Plasma aminofunctionalisation of polymeric membrane surfaces for tissue engineering applications
By
Peter Hamerli
A Dissertation
Presented to the Doctoral School of Chemical Engineering of the University of Veszprém
in Partial Fulfilment of the Requirements for the PhD Degree
Supervisors:
Dr Thomas Weigel Senior researcher of
GSK Research Centre GmbH.
Institute of Chemistry Departement for Membrane Research
and
Dr Gyula Marton Professor
at the University of Veszprém Institute of Chemical
Engineering
2004
ii
Plasma aminofunctionalisation of polymeric membrane surfaces for tissue engineering applications
Értekezés doktori (PhD) fokozat elnyerése érdekében Írta:
Hamerli Péter
Készült a Veszprémi Egyetem Vegyészmérnöki Doktori Iskolája keretében Témavezetők: Dr Marton Gyula
Dr Thomas Weigel Elfogadásra javaslom (igen/nem)
(aláírás) A jelölt a doktori szigorlaton ………. %-ot ért el,
Veszprém, ………
a Szigorlati Bizottság elnöke Az értekezést bírálóként elfogadásra javaslom:
Bíráló neve: ……….. igen / nem
………
(aláírás) Bíráló neve: ……….. igen / nem
………
(aláírás) Bíráló neve: ……….. igen / nem
………
(aláírás) A jelölt az értekezés nyilvános vitáján ………. %-ot ért el
Veszprém, ………
a Bíráló Bizottság elnöke A doktori (PhD) oklevél minősítése ……….
………..
az EDT elnöke
iii
Abbreviations
ν pulse frequency
λ free pathway of molecules in the plasma
ε electron energy
θ contact angle
A neutral particle
A* excited particle
AFM atomic force microscopy
AO Acid OrangeII
ATR attenuated total reflection
CA contact angle
Camine amine concentration on the surface
CAP competitive ablation and polymerisation
CASING cross-linking by activated species in inert gases
Cp-amine primary amine concentration on the surface
cw-plasma continuous wave plasma
E activating energy
E Binding electron binding energy
E Kinetic electron kinetic energy
ECM extra cellular matrix
ECR electron cyclotron resonance
EtOH ethanol
f ferquency
F monomer flow rate
FDA fluorescein diacetate
FTIR Fourier-transformation infrared spectroscopy h Planck-constant
I current
ICP inductively coupled plasma
IEP isoelectric point
LDH lactate dehydrogenase
M molecular weigh of monomer
iv
M• free radical MF microfilter
MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromid mw microwave
ne electron density
P pressure
p-amine primary amine
Pav average power input
PET polyethyleneterephtalat PPAa plasma polymerised allylamine
PPhS polyphenylensulfon
Ppulse input power
rf radio frequency
rf GD radio frequency glow discharge RSGP rapid step growth polymerisation S substrate SEM scanning electron microscopy Te electron temperature
Tg duty cycle for pulsed plasma
Ti ion temperature
tpulse plasma-on time
UF ultrafilter
W plasma power
XPS X-ray photoelectron spectroscopy
v
Table of contents
Abstract ... 1
Kivonat ... 3
Zusammenfassung... 5
1. Introduction ... 7
2. Theoretical backgrounds ... 9
2.1 Introduction to plasma state and plasma generation... 9
2.1.1 Plasma state... 9
2.2 Plasma surface modification... 12
2.2.1. Plasma treatment of surfaces by implantation ... 13
2.2.2 Plasma polymerisation... 15
2.2.3 Impact of process parameters on plasma processes... 18
2.3 Biomaterials and plasma surface engineering ... 21
2.3.1 Biomaterials ... 21
2.3.2. Impact of surface properties on biocompatibility ... 22
2.3.3. Plasma surface modification of biomaterials... 24
2.3.4. Future developments - biohybrid organs ... 25
3. Methods and materials ... 27
4. Results and discussion... 35
4.1 Ammonia plasma treatment of PET and PPhS membranes ... 35
4.1.1 Introduction... 35
4.1.2 Colorimetric staining of amine functionalities ... 36
4.1.3 Fluorescence staining of primary amines ... 40
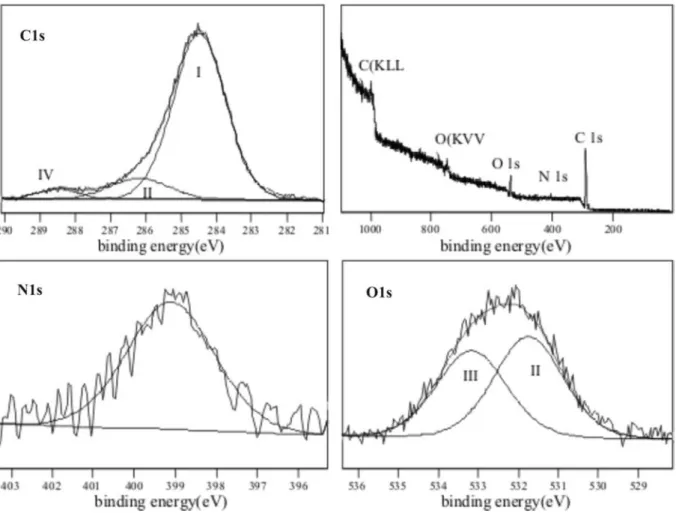
4.1.4 XPS measurements ... 42
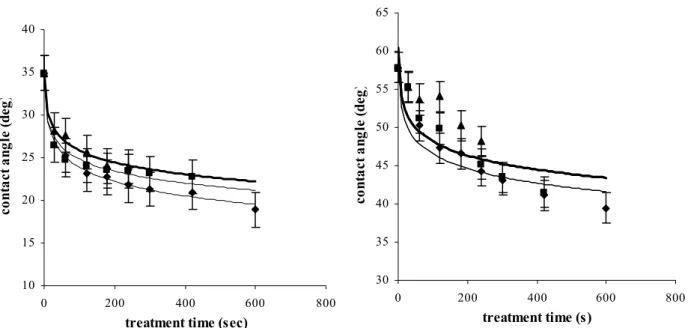
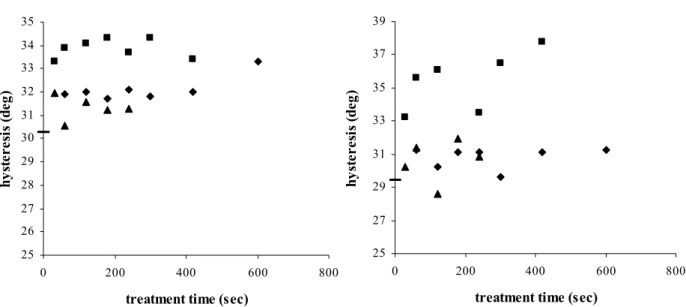
4.1.5 Wettability ... 44
4.1.6 Electrical properties ... 46
4.1.7 Surface morphology and pore structure... 47
4.1.8 Stability... 50
4.2 Plasma polymerisation of allylamine ... 52
4.2.1 Introduction... 52
4.2.2. Deposition rate... 53
4.2.3. Chemical composition ... 55
4.2.4 FTIR spectroscopy... 57
vi
4.2.5 XPS measurements ... 59
4.2.6 Wettability and electrical properties ... 63
4.2.7 Surface morphology and pore structure of allylamine coated membranes... 64
4.2.7 Stability... 65
4.3. Biocompatibility studies... 68
4.3.1 Cell adhesion... 68
4.3.2 Cell proliferation measurement ... 70
4.3.3 Cell morphology ... 71
4.4 Possible applications, ongoing research activities – Modification of intraocular lenses (IOL)... 76
5. Conclusions ... 80
5.1 Ammonia plasma modification ... 80
5.2 Allylamine plasma polymerisation... 82
5.3 Biocompatibility measurements ... 83
5.4 Summary... 85
Acknowledgements ... 86
References ... 87
vii
Plasma aminofunctionalisation of polymeric membrane surfaces for tissue engineering applications
Abstract
A rapid development in medicine and biotechnology, and parallel, an incremental need for biocompatible materials have been observed in recent years. Many efforts have already been taken to develop polymers with good mechanical properties and adequate blood or tissue compatibility. One way to increase cell adhesion on substrates with excellent mechanical properties is their amine functionalisation. Conventional methods are mostly inefficient while materials - meet former demands - are usually chemically resistive, what makes a surface modification difficult. Plasma technique however seems to be a promising method in this contrast.
Polyethylenterephtalat track etched membranes and a poly(phenylene-sulphon) ultrafilters were plasma-modified to increase cell adhesion and tissue compatibility. Amine functionalities were grafted to the membrane surfaces by means of ammonia plasma treatment, and plasma deposition of allylamine in continuous and pulsed processes. Changes in elemental composition were characterised, wettability, surface morphology and permeation characteristics were also investigated.
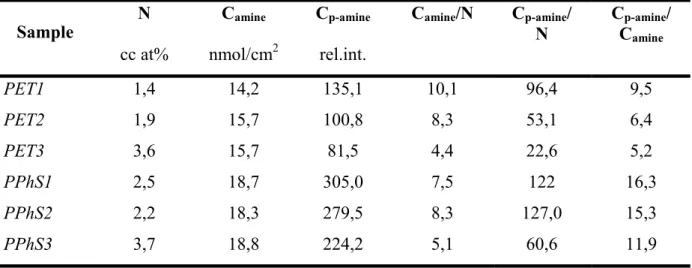
As a result of ammonia plasma treatment and allylamine plasma deposition relatively high amine concentrations (15-50 nmol/cm2) were measured on the surfaces. For ammonia plasma modified surfaces a maximum amine concentration was observed irrespective of the coupled energy. IR spectra of the plasma polymerised allylamine and the monomer indicated, that the monomer has undergone a reorganisation during plasma polymerisation and amine groups of the monomer were partially transformed into amide, imine, or nitrile functionalities. High energetic plasma exposure led to the recombination of amines into nitrile groups to a higher extent compared to low energetic plasmas. This effect however could be reduced by pulsed plasma operation, such as by increasing the monomer flow rates.
Samples treated by ammonia plasma were more hydrophilic, than the plain membrane and the wettability of the deposited allylamine differed also significantly from the substrate. The pore structure of the polysulfon membranes has undergone a drastic degradation while polyesters remained stable.
1
In vitro evaluation of biocompatibility was carried out by studying human skin fibroblast interaction with surfaces modified at different degrees of functionalisation. Cell adhesion was evaluated based on the number of adhering cells, but also on morphological criteria. It was found that fibroblasts adhere better on poly-allylamine coated surfaces with higher amine concentration than on ammonia plasma treated samples, but much better than on the original ones.
In frames of a cooperation silicone rubber intraocular lenses were modified by allylamine plasma with success and tissue compatibility tested. Both in-vitro and in-vivo results are very promising by fare.
2
Polimermembránok felületének módosítása plazma-technológiával biokompatibilitásuk javítása céljából
Kivonat
A gyógyászatban és biotechnológiában az elmúlt években tapasztalt jelentős fejlődés maga után vonta a biokompatibilis szerkezeti anyagok iránti kereslet növekedését is. A megfelelő mechanikai sajátságokkal, de nem megfelelő felületi tulajdonságokkal rendelkező anyagok sejtadhéziós képesség javításának egyik módja, amin funkciós csoportok felületre történő kapcsolása. Számos felületkémiai módszer közül a plazma technológia különösen hatékony eljárásnak ígérkezik ilyen, kémiailag ellenálló felületek funkcionalizálására.
A szerző poliszulfon és poliészter membrán felületek plazma funkcionalizálása során szerzett tapasztalatokat, és sejtadhéziós mérések eredményeit foglalja össze disszertációjában.
Az ammónia és allilamin plazmákkal módosított felületek kémiai tulajdonságainak vizsgálata röntgenfotoelektron-, illetve infravörös spektroszkópiás eljárással, valamint kolorimetriás mérésekkel történt. A felületek morfológiája, nedvesíthetősége, és a membránok pórusmérete szintén meghatározásra kerültek.
A felületi kezelés hatására 15-50 nmol/cm2 közötti felületi amin-koncentráció volt mérhető. Az ammóniás eljárásoknál az alkalmazott gerjesztő energiától függetlenül a felületi amin-koncentráció maximumot ért el. Az allilamin plazma esetén a képződött vékony plazma polimer film kémiai és fizikai tulajdonságai jelentősen eltértek az eredeti membrántól, és az ammónia plazmával módosított mintákétól is. A polimer monomerszerkezete a kiindulási monomer allilamintól jelentősen különbözött, az amin csoportok a plazma besugárzás hatására részben amid-, imid- illetve nitril csoportokká alakultak át. Az allilamin filmet nagy felületi amin-koncentráció és hidrofil karakter jellemezte. A poliészterek pórusszerkezete a plazma besugárzás ellenére is lényegében változatlan maradt, a poliszulfon membránoké viszont jelentősen megváltozott.
A felületek biokompatibilitását humán fibroblasztok felületi addíciójának tanulmányozásával határozta meg a szerző. A sejtaddíció minőségére az adszorbeált sejtek számából, valamint azok morfológiájából lehetett következtetni. A legintenzívebb sejt- szubsztrátum kölcsönhatás az allilaminnal módosított mintáknál volt megfigyelhető, de az ammóniával kezelteké is jobb volt, mint a kezeletlen membránok esetében.
3
Egy tudományos együttműködés keretében szilikonból készült intraokuláris lencsék allilamin plazmával történő módosítására is sor került. Mind az in-vitro, valamint in-vivo mérések eredményei eddig rendkívül bíztatóak.
4
Plasma Aminofunktionalisierung von Polymermembranen zur Erzeugung biokompatible Oberflächen
Zusammenfassung
Als Folge der rapiden Entwicklung der biomedizinischen und biotechnologischen Verfahren wächst die Nachfrage nach biokompatible Materialien ständig. Biomaterialien mit ausgezeichneten mechanischen Eigenschaften, gleichzeitig aber herausragender Blut- und Gewebeverträglichkeit sind heutzutage Ziel intensiver Forschung. Aminofunktionalisierung von Oberfächen ist unter anderen ein effektives Mittel, die zelladhesiven Eigenschaften zu verbessern. Chemische Methoden sind in diesem Bezug nur eingeschränkt geeignet, da die verwendeten Materialien oft sehr resistent sind. Deshalb bietet das Plasmaverfahren eine vielsprechende Alternative.
Membranen aus Polyethylenterephtalat und Polyphenylensulfon wurden im Mikrowellenplasma modifiziert, um ihre Gewebeverträglichkeit zu verbessern. Dabei wurden Aminogruppen mittels Ammoniak- und Allylamine Plasma an die Oberflächen gebracht. Die chemischen Veränderungen der Membranoberflächen wurden charakterisiert, ebenso wurden Benetzbarkeit, Porenstruktur und Morphologie untersucht.
Resultat der Behandlungen waren relative hochaminhaltige (15-50 nmol/cm2) Oberflächen wobei die Plasmapolymerisation von Allylamine significant effectiver war. Unabhängig von der Plasmaleistung ammoniak plasma Modifizierung führte zu eine maximum Aminkonzentration. Die IR-Spektren der im plasma polymerisierten und abgeschiedenen Allylaminschichten lassen eine chemische Veränderung während der Plasmabehandlung erkennen. Partiell wurden Amingruppen in Amide, Imide bzw. Nitrilgruppen umgewandelt.
Hochenergieplasma trägt zu einr Rekombination der Amingruppen in Nitrilgruppen jedoch vermindert das Pulsen des Plasmas diesen Effekt. Die modifizierte Oberflächen sind mit Wasser besser Benetzbar, im Vergleich zu den Unbehandelten. Die Porenstruktur der PET Membranen blieb nach Behandlungen unverändert, während die Morphologie von Polysulfonen sich sehr veränderte.
Die Gewebekompatibilität wurde anhand der Adhäsion von humanen Fibroblasten an unterschiedlich behandelten Membranen untersucht. Es wurde nicht nur Anzahl der adhierenden Zellen ermittelt, sondern auch die Zellmorphologie untersucht. Aus den
5
Ergebnissen geht hervor, dass die Zellen an Allylamine-modifizierten Membranen deutlich besser, als an mit Ammoniak behandelten und auch unbehandelten Proben haften.
Anschliessend wurden in Rahmen einer Zusammenarbeit intraoculare Linsen auf Siliconbasis mit Allylamine erfolgreich im Plasma beschichtet und deren Biokompatibilität getestet. Ergebnisse von in-vitro und in-vivo Untersuchungen sind weitgehend vielversprechend.
6
1. Introduction
The use of synthetic materials or special modified natural materials for medical applications and for bioengineering purposes is widespread. Biomaterials for artificial kidney contact lenses, heart valves, breast dental or orthopaedic prostheses etc. are well known, but besides the production of implants, even more interest is given to biotechnological applications (e.g.: cell culture substrata, bioreactive membrane etc.). A biomaterial has been defined as a “non-viable material used in a biomedical device intended to interact with biological systems” [Williams 1986,]. There are two main requirements biomaterials have to fulfil, namely they have to successfully perform the intended function and secondly they must not induce adverse responses i.e. have to be compatible with the living system and should not provoke dysfunction of the metabolism. It is relatively easy to meet requirements of functionality because design criteria are mostly well defined and many engineering materials are suitable. The biological responses of biomaterials and devices on the other hand are mainly controlled by their surface chemistry and structure, which have to be tailored according to special needs. [Ratner 1984].
Although intensive investigations were carried out studying the mechanism of cell adhesion and proliferation on a solid substratum, it is still fare from being well understood. It is widely accepted that besides other non-related forces physical and chemical properties of an adhesive surface like charge density, wettability, presence of certain functional groups and solidity are the most important factors to be considered [Koller 1995, Vacanti 1997, Chu 2002]. In many cases device design engineers cannot use the best materials because of their unfavourable biological surface properties. An obvious solution would be a surface post- treatment, to increase biocompatibility. The problem however is, that these materials are mostly chemical resistive, so conventional methods of chemistry (surface treatment with oxidising agents, strong acids, or alkali treatment etc…) are mostly too expensive or even impossible for large-scale operation, nevertheless very harmful for the personnel and for the environment [Ratner 1995].
In the last decades physical processes using electromagnetic field to induce chemical reactions are getting even more frequent. Such methods like magnetron sputtering, ion irradiation, plasma surface treatment, and plasma polymerisation are attractive to modify chemical resistive surfaces. For plasma processes depending on the process parameters, and
7
the used plasma gases several competitive reactions can take place. As a result etching of polymer, implantation of functional groups, or polymer deposition can occur on surfaces exposed to a plasma. Although chemical reactions in electrical discharges, and thin oily film formation by glow discharge polymerisation had already been observed in the late 19th century, the extensive investigation began only in the 1960s [Bell 1974, Yasuda 1978, 1985, Kay 1980]. First industrial applications in the 80s for etching and cleaning of surfaces, than later for thin film deposition in microelectronic are known. The advantages of plasma deposited layers (and plasma treated surfaces) for biotechnological applications has been recognised in the last decades. Blood compatible coatings, intraocular lenses, cell culture surfaces, and model surfaces for exploring interactions with biological systems are only some relevant examples.
The objective of this work was to prepare a high amine containing stable polymer surface with excellent cell adhesive properties for the invention of biohybrid organs or scaffolds for tissue cultures. In frames of this phD activity polyester (PET) microfiltration- and poly(phenylene-sulphon) (PPhS) ultrafiltration membranes were modified by ammonia and allylamine plasma. Both of these polymers are commonly used and relatively easy to produce.
Plasma parameters for different modification processes were optimised to get the highest surface amine concentrations possible, without changing the pore structure of the samples.
Additionally surface stability in different media was evaluated. The tissue compatibility of selected samples was studied in-vitro using human skin fibroblast as a model organism. Not only the number of seeded cells after several days of cultivation but also their morphology was investigated, which properties are related with tissue compatibility of a material. Finally, in a cooperation with the University of Aachen the surfaces of polysiloxane rubbers, and intraocular lenses were modified by allylamine plasma polymerisation.
8
2. Theoretical backgrounds
2.1 Introduction to plasma state and plasma generation
2.1.1 Plasma state
The name plasma was given by Irving Langmuir comes from the Greek “plasso”; self- forming material [Mott-Smith 1926]. Plasma is an ionised or partly ionised gas consisting of electrons, positively charged particles, exited molecules, radicals, photons, neutral atoms and molecules. Furthermore the whole plasma is basically neutral. Plasma is considered to be the fourth state of materials, which is more highly activated than other states.
A material composed of many atoms and molecules transfers from solid to liquid and further into gas state if its temperature increases (fig. 2.1.) As the temperature of a solid material rises, the thermal motion of atoms becomes so intensive, that finally they begin to leave the position determined by their potential energy, and the transition from solid state to liquid occurs. If this liquid is overheated so fare, that the kinetic energy of the molecules becomes higher than their
potential energy, molecules could escape from the liquid phase, evaporation occurs. When the temperature rises to more than few thousand degrees collision of atoms and photon absorption brings about the energy needed for the ionisation of atoms. This is the plasma state and as expected knowing its composition is at a very high energetic level compared to the
solid, liquid or gaseous state [Inagaki 1996, Goldston 1998].
Figure 2.1 Schematic drawing of transition of state
Actually plasma is the most common physical state, since more than 90% of the materials in our universe are in this state. Among others for instance the Aurora Borealis, the Van-
9
Allen-Belt, the Sun, the corn of the Earth, the Stars, the ionosphere, and natural gas discharges such as the lightning.
2.1.2 Plasma generation
To reach the plasma state energy must be transferred to gas atoms and molecules from an external energy source. As explained before excitation, ionisation, dissociation and recombination of particles in a low-temperature plasma occur mainly through inelastic collisions with electrons [Boenigh 1982, Haefer 1987]. The basic collision processes are listed as follows∗:
Excitation: A + e → A* + e (1.1)
Ionisation: A + e → A+ + 2e (1.2)
Dissociation: M + e → M• + •M’ + e (1.3)
Dissociative ionisation: M + e → M• + •M’+ + 2e (1.4) M + e → •M- + •M’ (1.5)
Recombination: M+ + e + S → M + S (1.6)
Dissociative recombination: M+ + e → M• + •M’ (1.7)
Recombination followed by photon radiation: A+ + e → A + hν (1.8) Elastic collision and photon absorption contributes also to the generation of plasma species. Association of free radicals, ions or excited molecules to molecule clusters with different structures are also typical reactions for a plasma.
In general a vessel filled with gas, cannot be heated directly to assure plasma state transition of the gas, because after a while the vessel itself would ionise and would itself be transited into plasma. Therefore technical plasma discharges are formed in the laboratory, by an electromagnetic field usually at low pressure (< 100 Pa). Free electrons gain energy from the electromagnetic field and by inelastic collisions, generate more electrons (as required for a self-sustaining discharge), ions, free radicals and excited particles. Thus for a continuous plasma operation a permanent external energy input, a suitable reaction chamber and usually, but not necessary a vacuum system, is needed.
∗ A: neutral particles, M: molecule, M•: free radical, A*: excited particle, A+, A-, M+, M-: ions, and S: substrate or reactor wall.
10
As indicated before because of convenience of handling generally electric energy is used operated at the International Telecommunications Union-approved industrial, scientific and medical frequencies as listed bellow:
direct current (DC);
•
•
•
•
•
•
commercial alternating current with a frequency of 50 or 60 Hz (AC);
alternating current with high frequency of more than 60 Hz 10 or 20 kHz audio frequency;
13,56 (or 27,12) MHz radio frequency (rf);
2,45 GHz microwave frequency (mw).
The most common plasma sources and operating conditions are presented in table 2.2 Table 2.2. Commonly used plasma sources and their characteristics
Plasma sources References:
Radio frequency glow-discharge (rfGD)
f = 13,56 MHz; P = 10-3 - 100 Pa; ne = 109 – 1012 cm-3; Te = several eV; Ti < 0,01 eV
large volume of highly uniform stable plasma; no impurities using inductively coupling (ICP)
Raizer 1995, Park 2000, Miyake 2000, Urano 1998, Microwave
plasma (mw)
f = 300 MHz - 2,45 GHz; P = 10-5-10-3 Pa; ne = 1011-1012 cm-3; Te>>Ti; high mean ion charge state;
not very uniform plasma distribution
Timmermans 1999, Ferreira 1993 Electron
cyclotron
resonance (ECR)
similar to mw but an additional magnetic field is applied to the plasma to ensure higher plasma density.
Zhao 2000, Lei 2000, Inagaki 2000, Corona discharge
plasma
Applied voltage = 2-5 kV; I = 10-10 – 10-4 A; ne = 1013 – 10 9 cm-3; Te ≈ 5 eV; Ti < 0,01 eV; limited application potential caused by the uneven plasma distribution
Yehia 2000, Raizer 1991
Atmospheric arc plasma
T > 8000 K; P = 105 Pa; ne = 1016-1019 cm-3; Te = 7- 9 eV; Ti = 0,3-0,9 eV; widely used for plasma spraying
Fauchais 1997, Kim 1995
Vacuum arc plasma
extremely high current (1012 A/m) and power density (1013 W/m) and ion kinetic energy in the range of 20-200 eV; ne = 1020 cm-3; ion charge state 1-3.
Boxman 1997, Yuskov 2000, Sanders 2000, Anders 1996, Laser plasma
source
Laser intensity 108-1010 W/cm2; ne = 1018-1020 cm-3; Te = 1-5 eV;
laser plasmas are difficult to operate
Chen 1996
11
For polymer surface modifications most commonly used plasma sources are nevertheless radio frequency (rf) and microwave (mw) ones. Low frequency units are in general different in size, coupling mode and shape from that of mw-frequency applications [Moisan 1993]. For rf-sources electric energy is generally coupled by inductive or capacitive coupling.
Furthermore rf-electrodes can be placed both inside and outside the plasma. For mw- excitations capacitive coupling would not be effective, because the mw-field would not be distributed evenly on the electrode plates, and the coupling with the generator would be inefficient also, since the wavelength of the plasma would be comparable with the plate dimension. Mw-powered systems are characterised by tubular quartz or Pyrex reactors and a resonant cavity coupled with a power supply. The plasma is than generated in the resonant cavity [Moisan 1992, Ferreira 1993]. Finally the operation frequency influences the composition of the plasma and the yield of plasma processes. The electron energy distribution of mw-discharges can be described better by Maxwellian-equation, while rf-plasmas can be characterised by the Druyvesteyn-distribution. Furthermore mw-plasmas are usually characterised by higher density of active species, electrons, and ions and can be operated at lower pressures. Additionally ion bombardment, thus the destruction of sample surface is more intensive using an rf-discharge.
2.2 Plasma surface modification
There are several methods developed for the modification of polymeric surfaces. One can for instance differentiate them by the art of energy coupling, the penetration intensity, or by the selectivity of functionalisation. Obviously the objective of the modification determines which method would be chosen. In several cases the task is to modify only a nanometer deep region of a polymer surface without influencing its structure and bulk properties. After these considerations different surface modification techniques will be introduced briefly, and the place of plasma technique in this relation.
An elegant method with minimal effects on the polymer morphology is the coupling of bi- functional agents on the surface with a subsequent fixation by photochemical excitation. The most known process is the Langmuir-Blodgett-technique, where the molecules on the surface are highly organised furthermore the modification has almost no effect on the polymer morphology. Disadvantages are however the very high cost of equipment, and the complicated synthesis.
12
In the second big group there are techniques, which operate with glow discharges or electromagnetic radiation. Among others most important are plasma techniques, magnetron sputtering, photo-induced polymerisation or grafting etc... Interaction between a plasma and a material will be discussed in the following section. By the photo-induced polymerisation or grafting surfaces are exposed to an irradiation at defined wavelength in the presence or without a photo initiator. The monomer can than attach at the excited places of the surface. A special advantage of this technique is that the wavelength can be adjusted in such a way that only special functional groups of the surface would be excited. The cost of equipment is however relatively high.
Conventional wet-chemical methods belong to the third group. Polymers are immersed in a solution of agents and the surface properties are changed by heterogeneous chemical reactions. There is no need for special equipment, and several chemical reactions can be carried out, but the method is not selective, and bulk properties are also often changed.
2.2.1. Plasma treatment of surfaces by implantation
The interaction between a plasma and the polymer could be considered as competitive reactions of functionalisation and degradation [Chappel 1991]. When modification effect dominates properties of the polymer will change due to implantation, plasma graft co- polymerisation or plasma deposition. When degradation is predominant etching will take place on the surface (fig. 2.2). The kind of interaction depends basically on the nature of plasma gases and process parameters chosen.
Etching and sputtering
Plasma deposition
Plasma implantation Cross linking Radical generation Figure 2.2. Interactions of plasma and membrane surfaces.
13
Inert plasma interaction with a substrate leads mainly to radical generation through chain scission or hydrogen elimination [Clark 1977, Clouet 1992, Ratner 1995]. Free radicals can also be generated by inelastic collision of electrons with polymer surface or UV irradiation emitted by the plasma [Penn 1994, Yasuda 1995, Iganaki 1996]. The recombination of these radicals yields a highly cross-linked polymeric surface. This was first observed by Hansen and Schonhorn [Hansen 1966,1967] and called this phenomena as cross-linking by activated species in inert gases (CASING). Besides cross-linking and radical generation as a result of plasma exposure atoms or molecular fragments of the very surface can be sputtered off.
Polymers with repeating unit of –CH2-CHR- undergo predominantly chain scission (e.g.
polyisobutylene, polytetrafluoroethylene, polymetacrylonitril etc.) and low molecular weigh products are formed (sputtering, cleaning, ablation) while on the other hand with a repeating unit of –CH2-CRR’- polymers undergo mostly cross-linking (e.g. polyethylene, polystyrene, polypropylene). Which of these reaction pathways are preferred is mainly determined by the resonance stability of radicals [Fettes 1964, Reich 1971, Charlesby 1960]. Though inert gases do not functionalise surfaces directly, most of the time incorporation of oxygen functionalities is observed. It was believed that this is alone due to subsequent reaction of radicals created by the plasma after exposure to atmosphere. By now it is proven that oxygen can also originate from water molecules absorbed on the reactor wall, reactivated by the plasma [Wheale 1998].
When a polymer is exposed to a reactive plasma and if the plasma density and treatment time are properly selected, many functionalities will be created near the surface and cross- linked polymer chains can be formed. In a typical implantation process, hydrogen is abstracted first so radicals are created in the midpoint of the polymer chains. These radicals could then recombine with each other or with simple radicals, and activated species of the plasma to form oxygen, nitrogen etc. functional groups. Which of the activated species in the plasma plays the major role in implantation reactions is not exactly cleared yet. It was however suggested by Inagaki et al. that radicals rather than ions contribute mainly to plasma induced reactions. Consequently implantation processes might be radical rather than ionic origin [Inagaki 1996].
Results of implantation reactions are significant changes of physical and chemical properties of the surface of a target polymer. Implantation reactions are used to improve wettability [Ganzarz 1999, Tufik 2002], adhesive properties [Vallon 1996, László 2001], biocompatibility, permeation properties [Wu 1997, Kumazawa 2000, Vidaurre 2001], or as pre-treatment for subsequent chemical reactions [Inagaki 1999, Moosheimer 1997]. Since this
14
work is mainly focused on biomedical applications examples for plasma implantation will be given in the next section.
2.2.2 Plasma polymerisation
In plasma polymerisation the transformation of low molecular weight molecules (monomer) into high molecular weight molecules (polymers) occurs with the assistance of energetic plasma species, such as electrons, ions and radicals. Plasma polymer deposition is one of the most important processes in plasma surface engineering, since layers with properties distinctly different from those of the bulk materials can be synthesized.
To understand effect of process variables (plasma composition) on plasma polymer film properties several investigations were already carried out. Plasma polymerisation is a very complex process involving reactions between plasma species, plasma species and surface, and between surface species. Three typical mechanism of plasma polymerisation had been suggested namely ionic mechanism, radical mechanism, and atomic polymerisation. Among others Williams and Hayes (1966), Haller and White (1963), Westwood (1971), and Thompson and Mayhan (1972) proposed ionic polymerisation. That means that chemical reactions of polymerisation steps are propagated by ionic species. On the other hand many investigators [Denaro 1968, Kobayashi 1974, Bell 1980] proposed radical mechanism where radicals, formed at the end of polymer chains initiate the propagating reaction of monomers.
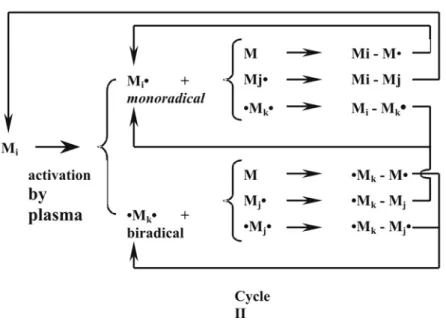
These considerations suggest also, that only one or other kind of species would initiate propagation reactions of polymerisation, as it is usual in conventional chemistry. Nowadays however the concept of atomic polymerisation mechanism proposed by Yasuda (1978), which involves fragmentation of monomer molecules, formation of activated species, and recombination reactions are the most accepted (fig. 2.3).
Based on the so-called “rapid step growth polymerisation” model (RSGP), molecules exposed to the plasma can be activated by C-C bound scission or hydrogen elimination. As a result mono Mi• or biradicals •Mj• are formed. These reactive species recombine with monomers or other radicals to form large molecules with or without radicals, as represented by “Cycle I” processes in figure 2.3. The new mono-, Mi-Mk•, and biradicals, •Mk-Mj• further recombine to form larger radicals which is the “Cycle II” process. As mentioned before ionic reactions can also contribute to plasma polymerisation.
15
Figure 2.3. Bicyclic gas-phase reaction scheme of plasma state polymerisation (RSGP- Model)
It should be considered that plasma polymerisation is chemically different from conventional ionic or radical polymerisation. Mostly polymers formed by plasma polymerisation have different chemical compositions as well as chemical and physical properties from those formed by conventional polymerisation of the same monomer [Yashuda 1985]. Furthermore two types of reactions have to be distinguished namely plasma state and plasma induced polymerisation, which however are accompanying processes of plasma deposition. First one is similar to the conventional free radical induced polymerisation of molecules containing unsaturated carbon bounds. Latter as indicated by the name is a polymerisation of activated molecules in plasma state.
If a substrate is exposed to a plasma its surface will be activated by plasma irradiation before plasma polymer is deposited, thus chemical bounds are likely to form between plasma polymer and the surface (adhesive coating) [Morosoff 1990]. Once a polymer is deposited, it is constantly irradiated by the plasma, until the polymerisation process is completed. This means that deposited polymers are subjected to further degradation and reorganisation (e.g.
cross-linking), and that etching, and polymer deposition take place in the reactor simultaneously. If the rate of polymerisation is higher than the rate of etching plasma polymer is deposited, but if the electron energy exceeds a certain value ablation processes become comparable to the polymerisation and the deposition rate decreases. This is the conception of
16
the widely accepted “competitive ablation and polymerisation” (CAP) scheme proposed by Yashuda in 1976 (fig. 2.4).
Figure 2.4. Competitive ablation and polymerisation mechanism of plasma polymerisation
As indicated before because of the complexity of plasma processes, and lack of knowledge, it is very hard to determine exactly how separate process parameters influence properties of deposited film. Furthermore processes must be optimised for each reactor and plasma source, and optimal parameters cannot be taken over for other units. The relation between process parameters and plasma polymerisation is depicted in figure 2.5 and some will be discussed below.
17
2.2.3 Impact of process parameters on plasma processes
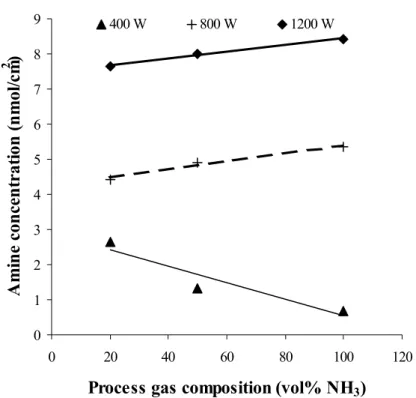
Effect of plasma frequency: Effects of activating frequency (rf- or mw-plasma) has not been intensively investigated yet therefore not completely understood. In general however higher deposition rate using the same monomer at the same activating energy was observed by mw-polymerisation. Higher cross-linking of deposited films was on the other hand observed while operating at low frequencies (e.g. rf-plasma) [Wertheimer 1985, Claude 1987].
Fig. 2.5. Relation between basic plasma parameters and plasma process parameters [Kay 1988]
Effect of plasma power and monomer flow rate: As described earlier the composition of a plasma polymer film is mainly dependent upon the fragmentation of the monomer by the plasma. This depends further on the electric energy coupled into the plasma, the density and
18
residence time of monomers, and the location of plasma monomer interaction in the reactor. One of the most frequently used parameters describing plasma polymerisation processes is the power to flow-rate ratio W/FM, where W is the plasma power, F the monomer flow rate and M the molecular weight of the monomer. It is assumed that deposition rate and physical, chemical properties of a plasma polymer remain constant when other parameters are the same (pressure reactor, activating frequency etc.); so the magnitude of W/FM is considered to be
proportional to the concentration of activated species in the plasma. Yasuda et al. identified two regions of plasma polymerisation [Yashuda 1983]. In the monomer-sufficient or energy- deficient region (low W/FM) activated species have a far lower concentration than the monomer, thus monomer molecules are subjected to less fragmentation, and plasma polymers with less rearrangement and small loss of functional groups are formed. In the monomer- deficient region however molecules are subjected to an extensive fragmentation and plasma polymers with much rearrangement and large loss of functional groups are formed (fig. 2.6).
Figure 2.6. Deposition domain for plasma polymerisation from Yashuda (1985).
Effect of processes time: Thickness of deposited plasma polymer films can be controlled by the duration of polymerisation processes. It should be noted however that after reaching a certain thickness the accumulation of intrinsic stress in the layer result in cracking and delamination of the film [Yashuda 1976].
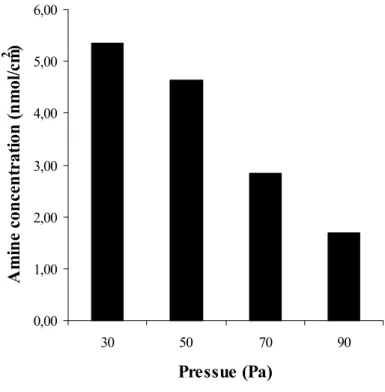
Effect of pressure: Pressure influences the plasma treatment processes in several ways. The density of gas molecules in the plasma is directly proportional to the pressure. The average electron energy ε is proportional to E/p, where E is the activating energy. So if the activating energy is constant at high pressure (many molecules) the average electron energy is lower, and less activated species can be formed. On the other hand at higher pressure the free pathway of molecules∗ (λ) is smaller, more collision occurs and more activated species can be formed if the plasma energy is high enough. It was indicated that the appearance of deposited
∗λ =¼πr2N, where r is the molecular radius, and N the gas density
19
polymers depends on the pressure [Tibbit 1977, Molinari 1974]. Operating at higher pressure, at constant flow rate and power, Molinari observed the formation of oily films of ethylene plasma polymer, whereas on the other hand powder-like polymers were deposited at low pressures.
Effect of substrate position: Depending on the sample position in the reactor we can distinguish direct and remote plasma treatments. In former case sample is placed directly in the plasma zone and interacts with all species (ions electrons and radicals) of the plasma. As a result degradation occurs more likely, and a higher variety of functional groups might be created. In remote position however the concentration of radicals – having a longer recombination time than ions - is higher, therefore functionalisation could be more effective and ion bombardment could be avoided or minimised [Goldman 1983, Inagaki 1996]. Plasma deposited films are more cross-linked and a more intensive rearrangement of monomer structure can be observed when substrates are placed directly in the plasma region.
Effect of substrate temperature: To reach a high deposition rate, products should have a low vapour pressure at room temperature, or substrates have to be cooled down. Otherwise the excited monomer escapes in the by-product gas stream. Both these methods lead to a rapid adsorption therefore plasma induced polymerisation mechanism is favoured. Products absorb namely fast on the surface, so the residence time in the active zone of the plasma is relatively short. Deposited polymers are weakly cross-linked, and the monomer structure is more or less retained. With the increase of temperature the deposition rate decreases logarithmically highly cross-linked surfaces are obtained and a loss or reorganisation of functional groups is observed also [Yashuda 1995].
Continuous wave and pulse plasma operation: Changing from continuous operation (cw- plasma) to pulse plasma, the fragmentation of monomer could be minimised. Pulse plasma operation means that the input power is switched off and on periodically over the treatment time. Pulse processes can be characterised by the pulse frequency (ν), and duty cycle (Tg).
ν
⋅
= pulse
g t
T
(1.9) where tpulse means the plasma “on time”. The average power input (Pav) can be expressed by the input power (Ppulse) and the duty cycle, as follows:
g pulse
av P T
P = ⋅
(1.10)
20
As a result of pulse plasma operation the time dependency of electron temperature and density differs in the ignition and in relaxation phase [Liebermann 1996]. In the ignition period the electron temperature increases rapidly and approaches through the pulse period almost the value of cw-processes, and relaxes rapidly when the power is switched off. The electron density however reacts significantly slower for the alteration of plasma power. In general the electron density is higher by pulse plasma than by cw-processes with the same average power input.
It has to be stressed again that considerations of impacts of process variables discussed above can only be guidelines to optimise plasma reactions, and are in many cases controversial.
Depending on the application for what the substrate is going to be used, plasma parameters can be tailored. In many cases it is desirable that the monomer structure is preserved in the resulted polymer, or in others physically or chemically resistant surfaces with high cross-link density are preferred. If the objective is to deposit polymers in which the monomer structure is preserved the degree of interaction between reactive species and monomer should be limited. This can be realised by avoiding ionic bombardment (remote plasma), limiting the residence time of the monomer in the plasma, limiting the power input, and working at relatively high pressures (decreasing the average electron energy) [Mas 1997].
Applications of plasma polymerisation are ranging from surface functionalisation [Hochart 2000, Inagaki 1998], permselective membrane preparation [Ulbricht 1996, Hyun 2001], over protective coatings [Ramachandran 1997, Bogaerts 2002], film transistors [Liebermann 1999], to optical coatings [Morosoff 1990, Bogaerts 2002] as described in the cited review articles.
2.3 Biomaterials and plasma surface engineering
2.3.1 Biomaterials
Artificial materials are widely used in the medical and biotechnological praxis (fig. 2.7).
These synthetic, or modified natural materials are often referred to as biomaterials. Although the use of medical devices is widespread, there is a continuous need for improvement to approach performance of natural organs. An exact definition of biomaterials is still controversial. A widely accepted definition is that they are “non-viable materials used in a
21
biomedical device intended to interact with biological systems” [Williams 1987]. This definition includes no considerations regarding to functional requirements, thus it must be completed as followed: a biocompatible material must satisfy all functional requirements, and it must not induce inflammatory and/or foreign body reactions [Ratner 1996].
To ensure that a material has appropriate mechanical properties, durability and functionality is the task of biomaterials engineers and can more or less be satisfied since there are several engineering materials available [Hill 1998, Enderle 1999]. A more complicated problem is to develop materials that will not stimulate inflammatory and foreign body responses. These biological responses to biomaterials and devices are largely controlled by their surface chemistry and structure [Williams 1987, Koller 1995, Hubell 1995, Castner and Ratner 2002,]. Usually surface properties of most construction materials are not adequate for biomedical applications therefore surface modification for improvement is self-evident. If a surface is modified properly, the mechanical properties and functionality of the device will not be changed, but the tissue interface related biocompatibility could be improved [Ratner 1996]
Before discussing the impact of individual surface characteristics two types of biomaterials have to be distinguished namely bioinert and bioactive ones. Bioinert materials can be characterised by weak interactions with the biological systems thus adsorption of proteins or adhesion of cells is very limited. These kinds of materials could be used in blood contacting applications. Bioactive ones on the other hand are designed to interact with the biological systems and adhere proteins and cells. They could therefore be used as soft tissue replacements or as implants for instance [Lanzer 1997].
2.3.2. Impact of surface properties on biocompatibility
Particularly, the biocompatibility of a material is determined by the interactions between implant and the biological system on the micro-, and nanometer scale, here the physicochemical surface properties of the materials, like chemical composition, wettability, surface charge play an important role [Ratner 1998, Chu 2002]. The biological response of a tissue to biomaterials is to a large extent cell specific consequently determined by adsorption of proteins to the surface [Norde 1991, Castner and Ratner 2002].
22
Already in the seventies an inhibited cell adhesion was observed on hydrophobic surfaces [Grinell 1973]. In the last decade however it was also indicated that cells do not attach onto very hydrophilic ones either [Tamada 1994]. As a result of intensive investigations in this field it was found that cells adhere on moderate hydrophilic surfaces having a water-air contact angle between 50° and 80° [Tamada 1994, Vogler 1998, Groth 1998, Massia 2000].
Figure 2.7. Polymeric biomaterials for medical applications [Silver 1994]
Cells adhere better on surfaces with high charge density, and studies of sulfonated polystyrene have shown that 2-5 negatively charged groups/nm2 promote maximum cell attachment and spreading [Maroudas 1977]. It is however generally accepted that adhesive proteins adhere - what in turn means cells attach - on surfaces that are either positively or negatively charged, and the proper charge density is more important than polarity [Panina 1985, Burns 1996].
Generally functional groups like carboxyl, amine and hydroxyl ones can be used for the engineering of biomedical surfaces to improve the spreading and growth of certain cells and/or immobilize biomolecules (enzymes, peptides, non-thrombogenic, etc.) by means of spacers [Daw 1999, Dewez 1999]. Naturally chemical composition determines at the end all above-mentioned surface properties, furthermore adhesive proteins can be covalently immobilised on functional groups what again improves cellular adhesion processes [Chu 1999, Altankov 2000]. Steel and co-workers (1994) observed for instance that fibronectin adsorbs better on moderate wettable surfaces containing nitrogen functionalities, while oxygen containing groups support adhesion of vitronectins.
23
The impact of surface morphology on cellular interactions cannot be neglected either. It was observed that cells adhere on smooth surfaces better than on extreme rough ones [Brunette 1988, Woerly 1997]. Random or geometrical patterns transferred at micron or nanometer scale at the surface of biomaterials are known for their ability of driving adhesion, motion, spreading and growth steps of cells at the interface. The effect is known as “contact guidance” and can be interesting for biosensor design, diagnostic kits, and tissue engineering [Carter 1965, Curtis 2001].
2.3.3. Plasma surface modification of biomaterials
Nowadays the application of plasma treated surfaces or deposited polymers in medicine and biotechnology is getting more important. This evolution is due to the fact that plasma polymers have advantageous properties for such applications, as follows:
Conformal, and pinhole free layers can be deposited.
•
•
•
•
•
•
•
•
•
Unique substrates with complex shape can be modified (metals, glasses, polymers, ceramics, carbons)
Good adhesion to the substrates.
Unique surface and film chemistries can be achieved.
Deposited films could serve as excellent penetration barriers.
Show low levels of extractables.
A well-developed simple technique for the modifications
Meanwhile a wide variety of characterisation methods are available.
Plasma modified surfaces are sterile, after preparation
Disadvantageous could be however that the exact chemical composition of the surface can often not be defined, further that plasma apparatus for industrial scale is still expensive, and contamination could be a big problem [Ratner 1995, Chu 2002].
One of the main approaches utilizing plasma chemistry in biomedical surface preparation is to create blood compatible surfaces to protect the material against fouling and protein adsorption (e.g. non-thrombogenic surfaces) by grafting hydrophilic polymers onto the surface such as polyethylenglycol, dextran, hydroxyethylmetacrylat or polyvinylpirrolidon [Mar 1999, Thom 2000, Palumbo 2001, Wu 2001]. Another approach is to prepare antithrombogenic surfaces by immobilisation of endothelial cells like they are in the inner walls of natural veins [Greisler 1996, Dee 1995, Ramires 2000] or by immobilisation of heparin [Steffen 2000] on plasma functionalised materials.
24
Another issue is to modify biomaterial surfaces to increase protein/cell adhesive properties making a surface tissue compatible. Plasma as indicated already is an excellent choice to invent tissue compatible implantable surfaces. There are many review articles written containing also several application notes in this field such as from Castner and Ratner (2002), Ratner (1990), Chu and co-authors (2002). Some examples are joint replacements, heart valves, ophthalmic implants, or biosensors.
2.3.4. Future developments - biohybrid organs
Possibly the greatest challenge in biomedical surface engineering is the invention of so called biohybrid organs. A biohybrid organ is a composition of an artificial material usually a membrane and immobilised organ cells on its surface, working as a small bioreactor. They should take over the function of a defected organ partly or totally, or support the regeneration of remaining tissue (fig 2.8). In biohybrid organs – e.g. bioartificial skin or biohybrid liver-, kidney-systems - membranes serve not only as cell support but also as barriers intended to protect the tissue from the environment and to assure sufficient delivery of oxygen and nutrients [Groth 2002, Krasteva 2002, Kuroyanagaki 1999].
Figure 2.8 Schematic model of a biohybrid organ.
In biohybrid liver or kidney support systems these membranes have to face two different environments. On the blood-contacting site they have to remain bioinert, thus not absorb blood proteins. Just on the contrary on the tissue-contacting site they have to support protein adsorption assuring excellent cell-surface and cell-cell contacts. Finally its transport properties have to be adjusted in the way to allow sufficient exchange of small molecular
25
weight solutes such as toxins and smaller proteins, but must also protect the immobilised organ cell from the immune system of the recipient [Colton 1995, Humes 2000, Legallais 2001, Fey-Lamprecht 2002]. Another more simple example is the development of bioartificial skin for deep burnt wound healing. In these applications the membrane serves as a mechanical stable support for keratinocytes and/or fibroblasts and works as a barrier after implantation, ensuring sterility and respiration of the new tissue [Sabolinsky 1996, Kuroyanagaki 1999, Horch 2000, Groth 2002].
Membrane surfaces used in these kinds of applications have to be modified according to special needs. To meet these requirements plasma technology offers an excellent solution, since it is easily maintained to modify only one side of the material at time, while properties of the other side remains unaffected.
26
3. Methods and materials
MW-plasma reactor
NH3
Ar O2
Allylamine
The plasma treatment was performed in a tubular MW-plasma reactor (fig. 3.1) operating at 2,45 GHz (modified Plaslan 500, JE PlasmaConsult, Wuppertal, Germany). The reactor consisted of a quartz bell jar (diameter of 16 cm, height of 50 cm) mounted on the reactor chamber (diameter and height of 50 cm). The MW-power (0,2-2 kW Muegge, Reichelsheim, Germany) which could be pulsed by an
external pulse generator (pulse frequency of 0,2-10 kHz, duty cycle 10 - 90 %) was coupled to the reactor by an angular slot antenna [Firma Plasma Consult 1996; Pehl 1988,1989; Werner 1997].
Figure 3.1. Schematic drawing of the plasma reactor
The plasma forming gases ammonia and argon were introduced directly on the top of the plasma chamber into the discharge.
For ammonia plasma modifications samples were placed in the post-discharge region 6-36 cm downstream from the plasma zone. After the samples were placed into the reactor the system was evacuated by a Roots- and a rotary vane pump (Leybold, Köln, Germany) down to
the base pressure of 10-3 mbar for 10 minutes, than purged with argon for another 10 minutes.
Than the process pressures indicated were adjusted. After stabilisation the plasma was ignited and modification performed.
For plasma polymerisation experiments argon was used as plasma carrier-gas. The monomer allylamine was introduced into the reaction chamber in the post discharge region.
To assure a better solute stability of the polymerised film, namely an extensive cross-linking a stainless steel sample holder with a diameter of 16 cm was heated up to different temperatures by water and fixed 10 cm downstream after the glow discharge region. The process pressure was than adjusted, the Ar-plasma was ignited, and substrates were activated for 30 sec to assure a better adhesion of the deposited plasma polymer layer to the original surface. Finally
27
the monomer was fed into the reactor 7 cm downstream from the plasma zone and deposition took place.
After treatment times were over, gas inlets were closed, and the reactor was pumped down again to the base pressure. Then the outlet valve was closed and the pressure was equalised by inert gas inlet.
Membranes
PET track-etched microfiltration (MF) membranes with an effective thickness of 20 µm and nominal pore diameter of 1 µm were purchased from Oxyphem (Großerkmannsdorf, Germany). PPhS ultrafiltration (UF) membranes (type Radell) with a molecular weigh cut-off of 30 kDa were produced by GKSS Research Centre (Geesthacht, Germany). Membranes were cleaned ultrasonically for 5 min in pure ethanol bath and dried in an exsiccator for 15 min before every treatment.
XPS measurements
The principle of all photoelectron spectroscopic methods is to determine the binding energy of an electron by measuring its’
kinetic energy released from an internal (XPS) or valence shell (UPS) by photon irradiation with a known energy of hν (fig. 3.2). The principle of the conservation of energy allows us to write the energy balance equation, valid for the absorption of a photon carrying energy of hν:
Valence shell
Internal shell MO
energy
Figure 3.2. Principle of X-ray photoelectron spectroscopy
hν = E Kinetic + E Binding + Ø Work function
Where
hν : X-ray beam incident energy
E Kinetic : Electron kinetic energy when leaving the specimen E Binding : Electron binding energy inside the atom
The surface chemical composition of the deposited layers was determined using an XR3E2 (VG Microtech) twin anode X-ray source and a Clam2 (X-ray photoelectron spectroscopy) hemispherical electron energy analyser. The base pressure of the analysis chamber was about
28
5×10-9 mbar. The used Mg Kα radiation (1253.6 eV) was not monochromatic. Wide scan spectra in the 0-1000 eV binding energy range were recorded with a pass energy of 50 eV for all samples. High-resolution spectra of the O1s, N1s, and C1s signals were recorded in 0.05 eV steps with pass energy of 20 eV. After the linear baseline was subtracted, the curve fitting was performed assuming a Gaussian peak shape.
FT-IR spectroscopy
Allylamine film was deposited on KBr-tablets transferred to the spectrometer and was purged by dry air for 10 minutes. The transmission spectra was collected by a Magna-IR 550 infrared spectrometer (Nicolet Instruments GmbH, Neu-Isenburg, Germany) mounted with an MTC/B detector of 0,4 cm-1 resolution. The apodisation was corrected by Happ-Genzel, and spectra were processed by the spectrometer software. Spectra of PPhS samples were collected in ATR mode, apodisation and ATR-correction was performed by the spectrometer software according to Happ-Genzel.
Colorimetric staining with Acid Orange II
For the determination of amine (primary, secondary, and ternary) concentration of the surfaces, samples with a diameter of 13 mm were immersed into a 500 µmol/l Acid Orange II (AO) solution solved in water with pH 3, set by HCl. After shaking overnight at room temperature, but at least 12 hours samples were washed twice with the acidic water. In order to dissolve the adsorbed AO they were shaken for15 min at room temperature in water with pH 12, set by HaOH. The AO concentration (which is similar to the amine concentration of the surfaces) of the solution was colorimetrically determined with an optical spectrometer at 485 nm [Uchida 1992].
Fluorescent labelling with Fluram®
A very sensitive semi-quantitative method to determine primary amine (p-amine) concentration is the fluorescent labelling with Fluram®. Samples with a diameter of 13 mm were immersed into a solution of 10 mg Fluram® (Fluorescamine, Fluka 47614) in 20 ml dry acetone. After shaking for 5 min at room temperature the samples were washed twice with water and once in absolute ethanol. The samples were dried for 15 min in air and placed into
29
the solid sample holder of a fluorescence spectrometer. Measurements were performed with excitation at 335 nm and emission at 467 nm [Ivanov 1996].
Surface morphology (SEM, AFM)
The morphology of the plasma-coated samples was investigated by scanning electron microscopy (SEM), and atomic force microscopy (AFM). For SEM the membranes were fractured in liquid nitrogen and coated with gold/palladium (80/20) at room temperature. The prepared samples were scanned in a JSM 6400-F field emission scanning electron microscope (Joel, Japan) at an acceleration voltage of 5 kV. AFM measurements were performed by a multimode scanning force microscope (NanoScope IIIa, Digital Instruments; Santa Barbara, CA, USA) in contact mode.
Contact angle (CA) measurements
Contact angle has been defined as the angle between the solid surface and the tangent of the liquid-vapor interface of a sessile drop [Zisman 1964]. For a water droplet on a polymer surface the contact angle approaches zero (the drop spreads out over the surface) for a hydrophilic surface and increases for a increasingly hydrophilic ones.
CAs were measured using the captive bubble method (fig 3.4), where an air bubble was injected from a syringe with a stainless steel needle onto the surfaces immersed into ultra pure water. While the needle remained inside the bubble advancing and receding angle measurement were realised with a goniometer fitted with a tilting stage (Karl Zeiss, Germany) by stepwise withdrawing/adding air from/to the bubble. At least 10 measurements of different bubbles on at least three different locations
were averaged to yield one CA value.
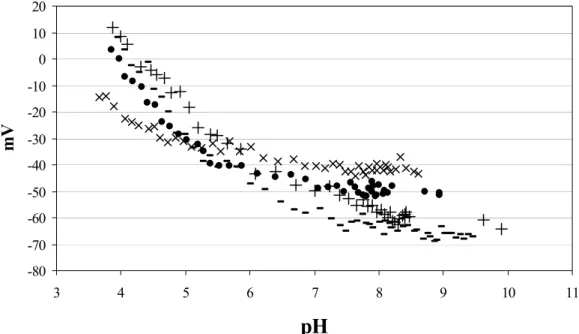
Zeta-potential measurement
Streaming potential (fig. 3.5) measurements were carried out with a computer controlled EKA Electro Kinetic Analyzer (Anton Paar KG., Austria) using a flat plate measuring cell [van Wagenen 1980, C. Werner 1995]. The potential difference was measured with reversible Ag/AgCl electrodes located in both
Θ°
Figure 3.4. Captive bubble method
30
electrolyte inlet and outlet of the cell and is referred to as tangential [surface; outer surface]
streaming potential.
Measurements were performed at 25.0±0.5 °C using aqueous KCl solution. The ionic strength was kept constant at I = 5x10-3 mol/l, the pH was adjusted with equimolar KOH and HCl solutions. Surface conductivity correction was taken into account according to Fairbrother and Mastin [Fairbrother 1924].
Conditioning of the samples was achieved by shaking them for 24 hours in 10-3 N HCl solution at room temperature. Subsequently, the samples were shaken for one hour in distilled water two times additionally equilibrated in the foreseen measuring solution for 24 hours.
The, zeta potential ζ was calculated according to Smoluchowski [Hunter 1981] using the
average slope of at least ten different streaming potential curves dU/dp over a range of pressure difference dp from 20 to 100 mbar along the channel. The pH of the measuring solution was changed stepwise in 0.1 units using a PC controlled titration unit RTU (Paar Physica, Austria), equilibration at each new pH was performed by circulation of the solution for at least 10 minutes before next measurement.
Figure 3.5. Schematic representation of the mechanism leading to the creation of streaming potential
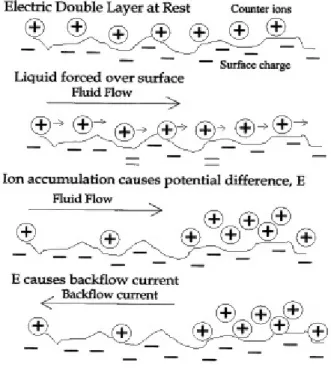
Porometry
Pore size distribution of PET microfiltration membranes was characterised by bubble point technique using a Coulter Porometer II (Beckman & Coulter Electronics, UK) and Porofil (Coulter) as displacement liquid. The bubble point technique is based on the measurement of pressure drop and nitrogen flow through the wetted and the dry membrane. The Young-
31
Laplace theory∗ gives the relation between the pressure the gas needs to pass the membrane and the pore size.
Membranes with a diameter of 25 mm were wetted than exposed to an increasing nitrogen pressure, its bubble point and the increasing nitrogen flow through the membrane until all pores were emptied were measured (wet run). Then the nitrogen pressure was raised again (dry run). Pore size distribution and separation curves were calculated by a device software [Weigel 1993].
Ellypsometry
Thickness of the deposited plasma polymer layers was determined by an ellypsometer SE 400 (Sentec Instruments, Berlin, Germany) mounted with three laser diodes of λ1 = 632,8 nm, λ2 = 839 nm, and λ3 = 1200 nm. Polymers were deposited on silicium wafers and irradiated from different entering angles between 50° and 70° in 5° steps. The thickness was calculated based on the path-length difference of the reflected waves by the device software.
Cell Culture
Human skin fibroblasts were obtained from Cell lining GmbH. (Berlin, Germany) and used up to the ninth passage. The cells were cultivated in Dulbecco’s Modified Eagle Medium (DMEM) containing 10 % Fetal Bovine Serum (FBS; Sigma, Taufenkirchen, Germany) in a humidified incubator with 5 % CO2 at 37,5 °C. Fibroblasts from nearly confluent cultures were harvested with 0,5% trypsin/0,6 mmol EDTA (Sigma), and trypsin was neutralised with FBS.
Since membrane samples removed from the reactor right after plasma treatment can be considered as sterile to remove adsorbed impurities and extractables present, they were immersed into 60% EtOH for 30 min additionally washed with ultrapure water two days. The sterile samples with a diameter of 13 mm were placed in 24-well tissue culture plates. A cell suspension with 2,5x104 cells/ml was filled into each well, and the samples were incubated at 37 °C for times indicated.
∗
P 4γcosd θ
= , where γ is the surface tension of the wetting liquid, θ the contact angle, P the applied pressure, and d the pore diameter
32
Cell proliferation
For the quantitative determination of cell proliferation MTT-tests and LDH tests were carried out. The measurement of cell viability (MTT) is based on the reduction of the yellow tetrazolium salt - 3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromid (MTT) (Boehringer Mannheim, Penzberg, Germany) - by metabolically active cells to form insoluble purple formazan crystals. For each cell type a linear relationship between cell number and absorbance was established, enabling quantification of changes in proliferation [Mosmann 1983, Denizot 1986]. After cultivation for one, three and six days the 100 µl MTT stock solution was given to the samples (four parallel) and were incubated for three hours. After the incubation period the produced formazan crystals were solved by 500 µl iso-propanol, and the solution was shaken for an hour. It was than pipetted into a 96 well-plate (double-detection) and the absorbance was measured by an optical spectrometer SPECTRAFluor Plus (TECAN, Germany) at 562 nm.
The proliferation of fibroblasts was determined by lactate dehydrogenase (LDH) assays.
The test is principally based on the measurement of LDH activity released from the cytosol of damaged cells [Tamada 1995]. Samples were washed and the plasma-membrane of the cells destroyed with 1% Triton-X-100 in phosphate-buffered saline (PBS) after an incubation for 1 h at room temperature. The solution was then centrifuged at 14 000 rot/min, and pipetted into a 96 well-plate (triple-detection). The LDH-test solution was afterwards incubated for 15 min by room temperature in the dark. The absorption of stained solutions was measured with an optical spectrometer (SPECTRAFluor Plus) at 492 nm.
Cell morphology
Overall cell morphology was studied by vital staining with fluorescein diacetate (FDA) (Sigma). 5 µl FDA stock solution (5 mg FDA solved in 1 ml acetone) was added to the samples with cells and incubated for 2 min at 37 °C, then the samples were washed twice with phosphate-buffered saline (PBS) fixed on a microscope slide and observed by a confocal laser scanning microscope LSM 510 (Carl Zeiss, Jena, Germany) with an excitation at 440…480 nm and emission at max. 520 nm [Netuschil 1988].
The cytoskeleton formation in fibroblast was detected by staining of actin, with phalloidin- FITC (Sigma). Attached cells were fixed with 3 % paraformaldehyde in PBS for 15 min and then permeabilized with 0,5 % Triton–X-100 in PBS for 15 min. Samples were incubated for
33
![Fig. 2.5. Relation between basic plasma parameters and plasma process parameters [Kay 1988]](https://thumb-eu.123doks.com/thumbv2/9dokorg/875256.47117/25.892.202.693.393.948/fig-relation-basic-plasma-parameters-plasma-process-parameters.webp)