ContentslistsavailableatSciVerseScienceDirect
Applied Surface Science
j o ur na l ho me p age :w w w . e l s e v i e r . c o m / l o c a t e / a p s u s c
Preparation of a boron nitride single layer on a polycrystalline Rh surface
János Kiss
a,b,∗, Károly Révész
a,b, Gábor Klivényi
a,b, Frigyes Solymosi
a,baDepartmentofPhysicalChemistryandMaterialsScience,UniversityofSzeged,Aradivértanúkt.1,H-6720Szeged,Hungary
bReactionKineticsandSurfaceChemistry,ResearchGroupofHungarianAcademyofSciences,RerrichB.t.1,H-6720Szeged,Hungary
a r t i c l e i n f o
Articlehistory:
Received30July2012
Receivedinrevisedform26October2012 Accepted28October2012
Available online 3 November 2012
Keywords:
Segregationofboron NOdissociationonRh FormationofB Nbond Boronnitride Augerfinestructures
a b s t r a c t
Thesegregationofboronanditsreactivitytowardnitricoxidehavebeeninvestigatedbymeansofhigh- resolutionAugerspectroscopy(AES),X-rayphotoelectronspectroscopy(XPS),ultravioletphotoelectron spectroscopy(UPS),andthermaldesorptionspectroscopy(TDS).ThesegregationofboronfromaRhfoil startedfrom700K.ItspresencealteredthesurfacebehaviorsofRh;theuptakeofNOincreasedbyabout 30–37%.WhereasthedissociationofNOwasabout3–10%onaclean,boron-freesurface,theextentof dissociation(atsaturation)athighestboronlevelwasalmost98%.Thisfeaturestronglysuggestadirect interactionbetweenNOandborononthesurface.Thepresenceofborongreatlystabilizedtheadsorbed nitrogenandoxygenformedinNOdissociation.Boronoxide(BO,B2O2)sublimatedfromthesurface below1000K.Clean,singleBNlayerformedonthesurfaceclosetoamonolayerregime,presumablein nanomashstructure.
© 2012 Elsevier B.V. All rights reserved.
1. Introduction
Graphenenanoribbons,atomicallythinstripsofgraphenethat arejustafewnanometerswide,areconsideredtobeexcellentcan- didatesfor futureelectronicsapplicationastheirpropertiescan beadjustedthroughwidthandedgeshape[1,3].Hexagonalboron nitride(h-BN)hasgreatpotentialforuseasthedielectriclayerin functionalheterostructureddevices,whichexploittheremarkable propertiesofgraphene[1,2].Thecombinationofgrapheneandh- BNopensuptheexistingpossibilityofcreatinganewclassofatomi- callythinmultilayeredheterostructures.Grapheneandh-BNshare thesamecrystalstructureandhaveverysimilarlatticeconstant but,unlikegraphene,h-BNisaninsulatorwithalargebandgap [4].Alonggraphene,boronnitridenanoribbonshaveattractedmore andmorefundamentalresearchinterest.Forinstance,researchers havefoundthatmagnetismcouldbeinducedinsuchribbonsby replacementofBorNwithBe,C,Al,Si,orwithvacancydefects.
Theyalsofoundthattheribbonscouldhaveanarrowedbandgap andimprovedelectricalconductivitytunedbyatransverseelectric fieldorspecialedgestructure.Thesefindingpromiseabrightfuture inoptoelectronicsandspintronicsforatomicallythinboronnitride nanoribbons.However,amajorchallengeinprovidingexperimen- talevidenceisthatthepreparationofatomicallythinboronnitride isverydifficult[5–7].SincefirstapplicationsofBNinfundamental researchthattheinterestinsinglelayerboronnitride(sBN)has
∗Correspondingauthorat:DepartmentofPhysicalChemistryandMaterialsSci- ence,UniversityofSzeged,Hungary.Tel.:+3662544803;fax:+3662546482.
E-mailaddress:jkiss@chem.u-szeged.hu(J.Kiss).
beenincreasingsteadily.Inthepastseveralexperimentalstudies havebeenperformedincludingTEM[8–10],AFM[11]andoptical andRamanspectroscopy[9]forcharacterizationofthematerial’s surface.ThemainexperimentalapproachesforproducingsBNhave beenbothmechanical[9,11]andchemical[10]exfoliation.
Oneofthesurfacesciencetoolsproducingboronsourceisthe dehydrogenationofdecaboraneonmetalsurfaces[12].Hexagonal boronnitride(h-BN)waspreparedbythermaldecompositionof borazine,B3H6N3onNi(111)[13],Cu(111)[14]andRh(111)[15].
Surfaceboroncanbepreparedbysegregationprocess,too.Boron segregatesfromthebulktothesurfacebyannealingthepolycrys- tallineRhsurfaceat750–1200K[16,17].Weearlierdemonstrated thatboronimpuritysegregatingtoanRhsurfacedramaticallyalters thereactivityoftheRhsurfacetowardN-andO-containingmoi- eties,suchas(CN)2[18],H2O[17],CO2[19,20].Apossiblereasonfor thisphenomenonisthatboronformsverystrongbondswithNand O,whichcanpromotetheprocessesofsurfacedissociationofthe adsorbedmolecules.Followingsurfacedissociation,wedetected theformationofdissociationproductsviathermaldesorption,and anewfeatureat9.4and7.4eVintheelectronenergylossspectrum duetoB OandB Nbond,respectively.Thesegregationofboron ontoaRhsurfacehasalsobeenobservedbyotherauthors[21–23].
Inpresentworkweexaminethesegregationofboronandinter- actionofsegregatedboronwithNObymeansofdifferentelectron spectroscopicmethods,suchashighresolutionAES,XPS,UPSand thermal desorptionmassspectrometry(TDS).Theprimaryaims weretodeterminethemonolayerBNformedduringthereaction betweenthedissociation productand segregated boron.Earlier theinteractionofNOwithcleanRh(111)surfacewasinvestigated widelybydifferentsurfacesciencetechniques[24–31].Theinitial 0169-4332/$–seefrontmatter© 2012 Elsevier B.V. All rights reserved.
http://dx.doi.org/10.1016/j.apsusc.2012.10.157
J.Kissetal./AppliedSurfaceScience264 (2013) 838–844 839 adsorptionofNOoncleanRhsurfaceisdissociativeat300K.At
higherNOcoveragetheadsorptionismolecular.
2. Experimental
Theexperimentswereperformedinanultra-highvacuumsys- tem,withabackgroundpressureinthelow-tomiddle10−10mbar range,producedbyturbomolecularandtitaniumgetterpumps.
Thesystemwasequippedwithahemisphericalanalyser(Leybold- HeraeusLHS-10)forUPS,XPSandhighresolutionAES,anArion gunforcleaningandaquadrupolemassspectrometerforTDS.
UPSwasperformedbyusingHeI(21.22eV)andHeII(40.81eV) radiation.TheanglesbetweenthesurfacenormalandtheUVlamp andbetweenthesurfacenormalandtheanalyserwere75◦ and 16◦,respectively.ThephotoelectronswereexcitedbyAlK␣radia- tion(1486.7eV)intheXPSregime.TheenergiesoftheXPSpeaks werecalibratedrelativetotheFermilevelofRh,metallicRh3d3/2 ispositionedat307.1eV.Thephotoemissionsweremeasuredby usingapassenergyof50eVinordertogettheoptimumresolution bymaintaininganacceptablesignal-to-noiseratioatmeasuring timeof60min.HighresolutionAugerelectronspectraweretaken indN(E)/dEmodeusingalock-inamplifier(Ithacho,Dinatrac391 A)with0.5–2eVpeak-to-peakmodulation.Attheseparameters theestimatedresolutionwas1.6–1.9eV.Inordertominimizethe electron-inducedchangesinourexperiments,weused0.2–1Aof incidentcurrentand2.5kVofincidentenergy.
ThepolycrystallineRhfoil(10mm×10mmand0.127mmthick 99.9%purity)waspurchasedfromHicolCo.Theinitialcleaningpro- cedurehasbeendescribedpreviously[19,20].Itisconsistedofion bombardmentandannealingat1270K.Themajorcontaminants ofRhfoilwereB,P,S,andC.TheP,CandSwereeasilyremoved, butnocompleteeliminationofboronwasachievedevenaftersev- eralcleaningcycles.Thefinalthermaltreatmentwasperformedat 700K.
Segregationof boronwasachieved byannealing the Rhfoil at750–1270K.Thelevelofsurfaceboronischaracterizedbythe relativeintensityoftheboronAugersignal,RB=B178/Rh302.B178
andRh302 representpeak-to-peakintensity ratiosof boronKLL andrhodiumMNNAugertransitionsdeterminedindifferentiated mode.Thepeakpositionsaretakenfromthenegativegoingofthe spectra.
3. Resultsanddiscussion
3.1. Generalfeaturesofsegregatedborononrhodiumsurfaces The segregation of boron was pointed out in earlier works [17–24,32].Ithasbeenobservedthatboronisamajorbulkimpurity (17ppm)inRh.Whenthesurfaceisheatedtheboronsegregates tothesurface.Fig.1showsatypicalsegregationcurve,RBvalues (relativeintensityofboronAugersignal)areplottedagainsttem- perature.Whenthefreshly sputteredsurfacewasheatedabove 700K boronsignalshowedup,it reacheda maximum valueat 1000K.(Experimentscannotbeextendedtohighertemperatures becauseoftheconstructionofoursampleholder.)Boroncoverages werecalculatedroughlybyusingtheratioofthetheoreticalXPS photoionizationcross-section[33]foradsorbedboronandoxygen andcomparingtotheXPSsignalfromtheknownoxygencoverage onpolycrystallineRhatsaturation(5.8×1014Oatoms/cm2)[34].
In thisway we found7.96×1014Batoms/cm2 athighest boron concentration,which abouthalfofa monolayer.Thisvaluecor- respondstoa relative Augerpeak ratio ofRB=0.103.Using the Langmuir–McLeanequation,assumingthatthesegregationprocess isinequilibrium,thesegregationenergyis−71.5kJ/mol.
Fig.1.PlotoftheB/RhAugerpeakratio(RB)versustemperature.
Thesegregationexhibits significantspacespecificity. Onthe Rh(100)face,thesegregatedboronformsa(3×1)LEEDpattern andasboronisdepletedfromthebulk,itthensegregatestoalesser extentandformsa(3×3)orderedoverlayer[23].Thesegregation onthe(755)faceismanytimeslargerthanthatontheRh(331) face[21].
AnanalysisofthewidthoftheHeIUPSuponsegregationof borondidnotrevealanychangesintheworkfunction,indicating thatthedipolemomentproducedbysurfaceboronisverysmall.
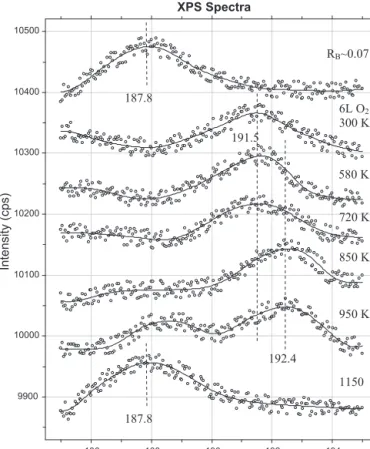
ThisisconsistentwiththesimilarPaulingelectronegativitiesof B(2.0)andRh(2.2),whichsuggestthatchargetransferbetween theseelementsisminimal.Independentlyofthecoverage,theB 1slevelappearedat187.8eVinXPS(Fig.2).Thepeakexhibited 3.5eVFWHMatsaturation.Thisvaluesignificantlylargerthanthat observedforB1sonMo(100)surfaceafteradsorptionofdiborane [35].WeassumethattheB1sphotoemissionsignaliscomposedof
186 188 190 192 194
BE (eV)
9900 10000 10100 10200 10300 10400 10500
Intensity (cps)
XPS Spectra
191.5 187.8
192.4
RB~0.07
6L O2
300 K
580 K
720 K
850 K
950 K
1150 187.8
Fig.2. XPspectraforB1slevelafterheatingthesample(RB≈0.075)exposedto6L O2at300Ktodifferenttemperatures.
Fig.3.UPspectraofadsorbedNOoncleansurfaceatdifferentexposuresat300Kandatdifferenttemperatures.
twoormoreoverlappingpeaks,indicatingdimersortheformation ofislands.
ThesegregationofborononRhfoilproducedaphotoemission peakat8.6eVindifferenceUPSspectra(notshown).TheB2plevel wouldbeexpectedataround4.0–5.0eV.Thedetectionofthispeak isdoubtful,presumableduetoitsverylowcross-section.Theinten- sityofthisphotoemissionat8.6eVincreasedanditshiftedslightly tohigherbindingenergywithincreasingofthesurfaceconcen- trationofboron[32].InaUPSstudyonironborides,peakdueto emissionfromtheboron2p,2sp2and2sstateswereidentified[36].
TheB2sp2levelislocatedat7.0eVforFe2Bandat10.0eVforFeB.
AccordingtothestudybyJoynerandWillis[36],the2sp2 emis- sionisduetoaB–Binteraction.AstheintensityoftheUPSpeakat 8.6–9.0eVincreasedwithboronlevelonRh,wemayassumethat B–Binteractionalsoexistinourcase,too.Stairetal.[35,37]reached asimilarconclusionfortheB/Mo(100)system.
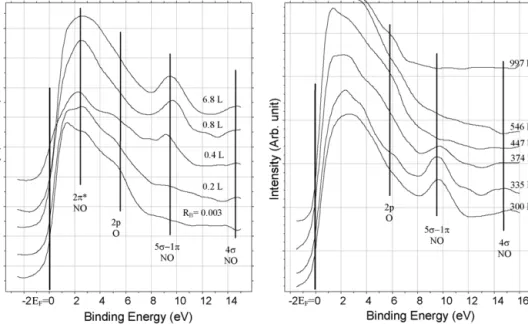
3.2. AdsorptionanddecompositionofNOonboroncontainingRh The chemical nature of adsorbed NO on boron-free poly- crystallineRh wasalmostidenticaltoRh(111) [30,38–41].The appearanceoftheO2pUPSsignalat5.6–5.8eVindicatesthedis- sociationofNOatlowcoverageat300K.Athigherexposuresthe adsorptionispreferentiallymolecular.Newphotoemissionswere producedat∼2.3,9.3and14.5eVbelowFermilevel(Fig.3).The NO-inducedemissionscanbeattributedtothe2*,1/5and4 molecularlevels,respectively.WhentheNO-saturatedsurfacewas heated,theintensitiesofthepeaksat9.3and14.5eVdecreased above354Kandthesepeaksdisappearedat 400–432K(Fig.3).
Theemissionat5.6–5.8eVwasmorestable;itwasdetectedup to997K.Thisemissioncanbeattributedveryprobablytothefor- mationofB Ospeciesathightemperatureswherethesegregation ofboronisdominating.MolecularNOdesorbsbetween350and 420KwithTp=381K.Nitrogendesorbsinthreestages(Tp=410, 550and640K).Somecharacteristicdatafordesorptionarecol- lectedinTable1.Theresultsobtainedsuggestthatthedissociation ofNOis limited.Theextentofdissociation issomewhathigher thanonRh(111)duetothehighernumber ofdefectsites.The calculationsofDeLouiseandWinograd[29],basedonXPSmea- surements,showedthatonly3%ofthesaturatedlayerdissociates at300KonsinglecrystalofRh.Itshouldbealsotakenintoaccount thatthedissociationofNOinthesaturatedlayerishampered,as
itrequiresfree adsorptioncentersinthevicinityofmolecularly adsorbedNO.
ThepresenceofboronalteredthesurfacebehaviorofRh;the uptake of NO onboron containing surface increased by about 30–37%.Theextentofdissociation(atsaturation)alsoincreased;
athigherboronimpuritylevelitwasalmost98%.Thepeaktem- peraturesfordesorptionofmolecularlyadsorbedNOfromboron containingRhwereunalteredsuggestingthatthisNOadsorbsand desorbsfromRhsitesnotinfluencedbyboronadatoms(Table1).
However,thepresenceofboron greatlystabilizedtheadsorbed nitrogenandoxygen.Thisstabilizingeffectwassogreatthatthe nitrogenformed in theboronpromoted dissociation ofNO did notdesorbbelow1200K.Thisfeaturestronglysuggestsadirect interactionbetweenNandborononthesurface.
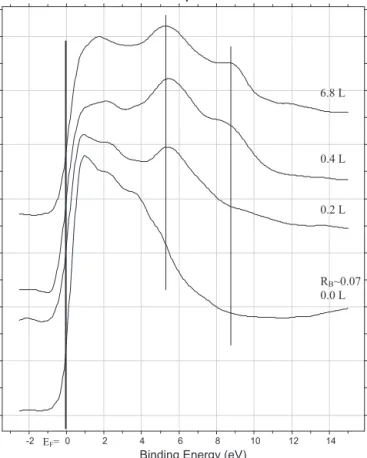
Fig. 4 shows the UPS spectra of NO as a function of NO exposureandthethermallytreatedNOcoveredsurfacedonboron- contaminatedsurfaces.Atlowcoverages,molecularlyadsorbedNO wasnotdetected;strongUPsignalwasobtainedat5.2–5.5eVdue totheadsorbedoxygenfromNOdissociation (Fig.4).Athigher coverages(from0.4L)aphotoemissionsdueto1/5orbitalsof adsorbedNOmoleculeweredetected.DuringheatingtheNOcov- eredsurface (Fig. 5)the emissionsfor molecular orbitalof NO disappearedfrom374K,theO2pintensified,andanewphotoemi- ssionappearedfrom546Kat9.0–9.5eV.Itcouldnotbeeliminated upto1270K.Thisphotoemissioncanbeattributedtotheformation ofbondbetweenBandN[42].
Inthenextstepswefollowedthesurfacechemicalchangesdur- ingadsorptionand thermaltreatmentbyhighresolutionAuger electron spectroscopy.In certain cases due totheloss features originated fromdifferentelectron-induced interband,intraband Table1
CharacteristicdataofdesorptionofNOfromcleanandboroncontainingRhsurfaces (RB≈0.075).
Surface Products Tp(K) Kineticsorder Ed(kJ/mol)
Clean
NO/NO 381 1 111
N2(1)/NO 410 1 120
N2(2)/NO 550 1 160
N2(3)/NO 640 2 –
Boroncontaining RB≈0.103
NO/NO 381 1 111
N2(1)/NO 402 1 117
N2(2)/NO 580 2 –
J.Kissetal./AppliedSurfaceScience264 (2013) 838–844 841
-2 0 2 4 6 8 10 12 14
Binding Energy (eV)
Intensity (Arb. unit)
UPS Spectra
EF=
0.2 L
RB~0.07 0.0 L 0.4 L 6.8 L
Fig.4. UPspectraofadsorbedNOonboroncontainingRhsurfacesatdifferentNO exposuresat300K.
transitionsandplasmonlosses,theAugerspectroscopycouldbe verysensitivetothechemicalnatureofadsorbedmolecules.Fig.6A showstheoxygenAESsignalswithincreasingNO exposuresat highest boron concentration. At lowest NO coverage two AES
-2 0 2 4 6 8 10 12
Binding Energy (eV)
Intensity (Arb. unit)
UPS Spectra
EF=
997 K 837 K 667 K 546 K
447 K 347 K 6.8 L NO 300 K
Fig.5.UPspectraofNOsaturatedboroncontainingRhsurfacesatdifferenttem- peratures.
transitionsweredetectedat513and519eV.Withincreasingexpo- suresbothintensitiesincreased.It isimportanttomentionthat onclean,boron-freesurfaceonestrongpeakappearedat519eV besidessomelossfeaturesatlow-energysideofthespectra.We
480 490 500 510 520 530
eV
dN(E)/dE
AES Spectra
490 500 510 520 530
dN(E)/dE
AES Spectra
0.2 L
0.4 L
0.8 L
6.8 L
Background
300 K
1020 K 374 K
547 K
740 K
880 K
B A
eV
Fig.6.O(KVV)AugerlineshapeafteradsorptionofNOonboroncontainingRhsurfaceatdifferentNOexposuresat300K(A).EffectofheatingonO(KVV)finestructuresof NOsaturatedboroncontainingRhsurfaces(B).Theintensitiesaredividedbythefactorof2.
Fig.7.N(KVV)AugerlineshapeafteradsorptionofNOonboron-freeRhsurfaceatdifferenttemperatures(A).EffectofheatingonN(KVV)finestructuresofNOsaturated boroncontainingRhsurfaces(B).
believethatthepeakat519eVbelongstoRh NObondwhilethe transitionat513eVcorrespondstotheB NObond.WhentheNO coveredsamplewasheatedthepeakat519eVdisappearedwhen themolecularNOdesorbedabove 374K.The intensityofother oxygenpeakdecreasedfrom740K,disappearedbetween880and 1020K(Fig.6B).TheformationofaB Obondisalsojustifiedby thefinestructureofB(KVV)andO(KVV)AESsignalobtainedinthe interactionofoxygenwithboroncontainingRhat300K[32].The AEStransitionofadsorbedoxygenonaB/Rhsurfaceappearedat 513eVatlowexposure.Withincreasingoftheoxygencoverage, theintensityofthispeakincreasedandatransitionat518eVfor adsorbedoxygenonRhsitesalsodeveloped.
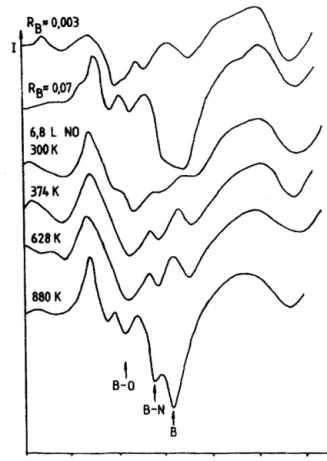
TheAugerfinestructureofnitrogenafteradsorptionanddecom- positionofNOonacleanandboroncontainingRhsurfaceswas somewhat more complex. The adsorbed nitrogen gives a very intense “four-peak”structure in AES[43,44]. Thisstrongstruc- tureoverlapswiththatofotherN-containingmolecules.Onclean RhafterNOadsorptionat300Kfourstructurefinestructurewas observed (Fig.7A).During heating, independentlyfrom theNO dissociationthisstructureremainedupto547–628K,abovethis temperaturetheAESpeakat383eVgained,above740K,where theboronsegregationstarttobedominant,practicallyonepeak remainedat383eV.OnepartofnitrogendesorbsfromRhatoms, other partof nitrogen migrates from Rh sites to boron, which startstosegregatefrom550K.TheformedB Nspeciesexhibits mainlyonepeakbetween380and400eVinAugerspectrum.Sim- ilarstructurewasreportedforhexagonalphaseofboronnitride andBNingraphitizedsteelsheet[45,46].Onboroncontainingsur- face(RB=0.075)atsaturatedNOcoveragethefourpeakstructure ispresentat300K.WhenthemolecularNOdesorbsat434Kand abovetheAESpeakat384–386eVwasonlypresentwithsome satellitesatlowerbindingside(Fig.7B).Thispeakremainedwith sameintensityupto1270K,indicatingthatBNstablesurfacecom- pound.
Fig.8 shows theAugertransitions in boronrange from 150 to 200eV. Without boron (so-called clean surface, RB≈0.003) this regime contains some weektransitions, which are always presentineveryAESspectraofRh.Whenthesurfacewasflashed to1050K, boronsignalappearedat179–183eV.WhenNOwas addedtosurfaceat300K,theintensityofelementalboronsignal
significantlydecreased,ashoulder at172eVgainedremarkably.
Whenthe surface washeated tohigher temperature this peak increased further. In addition, the oxygen Auger feature was shiftedfrom520to513.7eV.Thesame changeswereobserved when oxygenwas added to the boron-containing surface, and followingtheoxidationofboronformingB2O3[47,48].Nodoubt
Fig.8.EffectofheatingonB(KVV)AugerfinestructuresofNOsaturatedboron containingRhsurfaces.
J.Kissetal./AppliedSurfaceScience264 (2013) 838–844 843 theAEStransitionat172eVbelongs totheboronoxide,which
canbeeliminatedabove880K.AnewAESfeaturedevelopedat 176eVfrom300K. Inharmonywiththeliteraturedata[46,49], weattributedittotheB Nbond.Similarlytothecorresponding NAESsignal,itcannotbeeliminatedbelow1270K.Anextensive argonionbombardmentwasusedtogetbackthecleansurface.
AnalysingtheUPS,XPSandAESdataitturnedoutthatduring heattreatmenttheboronoxidedisappearedfromthesurfaceabove 800K.Furtherprocesses areindicatedby thedecrease intheO Augersignalabove850Kandbythereappearanceoftheelemental boronfeatureat187.8eVinXPS(Fig.2).Afteroxygenadsorptionat 300KtheB1sphotoemissionpeakmovedto191.5eV.Duringheat- ingashoulderdevelopedat192.4eV.Thesebindingenergieswere observedwhenB2O3wastreatedinreducingatmosphere,where transientlytwo-valenceboronwasobserved[50].Whentheoxygen coveredRhsurfacewasheatedabove850Kthepeakshiftedbackto 187.8eV.AswedonotexpectthedecompositionofthestableB2O3 species(thedissociationenergyofBOmoleculeis787kJ/mol[51]
andB Osinglebonddissociationenergyisconsideredtobelower, around540kJ/mol),weassumeafurthersegregationofboronand areactionbetweenB2O3andelementalboron
B(S)+B2O3(S)−B2O2(g)+BO(g),
theheatofreactionis141.1kJ/mol[51].Theoccurrenceofthisreac- tioninthepresentcaseissupportedbytheobservationthatupon heatingtheRhfoilcontainingtheB Ocomplextohightemper- aturewedetecteddesorbingspeciesat54and27amu(B2O2and BO,respectively);thepeaktemperaturewas1050K.Notethatpar- tialoxidationofbulkboronleadstotheformationof(BO)xwhich vaporizesat1300K,asB2O2,anddisproportionatesoncondensa- tion[52].
In the interaction of NO with boron-containing Rh surface above900Kwe prepareda cleansingle BNlayerinmonolayer orclosetomonolayerregime.Anh-BNlayerwasfoundtobind a quitelargenumber ofsubstrates,even when thelatticecon- stantofh-BNand thesubstratedonot match.Theprototypical example is the h-BN/Ni(111) interface,which has nice lattice matchingandformsasimpleepitaxial1×1structure[13,53].In thecaseofincommensurateinterfaces,themismatchcanleadto someextraordinarystructures[54].Corsoetal.[15]havereported the formation of a self-assembled nanostructure, the so-called nanomesh,onthelatticemismatchedRh(111)surfacewithaperi- odicityofabout3.2nm.Thiscorrespondstoasituationwherethe latticeof 12×12 Rh(111)matches 13×13 h-BN cells forming periodic nanomesh structure, which is caused by the lattice- mismatchbetweenRh(111)andh-BN.AlthoughourRhsample ispolycrystallinewecannotexcludethattheformedBNconstitute nanomeshonthesurface.Sincethefirstdiscoveryofnanomesh [15]onRh(111)surface,similarstructureshavealsobeenfound onRu(0001)[55],Pd(111)[56]andPd(110)[57].
4. Conclusions
ThesegregationofboronfromaRhfoilstartedat700K.Theseg- regatedboronproducedapeakfortheB1slevelonXPSat187.8eV andemissioninUPSat8.6–9.0eV,respectively.Thepresenceof borondrasticallyalteredthesurfacebehaviorofRh;theuptake ofNOincreasedbyabout30–37%.TheextentofNOdissociation (atsaturation)athighestboronlevelwasalmost98%.Thisfeature stronglysuggestadirectinteractionbetweenNOandborononthe surface.Thepresenceofborongreatlystabilizedtheadsorbedoxy- genandnitrogenformedduringNOdissociation.Boronoxides(BO, B2O2)sublimatedfromthesurfacebelow1000K.Thestabilizing effectwassogreatthatthenitrogenbondedwasnotreleasedbelow 1200K.Theformationofboronnitridewasdetectedat9.0–9.5eV
inUPS.Thisphotoemissioncanbeattributedtotheformationof bondbetweenBandN.Above400KnewintenseAugertransitions developedat176eV(B)and384eV(N),whicharetypicalforB N species.Aclean,singleBNlayerformedonthesurface,presumable innanomashstructure.
Acknowledgement
ThefinancialsupportoftheHungarianScientificResearchFund (OTKA)throughprojectsK81517,K81660andTámop-4.2.2/B-10/1- 2010-0012areacknowledged.
References
[1]Y.Zhang,T.W.Tan,H.L.Stormer,P.Kim,Nature438(2005)201–204.
[2] A.Geim,K.Novoselov,NatureMaterials6(2007)183–191.
[3]L.Britnell,R.V.Gorbachev,R.Jalil,B.D.Belle,F.Schedin,M.I.Katsnelson,L.Eares, S.V.Morozov,A.S.Mayorov,N.M.R.Peres,A.H.C.Neto,J.Leist,A.K.Geim,L.A.
Ponomarenko,K.S.Novoselov,NanoLetters12(2012)1707–1710.
[4]C.R.Dean,A.F.Young,I.Meric,C.Lee,L.Wang,S.Sorgenfrei,K.Watanabe, T.Taniguchi,P.Kim,K.L.Shepard,J.Hone,NatureNanotechnology5(2010) 722–726.
[5]M. Xie, J. Wang, Y.K. Yap, Journal of Physical Chemistry C 114 (2010) 16236–16241.
[6]Z.Liu,L.Song,S.Zhao,I.Huang,L.Ma,J.Zhang,J.Lou,P.M.Ajayan,NanoLetters 11(2011)2032–2037.
[7]R.M.Ribeiro,N.M.R.Peres,PhysicalReviewB83(2011),No.235312.
[8]W.-Q.Han,L.Wu,Y.Zhu,K.Watanabe,T.Taniguchi,AppliedPhysicsLetters93 (2008)223103–223106.
[9] J.C.Meyer,A.Chuvilin,G.Algara-Siller,J.Biskupek,U.Kaiser,NanoLetters9 (2009)2683–2689.
[10]J.H.Warner,M.H.Rümmeli,A.Bachmatiuk,B.Büchner,ACSNano4(2010) 1299–1304.
[11] C.Lee,Q.Li,W.Kalb,X.Liu,H.Berger,R.W.Carpick,J.Hona,Science328(2010) 76–80.
[12]J.Jones,M.Trenary,JournalofPhysicalChemistryC112(2008)20443–20450.
[13]B.Grad,P.Blaha,K.Schwarz,W.Auwarter,T.Greber,PhysicalReviewB68 (2003),No.085404.
[14] A.B.Preobrajenski,A.S.Vinigradov,N.Martensson,SurfaceScience582(2005) 21–30.
[15]M.Corso,W.Auwarter,M.Muntwiler,A.Tamai,T.Greber,J.Osterwalder,Sci- ence303(2004)217–220.
[16] J.Kiss,F.Solymosi,SurfaceScience135(1983)243–260.
[17]J.Kiss,F.Solymosi,SurfaceScience177(1986)191–206.
[18]F.Solymosi,L.Bugyi,AppliedSurfaceScience21(1985)125–138.
[19] F.Solymosi,J.Kiss,ChemicalPhysicsLetters110(1984)639–642.
[20] F.Solymosi,J.Kiss,SurfaceScience149(1985)17–32.
[21]D.G.Castner,G.A.Somorjai,SurfaceScience83(1979)60–82.
[22]S.Semancik, G.L.Haller, J.T.YatesJr.,AppliedSurfaceScience 10 (1982) 546–558.
[23]R.E.Hendershot,R.S.Hansen,JournalofCatalysis98(1986)150–165.
[24]L.Bugyi,J.Kiss,F.Solymosi,JournalofVacuumScienceandTechnologyA5 (1987)863–864.
[25] R.J.Baird,R.C.Ku,P.Wynblatt,SurfaceScience97(1980)346–362.
[26]C.T.Campbell,J.M.White,AppliedSurfaceScience1(1978)347–359.
[27]H.A.C.M.Hendrickx,B.E.Nieuwenhuys,SurfaceScience175(1986)185–196.
[28]T.W.Root,L.D.Schmidt,G.B.Fisher,SurfaceScience150(1985)173–192.
[29]L.A.DeLouise,N.Winograd,SurfaceScience159(1985)199–213.
[30]L.Bugyi,J.Kiss,K.Révész,F.Solymosi,SurfaceScience233(1990)1–11.
[31]F. Bondino,G.Comelli,A.Baraldi,JournalofChemicalPhysics119(2003) 12525–12533.
[32]J.Kiss,K.Révész,F.Solymosi,AppliedSurfaceScience37(1989)95–110.
[33]J.H.Scofield,JournalofElectronSpectroscopy8(1976)129–137.
[34]C.T.Campbell,S.-K.Shi,J.M.White,AppliedSurfaceScience2(1979)382–396.
[35]T.B.Fryberger,J.L.Gland,P.C.Stair,Langmuir3(1987)1015–1025.
[36]D.J.Joyner,R.F.Willis,PhilosophicalMagazineA43(1981)815–833.
[37]T.B.Fryberger,J.L.Gland,P.C.Stair,JournalofVacuumScienceandTechnology A5(1987)858–862.
[38]E.Umbach,S.Kulkarni,P.Feulner,D.Menzel,SurfaceScience88(1979)65–94.
[39]M.Kiskinova,G.Pirug,H.P.Bonzel,SurfaceScience136(1984)285–295.
[40] D.E.Ibbotson,T.S.Wittrig,W.H.Weinberg,SurfaceScience110(1981)294–312.
[41]H.Conrad,G.Ertl,J.Küppers,E.E.Latta,SurfaceScience65(1977)235–244.
[42]W.vonNiessen,TheoreticaChimicaActa29(1973)29–48.
[43]J.C.Fuggle,D.Menzel,Proc.7thIntern.Vac.Congr.,Vienna,1977,p.1003.
[44]F.Solymosi,J.Kiss,SurfaceScience104(1981)181–198.
[45]S.J.Simko,M.C.Militello,SurfaceScienceSpectra1(1992)288–291.
[46]T.Mega,R.Morimoto,M.Morita,J.Shimomura,SurfaceandInterfaceAnalysis 24(1996)375–379.
[47] D.J.Joyner,D.M.Hercules,JournalofChemicalPhysics72(1980)1095–1108.
[48]J.W.RogersJr.,M.L.Knotek,AppliedSurfaceScience13(1982)352–366.
[49]G.Hanke,K.Müller,SurfaceScience152(1985)902–910.
[50]I.Bertóti,R.Kelly,M.Mohai,A.Tóth,NuclearInstrumentsandMethodsB80–81 (1993)1219–1225.
[51]B.deB. Darwent,BondDissociationEnergiesin SimpleMolecule,National BureauofStandards,Washington,D.C.,1970.
[52]M.G.Ingram,R.F.Porter,W.A.Chupka,JournalofChemicalPhysics25(1956) 498–501.
[53]M.N.Huda,L.Kleinman,PhysicalReviewB74(2006),No.075418.
[54]H.-P.Koch,R.Laskowski,P.Blaha,K.Schwarz,PhysicalReviewB84(2011),No.
245410.
[55]A.Goriachko,Y.He,M.Knapp,H.Over,Langmuir23(2007)2928–2931.
[56]M.Morscher,M.Corso,T.Greber,J.Osterwalder,SurfaceScience600(2007) 3280–3284.
[57]M.Corso,T.Greber,J.Osterwalder,SurfaceScience577(2005)L78–L84.