Folic Acid, Biotin, and Pantothenic Acid
E . L . R . STOKSTAD AND SUSAN M . OACE
Department of Nutritional Sciences University of California
Berkeley, California
Part 1. Folic Acid 286 I. Introduction and Nomenclature 286
II. Structure, Properties, and Reactions of Folic Acid 287
A. Structure 287 B. Properties . . . . 2 8 7
C. Reactions 288 III. Metabolically Active Forms of Folic Acid 290
A. Conjugates of Folic Acid with Glutamic Acid 290
B. Formyl Folic Acid Derivatives 291 IV. Analysis and Assay of Folic Acid . 297
A. Chemical Analysis 297 B. Microbiological Assay 297 C. Preparation of Samples and Hydrolysis of Conjugates . . . . 300
D. Assay of Folic Acid in Blood 301 E. Identification of Metabolic Derivatives of Folic Acid . . . . 302
V. Coenzyme Functions 303 A. Purine Synthesis 304 B. Pyrimidine Synthesis 305 C. Amino Acid Metabolism . . 306
D. Enzymatic Oxidation-Reduction Reactions of Folic Acid . . . . 308
E. Formylation Reactions of Tetrahydrofolic Acid 309 F. Hydroxylation of Phenylalanine to Tyrosine 310 G. Oxidation and Reduction of 5,10-Methylene-FH4 310 VI. Production of Folic Acid Deficiency in Animals 311
A. Chickens and Turkeys 311
B. Rats 311 C. Mice 311 D. Guinea Pigs 311 E. Monkey 312 F. Dogs 312 VII. Symptoms of Folic Acid Deficiency 312
A. Blood Changes 312 B. Reproduction 312 C. Feather Growth and Pigmentation . 312
D. Resistance to Infection 313 E. Endocrine Relationship 313
Part 2. Biotin 313 I. History 313
285
Part 1. Folic Acid
I . INTRODUCTION AND NOMENCLATURE1
Folic acid is a vitamin which is essential for growth and hemopoiesis in animals and functions as a growth factor for a variety of micro
organisms. It was originally discovered as a factor essential for growth (1) and hemoglobin (2) formation in chicks and as an essential growth factor for lactic acid bacteria (3).
What is now referred to as folic acid has been variously designated
"Factor U" (1), "vitamin Bc" (4), "vitamin M" (antianemia factor for monkeys) (5), and "Lactobacillus casei factor (6). The name "folacin"
has been recommended by a joint nomenclature committee of the Amer-
JT h e following abbreviations are used in the manuscript: ADP = adenosine diphosphate; ATP = adenosine triphosphate; D P N = diphosphopyridine nucleotide;
D P N H = reduced diphosphopyridine nucleotide; FAD = flavin adenine dinucleo- tide; FADH2 = reduced flavin adenine dinucleotide; FIGLU = formiminoglutamic acid; FH2 = dihydrof olic acid; FH4 = tetrahydrof olic acid; Ρ ABA = p-aminoben- zoic acid; PABG = p-aminobenzoylglutamic acid; T P N = triphosphopyridine nu
cleotide ; T P N H = reduced triphosphopyridine nucleotide; UMP = uridine mono
phosphate.
II. Avidin—A Specific Biotin Antagonist 314 A. Characterization of Avidin 314 B. Avidin-Biotin Complex 314 III. Metabolic Function of Biotin 315
A. Carbon Dioxide Fixation 315 B. Fatty Acid Synthesis 315 C. Other Biotin Enzyme Functions 316
D. Indirect Involvement of Biotin with Ornithine Transcarbamylase and
the Malic Enzyme 318 IV. Mechanism for Carboxylation by Biotin Enzymes 318
V. Incorporation of Biotin into Enzymes—Apoenzyme Activation . . . 319
VI. Biotin Assays 320 A. Biological Assays 320 B. Microbiological Assays 321 C. The Use of Avidin for Estimation of Biotin 322
Part 3. Pantothenic Acid 323
I. History 323 II. Methods of Pantothenic Acid and Coenzyme A Assay 324
A. Biological Assays 324 B. Microbiological Assays 325 C. Chemical Methods 328 D. Coenzyme A Assay 328 III. Pantothenic Acid Antagonists 329
IV. Symptoms of Pantothenic Acid Deficiency 330 V. Metabolic Function of Coenzyme A 331
References 331
ican Institute of Nutrition and the Society of Biological Chemists. The chemical name "pteroylglutamic acid" (7) has been assigned on the basis of its chemical structure, which contains a pteridine and glutamic acid.
I I . STRUCTURE, PROPERTIES, AND REACTIONS OF FOLIC ACID
A. Structure
OH , ν COOH
I Η /Γ^\ Η I
H2N Ν
Ν — ^ ^)—CO—N—CH—CH2—CH2—COOH
Pteridine p-Aminobenzoic Glutamic acid acid
Pteroic acid
Pteroylglutamic acid Folic acid
B. Properties
Empirical formula: C i9H1 9N706 Molecular weight: 441
Crystal color and shape: yellow, spear-shaped needles Solubility in water (8)
Free acid: 10 /ig/ml, 0°C; 500 μg/ml, 100°C Disodium salt: 15 mg/ml, 0°C
Optical rotation (9) [ « ]D 2 0 = + 1 6 ° in 0.1 Ν NaOH
2 2 0 2 6 0 3 0 0 3 4 0 3 8 0 4 2 0 λ , σ μ ι
FIG. 1. Absorption spectra of folic acid and pteroic acid: (1) folic acid in 0.1 Ν NaOH, (2) folic acid in 0.1 Ν HC1, and (3) pteroic acid in 0.1 Ν NaOH.
pi£a values (10)
Carboxyl groups: 5.0 Enolic group: 8.2
Absorption spectra (11) (Fig. 1)
In 0.1 Ν NaOH, 256 m/x: c = 26,900 cm2/mole In 0.1 Ν NaOH, 282 m/x: c = 25,800 cm2/mole In 0.1 Ν NaOH, 365 τημ: € = 8,350 cm2/mole In 0.1 Ν HC1, 295 m/x: c = 20,680 cm2/mole C. Reactions
1. Alkaline Hydrolysis (12)
Hydrolysis in 1.0 Ν NaOH for 10 hours at 120° C under anaerobic conditions does not inactivate folic acid. Conjugates of folic acid, such as pteroyltriglutamic acid, may be hydrolyzed to pteroylglutamic acid and glutamic acid by anaerobic hydrolysis for 20 hours in 1.0 Ν NaOH at 100°C. Hydrolysis by 1.0 Ν NaOH for 1 hour at 100°C in the presence of oxygen or air results in biological inactivation with formation of p- aminobenzoylglutamic acid (PABG), and 2-amino-4-hydroxy-6-carboxy- pteridine. The p-aminobenzoylglutamic acid can be readily measured by the Bratton and Marshall method (13) for aromatic amines. This proce
dure forms a convenient method for detection of the degradation of folic acid in which PABG is formed.
2. Oxidation
Oxidation with permanganate or chloric acid (14) splits the bond between C-9 and N-10 and yields 2-amino-4-hydroxy-6-carboxypteridine.
The aromatic amine is oxidized and cannot be measured by the Bratton and Marshall method, but the resulting pteridine may be conveniently measured fluorometrically. This has been made the basis of a chemical method for the determination of folic acid (15). Oxidation with per
manganate resembles oxidation with oxygen in alkaline solution (12) in splitting the bond between C-9 and N-10 between the pteridine and PABG.
3. Reductive Cleavage
Reduction in acid solution also breaks the bond between C-9 and N-10 to give a pteridine and PABG. Folic acid can be reduced quantita
tively by zinc and hydrochloric acid to give a pteridine and PABG (16).
The latter can be readily estimated by the Bratton and Marshall method, and this reaction has been used as the basis for the quantitative esti
mation of folic acid (17). Reduction by zinc in acid solution yields
PABG plus a reduced pteridine, which, after reoxidation with manganese dioxide, yields 2-amino-4-hydroxy-6-methylpteridine. Catalytic reduc
tion with hydrogen (16) in acid solution also yields a pteridine and PABG. Hydrolysis with 0.5 Ν H2S 03 at room temperature yields PABG and a pteridine aldehyde (16).
4. Hydrogenation
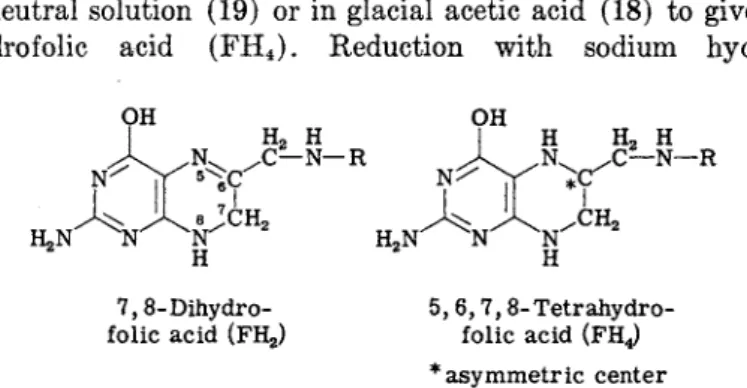
The pyrazine ring of folic acid can be readily reduced to give either dihydro or tetrahydro derivatives. In alkaline solution, folic acid is catalytically hydrogenated to give 7,8-dihydrofolic acid (FH2) (18, 19), and in neutral solution (19) or in glacial acetic acid (18) to give 5,6,7,8- tetrahydro folic acid (FH4). Reduction with sodium hydrosulfite
7,8-Dihydro- 5, 6,7,8-Tetrahydro- folic acid (FH2) folic acid (FHj
* asymmetric center
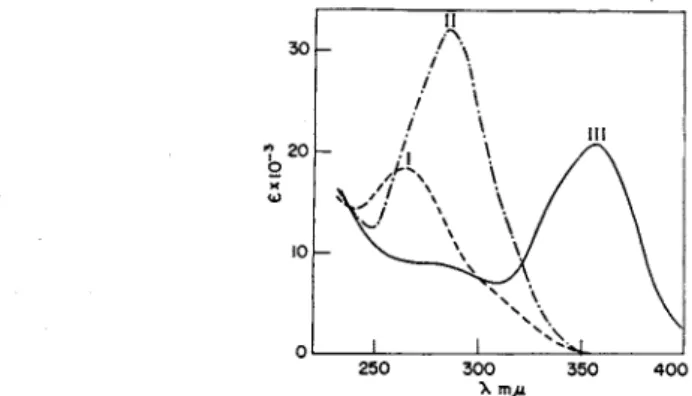
(Na2S204) in 7% ascorbate at pH 6 at room temperature for 5 minutes yields F H2 (20, 21), while similar reduction at 75°C for 90 minutes yields F H4 (22). The reaction is carried out in ascorbate to minimize reoxidation of the F H4 by atmospheric oxygen. The absorption spectra for F H2 and F H4 are shown in Fig. 2. F H2 has a maximum at 281 τημ (c = 21,000 cm2/mole) at pH 8, and F H4 has a maximum at 297 ηΐμ
2 5 0 3 0 0 3 5 0 λ , π μ ι
FIG. 2. Absorption spectra of folic acid derivatives at pH 8: (I) Folic acid, (II) FH2, and (III) FH*.
(c = 22,000 cm2/mole) at pH 8. Reduction to give F H4 produces an asymmetric carbon at the 6-position, and the chemically reduced mate
rial is therefore a mixture of the two diastereoisomers. Only one of these is biologically active in enzyme reactions involving F H4. Enzymatic reduction of F H2 to F H4 yields 1-L-FH4 (23), which is twice as active biologically as chemically prepared FH4. The optical rotation of enzy- matically prepared 1-L-tetrahydrofolic acid is [«]D2 0 = —16.9°, compared with a rotation of [ « ]D 2 0 = +14.9° for F H4 prepared by chemical reduction (24).
Both F H2 and F H4 are readily oxidized by atmospheric oxygen in neutral and alkaline solutions. Air oxidation of F H4 for 1 hour results in 50% breakdown of folic acid as measured by formation of PABG (19).
This property of F H4 to yield PABG in the presence of oxygen has been used as a basis for the estimation of F H4 during enzymatic reactions
(25). Oxidation of F H4 by atmospheric oxygen can be detected in 10 minutes on the basis of changes in the absorption spectra (26). Oxidation of F H4 can be prevented by the use of ascorbate at concentrations of 2 to 5 mg per milliliter (27) and by the use of mercaptoethanol (0.2 to 1.0 M) (28). Mercaptoethanol (0.2 M) may be used during fractionation work with naturally occurring reduced folic acid products to minimize reoxidation by atmospheric oxygen.
5. Photolysis
Folic acid is decomposed by light. Biological inactivation approxi
mately parallels the formation of PABG (29), showing that the initial reaction consists of the cleavage of the bond between the C-9 and the N-10 of the aromatic amine. This reaction proceeds under daylight lab
oratory lighting conditions, but very slowly in artificial light. Care must be taken to prevent exposure to direct or indirect sunlight when working with dilute solutions of folic acid.
III. METABOLICALLY ACTIVE FORMS OF FOLIC ACID A. Conjugates of Folic Acid with Glutamic Acid
1. Pteroyltriglutamic Acid
A folic acid derivative has been isolated from the fermentation product of a Corymebacterium, which contains three glutamic acid resi
dues (30, 31). This pteroyltriglutamic acid is as active as folic acid (pteroylglutamic acid) for Lactobacillus casei, but relatively inactive for Streptococcus faecalis R. Pteroyltriglutamates seem to be the more prevalent form of folic acid existing in microorganisms. Tetrahydro-
pteroyltriglutamic acid has been identified as the coenzyme involved in the metabolism of formiminoglycine by Clostridium acidiurici (32), in glycine-serine conversions by Clostridium cylindrosporum (33), and in the synthesis of methionine by Escherichia coli (34-36).
2. Vitamin Bc Conjugate (Pteroylheptaglutamic Acid) Binkley (37) has isolated from yeast a folic acid conjugate which contains seven glutamic acid residues (38, 39) and which has been designated vitamin Bc conjugate. This conjugate is 0.4% as active as folic acid for Lactobacillus casei and 0.2% as active as folic acid for S. faecalis R.
B. Formyl Folic Acid Derivatives
Four formyl derivatives of tetrahydrofolic acid have been found in natural products and are known to be involved in biochemical reactions.
These are iV5-formyltetrahydrofolic acid (leucovorin), iV1 0-formyltetra- hydrofolic acid, iV5,iV10-methenyltetrahydrofolic acid, and formimino- tetrahydrofolic acid.
1. N5-Formyltetrahydrofolic Acid ("Leucovorin,"
"Citrovorum Factor" "Folinic Acid")
This material was first recognized as a growth factor for Leuconostoc citrovorum (also known as Pediococcus cerevisiae) (40), and, on the basis of its activity for this organism, it has been called the citrovorum factor (CF). It has been isolated from liver (41, 42). It has been syn
thesized, and its structure has been established as iV5-formyltetrahydro- pteroylglutamic acid (5-formyl-FH4) by Brockman et al. (43) and by Shive et al. (44). The synthetic material has been designated "folinic acid SF" by Flynn et al. (45) and "leucovorin" by Broquist et al. (46).
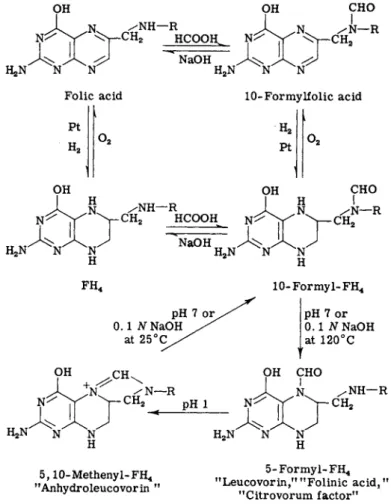
The synthesis involves formylation of folic acid to give iV10-formylfolic acid (Fig. 3). The latter, on catalytic reduction in formic acid, yields iV10-formyltetrahydrofolic acid (10-formyl-FH4) (40, 41). This rear
ranges to give 5-formyl-FH4 on heating at 125°C for 30 minutes in neutral solution in the presence of ascorbate (44), or anaerobically in 0.1 Ν NaOH (47). The 10-formyl-FH4 is labile to oxidation by atmos
pheric oxygen, and ascorbate is added to prevent reoxidation by atmospheric oxygen during its conversion to 5-formyl-FH4. Leucovorin (5-formyl-FH4) is stable to atmospheric oxygen in neutral and alkaline solutions because the reduced pyrazine ring has been stabilized by the presence of a formyl group on the N-5 ring nitrogen. It is stable to 0.1 Ν NaOH, but is hydrolyzed to F H4 by 5 Ν NaOH at 20°C (47). Its
absorption spectrum at pH 8, shown in Fig. 4, has a maximum at 285 m/x (c Χ lO- 3 = 32,100 cm2/mole) (24). Leucovorin contains an asym
metric center at the C-6 position, and the synthetically prepared mate
rial is a mixture of the two diastereoisomers. It is half as active for OH
XX J
K^N Ν Ν
/ N H — R
^>r-CH2 HCOOH^
NaOH Folic acid
Pt H2
H2N Ν
10-Formylfolic acid H2
Pt OH
N- C H/ N H - R 2
o2
Η I CHO Ν / Ν — R
Ν — C H2
)
FH4
pH 7 or^
0.1 Ν NaOH at 25°C
10-Formyl-FH4 pH 7 or 0.1 iVNaOH at 120°C OH CH
V C H , .NH—R
5,10-Methenyl-FH4
"Anhydroleucovorin "
5-Formyl-FH4
"Leucovorin,'1 MFolinic acid,"
"Citrovorum factor"
FIG. 3. Formylation reactions of folic acid and synthesis of leucovorin.
Leuconostoc citrovorum as the citrovorum factor isolated from liver (41, 42), which consists of only the one biologically active form. The syn
thetic material has been resolved by solubility differences into its two diastereoisomers (48). The less soluble form, calcium l,L-leucovorin, which has a rotation of [ « ]D 2 0 = —15.1° (compared with +15.3° for the original synthetic mixture), has the same biological activity as the natural CF obtained from liver (41, 42).
2 5 0 3 0 0 3 5 0 4 0 0
FIG. 4. Absorption spectra of tetrahydrofolic acid derivatives: (I) 10-formyl-FH4 at pH 8, (II) 5 - f o r m y l- F H 4 at pH 8, and (III) 5,10-methenyl-FH4 at pH 2.
2. N10-Formyltetrahydrofolic Acid
Catalytic reduction of folic acid, or of iV^-formylfolic acid in formic acid (49, 50), or formylation of F H4 in 90 to 100% formic acid (47) yields 10-formyl-FH4. No formylation of the N-5 position occurs unless acetic anhydride is also present in the reaction mixture (51). 10-Formyl- F H4 is readily converted into 5-formyl-FH4 by heating for 30 minutes at 120°C in neutral solution (pH 6-7) in the presence of ascorbate (0.2 to 7%) (49, 52) or by heating anaerobically in 0.1 Ν NaOH (47). The free energy of hydrolysis of the formyl group of 10-formyl-FH4 has been estimated to be ca. 4000 (53) to 5600 cal (27) greater than that of 5-
formyl-FH4, and thus the energy relationships of this reaction favor the formation of 5-formyl-FH4. The absorption spectrum of 10-formyl-FH4 at pH 8 is shown in Fig. 4 (24).
The extreme lability of 10-formyl-FH4 toward molecular oxygen is similar to that of F H4 and differs from that of 5-formyl-FH4 which is stable to atmospheric oxygen. Thus, it is necessary in the assay or chromatographic separation of 10-formyl-FH4 to protect against air oxidation with 0.2 to 0.6% ascorbate at pH 6-7 (27, 52). Mercapto- ethanol (0.2 to 1.0 M) can also be used to protect tetrahydro derivatives of folic acid (32) during extraction and purification procedures. Stabiliza
tion of 10-formyl-FH4 for assay purposes can also be achieved by heating it for 30 minutes at 120°C with 0.2% ascorbate at pH 6 (52) to convert it into 5-formyl-FH4, which is stable to oxidation and which may be assayed by Leuconostoc citrovorum. 10-Formyl-FH4 is active for L.
citrovorum only when it is protected against air oxidation by the use of ascorbic acid in the "aseptic plus ascorbate" procedure in which the sample is added aseptically to previously sterilized media containing ascorbate (27). In the conventional assay with L. citrovorum in the
absence of ascorbate, air oxidation occurring during sterilization of the media converts the 10-formyl-FH4 into 10-formylfolic acid which is inactive for L. citrovorum.
At pH 2 and 25°C, 10-formyl-FH4 is converted into N5,iV1 ( )-methenyl- F H4 (anhydroleucovorin) by elimination of water to give the bridge compound, as shown in Fig. 3. Anhydroleucovorin is stable to air oxida
tion since the pyrazine ring is stabilized by the substitution of the N-5 position.
10-Formyl-FH4 has been identified as a minor constituent of the folic acid fraction in mouse liver (52) and in chicken liver (54). Its oxida
tion product, A/ri0-formylfolic acid, has been isolated from horse liver autolyzed for 18 hours (55). When the liver is autolyzed for a longer period of time, 5-formyl-FH4 is formed instead of 10-formylfolic acid (55).
3. N5}N10-Methenyltetrahydrofolic Acid (Anhydroleucovorin) Treatment of 5-formyl-FH4 with 0.1 Ν HC1 or at pH 2 yields a product which has the properties of iV5,iV10-methenyltetrahydrofolic acid (5,10-methenyl-FH4). The half-time of the reaction at pH 1 is 8 min
utes; at pH 2 it is 60 minutes (56). This reaction involves splitting off a molecule of water to form an imidazolinium ring with a methenyl bridge between the 5- and 10-nitrogen atoms (Fig. 3). It has been termed anhydroleucovorin by Cosulich (51). Anhydroleucovorin is stable to atmospheric oxygen. The formation of an imidazolinium ring results in a shift of the absorption maximum from 282 τημ to a new peak at 358 τημ in acid solution (Fig. 4). This absorption peak in acid solution, which is unique for 5,10-methenyl-FH4, may be used for measuring anhydroleucovorin in the presence of other folic acid metabolites.
Although hydrolysis of anhydroleucovorin could yield either 5- formyl-FH4 or 10-formyl-FH4, the latter is the main product formed at neutral and alkaline solutions. At pH 10 the immediate product is 10-formyl-FH4, as indicated by rapid absorption of oxygen by the hy
drolysis product with the formation of 10-formylfolic acid (56). At pH 6.5 anhydroleucovorin is hydrolyzed to 10-formyl-FH4 in 2 hours (57).
This change can be followed by the change in absorption spectra from that of 5,10-methenyl-FH4 to that of 10-formyl-FH4. The resulting 10- formyl-FH4 may be converted back into 5,10-methenyl-FH4 by acidifica
tion to pH 2. The free energy of hydrolysis of the formyl groups of 5-formyl-FH4, 10-formyl-FH4, and 5,10-methenyl-FH4, has been esti
mated to be 2000, 7620, and 9070 cal, respectively (58).
Anhydroleucovorin is inactive for Leuconostoc citrovorum by con
ventional assay in which the sample is sterilized with the medium in the
absence of ascorbate, but is active for Lactobaallus casei and S. faecalis (51). Anhydroleucovorin is as active as leucovorin for Leuconostoc citrovorum when assayed by aseptic addition to sterile media containing ascorbate (27). These biological activities can be readily explained on the basis that anhydroleucovorin is converted to 10-formyl-FH4 which is oxidized by air to 10-formylfolic acid. The latter is inactive for Leuco
nostoc citrovorum because it is not in the reduced tetrahydro form, but is active for Lactobacillus casei and S. faecalis, which can use either the oxidized or reduced forms of folic acid.
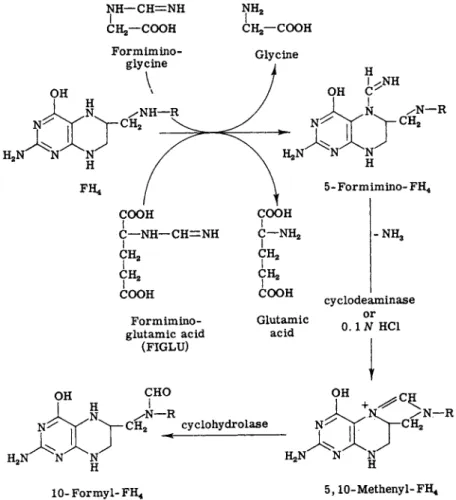
4. Formiminotetrahydrofolic Acid
Tetrahydrofolic acid reacts enzymatically with formiminoglycine (59- 61) and formiminoglutamic acid (62, 63) to form formiminotetrahydro-
N H — C H = N H CHjr-COOH I
Formimino
glycine
\
NH2
CHa—COOH Glycine
Ν / Ν — R N - C H2
Formimino
glutamic acid (FIGLU)
Glutamic acid
OH CHO
5-Formimino-FH4
- N H3
cyclodeaminase or 0.1 Ν HC1
OH / N — R
^ — CH2 cyclohydrolase
^N Η
10- Formyl- FH4 5,10-Methenyl- FH*
FIG. 5. Reactions of formiminotetrahydrofolic acid.
folic acid (Fig. 5). The formimino group is believed to be on the 5- nitrogen because the stability of formiminotetrahydrofolic acid
(formimino-FH4) toward atmospheric oxygen resembles that of 5-formyl- F H4 (59). Formimino-FH4 is rapidly converted by acid, according to the reactions shown in Fig. 5, into 5,10-methylene-FH4 and ammonia.
The half-time of this reaction in 2.3% perchloric acid is 10 minutes at room temperature, which is similar to the reaction rate for the cycliza- tion of 5-formyl-FH4 to 5,10-methenyl-FH4, but slower than the cycli- zation of 10-formyl-FH4 to 5,10-methenyl-FH4 which goes to completion in 8 minutes under the same conditions (62). The reaction of formimino- F H4 to 5,10-methenyl-FH4 and ammonia is also catalyzed by the enzyme cyclodeaminase (62).
5. Hydroxymethyltetrahydrofolic Acid
This has been identified by Deodhar et al. (64) and Jaenicke (65) as a product of the enzymatic reaction of serine with FH4. Hydroxymethyl
tetrahydrofolic acid (hydroxymethyl-FH4) is also formed nonenzy- matically from F H4 and formaldehyde (19, 65, 66). The formaldehyde- F H4 addition product is probably an equilibrium mixture of ^-hydroxy
methyltetrahydrofolic acid and iV5,N1 0-methylenetetrahydrofolic acid, with the ring structure as the predominant form (67).
5-Hydroxymethyl-FH4 5,10-Methylene-FH4
Maximum binding between formaldehyde and F H4 occurs only when both the N-5 and N-10 positions of F H4 are nonsubstituted (19, 67-71).
Hydroxymethyl-FH4 is more stable toward oxygen than FH4, being oxidized by molecular oxygen about one-third as rapidly as F H4 (19).
The complex dissociates appreciably to give formaldehyde and is not as stable as the formyl-FH4 derivatives.
6. N5-Methyltetrahydrofolic Acid {"Prejolic A")
A form of folic acid has been isolated from fresh liver (nonautolyzed) which is inactive for S. faecalis but active for Lactobacillus casei (72, 73). This has been identified as iV5-methyltetrahydrofolic acid (5-methyl- F H4) . It has been termed "prefolic A" (72) because it is converted into folic acid during prolonged autolysis of liver (74). The "prefolic A"
isolated from liver is also identical with a methyltetrahydrofolic acid derivative identified by Larrabee and Buchanan (75) as an inter
mediate in the synthesis of methionine by E. coli. The location of the methyl group on the N-5 position is indicated by the stability toward molecular oxygen and its nonidentity as determined by biological activity with synthetically prepared 10-methyl-FH4 (76). 5-Methyl-FH4 can be prepared by reduction of a mixture of F H4 and formaldehyde (5,10- methylene-FH4) with potassium borohydride for 1 hour at 45°C (77, 78).
IV. ANALYSIS AND ASSAY OF FOLIC Aero A. Chemical Analysis
Folic acid may be conveniently estimated chemically either by reduc
tive cleavage to give PABG, which may be measured colorimetrically, or by oxidation to give a fluorescent pterin which can be estimated fluorometrically.
Reduction of folic acid with zinc solution (17) or by titanous chlor
ide (79) in acid solution yields PABG, which can be measured by the Bratton and Marshall method for aromatic amines (13). The spe
cificity of the method is increased by measuring diazotizable amines be
fore and after reductive cleavage. The sensitivity of this method is high when compared with other colorimetric procedures since the molecular absorbancy is high (c = 37,200 cm2/mole). The absorbancy of the final colored reaction product of a solution equivalent to 1 μg of folic acid per milliliter is 0.085 in a 1-cm cell at 545 m/x.
Oxidation of folic acid by alkaline permanganate yields 2-amino-4- hydroxypteridine-6-carboxylic acid which can be estimated by fluoro- metric methods (15). This procedure has been used for assay of natural products, and gives results comparable to but slightly higher than those obtained by microbiological assay. The procedure offers the advantage that it measures all conjugates of folic acid irrespective of their bio
logical activities.
Folic acid has been measured directly spectrofluorometrically by Duggan et al. (80). However, the fluorescence of folic acid itself is low compared with that of the pteridine formed on permanganate oxidation, and the sensitivity and specificity of the procedure used by Allfrey et al. (15) could be greatly increased by use of the spectrofluorophotometer
(80).
B. Microbiological Assay
Folic acid is most frequently measured by microbiological assay using either Lactobacillus casei ATCC 7469 or S. faecalis ATCC 8043 (81, 82).
Leuconostoc citrovorum ATCC 8081, also known as Pediococcus ceri- visiae, is used for the assay of the citrovorum factor (40). The protozoan Tetrahymena geleii may also be used, and it has the advantage of responding to the higher pteroylpolyglutamates which are inactive for Lactobacillus casei and S. faecalis (83). The thermophile Bacillus co- agulans ATCC 12245, which responds to folic acid and to the tri- and
T A B L E I
RELATIVE ACTIVITY OF VARIOUS FORMS OF FOLIC ACID FOR ASSAY ORGANISMS'1
Organism
Leuco
nostoc citrovorum
(asceptic
Strepto Lacto Leuco plus Tetra
coccus bacillus nostoc ascorbate hymena Compound faecalis casei citrovorum method) geleii
Folic acid + b + c +
Pteroyldiglutamic acid
-
+- -
Pteroyltriglutamic acid — +
- -
+Pteroylheptaglutamic acid
- - - -
+FH4 + + — +
5-Formyl-FH4 + + + +
10-Formyl-FH4 + +
-
+5,10-Methenyl-FH5-Methyl-FH4 4 + +
-
+-
+ — —Pteroic acid + — — — —
β From Stokstad (86).
6 Plus ( + ) indicates activity of 70 to 100% of folic acid on a molar basis for S.
faecalis, Lactobacillus casei, and T. geleii, and 70 to 100% of 5-formyl-FH4 for Leuconostoc citrovorum.
c Minus ( —) indicates activity of less than 5%.
heptaglutamates, has also been suggested as an organism for folic acid assay (84). It has the disadvantage of responding to PABA and of under
going occasional mutations which eliminate its folic acid requirement (85). The activities of various folic acid derivatives for these assay organisms are shown in Table I (86).
Regarding the choice of an assay organism, Lactobacillus casei has the advantage of responding to more forms of folic acid than does S.
faecalis. Lactobacillus casei responds to pteroyltriglutamate (30, 31), but
not to higher polyglutamates such as pteroylheptaglutamate (37). 5- Methyl-FH4 is active for L. casei, but not for S. faecalis.
Lactobacillus casei is the most sensitive assay organism giving half- maximum growth with 0.02 to 0.04 m/Ag/ml of folic acid, while that required for half-maximum growth of S. faecalis is 0.06 to 0.4 m/Ag/ml of folic acid, depending on the assay conditions used (87). Half-maximum growth for T. geleii is obtained with 0.3 m/ig/ml, and for B. coagulans with 1.0 m/Ag/ml. Leuconostoc citrovorum requires 0.2 m/ig/ml of leucovorin for half-maximum growth.
Microbiological assays with Lactobacillus casei usually require 3 days, and the growth may be measured turbidimetrically or by titration of the lactic acid produced (82). Streptococcus faecalis grows more rapidly, and growth can be measured either turbidimetrically after 16 hours or by titration of lactic acid after 40 hours' incubation (88). More rapid growth with L. casei has been obtained by the use of a trypsin digest of casein which serves as a source of strepogenin (89). Strepogenin stimulates growth during the early growth phase, and by this procedure L. casei can be used for assay purposes with a 16- to 24-hour incubation period. Procedures which permit use of 20-hour incubation periods for both L. casei and S. faecalis have been described also by Toennies et aL (87), who used an enzymatic casein hydrolyzate as the source of strepo- genin and amino acids, and 0.025% of ascorbic acid to stimulate growth in the early growth phase. The effect of ascorbic acid in promoting early growth has been observed previously by Rickes et aL (90). Similar studies by Kihara and Snell (91) have shown that a combination of ascorbic acid, cysteine, glutamine, serine, guanylic acid, and spermine would replace strepogenin in stimulating early growth in L. casei. Cooper- man et al. (92) have developed a 16-hour L. casei assay for folic acid on a medium containing acid-hydrolyzed casein and no strepogenin source by using a special innoculum of bacteria in the fast, logarithmic growth phase.
Detailed procedures for microbiological assay with L. casei and S. faecalis and for the preparation of samples may be found in the review by Snell (82), in the published methods of the Association of Vitamin Chemists (93), and in the "Official Methods of Analysis" of the Association of Official Agricultural Chemists (94). Most of the media used are based on that described by Teply and Elvehjem (88) which uses additional quantities of glucose and phosphate buffer to give greater growth and a higher titratable acidity. A medium which can be mixed dry and dissolved in water when needed has been described by Toennies et al. (87, 95). Complete, dry, commercially made media for folic acid
assay are also available.2 Acid-hydrolyzed casein3 and enzymatically hydrolyzed casein4 may also be obtained commercially.
Agar plate-zone assays have been used for folic acid analysis. Bo
under et al. (96) used the agar cup-plate technique with Lactobacillus casei, S. faecalis, and Leuconostoc citrovorum and measured the size of the growth zone. Wieland et al. (97) used the pad-plate technique in which a paper disk is soaked with the solution to be tested, dried, and placed on an agar medium seeded with the assay organism. Such methods may not be as accurate as tube assays, but they are convenient and eliminate the need for aseptic addition of the samples when heat-labile tetrahydrofolic acid derivatives are being assayed.
Leuconostoc citrovorum is used for the assay of the citrovorum fac
tor. This organism responds only to tetrahydro derivatives of folic acid.
When no precautions are taken to minimize air oxidation of the oxygen- sensitive tetrahydro derivatives, only the citrovorum factor (5-formyl- FH4) is active. When the "aseptic plus ascorbate" procedure is used, in which ascorbate is added to the medium to prevent air oxidation and the samples are added aseptically, other tetrahydro derivatives, such as
F H4 and 10-formyl-FH4, are also active (27, 98). Anhydroleucovorin (5,10-methyenyl-FH4), which is stable to air oxidation in acid solution, is converted at pH 6 to 7 in the assay medium into 10-formyl-FH4, which is oxygen-labile. Anhydroleucovorin is relatively inactive for Leuconostoc citrovorum by conventional assay in which the sample is autoclaved with the medium (51), but is active for this organism when added aseptically to media containing ascorbate (27). 5-Methyl-FH4 is as active as folic acid for Lactobacillus casei, but is only 5-10% as active as folic acid for S. faecalis, and 5% as active as 5-formyl-FH4 for Leuconostoc citrovorum (99-101).
C. Preparation of Samples and Hydrolysis of Conjugates
Folic acid is known to occur in the form of conjugates in plant and bacterial cells. Pteroyldiglutamate is active for both Lactobacillus casei and S. faecalis (102). Pteroyltriglutamate is active for L. casei, but inactive for S. faecalis, while the higher conjugates, such as pteroylhepta- glutamate, are inactive for both organisms. Enzymes designated folic acid conjugases (103) are capable of hydrolyzing these conjugates into
2 Folic acid assay media available from Difco Laboratories, Detroit, Michigan, and General Biochemicals, Chagrin Falls, Ohio.
'Available from General Biochemicals, Chagrin Falls, Ohio, and Sheffield Chemical Co., Norwich, New York.
4 Available from General Biochemicals, Chagrin Falls, Ohio, and Sheffield Chemical Co., Norwich, New York.
folic acid. Three types of conjugases have been used. One, which is present in chicken pancreas, has a pH optimum at 7.5 (104) and splits conjugates down to the pteroyldiglutamate (105). The second type, such as that present in hog kidney and liver, has an optimum at pH 4.5 (103, 106). Another conjugase, obtained from the gas glands of Physalia physalts, has been reported to split various conjugates down to pteroyl- monoglutamates, as determined by chromatographic analysis (107). A more detailed account of the effect of various enzymes in releasing folic acid in tissues may be found in reviews on the assay of folic acid (81, 82, 108). Although both hog kidney and chicken pancreas have been recommended for assay (82, 93) and are widely used, chicken pancreas is the more active in the assay of various types of food materials (108).
Chicken pancreas also has the advantage that it may be prepared as a dry acetone powder which can be readily stored until used or may be obtained commercially in dry form.5
Procedures for the preparation of samples of food materials and enzymatic digestion with conjugase have been described by Toepfer et al. (108). The folic acid content of a wide variety of human foods is also reported (108).
D. Assay of Folic Acid in Blood
Special methods have been devised for the assay of folic acid in blood (85, 92). An important feature of these procedures is dilution of the blood or serum in pH 6.1 phosphate buffer containing 0.05 to 0.15%
ascorbate. The mixture is incubated at 37°C for 90 minutes (85, 92), then autoclaved and centrifuged to coagulate and remove blood pro- teins. Toennies et al. (95) have observed that blood hemolyzates contain a derivative of folic acid which is inactive for Lactobacillus casei and which is converted on incubation with a factor in plasma into a bio- logically active form. However, Herbert (109) finds that incubation with phosphate buffer and ascorbate is not necessary in the assay of serum. Paper chromatography shows the presence of four different folic acid derivatives active for Lactobacillus casei, none of which is identi- fied with folic acid or 5-formyl-FH4 (110). Most of the folic acid activity in blood is greater for Lactobacillus casei than for S. faecalis or Leuconostoc citrovorum (109). This was originally believed to indicate the presence of pteroyltriglutamates (109) which are known to be more active for Lactobacillus casei than for S. faecalis. However, the recent finding in liver of 5-methyl-FH4 (101), which is active for Lactobacillus casei, but relatively inactive for S. faecalis and Leuconostoc citrovorum,
has led to the suggestion that 5-methyl-FH4 is the primary folic acid
5 Chicken pancreas powder available from Difco Laboratories, Detroit, Michigan.
derivative in blood (99, 111). This is based on the observation that the S. faecalis activity of blood is not increased by pancreas conjugase and that most of the folic acid-active material of serum migrates the same as 5-methyl-FH4 on paper chromatography.
Detailed procedures for assay of folic acid in blood have been described by Cooperman et al. (92), Baker et al. (85), and Herbert et al. (109). For a more detailed account of the assay of folic acid in blood, the reader is referred to the recent review by Luhby and Cooper- man (112).
E. Identification of Metabolic Derivatives of Folic Acid
Both biological and chemical procedures have been used to identify the derivatives of folic acid which occur in natural products. Many studies have been made comparing the folic acid activities for the three main assay organisms, i.e., Lactobacillus casei, S. faecalis, and Leuco
nostoc citrovorum. It has been assumed that materials which are active for Lactobacillus casei but not for S. faecalis represent conjugates. How
ever, the finding that 5-methyl-FH4 is active for L. casei but not for S. faecalis (72, 73) has rendered comparative assays by L. casei and S. faecalis of little value in measuring folic acid conjugates. The amounts of 5-formyl-FH4 and of other tetrahydrofolic acid derivatives may be determined by assay with Leuconostoc citrovorum. Conventional assay procedures in which the supplements are autoclaved with the media measure only the oxygen-stable 5-formyl-FH4, while aseptic addition of the samples to previously sterilized media containing ascorbate per
mits the estimation of all tetrahydrofolic acid derivatives except 5- methyl-FH4 (27). The activities of different tetrahydrofolic acid deriva
tives for Leuconostoc citrovorum under both assay conditions are shown in Table I.
Chromatography on paper has been used for identification of folic acid derivatives (96, 97, 99, 110). The Rf values obtained by paper chromatography for various folic acid derivatives are shown in Table II.
Paper chromatography depends on the use of either Lactobacillus casei, S. faecalis, or Leuconostoc citrovorum for identification of active components, and therefore identification of activity is usually made by placing the paper strips on agar medium seeded with one of the three assay organisms. These procedures suffer from the limitation that the higher conjugates which are inactive for these assay organisms are not detected by this procedure. The higher conjugates can be measured only after hydrolysis with conjugase.
Column chromatography on DEAE-cellulose has proved the most effective procedure for separating and identifying folic acid derivatives
(28, 107). The separation of oxygen-labile tetrahydrofolate derivatives by this method permits the use of mercaptoethanol (0.2 ikf) (28) in the eluting material to stabilize oxygen-labile tetrahydro derivatives. Using this chromatographic procedure, Noronha and Silverman (107) studied the distribution of folic acid derivatives in an acetone powder of chicken liver. The folic acid was extracted from the dried tissue by a 1%
solution of ascorbate at pH 6. The ascorbate served to stabilize the
T A B L E II
SEPARATION OF FOLIC ACID DERIVATIVES BY PAPER CHROMATOGRAPHY
Rf values
Compound Solvent Ia Solvent I I6 Solvent I I P
Pteroic acid 0.04 0.05 —
Folic acid 0.19 0.30 0.14
Folic acid (G2) 0.35 — —
(pteroyldiglutamic acid)
Folic acid (G3) 0.34 0.58 —
(pteroyltriglutamic acid)
iV10-Formylfolic acid 0.55 0.80 —
5-Formyl-FH4 0.65 0.73 0.56
5-Formyl-FH4(G2) — 0.83 —
5-Formyl-FH4(G3) — 0.90 —
5-Methyl-FH4 — — 0.79
5-Methyl-FH4(G3) — — 0.90
° Solvent 1:5% citric acid + NH4OH + isoamyl alcohol (97).
6 Solvent II: 5% aqueous Na2HP04 in isoamyl alcohol (96).
c Solvent III: Saturated NaoHP04 (99).
reduced forms of folic acid present. Some of the fractions were poly- glutamates, since their activity for Lactobacillus casei was increased by hydrolysis with conjugase. Each active component was rechromato- graphed after hydrolysis with conjugase from Physalia physalis, which hydrolyzed the conjugates down to the monoglutamate. By this proce- dure, Noronha and Silverman (107) found that most of the activity of chicken liver is in the form of conjugates which are inactive for L. casei and are activated by conjugase treatment.
V . COENZYME FUNCTIONS
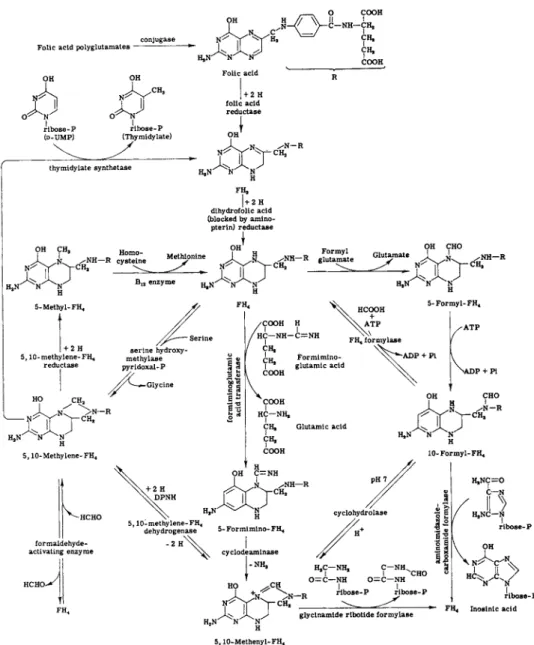
The coenzyme functions of folic acid are involved in purine and pyrimidine biosynthesis, amino acid metabolism, oxidation-reduction reactions, and hydroxylation reactions (outlined in Fig. 6).
Folic acid polyglutamates -
Η,Ν A ,
O COOH II I C—NH—CH,
CH, COOH
X
CH, folic acid 1 + 2 Ηreductase ribose-P
(d-UMP) ribose-P
(Thymidylate)
thyraidylate synthetase Η,Ν' N ^
Λ J
FH,
| + 2 Η dihydrofolic acid (blocked by amino- pterin) reductase
OH CH,
Η,Ν Η
ifv. / N H — R cysteine
I + 2 Η 5,10-methylene-FH,
— N ^ CH2
Η,Ν Ν Η 5,10-Methylene-FH«
formaldehyde- activating enzyme
Methipni OH Formyl
/ N H — R glutamate - C H ,
B „ enzyme
serine hydroxy- methylase pyridoxal-Ρ
> N — R -CH,
5,10-methylene-FH4 dehydrogenase
/COOH /HC—NH—
S S CH,
>gluta isferi
I COOH
ε *
β "2 \
COOH s -c HC—NH,
CH, CH, COOH
5,10-Methenyl-FH4
FIG. 6. Metabolic reactions involving folic acid.
A. Purine Synthesis
Folic acid participates at two stages in the biogenesis of purines. The first step involves the introduction of the 8-carbon of purine by the formylation of glycinamide ribotide (reaction 1) (113). The second step involves the introduction of the 2-carbon of the purine ring by the
Ο Ο II I I HaN— C HaN—C
C — Ν Η C—Ν
II \?H + 10-Formyl-FH4 0 = C II "c h
H2N C—Ν N - C - N
ribose-P ribose-P Aminoimidazole- Formylaminoimidazole-
carboxamide ribotide carboxamide ribotide
B. Pyrimidine Synthesis
Folic acid is involved in the biosynthesis of the methyl group of thymidylic acid (reaction 3) (115-117) and in the biosynthesis of the
thy midy late
.. synthetase N ' H
I] + 5,10-Methylene-FH4 J J + FH2 (3) Ο Ν
deoxyribose-P deoxyribose-P Deoxyuridylic acid Thymidylic acid
NHg N -
oV
+ 5'
ribose-P
10-Methylene-FH4
Cytidylic acid
N H2
CH2OH
Ο
ύ +
I ribose-P
F H4 (4)
5-Hydroxy methyl- cytidylic acid
formylation of aminoimidazolecarboxamide ribotide with 10-formyl-FH4, as shown in reaction 2 (114).
Η H2C—NH2 HjC—N— CHO I
O—C—NH + 5,10-Methenyl-FH4 0 = C — N H + FH4
ribose-P ribose-P Glycinamide Formylglycinamide
ribotide ribotide
hydroxymethyl group of 5-hydroxymethyldeoxycyticylic acid (reaction 4) (118).
It will be noted that, in the synthesis of the methyl group of thymine, F H4 functions not only as the carrier of the single carbon unit (active formaldehyde), but also serves as the hydrogen donor to effect the reduction of the hydroxymethyl to a methyl group (119-121). The re
sulting F H2 must be reduced by TPNH and dihydrofolic acid reductase to F H4 before it can participate again in the synthesis of thymine.
Thymidylate synthetase, which catalyzes the reaction of 5,10- methylene-FH4 with deoxyuridylate, is not inhibited by aminopterin (4- aminopteroylglutamic acid) (120, 121). Aminopterin inhibits thymine synthesis in vivo (122) by inhibiting the reduction of F H2 to F H4 by dihydrofolic acid reductase (123-125).
C. Amino Acid Metabolism
1. Serine-Glycine Interconversions
Folic acid serves as a coenzyme in the conversion of serine to glycine by serine hydroxymethylase, in which the ^-carbon of serine is converted into "active formaldehyde" in 5,10-methylene-FH4 (66, 126-129). Pyri
doxal functions as a cofactor.
pyridoxal
CH2OH—CH—COOH + FH4 > CH2—COOH + 5,10-methylene-FH4 (5)
I serine | N J J2 hydroxymethylase ]N^JJ2
Serine Glycine
2. Synthesis of the Methyl Group of Methionine
Homocysteine is methylated by 5-methyl-FH4 to give methionine (36, 76, 130-132). Two types of reactions for the methylation of homo
cysteine occur. The first is vitamin Bi2-independent (reaction 6a) and involves methylation by iV5-methyltetrahydropteroyltriglutamate [5- methyl-FH4(G3)] (36, 130). Only the triglutamate 5-methyl-FH4 serves as a methyl donor, and the monoglutamate 5-methyl-FH4 is inactive with this enzyme.
The second reaction which is capable of using 5-methyl-FH4 as the methyl donor (reaction 6b) is vitamin B1 2-dependent and requires ATP, FADH2, and magnesium as cofactors (133, 134).
M g+ +
5-methyl-FH4(G3) + homocysteine —> FH4(G3) + methionine (6a)
F A D H2, A T P , Mg++
5-methyl-FH4 + homocysteine » FH4 + methionine (6b)
B12 enzyme
The vitamin Bi2-independent system for methionine synthesis oper
ates in microorganisms such as wild type E. coli (135) and Aerobacter
aerogenes (136), while the vitamin B1 2-dependent system functions in cobalamin-dependent E. coli mutants and in liver (132, 137).
3. Formiminoglutamic Acid
Histidine may be catabolized in a series of reactions to give form- imino glutamate (138, 139). This reacts with F H4 to give glutamic acid and 5-formimino-FH4, according to reaction 7 (26, 62, 63).
COOH COOH
H C N H — C H = N H + FH4 -* HC—NH2 + 5-formimino-FH4 (7) CH2—CH2—COOH CH2—CH2—COOH
Formiminoglutamic Glutamic acid acid (FIGLU)
The catabolism of FIGLU is impaired in folic acid deficiency, and increased amounts of FIGLU are excreted in the urine (140-144). The excretion of FIGLU is increased in rats by the administration of histi
dine (145). A load test of histidine has been used by Luhby et al. (142) to increase the sensitivity of the FIGLU-excretion test for folic acid deficiency in man. Formiminoglutamic acid may be determined by microbiological, chemical, and enzymatic methods. The method that has found the widest use and has the greatest specificity and sensitivity is the enzymatic procedure developed by Tabor and Wyngarden (145a). It depends on the enzymatic reaction of FIGLU with F H4 to yield form- imino-FH4 which is converted on acidification to 5,10-methenyl-FH4. This may be estimated spectrophotometrically by its absorption at 350 τημ. A detailed description of this procedure and the preparation of the enzyme is given in the review by Luhby and Cooperman (112).
Although folic acid is the only vitamin known to be directly involved as a coenzyme in the metabolism of FIGLU, the excretion of FIGLU is also increased in vitamin B1 2 deficiency in rats (143) and in chicks (144).
The excretion of FIGLU on either a folic acid- or a vitamin B1 2-deficient diet is sharply reduced by feeding of 1 to 2% of methionine (143, 144, 146, 147). The reduction in excretion of FIGLU is accompanied by an increased conversion of the 2-carbon of histidine to respiratory carbon dioxide (147), which shows that the FIGLU is being metabolized and the formyl group converted into carbon dioxide.
4. Formiminoglycine
Formiminoglycine is formed during the fermentation of purine by Clostridium acidiunci. This reacts with F H4 to form glycine plus 5- formimino-FH4, as shown in reaction 8 (59-61).
COOH COOH
H2C N H — C = N H + FH4 -> CH2—NH2 + 5-formimino-FH4 (8) Formiminoglycine Glycine
D. Enzymatic Oxidation-Reduction Reactions of Folic Acid
The pyrazine ring of folic acid may undergo several types of enzymatic oxidation and reduction reactions. These reactions, which are reviewed in greater detail elsewhere (86, 148), may be summarized in the following manner.
1. Dihydrofolic Acid Reductase
Dihydrofolic acid reductase reduces F H2 rapidly to F H4 (reaction 9).
Both TPNH and DPNH may serve as hydrogen donors, but in most cases TPNH is the more effective (123, 149-153). Dihydrofolic acid re-
TPNH (DPNH)
FH2 > FH4 (9)
ductase from some sources such as chicken liver (123, 125, 148, 150) will reduce folic acid slowly, while purified dihydrofolic acid reductase from S. faecalis has no measurable effect on folic acid (153). Dihydrofolic acid reductase is strongly inhibited by aminopterin (4-aminopteroylglutamic acid) (124, 125). The binding of aminopterin by dihydrofolic acid re
ductase is so strong that the reaction is practically stoichiometric at concentrations of enzyme present in tissue (124, 154). The amount of aminopterin retained in a nondiffusible form may be used as a measure of the dihydrofolic acid reductase content of tissue (155). Methods for measurement of dihydrofolic acid reductase activity have been described by Futterman and Silverman (25), Blakley and McDougall (156), and Bertino (157).
2. Folic Acid Reductases
An enzyme has been separated from sheep liver which reduces folic acid to F H2 (reaction 10). This enzyme, in contrast to dihydrofolic acid reductase, is a flavoprotein and is relatively insensitive to aminopterin
(158).
FADH2
folic acid • FH2 (10)
A folic acid reductase has been found in Clostridium sticklandii which uses pyruvate (reaction 11) as the hydrogen donor and which is insen
sitive to aminopterin (159).
folic acid + pyruvate + HSCoA -> FH2 + C 02 + acetyl CoA (11)
E. Formylation Reactions of Tetrahydrofolic Acid
1. Tetrahydrofolate Formylase (Formate-Activating Enzyme) Enzymes which catalyze the reaction of FH4, formate, and ATP to give 10-formyl-FH4 (reaction 12) have been obtained from avian liver (160, 161), sheep liver (162-164), Micrococcus aerogenes (165-167), and Clostridium cylindrosporum (28).
M g++
HCOOH + FH4 + ATP ;=± 10-formyl-FH4 + AD Ρ + Pi (12)
This enzyme constitutes 3 % of the weight of C. cylindrosporum and may be readily obtained in crystalline form from this source (28).
2. Isomerization of 5-Formyl-FH4 to W-Formyl-FH^
Leucovorin (5-formyl-FH4) does not participate in formate transfer in purine biosynthesis until it has been converted to 10-formyl-FH4 by activation with ATP (160, 168-170). This reaction proceeds in two steps, as shown in reactions 13 and 14.
5-formyl-FH4 + ATP + H+ • 5,10-methenyl-FH4 + + H20 + A D P + Pi cyclodehydrase
(13)
5,10-methenyl-FH4 + + H20 > 10-formyl-FH4 + H+ (14) cyclohydrolase
The two enzymes cyclodehydrase and cyclohydrolase have been separated from liver by Greenberg et al. (171). The free energy of hy
drolysis of the two formyl derivatives of F H4 has been calculated to be 2000 cal for 5-formyl-FH4 and 9070 cal for 5,10-methenyl-FH4 (58), while that of 10-formyl-FH4 has been estimated to be 6000 cal by Huennekens and Osborn (10) and 7600 cal by Jaenicke (58).
3. Cyclodeaminase
Cyclodeaminase catalyzes the reaction of 5-formimino-FH4 to 5,10- methenyl-FH4 with splitting off of ammonia, as shown in reaction 15
(28, 59). The 5,10-methenyl-FH4 can then be converted by cyclo
hydrolase (reaction 14) into 10-formyl-FH4.
5-fonnimino-FH4 > 5,10-methenyl-FH4 + NH* (15) cyclodeaminase
4. Formylation of Glutamic Acid
The only formylation reaction in which 5-formyl-FH4 participates directly is the formylation of glutamic acid by an enzyme in liver to give iV-formylglutamate, as shown in reaction 16 (172).
glutamic acid + 5-formyl-FH4 ^± formylglutamic acid + F H4 (16)