
Res. Try/m ol. 0 0 0 0 0 0 0.7 0 0
References (1) K o n o su e t al. (1965) (2) P ech ère e t al. (1971) (3) P ir o n t et al. (1968)
(4) B h u sh a n a R ao e t al. (1969)
T a b le I I
Am ino acid composition of some P A of fish and amphibian white muscle
Carp Cod W h itin g P ike R a y Ray Frog/pl
Ammo acid II
ITT* IV
a + 1)
Л*
II III III* 11* III* (elav ata ) (inon tagúi)
Lys ] I 13 12 (14) 12 12 12 12 12 17 и 13 11
H is — 1 1 1 — 1 1 2 3 4
A r g 1 1 1 1 1 1 1 1 1 1 1 3
Asx 15 17 15 12 12 15 12 15 10 16 17 13
T h r 4 5 6 5 2 3 2 2 3 5 7 2
Ser 6 5 8 5 7 7 6 8 4 11 и 10
Glx 11 8 9 (8) 10 9 9 9 8 9 10 8 12
P r o 1 — — — — 1 — 1 — 1 1 —
Gly 9 8 9 (8) 12 9 9 10 7 7 8 7 9
Ala 20 20 16 19 23 19 22 20 19 12 12 15
Cys 1 1 1 1 2 1 3 1 — 2 2 —
Val 4 5 6 4 5 5 6 5 6 4 4 i
Met _ _ — 1 — i — 2 1 2 1 —
He C 5 5 7 5 7 5 4 5 6 о 7
Leu 9 9 9 8 8 8 8 9 10 9 10 9
T yr 1 — — — 1 1 1 1 1 1
Phe 9 10 10 10 10 10 10 10 9 8 8 9
T rp — — — 1 1 —
T otal 108 108 108 109 106 n o 107 107 109 110 113 108
R eferen ces in Pechère e t al. (1974) ex cep t for p ik e P A I I (G erday, 1974) a n d for w h itin g PA I I I (Jo assin , 1974). An a sterisk in d icates th e com pletely sequenced p ro tein s:
carp PA I I I (Coffee and B radshaw , 1973); h ak e PA H (C apony e t ah, 1973); p ik e PA I I I (F ra n k en n e e t al., 1973); p ik e PA I I (G erday, 1974); w h itin g PA I I I (Joassin, 1974).
F ig . 7. T ra n s v e rs e se c tio n s th r o u g h t h e ta il (a) a n d th e a b d o m e n (b) o f th e c a rp sh o w in g
th e la te r a l re d m u s c u la r reg io n
contains only negligible am ounts of PA. This conclusion is fu rth er rein
forced by recent observations on fish heart muscle Starch-gel electrophoreses (H a m o iret al., 1972) and immunological investigations (Gosselin-Rey, 1974) have shown th a t this muscle contains only trace am ounts of PA.
An unexpected observation has led to another suggestion about th e role played by these proteins. In th e course of amino acid analyses carried out in 1966 by Crokaert and m yself on th e two PA of Tilapia macrochir, we noticed th a t th e ash content of th e lyophilized PA powder, obtained after exhaustive dialysis of th e protein solution against w ater, was abnorm ally high; it reached 1 to 2%. The composition of the ash was completely different from th e salt composition of th e intracellular fluid: it contained traces of magnesium an d very large am ounts of calcium corresponding to 2 ions per molecule (Fleron, 1967-68). Pechère described similar observations in 1971 and has draw n th e atten tio n to th e fact th a t th e specific binding of Ca strengthens th e resem blance occurring betw een th e PA and th e calcium sensitizing factor, troponin-C or TN-C (Pechère et ah, 1971). The general occurrence of two calcium ions per molecule of PA is now well established.
L et us now compare some properties of PA and TN-C. The wide zoo
logical distribution of th e troponin-tropom yosin regulatory system is now well docum ented; it is found in all muscles except in molluscs (Lehman et ah, 1972; Szent-Györgyi e t ah, 1973), T h at of th e PA appeared until last year lim ited to fish, am phibia and some reptilia living in w ater; in fact these proteins seem to occur also in muscles of higher v ertebrates (cfr.
infra). Some characteristics of these two types of protein are shown in Table I I I . They have several properties in common; they are acidic, bind calcium strongly though with different affinities. They are highly antigenic:
passive transfer skin test reactions have always been positive with a cod
24
T a b le Г! I
Comparison of P A and TN-C
P ro p erties PA TN-C
A cidity Acidic Acidic
Molecular weight 11,500 17,850 (1)
. . .. Imoles/mole Ca + + -binding J
lK d
2 4
1 and 4 x 10-7 (2) 10“ 6 (3)
A ntigenicity - p a t 2 х Ю _ | " m g/m l (4) Strongly antigenic
N -term inus A cetylated (5) A cetylated (6)
C ontents (in residues per
mole) T rp 0 - 1 0 (6)
T yr 0 - 1 1- 2 (6)
Phe 8-10 8-10 (6)
H is 0 - 4 0 - 1 (6)
Arg 1 5 - 9 (6)
Met 0 - 2 6-10 (6)
Biological role N ot regulatory (6) R egulatory
C ontent (per kilo of
muscle) 0.7 mM (carp) 0.07 mM (rabbit)
References (1) Collins e t al. (1973) (2) B enzonana e t al. (1972) (3) P erry (1973)
(4) Aas (1967) (5) Pechère e t al. (1974) (6) D ém aillé e t al. (1974)
PA preparation a t the protein concentration of 2 - 10_1° mg/ml (Aas, 1967).
Their compositions in arom atic amino acids are very similar. On th e other hand, th e molecular weights and th e contents in some amino acids as arginine and m ethionine are very different. Furtherm ore, PA occur together with TN-C in fish muscle and are not able to replace TN-C in synthetic systems. The sim ilarities are still rem arkable; th ey share a common ancestry as we will see later.
Another relationship has also been recently suggested between th e PA and th e myosin LC. As the biological function of TN-C is fulfilled in mol
luscs by th e ED TA-light chain, it could be questioned if th e similarities found between TN-C and PA could be extended to th e myosin LC. R ecently 1 he LC have been purified and some analogies became obvious. B oth rabbit, alkali- and DTNB-LC have UV spectra rem iniscent of th e PA as shown by Perrie et al. (1973; Fig. 8). B oth alkali-LC have as th e PA, their N- term inus blocked (Frank and Weeds, 1974). This homology has been examined more closely by Collins (1974) who has compared the sequences o! rabbit TN-C and alkali-LC with those of 3 PA. He concluded from this comparison th a t the ex ten t of sim ilarity is too great to have occurred by chance. These proteins seem th u s to originate from a common rem ote ancestor.
As soon as it became obvious th a t the PA can be related to some con
tract i le proteins, th e determ ination of th e tertia ry stru ctu re of th e carp
F ig . 8. U V s p e c tr a o f th e m y o s in a lk a li (solid line) a n d o f th e D T N B (b ro k e n line) lig h t c h a in s o f r a b b i t s k e le ta l m u sc le (a f te r P e rr ie e t
a l„ 1973)
component I I I by m ethod of K retsinger received a great deal of attention.
In fact K retsinger sta rte d his X -ray stu d y before being aware of th e pres
ence of two calcium in PA. Now th e locations of these ions in th e molecule are well established (Kretsinger and Nockolds, 1973; Hendrickson and K arle, 1973).
Figure 9 is a picture of th e 1 cm-1 À model built by Dr. Gerday in Oxford in Prof. Phillips’ laboratory on th e basis of K retsinger’s data. The dim en
sions of th e protein are 3 0 x 3 0 x 3 6 À. The com pact stru ctu re does not contain groves or pits typical of active sites of enzymes. I t has a well- defined anhydrous hydrophobic core containing 8 of th e 10 phenylalanin residues of th e molecule; a t th e surface there are th e hydrophilic groups an d some hydrophobic ones which cannot be accom odated inside. The only polar internal groups are th e carboxylic groups associated w ith the two calcium ions and an internal salt-bridge between th e single A rg75 and G1u81. The chain contains six helicoidal regions: A, B, C, D, E and F in
cluding 52 of th e 108 residues. The first calcium is situated in th e loop between C and D; th e second one between E and F. K retsinger (1972) has discovered repeats in th e three-dim ensional stru cture which are particularly obvious a t th e two calcium sites and has suggested th a t a gene triplication had occurred associating three fragm ents of about 33-36 residues: the fragm ent AB (without C a+ + ), th e fragm ent CD (1st C a+ + ) and th e frag
m ent E F (2nd C a+ + ). The internal salt-bridge betw een Arg75 an d Glu81 occurs in a position relative to th e AB loop sim ilar to th a t of the Ca + + ions
26
F ig . 9. P ic tu r e o f a 1 cm -1 Â m o d e l o f th e c a rp P A I I I b u ilt o n th e b a sis o f K r e ts in g e r ’s d a t a (K re ts in g e r a n d N o c k o ld s, 1973) sh o w in g th e fa irly s p h e ric a l s h a p e o f th e
m o lecu le
in the two other fragm ents (Kretsinger, 1972). Selective m odification of the single arginine residue in pike component I I I greatly alters th e te rtia ry stru ctu re o f th e molecule as detected by immunochemical reactivity and leads to a reduced calcium -binding ability (Gosselin-Rey e t al., 1973). The chain can thu s be represented by th e following form ula indicating th e location of th e residues involved in calcium binding:
[A—B] [C -Ca51_ e2—D ] [E —Ca90_ 101—F].
A mere inspection of th e sequence reveals, however, no obvious hom ol
ogies. This in terpretatio n postulates th a t th e three-dim ensional structure of proteins evolves more slowly th a n th e amino acid sequence.
I f such a triplication of th e PA exists, TN-C can arise from a tetra- plication. This hypothesis has been p u t forw ard by Collins and cowork- ers (Collins et ah, 1973). The homologies found are sum m arized in Fig. 10. The PA sequence has been divided into three fragm ents: AB, CD and E F . A delation of two residues has been assum ed in ÄB. V ertical bars indicate helical regions and asterisks th e residues of the CD and E F loops involved in calcium binding. As you can see, th e CD calcium is co-ordinated by 6 well-defined oxygen atom s. The binding o f the E F calcium is n ot so well established by the work of K retsinger : 5 residues are involved, th e 6 th co-ordinate corresponds to a 2nd oxygen of Asp92 or to a w ater molecule.
27
PA T N -С
AB CD E F I II I I I IV
1 a 72 a 9 s 84 e
2 f ,y 34 g 73 d,g lO y 85 d
3 a,s 35 L 74 a 11 L 47 g 86 a 122 g
4 g 36 t,a,k 75 r 12 s 48 q 87 к 123 c
5 v ,i 37 s,g 76 a 13 e 49 t 88 g 124 h
6 L 38 к 77 L 14 e 50 p 89 к 125 V
7 n,a 39 s,t 78 t 15 m 51 t 90 s 126 t
8 d 40 a,p 79 d 16 i 52 к 91 e 127 d
9 a 41 d,a 80 g,a 1 7 a 53 e 92 e 128 e
10 d 42 d 81 e 18 e 54 e 93 e 129 e
U I 43 V ,I 82 t 19 F 55 L 94 L 130 I
12 a ,t 44 к 83 k,a 20 к 56 d 95 a 13 1 g
13 a 45 к 84 t,a 21 a 57 a 96 e 132 s
14 a 46 a 85 F 22 a 58 I 97 c 133 L
15 1 47 F 86 L 23 F 59 I 98 F 134 M
16 e,a 48 a 87 к 24 d 60 e 99 r 135 к
1 7 a 49 i 88 a 25 m I 61 e 100 i 136 d
18 c 50 I S 9g 26 F 1 62 V 101 F 137 g
19 k,e 51 d* 90 d* 27 d 63 d 102 d 138 d
20 a 52 q 91 s 28 a 64 e 103 r 139 k
21 a,e 53 d* 92 d* 29 d 65 d 104 n 140 d
22 d,g 54 к 93 g 30 g 66 g 105 a 141 n
23 s 55 s* 94 d* 31 g 67 s 106 d 142 d
24 F 56 g,d 95 g 32 g 68 g 107g 143 g
58 I,V 59 е*
97 I 98 g
34 I 35 s
70 I 71 fl
109 I 110 ti
145 I 140 d
26 h.e 60 e 99 V 36 V 72 f 1 1 1 a 147 f
27 к 61 d 100 d,e 37 к 73 e 112 e 148 d
28 a e 62 e* 101 e* 38 e 74 e 113 e 149 e
29 F 63 L 102 F 39 L 75 F 114 L 150 F
30 F 64 к 103 t,a i q 40 g 76 L 115 a 151 L
31 a ,s,t 65 1 104 a 2 d 41 t 77 V 116e 152 к
1 32 к 66 F 105 L,M 3 q 42 V 78 M 117 I 153 M
33 V 67 L 106 V 4 t 43 M 79 V 118 F 154 M
68 q 107 к 5 a 44 r 80 r 119 r 155 e
69 n 108 a,g 6 e 45 m 81 q 120 a 156 g
70 F 7 a 46 L 82 M 121 s 157 V
71 k,s 8 r 83 k 158 q
F ig . 10. R e la tio n s h ip b e tw e e n th e p rim a ry s t r u c tu r e s o f th e c a rp a n d h a k e P A (r e s id u es fro m c a rp c o m p o n e n t I I I f i r s t , follow ed b y c a rp c o m p o n e n ts I I , IV a n d h a k e I I I in t h e case o f s u b s titu tio n s ) a n d o f r a b b i t s k e le ta l T N -C . T h e a lig n m e n t in P A is a c c o rd in g to K re ts in g e r a n d N o c k o ld s (1973). V e rtic a l b a rs : h e lic a l re g io n s o f c a rp c o m p o n e n t I I I a n d p re d ic te d a -h elices o f T N -C . A s te ris k s : re sid u e s in P A in v o lv e d in c a lc iu m b in d in g . H o riz o n ta l lines a n d c a p ita ls : h y d ro p h o b ic co re re sid u e s
in P A a n d c o rre sp o n d in g re s id u e s in T N -C (a f te r C ollins e t a h , 1973)
TN-C has been, on th e other hand, divided into fonr regions I, I I, I I I and IV, each of which corresponds to a calcium binding site. The alignm ent chosen allows to get a good correlation with the 8 «-helical segments pre
dicted from th e am ino acid composition as shown by th e vertical bars, with t lie residues involved in Ca binding and with some hydrophobic core residues involved as shown by the horizontal lines. A structu ral sim ilarity seems th us to be exist between the CD and E F regions and TN-C. Tin;
F ig . 11. P ic tu r e o f a p a r t o f th e m o d el o f th e c a rp P A I I I o f F ig . 9 sh o w in g t h e c o o rd in a tio n o f th e tw o c a lc iu m ions a n d th e ir d if fe re n t a c c e ss ib ility
to th e so lv e n t
correlation with the AB region is less satisfactory. The location of th e two calcium is shown more clearly in Fig. 11. The CD calcium is n ot exposed to solvent an d th e E F one looks less tig h tly bound: it can be replaced by a terbium ion (Moews et ah, to be published).
Progress has also been made in the comparison of th e prim ary structures of th e PA. This field has been actively investigated by Coffee and Bradshaw in Saint Louis, by Capony and Pechère in Montpellier an d by G erday and his coworkers in Liège. The num ber o f prim ary structures determ ined increases rapidly. A review of th is com parative work appeared in System atic Zoology (Pechère et al., 1974). The comparison of the sequences allows to draw some interesting conclusions. The N -term inus portion corresponding to th e AB fragm ent varies m uch more th a n th e two fragm ents CD and EF.
The tw o calcium-binding acid regions (51-62 and 90-101) are invariable with regard to th e residues involved in th e co-ordination of the m etal as well as th e internal salt-bridge Arg75 Glu81. The internal phenylalanine residues which contribute largely to th e hydrophobic core, are also well preserved in all th e sequences studied so far. On th e other hand, th e calcula
tion of th e distances betw een 7 PA has allowed to detect a new feature.
An ancestral duplication has occurred; to lines of non-allelic genes have given rise to two genetic lines x an d ß . Similar calculations incorporating th e tw o new prim ary structures of pike P A I I (Gerday, 1974) and whiting PA I I I (Joassin, 1974) have allowed to build th e phylogenetic tree repro-
29
duced in Fig. 12 which agrees w ith th a t of Pechère and collaborators (Gerday, unpublished). As you can see, this duplication which dates back to Million Years, is responsible for th e occurrence of th e two pike PA investigated in Liège. The carp components II, I I I and IV have appeared m uch more recently.
On th e other hand, th e observation of Aas and Jebsen (1967) th a t the hypersensitivity of some persons to fish is due to PA, has draw n th e atten -
F ig . 12, P h y lo g e n e tic tr e e o f P A d ra w n o n t h e b a sis o f th e n u m b e r s o f r a n d o m e v o lu tio n a r y h it s (R E H ) . T h e l le n g th o f th e b ra n c h e s a re p ro p o r tio n a l to th e n u m b e r o f R E H
in d ic a te d (G e rd a y , u n p u b lish e d )
Species I Ж
Pa rv alb u m in s
Ш ж Y
Carp (Carpe) I
® ®
жBarbel (Barbeau) Ж Ж ж
N ase (Nase) Л Ж Y
Bream (Brème) I ж ж
Dace (Vandoise) Л ш Y
Chub (Chevesne) I
® @
YRoach (Gardon) П ж
F ig . 13. Im m u n o lo g ic a l re la tio n s h ip s b e tw e e n th e P A se v e n cy p - rin id a e . E a c h f r a m e encloses c o m p o n e n ts d is p la y in g a s im ila r im m u n o c h e m ic a l d is c rim in a tio n to w a rd s th e a n tis e r a t o th e fo u r e n c irc le d P A . T h e F r e n c h n a m e s o f th e sp ecies a re g iv en
b e tw e e n b ra c k e ts ( P ir o n t a n d G o sselin -R ey , 1974)