A N T I B I O T I C S IN L I C H E N S
K. O. VARTIA
II. Pharmacopoeias . . . III. Lichen Substances . . IV. Pharmacological Studies V. Antibiotics in Lichens References
I. Folklore 547
549 549 550 551 558
I. Folklore
Unofficially, in the folklore, the medicinal use of lichens has had a long history. Evernia furfur acea, for example, was apparently used for medicinal purposes in Egypt in the seventeenth and eighteenth centuries B.C., and is still brought to Egypt, in the present century, together with Iceland moss, as a foreign drug from Europe. Hippocrates recommended Usnea barbata
for uterine trouble; the same lichen (Usnea longissima) was employed by the Chinese, under the name of "Sun-Lo," as an expectorant, and its surface powder for the treatment of ulcers. The Manchurian "Shihoa" medicine contained obtusatic acid of the depside type. The Malayans still employ the Usnea species medicinally for colds and as a tonic.
In the fifteenth century A.D. lichens constituted an important commer- cial article in Europe. In the eighteenth century Peltigera canina was sold as
pulvus antilyssus, and the famous medicine Lichen quercinus virides contained mainly Evernia prunastri, E. furfuracea, and Parmeliaphysodes. Lichen island- icus, at that time, was a remedy held in high esteem, and Mucus cranii humanii,
lichen grown on the human skull, "cost its weight in gold." Iceland moss and
Lobaria pulmonaria were generally used for the treatment of catarrhal hem- optysis and pulmonary tuberculosis, e.g., in the form of cough-tea or "lichen chocolate," the former also being employed as a. laxative, Xanthoria parie- tina, Cladonia, and Pertusaria species also "helped" in the most varied range
547
of diseases: fever, jaundice, epileptics, convulsions, "gout and other diseases," edema, etc. Gilg-Brandt (1922), Hoffmann-Krauer (1927, 1929- 30) von Hovorka-Kronfeldt (1908, 1909), Jungbauer (1934), Lid-Storaker (1932), and Perez-Llano (1944).
In the Finnish folklore*—as an example which has been more carefully studied—the popular use of lichens as remedies seems to have been very widespread. Thus, for instance, yellow lichens (Xanthoria parietina, X. poly- carpa, Cetraria pinastri) have been recommended for the treatment of jaundice: similia similibus. Obviously, because of the exterior resemblance,
Lobaria pulmonaria has been used for pulmonary tuberculosis and cough and
Peltigera aphthosa as remedy for infantile aphthae.
Lichens with the widest range of medicinal uses in Finland are probably the beard mosses (genera Alectoria, Usnea, and perhaps also Ramalina thra-
usta). The advice given is to place a bunch of lichen on a fresh or infected wound, for athlete's foot or other skin eruptions. Oral doses have been used against sore throat and toothache. Cetraria islandica and Cladonia species have been used as remedies for pulmonary tuberculosis and cough, usually taken in the form of so-called lichen milk. Notes on so-called cup-lichens
(Cladonia coccifera, C deformis, etc.) and stone mosses (Parmelia, Stereo
caulon, etc.) are along similar lines.
The lichens have often been mentioned in cattle breeding, for instance, as a remedy against inflammation of udders.
The use of lichens in the folklore history of Sweden has been studied by Ahmadjian and Nilsson (1963).
As can be seen from the above, superstition and a primitive medical skill, based on nature and on rough empiricism, are always mixed up in the popular medicinal use of lichens. It is of interest to note that, in the use of lichens for coughs or expressly for pulmonary tuberculosis, old folklore exists from different parts of the globe and that present-day science, too, has found fairly strong antibiotics against the tuberculosis bacillus in the same species to some extent (Usnea sp., Cetraria islandica). As to the lichen species mentioned above, which have obviously been popular as medicines, no lichen acids proper have been found in Peltigera aphthosa, and the lichen substances contained in Lobaria pulmonaria and in the Xanthoria species have not been found to be active against many pathogenic bacteria and fungi; the attention devoted to these species, however, is readily explicable from the general rule similia similibus. But Cladonia alpestris, C. silvatica, C coccifera, C. deformis, Ramalina thrausta, Evernia prunastri, and all Usnea
species contain active usnic acid; Cetraria islandica contains rf-protoliche-
•Collection of Folklore Archives of the Finnish Literary Society and of the Finnish Dictionary Foundation.
sterinic acid; Parmelia physodes, and Evernia furfuracea contain physodic acid; and Cetraria pinastri contains vulpinic and pinastric acids (Zopf, 1907), all of which have been found to be chemically active. The concentration of the active substances, it is true, is not very high, but the effect is enhanced by the fact that the lichen substances primarily exist on the exterior surface of the thallus in the form of powder or crystals [the most common active substance, usnic acid is always there (Tobler, 1925)], and hence, although not readily soluble, they can diffuse into their surroundings.
II. Pharmacopoeias
Purely on the basis of their medicinal use in folklore, lichens have been listed in the pharmacopoeias. The oldest European pharmacopoeia (of 1546), however, includes no lichens among the drugs listed, whereas the
"Pharmacopoeia Universalis" of 1846 lists a great number of lichen medi- cines. The following species are mentioned: Cetraria islandica, C. nivale, Cladonia coccifera, Cladonia pyxidata, Usnea plicata, Peltigera canina, P.
venosa, P. horizontalis, P. polydactyla, Lobaria pulmonaria, Xanthoria parie- tina, and Evernia prunastri. According to this work, Iceland moss has been included among the drugs listed in 50 contemporary pharmacopoeias or dis- pensatories, recommended primarily as a cure for coughs. Lichens have gradually disappeared from the list of official medicines, paradoxically, just before the discovery of antibiotic properties in lichens. However, at least the Japanese pharmacopeia of 1922, the Estonian of 1937, and the French, Swiss, and German pharmacopoeias that are valid at present include Iceland moss as a drug, the French one both as a paste and as a potion. Lichen
islandicus is usually recommended for use as cough medicine and for its appetizing qualities. In Sweden about 600 pounds of dried Iceland moss are sold each year.
III. Lichen Substances
The explanation of the chemistry of lichens was begun by Pfaff (1826), when he found eine eigentumliche Saure from Iceland moss (Cetraria islan- dica). Pfaff s finding, evidently fumarprotocetraric acid (Hesse, 1904), was the beginning of the rapidly expanding field of lichen chemistry. Usnic acid was discovered in 1844 by Knop. The largest number of lichen substances has been isolated by Hesse (1861-1905) and Zopf (1907), and as a result of their investigations they totaled nearly 150 in number by 1907. Of this total, the structure of only a few had been studied; several of them later proved to be mutually identical or else impure.
The decisive work in the study of the structure of lichen substances has been carried out by Asahina and co-workers (1930-1942). Mainly on the basis of the classification worked out by Zopf, modestly termed by him as a
"temporary one, to provide a general picture," Asahina proposed a general system of known lichen substances as follows
1. Fatty acids and lactones
2. The zeorin group, neutral compounds not saponifiable by alkalis, the formulae of which are not well known
3. Pulvic acid derivatives
4. Cumarone derivatives, the only representative usnic acid with its deri
vatives 5. Depsides 6. Depsidones
7. Anthraquinone derivatives
In spite of the differences in structural formulae, lichen substances have a considerable number of properties in common. All lichen substances proper are crystalline and in most cases acid in character (which accounts for the widely used designation lichen acids), and even in the form of alkaline salts their solubility in water is very poor. Several lichen substances are optically active—this applies to all aliphatics; usnic acid even has an un
usually high specific rotation. The color of the crystals varies from colorless to white and reddish yellow. Several lichen substances have a very bitter taste.
The amount of lichen substances contained in the different species varies greatly. From Parmelia tinctorum, 23.5% of lecanoric acid alone was ob
tained; from Lepraria chlorina, 10.5% of vulpinic acid; from Alectoria ochro- leuca, 5.5% of usnic acid (Zopf, 1907), whereas the content of theprotoli- chesterinic acids in Iceland moss amounts to only a few promilles. The normal amount is possibly around 1%.
IV. Pharmacological Studies
Several lichen acids have been studied from the pharmacological point of view. Alms (1832) recommended picrolichenic acid (subsequently identified by Zopf) for the treatment of Wechselfieber, prompted in the first place by its taste, reminiscent of quinine, and similarly Lebail later (1853).
Ramm (1890) and Neuberg (1893) carried out animal tests with cetrarin, finding it relatively harmless for mammals (lethal dose 0.2 gm per kilogram of body weight). Injected in the form of sodium salt, the acid induced a specific acceleration in peristalsis, increased blood pressure, and secretion of
bile. According to Neuberg, accelerated peristalsis was also produced in the isolated bowels of a dog, and he recommended the substance as a remedy for anemia and lack of appetite. Later on, cetrarin was established to be ethylprotocetraric acid (Koller and Krakauer, 1929). Guesdon (1901) report- ed good results with cetraric and protocetraric acids as antiemetics in preg- nancy and tuberculosis.
The vulpinic acid occurring in Letharia vulpina, used as a fox poison, has been repeatedly studied toxicologically. Kobert (1892) and Neuberg (1893) gave the lethal dose for mammals as 20-30 mg per kilogram of body weight;
the same figure applies to the closely related pinastric acid. The toxicity of vulpinic acid was later found to be lower; Santesson (1939) obtained 78.8 mg per kilogram of body weight as the lethal dose for a cat, the most noticeable symptom of poisoning being acute dyspnea. Brodersen and Kjaer(1946) reported the lethal dose for a mouse as 75.0 mg per kilogram of body weight.
Mikoshiba (1933) published some investigations on the pharmacology of usnic acid and its derivatives. According to his studies, usnic acid has a papa- verine-like effect on the smooth muscles but is less poisonous than papaver- ine; the lethal dose for a mouse is 7.0 mg/10 mg subcutaneously and 0.25/
20 gm intravenously applied (= 0.7 and 0.025 gm/kg).
Fischer and Toth (1938) investigated certain effects of lichesterinic acid.
The hemolytic index obtained by them for defibrinatedblood was40,000;the index was found to drop to 5000 if equal amounts of lichesterinic acid and cholesterin were added. The lethal dose obtained for a frog was 200/ug/gm, and for a mouse, intravenously, 100 /ig/gm (0.2 and 0.1 gm/kg); the fish index was 25,000, normal drop number of 186, and foam value 1:25,000. Of the greatest interest is the ability of the acid, as found by them, to promote resorption greatly: with a frog, the symptoms of strychnine poisoning were produced percutaneously by 1/2-1/2.5 of the ordinary amount with a small simultaneous administration of lichesterinic acid. The absorption-increasing dose for lichesterinic acid, in micrograms per grams of a frog, was 3/ig after 45 minutes, obtained from the curare test. They presume that part of the medicinal effects of Iceland moss is based on this property of lichesterinic acid.
Fuzikawa found (1939) that the phenols and their derivatives, components of lichen depsides and depsidones, possess remarkably strong antiseptic qualities.
V. Antibiotics in Lichens
The study of lichens and lichen substances, from the antibiotic point of view, started in 1944, when Burkholdere/<z/.( 1944) published the first qualita- tive study of the antibiotic properties of lichens. They tested 100 American
lichen species in relation to Staphylococcus aureus and Bacillus subtilis,
by the Oxford Cup method, extracting a certain weight amount of lichen by phosphorus buffer solution. Fifty-two species (52%) prevented the growth of either one or both of the bacteria studied. With a few exceptions, the lichens studied had no effect at all on gram-negative bacteria. The usnic acid isolated from Cladonia mitis prevented the growth of B. subtilis,
but not of the Staphylococcus or the colon bacillus. Stoll (1947) and Stoll α/.
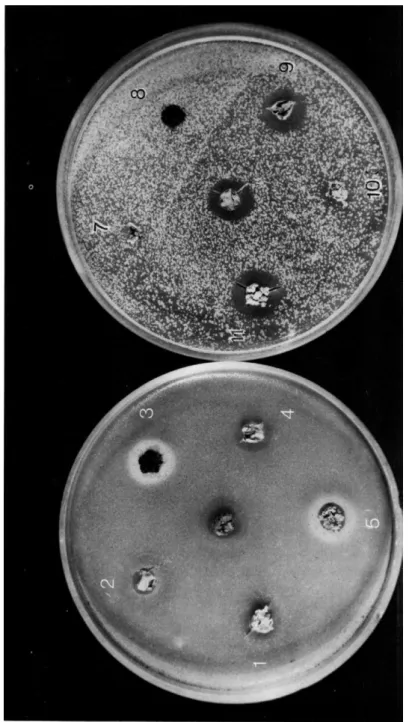
(1947) boiled some lichens for a short while with 5% alkaline glucose solution, and made corresponding plate tests with the extracts obtained: out of 58 Swiss species, 38 proved to be active against Staphylococcus (65.5%); clearly the most general active substance was usnic acid. In the plate tests with pieces of lichen by Vartia (1949,1950a,b) 75 of 149 Finnish lichen species investigated revealed properties preventing the growth of various gram-positive bacteria 50%* (Fig. 1).
Bargellini et al (1946) observed that usnic acid is active against the bac
terial species S. aureus, C diphteriae, and B. subtilis, but did not inhibit gram- negative bacteria.
Quantitative studies of crystalline lichen substances were initiated by Barry (1946). He found that the chlorine-containing lichen substance dip
loicin, previously isolated from Buellia canescens, inhibited the growth of human tuberculosis and diphtheria bacillus 1:100,000, and that of Mycobac
terium smegmatis 1:70,000 in vitro. He also pointed out that the only com
pound of the diphenyl ether type (such as the product of alkaline hydrolysis of diploicin) occurring in a normal organism is thyroxine, but he could not establish any definite physiological analogy between it and diploicin. Sub
sequent studies have primarily dealt with usnic acid, especially its inhibiting effect on the growth of Mycobacterium tuberculosis. Marshak (1947) isolated from the lichen Ramalina reticulata a crystalline substance, which he subse
quently found to be usnic acid, completely inhibiting the growth of different strains of human tuberculosis 1:20,000-1:50,000, and weakening their growth 1:200,000-1:2,000,000. The growth of bovine tuberculosis was completely inhibited in 1:20,000, and at this titer two avian strains were only partly inhibited. The growth of Staphylococcus, Streptococcus, and Pneu- mococcus was inhibited in 1:20,000. The effect was evidently bactericidal.
Stoll et al. (1947) obtained the following completely inhibiting titers for the usnic acid produced as by-product from Cetraria islandica: human tubercu
losis 1:64,000-1.800,000, bovine 1.500,000, an avian strain 1:125,000, Sta-
pholococcus aureus and Streptococcus pyogenes 1:100,000: no effect on colon bacillus, Salmonella typhimurium, and dysentery bacillus. In a third work
•Eight of 100 lichen species contained phytagglutinins; Peltigera aphtosa agglutinated human red cells in titer 1:400 as saline extract (Estola and Vartia, 1955).
FIG. 1. Pieces of lichens on petri dishes inoculated with Bacillus subtilis and Sarcina lutea. Some lichens inhibit the growth of bacteria, others seem to improve it (from Vartia, 1949).
published the same year, Bustinza and Lopez (1946) reported complete in
hibition by usnic acid isolated from
Usnea barbata
in the form of sodium salt on human tuberculosis cultivated on glycerine broth in 1:500,000, an avian strain 1:100,000. With Dubos' culture medium a 5-10 times greater concentration was necessary. Bustinza and Lopez tried to combine streptomycin and usnic acid into a salt but the result did not crystallize, possibly because it was a mixture of usnicates of very poor solubility. Shibata etal.
(1948) Shibata and Miura (1948, 1949) compared the activities of usnic acid and its derivatives including didymic acid and related compounds. With the different optic forms of usnic acid and their sodium salts and didymic acids with derivatives, the inhibiting titer was, with an avian tuberculosis strain,
1:160,000 and with
Staphylococcus
1:160,000-1/320,000. Acetylation of hydroxyl groups reduces the effectivity from one-half to one-quarter of that of the existing one. Marshak et al. (1949) studied the activity of usnic acid and 33 related compounds, as well as of vulpinic acid: only monoacetyl usnic acid against human tuberculosis obtained the same titer as usnic acid:1:100,000. Vartia (1949), for usnic acid isolated from
Cladonia alpestris,
obtained a completely inhibiting titer of 1:60,000, and a growth-retarding effect in 1:160,000. Klosa (1947, 1949) reported the inhibitory titer of 1:1,000,000 for usnic acid against
Mycobacterium tuberculosis, Streptococcus,
and
Staphylococcus.
Dopp and Bersch (1950) reported growth-weakening titer of 1:500,000 of usnic acid against the human tuberculosis bacillus.With reference to other lichen substances, Burkholder et al. (1945) con
firmed that atranorin and fumarprotocetraric acid are inactive. Asano (1945) determined the antibacterial action of lichesterinic acid and its deri
vative against
Staphylococcus.
Stollet al.
(1947) indicated that the effect of vulpinic, uf-protolichesterinic, lichesterinic, dihydrolichesterinic, physodic, and diffratic acids was somewhat similar to that of the usnic acid. Cavallito et al. (1948) reported on a dilution series made with aliphatic lactones, including protolichesterinic, lichesterinic, and dihydrolichesterinic acids:Mycobacterium tuberculosis, 1:600,000-1:1,000,000; Streptococcus pyogenes,
1:300,000-1:50,000; Staphylococcus, 1:100,000; and C welchii, 1:200,000- 2,000,000. The results were in compliance with those obtained with other aliphatic lactones. Klosa (1949) reported the inhibitory titer of evernic acid against tuberculosis bacillus,Streptococcus,
andStaphylococcus
at 1:1,000,000. Shibata and Miura (1948) published results of testing 23 lichen substances against avian tuberculosis andStaphylococcus aureus:
/-lichesterinic acid, /-dihydroprotolichesterinic acid and perlatoric acid inhibited growth of tuberculosis bacilli in 1:40,000-1:80,000 and, besides, proto
lichesterinic acid, spherophorin, divaricatic, anziaic acid, olivetoric, seki- kaic, ramalinolic, boninic, and lobaric acids had clear effects against staphyl
ococci. Caperatic and rangiformic acid, zeorin, atranorin, thamnolic,
salazinic, psoromic, fumarprotocetraric acids, pannarin, and endocrocin were inactive.
Vartia (1949-1950a,b) tested 20 lichen substances, of which five, liches- terylic and pinastric acids, anilide of pulvic acid, and gyrophoric acid, were active against Mycobacterium tuberculosis and gram-positive bacteria. Further rf-protolichesterinic, rf-lichesterinic, divaricatic acids, atranol, physodic acid, and diploicin were active against several gram-positive bacteria. Vartia also proved that nine lichen substances have an inhibitory effect against several pathogenic fungi.
According to the investigations referred to above, the following conclu- sions can be made.
1. More than 50% of the lichen species have antibiotic properties.
2. The antibiotic effects are based on lichen substances of which the most effective are usnic acid, the lichesterinic acid group, as well as orcinol- type depsides and depsidones.
3. In the folklore, lichens have been used for thousands of years in several parts of the globe, to a remarkable extent quite logically.
Figure 2 shows an overall view of antibiotics in lichen substances; it is approximate. One should note that the variable results, especially in the titers dealing with inhibition of tuberculosis strains, are due to many factors:
the culture medium used, the inoculation type and amount, the cultivation time, etc. According to the schematic illustration, the main effect is on the rapidly growing gram-positive bacteria, Mycobacterium tuberculosis, and some pathogenic fungi.
In vivo tests—with animals or human patients—are the next step. Exclud- ing folklore, the first report was made by Chiba (1898): he reported good results with Usnea tinctures taken per os with patients suffering from lymph- adenitis tuberculosa colli. Shibata et al. (1948), Marshak (1947), Marshak et al. (1947,1949), Marshak and Kuschner (1950) got somewhat contradictory results with inoculation of tuberculosis in guinea pigs treated with usnic acid.
Patiala et al. (1948) and Jantti (1952) found that usnic acid retarded the course and changed the type of tuberculosis in guinea pigs. In preliminary tests on human patients they found that a daily dose of 3 gm caused indefinite pains in the liver; 1 gm per day produced no symptoms of poisoning. Patiala(1949) has also published a case of lupus vulgaris treated with usnic acid.
The situation in vivo usually presents new problems and difficulties:
questions of resorption, toxicity, effect of serum albumin, allergic reactions and so on. The fate of the lichesterinic acid group was disturbed in vitro when serum albumin was present. Tests with mice gave no positive result (Vartia and Tervilla, 1952).
In the case of usnic acid the problem has been its very poor solubility
Caperatic acid Rangiformic acid Protolichesterinic acid Fatty acid-lactone group- Dihydroprotolichesterinic aci Lichesterinic acid Oihydrolichesterinic acid Lichesterylic acid Zeorin group Zeorin Vulpinic acid Pulvic-acid derivatives - P'nastric acid Anilide of pulvic acid Calycin Usnic acid Cumarone derivatives - Diacetylusnic acid (only distinctly active) Didymic acid deriv.
III
φ ω Ό to α α>
Ο
(Λ <D C Ο Ό ϋ) α φ
Ω
ο Ο
Λ. ϋ
Ό ο
UJ C
(Λ φ
ο C C
"5 σ CD
£ C
<
Lecanoinic acid Spherophorin Anziaic acid Perlatoric acid Φ Evernic acid Divaricatic acid .Ε .Ξ Gyrophoric acid £.g Umbilicaric acid Olivetoric acid SekikaiC acid Ramalinoric acid Boninic acid ο § Thamnolic acid *5> ο Atranorin r'Z Atranol Diffractic acid — *•*= Lobaric acid ο ο £.Ξ - Physodic acid ο·§ Diploicin es " Fumarprotocetraric acid ο 5 Salazinic acid 'ω ο " Acetate of salazinic acid ?*»- Psoromic acid Pannarin Mmnraquinuncs CNAOU r υυιπ • FIG. 2. Approximate diagram of the activity of the lichen substances studied on the different groups of microorganisms. The widt the ribbon symbolizes the strength of the antibiotic effect. no effect.
(usnic acid dissolves in water less than 1:5 ml, its sodium salt at approx.
1:600) (Heilala and Siintola, 1949a). It has been used, however, as ointments in infectious skin diseases and also per os. [In Austria with the name Usnia- kin as wound salve and powder, in Germany Evosin I (usnic and evernic acids), Evosin II (usnic, physodic, and physodalic acids) and in Switzerland Lichusnin.] The Finnish team headed by Virtanen et al. (1954; Virtanen, 1954, 1955; Virtanen and Karki, 1956; Virtanen and Niemi, 1955; Virtanen and Vahatalo, 1956; Korlekangas and Virtanen, 1956) has done extensive work to render usnic acid soluble, especially by combining the acid with a suitable base compound. Having made more than a hundred combinations with several amino compounds, sulfonamides, and known antibiotic sub
stances as isonicotinic acid hydrazide, streptomycin, ^-aminosalicylic acid, neomycin, and cycloserin, they finally arrived at a compound as follows:
benzylidimethyl-(2-[2-(/?-l, 1, 3, 3)-tetramethylbutylphenoxyethoxy] ethyl- ammonium hydroxide, which they named appropriately USNO. The LD5 0 of USNO is 125 mg/kg for a white mouse. It has given good results in skin diseases (Kilpio, 1956) and in veterinary use (mastitis in cows, Grignani and Tomaselli, 1957). Its antifugal effects are proved against yeasts (Capriotti, 1959, 1961) and in the treatment of Trichophyton gallinae (Virtanen and Kilpio, 1957).
Neither Usno nor other lichen medicines have, however, gained a per
manent position in the medical praxis: the fight for a place in the sun among antibiotics is too hard. Since the last decade the medicinal research of lichens has turned to new directions: the untiring Japanese group Nakazava, Ko- matsu, Hamada, Fujikawa, Hirai, Shibata et al. has studied the antitumor activity of lichens intensively since 1962. This activity obviously depends on the polysaccharide component in lichens, as found in Umbilicaria, Lasallia, Cetraria, Cladonia, Parmelia, and Usnea, and from psoromicacid.
References
Ahmadjian, V., and Nilsson, S. (1963). In "Swedish Lichens." (H. G. Leach, ed.), pp. 39-47.
Yearbook of the American Swedish Historical Foundation.
Alms, A. (1832). Uber einen neuen Stoff in der Variolaria amara. Ann. Pharm. 1, 61.
Asahina, Y. (1934). Zur Systematik der Flechtenstoffe. Acta Phytochim. 8, 33-45 (and more than one hundred articles on lichen substances 1930-1942, mostly in Ber. Deut. Chem.
Ges.)
Asano, M. (1945). Igakusora 1,41 (in Japanese) (see Shibata et al., 1948).
Asano, M., and Kanematsu, Τ. (1932). Uber die Konstitution der Protolichesterinsaure und Lichesterinsaure. Ber. Deut. Chem. Ges. 65, 1175-1178.
Bargellini, G., del Pianto, E., and Marini-Bettolo, G. B. (1946). Lull'attivita antibatterica di due accidi lichemici: Acido usnico et acido vulpinica, serie ottava. Atti Acad. Naz. Lincei.
Mem., CI. Sci. Fis. Mat. Natur., Sez. 3a [8] 1 (No. 12), 1252-1255.
Barry, V. C. (1946): The thyroid and tuberculosis. Nature (London) 158, 131-132.
Brodersen, R., and Kjaer, A. (1946). The antibacterial action and toxicity of some unsaturated lactones. Acta Pharm. Toxic. 2, 109-120.
Burkholder, P. R., and Evans, A. W. (1945). Further studies on the antibiotic activity of lichens.
Bull. Torrey Bot. Club. 72, 157-164.
Burkholder, P. R., Evans, Α., McVeigh, I., and Thornton, H. (1945). Antibiotic activity of lichens. Proc. Nat. Acad. Sci. U.S. 30, 250-255.
Bustinza, F., and Lopez, A. C. (1946). Contribucion al estudio de los antibioticos procedentes de liquenes. An. Jar. Bot. Madrid7, 511-533.
Capriotti, A. (1959). The effects of "Usno" on yeasts. G. Microbiol. 7, 187-206.
Capriotti, A. (1961). The effects of "Usno" on yeasts isolated from the excretions of tuber
culous patients. Antibiot. Chemother. 11, 409-410.
Cavallito, C. J., Fruehauf, D . McK., and Bailey, J. H. (1948). Lactone aliphatic acids as anti
bacterial agents. J. Amer. Chem. Soc. 70, 3724.
Chiba, K. (1898). J. Pharm. Soc. Jap. 258, 829 (in Japanese) (see Shibata et al., 1948).
Dopp, W., and Bersch, H. W. (1950). Uber die tuberkulostatische Wirkung einiger pflanz- licher Substanzen in vitro. Pharmazie 5, N o . 12, 603-604.
Estola, E., and Vartia, K. O. (1955). Phytagglutinins in lichens. Ann. Med. Exp. Biol. Fenn. 33, N o . 4, 392-935.
Fischer, R., and Toth, D . (1938). Uber einige Wirkungen der Agaricinsaure, Abietinsaure und Lichesterinsaure. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 190, 500-509.
Fuzikawa, H. (1939). The antiseptic action of phenols, phenolcarboxylic acids and the esters of lichen substances. VI. The antiseptic action of orcinol-carboxylic ester on soy cauce.
J. Pharm. Soc. Jap. 59, 615-616.
Gilg-Brandt, E. (1922). "Lehrbuch der Pharmakognosie" Berlin.
Grignani, Α., and Tomaselli, R. (1957). Die Wirkung des Praparates "Usno" in Fallen von Mastitis bei Rindern. "Studi Urbinatr Fac. Far. Ser. C. [N.S.] 31, No. 6.
Guesdon, H. (1901). See Perez-Llano (1944).
Heilala, P., and Siintola, S. (1949a). L-Usmiinihapon liukoisuudesta. Farm. Aika 58, N o . 10, 185-187 (in Finnish).
Heilala, P., and Siintola, S. (1949b). L-Usniinihapon eristamisesta Cladonia alpestris jakalasta.
Farm. Aikak. 58, N o . 11, 199 and 303 (in Finnish).
Hesse, O. (1861-1905). 20 articles on lichen acids. JustusLiebigs Ann. Chem., Ber. Deut. Chem.
Ges., J. Prakt. Chem.
Hesse, O. (1904). Beitrag zur kenntnis der Flechten und ihrer charakteristische Bestandteile.
Prakt. Chem. 70, 449-502.
Hoffmann-Krauer, E., and Bachtold-Staubli, H. (1927, 1929-30). Handwbrterbuch des deutschen Arberglaubens I; 1461; II; 1578.
Jantti, K. (1952). The effect of usnic acid on guinea-pigs infected with M. tuberculosis.
Farm. Aikak. 61, N o . 6, 139-140.
Jungbauer, G. (1934). "Deutoche Volksmedizin," p. 105. Berlin.
Kilpio, O. (1956). Iakttagelser over den antibakteriella effekten hos ett usninsy raderivat (USNO) och den kliniska anvandningen av detta vid pyodermifall. Nord. Hyg. Tidskr. 3 7 , 2 8 9 - 2 9 4 . Klosa, J. K. (1947). Penicillin und Apotheke. Pharm. Ztg. 83, 329-332.
Klosa, J. K. (1949). Antibiotische Stoffe aus Flechten. Pharm. Zentralh. Deut. 6, 165-167.
Knop, W. (1844). Chemisch-physiologische Untersuchungen uber die Flechten. Justus Liebigs Ann. Chem. 49, 103.
Kobert, R. (1892). Sitzungsber. Naturf. Ges. Vurjew p. 157 (See Zopf, 1907).
Koller, G, and Kraukauer, E. (1929). Uber die Konstitution der Cetrarsaure M. Chemie 52, 931-951.
Kortekangas, A. E., and Virtanen, Ο. E. (1956). The antibiotic activity of some amino com
pound derivatives of usnic acid. III. Suom. Kemistilehti Β 29, 2 - 4 .
Lebail, J. Β. (1853). "Des Lichens." Paris (See Perez-Llano, 1944).
Lid, N., and Storaker, Joh. Th. (1932). "Sygdom og Forgjorelse i den norske Folketro," p. 51.
Oslo.
Marshak, A. (1947). A crystalline antibacterial substance from the lichen Ramalina reticulata.
Pub. Health Rep. 62, 3-19.
Marshak, Α., and Kuschner, M. (1950). Action of streptomycin and usnic acid on development of tuberculosis in guinea-pigs. Pub. Health Rep. 65, 131-144.
Marshak, Α., Barry, G. T., and Graig, L. C. (1947). Antibiotic compound isolated from the lichen Ramalina reticulata. Science 106, 394-395.
Marshak, Α., Schaefer, W. B., and Rajagopalan, S. (1949). Antibacterial activity of </-usnic acid and related compounds on Mycobacterium tuberculosis. Proc. Soc. Exp. Biol. Med.
70, 565-568.
Mikoshiba, K. (1933). Tokyo Igaku Zasshi47,No. 8 and9(in Japanese)(seeShibataetal., 1948).
Neuberg, A. (1893). Dissertation Dorpat (Jurjew) (See Zopf, 1907).
Patiala, R. (1949). Application of usnic acid to a case of lupus vulgaris. Ann. Med. Exp. Biol.
Fenn. 27, 151.
Patiala, R., Patiala, J., Siintola, S., and Heilala, P. (1948). The effects of /-usnic acid in vivo.
Suom. Kemistilehti All, 127 (in Finnish).
Perez-Llano, G. A. (1944). Lichens. Their biological and economic significance. Bot. Rev.
10, 1-37.
Pfaff, C. H. (1826). Schweig. J. Chem. Phys. 47, 476-483 (see Tobler, 1925).
Ramm, W. (1890). Historische Studien aus die Pharmokologische Institut der Universitat Dorpat Bd. II, pp. 1—142 (see Zorpf, 1907).
Santesson. C. G. (1939). Einiges uber die giftige Fuchs- oder Wolfsflechte (Letharia vulpina (L.) Vain). Upsala Laekarefoeren. Foerh. 45, 1-8.
Shibata, S., and Miura, Y. (1948). Antibacterial effects of lichen substances. I. Comparative studies of various lichen substances. Jap. Med. J. 1, 518-521.
Shibata, S., and Miura, Y. (1949). Antibacterial effects of lichen substances. II. Studies on didymic acid and related compounds. Jap. Med. J. 2, 2 2 - 2 4 .
Shibata, S., Ukita, T., Tamura, T., and Miura, Y. (1948). Relation between chemical constitu
tions and antibacterial effects of usnic acid and its derivatives. Jap. Med. J. 1,151-155.
Shibata, S., Nishikawa, Y., Tanaka, M., Fukuoka, F., and Nakanishi, M. (1968). Antitumour activities of lichen polysaccharides. Z. Krebsforsch. 71, 102-104.
Stoll, A. (1947). The antibacterial effect of usnic acid on mycobacteria and micro-organisms.
Experientia 3, 115-117.
Stoll, Α., Renz, J., and Brack, A. (1947). Antibiotics from lichens. Experiential, 111-114.
Tobler, F. (1925). "Biologie der Flechten." Berlin.
Vartia, K. O. (1949). Antibiotics in lichens. I. Ann. Med. Exp. Biol. Fenn. 21, 4 6 - 5 4 . Vartia, K. O. (1950a). Antibiotics in lichens. II. Ann. Med. Exp. Biol. Fenn. 28, 7-19.
Vartia, K. O. (1950b). On antibiotic effects of lichens and lichen substances. Diss. Helsinki.
Ann. Med. Exp. Biol. Fenn., Suppl. 7.
Vartia, K. O., and Tervila, L. (1952). D-Lichesteric acid, effect in vivo on pigmented mice with inoculation tuberculosis. Ann. Med. Exp. Biol. Fenn. 30, 76-78.
Virtanen, Ο. E. (1954). The antibiotic activity of some amino compound derivatives of /-usnic acid. II. Suom. Kemistilehti Β 21, 6 3 - 7.
Virtanen, Ο. E. (1955). Derivatives of usnic acid with the most important tuberculostatic agents.
Suom. Kemistilehti Β 28, 125-126.
Virtanen, Ο. E., and Karki, N. (1956). On the toxicity of an usnic acid preparation with the trade name USNO. Suom. Kemistilehti Β 29, 225-226.
Virtanen, Ο. E., and Kilpio, Ο. E. (1957). On the in vivo fungistatic activity of an usnic acid preparation with the trade name USNO. Suom. Kemistilehti Β 30, 8 - 9 .
Virtanen, Ο. E., and Niemi, P. (1955). A reaction product of usnic acid with sulfaguanidine and N-methyl-L-glucosamine. Suom. Kemistilehti Β 28, 71.
Virtanen, Ο. E., and Vahatalo, M. L. (1956). Usnic acid derivative of 4-amino-3-isoxazolidone (cycloserine). Suom. Kemistilehti Β 29, 3 0 - 3 1 .
Virtanen, Ο. E., Viitanen, H., and Kortekangas, A. E. (1954). The antibiotic activity of some amino compound derivatives of /-usnic acid. I. Suom. Kemistilehti Β 27, 18-20.
von Hovorka, O., and Kronfeldt, A. (1908-1909). "Vergleichende Volksmedizin," Vol. I;
p. 225. Stuttgart. "Vergleichende Volksmedizin," Vol. II; pp. 20, 22, 26, 40, and 62.
Stuttgart.
Zopf, W. (1907). "Die Flechtenstoffe." Fischer, Jena.