ALKALI METAL TWO-PHASE HEAT TRANSFER FOR SPACE POWER: PRESENT STATUS
* / R.D. Brooks and S.G. Sawochka
General Electric Company, Cincinnati, Ohio Abstract
The selection of alkali metal working fluids for Rankine cycle space power systems has necessitated investigation of two-phase heat transfer and fluid flow characteristics. The theory and available experimental data related to boiling and condensing are reviewed. Nucleate boiling data, obtained in flat plate pool boiling experiments for potassium, are com- pared to several existing relationships. Forced convection boiling data in tubes have been observed * Heat fluxes up to 600,000 Btu/hr-ft have been achieved in stable, nucleate boiling. Data for condensation of potassium inside a horiz- ontal tube are also presented. Flow stability for two-phase flow is discussed briefly. Analogy methods for analysis using water may be useful in liquid metal systems. Typical instability observations for a boiling liquid metal loop are presented, and the method of correction is indicated.
Presented at the ARS Space Power Conference, Santa Monica, Calif., September 25-28, 1962. The authors are indebted to C.F. Bonilla of Columbia University for his helpful efforts in pool boiling and the interpretation of condensing results.
R.A. Fuller and W.R. Lloyd have made very important experi- mental and analytical contributions to this work. The
authors are grateful for the advice and support of J.W.
Semmel Jr. on those doubtful areas of metallurgical theory and practice for the L-605-potassium systems. This work has been supported by NASA under Contract 5-681; the assistance and guidance of S. Weiss and others of the NASA-Lewis
Research Center are acknowledged.
* R.D. Brooks, Manager, Heat Transfer Project, Space Power and Propulsion Section, Re-Entry Systems Department, Missile and Space Division*
/ S . G . Sawochka, Heat Transfer Project Engineer, Space Power and Propulsion Section, Re-Entry Systems Department, Missile and Space Division.
Introduction
A number of programs sponsored by different agencies now are underway to obtain data for a variety of alkali metals.
At this time, relatively few results have been reported. In the investigation at General Electric for NASA, experimental work is being conducted for the boiling and condensing of sodium and potassium. These data are to provide a basis for the practical design of heat transfer equipment used in high temperature space power applications. To explore the major regions of interest, four separate tests are utilized. Three forced convection facilities with test section heat input capabilities from 50 to 300-kw. Forced convection boiling is under study in a 300-kw facility. Measurements to a 1600 F saturation temperature with potassium have been made. Some condensing measurements also have been obtained in this test facility. A 100-kw test facility will obtain data for boiling of sodium in a Cb-lZr alloy system at temperatures to 2000 F.
A 50-kw test facility has been established to perform conden- sing tests with potassium in small diameter tubes. Infor- mation has been obtained on heat transfer characteristics, pressure drop, and flow stability from these facilities.
Research on pool boiling, under the direction of C.F. Bonilla, is being sponsored at Columbia University.
Alkali Metal Boiling
In many respects the boiling of alkali metals may be expected to behave similarly to more conventional fluids for which some understanding exists. In approaching the appli- cation to space power systems, it is interesting to consider the conditions of a once-through process, since it has
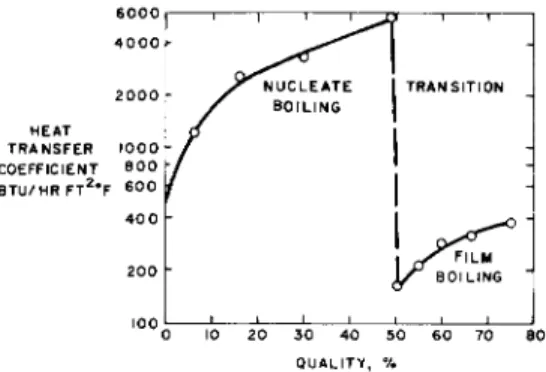
inherent simplicity that is attractive. Also, in an experi- mental investigation, it presents the opportunity to study the limits of nucleate boiling and hence defines the prob- able area of interest for recirculating designs. A typical relation , shown in Fig. 1, between heat transfer coefficient and quality has been suggested from studies utilizing water.
It is expected that the low liquid inlet velocities in the subcooled region will produce low convection heat transfer coefficients. At some point within this region nucleate boiling will begin, and the heat transfer coefficients are expected to be very high. As the region of liquid deficiency is reached, convective film boiling occurs, and the heat tran- sfer coefficients deteriorate to very low values.
Nucleate Boiling: Correlations
Analytical expressions derived by Forster and Zuber for 2 bubble radii and growth rates were applied in an analysis of nucleate boiling at high heat transfer rates. The authors show that the product of bubble radius and radial velocity is a constant, independent of bubble radius. This permitted the formulation of a Reynolds number for the flow in the thin, super-heated liquid layer adjacent to the heating surface.
The result of the analysis then was applied to the critical heat flux in pool boiling, and the following expression was derived:
A t w
where B = \ *
A/"
The Forster-Zuber correlation later was tested by Westwater on methanol over a wide range of Zit and by Camack on BonillaTs mercury data. In both tests, the correlation gave a good prediction of the experimental data. Combining like terms and making the appropriate substitutions, these equa- tions may be rewritten in the following final form:
q/A = K l A t 1.25 л р 0.75 1 w
0.458 /7T 0.5 ,_ 0.792 where K_ =0.1715 ~p
c °-
4 5 8A °-
5k
r> s
1 ) 0.25 ff 0.5 yO 0.25 ^ . 2 9 2
All liquid properties should be evaluated at the temperatures of the heating surface. Since, however, ò±t is generally small, K need be evaluated only once at each saturation temperature without introducing an appreciable error.
In a 1959 paper by Forster and Greif , which analyzes the 5 various proposed mechanisms of nucleate boiling, evidence is
presented in the form of a vapor-liquid exchange mechanism as opposed to microconvection and latent heat transport. The vapor-liquid exchange mechanism is shown to explain the
insensitivity of boiling heat flux to the level of subcooling.
A Reynolds analogy for nucleate boiling is presented in some detail. The authors derive an expression for the growth of a bubble in a highly super-heated liquid and apply this expres- sion in the deduction of two nucleate boiling correlations.
Because it has one coefficient for all liquid-surface com- binations, the second correlation is more useful than the first. The expression is
q/A = K ^ t Л Р 1 A
1 w -4 0 4 0 4
where K = 4 x 10 k C (y^LyPv) * t
' u л-> °- 8
Nucleate Boiling: Pool Boiling Results
Information on pool boiling is of interest for its value in the interpretation and correlation of forced convection nucleate boiling. In addition, investigation of particular aspects such as surface condition, wetting, additives, and pressure effects may be carried on readily in simple experi- ments .
The first set of runs has been carried out for pool boiling of potassium from a nickel plate. The equipment and pro- cedure are similar to those employed by N. Madsen in the study of the boiling of NaK. The present work has been con- ducted by C.F. Bonilla and M.M. Wiener. The maximum heat flux obtained was 106,800 Btu/hr/ft at 1532°F, the maximum saturation temperature investigated. The results are shown in Fig. 2. After evacuation of the boiling chamber, some runs were obtained at low pressure with no cover gas present.
Potassium pressure was obtained from the vapor-phase thermo- couples assuming that saturation temperature was being measured. In all the other runs, argon was present in the top of the boiling chamber to facilitate rapid equilibration of the system during operation. No measurable effect on the data was noticed with argon present. The pressure of argon was measured by mercury manometers to establish the boiling pressure. The difference between the low pressure and high pressure runs can be seen in Fig. 2.
The three nucleate boiling theories just discussed were computed for potassium saturation temperatures of 1400 F and are compared to the data in Fig. 2. Considering that the theories were developed for nonmetals, the agreement is good for the higher pressure data. Apparently any of these cor- relations would be satisfactory for approximating heat trans- fer coefficient or A t values in nucleate boiling at the higher pressures until more extensive data are available.
The comparison at low pressure is not, however, nearly as satisfactory. This can be explained by the fact that at these low pressures the large liquid to gas density ratio of potassium, i.e., 3.2 (10 ) at 700 F, no doubt leads to a sizeable portion of the heat transfer area being covered with
gas rather than liquid. This type phenomena would lead to a larger value of A t for a specific heat flux than that pre- dicted by theory, since gas coverage would serve to reduce drastically the heat transferred in any partially gas blan- keted region.
Forced Convection Results
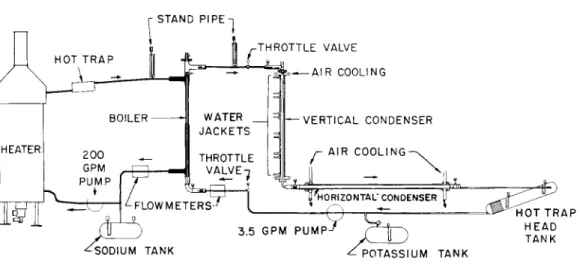
The 300-kw test facility used for this experiment is shown in Fig. 3. The primary loop pumps liquid sodium at tempera- tures to 1850 F and flow rates of 200 g/min. The gas fired heater has an output of more than 300 kw. The secondary loop contains the components for the investigation of potassium two-phase heat transfer characteristics. Temperature measure- ment for data purposes is accomplished with Pt-Pt 10 Rh alloy thermocouples. Pressure drop measurements were made at the boiling tube with diaphragm-type pressure gages. A head tank provides control of inventory to obtain stable operation with- in the secondary loop. In single-phase liquid operation, a flow of 30 g/min can be achieved, and in two-phase flow, 3.5 g/min with 100 psi pressure rise. A liquid-flow control valve maintains flow and pressure relationships at the
boiling test section entrance. An electromagnetic flowmeter measures the potassium flow rate.
The boiling test section is shown in Fig. 4. The hot sodium enters at the top right through the inlet. This fluid flows down through the annulus giving up heat to the center tube and exits at the bottom. Temperature measuring stations are located to give mixed-mean bulk temperatures of both fluids at the inlets and outlets. Outer wall temperatures are measured along the length of the test section. The potas- sium enters the test section at the bottom left, labeled
"potassium inlet" and flows up through the center tube taking heat from the sodium. The vapor is discharged through the outlet at the top left. Pressure measurements are made for the boiling fluid in the inlet plenum and at the exit of the tube. A bellows is provided to accommodate differential thermal expansions between the inner and outer tube. The heat transfer tube is constructed of Mo-0.5 Ti alloy selected for its high thermal conductivity which minimizes the tem- perature gradient in the wall.
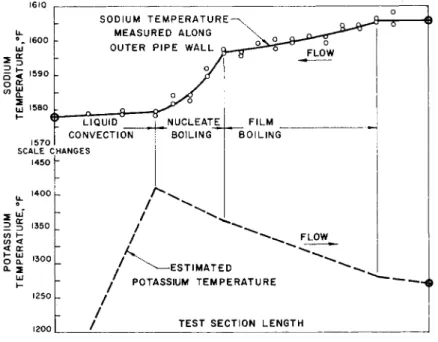
The wall temperature measurements and the potassium tem- peratures for a single test are shown in Fig. 5. This figure illustrates the liquid convection, nucleate boiling, and film-boiling regions for about 74.5% exit quality. The potassium temperature distribution is determined from the mixed mean inlet and outlet values.
Using the slope of the temperature curves, the heat flux as a function of length is determined. The temperature differ- ence between the wall and the boiling fluid then is obtained from the total temperature difference between the two streams using Lyon's heat transfer relation for sodium and a cal- culated resistance for the wall. In the film boiling region, the heat transfer resistance of the boiling film is an order of magnitude higher than that for the liquid and the wall.
In nucleate boiling, all heat transfer resistances are of the same order of magnitude. From these temperature measurements, the relationship between heat flux and T .,., - T . shown in
wall sat Fig. 6, is obtained. For the nucleate boiling values a com- parison with the work of Braunlich is shown. The potassium data of Hoffman and Krakoviak also are plotted and give good correlation using substantially different experimental techniques. The agreement of the liquid metal data obtained by different investigators is remarkable and should provide a satisfactory basis for preliminary design purposes where potassium heat transfer coefficients are required.
The relation between heat transfer coefficient and quality along the length of the tube for a sample run is shown in Fig. 7. The subcooled portion of the curve is not shown, but the similarity to the regions shown in Fig. 1 confirms the similarity to water in behavior. Two regions of heat trans- fer with the transition occurring at about 50% quality are shown in Fig. 7. The location of this transition is expected to vary with pressure and with mass flow rate. This point of transition required extensive investigation for the design of space power systems.
Alkali Metal Condensation
Numerous theories have been proposed for the correlation of condensing heat transfer results; the vast majority of these, however, are applicable only to vertical test sections or, at most, to ones that are inclined to the horizontal axis.
Although no data are available for direct comparison to these theories, a discussion of the main points of several of the proposed theories is presented. Correlation of low Prandtl number condensing data for vertical test sections would definitely tend to confirm the various analytical approaches that have been taken.
The characteristics of alkali metals can be supposed to result in film type condensations because of their excellent wetting of metal surfaces. In early work by Nusselt , the
film was assumed to be laminar and the local heat transfer
coefficient for vertical flow was given by
In this relation, it is assumed that the liquid film has the thickness of a steady film on a vertical flat plate and the heat is transferred by conduction without resistance between the liquid and the wall.
In a later work, Seban applied the Prandtl-Karman analogy 12 for conditions of a turbulent condensate layer also in
vertical flow. In this analysis an extension was made to low Prandtl number fluids such as alkali metals. It was noted that in determining the heat transfer coefficients for the low Prandtl case, the assumption was made that no vapor friction occurred at the vapor-liquid interface. SebanTs results are expressed in terms of the average heat transfer coefficient related to the Reynolds number of the condensate layer.
Rohsenow et al. presented an analysis of the effect of 13 vapor shear stress at the liquid-vapor interface on the aver- age heat transfer coefficient. Both laminar and turbulent films were considered; these were combined for a vertical plate with laminar flow at the top, followed by turbulent flow.
A recent paper by Dukler develops equations for velocity 14 and temperature distributions for falling films. In this instance, the Deissler relation is used for eddy viscosity and eddy conductivity near the wall. The results, presented by Dukler, apparently differ markedly from those obtained in other analytical investigations of this type. The predomin- ant reason for the deviation is the fact that Dukler does not allow for the buildup of a laminar film with a transition to a turbulent film occurring at some point down the tube, but rather allows a turbulent film to develop immediately. For this reason, the dimensionless values of the condensing ratio f 2 \ 1/3 are much lower than those obtained by
k U /
k
other investigators especially at low film Reynolds numbers, Previous alkali metal condensing data was reported for sodium by Misra and Bonilla . The values were low with respect to NusseltTs equation, and a derivation of kinetic theory limitations was made. The experiment was conducted with condensation on the outside of an inclined tube that
was surrounded by vapor generated., by a pool boiler. Results with mercury were shown by Dukler to occur in the region expected by his theory. Later work by Engelbrecht was conducted in a similar apparatus for potassium and rubidium on the outside of 0.5-in. and 1.75-in. diameter tubes. The results of this work were similar to Misra's, i.e., having very low values. Because of the difference between the mechanism for condensation on the outside of tubes in a semi-
infinite vapor environment and the flow inside tubes, no com- parison is presented here.
The test section used in obtaining data in this program is shown in Fig. 8. The two-phase mixture enters through the nozzle labeled condensate inlet and flows through the center tube. Cooling is provided by air flow through the annulus surrounding this tube. This unit has been used to obtain the results reported in horizontal orientation. Local heat tran- sfer coefficients were obtained at several stations along the length using wall thermocouples and thermocouples in the air stream. Inlet and outlet mixed mean temperatures of each stream and the flow rates were obtained.
The fluid mechanics of condensing potassium is not well known, but in the present experiment, wet vapor of undeter- mined liquid-vapor distribution enters a horizontal tube, where condensation occurs. Part of the liquid enters ent- rained in the vapor and presents homogeneous flow, while part of the liquid is on the wall and may not be distributed uniformly throughout the cross section.
In the design of condensors for space power systems, an empirical relation., based upon the local coefficients shown in Fig. 9 is recommended. The equation of this relation is
h / 2 11/3 „ „,„ лшт -0.438 x /v_] = 0.412 4_Г
k U /
Mwhere all quantities are liquid phase values:
h k X V
g
r
JUL />
4r/jbL
=
=
=
=
=
= -
=
Btu/hr- Btu/ft-
2 o -ft - F o -hr- F
*4i/3 kinematic ft/hr2
viscosity
lb mass/tube circumference lbm/ft--hr
lbm/ft3
local Reynolds number
ft
No attempt to determine the effect of shear stress for the various test conditions has been made. From pressure drop data, it may be possible to obtain an improved correlation.
Flow Stability
Since it raises problems for the experimentalist and must be understood by the system designer, flow stability is important in two-phase heat transfer. The nature of the boiling process can be considered a sequence in which heat is transferred alternately to liquid or to vapor. The resulting heat and mass transport causes a flow response that is
usually oscillatory within the boiling region that may be of sufficient magnitude to effect unstable behavior in a closed loop system. This subject has received much attention in connection with boiling water reactors for power generation purposes. A review of this subject is presented by
Anderson and Lottes
In space power systems and in test loops for development purposes, the problem is less complex because there is no nuclear interaction. An early recognition of the importance of flow stability on boiling frgat transfer results was reported by Lowdermilk et al. in connection with water burnout studies. A useful analog simulation of transient behavior for natural circulation systems, which successfully predicts oscillation of flow for unstable conditions, was proposed by Anderson et al. This method of analysis could be applied to a forced circulation system readily. The loop is divided into the necessary number of sensitive "lumps", and the equations for mass, energy, and momentum are estab- lished for each part. Successful utilization requires a knowledge of vapor-liquid slip ratio and the two-phase pres- sure drop characteristics for the loop under study. Using such an analog for alkali metal systems may be helpful in avoiding loop design problems and in analyzing some modes of instability which may occur. Caution must be used in apply- ing high pressure water instability results to liquid metals because of great differences in physical properties; e.g., the ratio of liquid to vapor density for potassium at 40 psia is 600, for water at 1000 psia, about 20. Also the ratio of C /H for potassium liquid is an order of magnitude less tnan That for water. The saturation temperature of potas- sium changes 66 F going from 40 to 30 psia when that of water at 1000 psia changes only 1.2 F for a similar 10 psi
A P. This suggests possible flashing in a potassium system which is not expected in a high-pressure water system. This difference cannot be overlooked in analog studies of liquid metals.
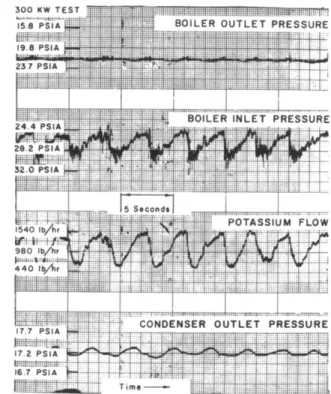
There are two important types of instability which have been identified in the present work. One is actually a steady-state of "equilibrium" condition in which flow, pres- sure, or temperature as variations at one or more locations will pulsate in a continuous way. A typical case is shown in Fig. 10 which represents an operating condition of the loop shown in Fig. 3. This condition is created by "over filling" the loop. In this condition an excess volume of liquid which is present is distributed among the components by the loop hydrodynamics, resulting in a percolation in the boiler. The variations of flow and boiler inlet pressure are the result of the variation in static head in the ver- tical column. A 4-sec transport time seems required for a liquid in slug flow to reach the top of the boiler. The pulsations of the condenser outlet pressure are thought to result from carryover of the liquid by the vapor. The magnitude of the variation of pressure at the boiler outlet
is questionable because of the damping effects of vapor con- tained in the pressure measuring lines. However, it is not likely that a variation nearly so large as that for inlet pressure would occur. An analysis as described by Levy and Beckjord is expected to predict the behavior of the loop under unstable conditions. To obtain reliable heat transfer data at stable operating conditions, it was necessary to reduce the liquid inventory by 20 - 25% from that used at the unstable conditions. The resulting values of the same para- meters are shown in Fig. 11.
A second type of instability is "drifting" equilibrium where there is no rapid oscillation but rather a given set of control points is not satisfied by the operation specified.
From the analog model of Anderson, a map of those areas of stable operation may be defined. In general, the use of a valve capable of large liquid pressure drop at the entrance to the boiler coupled with low two-phase pressure loss design should provide stable operating conditions. For the results reported in this paper, a criterion of - 1.0 F for a 1-hr period was used for the sodium and potassium inlet tempera- ture in defining steady-state acceptability.
References
Levy, S., Polomik, E#E# >and Sawochka, S.G#, "Boiling of Steam-Water Mixtures in Annular Flow at 800, 1100, and 1400 psi," Am. Soc. Mech. Engrs. Preprint 62-WA-136 (1962).
2 t, Forster, H.K# and Zuber, N., Dynamics of Vapor Bubbles and Boiling Heat Transfer, " Am. Inst. Chem. Engrs. J. _1, 531- 535 (1955).
3 „ Perkins, A.S. and Westwater, J.W. , Measurements of Bubbles
Formed in Boiling Methanol,M Am. Inst. Chem. Engrs. J. 2, 471-476 (1956).
4 t,
Camack, W.G., A Comparison of Forster and ZuberTs Theory of Boiling Heat Transfer with the Experimental Data on Pool Boiling of Mercury by Bonilla et al.,ff Research Memo, RM 62-20-10, Lockheed Aircraft Corporation (1956).
5 rt Forster, H.K. and Greif, R., Heat Transfer to a Boiling Liquid: Mechanism and Correlations," J. Heat Transfer 81, 43-53 (1959).
6 tr Chang, Y . P . and Snyder, N#W . , Heat Transfer in Saturated Boiling, TT Chem. Eng. Progr. Sym. 5^3, Ser. 30, 25-38 (1960).
7
Madsen, N. and Bonilla, C.F., "Heat Transfer to Sodium- Potassium Alloy in Pool Boiling," Chem. Eng. Progr. Sym. 56, Ser. 30, 251-260 (1960).
Lyon, R.N., "Liquid Metal Heat Transfer Coefficients,"
Chem. Eng. Progr. 47, 75-79 (1951).
9
Braunlich, R.H., Pool Boiling of Liquids at Reduced Pressures," Thesis in Chem. Eng., Mass. Inst. Tech. (1941).
10 TT
Hoffman, H.W. and Krakoviak, A.I., 2nd Annual High Tem- perature Tech. Meeting," Brookhaven Natl. Lab. (May 17-18, 1962).
Nusselt, W., Z. Ver. Deut. Ing. 60, 541 (1916).
12 ,,
Seban, R.A., Remarks on Film Condensation with Turbulent Flow," Trans. Am. Soc. Mech. Engrs. 7<в, 299-304 (1954).
13 tt Rohsenow, W.M., Webber, J.H#Jand Ling, A.T., Effect of
Vapor Velocity on Laminar and Turbulent-Film Condensation,"
Trans. Am. Soc. Mech. Engrs. 78, 1637-1643 (1956).
Dukler, A.E., "Fluid Mechanics and Heat Transfer in Vertical Falling-Film Systems," Chem. Eng. Progr. Sym. 56, Ser. 30, 1-10 (1960).
15 ,r
Deissler, R.G., Analysis of Turbulent Heat Transfer, Mass Transfer, and Friction in Smooth Tubes at High Prandtl and Schmidt Numbers," NACA TN 3145 (May 1954).
Misra, B. and Bonilla, C.F., "Heat Transfer in the Conden- sation of Metal Vapors: Mercury and Sodium up to Atmospheric Pressure," Chem. Eng. Progr. Sym. Í52, Ser. 18, 1-21 (1956).
17
Engelbrecht, J.C., Thesis in Chem. Eng., Columbia Univ.
(1961).
18
Anderson, R.P. and Lottes, P.A., "Boiling Stability,"
Progress in Nuclear Energy (Pergamon Press, London, 1961), Vol. 4, Chap. 1.
Lowdermilk, W.H., Lanzo, C.D. and Siegel, B.L., "investi- gation of Boiling Burnout and Flow Stability for Water Flowing in Tubes," NACA TN 4382 (September 1958).
20
Anderson, R.P«, Bryant, L.T., Carter, J.Ce)and
Marchaterre, J.F., "An Analog Simulation of the Transient Behavior of Two-Phase Natural Circulation Systems," Am. Inst.
Chem. Engrs. Preprint 27-A (August 1962).
21 ,, Levy, S. and Beckjord, E.S., Hydraulic Instability in a Natural Circulation Loop with Net Steam Generation at 1000 psia," Rept. AP 3215, General Electric Company (July 1959).
CONVECTION CONVECTIVE
BOILING CONVECTIVE FILM BOILING
NUCLEATE BOILING
BOILING LIQUID STARTS DEFICIENCY
MEDIUM HEAT FLUX
DRY VAPOR
SUB-COOLED1
LIQUID VAPOR-LIQUID SUPERHEAT
Fig. 1 Generalized relation for once-through boiling inside a tube
6 x io=
3 x io5 h
10=
6 X I 04h
4 X I04 3 X I 04
I 04
l
r-
- -
_
>
7/
/ / /- )
/
/ / —
<1P
! VI
! I " 7 ■
CHANG-SNYDER6 FORSTER-ZUBER2 - FORSTER-GREIF5 COMPARISON
AT 1400 °
/ / / / - /
M •' //
щ / / /
/ a ' / /
Л ! /
t///
PI/ \
i i i i / a
1 1 F
COMPARISON AT 700 °F
/
/ -
4°/
/
/ PRESSURE -Jo 770-1500 mm Hg
(ARGON OPERATION) -\
□ 2-10 mm Hg
(VACUUM OPERATION)
I I 1
10 2 0 4 0 70 100
T S A T . ° F
2 0 0 4 0 0
Fig. 2 Pool boiling results with potassium from the flat plate apparatus at Columbia University
HOT TRAP
r STAND PIPE-j
n I j- I MKUI I L THROTTLE VALVE
CO
HEATER
BOILER-
200 +_
GPM PUMP
ш ^ fc
WATER JACKETS THROTTLE
VALVE -j
FLOW METERS
f^i
AIR COOLING
h ~ VERTICAL CONDENSER AIR C O O L I N G - x
•A.
3.5 G
TJ^HORIZONTAL" CONDENSER
— 9 c:
PM P U M P ^ г п Л
SODIUM TANK POTASSIUM TANK
HOT TRAP HEAD TANK
Fig. 3 Flow sheet showing principle components and arrange- ment of the 300-kw test loop
TO O O 7s
>
z
oo
>
O n X
>
S0DIU4 A o INLET4 {* °
4 , » i I POTASSIUM
j ' OUTLET^
POTASSIUM
BOILER TEST SECTION
F i g . 4 T h e 3 0 0 - k w t e s t l o o p b o i l e r t e s t s e c t i o n
1610
5 < , 5 9 0 h o °£
Ш 1580
SODIUM TEMPERATURE- MEASURED ALONG OUTER PIPE WALL
1570 I SCALE CHANGES
CONVECTION
1400 3 Ш * rr
^ 3 1350 сл <
< oc S Ï '300
ш
FILM BOILING
FLOW
ESTIMATED
POTASSIUM TEMPERATURE
TEST SECTION LENGTH
Fig. 5 300-kw boiling test section temperature measurements for a high-quality (approximately 74.5%) run
, , + GENERAL ELECTRIC .929 INCH I.D. TUBE I □ OAK RIDGE NATIONAL LAB. 325 INCH I.D. TUBE
■ OAK RIDGE NATIONAL LAB. .87 INCH I.D. TUBE10 - BRAUNLICH9
30 50 100 300 500
TWALL * TSAT, ° F
Fig. 6 Forced convection boiling of potassium correlating heat flux with T wall sat
HEAT TRANSFER COEFFICIENT BTU/HR F T2'
2 0 0 0
1000 8 0 0 F 6 0 0
30 40 50 60 70 QUALITY, %
o
Fig. 7 Correlation of the boiling test section preliminary data relating heat transfer coefficient and quality along the tube length
CO CD - 3
CO -<
CO -H rn
CO
-mERMOWELL >
n O
IE
Fig. 8 The vertical condensing test section for the 300-kw
JÍ5
o
o EXPERIMENTAL DATA, Pr=.0033-35
— - EMPERICAL RELATION, ■£*" ( - J - ) " ^ ° '4 1 2П П 0.438
LIQUID FILM REYNOLDS NUMBER 4Г
Fig. 9 Local condensing heat transfer coefficients for potassium vapor
300 KW TEST
BOILER OUTLET PRESSURE
Fig. 10 Two-phase flow instability in 300-kw test obtained by introducing excess inventory of liquid phase potassium
3 0 0 KW TEST ш*у. Г::;|;;::г
15.8 PSIA
Fig. 11 Stable operating conditions of 300-kw test resulting from proper inventory control of liquid phase
potassium