— 1 3 —
I. Introduction 413
II. Definition 414
III. Preparation and Purification of Polyhedra and Viruses . . 414 A. Preparation and Purification of Polyhedra 414 B. Preparation and Purification of Virus Particles 415 IV. Structure of Polyhedra and Morphology of Viruses 417
A. Size and Structure of Polyhedra 417
B. Morphology and Size of Viruses 418
V. Physicochemical Properties and Chemical Composition of
Polyhedra 432
A. Physicochemical Properties 432
B. Chemical Composition 435
VI. Chemical Composition of Virus Particles 438 VII. Serological Properties and Relationship of Nuclear-
Polyhedron Proteins, Nuclear-Polyhedrosis Viruses, and
Insect Hosts 444
A. Serological Properties of Nuclear-Polyhedron Proteins 444 B. Serological Properties of Nuclear-Polyhedrosis Viruses 445 C. Serological Relationship between Nuclear-Polyhedron
Proteins and Nuclear-Polyhedrosis Viruses 446 VIII. Taxonomy of Nuclear-Polyhedrosis Viruses 447 I X . List of Nuclear-Polyhedrosis Viruses 448
References 450
I . INTRODUCTION
Representatives of the nuclear-polyhedrosis viruses were the first insect viruses investigated. T h e reason for this is that this group of insect viruses is characterized by the presence of inclusion bodies, the so-called
413
The Nature of Nuclear-Polyhedrosis Viruses
GERNOT H. BERGOLD
Instituto Venezolano de Investigaciones Cientificas (I.V.I.C.), Departamento de Virologia, Apartado 1827, Caracas, Venezuela
414 GERNOT Η. BERGOLD
polyhedra, in infected insects. These polyhedra can be readily seen with the light microscope. Maestri (1856) and Cornalia (1856) were the first to describe them. T h e nature and significance of these poly
hedra remained a mystery for a long time and is still not entirely under
stood. Von Prowazek (1907, 1912, 1913) was probably the first to observe particles within the polyhedra. Komärek and Breindl (1924) were the first who actually demonstrated with histological methods some particles within the polyhedra of Lymantria monacha (Linnaeus). Paillot and Gratia (1939) observed, in the dark field of a light microscope, during the alkaline dissolution of polyhedra, very small highly refractive gran
ules which they believed to be the infectious virus agent. However, it was not until the electron microscope was available that the virus parti
cles could be isolated and identified as the infectious viral agent by Bergold (1947).
I I . DEFINITION
According to our present-day knowledge, typical nuclear-polyhedrosis viruses occur only in Lepidoptera, in the family of Tenthredinidae of Hymenoptera, and in a species of Neuroptera. T h e polyhedrosis virus of Bombyx mori (Linnaeus) and Peridroma saucia (Hübner) have been successfully grown in insect tissue culture (Trager, 1935; Aizawa and Vago, 1959; Martignoni and Scallion, 1961).
In the following, the word ''polyhedra'' will designate inclusion bodies found in the nucleus of cells of insects as a result of virus infection.
T h e term "polyhedrosis virus" will be used for the infectious agent which causes nuclear polyhedroses in insects. Synonymous for the term
"polyhedrosis/' which was first proposed by Fischer (1906), is Poly
ederkrankheit suggested by Wahl (1909), which is sometimes used in German-speaking countries. Accordingly, Polyeder Virus is synonymous with polyhedrosis virus. In Italy, the terms giallume and poliedria, and in France the terms grasserie, jaunisse, and vir ose are used.
I I I . PREPARATION AND PURIFICATION OF POLYHEDRA AND VIRUSES
A. Preparation and Purification of Polyhedra
Bolle (1873, 1893) found first that polyhedra are insoluble in water.
Furthermore, they are not decomposed by ordinary bacterial putrefac
tion processes. For these reasons, it is comparatively easy to obtain gram quantities—up to 10 mg per larva—in a very pure form by differential centrifugation. All that is necessary is to suspend virus-diseased insects in water, allow the polyhedra to settle to the bottom, and purify them further by centrifugation. One has to watch, however, that the pH of the medium in which the polyhedra are suspended never drops below
13. N A T U R E O F N U C L E A R - P O L Y H E D R O S I S VIRUSES 415 prL 5, or rises above pH 8.5 to prevent possible dissolution of the poly
hedra. In some cases, treatment with fluorocarbons of suspensions of insects containing smaller quantities of polyhedra (Bergold, 1959a) is helpful for the purification process. A more rapid method of obtaining quantities of polyhedra in very pure form is to use only fresh hemolymph taken from virus-diseased larvae before their death. Such a hemolymph is of a milky appearance; its purification is very simple if carried out the next day. Polyhedra can also be purified readily from infected pupae (Vago and Atger, 1961). Because of their rather unusual physicochemical properties, polyhedra can be stored in form of a dry powder or in flame- sealed glass tubes for as long as twenty years without significant changes in their solubility as well as without loss of infectivity of the enclosed virus particles (Aizawa, 1953, 1954a; Steinhaus, 1960a).
B. Preparation and Purification of Virus Particles 1. Principal Considerations
Virus particles characteristic of nuclear polyhedroses, as well as of those causing granuloses (see Chapter 1 6 ) , are by far the easiest of all known viruses to purify. There are several reasons for this: first, the starting material consists of water-insoluble inclusion bodies which them
selves can be obtained in a very pure form. Second, as will be shown in Section I V , B , 2, except for their own protein, polyhedra contain nothing but virus particles. Third, the size and weight of the virus particles is so much greater than that of the polyhedron-protein mole
cules that they can easily be separated by centrifugation.
For liberation of the virus particles, the polyhedra must be dissolved in weak alkaline solutions. T h e concentration of alkali necessary to dissolve the polyhedra of different insects is not the same, and must first be determined. T o prevent destruction of the virus particles, it is very important not to use too much alkali. There is a definite relation
ship between the quantity of polyhedra, the volume of liquid in which they are suspended, and the amount of alkali used to dissolve them.
T h e alkali concentration has to be just high enough to dissolve all polyhedral bodies, but not so great as to destroy the virus particles.
Rather high concentrations of alkali are necessary, but it is not advisable to use buffers because if all polyhedra are dissolved, the pH will have dropped from an initial value of over 10 to about 8.5 at the most. I f the final pH is higher than 8.5, then many virus particles will have lost their infectivity. T h i s can easily be discerned by the complete change of their morphological appearance (see Section IV, Β , 1 ) . However, in every virus suspension from polyhedra, even when prepared most care
fully, there is a certain number of particles that have shed their develop-
416 GERNOT Η. BERGOLD
mental membrane; therefore, one can always find several empty develop
mental membranes in such preparations. T h e principal methods and detailed procedure for the isolation and purification of any insect virus were first described by Bergold (1947). Since then, this method for the isolation of nuclear polyhedrosis viruses is followed in principle, or with only minor changes, by virtually all insect virologists.
2. Detailed Procedure
Five milligrams of polyhedra are suspended in 1 ml of 0.004 to 0.03 Μ N a2C 03 plus 0.05 Μ NaCl. T h e polyhedra should dissolve at room temperature in about 1 to 2 hours with occasional or continuous gentle shaking. After the suspension has become somewhat opaque, it is centrifuged for about 5 minutes at about 2000 to 4000 g. If the polyhedra were not very pure, a small brownish sediment of insoluble material consisting of impurities will result. T h e supernatant has a bluish-white appearance, caused by the virus particles suspended in the clear poly
hedron protein solution. If part of the sediment is white, then the alkali concentration was not high enough to dissolve all polyhedra. T h e super
natant is then centrifuged for about 1 hour at 10,000 to 12,000 g in order to sediment the virus particles. These will collect in a bluish-white pellet. After discarding the supernatant, the virus pellet is resuspended in C02-free distilled water of the same volume, and centrifuged for another hour at about 10,000 to 12,000 g. T h e resulting clear super
natant is discarded, and the final pellet of virus particles dissolved in one-seventh of the original volume in C02-free distilled water. T h e resulting suspension of pure virus particles should have a whitish-blue appearance. If the virus particles do not suspend well, then too much of the water-insoluble polyhedron protein has been carried over, resulting in precipitation. T h e addition of extremely small amounts of alkali will resuspend the virus particles. However, this will often cause destruction of many virus particles with consequent loss of infectivity.
If the virus particles are washed further with distilled water, they become sticky and suspend less readily in distilled water. Addition of microliter amounts of 0.01 Μ N a2C 03 will dissolve the polyhedron protein again and will readily resuspend the virus particles. By these methods of liberation and purification of virus particles, one can obtain about 1 to 2 mg of highly purified virus particles from about 35 mg of polyhedra (Bergold, 1947). This corresponds to about 3 to 5 percent of the weight of polyhedra. T h e yield depends on the species of insect from which the polyhedra were harvested.
13. NATURE OF NUCLEAR-POLYHEDROSIS VIRUSES 417
IV. STRUCTURE OF POLYHEDRA AND MORPHOLOGY OF VIRUSES
A. Size and Structure of Polyhedra
T h e size and shape of nuclear polyhedra varies considerably not only between polyhedra from different insects, but often also within polyhedra of the same species. They crystallize as dodecahedra, tetra- hedra, cubes, or forms irregular in appearance. In some species of insects, like the silkworm, B. mori, the prevailing types of polyhedra are dodec
ahedra, whereas those of L. monacha consist chiefly of tetrahedra. T h e polyhedra from Porthetria dispar (Linnaeus) are irregular. In some cases, the shape of polyhedra depends on the strain of the virus, as was shown by Gershenson (1959, 1960), who was able to isolate from Antheraea pernyi Guerin-Meneville a mutant strain with a hexagonal shape instead of a tetragon-tritetrahedral shape.
T h e diameter of polyhedra varies from about 0.5 to 15 μ, depending on the insect species. However, in some insect species, the diameter of polyhedra varies considerably within the individual insect.
If polyhedra are dissolved in alkaline solutions, one can observe what appears to be some kind of limiting ''membranes'' around the poly
hedra. Such "membranes," if they exist, should also be visible in sections of polyhedra. This is, however, not the case, and it is believed that these
"membranes" are artifacts arising during the purification process of the polyhedra, as has been previously suggested (Bergold, 1943, 1962a;
Hughes, 1953).
Investigations of the fine structure of polyhedra were first carried out by Morgan et al. (1955, 1956). They revealed the crystalline lattice of the polyhedron protein molecules, which shortly afterward was con
firmed by Day et al. (1956). Furthermore, Morgan and co-workers observed that the distribution and position of virus particles within the crystalline lattice is completely at random, as assumed by Hughes (1953). It is quite surprising that the presence of these randomly dis
tributed virus particles does not disturb in any way the crystalline lattice.
This could be understood if the virus particles would act as centers for the crystallization of the inclusion bodies, but no evidence for this could be obtained, since in no case could a concentric growth of polyhedron protein molecules around the virus particles be observed. It was first assumed on the basis of pseudohexagonal arrangement of the polyhedron protein molecules that they are arranged in a hexagonal packing. A reinvestigation of the fine structure of polyhedra (Bergold, 1962a) has revealed that this is not t;he case. T h e polyhedron protein molecules are in fact arranged in a cufcic system, since 90-degree angles between the molecule rows could be found in polyhedra from many different insects.
418 GERNOT Η. BERGOLD
Furthermore, the distance between the molecule centers is shorter in 90-degree dot patterns than in 120-degree dot patterns. With the help of wooden molecule models, it could be shown that the 109 to 120 degree patterns frequently observed in sections of polyhedra can easily be explained by the appearance of sections through molecules arranged in a cubic system, but cut at certain angles. T h e line patterns observed by Morgan et al. (1955, 1956) were previously interpreted as a para- crystalline arrangement of the somewhat rod-shaped polyhedron protein molecules. However, a recent investigation (Bergold, 1962a) has shown that line patterns can easily be obtained when spherical molecules which are arranged in a cubic system are cut at a certain angle. T h i s is sup
ported by an electron microscopic investigation by Hall (1960), who found in solutions of polyhedron proteins only spherical molecules with a diameter of about 90 Ä. One must, therefore, conclude that all poly
hedra investigated so far are composed of spherical molecules arranged in a face-centered cubic system. In such a cubic system the molecules are not arranged in the closet possible packing. Therefore, special points of attraction may be assumed at six sites of the surface of each poly
hedron protein molecule in order to explain the loose arrangement of the cubic system. It is somewhat surprising that no dislocations have ever been found in the lattice of polyhedra.
From the 90-degree dot patterns, one can calculate the diameter of the polyhedron protein molecules, assuming that they are touching, or at least are very close to, each other. One arrives at diameters for molecules from polyhedra of different insects which are slightly smaller than those which can be calculated, on the basis of sedimentation and diffusion measurements of polyhedron protein solutions (see Section V). One may, therefore, conclude that shrinkage of the molecules in the lattice occurs as a result of dehydration during the electron microscopic prepa
ration.
B. Morphology and Size of Viruses
Because of their small size, only images of virus particles can be observed in the dark field of the light microscope. Electron microscopic observation is necessary in order to investigate their exact morphology.
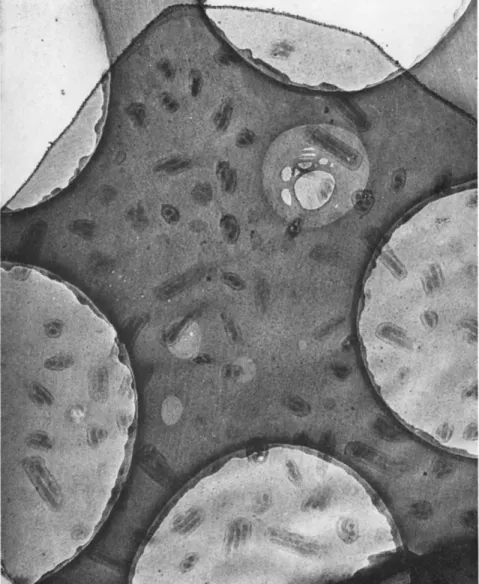
FIG. 1. Highly infectious preparation of nuclear-polyhedrosis-virus particles of Bombyx mori. T h e majority of rods are still surrounded by the developmental mem
brane. A double and a single rod, in the center, have shed their developmental mem
branes, and thin protrusions on one end are visible. Note empty spherical develop
mental membranes, as well as early spherical stages of virus particles. Magnification χ 50,000.
NATURE OF NUCLEAR-POLYHEDROSIS VIRUSES
1 3 . 4 1 9
420 GERNOT Η. BERGOLD
There are three ways to examine insect virus particles: (1) free virus particles isolated from polyhedra; (2) virus particles still enclosed in polyhedra; and (3) virus particles within infected cell nuclei.
1. Free Virus Particles Isolated from Polyhedra
T h e first of any insect viruses demonstrated with the electron micro
scope were the virus particles of B. mori, P. dispar, and L. monacha (Bergold, 1947). Since then, many virologists were able to illustrate numerous insect viruses of this group. Summarizing all these results, one can characterize free nuclear insect-virus particles as follows: they are rod-shaped, about 20 to 50 πιμ in diameter (including the develop
mental membrane), and about 200 to 400 πιμ long (Figs. 1 and 8 ) . T h e diameter is only about two-thirds that of virus particles still enclosed in polyhedra (see Section IV, B , 2 ) . This indicates that free virus particles shrink considerably in diameter, probably because of preparation tech
niques for electron microscopy. T h e length of the rods are, within one insect species, quite similar; however, the diameter varies considerably in some insect species. This is due to the fact that in some species, for instance, in P. dispar and L. monacha, there is more than one virus rod in the form of a bundle enclosed within the so-called developmental membrane (Fig. 2) (Bergold, 1948, 1962b). In some species, like B.
mori, the majority of virus particles have the same diameter, a fact indicating that there is only one virus particle within the developmental membrane; however, up to seven rods were found once in one bundle
(Bergold, 1962b).
Using the method of liberation and purification of virus particles mentioned above, one obtains highly infectious preparations of virus particles. Comparing the morphology of the virus particles, it becomes immediately obvious that considerable changes of the morphology of some virus particles occur during the alkaline solution of polyhedra.
These changes can be studied by controlled treatment with alkali. By such treatment, nuclear-polyhedrosis-virus particles disintegrate into different morphological structures (Bergold, 1953a). T h e concentration of alkali needed to reveal these substructures varies for viruses from different insects. Generally speaking, virus particles will begin to dis
integrate if they are suspended in an alkaline concentration about 30
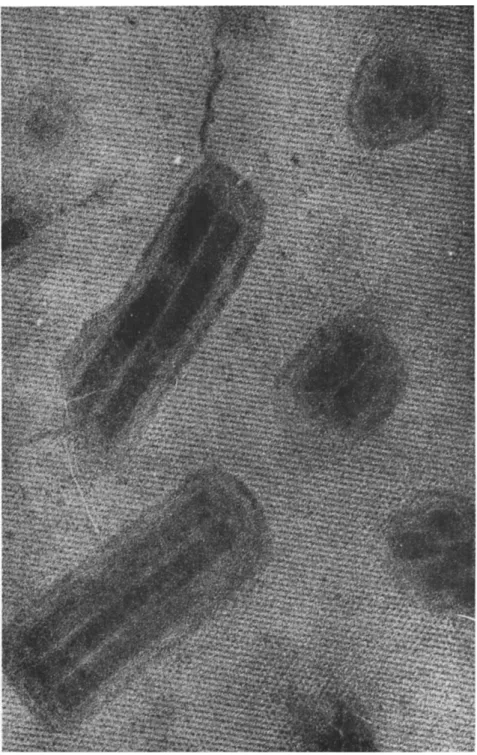
FIG. 2. Nuclear-polyhedrosis-virus particles of Porthetria dispar. T h e wide, very dense particles represent bundles still within their developmental membranes; the slender particles have shed their membranes. Note one, more or less spherical, empty developmental membrane and several rod-shaped, empty intimate membranes; two big spherical developmental stages and several spherical small subunits. Magnification
χ 50,000.
NATURE OF NUCLEAR-POLYHEDROSIS VIRUSES
1 3 . 4 2 1
422 GERNOT Η. BERGOLD
percent higher than that necessary to liberate them from their polyhedra.
Bombyx mori virus particles will begin to disintegrate when suspended in 0.0025 Μ N a2C 03. T h e first structural change seems to be the appear
ance of a short protrusion at one end of the virus rod. This protrusion is apparently attached to the intimate membrane and pierces through the developmental membrane (Figs. 1 and 3 ) . Part of the latter often remains attached to the end of the rod. In P. dispar virus bundles, all rods show their protrusion at the same end. T h e significance of this protrusion is not known, but it has been suggested that it is somehow related to the process of infecting susceptible cells (Bergold, 1951), as is the case with bacteriophages. This speculation is supported by investi
gations by Bird (1957, 1959), who observed an orientation of reinfecting virus rods of Diprion hercyniae (Hartig) with the protrusion pointing toward the chromatin material.
At a concentration of about 0.025 Μ N a2C 03, the external, or so- called developmental membrane, is shed leaving behind a slender rod. T h e empty developmental membrane has then the appearance of a spherical collapsed bubble. By further increasing the alkali concentration, the slender rods begin to disintegrate into about six to eight more-or-less spherical or disc-shaped subunits, leaving behind the tubular-shaped so- called intimate membrane (Bergold, 1952, 1953a) (Figs. 2 and 4 ) . It was shown by Krieg (1957, 1961a) that these subunits possibly have a hole in the center, which could form a central channel in the virus rods.
Investigations by the author have confirmed this. These holes are proba
bly the result of dissolution of some specific material by the alkaline treatment, since they are never found in intact virus particles (Section IV, B , 2 ) .
Frequently, one can observe that the virus rods run out into long threads at one end. It appears that these threads are mainly parts of disintegrating intimate membranes. During the preparation of virus membranes, one may notice that these threads aggregate readily to form monomolecular layers (Bergold and Wellington, 1954). T h e rod-shaped form of virus particles is completely changed into a spherical form by boiling briefly in water.
FIG. 3. (Top) Nuclear-polyhedrosis-virus particles of Bombyx mori. Two particles with, and others after having shed, their developmental membranes. One particle shows clearly the thin protrusion on one end. Magnification χ 75,000.
FIG. 4. (Bottom) Nuclear-polyhedrosis-virus particles of Bombyx mori. One particle still lies within the intimate membrane at the upper left. In the center, note one empty intimate membrane; at the upper right, one still containing a subunit. At the bottom is a particle that has released about half of its spherical subunits, one of which, in the lower left, shows clearly a central hole. Magnification χ 75,000.
NATURE OF NUCLEAR-POLYHEDROSIS VIRUSES
1 3 . 4 2 3
424 GERNOT Η. BERGOLD
A careful examination of virus suspensions reveals that apart from the rods, one can always observe a few spherical (Figs. 1 and 2 ) , and some kidney and V-shaped, particles. T h e spherical particles appear to have also a membrane as well as the V-shaped particles (Bergold, 1958a).
In preparations of virus particles from P. dispar, one can find large, somewhat irregular, spherical particles which seem to consist of several small spheres. It is believed that these large spheres are early stages of virus bundles, which was previously suggested by Bird (1959), because they are usually found in viruses which occur frequently in bundles (Bergold, 1962b). Serological investigations (Section V I I , B , 2) have shown that all particles isolated from inclusion bodies are unrelated to host materials; it is, therefore, believed by the author that all morpho
logically differentiated forms which can be found in such virus suspen
sions represent different stages of a life and multiplication cycle of the virus. One can arrange the various differentiated forms into a sequence and speculate on the following life and multiplication cycle of nuclear polyhedrosis viruses: the virus rods disintegrate into spherical subunits which grow, passing through the kidney and V-shaped forms, into rods which in turn disintegrate and initiate another cycle (Bergold, 1950a, b, 1953a, b ) . This multiplication cycle has been approved and extended by Bird (1959).
T h e electron microscopic investigations of sections of sedimented virus particles isolated from polyhedra confirm in general the structures described above, and in Section IV, B , 3, without revealing much addi
tional information (Bergold, 1962b).
Using the negative staining technique of Brenner and H o m e (1959), one can demonstrate some of the structures described above. It is, how
ever, surprising that this method, so useful for other viruses, has appar
ently a somewhat destructive effect on the virus particles. T h e latter are considerably distorted in comparison to their appearance after ordinary fixation with osmic acid, or to the appearance of sections of virus particles when still enclosed within polyhedra (Bergold, 1962b).
2. Virus Particles Still Enclosed in Polyhedra
It is obvious that the most reliable results can be expected from examination of virus particles still enclosed within polyhedra. Such virus particles lie embedded within the very homogenous polyhedron- protein lattice in their original state. Under these circumstances, no treatment can change their natural shape or size except that required to make the electron microscopic examinations.
Electron microscopic investigations of thin-sectioned polyhedra and virus particles enclosed therein reveal a very high degree of preservation
13. NATURE OF NUCLEAR-POLYHEDROSIS VIRUSES 425 and of resolution of the structure of the virus particles (Figs. 5, 6, 7 ) . A perfect check for the shape and external structure of the virus particles is provided by the degree of regularity of the lattice of the polyhedron- protein molecules surrounding any given virus particles. Deviation from the direction of the molecules can be checked within a few Angström
FIG. 5. Section of a polyhedral body of Lymantria monacha laid over a perforated supporting film. Cross- and longitudinally sectioned virus bundles are embedded randomly and are in random positions within the polyhedron-protein lattice (120- degree dot pattern). Magnification χ 40,000. (From Bergold, 1962b).
FIG. 6. Section of Lymantria monacha. Cross- and longitudinally sectioned virus particles within the linear pattern of polyhedron-protein lattice. Magnification χ 200,000. (From Bergold, 1962b.)
426
FIG. 7. High-resolution electron micrographs of a section of a polyhedral body of Bombyx mori; one cross-sectioned virus particle and parts of two longitudinally sectioned particles embedded within the face-centered cubic polyhedron-protein lattice of about 90 degrees. Magnification χ 500,000. (From Bergold, 1962b.)
427
428 GERNOT Η. BERGOLD
units, and, therefore, distortions of virus particles can be very readily detected. Generally speaking, the morphology of intact virus particles, as described in the preceding paragraphs, can be confirmed (Bergold, 1962b) : virus particles have two membranes, and occur singly or in bundles. However, in spite of the study of numerous electron micro
graphs, empty developmental and intimate membranes could never be found within polyhedra. This means that destruction of some virus particles takes place by the alkaline treatment of polyhedra during the liberation and purification of the virus particles. This is in agreement with virus infectivity titrations which show that suspensions containing many virus particles without developmental membranes are less infectious than those in which only very few particles had shed their developmental membrane. One must conclude, therefore, that the developmental mem
brane is important in preserving the infectivity of the virus particle.
Bird (1959) denies this importance of the developmental membrane, because he observed infective virus rods without them. One can argue, however, that these membranes are shed simultaneously with the in
fective process.
Furthermore, within polyhedra, protrusions were never found extend
ing from virus rods. Similarly, the spherical or disc-shaped subunits seen after alkaline treatment of liberated virus particles can never be seen in virus particles still embedded in polyhedra. This is certainly not due to a lack of resolution, because the resolution of the electron micrographs of sectioned virus particles within polyhedra is well below 10 Ä. There
fore, subunits, as well as their central holes mentioned earlier (Section IV, B , 1 ) , would show very clearly. T h e appearance of discs and the central channel in them must, therefore, be due to dissolving of materials not distinguishable by the electron microscope from the rest of the virus particle. It is most surprising that no molecules of a molecular weight of about 300,000 or higher can be discerned within the virus particles, although the polyhedron protein molecules with a molecular weight of about 300,000 are readily resolved around the virus particles. All mole
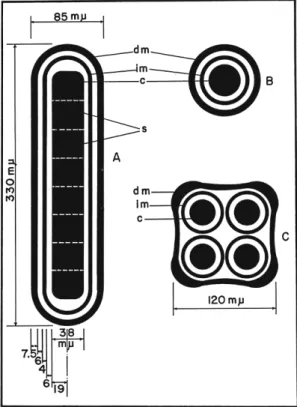
cules constituting morphological differentiations of the virus particles must be, therefore, closely packed, or tightly surrounded by very small molecules. This is in agreement with the greater density of the virus particles than that of the surrounding crystalline lattice of polyhedron protein molecules. T h e densest part of the virus particles appears to be the center, where a very homogenous corelike column is situated. Even with the very high resolution of the pictures, and in spite of its assumed molecular weight of several millions, no evidence for the location of the nucleic acid within the virus particles could be found. Figure 8 shows a schematic drawing of the morphology of nuclear-virus particles of B.
13. NATURE OF NUCLEAR-POLYHEDROSIS VIRUSES 429 mori. From the outside toward the inside, one can first discern the developmental membrane about 75 A thick, then some space between it and the intimate membrane of lesser density 60 Ä wide, then the intimate membrane about 40 Ä thick, then a space between the intimate membrane and the central part of the virus particle again of lesser
FIG. 8. Schematic drawing of nuclear polyhedrosis virus particles as they appear within polyhedra. ( A and B) Longitudinal and cross sections of a single Bombyx mori virus particle showing the developmental membrane (dm), the intimate mem
brane (im), and the dense central core (c) with 8 subunits (s). (C) Cross section through a bundle of 4 virus particles of Laphygma frugiperda showing the common developmental (dm) and 4 intimate membranes (im), and the 4 dense central cores (c).
(From Bergold, 1962b.)
density about 60 Ä wide, and finally, the central very dense core of the virus particle with a diameter of about 380 Ä. T h e distances between these structures are fairly regular, but vary somewhat between viruses from different insect species. T h i s diameter of about 850 Ä is considerably greater than that measured for free virus particles (see Section I V , Β , 1 ) . This is probably the true diameter of virus particles, since the poly-
430 GERNOT Η. BERGOLD
hedron-protein lattice provides a perfect check (Figs. 5-7) for distortion, shrinkage, and dimensions. However, no distortion or shrinkage of the virus particles within the polyhedron protein lattice can be detected.
Comparing the dimensions of free and enclosed virus particles in several insect species, it appears that this is generally true. This means that practically all diameters given in the literature for free insect nuclear polyhedrosis virus particles are smaller than the true diameters (Bergold, 1962b).
As mentioned above, virus particles occur singly or in bundles; this can be very well demonstrated in sections through polyhedra. Viruses of some insect species, for instance those of B. mori, have mainly single- virus particles, but occasionally up to seven rods within one bundle were found in polyhedra. Viruses from other species have different numbers of particles within one developmental membrane. Bird (1959) found a maximum of 12 rods in polyhedrosis of Choristoneura fumiferana (Clemens). T h e record, so far, is 19 virus particles within one common membrane in L. monacha. Comparing the cross sections of bundles containing different numbers of virus particles, one can see that the particles are always arranged in the closest possible packing, forming triangles, squares, pentagons, hexagons, etc. It is remarkable that the 19 particles are arranged in a perfectly symmetric layout. However, cross sections of bundles containing such numbers of virus particles that do not permit a symmetric pattern are accordingly very irregular (Bergold, 1962b). T h e distances between the virus particles within the bundles are, however, fairly constant. One must conclude from the above that during the formation of polyhedra, the virus particles in the bundles are sus
pended in a somewhat liquid medium within the developmental mem
brane which permits the shaping of symmetrical patterns by the poly
hedron-protein molecules. This means, further, that the enclosure of the virus particles progresses concentrically toward the surface of the parti
cles, until finally, the polyhedron protein molecules have completely surrounded the virus particles. Some pictures of Bird (1959) suggest such an event. I f this is indeed the mechanism of crystallization, one can
not explain why no other cell components are enclosed within the poly
hedra. T h e relationship between the polyhedron protein molecules and the virus particles is, therefore, still mysterious.
T h e developmental membrane surrounding the virus particles must be quite elastic. Within the polyhedra it has all kinds of shapes according to the number of particles within a bundle, but after the liberation from the polyhedra, and having been shed from the virus particles, it always appears spherical in spite of the tubular appearance when surrounding the virus bundles.
13. NATURE OF NUCLEAR-POLYHEDROSIS VIRUSES 431 One would also expect to find occasionally within polyhedra spherical virus particles which can be found in suspensions of liberated particles.
However, in serial sections (Morgan et al., 1956) no conclusive evidence could be found for the presence of such spherical particles within the polyhedra. However, a close examination of unpublished pictures of this investigation does indicate occasionally the presence of spherical particles, because the cross sections appear only in two or three consecu
tive sections; whereas, cross sections of typical virus rods in the same series appear in five or six consecutive sections. There are some indica
tions of the presence of V-shaped virus particles within polyhedra, because occasionally only the center part of a somewhat rod-shaped particle appears to be cut; whereas, on either side of this area, one can see the polyhedron-protein molecules deposited.
Summarizing the various methods for investigation of the structure of virus particles, one can state that the best way to study the morphology of virus particles in their original state is to section them while they are still embedded within the polyhedra; but to observe some of their sub
structures, one has to study particles liberated from the polyhedra with alkali.
3. Virus Particles within Infected Cell Nuclei
If one investigates free virus particles within infected cells, one has to search for them among all kinds of structures characteristic of infected- cell nuclei. It is obvious that this is the least reliable method of observa
tion.
Only few investigations have been carried out to demonstrate virus within infected cells. Generally speaking, there are so many different structures in infected cells that the identification of virus particles, or stages of them, is more difficult in comparison to the two methods mentioned previously. Furthermore, several multiplication cycles occur, out of phase, simultaneously in one cell, and this makes it difficult to coordinate the sequence of morphological differentiations. Characteristic forms like, for instance, an empty tubular-shaped intimate membrane, could be found at one occasion (Bergold, 1952). Similarly, Bird (1957) found spherical and rod-shaped stages in infected cells of Neodiprion pratti banksianae Rohwer. It appears that the diameter of the rod-shaped virus particles is considerably larger and much wider in the middle part of the particle, resulting in a rather wide space between the develop
mental membrane and the virus particle proper. T h i s space is of very low electron density. It may be mainly water which, later on, leaves the virus particle when it becomes enclosed within the polyhedron. How
ever, many rods have no developmental membrane, and it is not clear
432 GERNOT Η. BERGOLD
whether such rods have shed or are acquiring the developmental mem
brane (Hughes, 1953; Day et al, 1958; Bird, 1957, 1959).
V . PHYSICOCHEMICAL PROPERTIES AND CHEMICAL COMPOSITION OF POLYHEDRA
A. Physicochemical Properties
T h e physicochemical properties of polyhedra were first investigated by Bolle (1873, 1893). Polyhedra do not dissolve in hot or cold water, alcohol, ether, chloroform, benzol, or acetone. They dissolve, however, in aqueous solutions of NaOH, K O H , N H3, H2S 04, and C H3C O O H . For histological staining it is, therefore, necessary to pretreat polyhedra with weak acids
(Escherich and Miyajima, 1911). After such treatment, they will take several different stains. All histological methods proposed are based on this staining ability. After heat fixation, polyhedra are gram positive, but after treatment for 2 hours with glycocolic acid at 60°C, they become gram negative. It is somewhat surprising that polyhedra are not destroyed by bacterial putrefaction processes. Accordingly, polyhedra are not digested by proteinases, such as papain (at pH 8 . 3 ) , trypsin (at pH 6 . 8 ) , or pepsin (at pH 3.3 to 4 . 0 ) , but by pepsin at pH 2.0 to 2.9 and by trypsin and papain after alkali treatment (Zalmanzon, 1949, 1952).
Some investigators have found that polyhedra are insoluble in the hemolymph of insect larvae (Bergold, 1943; Ishimori and Osawa, 1952;
Gershenson, 1956a). But, others report that polyhedra lyse in the pupal lymph of B. mori (Roegner-Aust, 1949) and in the prepupae of Neo
diprion sertifer (Geoffroy) (Krieg, 1955). Eto (1956a, b) reports that 70 percent by weight of B. mori polyhedra dissolve in alcohol after treat
ment with trichloroacetic acid.
Polyhedra are heavier than water and, therefore, collect at the bottom of wet mounts on microscope slides, a characteristic which helps to distinguish them from fat droplets which always float on top. T h e density of B. mori polyhedra is 1.268 (Bergold, 1958a). They are highly refractive and light up very strongly in the dark-field microscope. They are not double refringent, and are clearly transparent. When dried, they remain unchanged for many years. T h e solubility of B. mori polyhedra in N a2C 03 has, for example, not changed after being stored in a desiccator over CaCl2 for 37 years (Aizawa, 1954a).
Bombyx mori polyhedra migrate in an electric field to the positive pole (von Prowazek, 1913; Dikasova, 1949) and have an isoelectric point at pH 5.2 (Tarasevich, 1945).
Using the standard methods for the isolation of virus particles [(mentioned in Section I I I , B , 2) (Bergold, 1947, 1958a, 1958b)] in weak solutions of alkali (0.005 Μ to 0.03 Μ N a2C Os + 0.05 Μ NaCl for
13. NATURE OF NUCLEAR-POLYHEDROSIS VIRUSES 433 1 to 3 hours), one obtains a slightly yellow, almost clear, solution of polyhedron protein after sedimentation of the virus particles at 10,000 to 12,000 g. Ultracentrifugation for 30 minutes at about 25,000 g removes the few remaining virus particles, and particularly the virus membranes, resulting in an exceptionally pure solution of polyhedron protein. T h e latter can be precipitated by lowering the pH with HCl or C H3C O O H , or by dialysis against distilled water. T h e precipitated protein can be washed repeatedly with distilled water and redissolved in dilute alkali.
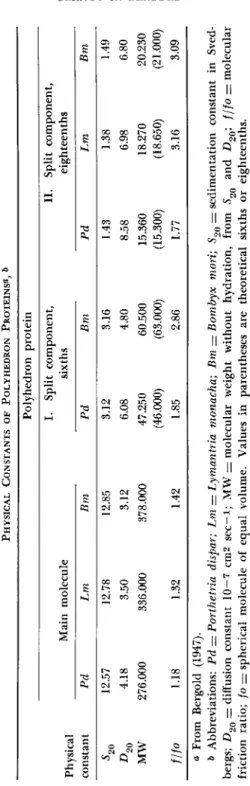
Under certain salt and pH conditions, such polyhedron-protein solutions sediment very homogenously in the ultracentrifuge, the sedimentation constant being around 12.5 Svedberg units ( S ) . T h e diffusion constant of several polyhedron-protein solutions was also measured, and molecular weights of 378,000 could be calculated for B. mori polyhedron protein, 336,000 for L. monacha polyhedron protein, and 276,000 for P. dispar polyhedron protein solutions. T h i s so-called main molecule of poly
hedron protein can be split reversibly into sixths by weak alkaline treatments, and irreversibly into eighteenths by further addition of alkali. Polyhedron proteins are very sensitive to pH changes and to the addition of neutral salts which will cause aggregation of the main molecules to doubles with 18 S. T h e various physical constants of this investigation are summarized in T a b l e I (Bergold, 1947).
T h e second split component has a molecular weight of about 20,000 which is in excellent agreement with the 22,000 calculated by Kratky (1943, cited by Bergold, 1947) on the basis of small-angle X-ray investigations. Kratky could estimate the dimensions of the smallest elementary cell to be 45.3 χ 28.0 χ 20.4 Ä. Panebianco (1895) reports that he recrystallized polyhedra by adding H2S 04. However, several attempts by the reviewer were unsuccessful, which is somewhat sur
prising since the polyhedron protein can be obtained in an exceptionally pure form and one should be able to recrystallize it. T h e second split component with a molecular weight of about 20,000 is not destroyed by boiling briefly in 0.5 Μ NaOH, and it crystallizes in bodies up to 5 μ in diameter similar to polyhedra (Glaser and Chapman, 1916; Bergold,
1947).
Polyhedron proteins are completely insoluble at their isoelectric points, which are at pH 5.7 for P. dispar, and between pH 5.6 and 5.3 for B. mori and L. monacha polyhedron protein (Bergold and Schramm, 1942). Yamafuji et al. (1953) found two components with different mobilities in nonpurified polyhedron solutions. According to Aizawa
(1955), polyhedron protein seems not to migrate on filter paper at pH 8.6. It spreads readily on water surfaces and takes twice the load (55 dynes/cm) as films of casein or egg albumen. T h e maximum area of
TABLE I PHYSICAL CONSTANTS OF POLYHEDRON PROTEINS«, & Polyhedron protein I. Split component, II. Split component, Physical constant
Main molecule sixths eighteenths Physical constant Pd Lm Bm Pd Bm Pd Lm Bm 5 20 12.57 12.78 12.85 3.12 3.16 1.43 1.38 1.49 4.18 3.50 3.12 6.08 4.80 8.58 6.98 6.80 MW 276.000 336.000 378.000 47.250 60.500 15.360 18.270 20.230 (46.000) (63.000) (15.300) (18.650) (21.000) f/fo 1.18 1.32 1.42 1.85 2.86 1.77 3.16 3.09 a From Bergold (1947). & Abbreviations: Pd = Porthetria dispar; Lm = Lymantria monacha; Bm — Bombyx mori; S90 = sedimentation constant in Sved- bergs; D2Q — diffusion constant 10—7 cm2 sec—i; MW = molecular weight without hydration, from 52Q and D20; f/fo = molecular friction ratio; fo — spherical molecule of equal volume. Values in parentheses are theoretical sixths or eighteenths.
13. NATURE OF NUCLEAR-POLYHEDROSIS VIRUSES 435 spread of B. mori polyhedron protein is around pH 5, and around pH 4 for P. dispar and L. monacha. T h e thickness of the spread protein films varies between pH 1 and 7 (Bergold and Brill, 1942).
B. Chemical Composition
T h e first chemical analyses of polyhedra were carried out by Bolle (1873, 1893). He found that polyhedra consist of protein and contain no lipids. In evaluating the results of chemical analyses of polyhedra, one should realize that polyhedra consist of essentially two components:
the polyhedron protein which constitutes about 95 percent of the total weight, and the virus particles which amount to 5 percent. All analyses of total polyhedra are, therefore, analyses of polyhedral protein plus the virus particles. T h e most important chemical analyses of polyhedra and purified polyhedron protein are summarized in T a b l e I I . This T a b l e shows that the nitrogen content of polyhedra and the purified polyhedron-protein preparations from different hosts does not vary much and is about 14 to 15 percent. However, the phosphorus content of polyhedra varies with the preparation of purified polyhedron protein.
It is doubtful whether the small amount of phosphorus (about 0.05 per
cent) that cannot be removed belongs indeed to the protein molecules, or whether it is only adsorbed phosphorus. There is a difference between the phosphorus content of polyhedra and of polyhedron protein of about 50 to 60 percent. This difference is due to free dialyzable phosphate which is liberated from the polyhedra by the alkaline treatment (Desnu- elle et al, 1943; Bergold, 1947). Faulkner (1962) found R N A in B. mori polyhedra. T h e base composition (expressed as moles percent of total bases) of all extractable R N A was 16.2 guanine, 36.8 adenine, 8.1 cytosine, and 38.9 uracil after phenol treatment, and 18.3, 45.7, 8.2, and 27.8 after perchloric acid treatment of polyhedra. T h e function of this R N A is not known. T h e base composition of this R N A differs from that of the host insect where 29 guanine, 24 adenine, 25 cytosine, and 22 uracil were found by Eto (1955). An investigation of the metal content has revealed that there is only 0.005 percent iron in B. mori polyhedra and polyhedron protein, and 0.083 percent magnesium in polyhedra, but not in poly
hedron protein (Holoway and Bergold, 1953, 1955).
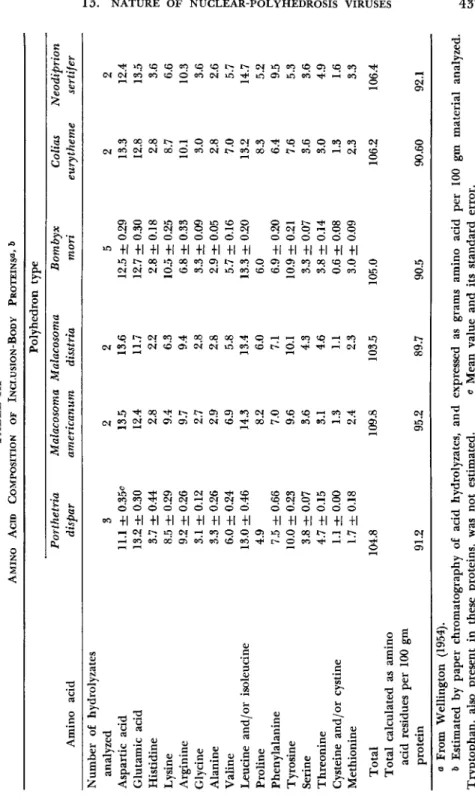
T h e first investigations of the amino acid content of B. mori poly
hedra were carried out by Desnuelle et al. (1943) and Desnuelle and Chang (1943). They found (expressed as percent of protein nitrogen), 0.4 cysteine and cystine, 4.6 alanine, 5.2 tyrosine, 4.7 histidine, 12.1 arginine, 3.8 phenylalanine, 3.0 tryptophan, 6.4 ammonia, and 3.8 humin nitrogen. T h e composition of amino acids of purified polyhedron protein from different insect viruses was investigated extensively by Wellington
436 GERNOT Η. BERGOLD
(1951, 1954). T h e results, summarized in T a b l e I I I , reveal that all analyzed inclusion-body proteins have a very similar amino acid composi
tion independent of whether the polyhedra were derived from a lepi- dopteron or a hymenopteron (N. sertifer). A comparison of any two of the proteins investigated show significant differences (P < 0.01) in the quantity of amino acids. No qualitative differences in amino acids were
T A B L E I I
COMPOSITION IN Bombyx mori OF INCLUSION BODIES, INCLUSION-BODY PROTEIN, AND VIRUS«
Substance Polyhedra
Polyhedron
protein Virus
Ν 14.73o-15.5c
14.5<*-14.29*
14.88-15.11/
14.9-15.2^
12.0Λ
15.16ft 15.0<*
14.53-15.81/
13.92 13.9
Ρ 0.191-0.243Ö
0.243^, 0.32*
0.22-0.350 0.21/-0.79Λ
0.062&, 0.064*
0.00-0.08/
0.915
Acid-soluble fraction DNA-P
RNA-P Ρ
0.00-0.03^
0.05-0.120 0.07-0.140
0.01-0.020 0.00-0.040 0.02-0.100 Nucleic Acid fraction
Ρ DNA-P RNA-P
0.10-0.170 0.07-0.100 0.00-0.090
0.07-0.140 0.06-0.110 0.00-0.040
N:P 60-77& 245ft 15.2*
C 54.37*
40.98-50.78/
51.67-52.33/
Η 7.02*
6.54-6.85/
6.81-7.31/
S 1.48, 0.92/
0.79&
0.83<*
C I 0.075*
Ash 1.51e
0.26-0.31/
0.67-0.98/
a Values are given as percentages.
& Bergold (1947).
* Bergold and Wellington (1954).
d Desnuelle et al. (1943).
* Glaser and Stanley (1943).
/ Ikeda (1946, 1951).
0 Yagi et al. (1951).
Ä Manunta (1940).
TABLE III AMINO ACID COMPOSITION OF INCLUSION-BODY PROTEINS^ δ Polyhedron type Porthetria Malacosoma Malacosoma Bombyx Colias Neodiprion Amino acid dispar americanum disstria mori eurytheme sertifer Number of hydrolyzates analyzed 3 2 2 5 2 2 Aspartic acid 11.1 ± 0.35c 13.5 13.6 12.5 ± 0.29 13.3 12.4 Glutamic acid 13.2 ± 0.30 12.4 11.7 12.7 ± 0.30 12.8 13.5 Histidine 3.7 ± 0.44 2.8 2.2 2.8 ± 0.18 2.8 3.6 Lysine 8.5 ± 0.29 9.4 6.3 10.5 ± 0.25 8.7 6.6 Arginine 9.2 ± 0.26 9.7 9.4 6.8 ± 0.33 10.1 10.3 Glycine 3.1 ± 0.12 2.7 2.8 3.3 ± 0.09 3.0 3.6 Alanine 3.3 ± 0.26 2.9 2.8 2.9 ± 0.05 2.8 2.6 Valine 6.0 ± 0.24 6.9 5.8 5.7 ± 0.16 7.0 5.7 Leucine and/or isoleucine 13.0 ± 0.46 14.3 13.4 13.3 ± 0.20 13.2 14.7 Proline 4.9 8.2 6.0 6.0 8.3 5.2 Phenylalanine 7.5 ± 0.66 7.0 7.1 6.9 ± 0.20 6.4 9.5 Tyrosine 10.0 ± 0.23 9.6 10.1 10.9 ± 0.21 7.6 5.3 Serine 3.8 ± 0.07 3.6 4.3 3.3 ± 0.07 3.6 3.6 Threonine 4.7 ± 0.15 3.1 4.6 3.8 ± 0.14 3.0 4.9 Cysteine and /or cystine 1.1 ± 0.00 1.3 1.1 0.6 ± 0.08 1.3 1.6 Methionine 1.7 ±0.18 2.4 2.3 3.0 ± 0.09 2.3 3.3 Total 104.8 109.8 103.5 105.0 106.2 106.4 Total calculated as amino acid residues per 100 gm protein 91.2 95.2 89.7 90.5 90.60 92.1 a From Wellington (1954). δ Estimated by paper chromatography of acid hydrolyzates, and expressed as grams amino acid per 100 gm material analyzed. Tryptophan, also present in these proteins, was not estimated. ο Mean value and its standard error.
438 GERNOT Η. BERGOLD
found between the membranes of P. dispar polyhedra and the poly
hedron protein (Wyatt, 1950).
It was shown by Desnuelle and Chang (1945) that treatment of B. mori polyhedra with anhydrous methanol and dried hydrogen chloride gas converts all carboxyl groups to methyl esters (111 groups), and the basic groups to hydrochlorides (67 groups).
VI. CHEMICAL COMPOSITION OF VIRUS PARTICLES
T h e first report that polyhedra (of P. dispar) give a positive Feulgen reaction was published by Breindl and Jirovec (1936). W e know, of course, that this reaction is due to the DNA content of the enclosed virus particles. Alkaline solutions of polyhedra have ultraviolet-absorp
tion spectra; these spectra suggest also the presence of nucleic acid (Dannenberg, cited in Bergold and Schramm, 1942). This was confirmed by chemical tests, but no uronic acid or free carbohydrates could be detected (Tarasevich, 1946). T h e first quantitative determinations are reported by Gratia et al. (1945), who found 0.48 percent DNA, but no RNA, in B. mori polyhedra. An analysis of purified virus particles revealed 13 percent DNA in virus particles of B. mori, and 16 percent in those of P. dispar (Bergold, 1947; Bergold and Pister, 1948). Careful analy
ses of purified virus suspensions of different insects showed no trace of RNA (Wyatt, 1952a, b). A chemical reinvestigation of very highly purified B. mori particles indicated that they contain about 7.9 percent DNA and 0.915 percent phosphorus, of which, however, only 87 percent is found in the DNA (Bergold and Wellington, 1954). T h e ratio of DNA basis to total phosphorus is in good agreement with this (Wyatt, 1952b). T h e remain
ing 13 percent phosphorus may originate from the surrounding virus membranes, which contain about 0.45 percent phosphorus. In the virus membrane, there is only about half as much nondialyzable phosphorus as there is in the virus. Nothing is known as to the kind of bond involved. Krieg (1956) found 9 percent DNA, but no RNA, in a purified preparation of the nuclear virus particles from A porta crataegi (Lin
naeus). Analyses of the nitrogen and phosphorus content of different virus particles and virus membranes are summarized in Tables I I and I I I . These tables indicate that virus membranes obtained by alkaline treatment and separation from the virus particles by ultracentrifugation have less nitrogen (12.5 percent) than have the virus particles (13.9 per
cent) (Bergold and Wellington, 1954).
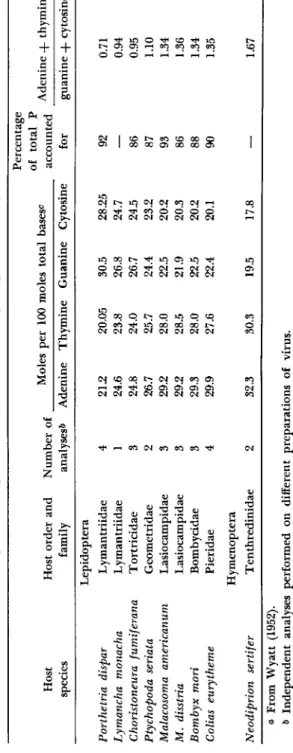
Manunta, in 1940, was the first to report the presence of purine bases in B. mori polyhedra. T h e results of intensive investigations (Smith and Wyatt, 1951; Wyatt, 1952b) of the bases of several insect viruses are summarized in T a b l e IV. This table shows that nuclear insect viruses