The Preparation of Long-Chain Carboxylic Acids from 1,3-Cyclohexanediones
H E R M A N N STETTER
Chemisches Institut der Universitdt Bonn*
I n t r o d u c t i o n
Dihydroresorcinol (1,3-cyclohexanedione) ( I ) undergoes a hydrolytic ring opening with alkali to form δ-oxocaproic acid ( I I ) . Vorlander had discovered this as far back as 1 8 9 7 (1).
ο
C II
/ \ ο H2C CH2 Cleavage ||
___ I I > C H3- C - C H2 CH2 CH2-COOH.
\ /
I CH2 II
With this reaction dihydroresorcinol proves to be a normal ^ - d i k e - tone, because it gives, on cleavage, the acids characteristic of such diketones.
Proceeding from these observations a new synthetic method for long- chain carboxylic acids was developed by H . Stetter and W . Dierichs
{2, 2a). This method proceeds in three steps:
T h e first step consists of introducing organic groups into the 2-posi- tion of dihydroresorcinol.
In the second step, the C-alkylated 1,3-cyclohexanedione is treated with alkali, and the δ-οχο acids, which now contain a 6-carbon atom chain plus R , are obtained. B y reduction of the carbonyl group in these 8- o x o acids to C H2, long-chain reduced carboxylic acids are obtained, as the third step.
With this reaction sequence the lengthening of carbon chains b y six carbon atoms becomes generally possible, according to the scheme:
The importance of the procedure lies in its wide applicability, which has been confirmed b y the synthesis of numerous long-chain carboxylic acids. Dihydroresorcinol, useful in lengthening the chain, in addition contributes its full molecular weight to the final acid. This fact m a y serve to emphasize the economical nature of the procedure.
* New address: Institut fiir Organische Chemie der Technischen Hochschule Aachen.
51
Ο Ο / \ / \
H«C CH, alkylation H2C CHR cleavage
I ! > I I >
H2C C= 0 H2C C= 0
\ H 2 C H2
reduction
R C H2C C H2 C HAC H2C O O H >
Ο
R C H2C H2C H2C H2C HAC O O H
M e t h o d s
1 , 3 - C y c l o h e x a n e d i o n e
Dihydroresorcinol was first obtained b y Merling (3) by the reduc
tion of resorcinol with sodium amalgam. T h e yield by this procedure is not satisfactory. Also, the procedure used b y Vorlander (4), the cycliza- tion of δ-oxocapronate ester, is not suitable for large quantities of dihydroresorcinol. I t first became readily available when Klingenfuss
(5) found that, whereas the catalytic reduction of resorcinol in a neutral or acidic medium leads to an uptake of six hydrogen atoms to form the isomeric resorcitol, in the presence of 1 mole of alkali the reduction stops at the dihydroresorcinol stage.
OH
OH
A
\ OH
The yield b y hydrogenation is over 9 0 % . In the laboratory the pro
cedure of R . B. Thompson ( # ) , who used Raney-nickel, has proved to be the best. T h e procedure can be simplified b y reducing at atmospheric pressure at 50° with agitation. With sufficiently intensive shaking the reduction takes 9-10 hr.
The closely related dimedone, 5,5-dimethyl-l,3-cyclohexanedione, is readily obtained b y the procedure of Vorlander ( 7 ) , using a Michael addition of malonic ester to mesityl oxide, followed b y cyclization of the ester (yield: 8 0 % ) .
CARBOXYLIC ACIDS FROM 1,3-CYCLOHEXANEDIONES 53
CH., C H3
" \
C - C H - C O - C H , F H2C ( C O O C2H5) , -> C - C H2C O C H3
/ / I C H , C H , C H - C O O C2H5
C O O CI 2H5
C H , / Η , C H ; J C H2
C C---0 C C- 0 / 1 Ι / ι J -> C H3 J J _^ C H3 I I
H C C H2 H,C C H2 / \ / ' \ /
C , H5O O C C C II I!
ο ο
In a similar manner other 5-substituted 1,3-cyelohexanediones, a 5- methyl-l,3-cyclohexanedione (8), and 5-phenyl-l,3-cyelohexanedione
(9) are readily obtained.
Dihydroresorcinol may exist in three tautomeric forms.
OH ο ο II II
C C c
/ X / \ / \ H , C C H H2C C H H2C C H ,
Ί I ^ Ί I I ^ I I H , C C O H H , C C O H H„C C- 0
" \ / " " \ / " \ /
C C H2 CHo
Η
A B C
Actually only the monoenol Β and the cyclohexanedione C exist in a solution of dihydroresorcinol (10). According to measurements of Schwarzenbach (11) the enol content in aqueous solution is 9 5 . 3 % . This strong enolization accounts for the strong acidic character of dihydro
resorcinol. Its p K is 5.25 and thus it is an only slightly weaker acid than acetic acid.
Whereas in the open-chain /?-diketones the formation of the enol form hinders free rotation and thereby entails an expenditure of energy, in practice this is not the case with 1,3-cyclohexanediones. This explains the great tendency toward enolization in contrast to the open-chain 0-diketones (12).
The high acidity is caused by the large resonance energy, which, b e cause of the planar configuration of the ring structure, is produced by the dissociation of a proton (13).
A further characteristic difference between open-chain and cyclic /?-diketones was found by Eistert and Reiss (H). The enols of open- chain /?-diketones, because of hydrogen bonding, show a chelate structure D , which restricts the possibility of forming the cis-structure of an enol.
R C — - Ο
\
HC Η
c Ο
These chelates are hydrophobic, as are the corresponding ketones. D R
From this it is evident that such β-diketones show less tendency for enolization in a strongly polar solvent, such as water, than in a nonpolar solvent such as hexane. In the case of cyclic β-diketones the chelate structure of enols is ruled out on steric grounds. In this case the enol form is "transfixed." Therefore it is more hydrophylic than the c y c l o - hexanedione form. Eistert and Reiss could confirm these predictions, finding for such 1,3-cyclohexanediones a higher enol content in the polar solvents than in the nonpolar ones.
On the same steric grounds the cyclohexanediones do not give an enol reaction with ferric chloride in absolute alcohol. The "transfixed" enols cannot form the iron complex necessary for the color reaction (15).
Altogether pure and dry dihydroresorcinol is practically stable without limit. Moist or slightly impure dihydroresorcinol undergoes extensive decomposition. Such decompositions are observed in more or less degree with almost all of the alkylated 1,3-cyclohexanediones. The cause is an autooxidation, which Toivonen and associates (16) found to be the case with 2,5,5-trimethyl-l,3-cyclohexanedione. As the first step they postu
late the formation of an enol peroxide ( E ) , which, in some unexplainable manner, is converted to β,β-dimethylglutaric acid and acetic acid. M o r e recent investigations with similar peroxides (17) seem to indicate that a keto hydrogen peroxide, formula F, is involved rather than the inter
mediate E.
ο
H2C CH, J
C CH
\ C - C H
3
» CH2
C O H
A / H ° H2C C—Ο
Ο
Jl C Γ Η / \ / ' H2C C-O-OH C H3 V
CH / \ / ι CHA
F C = 0
CH,x|
I
I c c—oΕ
CH8 CH.COOH
\ /
C + CH3COOH CH, C H/ \ 2C O O H
CARBOXYLIC ACIDS FROM 1,3-CYCLOHEXANEDIONES 5 5
T h r e e - s t e p P r o c e d u r e
FIRST STEP OF T H E REACTION: PREPARATION OF 2-SUBSTITUTED 1 ,3-CYCLOHEXANEDIONES
ο ο II I I c c / \ / \
H2C C H2 H2C CHR I I — • Ι Ι H2C C= 0 H2C C= 0
\ / \ /
CH2 CH2
The synthesis of carboxylic acids m a y be quite varied in the indi
vidual reaction steps. This is true especially in the first step which in
volves the introduction of an organic group into the 2-position of 1,3-cyclohexanedione. As /?-diketones these compounds, through their alkali derivatives, are directly alkylated with organic halides. A further possibility for the introduction of an organic group is through addition reactions, which are made possible by the presence of the acidic hydrogen.
T o the addition reactions belongs also the addition of carbonyl c o m pounds after the manner of an aldol condensation, as well as the Michael addition with ^-unsaturated ketones, esters, and nitriles.
Alkylation with Organic Halogen Compounds
As is the case in the alkylation of other β-dicarbonyl compounds, in the reaction of the metal derivatives of 1,3-cyclohexanedione with alkyl halides, the presence of enol ethers and C-alkylated cyclohexanediones must be expected because of the ionic mesomerism:
ΙΟ. .Ο. ,Ο, C C Η C / \ / \ • / X.
H2C CH H2C C| H2C CH I II - * - - > I I - < - > I I _ H2C C- 0 | H2C C= 0 H2C C - O
\ / - \ / ~ \ / -
CH2 C H2 C H2
Primarily enol ethers are formed, in keeping with the strong prefer
ence for the enol form in such cyclohexanediones. In agreement with this view Merling (18) obtained exclusively the enol ethers by treating the silver salt of dihydroresorcinol with alkyl iodides. The compounds could not be isolated in a pure state.
The first C-alkylations of dihydroresorcinol were described in 1 9 3 6 by Klingenfuss (19) and Hewett (20). Klingenfuss mentions the prepara
tion of 2-allyl-l,3-cyclohexanedione by the condensation of dihydro
resorcinol with allyl bromide. Hewett obtained 2- b e n z y l - l, 3- c y c l o h e x a n e -
dione by the reaction of the sodium derivative of dihydroresorcinol with benzyl chloride in absolute alcohol with an 1 8 % yield.
The alkylation of dimedone, 5,5-dimethyl-l,3-cyclohexanedione, was investigated more thoroughly. T h e methylation of this compound was first described by Toivonen (21). Desai (22) undertook alkylation with methyl iodide, ethyl iodide, η-propyl iodide, η-butyl iodide, isopropyl iodide, allyl iodide, and benzyl iodide; the corresponding C-alkylated dimedones were obtained only in small yields. As by-products enol ethers as well as dialkylated products were obtained.
Stetter and Dierichs (23) systematically investigated the alkylation of dihydroresorcinol from the standpoint of various factors. Alkylation was examined for its dependence upon solvents, salt-formation with various alkali metals, and the concentration and kind of alkyl halide used. T h e halogen compounds used were η-butyl bromide and n-butyl iodide. T h e separation of the resulting C-alkylated dihydroresorcinols from the enol ethers were readily achieved, since the C-alkylated dihydro
resorcinols still possess an acidic hydrogen and therefore are soluble in alkali, whereas the enol ethers are insoluble in alkali and can be extracted completely with ether.
The alkylation with η-butyl bromide with different alcohols as sol
vents showed that the proportion in which the C-alkylated products and T A B L E 1
REACTION OF THE SODIUM DERIVATIVE OF DIHYDRORESORCINOL WITH ΤΙ-BUTYL BROMIDE
Solvent C-alkyl O-alkyl Ratio C - 0 Cpd.
Methanol 1 5 % 3 6 . 5 % 1 : 2 . 4
Ethanol 1 2 % 3 0 % 1 : 2 . 5
<-Butyl alcohol 1 2 % 3 0 % 1 : 2 . 5
the enol ethers are formed, is independent of the kind of alcohol used (Table 1 ) . Only the higher combined yield in the case of methanol is noteworthy.
A stronger influence on alkylation is shown when different alkali metals are used for salt formation in methanol. In the progression from lithium to potassium a growing promotion of C-alkylation with increas
ing atomic weight is noted (Table 2 ) . T h e best yields were obtained with the potassium derivative. This discovery is in good agreement with the investigations of Brandstrom (24), who observed the same effect in the alkylation of acetylacetone with the series, sodium to cesium.
In order to study the influence of solvent concentration on alkylation, dihydroresorcinol was alkylated with η-butyl bromide in three different
CARBOXYLIC ACIDS FROM 1,3-CYCLOHEXANEDIONES 57
T A B L E 2
INFLUENCE OF ALKALI METAL ON THE ALKYLATION OF DIHYDRORESORCINOL WITH Π-BUTYL BROMIDE
Metal C-Alkyl O-Alkyl Ratio C : 0 Cpd
Lithium 6 % 2 1 . 0 % 1:3.4
Sodium 1 5 % 3 6 . 5 % 1:2.4
Potassium 1 7 . 5 % 3 6 . 4 % 1:2.1
It was known from previous work (25) with open-chain /?-diketones, that C-alkylation was favorably influenced in proportion to the reactivity of the halogen in the organic halogen compound used. If, in place of an alkyl bromide, the corresponding alkyl iodide is used, the yield of C-alkylated product increases. T h e same results are observed when under the same conditions η-butyl iodide is used in place of η-butyl bromide
T A B L E 3
INFLUENCE OF SOLVENT CONCENTRATION ON THE ALKYLATION OF POTASSIUM DERIVATIVE OF DIHYDRORESORCINOL WITH Π-BUTYL BROMIDE CH3OH
(ml)
Heating time
(hr) C-Alkyl O-Alkyl
Ratio C : 0 Cpd 30 5 1 8 . 2 % 4 2 . 4 % 1:2.3 50 5 1 7 . 5 % 3 6 . 4 % 1:2.3
200 15 6 % 2 5 % 1:4.2
in the alkylation of dihydroresorcinol. T h e yield of 2-butyl-1,3-cyclo
hexanedione increases from 18.2 to 2 8 . 4 % , while the yield of enol decreases from 42.4 to 3 6 . 4 % .
A totally different picture is presented when η-butyl p-toluenesul- fonate is used for alkylation in place of a η-butyl halide. T h e alkylation proceeds rapidly and gives almost exclusively the enol ether in 8 0 % yield. This exclusive enol ether formation is characteristic when using the esters of sulfonic acids and sulfuric acid for the alkylation of dihydroresorcinol and offers an excellent practical method for obtaining enol ethers.
The investigations just described have shown that the preparation of C-alkylated dihydroresorcinols is most favored when the potassium derivative of dihydroresorcinol is treated with an alkyl halide containing concentrations in methanol. T h e experimental results (Table 3) show definitely that C-alkylation is favored b y working in higher con
centration.
The closely related investigation of alkylation without a solvent showed that only enol ethers are formed.
the most active halogen, in the highest concentration possible, using methanol as solvent.
A theoretical interpretation of these investigations of Stetter and Dierichs (23), within the scope of a general theory concerning the C - and O-alkylation of β-carbonyl compounds, was presented by A. Brand- strom (26). According to Brandstrom the alkali salts of dihydroresorcinol exist, in the solvent, in a solvated form, the metal being attached to three solvent molecules and the resonance hybrid of the dihydroresorcinol ion.
In this complex one or more of the solvent molecules can be displaced by organic halogen compounds. These bound halides become strongly polarized. In the six-membered ring which is formed, the linkage of the R group can be joined to the carbon atom in position 2 of dihydro
resorcinol. T h e relations are summarized in the following scheme.
Solv. Solv. Solv. Solv.
\ / \ /
Κ Κ Ο Solv. Ο Xs ~
C C R8 I- / \ / \ H2C C H — > H2C C H H..C C O Η,ί C O
" \ / " \ / C Η 2 C Η 2
Solv. Solv.
\ / / \ Κ
Ο Χδ~ O H
C Rs+ C
/ ^ / V - > H2C C — Η — > H2C C - R
: I I I H,C C= 0 H , C C= 0
" \ / " \ /
C H2 C H ,
This theory explains the influences of solvent concentration on C - alkylation, since with a lower concentration of the solvent that of the alkyl halide increases, and the formation of the halogen-containing c o m plex, and with it C-alkylation, is favored. Similarly the theory explains the increase of C-alkylation with the increasing atomic weight of the alkali metal, since in the open-chain β-carbonyl compounds a reduction in the salt-like character of the alkali compound is also observed with increased atomic weight (27). The influence of the strongly polarized halides on the course of C-alkylation may be explained by the greater tendency to form the halogen-containing complex. On the other hand the formation of enol ether is explained by the reaction with the anion with
out the above-mentioned stabilization of the negative charge on the C-atom.
CARBOXYLIC ACIDS FROM 1,3-CYCLOHEXANEDIONES 59
Under the optimum conditions a series of simple alkyl iodides were reacted with dihydroresorcinol by Stetter and Dierichs (2). I t was shown that in the series from ethyl iodide to cetyl iodide the yields of C-alkyl- ated dihydroresorcinols are largely constant. T h e y are about 2 7 % . Only methyl iodide is an exception, giving 2-methyl-l,3-cyclohexanedione in 5 1 . 5 % yield. Also, a bromoacetate and allyl bromide gave higher yields
(51 and 3 3 % ) of the C-alkylated dihydroresorcinol, for in these c o m - pounds the halogen is activated by the adjacent double bond.
A significant improvement in C-alkylation was obtained when water or a mixture of water and methanol was used as a solvent in place of methanol. T h e alkylation with n-alkyl iodides under these conditions shows that the reaction velocity is strongly reduced in contrast to the situation in methanol solution. In the case of methylation with methyl iodide the yield of the C-alkylated product increases from 51.5 to 6 5 %
(28). T h e reaction with ethyl iodide gives the same yield as when methanol is used (29). With higher alkyl iodides the yield is lowered again. The lower yield is attributed to oxidation phenomena and the cleavage reactions by aqueous alkali which are brought about by the longer reaction time made necessary by working in aqueous solution at high concentration. The reaction products are quite impure and are purified with difficulty.
An essential improvement in the yields of C-alkylated compounds results when halogen compounds with strongly activated halogen are used for alkylation in an aqueous medium. In this case the yields of C-alkylated products increase greatly in contrast to working in methanol.
Thus the alkylation of dihydroresorcinol with allyl bromide in aqueous solution gives 2-allyl-l,3-cyclohexanedione in 7 5 % yield in contrast to a yield of 3 3 % in methanol solution. In the same manner the yield in alkylating with benzyl chloride increases from 18 to 7 0 % . I t is shown that in all cases in which a satisfactory reactive halogen is at hand the yields in C-alkylation in aqueous solution are significantly higher than in methanol.
In special cases aromatically bound halogen m a y be used for C - alkylation with success. Under consideration here are those halogen compounds in which the halogen is ortho to a carboxyl group. It had been found previously (30), that such o-halocarboxylic acids are useful in alkylations in the presence of copper salts. In this way Stetter and Siehnhold (31) have condensed, in aqueous solution, o-bromobenzoic acid and 2,5-dibromoterephthalic acid with dihydroresorcinol. Yields of 54 and 4 2 . 5 % of C-alkylation were readily obtainable. Owing to a very easy intramolecular lactonization with the formation of cyclic enol ester, it was possible to isolate both lactones X X I and X X V .
9
\\ I if
Ι o=c κ \ κ \ C CH,/ \ / \ / Ο
I
C CH.,H,C C ^ II / - \ / \ / "
\ / H X C Ι Ο CH2 Ο
XXI H X C C = 0
" \ / \ κ
CH, Ο X X V
X V + CH3OH
C,H5 CH2 CH CO CH2 CHA CH2 COOCH3 CH, X X I X
Since the 2-alkyl-l,3-cyclohexanediones still have an acidic hydrogen capable of salt formation, the possibility exists of alkylating again to form 2,2-dialkyl-l,3-cyclohexanediones. Stetter and Klauke (32) have obtained such dialkyl products through the condensation of 2- a l k y 1-1,3-
cyclohexanediones with halogen compounds in absolute alcohol. The products differ significantly from the monoalkylated products. Whereas the 2-alkyl-l,3-cyclohexanediones are enolizable because of the available acidic hydrogen, such an enolization is no longer possible with the dialkyl products. Therefore these compounds show a significantly better solubility in nonpolar solvents and in many cases m a y be distilled with
out decomposition. Moreover, these compounds prove to be especially sensitive toward aqueous alkali, with which the formation of acids is readily carried out (further details in the section on acid formation).
This sensitivity toward alkali leads to a partial cleavage to acid during alkylation in aqueous solvents, therefore the dialkylation is carried out preferably in water-free alcohols.
In one case (33) of alkylation in absolute methanol, an alcoholysis resulting in the methyl ester of the δ-oxocarboxylic acid was observed:
CARBOXYLIC ACIDS FROM 1,3-CYCLOHEXANEDIONES 61 in the methylation of 2-benzyl-l,3-cyclohexanedione, the ester, methyl 6-methyl-7-phenyl-5-oxoheptanoate ( X X I X ) , could be isolated directly.
Essentially, the conditions for alkylation of dihydroresorcinol are suitable for such 1,3-cyclohexanediones which possess alkyl groups in the 4- or 5-position as, for example, dimedone, 5-methyl-l,3-cyclohexane- dione, and 5-phenyl-1,3-cyclohexanedione. Table 4 gives a summary of the 2-alkylated derivatives of 1,3-cyclohexanedione which have been used in the preparation of carboxylic acids.
2. Alkylation with Carbonyl Compounds
A simple and important practical method for introducing an organic radical into the 2-position of dihydroresorcinol is shown by the reaction of dihydroresorcinol with aldehydes. The reaction proceeds with the splitting out of 1 mole of water from 2 moles of dihydroresorcinol and 1 mole of aldehyde. Alkylidenedi-1,3-cyclohexanediones having the gen
eral formula, G, are formed.
ο ο R ο
II IL I II
c c C H c
/ \ / \ H / \ H / \ 2 H2C C H2 + R C H O H2C C C C H2
Τ I 7 7 7 T > I I | | H2C C= 0 -H2 ° H2C C= 0 0- C C H2
\ / \ / \ /
C H2 C H2 G C H ,
T h e reaction goes without addition of a condensing agent and in aqueous or alcoholic solution. Since this condensation produces well- crystallized compounds, it is possible to use them for the characterization of aldehydes. The aliphatic aldehydes react most easily, formaldehyde best of all. Methylenedi- 1,3-cyclohexanedione ( X X X I I I ) is formed in practically quantitative yield. The reaction proceeds less completely with aromatic aldehydes.
The alkylated 1,3-cyclohexanedione reacts in a manner similar to dihydroresorcinol. I t is assumed that both hydrogen atoms are present at the 2-position. With 2 -alkyl-l,3-cyclohexanediones no condensation product with aldehydes could be obtained. The condensation products of dimedone form especially good crystals; for this reason dimedone instead of dihydroresorcinol (35) is used for the identification of aldehydes.
Another example of the introduction of an organic radical into the 2-position of dihydroresorcinol was found b y H . Stetter and co-workers (36). If dihydroresorcinol is heated for 10 hr in an aqueous buffered solution at p H 6, a good crystalline compound is obtained. Analytical data indicate that the molecular weight of the compound is double that for dihydroresorcinol. Apparently it is Γ-hydroxy [bicyclohexyl]-2,3',6-
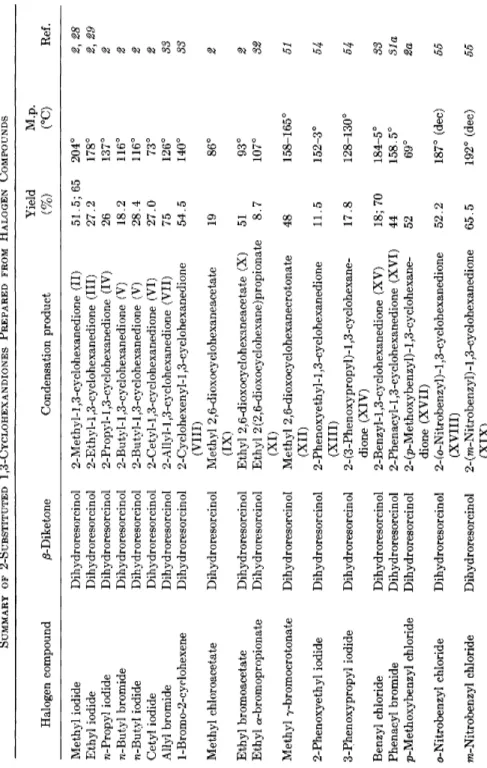
TABLE 4 SUMMARY OF 2-SUBSTITUTED 1,3-CYCLOHEXANDIONES PREPARED FROM HALOGEN COMPOUNDS Yield M.p. Halogen compound 0-Diketone Condensation product (%) (°C) Ref. Methyl iodide Dihydroresorcinol 2-Methyl-l ,3-cyclohexanedione (II) 51.5; 65 204° 2, 28 Ethyl iodide Dihydroresorcinol 2-Ethyl-l ,3-cyclohexanedione (III) 27.2 178° 2} 29 η-Propyl iodide Dihydroresorcinol 2-Propyl-1,3-cyclohexanedione (IV) 26 137° 2 η-Butyl bromide Dihydroresorcinol 2-Butyl-l ,3-cyclohexanedione (V) 18.2 116° 2 η-Butyl iodide Dihydroresorcinol 2-Butyl-l ,3-cyclohexanedione (V) 28.4 116° 2 Cetyl iodide Dihydroresorcinol 2-Cetyl-l ,3-cyclohexanedione (VI) 27.0 73° 2 Allyl bromide Dihydroresorcinol 2-Allyl-l,3-cyclohexanedione (VII) 75 126° 33 l-Bromo-2-cyclohexene Dihydroresorcinol 2-Cyclohexenyl-l,3-cyclohexanedione 54.5 140° 33 (VIII) Methyl chloroacetate Dihydroresorcinol Methyl 2,6-dioxocyclohexaneacetate /TV1! 19 86° 2 Ethyl bromoacetate Dihydroresorcinol Ethyl 2,6-dioxocyclohexaneacetate (X) 51 93° 2 Ethyl a-bromopropionate Dihydroresorcinol Ethyl 2 (2,6-dioxocyclohexane)propionate 8.7 107° 32 Methyl γ-bromocrotonate Dihydroresorcinol Methyl 2,6-dioxocyclohexanecrotonate 48 158-165° 51 (XII) 2-Phenoxyethyl iodide Dihy droresorcin ol 2-Phenoxyethyl-l,3-cyclohexanedione 11.5 152-3° 54 (XIII) 3-Phenoxypropyl iodide Dihydr oresorcin ol 2-(3-Phenoxypropyl)-l,3-cyclohexane-17.8 128-130° 54 dione (XIV) Benzyl chloride Dihydroresorcinol 2-Benzyl-l ,3-cyclohexanedione (XV) 18; 70 184-5° 33 Phenacyl bromide Dihydroresorcinol 2-Phenacyl-l,3-cyclohexanedione (XVI) 44 158.5° 31a p-Methoxybenzyl chloride Dihydroresorcinol 2- (p-Methoxybenzyl) -1,3-cyclohexane52 69° 2a dione (XVII) o-Nitrobenzyl chloride Dihydroresorcinol 2- (o-Nitrobenzyl) -1,3-cy clohexanedione 52.2 187° (dec) 55 (XVIII) ra-Nitrobenzyl chloride Dihydroresorcinol 2- (m-Nitrobenzyl) -1,3-cyclohexanedione 65.5 192° (dec) 55 (XIX)
p-Nitrobenzyl chloride Dihy droresorcin ol 2-(p-Nitrobenzyl)-l,3-cyclohexanedione (XX) 72.8 238° (dec) 55 o-Bromobenzoic acid Dihydroresorcinol Lactone (XXI) U 172° 81 1,2-Dibromoe thane Dihydroresorcinol 2,2/-Ethylenedi-l,3-cyclohexanedione 19 270-1° 51 1,2-Dibromoe thane (XXII) 1,4-Dibromo-2-butene Dihydroresorcinol 2,2'-(2-Butenylene)di-l,3-cyclohexane-69 224° (dec) 33 1,4-Dibromo-2-butene dione (XXIII) α,α'-Dichloro-p-xylene Dihydroresorcinol 2,2'-(p-Phenylenedimethylene)di-l,3- cyclohexanedione (XXIV) 52 300° (dec) 36a, 51 2,5-Dibromoterephthalic acid Dihydroresorcinol Dilactone (XXV) 42.5 380° (dec) 31 Ethyl bromoacetate Dimedone Methyl 4,4-dimethyl-2,6-dioxocyclo-32.5 110.5° 40 Ethyl bromoacetate h'6xanecarboxylate (XXVI) Benzyl chloride Dimedone 2-Benzyl-5,5-dimethyl-l,3-cyclohexane-80.4 155° 40 Benzyl chloride dione (XXVII) Benzyl iodide Dimedone 2-Benzyl-5,5-dimethyl-l,3-cyclohexane-36 155° 22 Benzyl iodide dione (XXVII) Methyl iodide Dihydroresorcinol 2,2-Dimethyl-l,3-cyclohexanedione (XXVIII) 15 40° 34 Methyl iodide 2-Methyl-l,3-cyclo-2,2-Dime thyl-1,3-cy clohexanedione 70.7 40° 34a Methyl iodide hexanedione (XXVIII) Methyl iodide 2-Benzyl-l ,3-cyclo- hexanedione (XV) Methyl 6-methyl-5-oxo-7-phenyl- heptanoate (XXIX) 71 b.p. 160°/6 mm 33 η-Butyl iodide 2-Benzyl-l ,3-cyclo- hexanedione (XV) 2-Benzyl-2-butyl-l,3-cyclohexanedione (XXX) 10 89° 32 Allyl bromide 2-Benzyl-l ,3-cyclo- hexanedione (XV) 2-Allyl-2-benzyl-l,3-cyclohexanedione (XXXI) 53.5 69° 32 Benzyl chloride 2-Benzyl-l ,3-cyclo- hexanedione (XV) 2,2-Dibenzyl-l,3-cyclohexanedione (LXV) 69.2 137° 32 Ethyl bromoacetate 2-Methyl-l,3-cyclo- hexanedione (II) Ethyl 2,6-dioxo-l-methylcyclohexane- acetate (XXXII) 56.5 67° 32 10-Undecenyl iodide Dihydroresorcinol 2-(10-Undecen-l-yl)l,3-cyclohexadione (CXXV) 20 34b (continued)
CARBOXYLIC ACIDS FROM 1,3-CYCLOHEXANEDIONES 63
TABLE 4 (Continued) Halogen compound /3-Diketone Condensation product Yield (%)
M.p. (°C) Rel Methyl iodide 5-Methyl-l ,3-cyclo-2,5-Dime thy 1-1,3-cy cl ohexanedione 60.7 175.5° 53 hexanedione (CXXVI) Benzyl chloride 5-Methyl-l ,3-cyclo-2-Benzyl-5-methyl-l ,3-cyclohexanedione 75 137° 53 hexanedione (CXXVII) Ethyl bromoacetate 5-Methyl-l ,3-cyclo-Methyl 2,6-dioxo-4-methylcyclohexane-29.7 94° 53 hexanedione acetate (CXXVIII) Phenacyl bromide 2-Methyl-l,3-cyclo-2-Methyl-2-phenacyl-l ,3-cyclohexane-44 125-6° 34c hexandione (II) dione (CXXIX) Allyl bromide 2-Allyl-l,3-cyclo-2,2-Diallyl-l ,3cyclohexanedione 70 b.p. 78°/2 mm 34d hexanedione (VII) (CXXX) Allyl bromide Dimedone 2-Allyl-5,5-dimethyl-l,3-cyclohexane-27 147-8° 3/td dione (CXXXI) Benz}d chloride 4,6-Dimethyl-l,3-2-Benzyl-4,6-dimethyl-l,3-cyclohexane-53 110-20° 34e cyclohexanedione dione (CXXXII)
—
2,2-Dibenzyl-4,6-dimethyl-l,3-cyclo-11 116-8° 34e hexanedione (CXXXIII) Benzyl chloride 4,6-Diethyl-l,3-2-Benzyl-4,6-diethyl-l,3-cyclohexane-54 60-3° 34e cyclohexanedione dione (CXXXIV) Methyl iodide 5-Phenyl-l ,3-cyclo-2-Methyl-5-phenyl-l,3-cyclohexane-53.5 214° 53 hexanedione dione (CXXXV) Benzyl chloride 5-Phenyl-l ,3-cyclo-2-Benzyl-5-phenyl-l,3-cyclohexane-62 172.5° 53 hexanedione dione (CXXXVI) Ethyl bromoacetate 5-Phenyl-l ,3-cyclo-Ethyl 2,6-dioxo-4-phenylcyclohexane-34 209° 9, 53 hexanedione acetate (CXXXVII) Methyl iodide 3,5-Dioxocyclo-3,5-Dioxo-4-methylcyclohexanecarboxylic 38 229° 53 hexanecarboxylic acid acid (CXXXVIII) Allyl bromide 3,5-Dioxocyclo-4-Allyl-3,5-dioxocycIohexanecarboxylic 51.8 169° 53 hexanecarboxylic acid (CXXXIX) acidC A R B O X Y L I C A C I D S F R O M 1 , 3- C Y C L O H E X A N E D I O N E S 65 trione ( X X X I V ) which is formed b y an aldol condensation between two molecules of dihydroresorcinol. With concentrated sulfuric acid water is split out intramolecularly. Formulas Η and J are possible. This m a y
O H
C H . - C O I C H - - C O
/ \ 1 / \
H2C C H - C C H .
\ / \ /
C Ha- C O C H2- C H . X X X I V
argue for a three-carbon tautomerism. Catalytic hydrogenation converts the compound to [bicyclohexyl]-2,3',6-trione ( X X X V ) , which is o b tained readily in good yield.
C H j - C O C Ha- C O
- Ha0 / \ / \
- + H4C C = C C H . ^
\ / \ / C Ha- C O C Ha- C Ha
Η
C H2- C O C H — C O
Hac ^ \ : H - C ^ \ H .
\ / \ /
C H2- C O C HA- C HA
C Ha- C O C H . - C O + 2 H / \ / \
— > H2C C H - C H C H .
\ / \ /
C Ha- C O C H . - C H . X X X V
Alkylation by Michael Addition
Another simple means for the preparation of 2-substituted dihydro- resorcinols exists because dihydroresorcinol, possessing an acidic h y d r o gen, m a y undergo Michael addition with ^^-unsaturated ketones, car
boxylic esters, and nitriles. A Michael adduct of dihydroresorcinol was first appeared by Mikhailov (37). Mikhailov obtained the expected adduct ( X X X V I ) by the addition of dihydroresorcinol to benzalaceto- phenone under the catalytic influence of piperidine.
ο
^ C « H5
/ \ I
H2C C H - C H C H2 CO CeH5
H2C C= 0
\ /
C H2 X X X V I
Stetter and Coenen (38) were able to obtain similar adducts with benzalacetone, ethyl acrylate, and acrylonitrile. The addition occurs in absolute alcohols in the presence of alcoholates. Because of the acidity
of dihydroresorcinol it is found necessary to use larger amounts of alkali as catalyst than is usual with the Michael addition reaction.
Addition of dihydroresorcinol t o benzalacetone gives l,6-dioxo-4a- hydroxy-8-phenyldecahydronaphthalene, which is formed b y cyclic aldol condensation of the primary adduct ( X X X V I I ) .
ο Ο
II
A C H2C H2C 0 C H3 /C\ /CH2CH2COCH3
H2C
X
H2C Cj J C H2C H2C O C H3 I I XH H,C C = 0 H2C C = 0
\ / \ / C H
2 X X X I X CH, X L
T h e y were able to obtain product X L I I I in small yield b y the addi
tion of 2 moles of 2-methyl-l,3-cyclohexanedione to 1 mole of divinyl ketone.
ο ο
Jl 11
/ \ CH C H , C
H2C C - C H2C H , C 0 C HiC Ha- C C H2 H2C C = 0 0 = C C H2
\ / \ / C H
2 X L I I I C H2
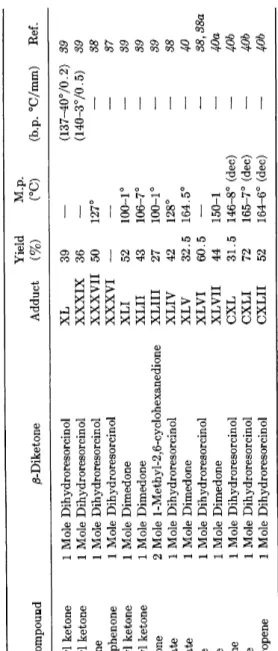
Dimedone also m a y be used in the Michael addition reaction. Such additions have succeeded with ethyl acrylate (40) and with methyl vinyl ketone (39). In Table 5 are listed the 2-substituted-1,3-cyclohexanediones prepared b y Michael addition.
The yields of adducts amount to 4 0 - 7 0 % .
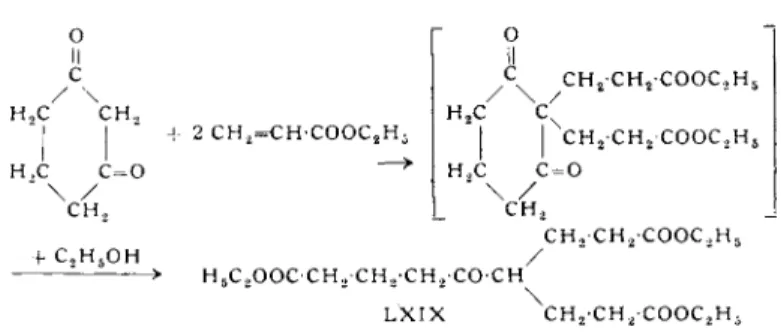
Nazarov and Zav'yalov (39), in the Michael addition of dihydro
resorcinol with excess methyl vinyl ketone in methanol and with the addition of potassium hydroxide, obtained the adduct X X X I X , formed from 1 mole of dihydroresorcinol and 2 moles of methyl vinyl ketone, as well as the simple adduct X L .
TABLE 5 SUMMARY OF MICHAEL ADDUCTS OF 1,3-CYCLOHEXANEDIONES Unsaturated compound /3-Diketone Adduct Yield (%)
M.p. (°C) (b.p. °C/mm) Ref. 1 Mole Methyl vinyl ketone 1 Mole Dihydroresorcinol XL 39 — (137-4070.2) 39 2 Mole Methyl vinyl ketone 1 Mole Dihydroresorcinol XXXIX 36 — (140-370.5) 39 1 Mole Benzalacetone 1 Mole Dihydroresorcinol XXXVII 50 127°
—
38 1 Mole Benzalacetophenone 1 Mole Dihydroresorcinol XXXVI — — — 37 1 Mole Methyl vinyl ketone 1 Mole Dimedone XLI 52 100-1° — 39 2 Mole Methyl vinyl ketone 1 Mole Dimedone XLII 43 106-7° — 39 1 Mole Divinyl ketone 2 Mole 1 -Methy 1-2,6-cy clohexanedione XLIII 27 100-1° — 39 1 Mole Ethyl aery late 1 Mole Dihydroresorcinol XLIV 42 128° — 38 1 Mole Ethyl aery late 1 Mole Dimedone XLV 32.5 164.5° — 40 1 Mole Acrylonitrile 1 Mole Dihydroresorcinol XLVI 60.5 — -— 38, 38a 1 Mole Acrylonitrile 1 Mole Dimedone XLVII 44 150-1 — 40a 2 Mole Nitroethylene 1 Mole Dihydroresorcinol CXL 31.5 146-8° (dec) — 40b 1 Mole Nitrostyrene 1 Mole Dihydroresorcinol CXLI 72 165-7° (dec) — 40b 1 Mole 1-Nitro-l-propene 1 Mole Dihydroresorcinol CXLII 52 164-6° (dec) — 40bCARBOXYLIC ACIDS FROM 1,3-CYCLOHEXANEDIONES 67
The Michael addition could also be realized between dihydroresor
cinol and nitroolefins (40b). B y using nitroethylene, 2,2-bis(2-nitro- ethyl)-l,3-cyclohexanedione ( C X L ) was the only reaction product obtained. The addition of dihydroresorcinol to /?-nitrostyrene and 1- nitro-l-propene gave crystalline reaction products which .contained one molecule of water less than that of the expected adduct. I t is shown that these compounds are 4-phenyl- and 4-methyl-5-oxo-5,6,7,8-tetrahydro-
1,2,4,-benzoxazine N-oxide ( C X L I and C X L I I ) which are formed from the initial adducts through spontaneous splitting out of water.
ο II
/ \ CeH5
I I + I C H = C H N Ot
Ο
CeH5
I C H j OH N O ,
H20
C , H6
CH
Ο C X L I
CH II NO
Other Methods
In this section reference will be made to other methods for the preparation of 2-alkyl-l,3-cyclohexanediones, although these methods have not been used in the synthesis of carboxylic acids.
B y reaction of dihydroresorcinol with carboxylic acid anhydrides in the presence of the alkali salts of the corresponding acid or in the pres
ence of pyridine, the 2-position of dihydroresorcinol is acylated. Such 2-acyl-l,3-cyclohexanediones can be obtained in reasonably good yields Smith (42) has subjected 2-acetyl-l,3-cyclohexanedione to catalytic hydrogenation under a variety of conditions. Hydrogenation with pal
ladium on charcoal furnishes l-acetyl-2-cyclohexanone as the main product, along with a small amount of 2-ethyl-l,3-cyclohexanedione
( I I I ) . If increasing amounts of sodium hydroxide are added to the hydrogenation mixture the amount of l-acetyl-2-cyclohexanone de
creases, and the quantity of 2-ethyl-l,3-cyclohexanedione ( I I I ) increases.
With a molar quantity of alkali the yield becomes 4 5 % of the theoretical.
I t is assumed that in a similar manner other 2-acyl-1,3-cyclohex
anediones can be reduced to 2-alkyl-l,3-cyclohexanediones. This offers another approach to the syntheses of 2-alkyl-l,3-cyclohexanediones.
The preparation of 2-alkylated dihydroresorcinols from the resorcinol dimethyl ether is also possible. Hydrogenation with sodium and alcohol in liquid ammonia gives the dimethyl ether of 2,5-dihydroresorcinol. The latter forms a potassium salt ( K ) on treatment with potassium amide