1 This manuscript is contextually identical with the following published paper:
1
Balogh Csilla; Vláčilová Alena; G.‐Tóth László; Serfőző Zoltán (2018) Dreissenid 2
colonization during the initial invasion of the quagga mussel in the largest Central 3
European shallow lake, Lake Balaton, Hungary. - JOURNAL OF GREAT LAKES 4
RESEARCH, 44: pp. 114-125.
5
The original published PDF available in this website:
6
https://www.sciencedirect.com/science/article/pii/S0380133017301910?via%3Dihub 7
8 9
Dreissenid colonization during the initial invasion of the quagga mussel in the largest 10
Central European shallow lake, Lake Balaton, Hungary 11
12
Csilla Balogha,b, Alena Vláčilovác, László G.-Tótha,b, Zoltán Serfőzőa,b*
13 14
aCentre for Ecological Research, Balaton Limnological Institute, Hungarian Academy of 15
Sciences Tihany, Hungary 16
bMTA Centre for Ecological Research, GINOP Sustainable Ecosystems Group, 8237 Tihany, 17
Klebelsberg Kuno u. 3.
18
cCentre for Popularization, Faculty of Science, Palacky University, Olomouc, Czech Republic 19
20
*Corresponding author 21
address: MTA CER BLI, Klebelsberg Kuno street 3, Tihany, H-8237 22
mailto: serfozo.zoltan@okologia.mta.hu 23
phone: +3687448244/202 24
2 ABSTRACT
25 26
The colonization progress of the invasive bivalve dreissenids, the formerly dominant 27
Dreissena polymorpha and the recently (2008) introduced Dreissena rostriformis bugensis 28
was studied between 2009 and 2013 in the largest Central European shallow lake, Lake 29
Balaton, Hungary. The density of dreissenid planktonic veligers, new settlers (post-veligers 30
and early juveniles), and the population structure (density, length frequency, relative 31
abundance) of the two species were monitored on experimentally introduced natural stone 32
substrata, on different time scales. Dreissenids started dynamic settling following a sudden 33
veliger bloom. As substratum saturation progressed, competition between species for places 34
was suggested, which, after two years, led to an increased number of large individuals (> 20 35
mm) and also recruits of D. r. bugensis. By contrast, the population of D. polymorpha 36
confined to middle size (11-18 mm) individuals of the first settler generation. On local 37
substrata, where the benthic community was already established, the replacement of D.
38
polymorpha by D. r. bugensis took longer, but it happened in a similar way. The invasion 39
speed of D. r. bugensis in Lake Balaton resembled the speed obtained in other European 40
water bodies where D. r. bugensis, similar to Lake Balaton, was introduced much later than 41
D. polymorpha. However, a longer replacement process was found in North America, where 42
both species invaded new habitats at the same time. This suggests that the speed, and 43
probably the success, of D. r. bugensis invasion depends on new surface availability, and 44
whether the two dreissenid species are introduced together or at different times.
45 46
Index words: biological invasion, colonization dynamics, dreissenid, quagga mussel, 47
population structure 48
3 Introduction
49 50
Invasive aquatic alien species, among them the freshwater bivalve dreissenids 51
(Dreissena polymorpha, Pallas, 1771 [D. polymorpha, zebra mussel]) and Dreissena 52
rostriformis bugensis, Andrusov 1897 [D. r. bugensis, quagga mussel]) are serious threats to 53
and provoke dramatic changes in community abundance, species diversity, resource 54
availability, nutrient cycling and functioning of the ecosystem (Crooks, 1998; Gutiérrez et al., 55
2003; Ward and Ricciardi, 2007; Ricciardi and MacIsaac, 2011). Due to their invasive nature 56
and intensive filter-feeding behavior, dreissenids are counted among the most invasive and 57
destructive invaders in North American and European freshwater ecosystems (OTA, 1993;
58
DAISE, 2003; Pimentel et al., 2005).
59
Due to the fast spread of invasive species nowadays, a new invader is more likely to 60
come into contact with a former dominant invader (van den Brink et al., 1993; van der Velde 61
et al., 1994; Dick and Platvoet, 2000; Platvoet et al., 2009; Ricciardi, 2001; Gallardo and 62
Aldridge, 2015; de Gelder et al., 2016). Although the occurrence and progression of D.
63
polymorpha in Europe has long been described in many different habitats (Bij de Vaate, 1991;
64
Pollux et al., 2003; Aldridge et al., 2004; Cianfanelli et al., 2010; Karatayev et al., 2010a;
65
Palau Ibars et al., 2010; Stańczykowska et al., 2010), in contrast to North America (Mills et 66
al., 1993; Watkins et al., 2007; Dermott and Dow, 2008; Nalepa et al., 2010; Karatayev et al., 67
2013a), and to the Ponto-Caspian region where dreissenids originated (Orlova et al., 2004;
68
Zhulidov, et al., 2010), the characteristics of its population dynamics during the invasion of 69
the new dreissenid, D. r. bugensis, has only rarely been observed and investigated (Bij de 70
Vaate, 2010; Aldridge et al., 2014; Heiler et al., 2013; Matthews et al., 2014).
71
The dispersal speed of D. polymorpha was found to be much faster than that of D. r.
72
bugensis throughout their invasion history (Karatayev et al., 2011). Therefore, in Europe, 73
4 apart from the connected river systems (Orlova et al., 2004; Zhulidov et al. 2010; Heiler et al., 74
2012; Heiler et al., 2013; Matthews et al., 2014) which are known as invasion pathways, in 75
lakes and reservoirs, D. r. bugensis has only appeared in the Kuybyshev, Saratov and Rybinsk 76
Reservoirs (part of the Ponto-Caspian Volga River invasion corridor, Orlova, 2004), some 77
Dutch lakes (Lake Ijsselmeer and Markermeer, Bij de Vaate et al., 2013), and recently in 78
Great Britain (Aldridge et al., 2014). In contrast to the spread over long distances, in 79
European water bodies where D. r. bugensis was introduced, its population increase rate was 80
26% per year within the dreissenid community, until the displacement of D. polymorpha 81
(Heiler et al., 2013), which suggests the significant spread of this species in local habitats.
82
Similar trends were reported from the Laurentian Great Lakes, where five years after the 83
initial introduction, D. r. bugensis represented 44% of the dreissenid population (Dermott and 84
Dow, 2008), and later increased up to 97% (Patterson et al., 2005), leading to total 85
replacement of D. polymorpha in the deep parts of the lakes, and the balanced co-existence of 86
the two dreissenids in shallow parts (Patterson et al., 2005; Watkins et al., 2007; Nalepa et al.
87
2010). It was also found that in some shallow lakes and reservoirs, or in shallow areas of deep 88
lakes, dreissenids could live together for longer periods of time in the same habitat (Tzeyeb et 89
al.1966; Zhulidov et al., 2010; Karatayev et al., 2011, 2013b), or exhibit a reversal, where D.
90
r. bugensis was displaced by D. polymorpha (Zhulidov et al., 2006). Thus, the interaction 91
between the two dreissenids can be characterized as competitive niche partitioning; however, 92
the dynamics and progression of the co-existence remain unknown, and they seem to be 93
influenced by environmental conditions.
94
The site of the present study, Lake Balaton, is the largest shallow lake in Central Europe 95
connected to the Danube River via the Sió channel (Fig. 1). In 1932, D. polymorpha was the 96
first Ponto-Caspian invader introduced from the Danube River to the lake, possibly via ship 97
transport (Sebestyén, 1938). Around 75 years later (2008), D. r. bugensis was discovered as a 98
5 new dreissenid invader in Lake Balaton (Majoros, 2009; Balogh and G.-Tóth, 2009; Benkő 99
Kiss, Á. personal communication). D. r. bugensis may have been imported in the same way as 100
D. polymorpha since the mussel was detected in the Hungarian region of the Danube River 101
much earlier than it was estimated to appear in Lake Balaton (Szekeres et al., 2008). The 102
recent introduction of D. r. bugensis to Lake Balaton makes this lake ideal to follow the 103
invasion dynamics in real time, and study the early consequences of the invasion on the 104
benthic community, including the dominant D. polymorpha population.
105
Studying the settlement and growth dynamics of the dreissenids would provide unique 106
data to help understand the population trends and invasion success of D. r. bugensis, thus 107
supporting the assessment and prediction of its environmental impact, ecological changes, and 108
management (Wong and Gerstenberg, 2011). Shallow lakes invaded by D. r. bugensis have 109
been reported by Karatayev et al. (2013b), who expected invasion dynamics to be different in 110
shallow versus deep lakes. For these reasons, we investigated the colonization progress of 111
dreissenids in Lake Balaton during the early invasion of D. r. bugensis. Colonization was 112
evaluated on early, short-term (daily), medium-term (monthly), and long-term (yearly) time 113
scales on experimentally introduced natural stone substrata by following the progression of 114
the number (relative abundance, density) and growth (size and covered substratum area) of 115
the two dreissenids. Data were compared with those sampled from the local stone substrata.
116
Such a complex research approach, providing high resolution data in a medium-term study, 117
has not yet been used for studying the invasion of D. r. bugensis. The objective was to 118
analyze the colonization dynamics of dreissenids, in the knowledge that D. r. bugensis has 119
been introduced to the lake recently, and the environmental conditions favor its settlement.
120 121
Materials and Methods 122
123
6 Study site with local conditions
124 125
The support structure for experimental substrata submerged in Lake Balaton was placed 126
near to the riprap zone at 1.9 m depth, in front of the shoreline of the Balaton Limnological 127
Institute, Tihany (Fig. 1). To compare the data obtained from the experimental substrata with 128
natural trends, three sampling locations (T1, T2, T3) in the riprap along the shoreline of the 129
Tihany peninsula were selected. The water characteristics of these local bottom substrata 130
(type, size, shape, depth of their location [T1: 1.5 m; T2: 1.9 m; T3: 1.1 m]) were similar to 131
those used for the experimental substrata. The only difference was that the local substrata had 132
already been coated by natural biofilm, including dreissenids.
133
During each substratum sampling, at the same depth where the support structure for 134
experimental substrata was placed, and the local sampling sites were situated, beside the 135
substrata, water characteristic variables were recorded in the conventional manner, using a 136
Horiba U-10 water multiparameter measuring instrument. In the first few months of the study, 137
between the summer and winter period, the measured values were as follows: temperature: 7–
138
29 °C; conductivity: 751–910 µS cm-1; pH: 8.0–8.9; chlorophyll-a concentration: 5.4±3.15 μg 139
L-1, the highest content of suspended material: 25 mg (dw) L-1. 140
141
Support structure for experimental substrata 142
143
To study the progression of surface colonization by dreissenids, stone holders were 144
created from a massive metal frame measuring 1×1 m in size, and joined with protruding 145
pipes, distributed at equal distances, and serving as holders (Fig. 2). Sixty palm-sized (surface 146
was 0.0191±0.0038 m2/ each) red sand stones of similarly irregular shape (hereafter referred 147
to as ‘stones’) were collected from the shoreline, scrubbed and dried. They were drilled, 148
7 inserted, and fixed onto metal pipes of uniform size with commercial glue. Stones with pipes 149
could be slipped into and easily taken out of the holder pipes, ensuring a secure fit on the 150
frame and sampling.
151 152
Sample collection 153
154
In each sampling session, three stones were randomly selected for retrieval by diving 155
from both natural and experimental substrata. Each stone was placed in a plastic bag for 156
protection, and transported to the laboratory, where the encrustation of the substrata was 157
removed by knife and soft brush, and sieved through a 60 m nylon net. The collected animal 158
specimens were preserved in 70% ethanol.
159
Veligers were sampled with a 50 cm high (volume: 34 L) Schindler–Patalas sampler 160
equipped with a 60 m mesh-sized collector funnel near to the site, where experimental 161
substrata were placed, on each occasion the substrata was sampled. Sampling was carried out 162
at 50 cm increments along the entire vertical depth. The samples were pooled together and 163
concentrated to 20 mL, then preserved with ethanol.
164 165
Sampling plan (dates and terms) and weather characteristics 166
167
The study began by submerging the instrument holding the experimental substrata on 3 168
August 2009. Sampling started from the second day of submersion and continued at a three- 169
day frequency in August (short-term scale), monthly from September to December of 2009 170
(medium-term), and annually, beginning in the summer of 2010 for the following three years 171
(long-term). In parallel, the local substrata were examined each August during the study 172
period, until 2013.
173
8 The average summer temperature was 0.6 and 1 °C higher in 2012 than in 2010 and 174
2011, respectively. The hottest water temperature (29.1 °C) was also recorded in 2012, when 175
a long dry period caused a significant decrease in water levels, which was low, as parts of the 176
shoreline had dried up. In summer 2012, a number of days with stormy winds and waves were 177
registered, which created harsh turbulences in the shallow water. Extreme weather 178
phenomena, such as heavy storms (wind speed was 80–90 km/h) on the 5th and the 14th of 179
August, and rapid cooling thereafter in September, occurred.
180 181
Density and size measurements 182
183
To calculate the surface area of sampled stones, the entire surface of the stones were 184
traced onto wrapping paper. An algorithm for cut paper weight vs. surface area was derived.
185
The density of dreissenids was represented as ind m-2 stone surface. The relative abundance of 186
D. r. bugensis within the whole dreissenid population was calculated and given as percentage 187
contribution in both introduced and local substrata.
188
The two dreissenids were distinguished from each other according to their unique 189
morphological features (Spidle, et al., 1995; van der Velde et al., 2010). The two species were 190
only differentiated if the dreissenid individual was >2 mm. New settlers were separated based 191
on their sizes: <0.5 mm (post-veligers – plantigrade) and 0.5< and <2 mm (early juveniles – 192
siphon-forming stage), according to Claudi and Mackie (1994), Kirpichenko (1964), and 193
Ackerman, et al. (1994).
194
The number of dreissenids >2 mm was counted with the naked eye, and their length was 195
measured with a digital caliper. Post-veligers and early juveniles were counted and measured 196
under a stereomicroscope using a mm scale underneath the counter dish. Length frequency 197
histograms were generated using 1 mm size classes to assess population size structure (Mills 198
9 et al., 1993; Orlova et al., 2004, 2013; Dermott and Dow, 2008; Karatayev et al., 2013a). To 199
count veligers, 2–5 mL multiple subsamples were taken from the concentrated 20 mL sample, 200
and examined with a Zeiss–Opton inverted microscope.
201 202
Calculation of substratum surface saturation by dreissenids 203
204
Cover of experimental substrata by dreissenids was calculated using the following 205
formula:
206
∑ length × [a × length + b] × π × n
(5−27) 4 Where,
207
length is the length of the individual in mm accuracy on a 5–27 mm scale;
208
a is the slope of the length–width regression line;
209
b is the intercept of the length–width regression line;
210
a × length + b is the calculated width;
211
length × [a × length + b] × π/4 is the surface of the ventral side of the animal;
212
n is the number of animals of a specific length;
213
Σ(5–27) is the total ventral surface of animals covering the surface.
214
The length– width correlation was obtained from the measurement of 30–40 D. polymorpha 215
and D. r. bugensis individuals, respectively, in each length group (between 5 and 27 mm with 216
1 mm difference). The longest distance of the rostro-caudal axis was measured as the length, 217
and the width was measured as the longest dimension in the direction perpendicular to the 218
length. Dreissenids face the attaching surface with their ventral side, which looks like an 219
ellipsoid in planar projection. The ventral side area of all mussels forming the dreissenid 220
population was therefore calculated using the formula for elliptical area (a × b × π, where a 221
and b are half of the length and width values, respectively), and finally, extrapolated to the 222
10 number of animals on the substrata. The sum of the surface area of differently sized 223
dreissenids colonizing the substrata simultaneously gave a total surface area, which would 224
ideally cover the substrata if the animals settled side by side. This score overestimates the real 225
surface area occupied by dreissenids, since animals attach to the substrata by their byssus and 226
thus do not occupy the surface with their total base (ventral surface). In addition, as 227
colonization progresses, many of the animals also use each other’s shells as settling sites 228
(multilayer aggregation). Nevertheless, with knowledge of these shortcomings, this 229
calculation allows to assess the dynamics of substratum occupation and estimate the time 230
when dreissenids reach total occupation.
231 232
Statistics 233
234
Before analysis, datasets were transformed to achieve homogeneity of variance and 235
improve normality. The normality of the data was checked with a normal Q-Q plot of the 236
model residuals (Sokal and Rohlf, 1995). A mixed model ANOVA was used to analyze 237
differences in density (log-transformed) and average length (log-transformed) between the 238
two species, on different time scales. The studied variables were species (within-subject, 239
repeated measures factor) and time (between-subject factor). Separate analyses were carried 240
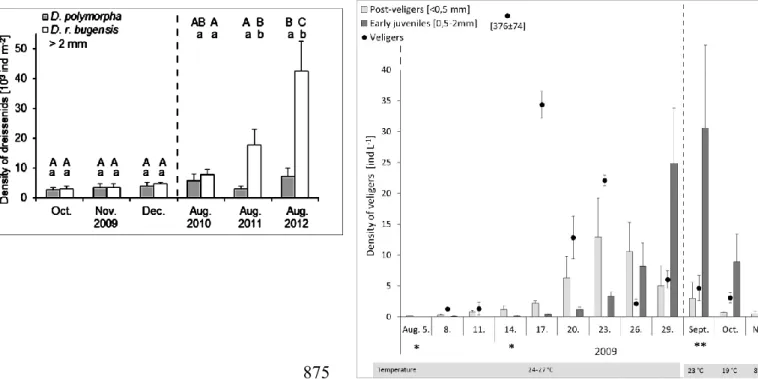
out for different time scales (months, years). Sequential Bonferroni-corrected t-tests were 241
used to show the significant interactions.
242
Yearly differences in the relative abundance of D. r. bugensis (percentage of D. r.
243
bugensis in the dreissenid population) were compared between sampling sites using GLM 244
ANOVA. The relative abundance of D. r. bugensis as a dependent (log-transformed) variable 245
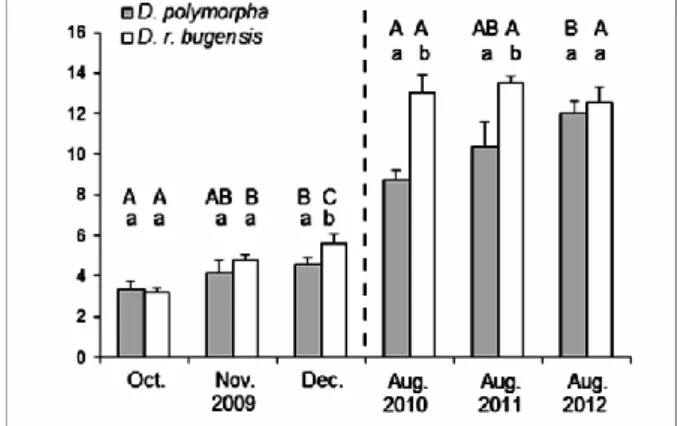
was analyzed with the independent categorical variables (fixed factors), time and sites 246
(including experimental and local substrata) in the model. A Tukey test was used as a post- 247
11 hoc procedure for evaluating the main effects and interactions (for differences between the 248
sites at a certain date, and differences between years at a given site).
249 250
Results 251
252
Densities of planktonic veligers and new settlers (size: 0.5–2 mm) on experimental substrata 253
254
Within a week of the onset of the settlement study, even though the veliger 255
concentration in the water was low (1-1.3 ind L-1), early colonization of post-veligers was 256
already observed (Fig. 3). Shortly thereafter, an extraordinary boom of veliger expansion was 257
detected (on the 14th August, 2009; 376±74 ind L-1), which was limited only to single day, 258
and could not be seen for the remainder of the month. A week after the veliger boom, on the 259
23rd of August, a peak appeared in the density of post-veligers (11500±6100 ind m-2) which, 260
within a month, showed a downward trend to a similar level as observed before the boom.
261
The curve of density dynamics of early juveniles was similarly shaped, peaking in September 262
with 27000±12000 ind m-2. As the temperature dropped, and autumn transitioned to winter, 263
veligers disappeared from the water sample, and post-veligers and early juveniles remained at 264
low density levels on the substratum surface (2009 December, post-veligers: 300±130 ind m-2; 265
early juveniles: 2600±300 ind m-2). In subsequent summers, veligers were consistently found 266
at moderate levels (14-25 ind L-1), whereas post-veligers and early juveniles showed a 267
decreasing tendency to colonize with a density between 400–1400 ind m-2,and 3400–11100 268
ind m-2, respectively.
269 270
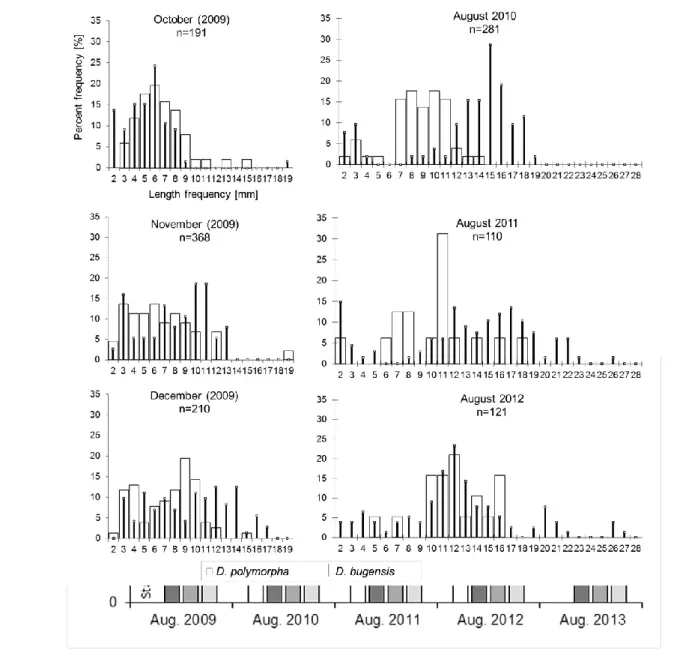
Dreissenid (size: >2 mm) density on experimental substrata 271
272
12 In the following months after substratum deployment (August 2009), no significant 273
differences in density data were found either between the two dreissenids, or between the 274
months (Fig. 4, Table 1a). The density of each species changed during this period, between 275
2652 and 2699 ind m-2. From 2010 to the end of the study (2012), the density of D. r.
276
bugensis significantly increased year by year (Fig. 4, Table 1b.), in contrast to D. polymorpha 277
for which density slightly fluctuated, but did not change overall. By 2012, D. r. bugensis 278
density reached up to 42453±10321 ind m-2, which was six times higher than that of D.
279
polymorpha.
280 281
Relative abundance of dreissenids (size: >2 mm) on experimental and local substrata 282
283
No difference in relative abundance was found on experimental substrata between the 284
two dreissenids after one year of substratum implantation (Fig. 5). From 2010 onward, with 285
increasing differences year by year, the abundance of D. r. bugensis (percentage of D. r.
286
bugensis in the dreissenid community) significantly exceeded that of D. polymorpha, which 287
was accompanied by the decline of the latter population (Fig. 5, Table 3).
288
Along with sampling from the experimental substrata during the study period (2009- 289
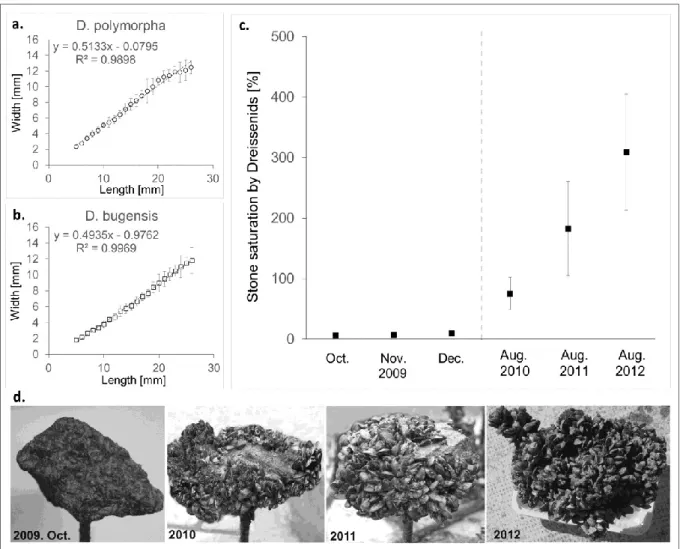
2012), in each August, and also in the upcoming year (2013), the dreissenid population was 290
also examined on natural (local) substrata at three points of the Tihany peninsula (see the 291
sampling site map in Fig. 1). In the year of substratum implantation, the relative abundance of 292
D. r. bugensis was significantly different at all three points (Fig. 5, Table 3). Percentage of D.
293
r. bugensis was 29.6±8% in T1, 48.6±7.1% in T2, and 16.4±4.4% in T3. By 2010, the 294
percentage of D. r. bugensis had significantly increased in all sampling sites, and a difference 295
was only found between the T2 and T3 sites. At the same time, except in the T2 site, relative 296
abundance was similar in the introduced as well as the local substrata. In 2011, the percentage 297
13 of D. r. bugensis was further increased in experimental substrata, and T1. Trends of relative 298
abundance equalization continued between the sampling sites. This resulted in around 80%
299
relative abundance of D. r. bugensis in all sites and no annual differences between the sites by 300
2012. The decrease in water level resulted in high mortality for the dreissenid populations on 301
the riprap, along which piles of shells could be traced in 2012; however, this had no 302
significant impact on the growing relative abundance of D. r. bugensis, which reached 98%
303
by 2013.
304 305
Size related composition of dreissenids (size: >2 mm) on experimental substrata 306
307
Fine resolution analysis of length distribution showed that both species were equally 308
represented on the surface after two months of substratum deployment (Fig. 6). The most 309
common sizes were within the 2–9 mm range, forming a bell shaped distribution.
310
Interestingly, some adult animals also appeared on the substrata at this very early stage of 311
colonization. In the subsequent months, the distribution of individuals within the 3–13 mm 312
range equalized further, which resulted in a smoother distribution pattern (Fig. 6). By the end 313
of 2009, five months after colonization started, the largest animals (> 12 mm) were mainly D.
314
r. bugensis. A year later (2010), and in 2011, it was again evident that D. r. bugensis was 315
more frequent among the largest animals (>12 mm), whereas the frequency of adult animals 316
belonging to the size range of 10–13 mm was higher in D. polymorpha. Following the annual 317
changes in length during the examination period, the whole size distribution pattern of D. r.
318
bugensis (ranging from 2 to 28 mm) was found to be much wider than that of D. polymorpha 319
(ranging from 2 to 17 mm). In 2010, the most abundant sizes found in the D. polymorpha 320
population were within a narrow range of 7–11 mm in length, which slowly shifted to 10–16 321
mm by 2012. In contrast, the size frequencies of D. r. bugensis showed a rather heterogeneous 322
14 distribution, resulting in a less coherent size distribution in the plot. In 2010 and 2011, 323
dreissenids 4–7 mm in size, were missing or underrepresented in the samples.
324
The average length of settled D. r. bugensis reached its maximum as early as in 2010, 325
whereas that of D. polymorpha in 2011-2012 (Fig. 7). As a straightforward consequence of 326
the difference in the size distribution observed between the two dreissenids (see Fig. 6), the 327
average length showed significant differences from December 2009 until 2011 (Fig. 7, Table 328
2a, 2b). By contrast, a difference in the average length was not seen in 2012.
329 330
Saturation of dreissenids (size: >2 mm) on experimental substrata 331
332
Both dreissenid species showed similar, linear correlations between length and width 333
(Fig. 8a, b). The total surface occupied by the dreissenid population was estimated according 334
to the ventral (attaching) surface of individuals and the quantity of settled animals of different 335
sizes (see Fig. 6). Two months after deployment of the support structure for the experimental 336
substrata, the total surface of colonizing dressenids occupied 10% of the available surface of 337
the implanted stones (Fig. 8c). In the following cold season, this area did not increase 338
significantly until the end of 2009. A year after deployment, the number of dreissenids 339
attached to the substrata represented 122±28 cm2 surface, which, supposing idealistic and 340
homogeneous distribution, covered the whole available surface (Fig. 8c). In the forthcoming 341
years (2011, 2012), the total surface of settled animals slightly increased, exceeding that of 342
the substrata. Considering that surface occupation was overestimated, dreissenids could have 343
saturated the whole surface around the summer of 2011. However, empty spots on 344
experimental substrata could be seen until 2012 (Fig. 8d). From that time, the stratified 345
appearance of dreissenid populations and phenomenon of multilayer aggregation could be 346
more frequently observed on the experimental substrata (Fig 8d).
347
15 348
Discussion 349
350
Dreissenid population structure and dynamics on experimental and local substrata 351
352
Shortly after the experimental substrata were submerged into the lake, a huge boom of 353
dreissenid larvae was observed, which could also be seen in the increasing number of post- 354
veliger and early juvenile individuals attached to the new surface in the subsequent week and 355
month. The spawning season of dreissenids lasts from late March to November depending on 356
the lake temperature. The frequency of dreissenid larvae release has not been studied so far in 357
Lake Balaton, but it is assumed to be influenced primarily by weather extremities, which 358
occur more often nowadays rapidly changing the physical conditions of the shallow lake.
359
Simultaneous release of a large number of larvae within days in the middle of the spawning 360
period assumes the presence of some triggering substances, which affect gonadal activity and 361
promote sudden rather than smooth production of new larvae. The veliger release coincided 362
with a storm, which evoked big waves and mixed up the whole water column, increasing the 363
amount of the suspended material. Nevertheless, correlation between the storm and the veliger 364
release cannot be established, which was supported by the low number of veligers observed 365
also at a stormy day just after the settlement. Taking into account that D. polymorpha larvae 366
stay in the plankton for at least 7–15 days (Marsden, 1992; Ackerman et al., 1994), and 367
because of the irregular larvae release, this implies that to address dreissenid larvae 368
propagation, at least weekly sampling frequency is required.
369
It can be deduced from the density and length frequency data of dreissenids, which have 370
been taxonomically identified (> 2 mm), that D. r. bugensis and D. polymorpha colonized the 371
experimental substrata with equal success in the first few months. This theoretically suggests 372
16 that at the beginning of the colonization study, veligers were distributed equally between the 373
two species. However, due to its stronger attachment, D. polymorpha is a better colonizer and 374
more often remain on the substratum (Peyer et al., 2009, Collas et al., 2016), also suggesting 375
that D. polymorpha might have represented more on the substratum, albeit its veliger is less 376
abundant in the water.
377
Some large individuals, found unexpectedly in the samples of early colonization, might 378
come from the neighboring riprap by detaching and transporting via water currents or by 379
active locomotion to the experimental substrata.
380
After an equalized abundance, from the first year of colonization, the D. r. bugensis 381
population significantly increased on experimental and local substrata, implying the success 382
of this dreissenid over the other species in the colonization process. However, the progression 383
of D. r. bugensis colonization on local substrata took longer than on experimental substrata at 384
the beginning of the study. This might be because of the established benthic community on 385
local substrata including D. polymorpha and C. curvispinum, which saturated the surface and 386
occupied the niche that is suitable for D. r. bugensis. In Lake Balaton, D. polymorpha was 387
also found to rapidly colonize new substrata before the appearance of D. r. bugensis (Balogh 388
et al., 2008), meaning that primarily the colonization speed is due to substratum saturation 389
and not dependent on the type of dreissenid species. Nevertheless, the presence of competitors 390
can significantly influence the process.
391
During the colonization process, the D. polymorpha population was mainly confined to 392
a mid-size range (8-14 mm) that grew slowly. On the spat side, the D. polymorpha population 393
could not be renewed, since after one year of colonization, D. polymorpha settlers were rarely 394
found. On the adult side, the higher density of large (> 20 mm) D. r. bugensis individuals 395
suggested more sexually mature D. r. bugensis in the sample. Pressure from both sides might 396
lead to a decline, if not collapse, of the population of D. polymorpha during the simultaneous 397
17 colonization of the two dreissenid species. Before D. r. bugensis was introduced to the lake, 398
D. polymorpha grew larger (up to 2.4 cm, Balogh et al., 2008), which, considering that the 399
general conditions of the lake have not changed in the past several decades, suggests that D. r.
400
bugensis negatively affects D. polymorpha population development. The possible influence of 401
the D. r. bugensis population on D. polymorpha growth may also be strengthened by the fact 402
that neither in our sampling points nor at other sites of the lake (Balogh C., unpublished 403
observation) can D. polymorpha individuals >2 cm be found.
404
From 2011, large D. r. bugensis individuals (between 20 and 28 mm), showing 405
heterogeneous frequency, were counted on experimental substrata. These animals must have 406
arisen from a compact group in which sizes were between 12 and 19 mm in 2010. The 407
reduction of the number of these large animals in 2012 could be due to the decline of the first 408
generation after the third year of settling, since, as found earlier in Lake Balaton (Balogh et 409
al., 2008) and other water bodies (Whitney et al., 1996), the lifetime of dreissenids often does 410
not exceed 3–4 years. However, different access to resources (food, oxygen), due to different 411
site positions of the individuals in the substratum (i.e. at the bottom of the multilayer 412
aggregation, or on that substratum side, which is relatively hidden from the water current), 413
may contribute to the heterogeneous size distribution observed in large animals.
414
By the end of the study (August, 2012), the average length of the two species had 415
equalized, which may be explained, on the one hand, by the decreasing number of large size 416
(> 20 mm) individuals and the increasing number of young settling (3-10 mm) D. r. bugensis 417
individuals, and on the other hand, by the smoothly growing population of the mid-size (10- 418
17 mm) first colonizer generation of D. polymorpha.
419
While the veliger density was not significantly different in the summers of 2010–2012, 420
the density of new settlers declined, suggesting that the progression of surface occupation, 421
and hence, increasing saturation by growing dreissenids did not favor new settling 422
18 generations. Parallel to the decrease in free settling places, the growing shell surfaces of 423
earlier colonized individuals provided novel surfaces for larvae to attach, as observed from 424
2011. According to the experimental study of Tošenovský and Kobak (2016), the lower initial 425
distances between settled mussels offered a higher possibility for aggregation, suggesting that 426
as habitats are narrowed during the D. r. bugensis invasion, multilayer aggregation is 427
facilitated. Substratum saturation by dreissenids was calculated to be completed around two 428
years after substrata implanted to the lake, after which the competition for surfaces becomes 429
more intense between the two dreissenids. This was confirmed recently in an experiment 430
(Dzierzynska-Bialonczyk et al., 2017), where the formation of D. polymorpha aggregations 431
was found as a consequence of the lack of available alternative attachment sites. On the other 432
hand, saturation progression and in contrast, uncovered sites on experimental substratum 433
found until the last year of the study (four years after substratum implantation), suggested that 434
the shell of dreissenids appeared as an alternative attachment surface that promotes multilayer 435
aggregation.
436 437
Abiotic and biotic factors that would explain the success of D. r. bugensis colonization over 438
D. polymorpha 439
440
As the temperature dropped in the late autumn of 2009, the density of veligers 441
decreased, and subsequently, the colonization of post-veligers was reduced. From 2010 442
onward, the new settlers were almost exclusively D. r. bugensis, which might be because D. r.
443
bugensis starts spawning earlier at lower temperatures (4–9°C, Claxton and Mackie, 1998;
444
Roe and MacIsaac 1997; Stoeckmann, 2003; Nalepa et al., 2010) than D. polymorpha (above 445
9°C, Sprung, 1987). In Lake Balaton, veligers could be found in the water column at 7.3 °C 446
(late November), but they were missing in December when the temperature dropped below 7 447
19
°C. Interestingly, before the D. r. bugensis invasion, veligers were usually missing in the cold 448
season, even in October, when the water temperature was higher than 8 °C (Balogh et al., 449
2008). The water temperature may explain the inability of D. polymorpha to produce larvae 450
between October and April in Lake Balaton, and could be an environmental factor providing 451
D. r. bugensis with an advantage for earlier spawning, and thus a settling opportunity.
452
From 2011 to 2012, the abundance of the two dreissenids on experimental substrata 453
remained unchanged. This can be explained by the weather extremities (high water 454
temperature, water level fluctuation, waves), which are less endurable for D. r. bugensis 455
(Karatayev et al., 2013b), partly due to the mild attachment strength and fragility of the shell 456
(Peyer et al., 2009; Casper and Johnson, 2010), and their low tolerance to temperatures above 457
30.5 °C (Spidle et al., 1995; Thorp et al., 1998; Karatayev et al., 1998). Conversely, in 2013, 458
the lake was spared from weather extremities. In spring, the water level became so high that 459
the local substrata suitable for colonization were submerged again. This allowed for the 460
ongoing domination of D. r. bugensis on the rocks of the riprap in our sampling sites, 461
resulting in almost total displacement of D. polymorpha.
462
Since there is no evidence of selective erasure from the substrata, or early death of D.
463
polymorpha the different size distribution found between the two dreissenids after one year of 464
substratum deployment could be due to the faster growth of D. r. bugensis. A similar 465
difference in the growth rate between the two dreissenids was reported from the Laurentian 466
Great Lakes (Jarvis et al., 2000; Diggins, 2001; Stoeckmann, 2003), which was attributed to 467
the lower respiration and higher filtration rate of D. r. bugensis. A lower respiration rate 468
enables D. r. bugensis to reduce the energetic expenditure on maintenance, and therefore 469
promotes faster growth and ensures better chances for survival (Baldwin et al., 2002;
470
Stoeckmann, 2003). D. r. bugensis grows faster and is heavier at the same shell length, so 471
generally has a larger shell length and body mass than D. polymorpha (Mills et al. 1996, 472
20 Jarvis et al. 2000, Diggins, 2001; Stoeckmann 2003; Karatayev et al., 2010b). It is known 473
from field (Karatayev et al., 1998; Stoeckmann, 2003; Orlova et al., 2005), and from 474
experimental studies (Stoeckmann and Garton, 2001; Baldwin et al. 2002) that the growth and 475
body mass of D. polymorpha declines more on a poor-quality diet and in the presence of high 476
suspended material concentration, than that of D. r. bugensis. Our experimental and local 477
sampling points are situated in the oligotrophic part of the lake, and are characterized by low 478
food (chlorophyll-a concentration was 5.4±3.15 μg L-1), and high suspended material 479
concentrations (25-600 mg dry weight L-1, G.-Tóth et al., 2011), which may explain the faster 480
growth of D. r. bugensis.
481
There is no evidence that D. polymorpha reaches sexual maturation at lower sizes than 482
D. r. bugensis, but if this is the case, this could also explain why it develops slowly thereafter.
483
In the years after substratum deployment, however, among the second and subsequent 484
generations of post-veligers attached on the experimental substrata, D. polymorpha 485
individuals were rarely found, suggesting that the aforementioned assumption might be false.
486
Instead, the recruitment of D. r. bugensis implies that more larvae, and a larger number of 487
individuals producing larvae belonging to this species, were present in the surroundings from 488
2010. Since local circumstances that would have influenced the selective depletion of D.
489
polymorpha larvae are not known, the declined colonization of this species may due be to the 490
decreasing number of sexually mature D. polymorpha individuals.
491
Fish and bird predation, which concerns mainly medium sized (8–17 mm) dreissenids 492
(Czarnołęski, et al., 2006), regulates the dreissenid population in Lake Balaton (Ponyi, 1985;
493
Specziár et al., 1997, Balogh et al., 2008). This, besides natural death, might cause the 494
heterogeneous size distribution of the larger/older (> 20 mm) D. r. bugensis individuals found 495
on experimental substrata. However, we do not know whether predation evokes any species 496
selectivity. Other factors such as ice scours (MacIsaac, 1996; Chase and Bailey, 1999; Balogh 497
21 et. al, 2008), and parasites (Molloy et al., 1997), considered as potential regulators of 498
dreissenid population dynamics (Strayer and Malcom, 2006), are less feasibly involved in our 499
study.
500 501
Comparison of dreissenid invasion dynamics in the eastern basin of Lake Balaton with other 502
lakes 503
504
The colonization dynamics of dreissenids and the replacement of D. polymorpha with 505
D. r. bugensis happened similarly in our experimental and local study sites to that found in the 506
entire eastern basin of Lake Balaton (Balogh C., unpublished), where oligo-mesotrophic 507
conditions are uniform (chlorophyll-a concentration: 2-3 μg L-1, Sebestyén et al., 2017), and 508
also in other European shallow reservoirs (Orlova et al., 2004; Heiler et al., 2013), and lakes 509
(Matthews et al., 2014; Bij de Vaate et al., 2013). In these water bodies, D. r. bugensis almost 510
entirely replaced D. polymorpha 3–4 years after its appearance. In the Laurentian Great 511
Lakes, the progression of D. r. bugensis, mainly in deep areas, was much slower: it took more 512
than 10 years, but finally led to a significant reduction of D. polymorpha population 513
(Patterson et al., 2005; Watkins et al., 2007; Dermott and Dow, 2008; Nalepa et al. 2010). By 514
contrast, in shallow areas of the Lakes, like the western basin of Lake Erie, the two 515
dreissenids have lived together for a long time (Karatayev et al., 2014). Recently, we also 516
found the co-existence of the two species in the western basin of Lake Balaton (Balogh C., 517
unpublished), where the water trophity (chlorophyll-a concentration: 5-7 μg L-1, Sebestyén et 518
al., 2017) similar to that of the western basin of Lake Erie (Barbiero and Tuchman, 2004), is 519
more eutrophic than the Eastern basin. Hence, in shallow lakes, it seems that rapid 520
replacement of D. polymorpha with D. r. bugensis more likely happens if food availability is 521
limited.
522
22 The difference between the colonization history of dreissenids in North America and in 523
Europe is that North America was invaded by the two species simultaneously (Carlton, 2008, 524
Mills et al., 1993), whereas in Europe, D. polymorpha colonized and became the dominant 525
macroinvertebrate in the benthic community well before the appearance of D. r. bugensis 526
(Van der Velde et al., 2010). Therefore, another possible explanation for the different duration 527
of the replacement found between the continents is that parallel invasion could evoke a longer 528
struggle for place and resources between dreissenids having similar ecological requirements.
529
Where D. polymorpha was the first and prevailing dreissenid for years, the algal biomass and 530
hence the trophic state of the habitat reduced, a condition that is much more tolerable for D. r.
531
bugensis than D. polymorpha. Therefore, if D. r. bugensis is introduced to a habitat where D.
532
polymorpha has already been colonized for a long time, the new invader has a competitive 533
advantage as it better tolerates poor food conditions (Karatayev et al., 1998; Baldwin et al., 534
2002; Stoeckmann, 2003; Orlova et al., 2005). Similarly, the invasion of D. polymorpha and 535
its competitor, the amphipod Chelicorophium curvispinum (Sars, 1895) gave different results 536
in Lake Balaton and the river Rhine. In Lake Balaton, where the two species were introduced 537
together (Sebestyén, 1938), they have lived side by side for a long time (Balogh et al., 2008).
538
By contrast, in the river Rhine, the much later introduced amphipod gradually suppressed the 539
mussel population over several years (van den Brink et al., 1993; van der Velde et al., 1994).
540
Hence, it is possible that the progression and fate of the D. r. bugensis invasion highly 541
depends on whether the invader comes simultaneously with or later than its competitors. In 542
summary, it can be predicted that if D. r. bugensis appears later than D. polymorpha,in a 543
shallow lake where food availability is low, then the replacement becomes rapid.
544 545 546
Conclusion 547
23 548
The colonization process of dreissenids (D. polymorpha and D. r. bugensis) on 549
experimental and local substrata, simultaneously, at the time of the D. r. bugensis invasion, 550
revealed that the new invasive species was very successful against its congener, the formerly 551
dominating D. polymorpha in a large European shallow lake, where environmental conditions 552
favor the settlement of the new invader. The differences found in the speed of replacement 553
process between habitats in Europe and the Laurentian Great Lakes raise the necessity of 554
running a cross-system analysis involving many lakes that have dreissenid population data.
555
This would support the relevance of our hypothesis that the habitat previously occupied and 556
modified by D. polymorpha facilitates the conduction of rapid invasion by D. r. bugensis.
557
Detailed population analysis revealed that the success of D. r. bugensis is due to the 558
increasing number of large (> 20 mm) reproducing individuals and the consequently recruited 559
generations. The introduction of new substrata (e.g. setting piers, ship and boat stations) more 560
likely favors the progression of D. r. bugensis invasion, which in turn implies that the proper 561
selection of substratum type, or coating them with material inhibiting dreissenid attachment, 562
might contribute to reducing or delaying the propagation of D. r. bugensis in newly invaded 563
habitats. Nevertheless, it is necessary to study the reason for competition (ability to 564
predominate, evidence of the impact of environmental factors, such as food availability and 565
combined abiotic status) in the future, so as to make predictions about the invasion of 566
dreissenids into shallow lakes.
567 568
Acknowledgements 569
570
Authors are indebted to Mrs. Henrietta Szabó, Mrs. Tünde Klein-Polgárdi, Ms. Judit 571
Nédli and Ms. Szandra Purgel for their excellent technical assistance in the sample processing 572
24 and to Mr. Géza Dobos and Mr. Péter Harmati for their generous help in sampling. The text 573
was language edited by the Proof-Reading-Service.com Ltd, Letchworth Garden City, 574
Hertfordshire, UK. Study was financially supported by the TÁMOP-4.2.2.A-11/1/KONV- 575
2012-0038, the GINOP-2.3.2-15-2016-00019, the Balaton monitoring framework of the 576
Hungarian Academy of Sciences, and the bilateral project of MTA (NKM-38/2014). Long 577
term temperature dataset was obtained from LIFE08 ENV/IT/000339.
578 579
References 580
581
Ackerman, J.D., Smith, S.J., Nichols, S.J., Claudi, R., 1994. A review of the early life history 582
of zebra mussels (Dreissena polymorpha): comparisons with marine bivalves. Canadian 583
Journal of Zoology 72, 1169–1179.
584
Aldridge, D.C., Elliott, P., Moggridge, G.D., 2004. The recent and rapid spread of the zebra 585
mussel (Dreissena polymorpha) in Great Britain. Biological Conservation 119, 253- 586
261.
587
Aldridge, D.C., Samantha, H., Froufe, E., 2014. The Ponto-Caspian quagga mussel, 588
Dreissena rostriformis bugensis (Andrusov, 1897), invades Great Britain. Aquatic 589
Invasions 9, 529–535.
590
Baldwin, B.S., Mayer, M.S., Dayton, J., 2002. Comparative growth and feeding in zebra and 591
quagga mussels (Dreissena polymorpha and Dreissena bugensis): Implications for 592
North American lakes. Canadian Journal of Fisheries and Aquatic Sciences 59, 680–
593
694.
594
Balogh, C., G.-Tóth, L., 2009. A Balaton bevonatlakó gerinctelen állatvilágának vizsgálata a 595
2008. évben. In Bíró, P., J. A. Banczerowsky (eds), Balaton kutatásának 2008. évi 596
eredményei. MTA, Budapest: 45-53 pp (In Hungarian).
597
25 Balogh, C., Muskó, I.B., G.-Tóth, L., Nagy, L., 2008. Quantitative trends of zebra mussels in 598
Lake Balaton (Hungary) in 2003-2005 at different water levels. Hydrobiologia 613, 57- 599
69.
600
Barbiero, R.P., Tuchman,M.L., 2004. Long-term dreissenid impacts on water clarity in Lake 601
Erie. Journal of Great Lakes Research 30, 557–565.
602
Bij de Vaate, A., 1991. Distribution and aspects of population dynamics of the zebra mussel, 603
Dreissena polymorpha (Pallas, 1771), in the Lake Ijsselmeer area (The Netherlands).
604
Oecologia 86, 40-50.
605
Bij de Vaate, A., 2010. Some evidence for ballast water transport being the vector of the 606
quagga mussel (Dreissena rostriformis bugensis Andrusov 1897) introduction into 607
Western Europe and subsequent upstream dispersal in the river Rhine. Aquatic 608
Invasions 5, 207–209.
609
Bij de Vaate, A., van der Velde, G., Leuven, S. E.W.R., Heiler, K.C.M., 2013. Spread of the 610
Quagga Mussel (Dreissena rostriformis bugensis) in Western Europe In Nalepa, T. F., 611
D. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control. CRC 612
Press, 83-92 pp.
613
Carlton J.T., 2008. The zebra mussel Dreissena polymorpha found in North America in 1986 614
and 1987. Journal of Great Lakes Research 34, 770–773.
615
Carpenter, S.R., Kitchell, J.F., 1993. The Trophic Cascade in Lakes. Cambridge University 616
Press, New York.
617
Casper, A.F., Johnson, L.E., 2010. Contrasting shell/tissue characteristics of Dreissena 618
polymorpha and Dreissena bugensis in relation to environmental heterogeneity in the 619
St. Lawrence River. Journal of Great Lakes Research 36, 184–189.
620
26 Chase, M.E., Bailey R.C., 1999. The ecology of the zebra mussel (Dreissena polymorpha) in 621
the lower Great Lakes of North America: I. Population dynamics and growth. Journal of 622
Great Lakes Research 25, 107–121.
623
Cianfanelli, S., Lori, E., Bodon, M., 2010. Dreissena polymorpha: current status of 624
knowledge about the distribution in Italy. In: G. van der Velde, S. Rajagopal, A. Bij de 625
Vaate (Eds), The Zebra Mussel in Europe, Backhuys/Margraf Publishers, 93-100.
626
Claudi, R, Mackie, G.L., 1994. Practical manual for zebra mussel monitoring and control.
627
Lewis Publishers, CRC Press, 227 pp.
628
Claxton, W.T., Mackie, G.L., 1998. Seasonal and depth variation in gametogenesis and 629
spawning of Dreissena polymorpha and Dreissena bugensis in eastern Lake Erie.
630
Canadian Journal of Zoology 76, 2010–2019.
631
Collas, F.P.L., Karatayev A.Y., Burlakova L.E., Leuven R.S.E.W., 2016. Detachment rates of 632
dreissenid mussels after boat hull-mediated overland dispersal. Hydrobiologia, doi:
633
10.1007/s10750-016-3072-4.
634
Crooks, J.A., 1998. Habitat alteration and community-level effects of an exotic mussel, 635
Musculista senhousia. Marine Ecology Progress Series 162, 137–152.
636
Czarnołęski, M., Kozłowski, J., Kubajak, P., Lewandowski, K., Müller, T., Stańczykowska, 637
A., Surówka, K., 2006: Crosshabitat differences in crush resistance and growth pattern 638
of zebra mussels (Dreissena polymorpha): effects of calcium availability and predator 639
pressure. Archiv für Hydrobiologie 165, 191–208.
640
DAISIE, 2003. Delivering Alien Invasive Species In Europe, funded by the European 641
Commission under the Sixth Framework Programme, Contract Number: SSPI-CT- 642
2003-511202.
643
De Gelder, S., Van der Velde, G., Platvoet, D., Leung, N., Dorenbosch, M., Hendriks, 644
H.W.M., Leuven, R.S.E.W., 2016. Competition for shelter sites: Testing a possible 645
27 mechanism for gammarid species displacements, Basic and Applied Ecology 17, 455- 646
462.
647
Dermott, R., Dow, J., 2008. Changing benthic fauna of Lake Erie between 1993 and 1998. In 648
Checking the Pulse of Lake Erie. In Munawar, M., R. Heath (eds), Goodwords Books.
649
New Delhi, India: 409–438 pp.
650
Dick, J.T.A., Platvoet, D., 2000. Invading predatory crustacean Dikerogammarus villosus 651
eliminates both native and exotic species Proceedings of the Royal Society London B 652
267, 977-983.
653
Diggins, T.P., 2001. A seasonal comparison of suspended sediment filtration by quagga 654
(Dreissena bugensis) and zebra (D. polymorpha) mussels. Journal of Great Lakes 655
Research 27, 457–466.
656
Dzierzynska-Bialonczyk, A., Skrzypczak, A., Kobak, J., 2017. Happy together? Avoidance of 657
conspecifics by gregarious mussels. Current Zoology, doi: 10.1093/cz/zox022 658
Gallardo, B., Aldridge, D.C., 2015. Is Great Britain heading for a Ponto-Caspian Invasional 659
Meltdown? Journal of Applied Ecology 52, 41-49.
660
G.-Tóth, L., Parpala, L., Balogh, C., Tátrai, I., Baranyai, E., 2011. Zooplankton community 661
response to enhanced turbulence generated by water-level decrease in Lake Balaton, the 662
largest shallow lake in Central Europe. Limnology and Oceanography 56, 2211-2222.
663
Gutiérrez, J.L., Jones, C.G., Strayer, D.L., Iribarne, O.O., 2003. Mollusks as ecosystem 664
engineers: the role of shell production in aquatic habitats. Oikos 101, 79–90.
665
Heiler, K.C.M., Brandt, S., Albrecht, C., Hauffe, T., Wilke, T., 2012. A new approach for 666
dating introduction events of the quagga mussel (Dreissena rostriformis bugensis).
667
Biological Invasions 14, 1311–1316 668
28 Heiler, K.C.M., Bij de Vaate, A., Ekschmitt, K., von Oheimb, P.V., Albrecht, C., Wilke, T., 669
2013. Reconstruction of the early invasion history of the quagga mussel (Dreissena 670
rostriformis bugensis) in Western Europe. Aquatic Invasions 8, 53–57 671
Jarvis, P., Dow, J., Dermott, R., Bonnell, R., 2000. Zebra (Dreissena polymorpha) and quagga 672
mussel (Dreissena bugensis) distribution and density in Lake Erie 1992–1998. Canadian 673
Technical Report of Fisheries and Aquatic Sciences 2304, 1-46.
674
Karatayev, A.Y., Burlakova, L.E., Padilla, D.K., 1998. Physical factors that limit the 675
distribution and abundance of Dreissena polymorpha (Pall.). Journal of Shellfish 676
Research 17, 1219–1235.
677
Karatayev, A.Y., Burlakova, L.E., Padilla, D.K., 2010a. Dreissena polymorpha in Belarus:
678
history of spread, population biology, and ecosystem impacts. Chapter 9 in G. van der 679
Velde, S. Rajagopal and A. Bij de Vaate (eds), The Zebra Mussel in Europe. Backhuys 680
Publishers, Leiden/ Margraf Publishers, Weikersheim. p. 101-112.
681
Karatayev, A.Y., Mastitsky, S.E., Padilla, D.K., Burlakova, L.E., Hajduk, M.M., 2010b.
682
Differences in growth and survivorship of zebra and quagga mussels: size matters.
683
Hydrobiologia. 668, 183-194.
684
Karatayev, A.Y., Burlakova, L.E., Mastitsky, S.E., Padilla, D.K., Mills, E.L., 2011.
685
Contrasting rates of spread of two congeners, Dreissena polymorpha and Dreissena 686
rostriformis bugensis at different spatial scales. Journal of Shellfish Research 30, 923–
687
931.
688
Karatayev, V.A., Karatayev A.Y., Burlakova, D.K.P., 2013a. Lakewide dominance does not 689
predict the potential for spread of dreissenids. Journal of Great Lakes Research 39, 622–
690
629.
691
Karatayev, A.Y., Burlakova, L.E., Padilla, D.K., 2013b. General overview of zebra and 692
quagga mussels: what we know and do not know. In Nalepa, T. F., D. Schloesser (eds), 693
29 Quagga and Zebra Mussels: Biology, Impacts, and Control, 2nd Edition. CRC Press, 694
Boca Raton: FL, 695–703 pp.
695
Karatayev, A.Y., Burlakova, L.E., Pennuto, C., Ciborowski, J., Karatayev, V.A., Juette, P., 696
Clapsadl, M., 2014. Twenty five years of changes in Dreissena spp. populations in Lake 697
Erie. Journal of Great Lakes Research 40, 550-559.
698
Kirpichenko, M.Y., 1964. Phenology, population dynamics, and growth of Dreissena larvae 699
in the Kuibyshev Reservoir. Tr. Inst. Biol. Vnutr. Vod Akad. Nauk SSSR 7(10),15-24 700
(in Russian).
701
MacIsaac, H.J., 1996. Population structure of an introduced species (Dreissena polymorpha) 702
along a waveswept disturbance gradient. Oecologia 105, 484–492.
703
Madon, S.P., Schneider, D.W., Stoeckel, J.A., Sparks, R.E., 1998. Effects of inorganic 704
sediment and food concentrations on energetic processes of the zebra mussel, Dreissena 705
polymorpha: implication for growth in turbid rivers. Canadian Journal of Fisheries and 706
Aquatic Sciences 55, 401-413.
707
Majoros, G., 2009. Invazív kagylófajok terjeszkedése a Balatonban: esetismertetés és a 708
probléma felvetése. Halászatfejlesztés - Fisheries and Aquaculture Development 32,57- 709
64 (In Hungarian).
710
Marsden, J.E., 1992. Standard protocols for monitoring and sampling zebra mussels. Illinois 711
Natural History Survey Biological Notes 138, 1-40.
712
Matthews, J. , Van der Velde, G., Bij de Vaate, A., Collas, F.P.L., Koopman K.R., Leuven, 713
R.S.E.W., 2014. Rapid range expansion of the invasive quagga mussel in relation to 714
zebra mussel presence in The Netherlands and Western Europe. Biological Invasions 715
16, 23-42.
716
Mills, E.L., Dermott, R.M., Roseman, E.F., Dustin, D., Mellina, E., Conn, D.B., Spidle, A.P., 717
1993. Colonization, ecology, and population structure of the ‘‘quagga’’ mussel 718
30 (Bivalvia: Dreissenidae) in the lower Great Lakes. Canadian Journal of Fisheries and 719
Aquatic Sciences 50, 2305–2314.
720
Mills, E.L., Rosenberg, G., Spidle, A.P., Ludyanskiy, M., Pligin, Y. May, B., 1996. A review 721
of the biology and ecology of the quagga mussel (Dreissena bugensis), a second species 722
of freshwater Dreissenid introduced to North America. American Zoologist 36, 271–
723
286.
724
Molloy, D. P., Karatayev, A.Y., Burlakova, L.E., Kurandina, D.P., Laruelle, F., 1997.
725
Natural enemies of zebra mussels: predators, parasites, and ecological competitors.
726
Reviews in Fishery Science 5, 27–97.
727
Nalepa, T.F., Fanslow, D.L., Pothoven, S.A., 2010. Recent changes in density, biomass, 728
recruitment, size structure, and nutritional state of Dreissena populations in southern 729
Lake Michigan. Journal of Great Lakes Research 36, 5–19.
730
Orlova, M.I., 2013. Origin and Spread of Quagga Mussels (Dreissena rostriformis bugensis) 731
in Eastern Europe with Notes on Size Structure of Populations Pp. 93-102 In: Quagga 732
and zebra Mussels: Biology, Impacts, and Control (T.F. Nalepa, D. Schloesser, eds.), 733
CRC Press.
734
Orlova, M.I., Muirhead, J.R., Antonov, P.I., 2004. Range expansion of quagga mussels 735
Dreissena rostriformis bugensis in the Volga River and Caspian Sea basin. Aquatic 736
Ecology 38, 561–573.
737
Orlova, M.I., Therriault, T.W., Antonov, P.I., Shcherbina, G.K., 2005. Invasion ecology of 738
quagga mussels (Dreissena rostriformis bugensis): A review of evolutionary and 739
phylogenetic impacts. Aquatic Ecology 39, 401–418.
740
OTA, (Office of Technology Assessment), 1993. Harmful non-indigenous species in the 741
United States. Publication No. OTA-F-565, US Government Printing Office, 742
Washington D. C.
743
31 Patterson, M.W.R., Ciborowski, J.J.H., Barton, D.R., 2005. The distribution and abundance of 744
Dreissena species (Dreissenidae) in Lake Erie, 2002. Journal of Great Lakes Research 745
31,223-237.
746
Palau Ibars, A., Cia Abaurre, I., Casas Mulet, R., Rosico Ramón, E., 2010. Zebra mussel 747
distribution and habitat preference at the Ebro basin (north east Spain), Chap. 10. In 748
Van der Velde, G., S. Rajagopal, A. Bij de Vaate (eds), The Zebra Mussel in Europe.
749
Backhuys Publishers, Leiden/Margraf Publishers, Weikersheim: 113–118.
750
Pimentel, D., Zuniga, R., Morrison, D., 2005. Update on the environmental and economic 751
costs associated with alien-invasive species in the United States. Ecological Economics 752
52, 273-288.
753
Petrie, S.A., Knapton, R.W., 1999. Rapid increase and subsequent decline of zebra and 754
quagga mussels in Long Point Bay, Lake Erie: possible influence of waterfowl 755
predation. Journal of Great Lakes Research 25, 772–782.
756
Peyer, S.M., McCarthy, A.J., Lee, C. E., 2009. Zebra mussels anchor byssal threads faster and 757
tighter than quagga mussels in flow. Journal of Experimental Biology 212, 2027–2036.
758
Platvoet, D., Dick, J.T.A., MacNeil, C., van Riel, M.C., van der Velde, G., 2009. Invader- 759
invader interactions in relation to environmental heterogeneity leads to zonation of two 760
invasive amphipods, Dikerogammarus villosus (Sowinsky) and Gammarus tigrinus 761
Sexton: amphipod pilot species project (AMPIS) report 6. Biological Invasions 11, 762
2085–2093.
763
Pollux, B.J.A., Minchin, D., Van der Velde, G., Van Alen, T., Moon-Van der Staay, S., 764
Hackstein, J.H.P., 2003. Zebra mussels (Dreissena polymorpha) in Ireland, AFLP 765
fingerprinting and boat traffic both indicate an origin from Britain. Freshwater Biology 766
48, 1127-1139.
767