CHAPTER 6
Survey of Microbial Electron Transport Mechanisms
M . I. D O L I N
Page
I. Introduction 319 II. The Respiratory Chain 320
A. Components 320 B. Reduction and Reoxidation of Pyridine Nucleotides 322
III. Subdivision of the Respiratory Chain 326 A. Reduction of Cytochrome System by Reduced Pyridine Nucleotides.. 326
B. Terminal Oxidases 333 C. Substrates as Reductants of the Respiratory Chain 336
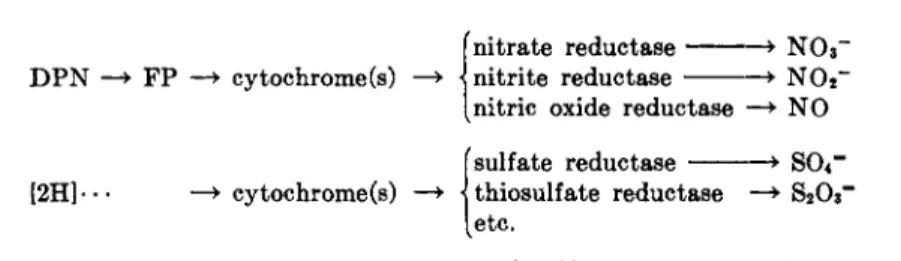
D . Inorganic Reductants of the Respiratory Chain 338 IV. Oxidants of the Respiratory Chain Other Than 02 339
A. Hydrogen Peroxide 339 B. Nitrate, Nitrite, Sulfate, etc 340
C. Artificial Electron Acceptors 341 V. Catalase and Peroxidase 342 VI. Determination of Respiratory Type 344
A. Determination of Reaction Sequences 344 B. Variation in Respiratory Chain with Change in Environmental Con-
ditions 345 VII. Electron Transport in Fermentation and Oxidation 346
A. Some Thermodynamic Considerations 346 B. Quantitative Aspects of Electron Transport 352
VIII. Coupled Oxidative Phosphorylation 355 A. Yield of "Energy-Rich" Phosphate ( ~ P ) 355
B. Bacterial Systems 357
References 358
I. Introduction
Three general processes are considered in the s t u d y of biological oxida- tion: (1) Dehydrogenation of a substrate, followed b y transfer of t h e hydrogen, or electrons, t o an ultimate acceptor. (2) Conservation, in a biologically available form, of t h e energy released in step 1. (3) Subsequent metabolism of t h e dehydrogenated (oxidized) substrate.
T h e present chapter is concerned with t h e enzyme mechanisms b y which bacteria accomplish t h e first two of these processes. I n biological oxidation t h e energy present in a n organic substrate is released b y successive de- hydrogenations of t h e carbon chain. Reducing equivalents are removed, two a t a time, and transferred t o a final acceptor, which m a y or m a y not
319
320 Μ. I. DOLIN
be oxygen, via a graded series of reversible oxidation-reduction systems.
T h e occurrence of several reversible oxidation-reduction systems between the initial reductant and final oxidant makes for smoother release of energy and provides loci for energy conservation steps. (The conflict between the terms hydrogen and electron transport has been clarified b y recent work, to be discussed in the next section. Quantitative treatment of electron transport is given in Section VII.)
I n general, knowledge of bacterial electron transport mechanisms h a s lagged behind t h a t of the mammalian systems. There are two reasons for this. First, the great diversity of metabolic types among bacteria makes for greater diversity in composition of the electron transport chain; even for a given organism, change in environmental conditions m a y alter t h e nature and concentration of the electron transport catalysts. Second, with bacteria one of t h e practical considerations has been, until recently, t h e problem of obtaining the large quantities of cells needed for enzyme purifica
tion work. An analytical difficulty common to the study of m a n y biological respiratory systems stems from the fact t h a t the catalysts comprising these systems m a y be bound to particulate structures.1"3 This feature makes it difficult to investigate individual steps in the sequential series of oxidation- reduction reactions catalyzed b y such preparations. T h e intact respiratory particle of animal systems, t h e mitochondrion, has now begun to yield individual subunits, which m a y be isolated and studied independently.2 Bacterial respiratory particles, as t h e y occur in disrupted cell preparations, are much smaller t h a n mitochondria and appear to consist primarily of broken cytoplasmic m e m b r a n e s .4 , 5
Although this chapter concerns only bacterial systems, reference will be m a d e to other microorganisms and to animal systems for purposes of orientation. T h e bacteria t o be considered here are t h e cytochrome-con- taining aerobes and facultative anaerobes. Respiratory enzymes of non- cytochrome-containing bacteria will be considered in more detail in Chapter 9.
II. The Respiratory Chain
A. COMPONENTS
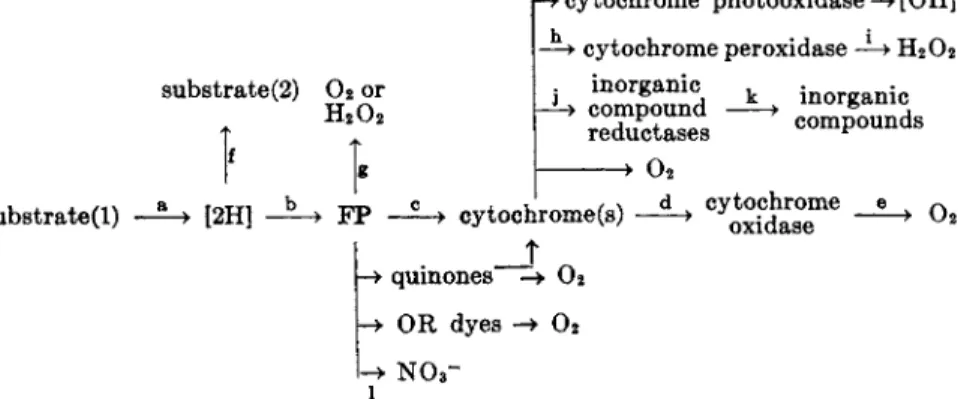
T h e sequence of electron transport reactions, as presently understood, is shown in Fig. 1. Hydrogen from substrate is transferred via pyridine nucleotides to flavoprotein, which in t u r n donates electrons to t h e cyto
chrome system. T h e reduced cytochrome is reoxidized b y cytochrome oxidase and the latter catalyzes the reduction of molecular oxygen t o water. Cytochrome and cytochrome oxidase are iron-porphyrin enzymes, discussed in detail in Chapters 7 and 8. I n intact animal mitochondria3 t h e
6. MICROBIAL ELECTRON TRANSPORT MECHANISMS 321 02
4 e | 4 H+ [H* + e]
-> T P N 2e
[ H * + e ] Τ [Η·
! i
2 H+ + e]
cytochrome oxidase
- I
substrate [ H + e l > D P N •
2 H +) ( ^ ^ ) 2 H+ ) cytochromes
2e 2e
2H+ 2 H+ - succinate
FIG. 1. Generalized electron transport chain. The number and nature of the flavo- protein ( F P ) and cytochrome components will depend upon the system under investi
gation. The formulation, [H* + e], indicates that the equivalent of a hydrogen atom plus an electron, or a hydride ion, is transferred. Transfer to and from flavoprotein is shown as a classic electron transport, for reasons discussed in the text.
reduction sequence for cytochrome is b, c, a, a3 (cytochrome oxidase). T h e identity and number of cytochrome components involved in bacterial sys
t e m s has purposely been left unspecified, since components v a r y from species to species and with growth conditions6 (Section V I , B ) . Further
more, bacterial pigments spectroscopically identical with mammalian cyto
chrome c, for instance, m a y have entirely different physicochemical and enzymic properties t h a n the animal counterpart.7 T h e electron transport catalysts, including diphosphopyridine nucleotide ( D P N ) in relatively in
t a c t preparations, m a y all be bound into an organized structure t h a t per
mits efficient interaction between the various components.
I n addition to the components mentioned, it becomes increasingly ob
vious t h a t lipids and lipid soluble substances have a significant function in electron transport systems. Since this is a relatively new development there is as yet little information on lipid function in bacterial systems. I n m a m malian respiratory preparations, lipids m a y act as a matrix in which the respiratory catalysts are imbedded,8 although a more active role in catalysis has been suggested.9 Among the fat-soluble substances, vitamin Ki can react with the respiratory chain of mammalian systems1 0 ·1 1 and bacteria (Mycobacterium phlei).12 Alpha-tocopherol appears t o be associated with mammalian respiratory particles1 8 ·1 4 and can function as an activator of a soluble pyruvate oxidase derived from an acetate-requiring m u t a n t of Escherichia coli.u&
Substituted p-benzoquinones (collectively designated the coenzyme Q group) have been isolated from beef heart mitochondria and various micro
organisms (Azotobacter vinelandii, Torula, Saccharomyces cerevisiae).lbh T h e nucleus of these quinones is 2,3-dimethoxy-5-methyl-p-benzoquinone, with an isoprene polymer occupying carbon 6 of t h e quinone ring. T h e length
322 Μ. I. DOLIN
of the isoprenoid chain differs depending upon the source of the quinone, and varies from a length of 10 units (Qio) for the beef heart quinone t o a length of 6 units (Qe) for the quinone isolated from S. cerevisiae. I t is sug
gested t h a t coenzyme Qi0 of mitochondria functions in electron transport from succinate to oxygen1 6 0 and t h a t it m a y be involved in oxidative phos- phorylation.1 5 d Apparently similar quinones, isolated from a variety of animal tissues, have been designated ubiquinone.1 5 6
Besides the components already noted, mammalian2 and bacterial (Azo- tobacter)1* respiratory particles contain nonheme iron and copper. A spe
cific role for the latter metals has not been established.
I t will be noted from Fig. 1 t h a t the role of oxygen in a typical respiratory system is t h a t of oxidizing the reducing equivalents removed from sub
strate. I n these reactions, any oxygen t h a t appears in the dehydrogenated substrate molecule is derived from water (i.e., through carbonyl oxidation systems and hydrases). There are enzymes, however, t h a t catalyze t h e direct introduction of either one or two atoms of oxygen from molecular oxygen into substrate. These reactions, which among bacteria have been studied mainly in the genus Pseudomonas, are probably not respiratory processes, b u t mechanisms for preparing specific compounds. T h e subject has been thoroughly reviewed b y Mason1 7 and will not be considered here.
T h e point of entry of a substrate into the respiratory chain is governed by thermodynamic considerations and by the enzymic constitution of t h e particular system under investigation. Some substrates, for instance, have t h e potentiality of reacting at t h e pyridine nucleotide level and also higher u p in the chain. Table I shows the entry point of various compounds into the respiratory chain and indicates those which can react at more t h a n one site. Theoretical treatment is given in Section V I I . Various enzyme systems have been described as "cytochrome-linked dehydrogenases'' because they were thought t o couple substrate dehydrogenation more or less directly with cytochrome reduction.1 8 For two such systems, however, succinic dehydrogenase1 9 and lactic dehydrogenase of y e a s t ,2 0*2 1 it is now known t h a t flavoprotein mediates the electron transport to cytochrome. These sys
tems m a y be prototypes for other cytochrome-linked dehydrogenases (i.e., those for α-glycerophosphate, choline, or malate).
I t should be stated at the outset t h a t the formulations of Fig. 1 and Table I do not imply t h a t a single flavoprotein is the electron acceptor for all t h e reactions shown.
B . REDUCTION AND REOXIDATION OF PYRIDINE NUCLEOTIDES
1. FORMATION OF D P N H AND T P N H
T h e bulk of hydrogen transport in main-line respiration passes through pyridine nucleotide coenzymes.2 2 I n this manner, reducing equivalents
6. MICROBIAL ELECTRON TRANSPORT MECHANISMS 323
T A B L E I
SITE AT WHICH VARIOUS COMPOUNDS ENTER THE RESPIRATORY CHAIN0
Pyridine nucleotide Flavoprotein Unknown Glyceraldehyde-3-P04
Isocitrate
Pyruvate 1
\ via lipoic acid α-Ketoglutarate J
Malate Lactate Alcohol Aldehydes
Glucose, glucose-6-P04
α-Glycerophosphate
β-Hydroxybutyrate, 0-hydroxy- butyryl-CoA
Formate Glutamate
Succinate Fatty acyl-CoA H2?
Lactate (yeast)
Glucose (Pseudomonas fiuorescens,
Acetobacter suboxydans) Gluconate (Pseudomonas
fiuorescens) Malate (yeast) α-Glycerophosphate Choline
β See: this chapter as well as H. A. Krebs and H. L. Kornberg Ergeb. Physiol., biol. Chem. u. exptl. Pharmakol. 49, 212 (1957), and T. P. Singer and Ε. B. Kearney, in "The Proteins" (H. Neurath and K. Bailey, eds.), Vol. 2, Part A, p. 123. Academic Press, New York, 1954.
drawn from a wide variety of substrates are funneled through a common p a t h w a y t o oxygen. T h e structure of D P N is shown in Fig. 2; triphospho- pyridine nucleotide ( T P N ) differs only in having a phosphate group on carbon 2 of t h e ribose linked t o adenine. Chemical or enzymic reduction and oxidation t a k e place a t t h e para position2 3 and are accompanied b y t h e appearance and disappearance, respectively, of an absorption band at 340 ταμ. T h e absorption change permits spectrophotometric assay of pyridine nucleotide-linked reactions. Classically,2 4 t h e reduction of D P N would be
A H2 -> A + 2H+ + 2e (la)
DPN+ + 2H+ + 2e -> D P N H + H+ (lb) Sum: A H2 + DPN+ -> D P N H + A + un
formulated as the electron transfer shown in equations ( l a ) and ( l b ) . However, a great deal of kinetic, isotopic and spectrophotometric evi
d e n c e2 5 ·2 6 now indicates t h a t t h e mechanism of pyridine nucleotide de
hydrogenase activity involves Η transfer within a ternary complex of enzyme, D P N , and substrate. A general formulation is shown in reactions (2a)-(2d). Experiments with deutero-labeled substrates or D P N H (i.e., D P N D ) have shown2 7 - 2 8 a t h a t with m a n y pyridine nucleotide-linked de-
324 Μ. I. DOLIN
Ε + DPN+ τ± E D P N + (2a) E D P N + + Α Η2 τ± E D P N + A H2 τ± E D P N H A + Η+ (2b)
E D P N H A τ± Ε D P N H + A (2c) Ε D P N H Ε + D P N H (2d) Sum: DPN+ + ΑΗ2 & D P N H + A + Η+
hydrogenases there occurs a direct and stereospecific transfer of hydrogen t o and from D P N . T h a t is, (1) t h e hydrogen transferred from substrate or pyridine nucleotide does not equilibrate with protons in the environ
m e n t and (2) the enzyme catalyzing the transfer shows specificity for t h e
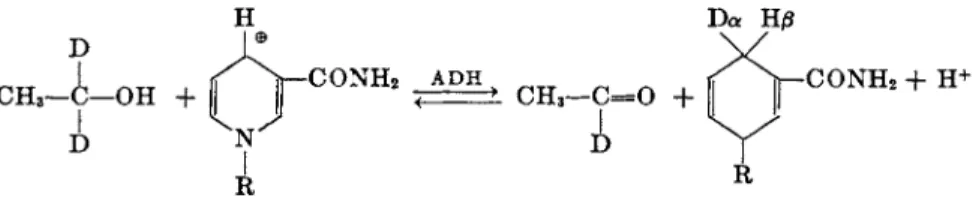
<*- or 0-side of the pyridine ring. T h e reduction of D P N b y deutero-labeled ethanol is illustrated in Fig. 3. T h e a and β specificities of a series of de-
C O N H2
FIG. 2. Diphosphopyridine nucleotide (DPN). The asterisk denotes the position of the third phosphate group of triphosphopyridine nucleotide (TPN).
Όα Έίβ
FIG. 3. The reduction of D P N by alcohol and in the presence of alcohol dehydro
genase ( A D H ) . The absolute a- and jS-conngurations are unknown. The α-configuration refers to the stereospecificity shown by yeast alcohol dehydrogenase, and the ^-con
figuration to the opposite stereospecificity.
6. MICROBIAL ELECTRON TRANSPORT MECHANISMS 325 hydrogenases have been summarized.2 8* These reactions are properly called hydrogen transfer, since t h e equivalent of one Η atom plus one electron [H' + e] or [H:]~ is transferred from donor t o acceptor, and equations ( l a ) and ( l b ) do not accurately represent t h e "activation" of either t h e electron donor or acceptor b y t h e enzyme. If, however, hydrogen transfer from donor t o acceptor is mediated b y a n enzyme-bound, reduced intermediate t h a t has a n appreciable acid dissociation, activation of substrate m a y , in a formal sense,2 4 conform t o equation ( l a ) . Flavoprotein enzymes such a s cytochrome c reductase, succinic dehydrogenase (see below), a n d di- hydroorotic dehydrogenase2 8 1* m a y offer examples of t h e latter mechanism.
2. INTERCONVERSION OF D P N H AND T P N H
Soluble enzymes have been prepared (pyridine nucleotide transhydro- genases) from Pseudomonas fiuorescens, P . aeruginosa, a n d Azotobacter s p .2 e which catalyze t h e following reactions:
T h e reversal of reaction (3a) requires 2'-adenylic acid; reaction (3b) was demonstrated with nicotinamide-labeled D P N . B y means of reaction (3a), TPN-specific dehydrogenases can be linked t o D P N H formation. Since evidence from animal systems indicates t h a t T P N H a n d D P N H m a y be reoxidized b y different pathways, with only D P N H oxidation resulting in oxidative phosphorylation, transhydrogenases m a y be of importance in regulating energy metabolism.2 9 I t h a s also been suggested t h a t trans- hydrogenase enzymes m a y be required for t h e reduction of particle-bound D P N b y free D P N H .2 6 Transhydrogenase enzymes of animal origin a r e particle-bound a n d have somewhat different properties from t h e bacterial enzymes.3 0 Bacterial transhydrogenase catalyzes direct a n d stereospecific Η transfer. A soluble transhydrogenase from placenta3 1* which catalyzes reaction (3a) in a freely reversible manner requires small amounts of a steroid hormone as cofactor, a finding which h a s obvious implications for metabolic control mechanisms. T h e transhydrogenase activity of mito
chondria, however, does n o t appear t o b e a steroid-dependent reaction.8 1 1 5 3. OXIDATION OF D P N H BY MOLECULAR OXYGEN
Particulate systems for t h e oxidation of D P N H b y oxygen are assumed to have t h e general composition [(DPN)-flavoprotein-cytochromes-cyto- chrome oxidase]. These oxidases have been studied extensively in animal systems (reviewed b y Chance3) a n d beginnings have been m a d e with bacteria. T h e over-all reaction conforms t o equation (4), a n d involves a
T P N H + DPN+ τ± TPN+ + D P N H (3a) D P N H + * D P N+ <=t DPN+ + *DPNH (3b)
326 Μ. I. DOLIN
2DPNH + 2H+ + 02 2DPN+ + 2 H20 (4)
4H+ + 4e + 02 2 H20 (5)
four-electron transfer, as shown in equation ( 5 ) . This balance has been documented for animal systems3 2 ·3 3 and is presumed to express the stoichio
m e t r y for apparently similar bacterial systems, although balances are not always presented. Particulate cytochrome-containing D P N H oxidases have been reported in Azotobacter vinelandii,1*·34·35 Escherichia coK,3 6 Pseudo
monas fluorescens,® Mycobacterium tuberculosis,™* Alcaligenesfaecalis,zshand Mycobacterium phlei.™ Other reports of particulate preparations (yeast, Proteus, Aerohacter),40 Serratia marcescens,41 and Bacillus megaterium5 do not mention D P N H oxidation specifically; however, the fact t h a t these preparations catalyze the oxidation of several Krebs cycle intermediates and other pyridine nucleotide-linked substrates suggests t h a t D P N H oxidases of the t y p e under consideration here m a y be present in the latter organisms. I t should be emphasized, however, t h a t conclusions regarding specific mechanisms should not be drawn merely from the fact t h a t bac
terial and mammalian D P N H oxidases resemble each other in gross com
position.
111. Subdivision of the Respiratory Chain
A. REDUCTION OF CYTOCHROME SYSTEM BY REDUCED PYRIDINE NUCLEOTIDES
1. RELATION TO FLAVIN
T h e sequence in which the bound catalysts operate in mammalian mito
chondrial preparations has been deduced in several ways3 (oxidation-reduc
tion potential of components; inhibitor studies; kinetics of oxidation and reduction of individual components, using sensitive spectrophotometric techniques; studies of partial reactions, catalyzed b y soluble components, or b y mixtures of soluble and particulate preparations). Since relatively few components have been p u t into true solution, t h e classic techniques of enzyme chemistry, which involve studies of single steps, each catalyzed b y a separate enzyme, are not fully applicable. T h e concept has arisen2*4 2 t h a t particulate systems can be fragmented in specific ways to give particle subunits of relatively constant composition and enzyme activity. Ideally, fragmentation would result in the preparation of truly soluble components:
(a) enzymes catalyzing the reduction of one or more cytochromes, (h) a series of soluble cytochrome components, and (c) a terminal (cytochrome) oxidase. According t o current concepts, therefore, t h e soluble respiratory catalysts t h a t have been isolated are presumed to have arisen from par
ticulate oxidases. Strict application of this concept, especially to bacterial
6. MICROBIAL ELECTRON TRANSPORT MECHANISMS 327 systems, m a y not be warranted, however. T h e question of the origin of a given enzyme m a y be difficult t o deduce and should be carefully evaluated for each system. I t is possible, for instance, t h a t some of the bacterial respiratory systems will require t h e participation, at one or more points, of enzymes t h a t are "normally" soluble.
Among the animal systems, cytochrome c and cytochrome c reductases3 have been obtained in soluble form; among the microorganisms, various cytochrome reductases (Table I I ) and a series of soluble cytochromes (Chapters 7 and 8) have been prepared.
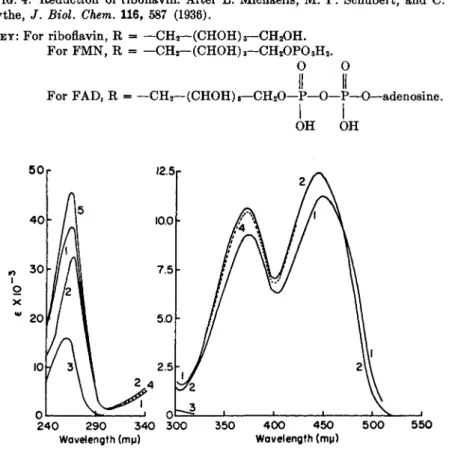
Enzyme-bound flavin was first implicated in the early steps of electron transport to oxygen or to iron carriers b y the work of W a r b u r g and Chris
t i a n4 3 and of Theorell.4 4 This work will be considered in more detail in Chapter 9. T h e structure of riboflavin and reduced riboflavin is shown in Fig. 4. I n its functional form, riboflavin occurs either as F M N (flavin mono
nucleotide) or F A D (flavin adenine dinucleotide). F M N has a phosphate group on the 5'-ribityl moiety of riboflavin; in F A D , 5'-adenylic acid is combined in a pyrophosphate linkage with F M N . T h e discovery and chemistry of these compounds have been r e v i e w e d .4 5 , 4 6
M a n y flavoproteins are now k n o w n .4 6 T h e bacterial flavoproteins not concerned primarily with cytochrome reduction are discussed in Chapter 9.
Proof of flavoprotein catalysis requires either (a) isolation of the pure enzyme in amounts sufficient t o demonstrate t h e flavin component, or (b) demonstration of reversible inactivation of the enzyme by removal and readdition of t h e flavin component.4 5 T h e latter approach is not always possible since some flavoproteins m a y be inactivated at t h e acid p H needed for removal of the bound flavin or, in some cases, because the reformation of active enzyme appears to require special conditions.4 7 Flavoproteins v a r y widely in the tenacity with which t h e y bind t h e flavin moiety and v a r y from nitrate reductase of Neurospora,** in which t h e flavin dissociates spontaneously during enzyme purification, t o succinic dehydrogenase,1 9 in which the flavin is combined with the protein b y strong, presumably co- valent, linkages. T h e absorption spectra of free flavins are shown in Fig. 5.
On chemical reduction by hydrosulfite, t h e yellow color of t h e flavin is discharged and the absorbance of t h e 450-ηΐμ band decreases approximately 9 0 % . Similar results are obtained with various flavoproteins;46 however, with some flavoprotein enzymes, addition of substrate results in incomplete reduction of t h e 450-ιημ band and formation of a new band in t h e long wavelength region.4 9' 5 0 T h e latter changes indicate t h e formation of en
zyme substrate complexes which in some systems m a y have a free radical nature.4 9- 5 1
Evidence t h a t flavoproteins are concerned in the reduction of cytochrome c came from the demonstration t h a t highly purified cytochrome c reduc-
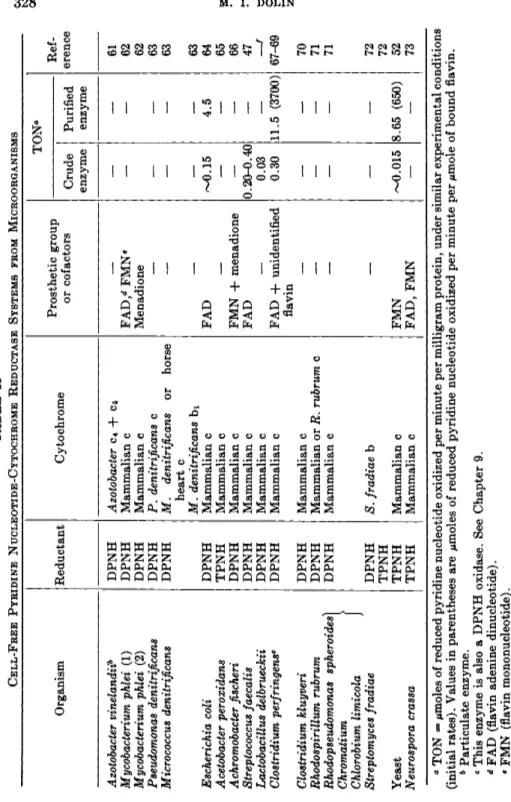
TABLE II CELL-FREE PYRIDINE NUCLEOTIDE-CYTOCHROME REDUCTASE SYSTEMS FROM MICROORGANISMS ΤΟΝ» Prosthetic group or cofactors Ref erence Organism Reductant Cytochrome Prosthetic group or cofactors Crude enzyme Purified enzyme
Ref erence Azotobacter vinelandiib DPNH Azotobacter c4 + C5 61 Mycobacterium phlei (1) DPNH Mammalian c FAD/* FMNe — — 62 Mycobacterium phlei (2) DPNH Mammalian c Menadione 62 Pseudomonas denitrificans DPNH P. denitrificans c — — — 63 Micrococcus denitrificans DPNH M. denitrificans or horse heart c
—r-— — 63 Μ. denitrificans bi — — — 63 Escherichia coli DPNH Mammalian c FAD ~0.15 4.5 64 Acetobacter peroxidans TPNH Mammalian c — — — 65 Achromobacter fischeri DPNH Mammalian c FMN -f- menadione — — 66 Streptococcus faecalis DPNH Mammalian c FAD 0.20-0.40 — 47 Lactobacillus delbrueckii DPNH Mammalian c — 0.03 — —f Clostridium perfringensc DPNH Mammalian c FAD -h unidentified 0.30 11.5 (3700) 67-69 Clostridium perfringensc flavin Clostridium kluyveri DPNH Mammalian c — — — 70 Rhodospirillum rubrum DPNH Mammalian or R. rubrum c — — — 71 Rhodopseudomonas spheroides) DPNH Mammalian c — — — 71 Chromatium > Chlorobium limicola J Streptomyces fradiae DPNH TPNH S. fradiae b — — — 72 72 Yeast TPNH Mammalian c FMN ~0.015 8.65 (650) 52 Neurospora crassa TPNH Mammalian c FAD, FMN — — 73 β TON μπιοΐββ of reduced pyridine nucleotide oxidized per minute per milligram protein, under similar experimental conditions (initial rates). Values in parentheses are μΐηοΐββ of reduced pyridine nucleotide oxidized per minute per μπιοΐβ of bound flavin. b Particulate enzyme. e This enzyme is also a DPNH oxidase. See Chapter 9. d FAD (flavin adenine dinucleotide). • FMN (flavin mononucleotide). ' Μ. I. Dolin, unpublished.
328 M. 1. DOLIN
+H++e ;
Oxidized (yellow)
- H+- e OH
Semiquinone
(green pH 2-7; red < pH 0, with binding of H+ to Ν
at position 10)
FIG. 4. Reduction of riboflavin. After L. Michaelis, M. P. Schubert, and C. V.
Smythe, / . Biol. Chem. 116, 587 (1936).
KEY: For riboflavin, R - — CH2— (CHOH)3—CH2OH.
For FMN, R - —CH2—(CHOH)3—CH2OP03H2. Ο Ο
II II
For FAD, R = — CH2— (CHOH)8—CH20—Ρ—Ο—P—O—adenosine.
I I
OH OH
240 290 340 300 350 400 450 500 550
Wavelength (mp) Wavelength (my)
FIG. 5. Absorption spectra of flavins and of AMP. 1, F A D ; 2, riboflavin; 3, AMP;
4, FMN (interrupted line); 5, theoretical spectrum of AMP + F M N ; between 290 and 510 πΐμ the theoretical spectrum is the same as the spectrum of FMN. From L.
G. Whitby, Biochem, J. 64, 437(1953).
329
330 Μ. I. DOLIN
t a s e s4 6-5 2 ·5 3 (enzymes t h a t catalyze cytochrome c reduction, b u t t h a t do not react at any appreciable rate with oxygen) contain flavin prosthetic groups. T h e reaction catalyzed by these enzymes can be formulated as follows:
ΡΝΗ + H+ + FP -» F P H2 + PN+ (6a)
F P H2 + 2 cyt. c ( F e+ + +) -> FP + 2 cyt. c (Fe++) + 2H+ (6b) Sum: P N H + 2 cyt. c ( F e+ + +) PN+ + 2 cyt. c ( F e+ +) + H+
This reaction sequence is not intended to show the mechanism, b u t merely t o indicate over-all stoichiometry. I t is with reactions of this type t h a t the link between two-electron and one-electron transport takes place, since two reducing equivalents from reduced flavoprotein are transferred to one- electron acceptors. I t has been postulated5 4 t h a t such transfers t o one- electron acceptors are specifically catalyzed b y metalloflavoproteins (those t h a t contain a metal in addition to a flavin prosthetic group, i.e., the iron- containing cytochrome c reductase of pig h e a r t .5 5 Ordinary flavoproteins were thought to catalyze only two-electron transport. However, it is now known t h a t various flavoproteins which do not contain significant amounts of m e t a l s4 9 , 5 0 are capable of catalyzing efficient one-electron transfer re
actions. Recent spectrophotometries and potentiometric5 7 evidence ex
tends and confirms the theory of Michaelis5 8 a t h a t oxidation and reduction of flavin (as well as other redox dyes) involves the formation of a semi- quinone, as shown in Fig. 4. This one-electron intermediate is presumed t o facilitate the link between two- and one-electron transfer reactions. (It has been pointed out, however,5 8 b t h a t the occurrence of a one-electron inter
mediate does not necessarily implicate it in the mechanism of electron transfer. T h e sequiquinone m a y form through dismutation of one fully reduced and one fully oxidized molecule.) Beinert has presented spectro- photometric evidence t h a t semiquinoid intermediates m a y form not only with free flavin56 b u t also with enzyme-bound flavin.59 Another mechanism for linking flavoprotein oxidation to cytochrome reduction would require the binding of two cytochrome molecules to one molecule of cytochrome reductase.
T h e reaction between reduced pyridine nucleotide and cytochrome c reductase appears to be stereospecific. W i t h D P N H - c y t o c h r o m e c re
ductase of pig heart it hag been shown t h a t both t h e oxidation of D P N H b y cytochrome c and an exchange reaction t h a t takes place between D P N H and D20 are specific for the 0 H of the pyridine r i n g .6 0 T h e exchange re
action suggests t h a t the "activation" of D P N H by cytochrome reductases m a y be represented formally b y the reversal of equation ( l b ) .
6 . MICROBIAL ELECTRON TRANSPORT MECHANISMS 3 3 1
2 . SOLUBLE CYTOCHROME REDUCTASES FROM MICROORGANISMS
T h e first cytochrome reductase obtained in highly purified form was isolated from y e a s t6 2 (mol. w t . 7 5 , 0 0 0 on basis of 1 bound flavin per mole enzyme). F M N is t h e bound flavin and T P N H t h e reductant. Although m a n y cytochrome c reductase activities have been reported in bacteria, few enzymes have been extensively purified and t h e latter have been obtained in relatively dilute solution. T h e difficulties involved in preparing large amounts of purified enzyme from bacterial sources h a s been mentioned.
Table I I lists various pyridine nucleotide-cytochrome c reductase activities t h a t have been found in microorganisms. These reactions were dem- onstrated with cell-free enzymes and soluble cytochrome components.
Turnover numbers are recorded for t h e purified enzymes (yeast, E. coli, Clostridium perfringens)) it will be noted t h a t t h e activity of t h e bacte- rial enzymes compares very favorably with t h a t of t h e yeast reductase.
I n fact, on a protein or flavin basis, t h e turnover of t h e enzyme obtained from t h e obligate anaerobe C. perfringens exceeds t h a t of t h e yeast en- zyme. T h e activity of t h e Clostridium kluyveri enzyme is also reported t o be high.2 6 Since Clostridia and Streptococcus faecalis do not contain cyto- chromes, t h e presence in these organisms of potent cytochrome c reduc- tases indicates t h a t t h e occurrence of such enzymes in a n y given system does not, in itself, furnish evidence for t h e existence of a functional cytochrome- linked respiratory system. T h e relation of these findings t o t h e physiology of cytochrome-free facultative a n d obligate anaerobes will be considered in Chapter 9 .
M a m m a l i a n cytochrome c, which is readily available, h a s been used as t h e oxidant for most of t h e reactions shown in Table I I . Results obtained in this w a y can be misleading, however, since bacteria m a y show preferential activity toward their own cytochromes.7 Some of t h e recently isolated soluble bacterial b a n d c cytochromes have been tested, as shown, b u t little information about t h e nature of t h e reductases is available.
3 . ARTIFICIAL OR M O D E L CYTOCHROME REDUCTASES
I n addition t o t h e enzymes which resemble t h e original yeast reductase, various artificial cytochrome reductases can be reconstructed. These re- actions depend upon t h e fact t h a t cytochrome c can be reduced nonen- zymically b y reduced compounds, such as hydroquinone,7 4 reduced mena- dione,6 6 free reduced flavins,76'76 reduced phospho- or silicomolybdate,7 7 and slowly b y ferrous citrate chelates.7 8 Therefore, enzymes which can catalyze t h e reduction of these compounds, with reduced pyridine nucleo- tide as electron donor, can also cause pyridine nucleotide-dependent cyto- chrome reduction. Such enzymes are t h e quinone and menadione reductases of E. coli,79 t h e menadione reductase of M. phlei,*2 and t h e flavin-dependent
332 Μ. I. DOLIN
menadione reductases of S. faecalis?0 and Achromobacter fischeri™ Quinones, in general, m a y act as electron acceptors for a variety of flavoproteins.81 Free flavin can serve as acceptor for D P N H oxidation in crude extracts of C. perfringens*7,82 and C. kluyveri.10 Various flavoproteins, including t h e diaphorase of P. fluorescens, can catalyze the reduction of ferric citrate chelates.7 8 (Aldehyde dehydrogenase can use phospho- or silicomolybdates as electron acceptors and thereby cause cytochrome c reduction;7 7 pyridine nucleotide-dependent reduction, in such a system, has not been reported.) Reduction of noncytochrome iron (Fe*"1"*, methemoglobin, ferric 8-hydroxy- quinoline chelate)7 6 and ferric citrate7 8 can also be achieved through free reduced flavin and therefore through enzymes t h a t can reduce free flavin.
Pig heart diaphorase (recently identified as lipoic dehydrogenase8 3) catalyzes cytochrome c reduction b y D P N H in the presence of lipoic acid. T h e lipoic dehydrogenase reaction (Chapter 9) yields reduced lipoic acid which, presumably in a spontaneous reaction, reduces cytochrome c.
As an example of one model reaction, the menadione-dependent reduction of cytochrome c6 2 > 6 6·8 1 is shown in equations (7a) and (7b).
D P N H + H+ + menadione -+ DPN+ + menadione H2 (7a)
menadione-H2 + 2 cytochrome c ( F e+ + +) —*
menadione + 2 cytochrome c (Fe**) •+- 2 H+ (7b) Sum: D P N H + 2 cytochrome c ( F e+^J - +
DPN+ + 2 cytochrome c (Fe++) + H+ (7c) T h e artificial reactions, in general, show low activity compared to the purified cytochrome reductases; some of t h e menadione reductases, how
ever, support rapid cytochrome reduction.
These reactions m a y have no physiological significance, except as pos
sible bypasses (Sections IV, C and VI, A), although it is tempting to think t h a t menadione (vitamin K3) m a y act in some cases as a water-soluble model for vitamin K, and not merely as a nonspecific quinone. Vitamin Κ is found in m a n y bacterial species8 4 and has been directly implicated in t h e respiratory chain of Μ. phlei.12*39 T h e site of action has not yet been estab
lished, however, with a mammalian respiratory chain preparation, reduced menadione apparently reacts a t the flavoprotein level8 5 and does not spon
taneously reduce bound cytochromes.
T h e biological consequence of some of these model reactions is illustrated b y the luminescent system of A. fischeri. Compounds which reoxidize reduced flavin spontaneously (cytochrome c, F e+ + +, methemoglobin) in
hibit luminescence b y competing with the luciferase for F M N H2.7 6
6. MICROBIAL ELECTRON TRANSPORT MECHANISMS 333 4. OTHER COMPONENTS OF CYTOCHROME REDUCTASE
Purified pig heart cytochrome c r e d u c t a s e5 5 contains nonheme iron;
α-tocopherol has been identified in solubilized beef heart DPN-cytochrome c reductase.8 6 There is no information on the occurrence of bound metals or fat-soluble vitamins in t h e purified bacterial reductases.
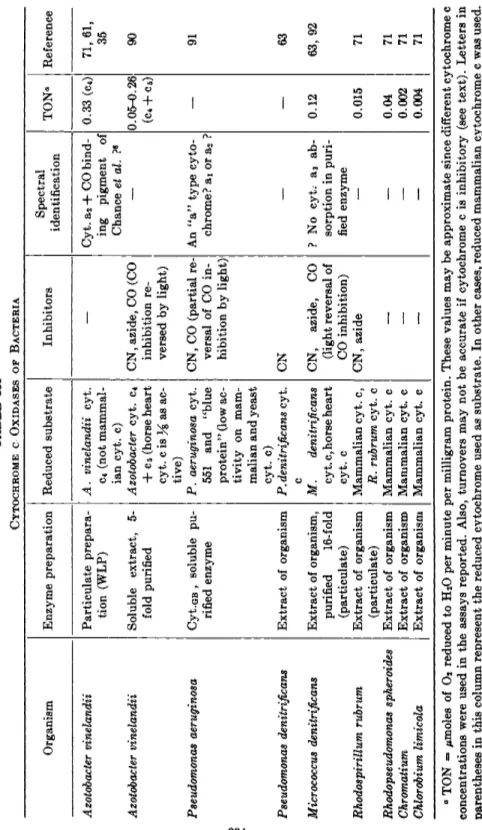
B . TERMINAL OXIDASES
Reducing equivalents from flavoprotein are transferred through t h e cyto
chrome system in a series of one-electron steps. These reactions, which comprise true electron transport, involve a ferri-ferro cycle for each of t h e intermediate cytochrome components. T h e oxidation-reduction reactions can be followed b y spectrophotometric observation of t h e characteristic cytochrome bands (see Chapter 7). Chance8 7 believes t h a t thermal collisions between neighboring cytochromes can account for t h e observed kinetics of electron transport in this region of t h e respiratory chain. T h e ultimate
4 cyt. c (Fe++) + 02 + 4H+ c y t' o x i d a a e > 4 cyt. c (Fe+++) + 2 H20 (8) cytochrome component (cytochrome c oxidase in mammalian and yeast systems)6 can react with oxygen and catalyze t h e four-electron reduction of 02 to 2 H20 , as shown in equation (8).
Possible mechanisms for reaction (8) have been c o n s i d e r e d .8*1 7·8 8 Briefly, these require either (a) a series of 4 one-electron steps with free radical forms of oxygen as intermediates, (6) a two-electron reduction of oxygen t o a peroxide, followed b y a two-electron reduction of t h e peroxide t o 2 H20 - higher valence states of the iron, i.e., F e4 +, m a y occur in this reaction, (c) participation of a tetraheme cytochrome c oxidase in a simultaneous four- electron reduction of oxygen.
Cytochrome c oxidase from mammalian sources has not been obtained in true solution, although t h e enzyme can be "solubilized" in deoxycholate and various particulate preparations have been studied.8 9 Particulate preparations with cytochrome oxidase activity have also been obtained from y e a s t6 and bacteria; "soluble" or a t least small particle preparations have been described for P. aeruginosa and Azotobacter vinelandii (Table I I I ) .
Table I I I summarizes the characteristics of various bacterial cytochrome c oxidase preparations. I t is rather difficult a t present to assess the sig
nificance of these reactions since t h e turnover of some, a t least, of these enzyme preparations appears t o be too low t o account for the respiration of t h e intact organism. T h e discrepancies may, in part, be traced to technical difficulties in the assay procedure. If it is assumed t h a t the oxidase reactions t h a t have been demonstrated constitute a major physiological p a t h w a y and t h a t t h e enzymes have not been damaged in preparation, low activity
TABLE III CYTOCHROME c OXIDASES OF BACTERIA Organism Enzyme preparation Reduced substrate Inhibitors Spectral identification TON-Reference Azotobacter vinelandii Particulate preparaA. vinelandii cyt. — Cyt. a2 + CO bind0.33 (c4) 71, 61, tion (WLP) c4 (not mammaling pigment of 35 ian cyt. c) Chance et al. ?e Azotobacter vinelandii Soluble extract, 5-Azotobacter cyt. c4 CN,azide,CO (CO — 0.05-0.26 90 fold purified 4- C5 (horse heart inhibition re(c4 + c5) cyt. c is % as acversed by light) tive) Pseudomonas aeruginosa Cyt.GB , soluble puP. aeruginosa cyt. CN, CO (partial reAn "a" type cyto— 91 rified enzyme 551 and "blue versal of CO inchrome? ai or a2 ? protein" (low achibition by light) tivity on mam malian and yeast cyt. c) Pseudomonas denitrificans Extract of organism P. denitrificans cyt. CN — — 63 Micrococcus denitrificans Extract of organism, c M. denitrificans CN, azide, CO ? No cyt. a3 ab0.12 63, 92 purified 16-fold cyt. c, horse heart (light reversal of sorption in puri (particulate) cyt. c CO inhibition) fied enzyme Rhodospirillum rubrum Extract of organism Mammalian cyt. c, CN, azide — 0.015 71 (particulate) R. rubrum cyt. c Rhodopseudomonas spheroides Extract of organism Mammalian cyt. c — — 0.04 71 Chromatium Extract of organism Mammalian cyt. c — — 0.002 71 Chlorobium limicola Extract of organism Mammalian cyt. c — — 0.004 71 β TON = ^moles of 02 reduced to H20 per minute per milligram protein. These values may be approximate since different cytochrome c concentrations were used in the assays reported. Also, turnovers may not be accurate if cytochrome c is inhibitory (see text). Letters in parentheses in this column represent the reduced cytochrome used as substrate. In other cases, reduced mammalian cytochrome c was used.
334
6. M I C R O B I A L E L E C T R O N T R A N S P O R T M E C H A N I S M S 335 m a y be explicable on the following grounds, (a) T h e specific substrate for the oxidase m a y not always be available (specificity for bacterial cyto
chromes is illustrated in Table I I I ) . T h e absence in a variety of cyto- chrome-containing bacteria6 of oxidases for reduced mammalian cyto
chrome c m a y be understandable on this basis. (6) If bacterial cytochrome oxidases resemble the mammalian counterpart, the reactions will show first-order kinetics for all reasonable concentrations of cytochrome c; how
ever, the rate constant will decrease with increasing cytochrome c (reduced or oxidized) concentration.9 3 T h e inhibition reaction makes it difficult to extrapolate back to the situation in intact cells and t o calculate t h e " t r u e "
turnover characteristic of the bound cytochrome and cytochrome oxidase components, (c) If some of the bacterial cytochrome oxidases t h a t have been prepared resemble the relatively intact mammalian respiratory par
ticle, special "opening" techniques2 m a y have to be used in order to facilitate contact between an exogenous cytochrome component and the bound cyto
chrome oxidase.
I t has been pointed out7 t h a t even when cytochrome oxidase activity is demonstrable, the reaction m a y not be direct (as shown in equation 8), b u t m a y consist of a series of model reactions in which some autoxidizable
component other t h a n t h e classic cytochrome oxidase is the terminal catalyst.
Artificial substrates such as p-phenylenediamine or p-phenylenediamine plus α-naphthol (Nadi reagent) are sometimes used as reductants of the cytochrome c-cytochrome oxidase system; however, the nonspecificity of this reaction, when used as a criterion for cytochrome oxidase activity, has been emphasized.6
" T y p i c a l " cytochrome c oxidases are inhibited b y C N , azide, and C O .3'6 Classically, t h e spectrophotometric identification of a terminal oxidase is m a d e b y examining the action spectrum for reversal of CO inhibition8 7 b y light. If the latter corresponds with the photochemical dissociation spectrum of the CO-inhibited system, the spectrum is t h a t of the CO compound of the oxidase. Although m a n y bacterial oxidases are inhibited b y typical cytochrome oxidase inhibitors, there is m u c h variability in inhibition pat
tern and in t h e extent of CO inhibition and its reversibility by light.6 Spectrophotometric techniques6 have demonstrated t h a t : (a) Yeast con
tains a cytochrome of the classic a 3 type, (b) Bacillus subtilis contains a cytochrome closely resembling cytochrome a 3 , b u t the organism has no oxidase activity for reduced mammalian cytochrome c. I t is questionable whether a n y bacteria yet examined contain the classic cytochrome a3. (c) T h e terminal oxidase of Acetobacter pasteurianum appears to be cytochrome ai. (d) A variety of bacteria m a y use an enzyme similar to t h e Rhodo- spirillum heme protein ( R H P )7'9 4 as a terminal oxidase. T h e latter enzyme has been obtained in soluble, purified form from R. rubrum and appears t o
336 Μ. I. DOLIN
be an autoxidizable variant of cytochrome c.9 4 R H P can be reduced by mammalian cytochrome c reductase; however, the physiological reductant is unknown. Apparently it is an open question whether autoxidation of R H P results in the formation of water or peroxide.7 Since the enzyme does not form complexes with C N or azide, it cannot account for the cyanide sensitive terminal oxidase activities t h a t have been reported.
Several autoxidizable CN-insensitive cytochrome b components have been found in b a c t e r i a6 3'7 2 b u t the extent to which they m a y be able to function as terminal oxidases is not known. T h e possible function of flavo- proteins as direct oxidases for reduced pyridine nucleotide will be con
sidered in Section V I .
C. SUBSTRATES AS REDUCTANTS OF THE RESPIRATORY C H A I N
1. SUCCINATE OXIDATION
One member of t h e citric acid cycle, succinate, enters the cytochrome chain at the flavoprotein level (Fig. 1). Succinate oxidation has been studied extensively with various particulate preparations;3 however, Singer and Kearney showed t h a t the primary dehydrogenase (assayed with phenazine methosulfate as electron acceptor) can easily be isolated in soluble form from m a n y sources.1 9 T h e enzyme, highly purified from beef heart or y e a s t1 9 is an iron-flavoprotein (mol. wt. ~ 2 0 0 , 0 0 0 ; 4 nonheme iron atoms and 1 mole of flavin per mole enzyme). T h e flavin ( F A D or derivative thereof) is covalently linked t o the protein. Succinic dehydrogenase (animal and yeast) also functions as a fumarate reductase. W i t h t h e proper reductants (e.g., F M N H2 or various leuco dyes) t h e enzyme catalyzes fumarate re
duction to succinate. Catalysis is more rapid, however, in t h e direction of succinate oxidation. Particulate heart preparations will, under anaerobic conditions, catalyze a fumarate-stimulated, nonstereospecific exchange be
tween the methylene hydrogens of succinate and deuterium of D20 .9 5 a This exchange is probably best explained b y t h e reversibility of t h e succinic
succinate + fumarate + Ε · Η2 (9)
dehydrogenase reaction, as shown in equation (9), since reduced enzyme could exchange protons with water (see discussion G u t f r e u n d9 5 b) .
Succinic dehydrogenase has also been purified from t h e obligate an
aerobe Micrococcus lactilyticus.9* ·9 7 T h e enzyme is an iron-flavoprotein containing 1 mole of F A t ) and 40 atoms of nonheme iron per 460,000 g.
of protein (mol. wt. several million). T h e flavin, unlike t h a t of the yeast and animal enzymes, is easily removed on heating. Reversibility of t h e bacterial succinic dehydrogenase can be demonstrated with F M N H2 or leucomethylviologen as reductants and, in fact, t h e reaction is more rapid