AUTOGENOUS PRESSURIZATION SYSTEM ANALYSIS OF PROPELLANT TANK PRESSURIZATION
T. F. Morey and M. M. Koshar The Martin Company
Denver Division Denver, Colorado
Abstract
An analytical model for propellant tank pressurization i s presented. I t permits the prediction of the time curves for tank gas pressure, temperature, weight, volume, and other pertinent parameters in a propellant tank during missile operation, when the propellant may be volatile and i t s vapors may dissociate.
I n i t i a l pressurization i s obtained from an inert gas plus the propellant vapor pressure. Additional pressurization during propellant outflow may be obtained by the inflow of additional inert gas, or a self-generated (autogenous) gas, or both. Heat transfer i s considered between the gas and liquid phases, between the gas phase and the tank wall, and between the tank wall and the atmosphere. External heat transfer i s considered for a stationary missile or for a missile in f l i g h t . Mass transfer i s considered for surface condensation or evaporation at the gas-liquid interface, for bulk boiling within the liquid phase, and for cloud conden
sation within the gas phase.
Introduction
During the operation of a liquid propellant rocket, the tank top pressure in each propellant tank must be main
tained between the minimum pressure curve set by minimum pump and structural requirements and the maximum pressure curve set by maximum structural requirements. These pressure requirements are met by prefiring pressurization of the i n i t i a l gas or ullage space alone or together with the inflow of
additional pressurization gas during propellant outflow.
The i n i t i a l pressurization i s usually obtained by some inert gas such as helium or nitrogen in addition to the propellant vapor pressure. Additional pressurization during rocket operation may be by additional inert gas or by some
85
self-generated or autogenous gas such as propellant vapors or combustion products.
To estimate the desired i n i t i a l gas volume and pressure and the feed gas flow rate and conditions, and to predict the resulting pressure curve, some type of mathematical model i s required. The complexity of this model depends on the type or types of pressurization systems to be analyzed and the number of factors to be considered.
To indicate what can be accomplished with this type of analysis, the mathematical model for an autogenous pressuri
zation system i s presented. For the particular system considered, the tank i s i n i t i a l l y pressurized with an inert gas, the propellant i s volatile, vaporized propellant i s used as the added pressurant, and the vapors are dissociating.
For the simplest case of a" nonvolatile propellant pressurized with a single inert gas, the fraction dissociated and the propellant vapor pressure are set equal to zero, and the added pressurant has the same properties as the i n i t i a l pressurant.
Tank Gas Thermodynamics
At the relatively low tank pressures used with pump fed propeliants, use of the ideal gas law i s generally valid, although a compressibility factor can be readily introduced i f desired. Therefore, the tank pressure at any time may be expressed as a simple function of the total tank gas weight, temperature, volume, and average molecular weight, and the universal gas constant. (See Figure 1 for tank schematic.)
P4.tg tg tg o' tg tg = W. T, R / V M, (1) The values for these parameters at the end of any time
increment (n) are also accurately represented for small time increments ( Δ t ) by simple rate equations. The average molecular weight at any time may also be expressed in terms of total gas weight and moles of "autogenous" gas and inert
"residual" gas.
Wt (n) = Wt (n-1) + Wt (n) A t (2) Tt g( n ) = Tt g (n-1) + Tt g( n ) A t (3) Vt g( n ) = Vt g (n-1) + Vt g( n ) A t (4) Mt g( n ) = Mt (n-1) + Mt g( n ) A t (5)
Mt g( n ) - Wt g ( n ) / ( Wr g/ Mr g + ^ & g ) (6) In the above equations, W i s obtained from the flow
rate of autogenous gas entering 'fhe tank plus the mass transfer between liquid and gas phases. i s obtained from the
liquid volumetric outflow rate plu§ the volume rate of liquid lost through boiloff. The molecular weight change (M^ ) i s the result of the change in proportions of the residua? gas and the autogenous gas, plus the change in average molecular weight of the autogenous gas due to dissociation.
W. = W + Wtg age agv (7)
Mt g - 0 ^ t) [ wt g/ ( Wr g/ Mr g + Wa g/ Ma g) ]
= W. M.tg tg' tg tg tg ag tg /W. - W. M. 2/ M W, +
W M.ag tg 2
ag
M /M ag tg 2 W+ ( 9 ) The value for i s obtained from an energy balance on the total pressurizing gas in the tank ullage space. The energy terms involved in this balance are related to a number of possible factors.a. Pressurant entering enthalpy and flow rate b. Heat transfer between tank gas and tank wall c. Heat transfer between tank gas and tank liquid d. Heat transferred to tank gas by cloud condensation e. Mass transfer between gas and liquid phases
f. Expulsion work on the propellant
g. Change in internal energy of the total gas phase
H = U + PV/J (10) H = Û + PV/J ( i f Ρ i s assumed relatively constant) (11)
Q + Q + Q + h W + h W = W u +
^gs χ gw age age agv agv rg rg
W u + u W + u W +ag ag ag ag rg rg tg tg' P. V. /J (12) With an inert gas the enthalpy can be represented by
CpT, internal energy by C^T and internal energy change by CyT, because C„ and Çp can be considered constant. For a dissociating gas, CyT can be used for changes in internal
energy over small increments, but enthalpy and internal energy must be calculated by more complex means that consider the dissociation. The vapor enthalpy i s computed as a function of temperature and fraction dissociated ( 1 ) , and the internal energy i s expressed in terms of the enthalpy. The energy balance can then be rearranged to obtain an expression for T.
Q + Q + Q + h W + h W = T, (W C,^gs χ ^gw age age agv agv tg rg Vrg r +
W C„ ) + (W + W )(h - R Τ. /Μ ) + Ρ, V, /Jag Vag age agv ag ο tg' ag tg tg' (13) T. = Q + Q + Q + h W + h W - W (h
tg L 6S c Sw aSe aSe agy aEv ag a£
V t A g ' -
pt
sV
J]
/ wtg
cvt
gThe solutions to the above equations for A and Î require a knowledge of the thermodynamic properties of the gases con
cerned, the heat transfer rates, mass transfer rate, and
inflow and outflow rates. Properties of the inert pressurizing gases are readily available as constants or simple curves while the propellant vapor properties including molecular weight must be calculated for each set of conditions ( 1 ) ·
The i n i t i a l conditions in the propellant tank are also required before the above equations can be solved. For the case considered, the i n i t i a l autogenous gas pressure i s the propellant vapor pressure, and the residual or inert gas pressure i s the difference between total pressure and vapor pressure. The i n i t i a l weights of these gases are obtained from their i n i t i a l pressures and molecular weights and the i n i t i a l gas temperature and volume.
Ρ = Ρag ν (15)
Ρ = Ρ,. - Ρrg tg ag (16)
W = Ρ V, M /R Τ,rg rg tg rg ο tg (17) W = Ρ V,. M /R T.ag ag tg ag' ο tg (18) For pressurization with additional inert or residual
gas, the mass of this gas becomes a variable, the rate of change in tank gas weight includes the inert gas inflow rate, and the temperature rate change equation includes the inert gas inflow rate and temperature. I f this flow rate i s con
trolled by a pressure regulator, which is often required for 8 9
certain static firings, the inflow rate i s varied by an i t e r a tion process at each computation point, until the tank pressure f a l l s within the regulated pressure band. A maximum allowable flow rate can also be used to simulate maximum flow operation of a regulator when making large adjustments in pressure.
Heat and Mass Transfer
Convective heat transfer may occur at the liquid surface and at the tank wall. The heat transfer rates are complicated by a large number of variable factors. To arrive at a reason
able solution to the effects of heat transfer without unduly complicating the analysis, the following simplifying assump
tions were made.
a. Temperature of tank gas i s uniform.
b. Composition of tank gas i s uniform.
c. Temperature of tank metal exposed to gas i s uniform.
d. Temperature of tank metal below liquid level, and of metal as i t i s exposed, i s equal to liquid temperature.
It is believed that while these assumptions are not strictly true, they w i l l not introduce appreciable errors, because the temperature differences between gas and wall are relatively uniform and the heat transfer rates contribute only a fraction of the gas temperature change.
The heat transfer rates between the gas phase and the tank wall and between the gas phase and the liquid surface are obtained from the gas and metal or liquid surface tempera
tures, the area exposed to the gas, and suitable heat transfer coefficients. The heat transfer between the gas phase and a surface should be predominantly be free convection, for which heat transfer coefficients can be obtained from a standard correlation ( 2 ) .
Q„g g s s tg = h Ae (Τ - T, ) (19) h = C k / L [ c L 3 (3 (i 2a (T - T. )/k μ Ί η (20)
g g s [ pg s rg g s tg ' g ^gj
For heat transfer between a fluid and a vertical surface such as the tank wall, L i s the vertical length or height, A i s the tank wall area exposed to thé tank gas, and Τ i s tie average temperature of the metal wall exposed to the tank gas. C and η are 0.59 and 0.25 respectively for laminar conditions and are 0.13 and 0.333 for turbulent conditions.
The transition point occurs when the Prandtl-Grashof product (terms in brackets) in 1 χ 10 ( 2 ) .
For heat transfer between a fluid and a cooler horizontal surface such as the gas-liquid interface, L i s the horizontal length or tank diameter, A i s the area of the liquid surface,
Τ i s the temperature at the interface, and C and η are 0.27 and 0.25 respectively (3)·
The liquid phase heat transfer i s required to determine the surface mass transfer. The heat transfer between the bulk liquid and the surface may be represented by the same free convection correlation used for the gas phase.
*1» • hl As ( T S - Tl > ( 2 1 )
hi - c V Le [CP I L s 3 β
ι
pi2 a ( Ts " τι
) Αι
μι ]
Π ( 2 2 )For free convection below a horizontal warm surface C and η are again 0.27 and 0.25 respectively, L i s the tank diameter, A i s the surface area, and Τ i s tie surface temperature.
Because the heat transfer at the surface i s accompanied by mass transfer, the surface temperature i s taken as the saturation temperature corresponding to the partial pressure of the autogenous gas at the surface. When most of the gas phase i s autogenous gas, i t s average pressure in the gas phase should be very close to the pressure at the surface.
The average properties in the gas film are calculated from the individual gas properties and the gas composition.
The individual gas properties are evaluated at the average gas film temperature, which i s taken as the average between the gas and surface temperatures. With a dissociating gas, the p a r t i a l pressures and mole fractions of the two gases are considered equal to the average tank gas values. The weight fractions, average molecular weight, and density are calculated using the average vapor molecular weight at the average film temperature. The liquid properties are evaluated at the average liquid film temperature, which i s taken as the average between bulk liquid and surface temperatures.
The tank wall temperature depends on the internal and external heat transfer rates; the temperature, mass and specific heat of the previously exposed metal; and the temperature, mass, and specific heat of the newly exposed metal. The average temperature of the metal exposed to the tank gas at the end of any time increment (n) i s obtained from a heat balance around the metal.
Vn ) = Tm(n-D Wm(n-1) Cp. + 0 » , t - Qg w t + ^ Wm Cp m
V
n ) °Ρπ, (23)The external heat transfer between the tank wall and the atmosphere i s by forced convection produced by motion of the rocket through the a i r , or by motion of the a i r as
91
wind against a stationary rocket.
Q = h Α (Τ - Τ ) (2*0
^aw aw w aw m
With the wind providing the convection currents, the heat transfer coefficient depends on the tank diameter and wind velocity (k) ·
h = 0.00021 U ° ·aw a 6t aw /0 for h in BTU/sec f t2 °R (25)
With the rocket trajectory providing the convection, the heat transfer coefficient can be obtained from a more complex correlation involving the wall temperature, a i r temperature and pressure, velocity, and distance from missile nose to propellant tank in question (5)·
Τ = Τ + 0.00007^9 Uaw a t 2 (26)
Τ = 0Λ2Τ + 0.58 Τ + 13.32 x 10""r a m t 6 U 2 (27) h = 0.00037 Ρ U °aw ^ a t η r # 8/ L 0 β 2Τ 0 # 5 1 (28)
The mass transfer may be either condensation or evapora
tion and may occur in any one of three ways. The free con
vection heat transfer between the gas and the liquid produces mass transfer at the surface that depends on the difference between the heat into the gas and the heat out of liquid and on the required change in enthalpy between liquid and gas phases.
W = (Q- - Q ) / ( h - h-)agv ^ls ^gs ag 1 (29)
Bulk boiling in the liquid also occurs whenever the total pressure of the tank gas drops to the vapor pressure of the bulk liquid. The rate of boiling i s that sufficient to maintain the tank gas pressure equal to the liquid vapor pressure. The heat of vaporization i s removed primarily from the liquid, gradually lowering the temperature and vapor pressure.
W = (P - P. ) V. M /Ragv ν tg tg ag' ο tg T. A t (30)
Τχ( η ) = T± (n-1) - Wa g y A hy A t / Cp l V1 ρχ (3D The third type of mass transfer between phases may occur
i f the i n i t i a l gas i s cooled by expansion without added pressurizing gas. When i t i s cooled to the autogenous gas dew point, cloud type condensation occurs at a rate sufficient to maintain the autogenous gas pressure equal to i t s saturation pressure. This condensation removes the mass condensed from the gas phase and releases the heat of condensation to the gas phase.
Φ = (Ρ - Ρ ) V. M /T. R A tage ag ν tg ag' tg ο (32)
Q = W A hχ age ν (33)
W (net) = W (surface) - W (3*0 agv agv age
Analysis Verification
Experimental data from a number of tests have been com
pared with the corresponding analytical results and showed agreement ranging from f a i r to excellent. The principle parameter for comparison i s the tank top pressure curve. In general, excellent agreement has been obtained when the experimental data are accurate and correctly interpreted and the test conditions are adequately represented by the analysis.
Good agreement can not be obtained when these requirements are not or cannot be met.
Figure 2 shows the experimental and analytical tank pressure curves for a propellant tank pressurization system using nitrogen tetroxide as the propellant, nitrogen gas as the i n i t i a l pressurant, and hot Np0, vapors as the autogenous gas supplied during propellant outflow. The agreement between the two curves i s about as good as the test data accuracy would allow. Figure 3 shows the corresponding curves for a propellant tank pre-pressurized to a relatively high pressure in a relatively large i n i t i a l ullage volume. Again the propellant was NpO^, and the i n i t i a l pressurant was nitrogen, but no gas was added during outflow. In this quite different case, excellent agreement was also obtained.
Figure k- i s an example of rather poor agreement between the pressure curves that may occur when the existing test
conditions are not adequately represented by the analysis. In this case a large heavy diffuser was installed at the gas i n l e t . I t absorbed so much heat from the entering vapors that they were i n i t i a l l y cooled and even condensed producing a decided dip in the tank pressure. The experimental and analytical curves could not agree because neither the tempera
ture nor the mass flow rate of the gas actually entering the tank was accurately represented by the values measured in the
9 5
Time Figure 2. Tank Pressure Curve with Autogenous Gas Inflow.
Figure 3· Tank Pressure Curve with No Gas Inflow.
95
1 1 1 ι 1 I I » I 1 ' 1 1 I I L I 1 1 1 » Time Figure k9 Tank Pressure Curve with Autogenous Gas Inflow through a Heavy Diffuser.
line to the tank and used in the analysis. On removal of this diffuser, good agreement was again obtained as shown in Figure
5.
Analysis Application
The type of analysis presented can be used in the design, development, and testing of a missile pressurization system in a number of ways. For instance, i t can be used for comparing different pressurization systems, for optimizing a particular system with regard to i n i t i a l gas volume and pressure and pressurant flow rate and temperature, for establishing the allowable variation in these and other parameters, for pretest analysis of a proposed configuration and set of conditions, for interpreting the results of tests, and for comparing the results of similar tests.
The originally reported pressurizing gas flow rate for the test represented in Figure 5 produced analytical results quite different from the test results. The flow rate of gas entering the tank was then recalculated and found to differ considerably from the reported values. The correction of this discrepancy for the whole set of tests concerned showed them to be consistent with previous tests and eliminated a major difficulty in interpreting the test data. As shown in Figure
a comparison of experimental with the corresponding analytical results that do not agree often reveals important factors which might otherwise have been overlooked. In this case the heavy diffuser which caused the trouble was eventually replaced by one of a much lighter and simpler design.
For the particular system described above, the effects of a number of parameters on the tank pressure have been studied.
Figure 6 presents the partial derivatives of tank gas pressure with respect to i n i t i a l gas volume, i n i t i a l gas pressure, i n i t i a l tank temperature, gas inflow rate, gas inflow tempera
ture, and liquid outflow rate as they vary with time for a particular set of nominal conditions.
Each of these partials i s given as the ratio of
fractional change in tank pressure to corresponding fractional change in the pertinent parameter. This method of presentation i s used to indicate the relative importance of the various parametersο
The effects of these parameter differ considerably from each other in magnitude, direction, and variation with time.
For instance the i n i t i a l pressure and volume decrease in significance with time, while the inflow and outflow rates increase in significance. The two temperature curves show a maximum or minimum because of the interaction of heat transfer, vapor pressure, and gas mass effects.
97
Figure 5· Tank Pressure Curve with Autogenous Gas Inflow with No. Diffuser.
99
Figure 6. Effects of Various Parameters on Tank Pressure.
These partials can be used, together with the other available information, to arrive at the optimum pressurization system conditions and allowable variations in these conditions.
Figure 7 i s an example of the pressure requirements for pro
pellant tank and the anticipated range of tank pressures produced by the chosen nominal values and allowable ranges for the operating conditions. The NPSH and structural require
ments w i l l vary considerably for different missiles, stages, propellants and pressurization systems. The optimum nominal values for a l l parameters, and the associated partials and allowable ranges of values w i l l likewise vary with these requirements. However, for any liquid propellant rocket pressurization system, this type of analysis and these techniques for using i t should prove most profitable.
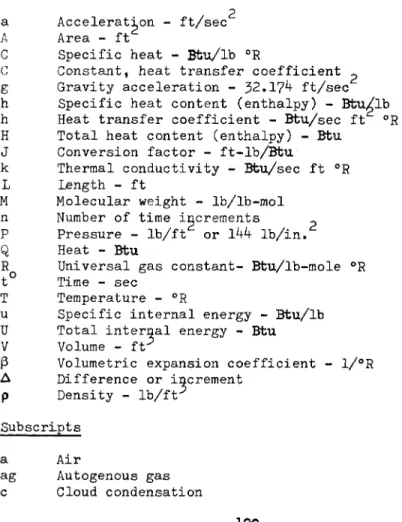
Table of Nomenclature Principal Concepts
a Acceleration - ft/sec A Area - ft
C Specific heat - Bbu/lb °R
C Constant, heat transfer coefficient ^ g Gravity acceleration - 32.17*+ ft/sec h Specific heat content (enthalpy) - Btu/lb h Heat transfer coefficient - Btu/sec ft °R H Total heat content (enthalpy) - Bbu
J Conversion factor - ft-lb/Bfcu
k Thermal conductivity - Btu/sec ft °R L Length - ft
M Molecular weight - lb/lb-mol η Number of time increments Ρ Pressure - l b / f t or lkk l b / i n . Q Heat - Btu
R Universal gas constant- Btu/lb-mole °R t Time - sec
Τ Temperature - °R
u Specific internal energy - Bfcu/lb U Total internal energy - Btu V Volume - ft*
β Volumetric expansion coefficient - 1/°R Δ Difference or increment
ρ Density - l b / f t Subscripts
a Air
ag Autogenous gas c Cloud condensation
Figure Flight Tank Pressure Profile.
101
w g
1
m Ρ rg
ν s
V t t
e Entering Gas
liquid Metal
Constant pressure Residual gas liquid-gas surface Total
Tank
Vaporization Constant volume Wall
Superscripts
η
Time rate of change - 1/sec
Heat transfer correlation exponent References
1· Morey, T. FM "Thermodynamic Properties of Nitrogen Tetroxide," Martin-Denver TM*f31-302, Rev. A , Feb. 2 1F
1 9 6 1 .
2 . McAdams, W. H., Heat Transmission, 3rd ed., McGraw-Hill, New York, 195^, p. 1 7 2 .
3 · Ibid, p. l80.
*f. Ibid, p. 2 6 1 .
5. Eckert, E.R.G., "Survey on Heat Transfer at High Speeds,"
WADC T R 5 0 - 7 0 , April 1 9 5 ^ ·