CHAPTER 7
Cytochrome Systems in Aerobic Electron Transport
LUCILE SMITH
I. The Nature of the Cytochrome Pigments 365 II. Nomenclature of the Cytochromes 366 III. Methods of Studying the Respiratory Chain Pigments 368
A. Observations of Absorption Spectra 368 B. Measurements of Reaction with Oxygen 369
C. Use of Inhibitors 370 IV. Functions of the Cytochromes 370
A. Oxidases 371 B. Functions of b- and c-Type Cytochromes 374
V. The Particulate Nature of the Bacterial Respiratory System 375
VI. Oxidative Phosphorylation 378 VII. Bacterial Cytochromes 380 VIII. Soluble Bacterial Cytochromes 386
IX. The Effect of Environmental Factors during Growth on the Cytochrome
Content of Bacteria 390 A. Effect of Oxygen Tension 390
B. Effect of Growth Rate 391 C. Effect of the Iron Content of the Medium 391
D . Other Effects 392 X. Summary 392
References 393
I. The Nature of the Cytochrome Pigments
T h e cytochrome pigments are hemoproteins. This means t h a t t h e y are composed of an iron-porphyrin prosthetic group attached b y one or more linkages t o t h e protein p a r t of the molecule.1 ·2 I n the different hemopro
teins the porphyrin or the protein p a r t m a y differ, or there m a y be different kinds of bonds between t h e porphyrin and t h e protein parts. T h e cyto
chromes are rapidly reversible redox systems, and the valence of t h e iron appears t o change on oxidation and reduction.3* W h e n t h e cytochromes are in t h e reduced (ferrous) form, they show sharp bands in the absorption spectrum in t h e region from 500 t o 650 πΐμ; in t h e oxidized form (ferric) only broad diffuse bands are apparent in this region of t h e spectrum. I n b o t h the oxidized and reduced forms t h e absorption spectra of t h e cyto
chromes show intense bands in t h e so-called " S o r e t " region of t h e spectrum (400 t o 450 ιημ), these absorption bands being related t o t h e presence of an intact porphyrin ring. .When oxidized cytochromes are reduced, there is a shift of 7 to 20 πΐμ in t h e position of t h e Soret band toward longer wave-
365
366 LUCILE SMITH
lengths. Figure 1 shows the absorption spectra of purified cytochrome c from horse heart in both oxidized and reduced forms.3 b
T h e characteristic changes of absorption spectra of the cytochrome pigments during oxidation and reduction led t o the recognition of their involvement in cellular respiration. B y observations of m a n y kinds of cells with a spectroscope, Keilin4 saw the cytochrome absorption bands appear and disappear as conditions changed from anaerobic to aerobic. All the known functions of the cytochrome pigments are based on their capacity readily t o undergo oxidation-reduction reactions. As discussed below, it now appears t h a t some of t h e cytochromes function in aerobic oxidations b y forming part of a chain of enzymes t h a t transport hydrogen or electrons from t h e substrate being oxidized t o molecular oxygen. I t is these respir- atory chain cytochromes which will concern us in this chapter. Other cyto chromes have been characterized t h a t perform other functions in cells, b u t these either have not been found in bacteria or are discussed in Chapter 8.
II. Nomenclature of the Cytochromes
From his original observations of the cytochromes involved in reaction with oxygen, K e i l i n4'6 recognized t h a t there were a t least three such pig-
FIG. 1. Absorption spectra of mammalian cytochrome c in the oxidized and re- duced forms.3 b Data are reprinted through the courtesy of the British Medical Bul- letin.
7. CYTOCHROMES IN AEROBIC ELECTRON TRANSPORT 367
merits present, which he designated components a, b, and c. Further work has indicated t h a t these different components have a number of properties which distinguish them, and as additional components have been found, they have often been named as cytochromes of type a, b, or c, according to the following characteristics:
(1) Cytochromes of type a have absorption bands in the reduced form in the red region of the spectrum, t h a t is, from about 590 t o 635 ιημ. T h e absorption spectra of reduced cytochromes a and a3 are different from those of b - or c-type cytochromes in t h a t the former have only one band in the visible region of the spectrum, while the latter have two. T h e porphyrin rings of the prosthetic groups of the a-type cytochromes have been shown to be different from those of types b and c .6 , 7 T h e compound of the heme of cytochromes a, ai, and a3 with pyridine (known as t h e pyridine hemo- chromogen) has absorption bands at 587 and 430 πΐμ in t h e reduced form.
Some of t h e a-type cytochromes react rapidly with oxygen and will form compounds with carbon monoxide.
(2) Cytochromes of type b show absorption spectra in the reduced form similar to t h a t characteristic of a protohemin hemochromogen, which is a compound formed b y linkage of nitrogenous compounds to the iron of the hemin nucleus.8 These have α-, β-, and γ-absorption bands around 560, 530, and 430 ιημ. T h e b-type cytochromes can be easily split into heme and protein parts by treatment with alkali. T h e n after splitting, a characteristic hemochromogen, such as t h a t with pyridine, can be prepared as a means of identifying protohemin as t h e prosthetic group.
(3) T h e c-type cytochromes are characterized b y somewhat greater stability t o heat and t o acid and alkali t h a n those of t y p e a or b . T h e prosthetic group is protohemin with additional linkages on the two vinyl groups t o cysteine residues in the protein p a r t .9 I t is more difficult to hydrolyze t h e heme-protein linkages in cytochromes of type c, b u t some of these can be split with alkali so t h a t characteristic hemochromogens can be formed with pyridine or cyanide. T h e c-type cytochromes are very slowly autoxidizable, if at all.
Numerous cytochromes have been discovered and designated according t o this system of nomenclature. There have now been described cytochromes a through , b through b ? , and c through c6. Cytochromes f and h do not occur in bacteria and t h u s do not concern us here.
Another t y p e of cytochrome nomenclature has been suggested b y Scaris- brick.1 0 Following this system the pigment is named according to the source of the cytochrome and the wavelength of the alpha absorption band of the reduced pigment. T h u s Azotobacter vinelandii cytochrome 554 would refer to a pigment in Azotobacter vinelandii having a n alpha absorption b a n d at 554 πΐμ. This pigment is usually referred to as cytochrome c6.
368 LUCILE SMITH
Recently another system of nomenclature of the cytochromes has been suggested by Egami et al.,11 and a committee of t h e International Union of Biochemists is at present working on still another. However t h e cyto- chromes are named, the difficulty remains t h a t t h e physiological reactions of most of these pigments are not known, and the nomenclature rests mainly upon characteristics of the absorption spectra.
III. Methods of Studying the Respiratory Chain Pigments
A. OBSERVATIONS OF ABSORPTION SPECTRA
T h e cytochrome pigments and also the pyridine nucleotides and fla- voproteins which make u p the respiratory chain all show changes of ab- sorption spectrum on oxidation or reduction; thus they can be studied by observing these changes. T h e changes in absorption spectra of the cyto- chrome pigments, b u t not the pyridine nucleotides or the flavoproteins, can be followed by observations with a spectroscope. After the original work of Keilin, numerous observations were made of the cytochrome ab- sorption bands in different kinds of bacteria.1 2 - 1 4 I n making these direct spectroscopic observations of cells, it is important to have a good source of illumination. T h e spectroscopic technique has been reviewed b y H a r t r e e .1 6 T h e absorption spectra of the cytochromes have also been investigated with spectrophotometers. T h e cytochrome content of most cells is rather low, and spectrophotometric measurements of the absorption spectra of cytochromes in intact cells are difficult because of the considerable light scattering and the presence of other pigments. T h u s special precautions must be observed to eliminate these interferences and to ensure t h a t enough light is transmitted through the turbid suspension to be able to maintain a narrow band width. A spectrophotometer has been specially designed for this purpose;1 6 it can measure small changes in absorption spectra which occur when aerobic bacteria exhaust t h e oxygen in solution b y their respira- tion. If there are no changes in light scattering during this period, t h e difference spectra obtained result from oxidation-reduction changes of t h e enzymes which are involved in t h e reaction with oxygen. Actually it has always been possible to eliminate effects on absorption spectrum resulting from changes in light-scattering properties of bacteria or from other non- specific reactions;1 7 with t h e specially designed spectrophotometers it has been possible t o measure anaerobic minus aerobic difference spectra of m a n y kinds of microorganisms,1 8 as well as difference spectra obtained on reaction of pigments with reagents such as carbon monoxide, cyanide, or azide. T h e recent development of double-beam spectrophotometers has greatly minimized interference b y nonspectral changes in signal.
There is one report of a difference spectrum of bacteria m a d e in an
7. CYTOCHROMES IN AEROBIC ELECTRON TRANSPORT 369 ordinary spectrophotometer;1 9 t h e organism, Nitrobacter, appears to have an unusually high cytochrome content. I n a n u m b e r of instances difference spectra have been obtained in ordinary spectrophotometers with particulate preparations derived from bacteria, in which the light scattering is less t h a n in whole cells.2 0 - 2 3
Another method sometimes used t o decrease interference from light scat
tering is to increase the refractive index of t h e suspending medium. Wei- bull2 4 and Stanier et al.2b used glycerol for this purpose in measuring the absorption spectra of insoluble particulate fractions of Bacillus megaterium and Pseudomonas fiuorescens. Although B a r e r2 6 found high concentrations of proteins successful in decreasing light scattering, no cytochrome a b sorption spectra have been determined in this way.
There has been one report of measurements of cytochromes of intact Escherichia coli b y collection of the scattered light in an absorbing sphere with diffusely reflecting walls.2 7
Keilin and H a r t r e e2 8 devised a method of examining cytochrome absorp
tion bands a t the temperature of liquid air, where t h e absorption bands of t h e cytochromes will be intensified and sharpened and will sometimes split into two or more bands. I t is only in this manner t h a t the α-absorp
tion bands of cytochromes c and Ci can be seen separately. Using this method, Tissi&res could identify two c-type cytochromes in Azotobacter vinelandii with absorption bands which overlapped a t room temperature.2 2 I n Acetobacter suboxydans28 ·2 9 and Bacillus subtilis?*'*0 a large single a- absorption band seen at room temperature splits into several bands at the temperature of liquid air.
B . MEASUREMENTS OF REACTION WITH OXYGEN
Since t h e cytochromes are p a r t of t h e chain of enzymes carrying out aerobic oxidation of substrates, the measurement of oxygen u p t a k e in t h e presence of various substances is sometimes used to test for the presence of this system of enzymes. Since a whole chain of enzymes is involved in the reaction of substrates with oxygen (see below), the observed rates of oxygen u p t a k e will depend upon a number of factors, b u t most directly will be a measure of the rate-limiting reaction in the chain and t h u s m a y not demonstrate the full capacity of the cytochromes present.
T h e part of the cytochrome chain which reacts with oxygen can also oxidize a number of dyes and other redox systems with oxygen (for ex
ample, p-phenylenediamine or hydroquinone), and this t y p e of reaction is sometimes used as a measure of "cytochrome oxidase" activity. Either the oxygen u p t a k e or the oxidation of the dye can be measured. I t should be pointed out t h a t the method is a quite nonspecific one and should be used only in conjunction with other d a t a .1 5 , 3 1
370 LUCILE SMITH C . U S E OF INHIBITORS
One approach to the study of the respiratory chain enzymes involves the use of inhibitory substances which act at different points along the chain to interrupt the electron transport. For example, cytochrome oxidase ac
tivity is inhibited by cyanide, azide, and carbon monoxide. As discussed below, light-reversible carbon monoxide inhibition of respiration is the most specific test for cytochrome oxidases. T h e respiration of m a n y bacteria and of cell-free extracts is inhibited by 0.001 Μ cyanide,1 3 -3 2 ·3 3 b u t in bacterial extracts azide sometimes appears t o be a less effective i n h i b i t o r .2 0*3 3·3 4 a b There is evidence t h a t some bacterial extracts contain cyanide-insensitive reduced diphosphopyridine nucleotide ( D P N H ) and reduced triphospho- pyridine nucleotide ( T P N H ) oxidases.3 4* ·3 5
Narcotics inhibit the reduction of t h e cytochromes, possibly b y affecting t h e m u t u a l accessibility of t h e enzymes in t h e chain.3 6 I n the mammalian respiratory chain antimycin A reacts with an unidentified factor to inter
rupt electron transfer.3 7 This inhibitor has no effect upon t h e respiratory chains of bacteria,1 7*2 0 ·3 8 b u t some quinoline iV-oxime derivatives appear to have inhibitory effects in bacteria similar to t h a t of antimycin A in m a m malian cells.3 8
There are a number of difficulties involved in inhibitor studies. These have been r e v i e w e d1 5*3 9*4 0 and can be summed u p as follows:
(1) W i t h intact cells, there m a y be a permeability barrier t o t h e in
hibitor. This is true of azide, which penetrates some cells only in t h e form of t h e undissociated acid.
(2) Some inhibitors m a y be bound in an inactive form b y substances present in cells or extracts. For example, keto acids react rapidly with cyanide to form cyanohydrins and proteins will bind antimycin A.4 1
(3) T h e inhibitor m a y not react with t h e member of the enzyme chain which is the rate-limiting step. I n this case, considerable inhibition of t h e enzyme will not affect the rate of t h e over-all reaction.
IV. Functions of the Cytochromes
T h e respiratory chain of mammalian tissues has been m u c h more ex
tensively studied t h a n the corresponding system of microorganisms. T h e components and sequence of reactions in the mammalian respiratory chain is usually given as follows:4 2
succinate
\
substrates —* D P N - or ΤΡΝ-linked dehydrogenases —* flavoprotein cytochrome b —*· cytochrome Ci —j-
cytochrome c —* cytochrome a —* cytochrome a3 —* oxygen
7. CYTOCHROMES IN AEROBIC ELECTRON TRANSPORT 371
where t h e arrows indicate the direction of passage of hydrogen or electrons.
I n intact cells or with cellular extracts, the pigments which become rapidly oxidized at low oxygen tension and are rapidly reduced as the oxygen ten
sion is decreased to a value near zero are considered to participate in t h e respiratory chain. All cytochrome oxidases, including t h e mammalian en
zyme and those of bacteria, react rapidly with oxygen and have very high affinities for o x y g e n ;4 3 4 4 the concentration of oxygen giving half of t h e maximum rate of respiration is of the order of 10~7 to 10~6 Μ oxygen.
I n intact mammalian liver and heart mitochondria, this chain of hy
drogen and electron transport is coupled in a t least three places to enzyme systems which can bring about t h e phosphorylation of adenosine diphos
phate (ADP) to adenosine triphosphate ( A T P ) . T h u s the ultimate function of the enzyme chain which brings about the aerobic oxidation of substrates is to furnish a source of energy for t h e cells, since A T P can be used in energy-requiring reactions.
A. OXIDASES
As indicated in the above scheme, cytochrome a3 is the component in mammalian tissues and in yeast t h a t reacts rapidly with o x y g e n .4 5'4 6 I t then reacts with cytochrome a, which then oxidizes cytochrome c, etc.
Together cytochromes a and a3 comprise the combination of pigments which can rapidly oxidize reduced cytochrome c with oxygen. This can be demonstrated with added cytochrome c, which is one of the cytochromes t h a t has been obtained in purified form. T h e mixture of cytochromes a and a3 is t h u s often referred to as cytochrome c oxidase.
T h e classic method for demonstrating which enzyme in the chain is the terminal oxidase is based upon t h e fact t h a t carbon monoxide competes with oxygen for reaction with t h e oxidase, and the carbon monoxide com
pound can be decomposed b y light. T h e pigment with which carbon mon
oxide and oxygen compete for combination is the one t h a t reacts directly with oxygen, and t h e absorption spectrum of the carbon monoxide com
pound of this pigment can be determined from the action spectrum, t h a t is, from measurements of the relief of inhibition of the respiration b y light of different wavelengths.4 7 ·4 8 T h e action spectrum can then be compared with t h e absorption spectrum measured in the presence of carbon monoxide and with the photochemical dissociation spectrum (absorption spectrum of the carbon monoxide compounds dissociated b y light).4 9 From measurements of this kind on a number of b a c t e r i a ,4 6 , 4 8· 5 0 some information has been gleaned about t h e n a t u r e of t h e terminal oxidases present. This is sum
marized in Table I.
Although some bacteria contain a cytochrome with spectroscopic prop
erties similar t o those of mammalian cytochrome a3 (for example, B.
TABLE I TERMINAL RESPIRATORY ENZYMES OF BACTERIA*1 Terminal oxidase Organisms Peaks of absorption spectrum of carbon monoxide compound (ταμ) Peaks in absorption spectrum of reduced oxidase (Πΐμ)
Turnover number at 25° C. (sec.-1) Cytochrome a3 Cytochrome ai Cytochrome a2 "Carbon-monoxide bind ing pigment" (cyto chrome o)
Bacillus subtilis Sarcina lutea Only terminal oxidase in: Acetobacter pasteurianum One of several oxidases in: Proteus vulgaris Azotobacter vinelandii One of several oxidases in: Escherichia coli Proteus vulgaris Aerobacter aerogenes Only terminal oxidase in: Micrococcus pyogenes var. albus Acetobacter suboxydans One of several oxidases in: Escherichia coli Proteus vulgaris Aerobacter aerogenes Azotobacter vinelandii
590-91, 547-50, 430-31 585-92, 548, 427-28 636-37 567-68, 535-37, 416-18
605, 445 About 590, about 440 628-630 430, ? ?
76 (in B. subtilis oxidiz ing glucose) 620 (in A. pasteurianum oxidizing alcohol) β The identity of the terminal oxidases has been established in a number of bacteria by measurement of the carbon monoxide action spectra of the organisms. In some instances the photodissociation action spectra have also been measured. This information has been obtained only for the organisms listed in the table. Terminal respiratory enzymes are those that react with oxy gen.46 · 48"w- 67
7. CYTOCHROMES IN AEROBIC ELECTRON TRANSPORT 373
subtilis), t h e bacterial cytochrome a3 is not identical with the mammalian enzyme.4 6 -5 1 T h e bacterial cytochromes a plus a3 do not rapidly oxidize mammalian cytochrome c.5 2 I n fact, most bacterial extracts will oxidize mammalian cytochrome c only very slowly, if a t a l l .5 2 - 5 4 Extracts of Micro
coccus denitriiicans appear to oxidize mammalian cytochrome c,3 4 a b u t it has not been established whether the rate is as high as with the mammalian oxidase.
T h e specificity of the bacterial oxidases for the cytochrome substrates also extends to the c-type cytochromes. For example, two purified c-type cytochromes from Azotobacter vinelandii are rapidly oxidized b y oxidases of these bacteria, b u t not b y the mammalian oxidase or b y those of a number of other species of bacteria.5 5 Although the c-type cytochrome isolated from M. denitrificans is oxidized b y mammalian cytochrome c oxidase,3 4* ·5 4 t h e r a t e appears t o be low.
Great caution m u s t be executed in deciding whether a given reaction observed in vitro has physiological importance for the cells until quantita
tive measurements are made to ascertain t h a t t h e reaction is rapid enough t o fulfill t h e required function. I n the case of the cytochrome oxidases, as pointed out b y K a m e n ,5 6 a somewhat autoxidizable cytochrome t h a t can couple with mammalian cytochrome c would appear t o simulate cytochrome c oxidase activity.
Several different cytochrome oxidases are found among the bacteria. T h e pigment designated cytochrome ai of Acetobacter pasteurianum has been shown t o be t h e terminal oxidase of this bacterium4 8 and is t h e only pig
ment in the strain studied t h a t combines with carbon monoxide.5 7 Cyto
chrome ai has an absorption peak at 590 πΐμ in t h e reduced form. Evidence for a high concentration of a spectroscopically similar pigment is seen in t h e difference spectrum of Nitrobacter.19 I t is also found in a number of other bacteria in combination with one or more other cytochromes t h a t can re
act with carbon monoxide; it is often seen in combination with cytochrome a 2, which can also act as a terminal oxidase.5 0 Cytochrome a2 has so far never been found as t h e only terminal oxidase present in a bacterium.
Castor has s h o w n5 0 t h a t the concentration of cytochrome a2 is low in cells in the logarithmic phase of growth. I n preparing cell-free extracts t h e cytochrome a2 of t h e cells is sometimes lost,2 2 »5 8 b u t the respiration is still active in its absence, because of the presence of another oxidase.
T h e terminal oxidase of some bacteria is a pigment which, unlike the other oxidases, is not an a-type cytochrome.4 8 , 4 9 I t is found in quite a number of bacteria,5 0 sometimes as t h e only oxidase (for example in M.
pyogenes var. albus and Acetobacter suboxydans) and sometimes in com
bination with cytochromes ai and a2 (in Aerobacter aerogenes, E. coli, Azotobacter vinelandii). T h e carbon monoxide compound of this pigment is
374 LUCILE SMITH
similar to t h a t of a pigment from photosynthetic b a c t e r i a6 9 which seems t o have a prosthetic group like t h a t of cytochrome c, b u t some of t h e linkages between the iron and the protein part are missing. I t t h u s a p - pears t h a t different kinds of hemins m a y act as prosthetic groups of oxi- dases. The new type of oxidase has so far been referred to as t h e "carbon monoxide binding pigment,'' b u t will in the future be named cytochrome T o sum up, several cytochromes have been characterized as terminal oxidases of bacteria: cytochromes ai, a 2, a3, and the "carbon monoxide binding pigment." Some bacteria have only one oxidase, others m a y have two or three. Castor has s h o w n5 0 t h a t when more t h a n one oxidase is present, any one of these m a y act as the terminal oxidase. I t has not yet been possible to show to what extent t h e different oxidases are operative under different conditions. T h e respiration of some bacteria, such as E.
coli and Azotobacter, is less sensitive to inhibition b y carbon monoxide t h a n t h a t of others, and the inhibition is relieved only b y relatively strong il- lumination. I t m a y be t h a t in these bacteria, which have several oxidases, there is a relatively greater excess of oxidase activity.
Although there are other oxidases in cells besides t h e cytochrome oxi- dases, the affinity of t h e other oxidases for oxygen is usually m u c h lower.
Studies with inhibitors and kinetic studies show t h a t in mammalian cells and in most bacteria which contain a cytochrome system, t h e bulk of t h e respiration goes via the cytochrome pathway.
B . FUNCTIONS OF b - AND C-TYPE CYTOCHROMES
T h e cytochromes described above as terminal oxidases pass electrons directly to molecular oxygen. From our present knowledge it appears t h a t the rest of the respiratory chain cytochromes serve as a p a t h w a y for elec- tron transport between flavoproteins and t h e terminal oxidases. T h u s t h e y are assumed to undergo alternate oxidation and reduction during t h e oxida- tion of the substrates and somehow to link these oxidations to t h e forma- tion of A T P . Unfortunately t h e mechanism of electron transport through this chain of enzymes, which are all anchored to cellular particles, although often discussed, is still not known.
Several c-type cytochromes of bacteria have been isolated and purified (see Section V I I I ) . T h e cytochrome C2 of the photosynthetic bacteria is not a member of the respiratory chain of these bacteria.6 0 B u t t h e other purified bacterial c-type cytochromes are rapidly oxidized and reduced b y particulate preparations from t h e corresponding bacteria; t h u s t h e y can act as intermediary electron carriers.3 4*1 ·5 4 ·5 5 I t has been suggested t h a t a c-type cytochrome of Nitrobacter is reduced directly b y nitrite,1 9 since no cytochrome b was seen in these organisms. However, t h e presence of a
7. C Y T O C H R O M E S I N A E R O B I C E L E C T R O N T R A N S P O R T 375
small a m o u n t of cytochrome b could be masked b y a large a m o u n t of cytochrome c in spectrophotometric observations, so t h a t observations of t h e absorption spectrum at liquid air temperature would be necessary to rule out the presence of cytochrome b .
Only two bacterial cytochromes of t y p e b have been isolated.3 5 B o t h were rapidly reduced b y extracts of the corresponding bacteria in the presence of succinate or D P N H . T h e cytochromes b2 of yeast and b5 of mammalian microsomes show lactate and D P N H cytochrome c reductase activities, respectively.6 1' 6 2 There is so far no evidence in bacteria for t h e presence of cytochromes which are not a part of the respiratory chain, such as the cyto- chrome b5 found associated with mammalian microsomes.
Bacterial cytochromes have also been shown to react in oxidation-reduc- tion reactions t h a t pass electrons to other oxidants t h a n molecular oxygen, such as nitrate, sulfate, t h e oxidant formed on illumination of photosyn- thetic cells, and possibly an oxidant formed during t h e fixation of nitrogen.
I n some nitrate-reducing bacteria t h e same cytochrome chain can react with either nitrate or o x y g e n .6 3*6 4 These reactions are discussed in Chap- ter 8.
V. The Particulate Nature of the Bacterial Respiratory System T h e remarkable multienzyme system which mediates respiration and the associated A T P formation appears to be attached to insoluble particulate material within the cell in an orderly arrangement t h a t allows rapid re- actions to t a k e place.3 6 ·4 2 I n mammalian liver, the enzyme systems of oxidative phosphorylation are a p a r t of the mitochondria, as are t h e en- zymes of the tricarboxylic acid cycle. This has been established b y the examination of mitochondria isolated in a relatively intact form. When mammalian cells are broken u p in such a way t h a t the structure of the mitochondria is not maintained, fragments of the mitochondria can be separated because of their insolubility. For example, the succinoxidase preparation of Keilin and H a r t r e e6 5 is a suspension of small, insoluble particles derived from t h e disintegration of the mitochondrial m e m b r a n e .6 6 These particles are not capable of phosphorylation of A D P , and only suc- cinate and D P N H are oxidized at an appreciable rate.
Examination of broken-cell extracts of bacteria has not revealed t h e presence of particles with all of the biochemical properties of mammalian or plant mitochondria. When t h e cell-free extracts are centrifuged, several fractions can be separated by their ease of sedimentation. Usually all frac- tions possess similar enzymic activities,2 5 -3 1 · 6 7"7 0 the particles differing only in size.
T h e cytochrome system has always been found t o be associated with particulate m a t t e r in bacterial extracts. Usually t h e absorption spectrum
376 LUCILE SMITH
of the cytochromes seen in the particulate fractions is the same as t h a t observed in the intact cells;2 5 -3 3·7 1 - 7 3 t h e one exception is the low level or absence of cytochrome a2 in the particulate material. T h e particle-bound cytochromes can also become reduced on addition of an oxidizable sub
strate and then are reoxidized by aeration.2 5 , 5 5- 6 7
T h e bacterial particulate m a t t e r also contains some dehydrogenases.
Washed, insoluble particles from Azotobacter vinelandii™ ·6 7 were observed t o oxidize succinate or D P N H , while preparations from Aerobacter aer
ogenes could oxidize in addition lactate.7 4 ·7 5 Fractions from other bac
teria were also able to oxidize formate7 and ^-glycerophosphate3 3 or m a late.2 5- 6 9 ·7 0 -7 6 However, when the particulate fractions were supplemented with nonsedimentable fractions, additional substrates could be oxidized,7 1-
7 5 , 7 7, 7 8 although it was not possible to demonstrate complete oxidation of substrates of the tricarboxylic acid cycle to C 02 and H20 .6 7 Linnane and Still7 9 subjected suspensions of Serratia marcescens in sucrose to rapid shak
ing with glass beads for varying time intervals in the shaker designed by Nossal.8 0 Some of the dehydrogenases and the tricarboxylic acid cycle en
zymes—isocitric and malic acid dehydrogenases and aconitase and fu- marase—were very easily dissociated from the particles during the shaking procedure. I n contrast, succinic, lactic, formic, and α-ketoglutaric acid dehydrogenases, like the cytochrome system, remained firmly bound to the insoluble material, even with increased periods of shaking. T h e hydro
genase of Hydrogenomonas facilis is attached to insoluble particles if t h e bacteria are given only brief treatment in a sonic oscillator, b u t can be re
leased in soluble form b y increased periods of exposure to sonic oscillation.8 1 I t t h u s appears t h a t the cytochrome system is more firmly bound to the particulate material t h a n the dehydrogenases, except those for succinic, malic, and lactic acids. T h e dehydrogenases which remain bound to the particles will depend to some extent upon t h e method of breaking t h e bac
teria. When the dehydrogenases are released from t h e particles into t h e soluble fraction, they are still able to interact with t h e particulate enzymes.
When Schachman, Pardee, and Stanier8 2 centrifuged extracts of several kinds of bacteria broken in a number of different ways, they could dis
tinguish in all of the extracts fractions with sedimentation constants around 40, 29, and 5 S. As discussed above, the cytochrome system and other oxidizing enzymes have been found t o be present in fractions sedi- menting a t varying centrifugal fields. Although fractions from a number of bacteria which contained the 40 S particles were often observed to exhibit considerable succinoxidase a c t i v i t y ,7 0 ·7 5 ·7 7 the activity was never localized in these fractions. Sometimes t h e smaller particulate fractions showed greater oxidizing activity, expressed in terms of the protein c o n t e n t .6 7 - 7 0 Tissi&res et al.67 even found t h a t they could increase the specific activity of a larger particulate fraction from Azotobacter vinelandii to t h a t of a smaller
7. CYTOCHROMES I N AEROBIC ELECTRON TRANSPORT 377
fraction b y exposing t h e former briefly t o sonic oscillation. I n some in
stances the smallest particulate material obtained could be sedimented only after several hours centrifugation at 140,000 g25> 3 3 ·6 8 1 7 6 T h u s in cell-free extracts of bacteria there exists a whole range of particle sizes, all of which show similar oxidative activities and some of which are very small. T h e smallest particles have usually been obtained from extracts m a d e by break
ing the bacteria b y exposure to sonic o s c i l l a t i o n .3 3*6 8·8 3 Electron micro
graphs of actively oxidizing particulate preparations also show a hetero
geneous mixture of p a r t i c l e s .2 5 , 7 6·7 7 I n a coarse particulate fraction obtained from Pseudomonas fiuorescens25 t h e particles ranged in diameter from 10 to 100 ιημ. As calculated b y Tissiferes,7 5 particles with a diameter of 150 A.
would have a volume only one-millionth t h a t of a liver mitochondrion.
More recent work has shown t h a t particles with a sedimentation con
stant around 40 S and which are high in ribonucleic acid (RNA) content can be separated from t h e particles containing t h e oxidative enzymes b y starch electrophoresis7 6 or b y ultracentrifugation.8 4 On breaking Azotobac
ter vinelandii in a sonic oscillator the R N A particles and a soluble dehydro
genase were released at the same rate as t h e rupture of the cells;8 5 thus the RNA-containing particles are assumed t o exist as such in the cytoplasm.
T h e rate of release of the oxidizing enzymes and the phospholipids was, how
ever, slower and similar to the decrease of turbidity, suggesting t h a t these particles are derived from the breakdown of a larger structure, probably t h e cell envelope. On brief exposure to sonic oscillation large fragments re
mained which were called ' b u l l s ' ' and were thought to contain cell wall and possibly an adhering m e m b r a n e .8 5 Separation of the R N A particles from the oxidizing particles by starch electrophoresis7 6 showed t h a t the for
mer fraction contained particles of diameter 30 πΐμ which contained 50 % R N A and 50 % protein and no enzymic activities. T h e main component of this fraction had a sedimentation constant of 42 S. T h e other particles, which represented a very heterogeneous fraction, contained all of t h e en
zymic activities and were high in phospholipid. Exposure of the isolated hulls to more extensive sonic oscillation produced the oxidizing particles of irregular size and shape.
Weibull8 6 ·8 7 digested the cell wall of Bacillus megaterium with lysozyme in t h e presence of a sucrose concentration which prevented t h e osmotic lysis of t h e protoplasts formed. When t h e protoplasts were lysed osmoti- cally, a " g h o s t " fraction was derived from t h e cytoplasmic membrane plus possible adhering particles. This ghost fraction could be separated b y low centrifugal force and was found t o contain all of t h e cytochrome system of t h e cell. T r e a t m e n t of this fraction in a sonic oscillator gave rise t o insoluble particulate material like t h a t usually obtained on rupture of t h e bacteria.
Militzer et a Z .8 8 , 8 9 appear t o have obtained a ghost fraction with similar properties b y t r e a t m e n t of thermophilic bacteria with lysozyme.
378 LUCILE SMITH
T h u s there is now good evidence t h a t the particles of broken-cell extracts of bacteria which show respiratory activity are particles of varying sizes which are derived from a larger structure. This structure seems t o be t h e cytoplasmic membrane, possibly with some attached structures. T h e re
spiratory particles do not exist as such in the intact cells, in contrast to t h e RNA-containing particles which exist preformed in t h e cytoplasm. I n this respect, it is interesting t h a t the respiratory particles of t h e bacteria re
semble in activity either the succinoxidase particles obtained by extensive disruption of heart muscle4 5 or t h e small particles derived from liver or heart mitochondria which can oxidize a limited number of substrates and can carry out some oxidative phosphorylation.9 0'9 2
Storck and W a c h s m a n9 3 found t h a t the ghost fraction obtained b y lysis of B. megaterium protoplasts represents 1 5 % of the total lysate; this would seem t o be high if it comprises only t h e cytoplasmic membrane. T h e y did not obtain a clear-cut localization of the various oxidizing capacities either in t h e ghost fraction or in t h e supernatant obtained from centrifuging down t h e ghosts, but the succinic, lactic, and α-ketoglutaric acid dehydrogenases were concentrated in the ghost fraction. T h e supernatant fraction contained some structures which looked like pieces of ghosts. These experiments ap
pear to demonstrate how easily t h e ghost fraction is disrupted into smaller pieces.
One difficulty yet to be explained is t h e considerable loss of respiratory activity sometimes observed on rupturing the c e l l s .7 5 , 7 8· 9 4 This is not al
ways the case. Broken-cell extracts of Azotobacter vinelandii in buffer9 5 or in sucrose or lactose6 9 ·7 0 are about as active metabolically as t h e original organisms. Although Linnane and Still7 9 found t h a t the amount of some dehydrogenases released from the particles during shaking with glass beads depended somewhat upon t h e concentration of sucrose in the suspending medium, it has usually been observed t h a t the activities of the particles prepared from ruptured bacteria are the same irrespective of t h e medium in which t h e y are broken.6 7- 9 6· 9 7 This is in contrast to the situation in preparing intact animal mitochondria, where hypertonic sucrose solutions must be used. These observations agree with the suggestion t h a t t h e parti
cles derived from the bacteria do not represent cell structures.
A t t e m p t s to increase the specific activities of the oxidizing systems of isolated bacterial particles have so far m e t with only limited s u c c e s s .2 0 , 2 1 ·
6 8 ·9 8 Also no separation of the particles into subfractions with a separation of the oxidative activities has been o b t a i n e d .2 0 , 6 8
VI. Oxidative Phosphorylation
I n some broken-cell suspensions of bacteria the formation of A T P from A D P has been obtained during t h e oxidation of substrates b y oxygen.
7. CYTOCHROMES IN AEROBIC ELECTRON TRANSPORT 379 Usually the ratio of A T P formed to 02 consumed was found to be low compared with similar ratios obtained with mammalian or plant mito
chondria. I n bacterial extracts, P : 0 ratios of 0.4 t o 1 have been ob
tained with fractions of Proteus vulgaris,74 Corynebacterium creatinovorans,"
Azotobacter vinelandii,97 ·72 ·99"101 Micrococcus denitrificans,*4& and E. coli.102 I n experiments with Alcaligenes faecalis,10*'104 Aerobacter aerogenes,74 and Mycobacterium phlei,96'97·105 P : 0 ratios greater t h a n 1 have been reported.
However, in some cases the values for the inorganic phosphate disappearing did not agree with t h e A T P formed (measured as acid-labile phosphate).
T h e bacterial systems studied differ in a number of properties from the system of intact liver mitochondria which carries out oxidative phosphoryl
ation. For one thing, t h e bacterial systems were found to be relatively insensitive t o t h e inhibitor d i n i t r o p h e n o l ,7 4 , 9 9 ·1 0 0 ·1 0 4 ·1 0 6 ·1 0 7 which acts in low concentrations (10~6 M) to "uncouple" the phosphorylation from t h e oxidative reactions in mammalian mitochondrial systems. T h e Μ. phlei sys
tem seems to be different from the other bacterial systems, being completely uncoupled b y 5 Χ ΙΟ^6 Μ dinitrophenol, even when α-ketoglutarate was t h e s u b s t r a t e .1 0 6 I n mammalian systems, there is one substrate-linked phos
phorylation with this substrate t h a t is insensitive t o t h e inhibitor.1 0 8* T h e bacterial systems also differ from t h e mammalian ones in t h a t t h e bac
terial preparations do not show "respiratory control." I n mammalian mitochondria this respiratory control means t h a t respiration is inhibited unless there is a phosphate acceptor system present so t h a t phosphoryla
tion can proceed. I t is not known whether this difference between the bac
terial and mammalian systems is due t o t h e presence of an endogenous phosphate acceptor system in t h e bacterial preparations or t o a different t y p e of coupling between the respiration and the phosphorylation. As dis
cussed above, t h e particles derived from broken-cell extracts of bacteria represent pieces derived from t h e breakdown of a larger structure, while isolated liver mitochondria represent intact structures in the cytoplasm.
Evidence has been reported for the separation of the enzyme systems carrying out oxidative phosphorylation in A. faecalis10*'104 and M. phlei97 into several components, one of which is in t h e soluble fraction. B o t h Tis- siferes et al10* and Rose and O c h o a1 0 0 obtained oxidative phosphorylation with washed particles from Azotobacter vinelandii. However, when t h e su
p e r n a t a n t fraction from centrifugation a t 120,000 g for 90 minutes was added t o t h e washed particles, more rapid phosphorylation was observed t h a n expected from the separate activities of the particles and the super
n a t a n t fraction.1 0 6 T h e effect of t h e soluble fraction was not so pro
nounced with the particles from Azotobacter as with those from A. faecalis or Μ. phlei.
T w o fractions of particles can be separated b y centrifugation from Azo-
380 LUCILE SMITH
tobacter vinelandii extracts prepared b y grinding the bacteria with powdered glass. T h e smaller particles have the greatest phosphorylating activity, expressed on a d r y weight basis, b u t qualitatively t h e two kinds of parti
cles seem to be identical.6 7 These d a t a agree with t h e above suggestions about the source of t h e bacterial particles. Also t h e same phosphorylating activity was obtained when bacteria were broken in water, buffer, or sucrose.
Finally, no experiments similar to those done with liver mitochondria4 2 have been carried out with phosphorylating bacterial particles to show t h e oxidation-reduction reactions of the respiratory chain enzymes accompany
ing initiation or cessation of phosphorylation. This will be difficult t o ac
complish in the bacterial systems because of the lack of respiratory control.
VII. Bacterial Cytochromes
As discussed in previous sections, t h e bacteria have been shown to pos
sess a-, b-, and c-type cytochromes plus cytochrome o. As far as is known, these cytochromes have properties similar t o the cytochromes of yeast and mammalian tissues, which are often referred to as the " t y p i c a l " cyto
chrome system. T h e anaerobic minus aerobic difference spectrum of some mammalian cells, pictured in Fig. 2, shows the changes in absorption spec
t r u m of t h e pigments which react with oxygen. T h e α-, β-, and γ-cyto- chrome peaks are marked accordingly. Several difference spectra of bac
teria are plotted in Figs. 3 through 7. T h e qualitative observations on absorption spectra of bacterial cytochromes can be summarized as follows :
(1) Some bacteria show cytochrome absorption bands at the same wave-
+.20 i
Ε t . 1 0
5 - 1 0
2 " 2 0 i
310 3 5 0 3 9 0 430 470 510 550 590 630
FIG. 2. Difference in absorption spectrum between anaerobic and aerobic guinea pig liver mitochondria. Different dilutions of the suspension were used for the two wavelength regions and the optical density scales are also different.
390 420 450 480 500 550 600
FIG. 3. Anaerobic minus aerobic difference spectrum of B. subtilis.31 The bacterial suspension was diluted 2-fold for measurements between 500 and 600 ταμ and 5-fold for measurements between 390 and 490 πΐμ.
Sorcino luteo
" 4 0 0 4*30
λ<7<> 5 5 0 6 0 0 6 5 0 Μ pyogenes vor olbus
FIG. 4. Anaerobic minus aerobic difference spectra of Sarcina lutea and Micro
coccus pyogenes var. albus.17
382 LUCILE SMITH
Bact./I 2 Bact./ 2
λ(π\μ) Τϋημ)
FIG. 6. Anaerobic minus aerobic difference spectra of Aerobacter aerogenes and Escherichia coli.11 The E. coli suspension was diluted 2-fold for measurements be
tween 490 and 670 ιημ and 6-fold for measurements in the Soret region of the spectrum.
FIG. 5. Anaerobic minus aerobic difference spectrum of Acetobacter suboxydanz*1
7. CYTOCHROMES IN AEROBIC ELECTRON TRANSPORT 383
B o d . / 6 Boct. Undil.
Γ + 0 . 0 2
+ 0 . 0 1
- 0 . 0 1
- 0 . 0 2
5 5 0 6 0 0 6 5 0
FIG.
3 9 0 4 2 0 4 5 0 4 8 0 5 0 0 λ ( π ι μ )
7. Anaerobic minus aerobic difference spectrum of Azotobacter chroococcum.in
lengths as those of yeast and heart muscle; examples are B. subtilis and Sarcina lutea. As discussed in Section IV, these cytochromes are not identi
cal with their mammalian equivalents. M. pyogenes var. albus shows a difference spectrum between 500 and 650 πΐμ similar to t h e typical one, b u t t h e peak of cytochrome a3 in t h e Soret region is missing. T h u s these bacteria contain cytochrome a, b u t no cytochrome a 3 .
(2) A number of bacteria lack t h e cytochrome a peak which m a y be replaced b y cytochrome ai (in Acetobacter pasteurianum) or b y a mixture of cytochromes ai plus a2 (E. coli, Aerobacter aerogenes, Azotobacter chroococ- cum). There is no evidence of an a-type cytochrome in Acetobacter suboxy- dans or Rhodospirillum rubrum.
(3) Several bacterial species contain t h e α-absorption band of cyto
chrome bi in place of the similar band of cytochrome b . This is sometimes t h e only α-band seen in the spectral region characteristic of b - and c-type cytochromes even at liquid air temperature (example: E. coli, A. aerogenes).
I n other bacteria, cytochrome bi is seen in combination with c-type absorp
tion bands (example: Azotobacter). In some strains of B. subtlis, Keilin a n d H a r t r e e2 8 found t h a t at liquid air temperature the cytochrome bi absorption band split into three bands corresponding t o cytochromes b , c, and C i . Chaix and P e t i t3 0 observed four absorption bands.
(4) Two strains of Acetobacter show t h e difference spectra at room tem
perature with a large α-absorption band at 554 ταμ. At the temperature of liquid air, this band is found to have one main and two smaller components, t h e former resembling cytochrome Ci and the latter at positions correspond
ing to cytochromes c and b .2 8 , 2 9
384 LUCILE SMITH
(5) Numerous assortments of t h e various cytochromes m a y be seen in different bacteria. The d a t a are summarized in Tables I I and I I I .
T o sum up, the cytochrome systems of various bacteria m a y include pigments like the " t y p i c a l " cytochromes a, a3, b , c, and C i. Or, these to
gether with cytochromes ai, a2, a 4,1 0 8 b b i , b4, c3, c4, and c6 m a y be present in various combinations. T h e latter group of cytochromes has so far only been observed in bacteria. In spite of t h e great variety of cytochromes found among the bacteria, there is no evidence t h a t they perform functions different from those of mammalian tissues. T h e main difference between the bacterial and the mammalian cytochrome systems seems to be the presence of several oxidases in some bacteria.
The studies of the bacterial cytochrome systems, with their great variety of pigments, have given some insight into the cytochrome requirements for an electron transport chain.
(1) T h e combination of cytochromes a and a3 is not necessary, and the ratios of the two differ with the organism.
(2) The ratio of cytochromes c and Ci varies over a wide range.
(3) At least one organism has failed to show evidence of a c-type cyto
chrome.
T A B L E II
PEAKS IN DIFFERENCE SPECTRA OF BACTERIAL CYTOCHROMES'*
Bacteria
Visible6 Soret6
Bacteria
a β 7
Bacillus subtilis 604 564 552 523 444 422
Micrococcus pyogenes var. albus 604 565 552 523 — 427
Sarcina lutea 605 562 552 523 444 430
Micrococcus lysodeikticus 600 552 520 440 432
Aerobacter aerogenes 628 592 560 530 435 430
Escherichia coli 630 593 560 533 437 432
Proteus vulgaris 630 595 560 533 440 430
Azotobacter chroococcum 628 590 560 552 530 440? 428 A. chroococcum after several 590 552 522 440? 427
transfers
Acetobacter pasteurianum 588 554 523 445 428
Acetobacter suboxydans 554 525 — 422
Pseudomonas fiuorescens 580 560 552 523 — 424
Streptococcus faecalis No cytochrome peaks
Yeast 605 563 552 525 445 426
Heart muscle particles 605 563 552 525 445 426
• See Smith.3 1
6 Wavelengths in millimicrons.
7. CYTOCHROMES I N AEROBIC ELECTRON TRANSPORT 385
TABLE III
COMBINATIONS OF CYTOCHROMES OCCURRING IN BACTERIA41
Bacterium Cytochrome components Bacillus subtilis a, a3 , b, c, CI
Sarcina lutea a, AE , b, c6
Micrococcus pyogenes var. albus a, b, c,6 ο Acetobacter suboxydans b, c, CI , ο Acetobacter pasteurianum AI , b, c, CI
Acetobacter peroxydans c b, c, CI
Azotobacter vinelandii AI , A2 , bi , C4 , C5 , O
Aerobacter aerogenes AI , A2 t bi , 0
Escherichia coli AI , A2 , bi , 0
Proteus vulgaris AI , A2 , bi , 6 0
β The table represents a compilation of information from a number of sources.
Complete data on cytochrome components are obtained only by examination of the absorption spectra both at room temperature and at liquid air temperature plus tests for the oxidases present by measurements of carbon monoxide difference spectra or action spectra. Complete data have been obtained for only a relatively small number of bacteria. The purpose of the table is to illustrate the various combinations of cytochromes which occur in bacteria.
6 The absorption spectra of these bacteria have not been examined at liquid air temperature.
c Cytochrome a4 has only been observed in these bacteria;1 0 8 6 its function has not been investigated.
(4) All cytochrome-containing bacteria studied show several of these pigments. These presumably m a k e u p t h e organism's electron t r a n s p o r t chain. N o requirement for a given combination of t y p e s can yet be dis
cerned. This m a y suggest t h a t t h e only requirement m a y be a combination of cytochromes with enough difference in redox potential to give t h e energy for A T P synthesis.
T h e possible p a t h w a y s observed among t h e different bacteria a r e sum
marized in Fig. 8.
Bacteria like Acetobacter a n d Azotobacter contain relatively high con
centrations of cytochrome pigments. M o r e pertinent is a measurement of t h e t u r n o v e r numbers of t h e bacterial cytochromes. T a b l e I V lists
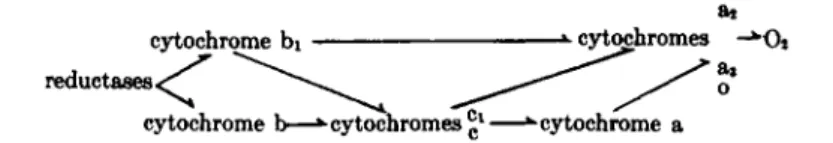
ai as cytochrome bi cytochromes -*0*
reductases <
<
cytochrome b—* cytochromes £l—^cytochrome a
FIG. 8. Possible pathways of electron transport in bacterial cytochrome systems.
386 LUCILE SMITH T A B L E I V
RATE OF TURNOVER OP BACTERIAL CYTOCHROMES
Organism Substrate
O2 uptake (μΜ/sec.) Δ O Da main
Soret peak (424-430
ΠΐΜ)
02 uptake (μΜ/sec.) Δ OD* Soret
peak of cytochrome a3 or a i (445
or 440 ΠΐΜ)
Turnover number6 of cytochrome a3 or a i (sec. _ 1)
Yeast Alcohol 186 65 Heart muscle particles Succinate — 80 29 Acetobacter pasteurianum Alcohol 1291 4500 620
Aerobacter aerogenes Lactate 160 218 —
Azotobacter chroococcum Glucose 185 — —
Micrococcus pyogenes var. Glucose 89 — —
albus
Sarcina lutea Succinate 847 2130 —
Escherichia coli Succinate 411 454 —
Micrococcus lysodeikticus Lactate 853 — —
Acetobacter suboxydans Alcohol 1530 — —
Bacillus subtilis Glucose 82 760 76
Pseudomonas fluorescens Glucose 209 — —
0 Difference in OD between anaerobic and aerobic states.
6 ( 02 uptake)/(Δ OD) X 4 Χ Δ extinction coefficient for cytochrome a3 or a i . the ratios of oxygen u p t a k e to cytochrome peaks in t h e difference spectra for yeast, heart muscle particles, and a number of bacteria. This ratio is an expression of the turnover numbers of the bacterial cytochromes, and can be calculated as turnover numbers for those cytochromes where the differ
ence in extinction coefficients between the oxidized and reduced forms is known. T h e d a t a show t h a t some of the bacterial cytochromes have quite high rates of turnover.
VIII. Soluble Bacterial Cytochromes
Recently a number of bacterial cytochromes have been obtained in soluble form, purified to varying degrees, and some of the chemical and physical properties studied. I t is not surprising t h a t most of t h e bacterial cytochromes which have been obtained in soluble form are those of t y p e c, since these are the most stable. Sometimes methods used for the isolation of cytochrome c from mammalian tissues were unsuccessful in work with t h e bacterial pigments; consequently several other types of extraction pro
cedure have been d e v e l o p e d .2 2 , 3 5'5 , 4·1 0 9·1 1 0
T h e first cytochrome to be isolated from bacteria was one from a photo-
7. CYTOCHROMES IN AEROBIC ELECTRON TRANSPORT 387 synthetic bacterium, Rhodospirillum rubrum.111 This cytochrome appears t o be involved in light-induced reactions in these bacteria.1 1 2 Cytochrome pig
ments have also been isolated from other photosynthetic bacteria and from anaerobic sulfate and from nitrate-reducing organisms. These bacteria all h a v e an oxidative metabolism, although the oxidant is not oxygen. T h e cytochromes of anaerobic metabolism are described in Chapter 8.
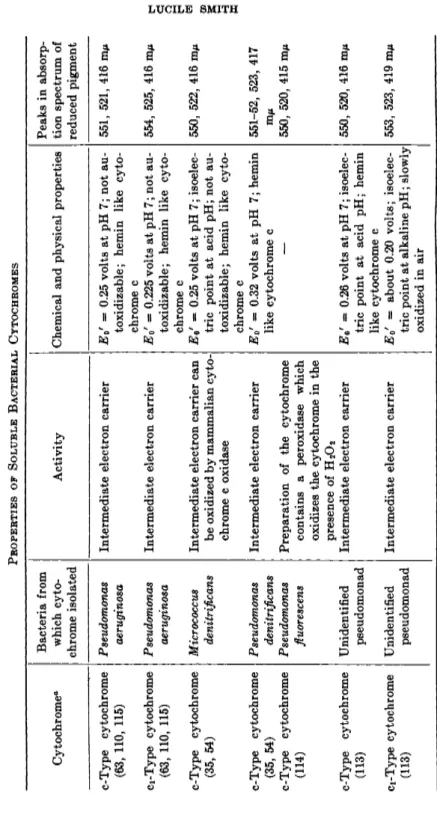
Cytochromes of type c have been isolated from Azotobacter vinelandii,22' 55 Micrococcus denitrificans,35' 54 Pseudomonas aeruginosa,63'109 Pseudomonas denitrificans,35'54 an unidentified pseudomonad,1 1 3 and from Pseudomonas fluorescens.m As discussed in Section IV, there is considerable specificity in the interaction between the c-type cytochromes and t h e cytochrome oxidases. Of the c-type cytochromes isolated, only t h a t from M. denitri
fieans was observed t o be oxidized b y mammalian cytochrome c oxidase, and this reaction appeared t o have a rather low r a t e .5 4 I n all cases where observations were made, the bacterial c-cytochromes were rapidly oxidized and reduced b y oxidases and reductases on particles prepared from t h e corresponding b a c t e r i a .2 2 , 3 5-5 4 ·6 3 T h e purified cytochromes from Azotobac
ter vinelandii were not oxidized b y particulate preparations from E. coli or Acetobacter peroxydans22 T h e preparation of cytochrome c from Pseudo
monas fluorescens contains a peroxidase which will oxidize the reduced cyto
chrome c on addition of H202.1 1 4 T h e relationship between t h e cytochrome c peroxidase and the cytochrome c oxidase of these bacteria is not clear.
Two c-type cytochromes were isolated from Azotobacter vinelandii?2 and Pseudomonas aeruginosa,109'115 and there is evidence for two in an unidenti
fied pseudomonad.1 1 3 These are reminiscent of the mixture of cytochromes c and Ci in mammalian and yeast cells. As with cytochromes c and C i , t h e wavelengths of the α-absorption peaks of the two c-type cytochromes are so close t h a t only one fused peak is observed in the mixture a t room temperature. T h u s they are recognized as separate entities only b y physical separation or b y observations of the absorption spectra a t the temperature of liquid air.2 2 I n Pseudomonas aeruginosa it is claimed t h a t electron trans
port has been observed between t h e two c-type cytochromes.1 1 5
Some of the properties of the isolated c-type bacterial cytochromes are summed u p in Table V (the cytochromes of t h e photosynthetic bacteria are excluded). I n all cases these cytochromes were shown to have absorp
tion spectra, chemical reactions, and stability similar t o mammalian cyto
chrome c and to form t h e same pyridine and cyanide hemochromogens.
T h e y t h u s have t h e same prosthetic group as mammalian cytochrome c.
T h e isoelectric points are usually quite different from t h a t of the m a m malian pigment, indicating a different protein moiety. K a m e n and T a k e d a1 1 0 showed t h e amino acid composition of t h e cytochrome c from Pseudomonas aeruginosa to be quite different from t h a t of mammalian cytochrome c.