II. CONVERSION OF PROGESTERONE TO TESTOSTERONE IN VITRO
HAROLD ff. LEE LORETTA LIANG-TANG*
NORIMOTO KAZAMA**
Department of Biology University of Toledo
Toledo, Ohio
ABSTRACT
Radioactive 17a-hydroxyprogesterone, androstenedione and testosterone were definitively identified by recrystallization to constant specific activity when monolayer cultures of testicular cells from juvenile cockerels were incubated with progesterone-
3H . These results suggested that the testicular cells retained their differentiated characteristics for as long as 11 days in vitro and that they possessed all the necessary enzymes for the conversion of progesterone to testosterone. Results from double-
*Present address: Department of Obstetrics and Gynecology, University of Rochester Medical School, Rochester, New York.
**Present address: Division of Reproductive Biology, C. S. Mott Center for Human Growth and Development, Wayne State University, Detroit, Michigan.
347
348 H A R O L D H . L E E étal.
labelled experiments suggested that pregnenolone, dehydroepiandro- sterone and andros-5-ene-33, 1 7 3- d i o l might be formed. However, recrystallization data did not support those of the chromatog- raphy. This study failed to demonstrate the reversibility of the 33-hydroxysterοid dehydrogenase-isomerase reaction.
I· INTRODUCTION1
One of the advantages in using cell culture over in vivo ap- proaches for endocrinological studies is the preclusion of endo- crine contributions from other organs. However, one should be aware that cells in vitro may change, or dedifferentiate, although transformation from one differentiated cell type to another (Gur- don, 1974) is rare. Testis is not only an endocrine organ but also a developing system throughout most of the life span of the animal. The germinal population proliferate and differentiate continuously while the steroidogenic cells, Leydig and Sertoli, produce androgens. These cellular characteristics are not shared by most organ systems whose proliferative abilities either cease or decrease to a level necessary for the maintenance of a stable population of differentiated cells.
Compared to investigations on the physiology of the testes in vivo, studies using cell cultures have been few. Cultures derived from mouse Leydig cell tumors failed to synthesize androgens
(Shin, 1967). Monolayer cultures of immatured rat testes, previ- ously primed in vivo with HCG, were unable to synthesize testost-
^The following trivial names and abbreviations are used: pregn- enolone (Pnl), pregn-5-ene-3$-ol-20-one; progesterone (Ρ), pregn- 4-ene-3, 20 dione; 17a-hydroxyprogesterone (17aP), pregn-4-ene- 17a-ol-3, 20 dione; androstenedione (A4-dione), androst-4-ene-3, 17-dione; dehydroepiandrosterone (DHEA), androst-5-ene-3ß-ol-17- one; testosterone (Τ), androst-4-ene-173-ol-3-one; androstenediol
(A5-diol), androst-5-ene-33, 173-diol; TLC, thin-layer chromato- gram; SA, specific activity; dpm, disintergrations per minute;
cpm, counts per minute.
erone unless androstenedione, an immediate precursor of testost- erone, was supplied as substrate (Steinberger et al., 1970). Re- cently Steinberger and coworkers (1975) were able to isolate and maintain a viable, although not proliferative, population of Ser- toli cells. The steroidogenesis of these cultures have not been elucidated.
In view of the fact that testicular cultures would lend them- selves in the investigation of not only steroidogenesis but also spermatogenesis, we established a primary monolayer culture sys- tem derived from juvenile cockerel testes. Previous publications showed that this cockerel testis culture had histoformative abili- ty and were capable to synthesize androgens (Lee, 1971; Tang and Lee, 1973). The primary cultures reach confluency in about 10-12 days after seeding.
We now report that the primary cultures from cockerel testes are able to convert progesterone to testosterone via the Δ^-path- way. Therefore, the differentiated characteristic of the ster- oidogenic cell population are retained without added hormonal
stimulation.
II. MATERIALS AND METHODS
A. Cell Cultures
The method of cell culture has been reported previously in detail. Briefly, testes aseptically removed from 10-12-week-old fowl (White Leghorn variety) were decapsulated, minced and dis- sociated into single cell suspensions with 0.125% trypsin (Nutri- tional Biochemical Corp., grade 1:125) in saline. The cells, at an initial density of 2 χ 1 06 per dish were cultured in 60-mm polystyrene plastic tissue culture dishes (Falcon Plastics) at
37°C in an atmosphere of 5% C 02 in air. Each dish contained 5 ml of modified Ham's F12 nutrient mixture supplemented with 1% horse serum, 10% tryptose phosphate broth (DIFCO Laboratories) and anti-
350 H A R O L D H . LEE et al.
biotics. Monolayers with distinct reorganized patterns, i. e., 3 or 4 days after seeding, were used.
B. Incubation with Steroids
Radioactive steroids, 1, 2-3H-progesterone (SA = 47.8 Ci/m mole or 8.3 Ci/m mole), progesterone-4-1 4C (SA = 52.8 Ci/m mole), DHEA-7-3H (SA = 2 5 Ci/m mole) were purchased from New England Nu- clear Company. Their radiochemical purity was examined by thin layer chromatography (TLC) with a solvent system of benzene-hep- tane-ethyl acetate (5:2:3). For recrystallization studies, pro- gesterone was purified prior to use by TLC with a solvent system of chloroform:acetone (92:8). Steroid substrates were added di- rectly to the cultures in a volume of 10 μΐ containing various quantities of isotope as indicated in each experiment. The dura- tions of incubations varied from 15 minutes to 24 hours depending on experiments.
C. Extraction of Steroids
At the end of the incubation, cells were scraped off the bot- tom of the culture dishes with a rubber policeman. The cells to- gether with the culture medium were transferred to a 50 ml capped conical centrifuge tube. Steroids were extracted with either 9 volumes of chloroform-methanol (5:4) or 24 ml of methylene di- chloride with or without carrier steroids. The organic phase was concentrated or dried over anhydrous sodium sulfate, both under N2. Aliquots of these extracts were analyzed for recovery with a scintillation spectrometer. The recovery at this stage was 85-93% of the initially added radioactivity. Depending on subse- quent steps, the above procedures varied slightly as indicated.
D. Fractionation by TLC
As a preliminary identification of the conversion products from progesterone, the concentrated samples were transferred to silica gel G TLC plates and subjected to ascending chromatography in benzene-heptane-ethyl acetate (5:2:3) according to Nugara and Edwards (1970). Fractions corresponding to authentic steroids
(purchased from either Sigma Chemical or Steroloids, Inc.) were eluted with acetone and the radioactivities were determined.
E. Further Identification of Conversion Products by Acetylation
For the definitive identifications of products converted from progesterone, authentic steroid of Ρ, 17aP, A4-dione, Pnl, 17aPnl, DHEA or Τ at 40 yg each was added to the first extract. The mix- ture was then analyzed with TLC with a solvent of chloroform- acetone (92:8). TLC twice developed in the same direction ef- fectively separated Ρ and -dione from each other and from other neutral steroids; other neutral steroids were also separated.
The steroid spots, detected by a germicidal lamp and by Rhodamine 6G (0.015% in absolute ethanol), were aspirated onto a Pasteur pipette packed with glass wool. The steroids were then eluted with a mixture of chloroform-methanol (1:1).
The steroids were again dried and were acetylated by incubat- ing with 200 μΐ of pyridine and 40 μΐ of acetic anhydride for 18 hours at room temperature (Dominquez et al., 1963). The acety- lated extracts were then chromatographed for further purification prior to recrystallization. The solvent systems were as follow:
I. Chloroform-acetone, 92:8
II. Heptane-benzene-ethyl acetate, 20:50:30 III. Hexane-ethyl acetate, 50:20
IV. Benzene-diethyl ether, 80:20
V. Heptane-benzene-ethyl acetate, 20:60:20 VI. Hexane-ethyl acetate, 50:50
352 H A R O L D H . L E E et al.
The solvent systems used for individual acetylated derivative will be described in the Result section. The TLC plates used for this procedure were silica gel 60F-254 (Brinkman Instruments, Inc.).
F. Final Identification by Recrystallization
A final semi-purified fraction was mixed with 13-22 mg of authentic carrier compound. Repeated crystallizations were per- formed with appropriate solvent pairs (in Results) and the SA in both the crystals and mother liquor solids were determined.
Crystals of approximately 600-700 ]ig were weighed on a Cahn elec- trobalance model G2. Radioactivities of each sample were again analyzed with a scintillation spectrometer. When the values of the SA of 3 successive recrystallizations were within ±5% of the average of the three values, the purity, i. e., the definitive identification, of an individual compound is established.
III. RESULTS
A. Time Course Studies
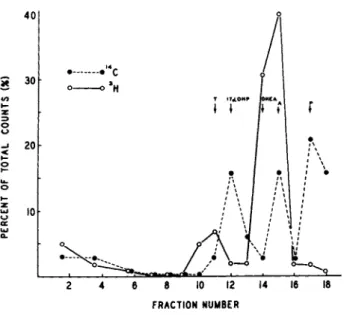
Progesterone-3H (SA = 8 . 3 Ci/m mole) at 0.5 yCi/ml of medium was incubated with 11-day cultures for 7 intervals, 15 min., 30 min., 1, 2, 3, 6 and 24 hours. The first steroid extracts were concentrated and analyzed by TLC with a solvent system of benzene- heptane-ethyl acetate (5:2:3). The data (Fig. 1) was calculated as percent of total cpm. Two major metabolites appeared in the fractions corresponding to androstenedione and DHEA at the 15 minute incubate. With increase in time of incubation, there was a substantial increase of radioactivity in fractions correspond- ing to testosterone and an unidentified compound X. By 3 hours or longer, essentially all the added progesterone had been con- verted to testosterone, DHEA, androstenedione and compound X.
FRACTION NUMBER
FIGURE 1 Rate of metabolism of progesterone-1, 2-3H by tes- ticular cells in monolayer cultures. For details of incubation see text. Areas corresponding to authentic steroids on the TLC are indicated.
B. Double-labelled with P -1 4C and DHEA-3H
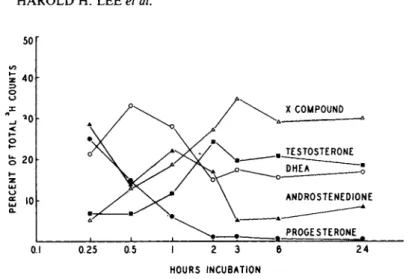
Equimolar concentrations (2.5 ymole) of progesterone-1 4C and DHEA-3H both at 0.5 yCi/ml of culture medium were added to 5-day testicular cultures. Preliminary identifications of the metabo- lites were carried out with TLC by the solvent systems, benzene- heptane-ethyl acetate (5:2:3). The percents of radioactivities of either ^4C or 3H present in fractions corresponding to authen- tic steroids were presented in Fig. 2. The 2 major metabolites labelled with 1 4C were 17a-P and A4-dione. The major metabolites with 3H appeared to correspond to androstenedione and testost- erone .
C. Identifications of Intermediates
Spots in solvent system II corresponding to Pnl was eluted, acetylated and chromatographed again in System III in the same
354 H A R O L D H . LEE et ai
FIGURE 2 Distribution of radioactivity on thin layer chrom- atograms of steroid extracts from monolayer cultures incubated with P-14C and DHEA-3H at 37°C for 3 hours. Solvent system in text. Areas corresponding to authentic steroids on the TLC are indicated by arrows.
direction twice and then in System IV in the second dimension.
The Pnl spot was then eluted and chromatographed with System V.
However, recrystallization with authentic Pnl-acetate failed to achieve constant SA although the first crystal contained 2101 cpm/mg. The solvent pairs were acetone alone, acetone-heptane, chloroform-heptane, methanol-water and ethyl acetate-heptane, in that order.
For the identification of DHEA, the same solvent systems and procedures for TLC were used as for Pnl. The solvent pairs were acetone alone, acetone-70% aqueous methanol, chloroform-heptane, acetone-70% aqueous methanol, and methanol-50% aqueous methanol, in that order. Although the TLC purified fraction had SA = 1670 dpm/mg, the first crystal had a gross cpm less than twice the background.
To identify Δ^-diol, the preliminary TLC spot correspond to authentic Δ^-diol was eluted, acetylated and chromatographed in two dimension with solvent system VI. The area corresponding to
authentic A5~diol*-diacetate was eluted and recrystallized five times with added authentic A5-diol diacetate. The solvent pairs for recrystallization were acetone-70% aqueous methanol, methanol alone, methylene dichloride-heptane, chloroform-heptane, and ace- tone-50% aqueous methanol, in that order. Although the initial steroid mixture had SA = 24,696 dpm/mg, the first crystal had only a gross count less than twice the background.
Biochemical determinations to establish the identities of the possible Δ 5 intermediates, Pnl, DHEA and A5-diol, leading to testosterone from progesterone have yielded negative results.
The reverse reaction, i. e., Ρ •> Pnl, did not exist.
Attempts to identify the compound X in Fig. 1 were not suc- cessful. Radioactivity disappeared when a second TLC was carried out.
D. Identifications of Δ Intermediates 4
After a 20 minute incubation with P-3H, only 40% of the original radioactivity remained with the Ρ as revealed by TLC
with solvent system I. The areas corresponding to 17aP, -dione, and testosterone were eluted for intensive purification as de-
scribed below.
1. 17aP
Areas corresponding to authentic 17aP was eluted, acetylated and the acetylated compound was chromatographed again in solvent system VI two dimensionally. For recrystallization, authentic 17aP was added. Constant SA for both crystal and mother liquor were obtained for the last three solvent pairs (Table I ) .
2. L4-dione
The area corresponding to authentic Δ* -dione was eluted from the preliminary TLC and was acetylated. Although no acetylation occurred, a radioactive product corresponding to authentic Δ4-
τι
• Η P H ο
w
u
Ο
&
• Η
Φ -Ρ
• P
en
U
•H o
• P
•H fd
>
Q M-l Φ
< s
•H φ O §
Q MH
- P c
> φ H O W
id ft
σ» C N oo
η H C N
o m co o ^ oo r**
C0 rH o o
m C N C M m o o o o
+ 1 + 1
^ vo H n
Γ*^ Vû Γ-» VO O O O O
Φ C rd + J
ft
+ > - P ft ft φ φ
υ υ υ υ (Ν
ο
CM +> φ +» fd ϋ , φ , , fd Φ
CN ΓΟ β Η rH Η Ο
> ι -Ρ
r C Φ
•Ρ ϋ
Φ fd
η cm en *r oo ο
fd
356
Ο W
U Ο
&
• Η
<υ
rfi •Ρ
•Ρ fd en
>1
U rH
Q <W
w a
c fi
Ο fd
• H ω
' H g
Q <H
if
o o + l
OD OO VO rH 00 O rH vO CN CN (T\ 00 00
CN O O o O
rH H rH rH H
CN O CN LO Γ 0 O O O + l +
H CN 0 0 «tf
LO i n
rH CM vo 00 rH
00 oo 00
o o o o O rH rH rH rH rH
fi
•P fd
ft Q) ω fi
ω ,fi fd
fi
ω \ -P(Tj
c <D O
•p fd •Pft -P fd o 0
ω ft -P w r fi <u
Q)
- H\ r fi ϋ \
\ fd
Q)
fi
CNfi
0 rH rH 0
•P U >i -P
<U C N Ä <U ϋ
a
-Ρ ϋid u <u fd
fi fd
357
T l - Η RH Ο CO
u
Ο
&
- Η Γ Η D)
4-) Ο
4-» rd ω >ι U R H
Û ΜΗ
< s
CO g T 5
Ο
•rH
•Ρ RD
RÖ Φ
Q ΜΗ
g
1< s
Ό
rH Ο O + l I
C N vo oo
^ oo oo vo vo vo σ» co L D
oo m oo
vo L O vo vo ο CM rH O + + l CN
l
^ h ΓΟ ^ Ο VD VO 0 0 L T ) 0 0 L O Γ 0 L O 0 0 0 0 CN CN CN 0 0 0 0 0 0 0 0 0 0 0 0
rH CN 0 0 L O VO
CD -Ρ C Ο Φ Ο U Φ
•Ρ CO -Ρ Ο CO Φ
•Ρ ϋ
• Η
•Ρ Φ -Ρ
ο
-Ρ C J>
- Η Τ Ί Ο ft CO Φ u u ο ϋ c ο
RD · Η CO Φ
• Η
•Ρ - Η
>
- Η
•Ρ ϋ FD ϋ CO · Η Φ ΜΗ
• Η · Η -Ρ
• Η
>
• Η
•Ρ ϋ RD ϋ
• Η ΜΗ
• Η
Ο Φ ft CO Φ
>
• Η
Φ Γ Η RD Φ
•Ρ
§
ΜΗ Τ $ Φ β
CT1 ΜΗ RD Ο rA°
Φ
ο
-Ρ Φ Ϋ ΜΗ RD
-Ρ U Ul ΜΗ rH Ό RD Φ
>
• Η R H Φ Τ Ί co
Ό Φ Ό
> · Η
• Η ΜΗ RD Φ
£
ΛΟ Ό
> Φ
• Η -Ρ
ϋ RD Ο
• Η Τ Ι RD U
Φ Ε Η RD
ft ω RD
•Ρ Ê Φ co
> rH
5
ο
CO R H
• Η
I
S Î H Q
CO Ο ϋ g RD U Ss M H M H M H U T l O O rH" Φ < <
E H N W W
> ( H•H ^ | M R H rH Ο O RD RD M H M H
fi 4-) ' co fi fi
>i FD FD R H Φ Φ rH y S S Φ Λ ϋ τι
u ft
358
dione was found. Recrystallization with authentic A4-dione as carrier to constant SA was obtained for the last three solvent pairs (Table I I ) .
3. Testosterone
The area corresponding to authentic testosterone on the pre- liminary TLC was eluted, acetylated and chromatographed in the same direction twice with system VI. Recrystallization with au- thentic T-acetate to constant SA with one solvent pair was ob- tained after six times (Table III).
Therefore, the 2 Δ pathway intermediates and testosterone 4 were definitively identified according to the accepted criteria
(Axelrod et al., 1965).
IV. DISCUSSION
Our present findings demonstrate that monolayer cultures of cockerel testes are capable of converting progesterone to test- osterone. The double labelled experiment (Fig. 2) indicates the possibility that both Δ4 and may exist in cockerel testis in vitro. Although multiple TLC analyses suggest that the Δ 5 path- way may be present with labelled progesterone as substrate, ex- periments on recrystallizations of several Δ^ intermediates failed to yield constant SA. Therefore, unlike the rabbit testes and sheep adrenal (Rosner et al., 1965; Ward and Engel, 1966) there is no reversibility of A5-33-hydroxysteroid dehydrogenase- isomer ase in the cockerel testes in vitro converting Ρ -> Pnl.
However, conversion of DHEA to A4-dione is still feasible.
Conversion of P-3H to testosterone via the Δ pathway has 4 been firmly established from the results of the present investi- gation. Authenticities of 17α-Ρ, Δ4-άΐοηβ and Τ from H3- P have been verified radiochemically according to the criteria of Axel- rod (1965). Therefore, the 3 enzyme complexes, 17a-hydroxylase,
360 H A R O L D H. L E E et al.
c1 7 - 2 0 lyase and 17BOHSD, for steroidogenesis are present in the primary monolayer cultures of cockerel testis. Unless the Δ^
pathway exists in vivo, one can conclude thst the monolayers de- rived from cockerels retain the differentiated characteristics for as long as 11-days in vitro. The testes for the primary cul- ture are at the stage prior to the onset of spermatogenesis when steroid synthesis of the testis is at the highest level. Prior to this stage, going back to embryonic gonadol development, the biochemical differentiation with respect to steroidogenesis might, however, differ. The difference might exist in the metabolic pathways whose influence on the gonadol development may be signi-
ficant. The present culture system offers an opportunity to in- vestigate this problem and that of the steroidogenic cell-germ cell interactions.
ACKNOWLEDGMENTS
This study was supported by a grant (HD 06725) from the National Institute of Child Health and Human Development, USPHS.
One of us (Harold H. Lee) is recipient of a University of Toledo Trustee's Research Award (1974 and 1975).
REFERENCES
Axelrod, L. R., Matthijssen, C , Goldzieher, J. W. and Pulliam, J. E. (1965). Acta Endocrinol (Kbh) Suppl. 99, 3.
Dominquez, Q. V., Seely, J. R. and Gorski, J. (1963). Anal. Chem.
35, 1243.
Gurdon, J. B. (1974). "The Control of Gene Expression in Animal Development," Harvard University Press, Cambridge, Massachu- setts.
Lee, H. H. C1971). Develop. Biol. 24, 322.
Nugara, D. and Edwards, Η. Μ., Jr. (1970). J. Nutr, 100, 539.
Rosner, J. W,, Hall, Ρ? F. and Eik>Nes, Κ. B. C1965). Steroids 5, 199.
Shin, Seung-il. C 1 9 6 7 ) . Endocrin. 81, 440.
Steinberger, Α., Heindel, Α. Α., Lindsey, J. Ν., Elkington, J. S.
Η., Sanborn, Β. M. and Steinberger, E. (1975). Endo. Res. Comm.
3 (In Press).
Steinberger, Ε., Steinberger, A. and Ficher, M. (1970). Recent Progr. Horm. Res. 26, 547.
Tang, F. Y. and Lee, H. H. (1973). Endocrin. 92, 318.
Ward, M. G. and Engel, L. L. (1966). J. Biol. Chem. 241, 3147.