Section LB
Drug Transference: Drug Metabolism*
Introduction . . . . . . . . . . . . 54
I.B.I. Dissimilation of Drugs . . . . . . . . . 54
1.1. Oxidation . . . . . . . . . . 54
1.2. Reduction . . . . . . . . . . 58
1.3. Hydrolysis 58 1.4. Conjugation . . . . . . . . . . 60
1.5. Chemical Antagonism or Antagonism by Neutralization. . . 64
1.6. Multiple Metabolic Pathways . . . . . . . 65
I.B.2. Factors Influencing Drug Metabolism . . . . 6 6 2.1. Chemical Properties . . . . . . . . . 66
2.2. Effect of Dosage 68 2.3. Route of Application . . . . . . . . 69
2.4. Diet and Drugs 69 2.5. Species Effect 70 2.6. Effect of Sex Differences 73 2.7. Individual Variations . . . . 7 4 I.B.3. General Aspects of Drug Metabolism . . . . . . . 75
3.1. Bio-Inactivation and Detoxication . . . . . . 76
3.2. Bio-Activation . . . . . . . . . . 77
3.3. Evolutionary Aspects . . . . . . . . 82
3.4. Some Practical Consequences . . . . . . . 83
I.B.4. Inhibitors of Drug Metabolism . . . . . . . . 89
4.1. Inhibition of Bio-Inactivation and Detoxication . . . . 89
4.2. Inhibition of Bio-Activation . . . . . . . 96
I.B.5. Assimilation of Drugs . . . . . . . . . . 98
5.1. Metabolites and Parametabolites . . . . 9 8 5.2. Antimetabolites . . . . . . . . . 102
* By E. J. Ariens and A. M. Simonis.
5 3
54 Ε . J . A R I E N S AND Α. Μ. SIMONIS
I N T R O D U C T I O N
The general introduction to this volume outlined t h e factors which govern t h e concentration of a drug in t h e biophase. The preceding chapter discussed t h e processes of t r a n s p o r t of drugs across membranes. This present section will discuss processes of equal importance in t h e maintenance of a d e q u a t e concen
trations of drug in t h e biophase—the effects of metabolism.
Metabolism is usually divided into catabolism and anabolism. Catabolic processes lead to a dissimilation, t h e degradation or breakdown of t h e meta
bolites, which in this way are eliminated. Anabolic processes lead to an assimi
lation, t h e incorporation of t h e metabolites in t h e body constituents. For drug metabolism a similar differentiation is useful: (a) Dissimilation leads t o bio
chemical changes in t h e drug molecule with, as a final result, t h e elimination of t h e drug; (b) Assimilation leads to an incorporation of t h e drug in t h e body constituents and, therefore, a fixation of t h e drug.
Dissimilation as a rule leads t o a decrease in t h e effective concentration of t h e active drug. However, a temporal increase as a result of bio-activation of originally inactive, or less active, compounds can t a k e place. Assimilation of t h e drug molecules means t h a t there will be a prolonged action even after t h e free drug has disappeared from t h e biophase. Assimilation of t h e drug and change in, or inhibition of, enzyme action by t h e drug in a number of cases is responsible for its pharmacological action (17a, 47,128,129).
I.B.1. DISSIMILATION OF D R U G S
The main routes of drug catabolism will be stressed. They can be categorized into 4 general types of chemical alteration: (a) oxidation; (b) reduction;
(c) hydrolysis; and (d) conjugation or synthesis.
I.B.1.1. Oxidation
Many of t h e oxidative changes of drugs are brought a b o u t by r a t h e r non
specific enzymes. These are located in t h e endoplasmatic reticulum of t h e liver cells. After homogenization of t h e cells, t h e microsomes are obtained from this reticulum (145). I n some reactions t h e more specific oxidoreductase systems of t h e mitochondria t a k e p a r t . Many of t h e rather nonspecific oxidative sys
tems, such as " h y d r o x y l a s e s " in t h e microsomes of t h e liver cells, require
I.B.6. Drugs Acting Indirectly . . . . . . . . . 105
6.1. Protection of Endogenous Compounds. . . . 1 0 5 6.2. Release of Endogenous Compounds . . . . . . 105 6.3. Release or Displacement of Drugs from Silent Receptors . . 107 Concluding Remarks . . . . 1 0 9
References . . . . . . . . . . . . . 109
Ι , Ι . Β . DRUG TRANSFERENCE: DRUG METABOLISM 55 oxygen a n d reduced triphosphopyridine nucleotide (NADP). * An intermediate formation of activated oxygen in t h e form of a peroxide, which m a y serve as a
" h y d r o x y l donor," is assumed (33, 37, 38a, 51, 77, 78a).
The most common t y p e of oxidation of a drug molecule is t h e a t t a c k on t h e alkyl chains, including those bearing carbonyl, aldehyde, carboxyl, a n d amino groups. As a rule, t h e oxidation starts as an ω-oxidation, resulting in a conver
sion of t h e terminal carbon a t o m to a carboxyl group, followed by shortening of t h e chain b y j8-oxidation, also called (ω — 1) or penultimate oxidation (76b, 214.)
I n t h e case of amines, oxidative deamination occurs (17, 22). This deamina- tion is catalyzed by different types of enzymes. Phenylethylamine, t y r a m i n e , dopamine, normethanephrine a n d 5-hydroxytryptamine are deaminated by monoamine oxidase. The α-methyl-substituted amines, like a m p h e t a m i n e a n d ephedrine, are resistant against t h e action of this enzyme. They m a y be de
aminated by a microsomal deaminase (11,15). Histamine is deaminated b y a diamine oxidase.
A second t y p e is t h e oxidative dealkylation. B y this process, m e t h y l a n d ethyl groups are eliminated from amines a n d alkyl ethers. Examples are t h e conversion of morphine into normorphine, of codeine into morphine, a n d of phenetidine into p-aminophenol. Also, various iV-methylated cyclic ureides, e.g., iV-methylbarbiturates are demethylated. An example of this t y p e of reac
tion is t h e conversion of mephobarbital t o phenobarbital (73,133b, 150).
A t h i r d t y p e of oxidation is t h e introduction of O H groups into aromatic rings, for instance, t h e formation of p-aminophenol from aniline.
I n drug catabolism, t h e oxidative reactions seem t o prevail. As far as ali
phatic chains are concerned, oxidation runs most smoothly when straight chains are involved. Branching brings on difficulties, while tertiary-substituted carbon atoms form a nearly insurmountable barrier t o t h e oxidative process (108, 212). Secondary a n d t e r t i a r y alcohols or alcohols with t h e carbonyl group on a t e r t i a r y carbon a t o m are h a r d t o oxidize. They are usually conjugated t o glucuronides (see Table I). F u r t h e r examples are pinacol, trichloro- a n d tri- bromoethanol, a n d 2,2,3-trichlorobutanol. The analogous aldehydes t e n d t o be reduced instead of oxidized. Chloralhydrate a n d butylchloralhydrate are con
verted t o trichloroethanol a n d 2,2,3-trichlorobutanol, respectively. Analogous, too, is t h e reduction of 1,3-dichloroacetone t o 1,3-dichloroisopropyl alcohol a n d t h e reduction of other ketones (212).
The conclusion m a y be t h a t , as a rule, " p a c k e d " alcohols are less vulnerable t o oxidative processes. I n regard t o this, it is interesting t o note t h a t m a n y of
* In accordance with the recommendations of the Commission on Enzymes of the International Union of Biochemistry, 1960, NAD (nicotinamide-adenine dinucleotide) has been substituted for DPN (diphosphopyridine nucleotide); NADH2, the reduced form, for DPNH; NADP (nicotinamide-adenine dinucleotide phosphate) for TPN (triphospho
pyridine nucleotide; NADPH2, the reduced form, for TPNH.
56 Ε . J . A R I E N S AND A. M. SIMONIS
the so-called minor tranquilizers are alcohols of t h e t y p e just mentioned or their carbamates (see Table I I ) .
E t h y l alcohol also is a tranquilizer. I t is difficult, however, to maintain an effective ataractic level of this drug in body fluids. This can only be attained by frequent medication with small doses. If one tries t o cover a longer period with a single dose, t h e required concentration is exceeded a n d instead of being
T A B L E I
GLUCURONIDE CONJUGATION OF PRIMARY, SECONDARY, AND TERTIARY ALCOHOLS IN RABBITS"
Primary G.C.6 Secondary G.C.6 Tertiary G.C.*
alcohol (%) alcohol (%) alcohol (%)
—If:— OH 10 C—(ί—C C—C—OH 0.5 C—G—OH 10 C—C—OH 24
J — C — O H 14 C — C — C C—C—G—OH 0.9 C—C—G—OH 14 C—C—<J—OH 58
I
c
— O H 45 C — C — C — C C—C—C—C—OH 1.8 C—C—C—G—OH 45 C—C—C—C—OH 57
C—C—C—C —C —OH 6.7 C—C—C—C—G— OH 54 — c — c — c — C
a Selected from data presented by Williams (214).
b G.C. = Glucuronide conjugation.
tranquilized, t h e biological object becomes intoxicated. The more oxidation- resistant alcohols, which are also more fat-soluble t h a n ethyl alcohol, seem t o be more suitable in this tranquilizing ability.
For drugs with chains bearing carboxyl groups, blockade of β-oxidation, which can be realized by α-substitution, decreases t h e vulnerability of t h e drug to oxidation. Substitution on t h e α-carbon atom m a y be considered as a
" p a c k i n g " of t h e carboxyl group. A higher fraction of t h e acid then appears in t h e conjugated form in urine. Examples are trimethylacetic acid (61), α-ethylbutyric acid, and α-ethylhexanoic acid (109), benzoic acid, a-phenyl- acetic, and α-diphenylacetic acid (212). I n the case of phenylalkyl derivatives,
Ι , Ι . Β . DRUG TRANSFERENCE: DRUG METABOLISM 57 depending on an odd or even n u m b e r of carbon atoms in t h e chain, benzoic acid or phenylacetic acid will be t h e respective end-products.
For t h e amines, an analogous situation exists. While most aliphatic amines are deaminated oxidatively, heavily " p a c k e d " amines, as for instance mec- amylamine a n d pempidine, are excreted largely unchanged in t h e urine (4, 135,183a, 185,196,199) (see Table I I I ) .
TABLE II
"PACKING" OF THE CARBINOL GROUP IN VARIOUS MINOR TRANQUILIZERS
Ϊ
C I — C — C — O H
h
Trichlorethanol
Η
?
C
C—C—C—A—C—OH h
Meprobamate (carbarn.)
C — C — ^ — O H I
c
Amylenehydrate Phenaglycodol
— O H
C — C — £ — O H
Methylparafynol
III c
O H
Ethinamate (carbarn.)
0 —C \ C — ( J — O H
h III Br
c
Repocal
I n all t h e examples given, a steric hindrance of t h e enzymes concerned with drug catabolism is feasible. The principle of " p a c k i n g " m a y be tried as a means of preparing more stable drugs, a t least as far as oxidative breakdown is concerned. An objection is t h a t t h e steric hindrance m a y concern n o t only the catabolic enzymes, b u t also t h e specific receptors with which t h e drugs have t o interact in order t o produce their effect (193a). For certain biological actions, a " p a c k i n g " of t h e active group in t h e drug seems t o be of a d v a n t a g e . Examples are t h e blood-pressure-lowering a n d t h e ganglion-blocking amines represented in Table I I I (185,196,199) a n d t h e minor tranquilizers represented in Table I I .
58 Ε . J . ARIENS AND Α. Μ. SIMONIS
T A B L E I I I
"PACKING" OF THE AMINO GROUP IN VARIOUS HYPOTENSIVE DRUGS
c
c
Mecamylamine Dimecamine
C — Ν — C
c
4
Penbutamine Pempidine
I.B.1.2. Reduction
The organism makes use of reduction in some cases where t h e oxidative enzymes fail. The conversion of chloralhydrate and butylchloralhydrate into t h e corresponding alcohols are examples. Many ketones, especially cyclic ketones, are reduced, for instance, progesterone into pregnanediol (105). For some molecular configurations, reduction seems to be t h e only possible cata- bolic route, viz., for t h e azo configuration, e.g., t h e conversion of prontosil into sulfanilamide, and for t h e nitro groups, e.g., t h e conversion of nitrophenols into aminophenols.
Many esters foreign t o t h e body are hydrolyzed by t h e enzymes of blood plasma and cells. Where serum esterases are concerned, t h e corresponding amides are frequently more stable t h a n t h e esters. Procainamide is more stable t h a n procaine, for instance. Substitutions on t h e α-carbon atom of t h e alcohol and/or the acid portion of t h e ester, tend t o stabilize it against hydrolysis by plasma esterases (123). This is again an example of packing of t h e essential group (see Fig. 1). Here, too, a steric hindrance with respect t o t h e active site on t h e esterase is feasible.
The resistance of lidocaine (Xylocaine) and 2,6-dimethoxybenzoylcholine against enzymatic hydrolysis is a further example of t h e influence of packing of t h e vital points in drugs on their stability (124, 194).
Thomas and Stoker (194) studied t h e enzymatic (acetylcholinesterase) and nonenzymatic (OH ions) hydrolysis of various or^o-substituted benzoyl- cholines and t h e inhibition of t h e hydrolysis of acetylcholine by acetylcholin
es.*.3. Hydrolysis
Ι , Ι . Β . DRUG T R A N S F E R E N C E ; DRUG METABOLISM 59 esterase b y means of these benzoylcholines. This s t u d y gives a good basis for t h e discussion of some factors t h a t play a role in t h e relation between t h e chemical structure a n d t h e r a t e of hydrolysis. The change in t h e nonenzymatic hydrolysis as a result of t h e various substitutions in benzoylcholine is due t o a change in t h e stability of t h e ester bond as such. I t m a y result from inductive effects a n d other effects leading t o a change in t h e intramolecular distribution of charges. The enzymatic hydrolysis is also dependent on t h e ester bond stability. Here a steric hindrance on t h e enzyme surface, especially with respect to t h e approach between t h e vital spot (the ester group in t h e drug) a n d its complement on t h e enzyme, a n d a change in t h e affinity between t h e drug a n d its receptors on t h e enzyme, have t o be t a k e n into consideration.
C ι C— C — O - C - C — N - C
II ι I
Ο A C
acetylcholine j ACh esterase
ι ι
c — c —o- c II I
ο
Γ<5Ί ι 1c I - N - C
I c
methacholinermore resistant to the esterase
H2N I
c- o —c
11 ι I
•C—Ν ' L Jj
c — c
c - c ι
rate of hydrolysis in human serum
R2 y / m l / h r
Η Η 500
Η CH3 15
CH3 CH3 0
FIG. 1. Influence of "packing" of the ester group on the stability of drugs.
F r o m Table IV.A it m a y be seen t h a t t h e nonenzymatic hydrolysis, t h e en
zymatic hydrolysis, and t h e affinity of t h e benzoylcholines t o t h e enzyme acetylcholinesterase, as represented by anti-acetylcholinesterase activity, are not strictly correlated. This means t h a t each of t h e three processes depends on t h e structure in its own way. The decrease in t h e r a t e of hydrolysis does not necessarily lead t o a decrease in cholinergic a c t i v i t y ; this m a y even increase (194a, 194b).
Possibly also t h e resistance of certain penicillins against penicillinase is based on such factors as mentioned for t h e benzoylcholines. 2,6-Dime- thoxyphenylpenicillin is not only penicillinase-resistant b u t it is also found t o act as a competitive inhibitor of t h e B. cereus penicillinase (161). The bond in penicillin split by penicillinase is t h e lactam bond which, although situated closely t o t h e 2,6-substituted ring, is not directly adjacent t o it as in t h e case of t h e benzoylcholines mentioned. The bis-or^o-substituted derivative, 5-methyl-3-phenyl-4-isoxazolyl-penicillin is also a competitive inhibitor of
60 Ε. J . A R I E N S AND A. M. SIMONIS
penicillinase. Experiments of Citri etal. (48a, b, 75b) m a k e it probable t h a t as a result of the or^o-substitution in t h e side chain of penicillin t h e conformation of t h e active site on t h e penicillinase is changed in such a way t h a t t h e enzymatic hydrolysis cannot take place. I n general t h e character of t h e side-chain in t h e various penicillins, especially α-substitution, clearly influences the rate of hydrolysis by penicillinase (10a, 48a, 54a, 75b).
T A B L E I V . A
NONENZYMATIC HYDROLYSIS, ENZYMATIC HYDROLYSIS ( A C H ESTERASE), AND ANTI- A C H ESTERASE ACTIVITY IN VARIOUS Ortho-SUBSTITUTED BENZOYLCHOLINE COMPOUNDS'*
^ T J- O - O - C - C .
?
+—Ν—C 1 c
Nonenzymatic
hydrolysis6 Enzymatic hydrolysis6
Anti-ACh esterase activityc
2-H 1.00 1.00 1.00
2-CH3 0.65 0.24 3.10
2-C1 1.40 1.30 8.81
2-Br 1.02 0.70 4.20
2-J 0.74 0.24 14.50
2 - N 02 1.40 0.19 8.58
2-OCH3 0.77 0.73 6.64
2,6-diCl 0 0 6.26
2,6-diCH3 0 0 —
2,4,6-triBr 0 0 5.80
2,4,6-triN02 3.40 0 8.23
° The ratio enzymatic: nonenzymatic hydrolysis for benzoylcholine is 5.30.
After Thomas et al. (194)
b Relative rate; benzoylcholine = 1.00.
c Relative activity tested on horse serum cholinesterase; benzoylcholine = 1.00.
Interesting studies on t h e relations between t h e chemical structure of amides and t h e rate of hydrolysis by amidases from r a b b i t liver were performed by B r a y et al. (27). Some of their results are summarized in Table I V . B .
The decrease in t h e velocity of hydrolysis for compounds with higher values for n, m a y be ascribed t o a decrease in t h e concentration of free molecules.
This results from t h e increasing tendency of these compounds t o form micelles (91).
I n addition to esterases and amidases, glucosidases also t a k e p a r t in enzym
atic hydrolysis.
I.B.1.4. Conjugation
I n conjugation, drug molecules are bound t o other molecules by elimination of water a n d formation of esters, amides, and other substitution products (26).
Ι , Ι . Β . DRUG T R A N S F E R E N C E : DRUG METABOLISM 61
C H3( C H2)NC O N H2 / \ _ ( C H2)N— C O N H2
η (%) (%)
0 2 4 4
1 3 1 7
2 1 3 1 0 5
3 5 5 5 8
4 9 0 2 6
5 9 0
6 6 3
7 3 8
8 1 8
9 8
1 0 8
1 1 3
1 2 8
1 3 2
1 4 3
a From Bray (27).
One or both reacting molecules t a k e p a r t in a n activated state. Usually, t h e active molecule is derived from metabolic processes of t h e organism. Well- known examples are *' active " acetate, sulfate, a n d glucuronic acid. Sometimes t h e drug or its metabolites, e.g., benzoic acid a n d phenylacetic acid, are acti
vated, t h a t is, bound t o coenzyme A (CoA). The active products are usually formed b y mitochondrial intervention in t h e liver cells. A transferase accom
plishes t h e transfer of t h e activated molecule t o t h e other members of t h e conjugation pair.
These transferases sometimes demonstrate a certain degree of specificity with respect t o t h e drug a n d are also located in t h e mitochondria of t h e liver ceUs (35, 60, 121a,b).
1. Conjugation with glucuronic acid occurs with alcohols resisting oxidation (demonstrated in Table I) a n d with phenolic OH-groups. Carboxyl groups, especially if t h e y are located on a n aromatic nucleus or close t o it, are in some cases converted t o glucuronides, e.g., benzoic acid, phenylacetic acid a n d diphenylacetic acid. T h e same holds t r u e for carboxyl groups with a heavy a-carbon-substitution. Take, for instance, trimethylacetic acid a n d t e r t i a r y butylacetic acid (61). " A c t i v e " glucuronic acid, uridine diphosphoglucuronic acid, is t h e source of glucuronic acid (Fig. 2) (60, 87). Some glucuronic acid
T A B L E I V . Β
PERCENTAGE OF AMIDES HYDROLYZED IN 5 H R AT P H 7 . 4 BY RABBIT-LIVER EXTRACT"
62 Ε. J . A R I E N S AND A. M. SIMONIS
transferases in liver microsomes effect t h e transfer under formation of ether-, ester-, and other glucuronides, depending on t h e substrate (60, 60a, 214).
2. Like glucuronic acid, amino acids are conjugated with acids. The foreign
COOH n N
Κ ° ° V Y
(oh Y — O — P — O — P — O — C ^ ^ O ^ ^ N ^ ^ HtT f OH OH
OH
HO OH uridine diphosphoglucuronic acid
OH CI
+ H O - C - C — C I I I
ci
trichloroethanol
COOH
Μ i1 jfoH ) 2- 0 - C — c— CI + uridine-diphosphate
HO ( CI OH
trichloroethyl glucuronide
FIG. 2. Glucuronic acid conjugation of trichloroethanol.
acids, activated as acyl-CoA, react with t h e amino groups of an amino acid.
Mammals usually conjugate with glycine, b u t m a n a n d ape sometimes with glutamine. Examples are t h e formation of hippuric acid, phenaceturic acid,
Ο
// \\
C O O H + A T P + HS — R - A M P + pyrophosphate benzoic acid coenzyme A benzoyl-coenzyme AΟ , V Ο
C - S — R + H?N — C — C O O H γ \ ) C~ N H — C — C O O H + H S — R
benzoyl-coenzyme A glycine hippuric acid coenzyme A FIG. 3. Glycocol conjugation of benzoic acid.
nicotinuric acid from t h e corresponding acids, benzoic acid, phenylacetic acid, and nicotinic acid (Fig. 3). Birds use ornithine instead of glycine; t h e y convert benzoic acid to ornithuric acid, which is t h e analog of hippuric acid. Certain spiders use glutamic acid and arginine for conjugation of aromatic acids (166a).
Ι,Ι.Β. DRUG TRANSFERENCE: DRUG METABOLISM 63
Ο Ο II II HO—S—Ο—Ρ—Ο—C
Ο OH I I
Ο OH HO—l>-OH
ο
IIadeno sine - 3' - phosphate - 5' - phospho sulfate
HO—S— Ο - γ y + a d e n o s i n e - 3 ' , 5'-diphosphate Ο
\=s
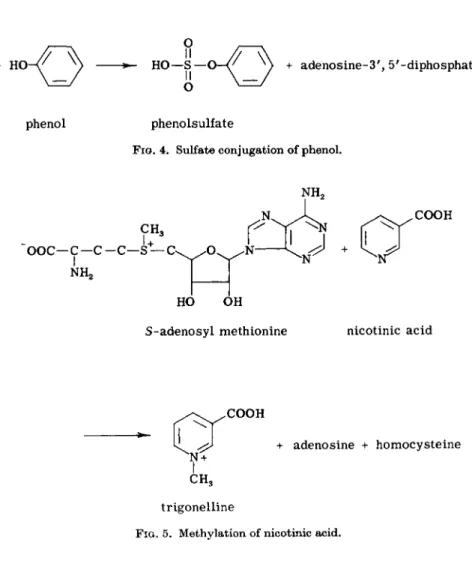
phenol phenolsulfate
FIG. 4. Sulfate conjugation of phenol.
CH3
u
ooc—c—c - C —
NH2S —
COOH
HO OH
S-adenosyl methionine nicotinic acid
COOH
+ adenosine + homocysteine
trigonelline
FIG. 5. Methylation of nicotinic acid.
64 Ε . J . ARIENS AND Α. Μ. SIMONIS
3. Acetylation is another t y p e of conjugation. Foreign compounds bearing amino groups not suitable for oxidative deamination, mainly t h e aromatic amines, e.g., t h e anilides, are conjugated with acetic acid ( " a c t i v e " acetate) derived from acetyl-CoA. The acetylation of various sulfonamides m a y serve as an example (120a).
4. Phenolic hydroxyl groups m a y be conjugated with glucuronic acid b u t also with sulfuric acid, which results in t h e formation of ethereal sulfates.
" A c t i v e " sulfate, 3'-phosphoadenosine-5'-phosphosulfate serves as a source for sulfate (Fig. 4) (23,82b). A sulfuric acid transferase brings about t h e transfer to t h e foreign compound. The formation of paracresylsulfuric acid from para- cresol is an example.
/ \
iOOH Ο iodobenzol c y s t e i n e acetyl c o e n z y m e A
I ft \ \ — S — C — C — N H — C — C H3 + R—SH COOH Ο
/>-iodophenylmercapturic acid c o e n z y m e A FIG. 6. Cysteine conjugation of iodobenzol.
5. Methylation is another t y p e of bio-transformation. I t takes place with nitrogen in aromatic heterocyclic rings, for instance, t h e formation of iV1- methylnicotinamide from nicotinamide (165). Phenolic OH-groups in t h e catecholamines, e.g., epinephrine (13,14, 82), and in steroids, e.g., estrone a n d estradiol, m a y be methylated t o m e t h o x y compounds (71). #-Adenosylmeth- ionine probably serves as a source for t h e methyl groups in this case (Fig. 5) (12, 16a).
6. Finally, mercapturic acid synthesis m a y be mentioned, whereby cysteine is substituted for hydrogen on aromatic rings, or for halogen atoms on aromatic rings or aliphatic chains (Fig. 6). This is of importance in t h e dehalogenation of foreign compounds, for example, halogenobenzene derivatives (18, 26b, 30).
I.B.1.5. Chemical Antagonism or Antagonism by Neutralization This t y p e of antagonism has something in common with conjugation. Here, too, t h e inactivation of a drug takes place as a result of its coupling with other molecules. This time t h e molecules are not of endogenous origin, as were glucuronic acid a n d acetic acid, nor are enzymes concerned with their binding
Ι,Ι.Β. DRUG TRANSFERENCE: DRUG METABOLISM 65 t o t h e drug. B o t h reacting compounds are drugs; one as a rule is a toxin, t h e other an antidote. The t y p e of reaction is often chelation. I n t h e paragraph concerned with chelation as a mechanism of t r a n s p o r t (Section I.A.1.3.C), various examples of this t y p e of bio-inactivation are mentioned: Ca++<-» cit
r a t e , Hg++<->BAL,* Pb++<->EDTA,f Cu++<->penicillamine. Some more ex
amples, curare<-> congo red, a n d curare<->germanin, will be mentioned in t h e following chapters. A q u a n t i t a t i v e theory on this t y p e of bio-inactivation is proposed by Gaddum (75) a n d Ariens (9). Because of t h e similarity of t h e dose- response curves for t h e competitive antagonism a n d for t h e chemical antagon
ism, it will get special a t t e n t i o n in Section I I . B . 3 . I.B.1.6. Multiple Metabolic Pathways
Compounds foreign t o t h e body m a y be catabolized simultaneously along different roads.
1. One chemical group in t h e drug m a y be processed in different ways. Take, for instance, phenol. I t is p a r t l y converted t o its glucuronide a n d t o its sulfuric acid ether. Benzoic acid given orally t o h u m a n s , dogs, or pigs m a y be recovered from t h e urine p a r t l y conjugated with glycine t o hippuric acid a n d p a r t l y as t h e glucuronide (212).
2. A drug molecule m a y be a t t a c k e d a t different points. An example is
^-aminosalicylic acid. This compound contains 3 groups suitable for conjuga
tion ; an amino group bound t o t h e ring; a carboxyl group bound t o t h e ring;
a n d a phenolic OH-group. After oral administration, a large fraction (42%) is excreted conjugated a t t h e carboxyl group with glycine, 2 9 % is found in t h e urine as an iV^-acetylated derivative, 2 5 % is excreted unchanged, while little if a n y of the drug is conjugated a t t h e phenolic OH-group (120).
N o t only conjugations b u t also different types of metabolic processes m a y t a k e place simultaneously. Salicylic acid is excreted in t h e urine, mainly conjugated with glycine a t t h e carboxyl group, as salicyluric acid ( 5 0 - 6 0 % ) ; a fraction is conjugated with glucuronic acid (20-25%). Two types are found, one probably conjugated in t h e carboxyl group, t h e other in t h e phenolic OH- group. A small fraction appears as oxidation products. One of these is gentisic acid (2,5-dihydroxybenzoic acid) (5, 84, 181). Also, for morphine and related compounds, a variety of metabolic changes occur in t h e body (121). Blockade of one metabolic p a t h w a y can lead to an increased metabolism of t h e drug along other routes (165a).
For more detailed and extensive information on drug metabolism t h e reader is referred t o William's well-known monograph (212, 214) a n d t o various review articles (15, 26a, 34, 35, 68, 68a, 78b, 121,122, 138a,b, 164,189, 208). A valuable discussion of t h e kinetics of metabolism of foreign organic compounds is given by Teorell (193) a n d by B r a y (28).
* British Anti-Lewisite.
t Ethylenediaminetetraacetic acid.
66 Ε . J . A R I E N S AND Α. Μ. SIMONIS
I.B.2. FACTORS I N F L U E N C I N G D R U G METABOLISM The relation between t h e dose of a drug and t h e effective concentration of t h e active drug is t h e resultant of a n u m b e r of detail processes. The various catabolic routes for a drug can be considered as parallel flow systems. The drug will be divided among these systems according to t h e capacity of each of t h e m . The relative capacity of t h e various catabolic systems for a drug, a n d therefore t h e fraction of t h e drug processed by each of t h e m , depends on various factors, as for instance: (a) t h e chemical properties of t h e d r u g ; (b) t h e dose; (c) the route of administration; (d) t h e diet a n d drugs given t o t h e a n i m a l ; (e) t h e animal's species; (/) its sex; a n d (g) its individual variations.
I.B.2.1. Chemical Properties
The way in which a certain group in a compound, e.g., a phenolic OH-group, is processed varies with t h e place of such a group in t h e molecule. Table V gives
T A B L E V
INFLUENCE OF RING SUBSTITUTIONS ON METABOLISM OF HYDROXYBENZALDEHYDE AS S E E N BY THE EXCRETION PRODUCTS IN URINE OF RABBITS"
% of dose* excreted as:
Ether-soluble Ester Ether Ethereal acid glucuronide glucuronide sulfate
2-Hydroxybenzaldehyde 75 18 9 3 3-Hydroxybenzaldehyde 75 9 9 7 4-Hydroxybenzaldehyde 67 4 16 9
* From Bray (28).
b Dose level 0.4 gm/kg.
examples of t h e influence of t h e substitution of OH-groups on t h e ortho, meta or para place on t h e t y p e of conjugation of hydroxybenzaldehyde in t h e r a b b i t (28). Table V I demonstrates t h e influence of ortho-, meta- a n d para-chloro- substitution in acetanilide on metabolism of t h e drug in r a t s (141).
Differences in charge distribution on the drug molecules often play a role in drug metabolism (95a). I t has been observed, for instance, t h a t t h e r a t e of acetylation of amines is related to the electronic charge on the amino nitrogen (if not protonated). The rate of deacetylation of iV^-acetyl amines is reported as correlating with the dipositivity (the positive charge on the nitrogen atom and on the carbonyl carbon atom) of the iV-acetyl bond (146c).
Differences in t h e biological activity of t h e stereoisomers of a compound strongly suggest t h e implication of drug-receptor interactions. Such a stereo-
Ι , Ι . Β . DRUG T R A N S F E R E N C E : DRUG METABOLISM 67 specificity m a y have its origin not only in t h e interaction of t h e drug with t h e receptors essential t o t h e effect studied, b u t in t h e processes concerned with t h e drug transference as well. Serum esterases only hydrolyze t h e L( + ) isomer of acetyl-jS-methylcholine (79). After a dose of racemic mepacrine, an optically active mepacrine appears in t h e urine (85). The antipodes in d^glutethimide are degraded in different ways (42b, 113m). Certain permeases, specific penetra
tion systems found in bacteria, exhibit stereo-specificity (50). The same m a y be t r u e for t h e displacement of drugs from silent receptors (19).
T A B L E V I
INFLUENCE OF RING SUBSTITUTION ON METABOLISM OF CHLOROACETANILIDES AS SEEN IN THE EXCRETION PRODUCTS IN THE U R I N E OF RATS AFTER INJECTION OF 2-, 3-,
AND 4-CHLOROACETANILIDE ( C l8 e)a
Products
-C—CH8
ο
II2-chloroacetanilide 3-ehloroacetanilide 4-chloroacetanilide (%) (%) (%)
4-OH-Derivatives6 45.10 57.32 —
2-OH-Derivatives* — — 61.96 6-OH-Derivatives* 8.53 26.46 — Deacetylation 28.28 8.26 2.52 Unchanged 3.18 1.34 4.63 Chloride ions 0.62 1.98 1.18
"From Newell (141).
b After acid hydrolysis of the glucuronides, ethereal sulfates, etc.
The relatively high, in vivo amine-oxidase-inhibiting activity of isopropyl- hydrazine with respect t o n a t u r a l amino acids, as compared with t h e u n n a t u r a l , is t h o u g h t t o be related t o specific t r a n s p o r t mechanisms n o t present in in vitro experiments. I n t h e latter, t h e differences in amine-oxidase-inhibiting activity toward n a t u r a l a n d u n n a t u r a l compounds are m u c h smaller (15).
The d-isomers of various amino-acid derivatives inhibit, in a competitive way, t h e hydrolysis of t h e Z-isomers b y a-chymotrypsine (208). The d-isomer of a-phenoxyethyl phenylpenicillin inhibits t h e hydrolysis of t h e Z-isomer by staphylococcal penicillinase (186). The breakdown of Z-histidine b y liver histi- dase is inhibited by d-histidine (65). There is a competition between t h e enan- tiomorphs of methionine during intestinal absorption (9p, 100).
I n t h e isomer which is not or only slowly metabolized, one of t h e groups concerned m a y have a wrong orientation with respect to t h e active site on t h e enzyme. A group essential for the reaction m a y point in t h e wrong direction, a
68 Ε. J . ARIENS AND Α. Μ. SIMONIS
group not essential for the reaction m a y cause a steric hindrance, etc. As mentioned before, a " p a c k i n g " around the vital group in a drug molecule like α-methyl substitution and or£/io-substitution often strongly influences drug metabolism (see Table I, Fig. 1, a n d Table IV.A). The cause of this m a y be steric hindrance; however, in oxidative processes the absence of α-Η atoms which possibly play an essential role in the process m a y be the cause as well (20).
Most catabolic changes of drugs, especially oxidations, reductions, and con
jugations, t a k e place intracellularly. Many types of hydrolysis occur mainly in t h e plasma. Penetration of t h e cell and t h e functional units of t h e cell, microsomes and mitochondria, is essential for m a n y metabolic processes. This means t h a t a certain degree of lipid solubility will be an advantage t o these processes. Take, for instance, t h e compounds chlorothiazide and flumethiazide as compared t o t h e more lipophilic hydrogenated compounds, dihydrochloro- thiazide and dihydroflumethiazide. These lipophilic compounds are absorbed more completely from the g u t ; t h e volume of distribution is larger for dihydro- chlorothiazide t h a n for chlorothiazide. The " d i h y d r o " compounds are reab
sorbed in t h e tubules and, therefore, are excreted less rapidly in t h e urine.
Chlorothiazide is excreted in urine completely unchanged, while t h e " d i h y d r o "
compounds are excreted partly metabolized (132).
Many strong bases, e.g., a, ω-bis-trimethylammonium compounds (hexa- methonium, decamethonium, etc.) a n d strong acids, e.g., sulfonic acids, ethereal sulfates, and phthalic acid are slowly absorbed from t h e gut, while t h e fraction reabsorbed in t h e tubules is small. These drugs are excreted mainly unchanged in t h e urine.
/.β.2.2. Effect of Dosage
The fraction of t h e dose of a drug subjected t o bio-transformation by a certain metabolic route m a y v a r y with t h e dose. I n h u m a n s when a dose of 400 mg andosterone is given orally, 4 . 4 % is recovered as t h e sulfate, a n d 4 8 % as t h e glucuronide. When a dose of 4000 mg is given, t h e recoveries are 2 1 % and 47 %, respectively (167). An increase in t h e doses of phenol given t o rabbits results in a decrease in the ratio sulfate conjugation .glucuronide conjugation.
This is probably because t h e sources of sulfate are restricted (29).
At a certain dosage the optimal capacity of a metabolic system m a y be reached. A further increase of t h e dosage will lead t h e n t o a switch t o other systems.
I t m a y be expected when combinations of drugs are given which are proc
essed by t h e same metabolic system, t h a t a m u t u a l interference will occur on t h e basis of competition for active sites on enzymes, etc. p-Aminobenzoic acid is found t o compete with salicylic acid and with benzoic acid for t h e glycine conjugation system (181).
Drugs can compete for common metabolic systems. Probenecid reduces
Ι , Ι . Β . DRUG T R A N S F E R E N C E : DRUG METABOLISM 69 in vitro conjugation with glycine of p-aminobenzoic acid (PABA). The reduc
tion in excretion of t h e PABA-glycine conjugate by probenecid m a y be p a r t l y due to t h e interference with t h e conjugation (20a).
I.B.2.3. Route of Application
This will be influential on drug metabolism for drugs which are rapidly metabolized in t h e liver, especially if oral a n d parenteral administration are compared. The liver plays an i m p o r t a n t role in drug metabolism. Examples of differences in drug metabolism dependent on t h e route of application have been described for ^-aminosalicylic acid (134), for cortisone a n d Prednisone (149), a n d for p a r a t h i o n a n d paraoxon (93). If drug metabolism in t h e liver leads t o a bio-inactivation, t h e parenteral application will be t h e most effective one. If bio-activation takes place in t h e liver, t h e oral route m a y be preferable (Section I.B.3.2).
Ι.Β.2Λ. Diet and Drugs
The fractions of benzoic acid excreted as hippuric acid, a n d as benzoylglu- curonide by t h e pig, vary with t h e supply of glycine in t h e diet. W i t h a protein- free diet, 6 0 % of t h e excreted benzoic acid is in t h e hippuric acid form; t h e addition of gelatin, rich in glycine, increases t h e hippuric acid fraction t o 8 9 % (53).
Animals on diets poor in sulfur-containing compounds, have difficulty in t h e bio-transformation of materials usually excreted as mercaptans. The ad
dition of cysteine, cystine, or methionine leads t o an increased excretion of mercapturic acid conjugates. Sulfate conjugation of phenols given in high doses is increased by feeding t h e animals sulfate precursors like I-cystine a n d sodium sulfite. Glucuronide conjugation is t h e n decreased (29). A counterpart of these dietary requirements is t h e induction of a deficiency in cysteine as a result of t h e administration of high doses of compounds t h a t require cysteine for their conjugation. An instance of this process is demonstrated when bromo- benzene is given t o young animals on diets poor in t h e sulfur-containing amino acids (187,188).
Injection of glycine or glucuronic acid strongly decreases t h e toxicity of salicylic acid in dogs; t h e excretion of salicylglucuronic acid increases (78).
Administration of glucose, fructose, or glucuronolactone can accelerate glucuronide formation a n d excretion (18a, 94a).
Adaptive responses of t h e body t o drugs by increasing t h e metabolizing enzymes are common (114c, 134a). Such an a d a p t a t i o n is " crossed" for drugs processed along t h e same metabolic way. P r e t r e a t m e n t of r a t s with pheno- barbital increases t h e liver activity with respect to t h e metabolism of hexo- barbital and other drugs. A decrease in duration of anesthesia for barbiturates like hexobarbital is t h e result (50a, 113b, 115a, 118,158,158b). This is found to be due t o an increased inactivation of t h e drug.
70 Ε. J . ARIENS AND Α. Μ. SIMONIS
Changes in drug metabolism induced by p r e t r e a t m e n t with drugs can cause a tolerance for other drugs (113d, e,f, g,j, k, 114b, 158a, c). This will be the case if the metabolic process results in a bio-inactivation of these drugs. If, however, a bio-activation takes place, the p r e t r e a t m e n t m a y result in an increased sensitivity (see Sections LB.3.1 and 3.2.) As a m a t t e r of fact, application of drugs can lead to a change in metabolism of endogenous compounds, too, e.g., steroid hormones (76a; see also 21 and 156).
An adaptive increase of drug metabolism induced by drugs such as pheno
barbetal and methylcholanthrene can be suppressed by inhibitors of protein synthesis like ethionine (50b, 113h).
Administration of various drugs stimulates t h e conversion of glucose t o glucuronic acid. This is often associated with increased excretion of ascorbic acid. This phenomenon is absent in t h e guinea pig, monkey, and m a n , species which lack t h e ability to convert glucuronic acid to ascorbic acid (42).
I n r a t s t h e increased excretions of ascorbic acid after drugs like Chloretone is attended with an increase in t h e concentration of lactonase (substrate D-glucuronolactone) in liver microsomes (113c).
Induction of enzyme synthesis by substrates is a common phenomenon in biochemistry. N o t only drugs, b u t also biological substrates are known to lead to adaptive increase in enzyme production. An interesting aspect is the specifi
city of the induction (208a). I t appears t h a t the inducer does not necessarily have to be a substrate for the enzyme whose production is induced. For the induction of ^-glucuronidase, it is found t h a t bad substrates m a y be good inducers a n d t h a t good substrates m a y nevertheless lack t h e capacity of induction (84a). Enzyme-inducing drugs have often also a direct inhibiting action on t h e enzymes concerned. B u t there is no direct relation between the two properties (113g).
/.β.2.5. Species Effect
There are wide variations between species in t h e metabolism of substances.
Knoefel et al. (115) made extensive studies of t h e metabolic changes, especially t h e conjugation with glycine and glucuronic acid of ο-, πι-, and p-aminobenzoic acid salts. Table V I I summarizes some of their results for species differences in renal clearance. The hippuric acids have a high, t h e glucuronides a lower, clearance in t h e rabbit. The latter results in a larger circulating fraction of t h e glucuronide formed.
Chemical analogs of nicotinic acid, e.g., 3-acetylpyridine, are known t o act as antimetabolites. I n certain enzyme reactions, however, these analogs can substitute for nicotinic acid in a functional way. N A D , substituted with 3-acetylpyridine or thionicotinic acid amide is effective in t h e case of lactic acid dehydrogenase of various sources. The efficacy varies with t h e animal species and t h e organ used as a source for t h e enzyme (42a, 110, 111).
Acetylation of p-aminobenzoic acid, sulfonamides, a n d other aromatic
Ι , Ι . Β . DRUG TRANSFERENCE: DRUG METABOLISM 71 amines, occurs in most mammals with t h e exception of t h e dog. This animal excretes t h e drugs with t h e amino groups unchanged. A m p h e t a m i n e is deamin
ated by t h e rabbit, while in t h e dog a n d r a t , ring hydroxylation is t h e principle metabolic route (11,12). The well-known a n t i r h e u m a t i c drug, phenylbutazone, is metabolized very rapidly in t h e mouse, r a b b i t , a n d dog. Therefore, large doses are required in these animals in order t o demonstrate anti-inflammatory
T A B L E V I I
CONJUGATION OF AMINOBENZOATES AND EXCRETION OF THEIR CONJUGATED DERIVATIVES IN RABBITS AND DOGS°
Conjugated products of aminobenzoate (/xM/kg/4.5 hr)
Ortho Meta Para
Hipp* Glucc Hipp Glue Hipp Glue
Rabbit
Excreted in urine 108 84 93 21 32 16
Excreted in bile 3 0.03 1 0 1 0
Circulated in body 0 24 0 34 6 0
Total conjugated 111 108 94 55 39 16
% of the dose 25 24 21 12 9 4
)og
Excreted in urine 23 140 119 53 10 36
Excreted in bile 0 10 0.1 1 0.03 1
Circulated in body 0 21 4 4 0 117
Total conjugated 23 171 124 58 10 154
% of the dose 5 38 28 13 2 34
° From Knoefel (115).
b Hippuric acid product.
c Glucuronide product.
effects. I n m a n , t h e compound is metabolized slowly a n d t h e anti-inflammatory action is demonstrable with much lower doses. Procaine is readily hydrolyzed b y m a n b u t appears unchanged in t h e urine of horses (106). On t h e other hand, procaine is reported to inhibit succinylcholine hydrolysis. This probably occurs as a result of a substrate competition for t h e esterase concerned (164a).
Often t h e differences are n o t qualitative b u t q u a n t i t a t i v e . An interesting study on comparative drug metabolism has been m a d e b y Sheppard et al. (176).
They studied t h e degradation of reserpine labeled with C1 4 in t h e 4-methoxy carbon position of t h e trimethoxybenzoic acid moiety of t h e drug, b y slices of the liver of various animals, under aerobic a n d anaerobic conditions. Among
72 Ε . J . ARIENS AND A. M. SIMONIS
the degradation products studied were reserpine-like substances, trimethoxy- benzoic-acid-like substances, and C 02. The results, summarized in Table V I I I , demonstrate t h a t O-demethylation (the production of C 02) occurs especially in t h e rat, mouse, and dog. The guinea pig has a remarkable hydrolytic capacity (the formation of trimethoxybenzoic-acid-like substances). The mouse also has such properties. The sleeping time of various species following t r e a t m e n t with hexobarbital varies greatly. I t is about proportional t o t h e half-life of t h e
T A B L E V I I I
DEGRADATION OF RESERPINE C14 BY THE LIVER OF VARIOUS ANIMALS"
No. of Gas Total
Species animals phase Ab BC recovery
Pigeon 5
o
2 0 89.1 1.25 90.41 N2 0 86.0 1.28 87.3
Dog 5
o
2 8.51 83.4 1.21 93.11 N2 0 96.2 0.34 96.5
Rabbit 5
o
2 0 80.1 15.7 95.81 N2 0 93.8 10.3 104.1
Mouse 5
o
2 5.6 65.0 31.0 101.7Rat 5 02 2 19.7 58.1 1.37 79.27
1 N2 1.3 92.8 2.09 96.25
Guinea pig 5 o2 2.67 3.19 80.6 87.97
1 N2 3.08 2.25 91.8 97.75
a Liver sections (500 mg) incubated with reserpine-C14 in Krebs-Ringer bicarbonate buffer at 37° C for 3 hr.
From Sheppard (176).
b Fraction A : C 02
c Fraction Β: reserpine-like substances
d Fraction C: trimethoxybenzoic acid-like substances.
drug in these species. "Half-life" is t h e t i m e necessary t o reduce t h e concen
tration of t h e compound in plasma t o 5 0 % of its maximal concentration. The half-life is found t o be inversely proportional t o t h e activity of those enzymes in t h e liver-cell microsomes t h a t bring a b o u t inactivation (34, 35, 37, 38a).
H e a r t glucosides are degraded a t different rates in various species. These differences correlate well with t h e differences in duration of action in these species (158d).
Even in one species, variations of drug metabolism are found. I n a number of cases this appears t o be genetically determined. Certain strains of r a b b i t s have enzymes in their blood t h a t can rapidly hydrolyze t h e alkaloid atropine.
This property is probably inherited as a partially dominant factor (205, 213).
Ι , Ι . Β . DRUG T R A N S F E R E N C E : DRUG METABOLISM 73
Relative Sleeping time Plasma level enzyme activity'
Sex (min) at 60 min (μ%)
Male 22 23 682 Estradiol-treated male 84 62 177 Female 90 65 134 Testosterone-treated female 38 37 543
a From Brodie (34).
b /xg of drug metabolized by liver microsomes per hour under standard conditions.
one longer branched side-chain (92, 131). The difference in sleeping time is reflected by a more rapid disappearance of t h e drug in t h e males. P r e t r e a t m e n t of t h e males with estrogens eliminates t h i s difference between t h e sexes (35,37).
Castration of t h e males increases their sensitivity to b a r b i t u r a t e action; in
jection of testosterone decreases it again (34,92,113,113a, 131) (see Table I X ) . Interesting in this respect is t h e prolonged action of hexobarbital after treat
m e n t with various malonic acid derivatives (Sch-5712 a n d -5715) (Fig. 14) which inhibit b a r b i t u r a t e metabolism (118). The difference between males a n d females is intriguing in this respect. I n t h e males, t h e sleeping time with a given dose of hexobarbital is increased 2 - 3 times after Sch-5712 b u t in t h e females, 10-15 times. Castration of t h e males a n d females vitiates this difference; t h e Great differences in drug metabolism are reported for certain inbred strains of r a t s (154a). Another well-known example is t h e inability of t h e Dalmatian dog t o reabsorb uric acid in t h e kidney tubules, while other dogs reabsorb it t o a great extent. The result t h e n is t h a t most dogs excrete allantoin as t h e meta
bolic end-product while the Dalmation excretes uric acid (205). (For reviews on pharmacogenetics, see Kalow (106a,b,c) a n d Clark (48c).
N o t only does drug metabolism as such v a r y for various species, b u t also drug-induced a d a p t i v e changes in this metabolism vary. According t o H a r t et al. (86b) adult rabbits, unlike r a t s , have drug-metabolizing hepatic enzymes which can be strongly stimulated b y phenobarbital.
I.B.2.6. Effect of Sex Differences
Not only differences in species, b u t also differences in sex affect drug meta
bolism. The sleeping time induced by b a r b i t u r a t e s is much shorter in male t h a n in female rats. This is especially t r u e for t h e barbiturates with one shorter a n d
T A B L E I X
SEX DIFFERENCES IN DURATION OF ACTION AND IN METABOLISM OF HEXOBARBITAL IN RATS'*
74 Ε . J. ARIENS AND A. M. SIMONIS
males become more, t h e females less, sensitive. P r e t r e a t m e n t of t h e males with estradiol increases, and p r e t r e a t m e n t of t h e females with testosterone de
creases, t h e prolongation of t h e hexobarbital sleeping time produced by Sch- 5712. The difference in sensitivity t o Sch-5712 is clear in vivo b u t absent in liver homogenates.
I n certain strains of mice, t h e males are much more sensitive t o t h e nephro
toxicity of chloroform t h a n t h e females. Castration of male mice abolishes this difference (207).
For male r a t s procaine toxicity is smaller t h a n for females (60 days old). A corresponding sex difference was found in procaine esterase activity in liver homogenates. This is larger in males. Castration of t h e males eliminates t h e difference, it is not restored by testosterone. I n younger animals, too, there was a sex difference in procaine esterase activity, b u t no difference in procaine sensitivity (135a). Sex differences for toxicity, etc., m a y vary for various species. The antibiotic acetoxycycloheximide is more toxic in female r a t s and mice t h a n in males. I n dogs no sex differences in toxicity occur (145b).
Sex and species differences are observed for drug-induced a d a p t a t i o n s in enzyme activity, too. Glucuronide formation from the substrate ortho- aminophenol is greater in male t h a n in female r a t liver microsomes. I n male r a t s estradiol decreases, in female r a t s testosterone increases glucuronide transferase activity (95b).
I.B.2.7. Individual Variations
Besides t h e biological variations, there are particular individual variations in drug metabolism. I n newborns t h e enzyme systems for drug metabolism are not fully developed yet (63a). Conjugations with glucuronic acid are im
paired (202, 203). This results in a relatively high toxicity of certain drugs, such as chloramphenicol, progesterone, and iV-acetyl-^-aminophenol. Prob
ably, neonatal bilirubinemia is also due t o insufficient conjugation with glucuronic acid (63, 209). Also, acetylation of sulfanilamides is impaired (67).
Jondorf (101) proved t h a t t h e newborn mouse and guinea pig show an insuffi
ciency in glucuronide conjugation as well as in a number of oxidative degra
dations of drugs. Fouts (215) found t h a t t h e newborn r a b b i t is unable to metabolize drugs such as hexobarbital, amphetamine, acetanilide and p-nitrobenzene.
An adaptive increase of hepatic microsomal drug metabolism in fetal a n d newborn rabbits can be induced by injecting the pregnant animal or t h e new
born animal with phenobarbital. No increase was observed in fetuses t a k e n from the pre treated doe for 4-8 days prior to term. Concurrent application of ethionine blocks the increase in enzyme activity (86b). The absence of drug metabolism in newborn animals is due possibly to t h e absence of inducing stimulants as a result of the relatively protected situation in utero. The lack of drug metabolism in early fetal life can be ascribed to such factors as a lack of the
Ι , Ι . Β . DRUG T R A N S F E R E N C E : DRUG METABOLISM 75
systems for enzyme synthesis or a lack of t h e mechanism necessary for the induction of t h e enzyme synthesis by t h e drug (86b, 114c).
I n this respect m a y be mentioned t h e extreme differences in sensitivity to drugs, found for maternal a n d fetal tissues. P o t e n t teratogens, such as folic acid antagonists a n d other antivitamins, are often relatively harmless to t h e mother. I n fetal tissue t h e need for vitamins is probably relatively high (207b, 216a). The teratogenic action (66b) of t h e drug thalidomide is supposed to be due to the formation of metabolites with an a n t i vitamin activity.
Occasionally, individual hypersensitivity, an idiosyncracy, for a particular drug is observed, which is due t o a deficiency in t h e formation of degradative enzymes. Such deficiencies m a y be caused b y liver damage (70a). Sometimes t h e y appear t o be genetically determined: '' inborn errors of m e t a b o l i s m ' ' (48c, 66a, 106b). The excessively prolonged action of succinylcholine, observed in some individuals, is correlated with a low serum-cholinesterase activity (77a, 86a), which is probably genetically determined.
Chronic administration of drugs m a y lead t o changes in t h e metabolism of t h e drug concerned. During tolerance developed against narcotics like mor
phine, jV-demethylation of morphine a n d other narcotic drugs is strongly reduced (15). This might suggest t h a t iV^-demethylation leads t o a bio-activa
tion of t h e drug a n d t h a t tolerance is t h e result of an impairment of it. The nor compounds, however, have little or no analgesic or narcotic activity. Although there is a correlation between tolerance a n d t h e decrease in i^-demethylation, this relation is n o t causal (121).
I.B.3. G E N E R A L ASPECTS OF D R U G METABOLISM
Summarizing, it m a y be concluded t h a t :
1. A certain lipid-solubility is necessary for a drug in order t o reach t h e intracellular enzyme systems for drug metabolism.
2. As a rule, chemical changes in a drug molecule will t a k e place on t h e sites of great reactivity. Oxidative processes, for instance, will t a k e place a t spots where alcoholic hydroxyl groups or amino groups are present, especially if t h e y are situated on a terminal carbon of alkyl chains. For reductions, hydrolysis, a n d conjugations, analogous conditions are required for t h e appropriate reactions.
3. Oxidations prevail; reductions t a k e over where oxidations fail. Hydroly
sis is common for esters a n d glucosides; amides, as a rule, are more resistant.
4. The oxidation and reduction processes change t h e molecule. New polar groups are introduced which, as a rule, are suitable for conjugation. Hydrolysis liberates polar groups suitable for conjugation or for oxidation followed b y conjugation.
5. Conjugation is, as a rule, a final step in drug metabolism.
76 Ε. J . ARIENS AND A. M. SIMONIS
6. Oxidation often results in a decrease of the number of atoms in the mole
cule and consequently in a reduction in size. Metabolic processes generally increase t h e hydrophilic character of t h e drug molecule.
7. Packing of t h e " v i t a l " groups by adjacent methyl substituents, as a rule, protects such groups against metabolic a t t a c k .
8. Reactive spots in a drug molecule are often essential t o its pharmaco
logical properties. The specificity of action is closely related t o t h e distribution in t h e molecule of spots with a high or low electron density and their spatial relations. Consequently metabolic changes in t h e drug molecule will frequently result in a change in its pharmacological properties, not only in a quantitative, b u t also in a qualitative sense.
Drug metabolism, especially oxidations, reductions, and hydrolyses, may lead to a bio-activation as well as to a bio-inactivation. As most of t h e studies on drug metabolism are done with pharmacologically active compounds, inacti- vation studies dominate the literature. A closer study of t h e metabolism of pharmacologically active compounds reveals t h a t a number of t h e m are only active because t h e y are converted in t h e body into active compounds (bio- activation) .
I.B.3.1. Bio-Inactivation and Detoxication
The oxidation of fat-soluble compounds will often result in t h e formation of acids with a decreased number of atoms. Smaller, more polar molecules are formed. I t is evident t h a t an increase in water-solubility will usually be t h e result of t h i s t y p e of bio-transformation; it effects a conversion of lipophilic compounds into more hydrophilic ones. The formation of carboxylic acids results, as a rule, in a loss of action. Among t h e m a n y drugs in use, such acids are relatively scarce. Ammonium compounds and amines are much more frequent.
The hydrolysis of various drugs also results in more water-soluble products.
The acids and alcohols derived from esters, and t h e acids a n d amines derived from t h e amides, are generally more water-soluble t h a n t h e esters and amides themselves. They become more easily metabolized to products t h a t are still more water-soluble. A loss of activity is t h e rule. Acetylcholine, procaine, suxamethonium, etc., are examples.
Deamination of a drug, too, as a rule, brings a b o u t bio-inactivation. Hista
mine, mescaline, dopamine, etc., are examples.
I n cases of reduction, especially if nitro groups are converted t o amines, hydrophilic properties also increase. The same is true for m a n y conjugations, especially coupling with glucuronic acid, sulfuric acid, and amino acids, which often leads to products with a greater polarity and, t h u s , to a better solubility in water t h a n t h e original compounds. An exception to this rule is t h e acetyl- ation of aromatic amines, for instance, t h e production of acetanilides. Here, t h e polarity of t h e compounds decreases as a result of t h e coupling. Conjugation