ORIGINAL ARTICLE
Hereditary and non-hereditary etiologies associated with extensive brain calcification: case series
András Salamon1&Dénes Zádori1&Anikó Ujfalusi2&László Szpisjak1&Melinda Lukács1&Brigitta Bihari3&
Noémi Szépfalusi1&Viola Luca Németh1&Zoltán Maróti4&Emese Horváth5&István Balogh2&Csaba Bereczki4&
Péter Klivényi1&Tibor Kalmár4
Received: 23 February 2021 / Accepted: 1 July 2021
#The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature 2021
Abstract
Cerebral calcification may be caused by several potentially treatable conditions, however, in most cases it does not receive special attention in clinical practice. From the point of view of etiology, the diseases associated with cerebral calcification can be divided into two main groups: idiopathic (mostly incurable) and secondary (potentially treatable). The first group includes mainly the hereditary diseases identified before 2021 (primary familial brain calcification subtypes, previously known as Fahr’s disease or Fahr’s syndrome). In contrast, the second group includes diseases with cerebral calcification that develop generally as a conse- quence of metabolic/endocrine/autoimmune abnormalities. The aim of our research was to present hereditary and non-hereditary etiologies associated with extensive brain calcification. We compare the detailed clinical, radiological and laboratory results of 6 patients with prominent cerebral calcification identified in our clinic in the last 3 years (idiopathic and secondary etiologies as well). Our research draws attention to the complexity of the etiologies in the context of cerebral calcification. We recommend, beside NGS-based sequence analyses, the application of array comparative genomic hybridization as well, to identify potential genetic etiologies associated with brain calcification.
Keywords Fahr’s syndrome . Genetic . Idiopathic basal ganglia calcification . Primary familial brain calcification . Brain calcification . Mosaicism
Introduction
Central nervous system calcification may be associated with several different underlying etiologies (Batla et al. 2017;
Donzuso et al.2019; Saleem et al.2013; Westenberger et al.
2019). However, the clarification of the underlying diseases is often neglected in the daily clinical practice, even though up to 20% of the head CT scans in the elderly population reveal some degree of brain calcification (Yamada et al. 2013).
There have been several attempts to date to classify the brain calcifications, based on location, etiology, and symptomatol- ogy (Oliveira et al.2013; Savino et al.2016). Manyam (2005) proposed the following classification on anatomical basis: (1) strio-pallido-dentato calcinosis, (2) bilateral strio-pallidal cal- cinosis and (3) bilateral cerebellar calcification. The use of this classification is complicated by the fact that there is no strong correlation between the localization of calcification and the underlying etiology. From this perspective the main groups are the followings: (1) physiologic/age-related, (2) vascular, (3) neoplastic, (4) infectious, (5) metabolic/endocrine, (6) András Salamon and Dénes Zádori contributed equally to this work.
* Tibor Kalmár
kalmar.tibor@med.u-szeged.hu
1 Department of Neurology, Faculty of Medicine, Albert Szent-Györgyi Clinical Center, University of Szeged, Szeged, Hungary
2 Division of Clinical Genetics, Department of Laboratory of Medicine, Faculty of Medicine, University of Debrecen, Debrecen, Hungary
3 Department of Neurology, Békés County, Orosháza, Hungary
4 Department of Pediatrics, Faculty of Medicine, Albert Szent-Györgyi Clinical Center, University of Szeged, Korányi fasor 14-15, Szeged H-6725, Hungary
5 Department of Medical Genetics, Faculty of Medicine, Albert Szent-Györgyi Clinical Center, University of Szeged, Szeged, Hungary
https://doi.org/10.1007/s11011-021-00790-9
/ Published online: 21 July 2021
inflammatory/autoimmune, (7) genetically determined disor- ders and (8) toxic diseases (Kıroğlu et al.2010; Saade et al.
2019; Saleem et al.2013; Taborda et al.2017) (Table1). A serious shortcoming of the above etiological classification is that individual diseases can even be classified into several groups. Depending on the localization and the etiology of brain calcification, a wide range of potential neurological (e.g. movement disorder), psychiatric and musculoskeletal symptoms may appear, however, these symptoms are not re- ally helpful during the differential diagnostic process (Batla et al.2017; Kostićand Petrović2017). From the etiological perspective, the greatest challenge in adult neurological pa- tient care is to differentiate between endocrine/metabolic (po- tentially treatable) and the genetically determined (mostly in- curable) forms of brain calcification. Therefore, diagnostic algorithms have been presented to help in distinguishing (Bonazza et al.2011; Saleem et al.2013).
Extended genetic studies revealed new genes responsible for primary familial brain calcification (previously known as idiopathic basal ganglia calcification /IBGC/ or Fahr’s disease or Fahr’s syndrome) (Table 2) (Batla et al. 2017; Westenberger et al.2019). These are mostly autosomal dom- inantly inherited (e.g.SLC20A2), however, some types show autosomal recessive inheritance (e.g.MYORG).
The aim of this case series is to compare the clinical, lab- oratory, and radiological features of hereditary and non- hereditary etiologies identified in association with extensive cerebral calcification.
Case reports
Case 1–Mos 46,XY,der(11)t(X;11)(p21.1;q14.2)[152]
/46,XY[48]
The 57-year-old male patient was admitted to the outpatient clinic due to severe gait disturbance, swallowing difficulty, and speech disorder. It should be noted from the previous medical history of the patient that he suffered from an ische- mic stroke in the territory of the right middle cerebral artery 2 years prior to admission. The following neurological symp- toms were observed during the detailed examination: upward gaze palsy, abnormal saccadic eye movements, severe dysar- thria and dysphagia, prominent four-limb ataxia, severe trunk ataxia, tremor (resting and postural), urinary incontinence (Supplementary video1). No signs of dysmorphia were ob- served on the patient. The performed detailed laboratory tests demonstrated normal results except for slightly elevated se- rum alkaline phosphatase and urine calcium levels (Table3).
Routine electroencephalography (EEG) found no epileptiform abnormalities, however, focal slowing in the right frontal-, temporal- and left frontal regions was detected as well. The Addenbrooke’s cognitive examination (ACE) detected a mild cognitive decline (76/100), principally the anterograde mem- ory and the attention-related functions were involved.
Extensive intracerebral calcification was revealed by head CT scan (Fig.1–Case 1). It showed primarily the involve- ment of the basal ganglia, cerebellum, deep white matter, and
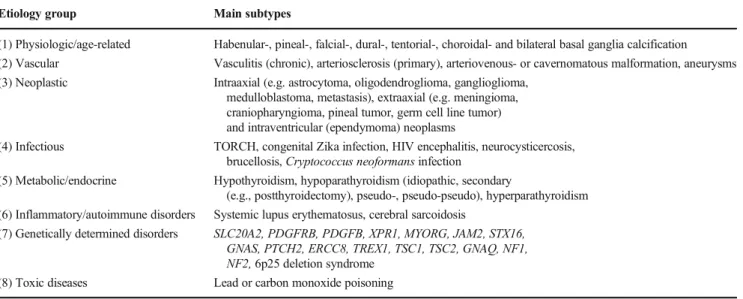
Table 1 Etiologies of brain calcification (Based on the following articles: Kıroğlu et al.2010; Saade et al.2019; Saleem et al.2013, Taborda et al.,2017) (Abbreviations:ERCC8–excision repair cross- complementing, group 8; GNAQ–guanine nucleotide-binding protein, Q polypeptide;GNAS–GNAS complex locus; HIV–human immuno- deficiency virus;JAM2–junctional adhesion molecule 2; MYORG– myogenesis-regulating glycosidase;NF1– neurofibromin 1;NF2–
neurofibromin 2;PDGFB–platelet-derived growth factor, beta polypep- tide;PDGFRB–platelet-derived growth factor receptor, beta;PTCH2– patched 2;SLC20A2–solute carrier family 20 (phosphate transporter), member 2;STX16–syntaxin 16; TORCH–toxoplasmosis, other agents, rubella, cytomegalovirus, herpes simplex;TREX1–3-prime repair exonu- clease 1;TSC1–TSC1 gene;TSC2–TSC2 gene;XPR1–xenotropic and polytropic retrovirus receptor)
Etiology group Main subtypes
(1) Physiologic/age-related Habenular-, pineal-, falcial-, dural-, tentorial-, choroidal- and bilateral basal ganglia calcification (2) Vascular Vasculitis (chronic), arteriosclerosis (primary), arteriovenous- or cavernomatous malformation, aneurysms (3) Neoplastic Intraaxial (e.g. astrocytoma, oligodendroglioma, ganglioglioma,
medulloblastoma, metastasis), extraaxial (e.g. meningioma, craniopharyngioma, pineal tumor, germ cell line tumor) and intraventricular (ependymoma) neoplasms
(4) Infectious TORCH, congenital Zika infection, HIV encephalitis, neurocysticercosis, brucellosis,Cryptococcus neoformansinfection
(5) Metabolic/endocrine Hypothyroidism, hypoparathyroidism (idiopathic, secondary
(e.g., postthyroidectomy), pseudo-, pseudo-pseudo), hyperparathyroidism (6) Inflammatory/autoimmune disorders Systemic lupus erythematosus, cerebral sarcoidosis
(7) Genetically determined disorders SLC20A2, PDGFRB, PDGFB, XPR1, MYORG, JAM2, STX16, GNAS, PTCH2, ERCC8, TREX1, TSC1, TSC2, GNAQ, NF1, NF2,6p25 deletion syndrome
(8) Toxic diseases Lead or carbon monoxide poisoning
brainstem (pons). Abdominal MRI detected cavernomatous hemangiomas, which may partly explain the mild elevation of alkaline phosphatase. Chest X-ray confirmed pneumonia, which was presumably associated with elevated urinary calci- um level. Extensive genetic studies (clinical exome sequenc- ing searching for the known genes behind idiopathic basal ganglia calcification, then G-banded karyotyping, array com- parative genomic hybridization (aCGH), fluorescence in situ hybridization and multiplex ligation-dependent probe ampli- fication) were performed on DNA isolated from blood and oral mucosa cells, respectively. Clinical exome sequencing indicated a deletion in chromosome 11, which prompted aCGH analyses. A mosaic form of presumed unbalanced translocation between chromosome X and 11 was detected.
The karyotype of the patient was the following: mos 46,XY,der(11)t(X;11)(p21.1;q14.2)[152]/46,XY[48] that re- sulted in Xpter→21.1 duplication and 11qter→14.2 deletion.
The gene content of the affected chromosomal regions was determined by UCSC Genome Browser, Ensembl BioMart and OMIM databases and is included in the Supplementary file1. Given the newly identified genetic abnormality, it was
not possible to initiate specific treatment in the absence of scientific data. The patient has 5 asymptomatic children (3 daughters and 2 sons) in whom the chromosomal rearrange- ment was not found (only blood samples were analyzed; no calcification was observed in the performed skull CT scans) (Supplementary file2). The father of the proband died of a lung neoplastic process; however, he did not suffer from a similar disease. Her mother was not facilitated to genetic test- ing due to her old age (82 years).
Case 2–SLC20A2
The 68 years old female patient was admitted to our clinic with prominent articulation difficulty and gait abnormality.
Her symptoms appeared first at the age of 42, and then progressed gradually until she was only able to walk safely with a rollator. The major features demonstrated by neurolog- ical examination of the patient (Supplementary video2) were muscle hypotonia and mainly left-sided bradykinesia.
Choreiform dyskinesis was observed in the right upper limb.
Her gait was unstable lacking the synkinesis of upper limbs Table 2 Genetic subtypes of primary familial brain calcification
(Abbreviations: AD–autosomal dominant; AR–autosomal recessive;
BBB–blood brain barrier;JAM2–junctional adhesion molecule 2;
MYORG–myogenesis-regulating glycosidase; N.D.–no data;PDGFB
–platelet-derived growth factor, beta polypeptide;PDGFRB–platelet- derived growth factor receptor, beta; PFBC–primary familial brain cal- cification;SLC20A2–solute carrier family 20 (phosphate transporter), member 2;XPR1–xenotropic and polytropic retrovirus receptor)
Gene [MIM] Location Inheritance Function Disease name [MIM] Red flag clinical symptom (Batla et al.
2017)
Reference
SLC20A2[158378] 8p11.21 AD Inorganic phosphate transporter type III
PFBC-SLC20A2 (Idiopathic basal ganglia calcification 1) [213600]
Parkinsonism Wang et al.2012
PDGFRB[173410] 5q32 AD BBB integrity PFBC-PDGFRB (Idiopathic basal ganglia calcification 4) [615007]
N.D. Nicolas et al.2013b
PDGFB[190040] 22q13.1 AD Pericyte regulation, BBB integrity
PFBC-PDGFB (Idiopathic basal ganglia calcification 5) [615483]
Headache Keller et al.2013
XPR1[605237] 1q25.3 AD Inorganic phosphate
transporter type III
PFBC-XPR1 (Idiopathic basal ganglia calcification 6) [616413]
N.D. Legati et al.2015
MYORG[618255] 9p13.3 AR Ion homeostasis regulation via the glycosidase domain
PFBC-MYORG (Idiopathic basal ganglia calcification 7) [618317]
Dysarthria Bauer et al.2019
JAM2[606870] 21q21.3 AR Cell-cell adhesion, maintaining the integrity of neurovascular unit
PFBC-JAM2 (Idiopathic basal ganglia calcification 8) [618824]
N.D. Cen et al.2020
Table3Theclinicalandlaboratorycharacteristicsofthenovelgeneticalterationinthecontextofsomeotheretiologies(Abbreviations:24h–asampleofurinecollectedfor24h;ALP–alkaline phosphatase;d.p.–duringpregnancy;PTH–parathyroidhormone;random–randomlytakenurinesample;SLC20A2–solutecarrierfamily20(phosphatetransporter),member2;STX16–syntaxin16; TSH–thyroid-stimulatinghormone;t(X;11)–mos46,XY,der(11)t(X;11)(p21.1;q14.2)[152]/46,XY[48];*–orientationmeasurements;#–thepatientreceivedvitaminDsupplementation) Case1 -t(X;11)Case2 -SLC20A2Case3-SLC20A2Case4 -STX16Case5 -SYPHILISCase6 -THYROIDECTOMY SerumlaboratoryvaluesCalcium(2.20–2.55mmol/l)2.322.312.461.852.341.51 Correctedcalcium(2.20–2.55mmol/l)2.342.312.371.852.401.45 Magnesium(0.70–1.05mmol/l)0.810.870.820.870.700.83 Phosphate(0.87–1.45mmol/l)1.1901.331.0801.2401.0802.180 PTH(1.6–6.9pmol/l)2.704.403.70177.7(d.p.<0.6)1.60<0.6 Osteocalcin(<43–46ng/ml)12.5029.3026.4014.3019.907.80 25-OHD3/D2-vitamin(>75nmol/l)75.4033.5037.80191.0#52.4097.100 TSH(0.27–4.2mIU/l)2.831.1702.5303.861.2102.200 ALP(35–104U/l)1474911965.09184 Calcitonin(0.0–7.5pg/ml)<2.0<2.0<2.0105.0<2.0<2.0 Lactate(<2.40mmol/l)0.640.7601.2301.0500.920.73 Creatinine(53–106umol/l)65657280.08697 Urinelaboratoryvalues*Calcium(1.25–3.75mmol/l)6.79(24h)6.480(random)0.80(random)1.2(24h)0.940(random)0.42(random) Phosphate(mmol/l)45.5032.1005.5019.503.4021.40 ClinicalfeaturesCognitiveimpairmentPresentPresentPresentPresentPresentPresent EyemovementabnormalityPresentAbsentPresentAbsentAbsentAbsent DysarthriaPresentAbsentPresentAbsentPresentAbsent DysphagiaPresentAbsentAbsentAbsentPresentAbsent ParkinsonismAbsentPresentPresentAbsentAbsentAbsent HyperkineticmovementdisorderPresentPresentPresentPresentPresentPresent CerebellarsymptomsPresentAbsentPresentPresentPresentPresent SeizureAbsentAbsentAbsentAbsentAbsentPresent
and turning from 6 steps. No other pathological abnormalities other than low 25-OH D3/D2-vitamin levels were detected in the performed detailed laboratory tests (Table3). The routine EEG showed normal brain activity without the evidence of epileptic discharges. The neuropsychological assessment re- vealed the signs of mild cognitive impairment (ACE: 81/100 points). In the background of this deterioration, working memory and executive dysfunction were identified.
Regarding attention-related tasks, a slowing was detected as well. The performed head CT scan showed an extensive sym- metrical cerebral calcification with the involvement of basal ganglia, cerebellum and deep white matter (Fig.1–Case 2).
The performed NGS-based genetic test (clinical exome se- quencing and deletion/duplication analysis) revealed a variant of unknown significance (class 3; PolyPhen: probably dam- aging; Align-GVGD: C0; SIFT: deleterious; MutationTaster:
disease causing) in heterozygous state in theSLC20A2gene (c.1535G > A; p.Gly512Asp). The detected heterozygous mu- tation affecting a single nucleotide inSLC20A2gene is very rare and was not previously described in 360 Hungarian con- trol samples or in the gnomAD, ClinVar and HGMD data- bases, which supported the clinical diagnosis. In view of the patient’s parkinsonism, we tried rasagiline, levodopa + benserazide and amantadine sulphate treatments, without marked clinical benefit. The proband has only one son (asymptomatic), whose head CT scan did not show
intracranial calcification, although he had reached the predi- lection age (Supplementary file 2). He is not carrier for his mother’sSLC20A2mutation.
Case 3–SLC20A2
A 57-year-old female patient was referred to our clinic with the followings. The patient noticed that her right hand was shaking at rest and she was unable to express herself period- ically for the last two months. The patient was undergoing psychiatric care due to depression. The following abnormali- ties were observed in the neurological status of the patient:
psychomotor slowness, cognitive deficit symptoms, square wave jerks and gaze-evoked nystagmus, dysarthria, cerebellar symptoms (dysmetria on the right hand, dysdiadochokinesis, intentional tremor), postural and resting tremor, bradykinesia predominantly at the right side, antalgic gait, absent synkinesis in the right side, tactile hypesthesia in the right body side (Supplementary video3). The laboratory tests de- lineated no abnormalities except for low vitamin D and slight- ly elevated alkaline phosphatase levels (Table3). The result of routine EEG was normal. The conducted neuropsychological assessment revealed signs of psychomotor slowness and low- er working memory capacity. The previously performed head CT scan confirmed extensive periventricular, basal ganglia, and cerebellar calcification (Fig.1 – Case 3). The genetic Fig. 1 Head CT images (3 sections: centrum semiovale, basal ganglia,
posterior fossa) of the probands (Abbreviation: 1–6–the number of the case;SLC20A2–solute carrier family 20 (phosphate transporter),
m e m b e r 2 ; S T X 1 6 – s y n t a x i n 1 6 ; t ( X ; 1 1 ) – m o s 46,XY,der(11)t(X;11)(p21.1;q14.2)[152]/46,XY[48])
analysis (bidirectional sequencing of the 4 most commonly involved genes (SLC20A2, PDGFB,PDGFRB and XPR1)) detected a known pathogenic mutation in heterozygous state in theSLC20A2gene (c.82G > A, p.Asp28Asn) (Chen et al.
2013). The detected heterozygous mutation affecting a single nucleotide inSLC20A2gene is very rare and was not previ- ously described in 360 Hungarian control samples or in the gnomAD databases. It is classified as“likely pathogenic– idiopathic basal ganglia calcification type 1” according to the ClinVar and HGMD databases. In the treatment of the patient, the primary goal was to manage the psychiatric symp- toms, therefore venlafaxine, clonazepam and quetiapine drugs were applied. During the segregation analysis of the family, we also found the mutation in the so far asymptomatic brother of the proband (receiving anticoagulant therapy for deep vein thrombosis), which correlated with the positive head CT result (Supplementary file2).
Case 4–STX16
The 47-year-old male patient was admitted to our clinic with the history of progressive, pronounced gait disorder for 2 years. He also fell several times in the year before his ad- mission and suffered from a fracture of right wrist in connec- tion with one such fall. Detailed neurological examination detected severe cerebellar symptoms (four-limb ataxia, dysdiadochokinesis, disruption of alternating movements, d y s a r t h r i a ) a s w e l l a s b i l a t e r a l p y r a m i d a l s i g n s (Supplementary video4). There were abnormalities in labora- tory results consistent with secondary hyperparathyroidism (Table3). The routine EEG did not detect epileptiform dys- function; however, it showed a slow wave activity in the right occipital region. The neuropsychological assessment raised the possibility of major neurocognitive disorder (ACE: 55/
100 points). A marked decrease was observed in the tasks examining attention- and memory- and language-related func- tions. The head CT scan showed pronounced bilateral basal ganglia, cerebellar and brainstem calcification (Fig.1–Case 4). The genetic analysis (clinical exome sequencing confirmed by Sanger sequencing) detected a large, previously not pub- lished deletion (NM_001001433.3(STX16):c.393 + 557_792 + 364del) in the STX16gene (chr20:57243567–
57,246,544 in GRCh37 or chr20:58668511–58,671,488 in GRCh38). This alteration was also indicated as pathogenic in ClinVar database (VCV000978043.1) submitted by Undiagnosed Diseases Network, NIH in 2020. The patient received cholecalciferol and calcium supplementation. The proband has an asymptomatic sister whose head CT scan revealed cerebral calcification identical to her brother and the same mutation was also found. (The rest of the family refused to undergo a detailed medical examination.) (Supplementary file 2).
Case 5–Infiltration of the parathyroid gland
The 34-year-old female patient was admitted due to progres- sive speech disorder, gait disturbance, and intermittent blurred vision in the left eye. Based on heteroanamnestic data, it was hard to express herself for half a year and counting problems were reported as well. Concurrently, she presented forgetful- ness, emotional lability, lack of motivation, and clumsiness in her hands as well. Regarding the previous medical history, antecedent previous syphilis infection should be noted (no documentation was available). In an earlier laboratory study of the patient, anti-dsDNA antibody level was significantly elevated (79 U/ml), however, the level of antinuclear antibod- ies was normal. The patient had two miscarriages. The neuro- logical phenotype was dominated by cerebellar symptoms, dysarthria, dysphagia, gait-activated dystonia and emotional instability (Supplementary video5). The endocrine laboratory tests showed alteration compatible with the diagnosis of normocalcemic hypoparathyroidism (Table 3). The result of the routine EEG was normal. The neuropsychological assess- ment revealed significant cognitive involvement (ACE: 61/
100 points) as well as impairment of language functions and abnormalities in retrograde memory. Head CT scan of the patient showed extensive calcification in the cerebellum, pons, basal ganglia, and subcortical regions (Fig.1–Case 5). No abnormality was demonstrated in thyroid ultrasound. A de- tailed dermatological examination revealed no abnormality suggestive of systemic lupus erythematosus. The performed whole exome sequencing did not identify any known or po- tential genetic abnormality underlying the clinical phenotype.
Given that normocalcemic hypoparathyroidism may be due to a decreased reserve capacity of the parathyroid gland (Cusano et al.2013), we considered the infiltration of the parathyroid gland by syphilis as an etiological factor, however, the possi- bility of neurolupus cannot be ruled out with absolute certain- ty (Andres et al.2009). The patient required the initiation of tiapride and pyridostigmine therapies for progressive chorea and dysphagia. Tiapride resulted in a significant improvement of chorea.
Case 6–Secondary hypoparathyroidism
The 72-year-old female patient was admitted to our depart- ment due to a temporary motor aphasia. After admission, the speech disorder was no longer observed, however, four limb hypotonia as well as cerebellar alterations (disturbance of al- ternating movements, intentional tremor, ataxic gait) and bi- lateral upper limb postural tremor were d etected (Supplementary video 6). Regarding her previous medical history, total thyroidectomy due to follicular adenoma (oncocytic type; histological specimens also included the parathyroid glands) should be highlighted. The laboratory test abnormalities were consistent with hypoparathyroidism
(Table3). The routine electroencephalography was negative.
Regarding cognitive alterations, mild impairment of executive functions and verbal fluency was found. The performed head CT scan showed extensive deposition of the calcium bilater- ally in the basal ganglia and cortical regions (Fig.1–Case 6).
Secondary (postthyroidectomy) hypoparathyroidism was con- sidered in the background of symptoms of the patient. The patient was treated with cholecalciferol and calcium supplementation.
Discussion
As the scientific literature expands, identifying an etiology underlying cerebral calcification is a real diagnostic challenge, especially in the adult care. Occasionally, a definitive diagno- sis can be made by analyzing only clinical symptoms or ra- diological abnormalities. However, in most cases, extensive clinical and laboratory studies are needed to find the diagno- sis. Investigations should focus on potentially treatable etiol- ogies, most often due to endocrine causes. Nevertheless, the identification of genetically determined diseases is essential mainly in terms of future clinical trials based on targeting the identified genetic alterations. The main goal of our re- search was to compare the clinical, laboratory and radiological characteristics of six patients identified with extensive cere- bral calcification. Four hereditary and two non-hereditary eti- ologies were found associated with brain calcification.
In case 1 (t(X;11)), we identified a novel, previously not r e p o r t e d u n b a l a n c e d c h r o m o s o m a l t r a n s l o c a t i o n (46,XY,der(11)t(X;11)(p21.1;q14.2)[152]/46,XY[48]) with unknown significance. The clinical symptoms correlated with the head CT pictures, namely, dysphagia could be explained by brainstem involvement, ataxia by cerebellar involvement.
The laboratory examination of endocrine parameters of our patient did not contain any relevant pathological abnormalities similarly to other hereditary etiologies (Bonazza et al.2011).
Despite, the lack of specific complaints (e.g. headache - PDGFB) and signs (e.g. parkinsonism -SLC20A2; seizure - endocrine disorder) (Batla et al.2017; Nicolas et al.2013a,b) we made clinical exome sequencing, which indicated a dele- tion in chromosome 11, which prompted aCGH analyses.
Regarding the pathogenicity of the genetic abnormality found in our patient, it was important to exclude other hereditary causes with high certainty. Therefore, we examined all the genetic alterations associated with cerebral calcification re- ported in the scientific literature (single nucleotide variations, deletions and duplications), but no pathogenic alteration was found (Batla et al.2017; Tadic et al.2015). The performed aCGH test detected a novel unbalanced translocation between chromosomes X and 11 in mosaic form and it was confirmed by FISH and MLPA analyses as well. This difference was not found in the offsprings (non-germline). The mechanism
leading to this genetic alteration is still unknown. The disease may be caused by an increase in the dosage of gene products formed in connection with the partial duplication of the pseudoautosomal region of the short arm of chromosome X or a decrease in the dosage of gene products in the partially deleted region of the long arm of chromosome 11. However, the pathogenicity of the alteration cannot be clearly stated, further studies/case reports are needed to clarify this question.
Genes affected by this translocation have not been clearly associated with cerebral calcification to date. The interpreta- tion of the genetic abnormality observed in the patient is fur- ther complicated by the fact that the genetic abnormality was found only in the blood and not in the sample taken from the oral cavity. We have no knowledge of whether the genetic abnormality is present in other tissues (e.g. in the central ner- vous system). Consequently, it is very difficult to give a genotype-phenotype correlation, as it can be very different depending on the tissue-specific distribution. Cases 2 and 3, although the genetic mutation identified in the background involved the same gene (SLC20A2), the clinical picture was only partially matched. Although parkinsonism was observed in both cases (consistent with the literature data (Batla et al.
2017)), choreiform symptoms predominated in Case 2, where- as cerebellar symptoms dominated in Case 3. A detailed lab- oratory examination did not show significant difference be- tween the cases. Interestingly, the pontine calcification (ob- served in Case 1, 4, 5) was not detected in our twoSLC20A2 cases, in line with the literature (Nicolas et al. 2013a, b);
Chelban et al. (2020) reported that pontin calcification on head CT may refer toMYORGmutation. These two cases point out that involvement of the same gene, partly depending on the different site of mutation, may also have different phenotypes.
The clinical picture of Case 4 was dominated by cerebellar involvement partially consistent with the head CT picture. The laboratory results showed alterations consistent with second- ary hyperparathyroidism. This case is a good presentation of the clinical situation where, although there is an alteration in endocrine laboratory parameters, hereditary etiology still pre- vails (STX16). Although the STX16deletion detected in our case affected exons 5–7, the clinical and laboratory picture (high PTH level, low calcium level, low or normal TSH level, absence of Albright osteodystrophy) was consistent with the findings observed in different mutations described previously (3 kb incl. ex. 4–6; 24.591 kb incl. ex. 2–8 (g.57235162–
57259753del); 4.4 kb incl. ex. 2–4). Interestingly, a case with a more complex phenotype was described in the ClinVar da- tabase, in which the same genetic difference was identified as in our case (NM_001001433.3(STX16):c.393 + 557_792 + 364del - sleep apnea; short nasal bridge; overgrowth; obesity;
exotropia; eczema; Arnold-Chiari malformation; 2–3 toe syn- dactyly) (Bastepe et al. 2003; Elli et al.2014; Laspa et al.
2004; Linglart et al.2005; Mantovani et al.2007). The expla- nation for this could be that there is a second unidentified
pathogenic alteration which could be resposible for these non STX16related phenotypes, while the development of the ce- rebral calcification in later years could not be ruled out.
The fifth case illustrates the difficult clinical situation where, although endocrine abnormalities can be identified, they cannot be clearly associated with any known hereditary or non-hereditary disease (Ramos et al.,2017). The young age of the patient and extensive cerebral calcification would be primarily in favor of hereditary etiology, however, the change in parathyroid hormone levels observed may be related to the pregnancy and the anamnestic syphilis and the elevated anti- dsDNA antibody level raise the possibility of a secondary etiology. Deeper laboratory (e.g. autoimmune, serology) and genetic analysis (whole genome sequencing) could be a for- ward step in resolving the case with higher certainty, but the necessity of the even more detailed laboratory evaluation is questionable and forms a limitation of our study. In contrast, the sixth case presents a very common and easily diagnosable etiology. Unfortunately, in clinical practice, follow-up and adequate treatment (calcium and hydroxy-vitamin D supple- mentation) of patients with permanent hypocalcemia after thy- roidectomy is often missing (Miccoli et al.2012; Petrarca et al.2017).
The aim of the present study was to describe and clinically characterize and compare five different etiologies associated with cerebral calcification. We conclude that accurate clinical, radiological and laboratory analyses lead to diagnosis in most cases. Furthermore, in well-selected cases, aCGH method can provide more complex information about the potentially rele- vant genetic alterations.
Supplementary Information The online version contains supplementary material available athttps://doi.org/10.1007/s11011-021-00790-9.
Authors’contributions AS and DZ examined the patients and wrote the manuscript. VLN and NSZ performed the neuropsychological examina- tion of the patients. Genetic tests and consultations were performed by AU, ZM, EH, IB and TK. PK and CSB had important recommendations to the manuscript. LSZ, ML and BB provided essential assistance in patient involvement and investigation.
Funding GINOP-2.3.2–15-2 grant (TK and ZM) provided by the National Research, Development and Innovation Office (Hungary).
Hungarian Brain Research Program [2017–1.2.1-NKP-2017-00002 NAP VI/4] (PK).
Data availability The datasets used and/or analyzed in the current study are available in this paper.
Declarations
Ethics approval Written informed consent was obtained from all indi- vidual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying infor- mation is included in this article (Regional Human Biomedical Research Ethics Committee of the University of Szeged registration numbers are
150/2014. and 44/2016., respectively). All procedures performed in this study involving human participants were in accordance with the ethical standards of the Regional Human Biomedical Research Ethics Committee of the University of Szeged and with the 1964 Helsinki dec- laration and its later amendments or comparable ethical standards.
Consent to participate Not applicable.
Consent for publication Not applicable.
Conflicts of interest/competing interests The authors declare that they have no competing/conflict of interests.
References
Andres RH, Schroth G, Remonda L (2009) Extensive cerebral calcifica- tion in a patient with systemic lupus erythematosus. BMJ case re- ports 2009:bcr2007125393.https://doi.org/10.1136/bcr.2007.
125393
Bastepe M, Fröhlich LF, Hendy GN, Indridason OS, Josse RG, Koshiyama H, Körkkö J, Nakamoto JM, Rosenbloom AL, Slyper AH, Sugimoto T, Tsatsoulis A, Crawford JD, Jüppner H (2003) Autosomal dominant pseudohypoparathyroidism type Ib is associ- ated with a heterozygous microdeletion that likely disrupts a putative imprinting control element of GNAS. J Clin Invest 112:1255–1263.
https://doi.org/10.1172/JCI19159
Batla A, Tai XY, Schottlaender L, Erro R, Balint B, Bhatia KP (2017) Deconstructing Fahr's disease/syndrome of brain calcification in the era of new genes. Parkinsonism Relat Disord 37:1–10.https://doi.
org/10.1016/j.parkreldis.2016.12.024
Bauer M, Rahat D, Zisman E, Tabach Y, Lossos A, Meiner V, Arkadir D (2019) MYORG mutations: a major cause of recessive primary fa- milial brain calcification. Curr Neurol Neurosci Rep 19:70.https://
doi.org/10.1007/s11910-019-0986-z
Bonazza S, Morgia CL, Martinelli P, Capellari S (2011) Strio-pallido- dentate calcinosis: a diagnostic approach in adult patients. Neurol Sci 32:537–545.https://doi.org/10.1007/s10072-011-0514-7 Cen Z, Chen Y, Chen S, Wang H, Yang D, Zhang H, Wu H, Wang L,
Tang S, Ye J, Shen J, Wang H, Fu F, Chen X, Xie F, Liu P, Xu X, Cao J, Cai P, Pan Q, Li J, Yang W, Shan PF, Li Y, Liu JY, Zhang B, Luo W (2020) Biallelic loss-of-function mutations in JAM2 cause primary familial brain calcification. Brain 143:491–502.https://doi.
org/10.1093/brain/awz392
Chelban V, Carecchio M, Rea G, Bowirrat A, Kirmani S, Magistrelli L, Efthymiou S, Schottlaender L, Vandrovcova J, Salpietro V, Salsano E, Pareyson D, Chiapparini L, Jan F, Ibrahim S, Khan F, Qarnain Z, Groppa S, Bajaj N, Balint B, Bhatia KP, Lees A, Morrison PJ, Wood NW, Garavaglia B, Houlden H (2020) MYORG-related disease is associated with central pontine calcifications and atypical parkin- sonism. Neurol Genet 6:e399. https://doi.org/10.1212/NXG.
0000000000000399
Chen WJ, Yao XP, Zhang QJ, Ni W, He J, Li HF, Liu XY, Zhao GX, Murong SX, Wang N, Wu ZY (2013) Novel SLC20A2 mutations identified in southern Chinese patients with idiopathic basal ganglia calcification. Gene 529:159–162.https://doi.org/10.1016/j.gene.
2013.07.071
Cusano NE, Maalouf NM, Wang PY, Zhang C, Cremers SC, Haney EM, Bauer DC, Orwoll ES, Bilezikian JP (2013) Normocalcemic hyper- parathyroidism and hypoparathyroidism in two community-based nonreferral populations. J Clin Endocrinol Metab 98:2734–2741.
https://doi.org/10.1210/jc.2013-1300
De Oliveira MF, Silva EBE, de Oliveira JRM (2013) Prevalence of brain calcifications in a Brazilian cohort: a retrospective study in radiology
services. Dement Neuropsychol 7:210–215.https://doi.org/10.1590/
S1980-57642013DN70200012
Donzuso G, Mostile G, Nicoletti A, Zappia M (2019) Basal ganglia calcifications (Fahr's syndrome): related conditions and clinical fea- tures. Neurol Sci 40:2251–2263.https://doi.org/10.1007/s10072- 019-03998-x
Elli FM, Sanctis LD, Peverelli E, Bordogna P, Pivetta B, Miolo G, Beck- Peccoz P, Spada A, Mantovani G (2014) Autosomal dominant pseudohypoparathyroidism type Ib: a novel inherited deletion ablat- ing STX16 causes loss of imprinting at the a/B DMR. J Clin Endocrinol Metab 99:E724–E728.https://doi.org/10.1210/jc.2013- 3704
Keller A, Westenberger A, Sobrido MJ, García-Murias M, Domingo A, Sears RL, Lemos RR, Ordoñez-Ugalde A, Nicolas G, da Cunha JEG, Rushing EJ, Hugelshofer M, Wurnig MC, Kaech A, Reimann R, Lohmann K, DobričićV, Carracedo A, PetrovićI, Miyasaki JM, Abakumova I, Mäe MA, Raschperger E, Zatz M, Zschiedrich K, Klepper J, Spiteri E, Prieto JM, Navas I, Preuss M, Dering C, JankovićM, Paucar M, Svenningsson P, Saliminejad K, Khorshid HRK, NovakovićI, Aguzzi A, Boss A, Le Ber I, Defer G, Hannequin D, KostićVS, Campion D, Geschwind DH, Coppola G, Betsholtz C, Klein C, Oliveira JRM (2013) Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice.
Nat Genet 45:1077–1082.https://doi.org/10.1038/ng.2723 Kıroğlu Y, CallıC, Karabulut N, Oncel C (2010) Intracranial calcifica-
tions on CT. Diagn Interv Radiol 16:263–269.https://doi.org/10.
4261/1305-3825.DIR.2626-09.1
KostićVS, PetrovićIN (2017) Brain calcification and movement disor- ders. Curr Neurol Neurosci Rep 17:2.https://doi.org/10.1007/
s11910-017-0710-9
Laspa E, Bastepe M, Jüppner H, Tsatsoulis A (2004) Phenotypic and molecular genetic aspects of pseudohypoparathyroidism type Ib in a Greek kindred: evidence for enhanced uric acid excretion due to parathyroid hormone resistance. J Clin Endocrinol Metab 89:5942– 5947.https://doi.org/10.1210/jc.2004-0249
Legati A, Giovannini D, Nicolas G, López-Sánchez U, Quintáns B, Oliveira JRM, Sears RL, Ramos EM, Spiteri E, Sobrido MJ, Carracedo Á, Castro-Fernández C, Cubizolle S, Fogel BL, Goizet C, Jen JC, Kirdlarp S, Lang AE, Miedzybrodzka Z, Mitarnun W, Paucar M, Paulson H, Pariente J, Richard AC, Salins NS, Simpson SA, Striano P, Svenningsson P, Tison F, Unni VK, Vanakker O, Wessels MW, Wetchaphanphesat S, Yang M, Boller F, Campion D, Hannequin D, Sitbon M, Geschwind DH, Battini JL, Coppola G (2015) Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat Genet 47:579–581.
https://doi.org/10.1038/ng.3289
Linglart A, Gensure RC, Olney RC, Jüppner H, Bastepe M (2005) A n o v e l S T X 1 6 d e l e t i o n i n a u t o s o m a l d o m i n a n t pseudohypoparathyroidism type Ib redefines the boundaries of a cis-acting imprinting control element of GNAS. Am J Hum Genet 76:804–814.https://doi.org/10.1086/429932
Mantovani G, Bondioni S, Linglart A, Maghnie M, Cisternino M, Corbetta S, Lania AG, Beck-Peccoz P, Spada A (2007) Genetic analysis and evaluation of resistance to thyrotropin and growth hormone-releasing hormone in pseudohypoparathyroidism type Ib.
J Clin Endocrinol Metab 92:3738–3742.https://doi.org/10.1210/jc.
2007-0869
Manyam BV (2005) What is and what is not ‘Fahr’s disease’. Parkinsonism Relat Disord 11:73–80.https://doi.org/10.1016/j.
parkreldis.2004.12.001
Miccoli P, Minuto MN, Miccoli M (2012) Incidence of morbidity follow- ing thyroid surgery. Thyroid surgery: preventing and managing complications. John Wiley & Sons, Ltd 2012:6–7
Nicolas G, Pottier C, Charbonnier C, Guyant-Maréchal L, Le Ber I, Pariente J, Labauge P, Ayrignac X, Defebvre L, Maltête D, Martinaud O, Lefaucheur R, Guillin O, Wallon D, Chaumette B, Rondepierre P, Derache N, Fromager G, Schaeffer S, Krystkowiak P, Verny C, Jurici S, Sauvée M, Vérin M, Lebouvier T, Rouaud O, Thauvin-Robinet C, Rousseau S, Rovelet-Lecrux A, Frebourg T, Campion D, Hannequin D, French IBGC Study Group (2013a) Phenotypic spectrum of probable and genetically-confirmed idio- pathic basal ganglia calcification. Brain 136:3395–3407.https://
doi.org/10.1093/brain/awt255
Nicolas G, Pottier C, Maltête D, Coutant S, Rovelet-Lecrux A, Legallic S, Rousseau S, Vaschalde Y, Guyant-Maréchal L, Augustin J, Martinaud O, Defebvre L, Krystkowiak P, Pariente J, Clanet M, Labauge P, Ayrignac X, Lefaucheur R, Le Ber I, Frébourg T, Hannequin D, Campion D (2013b) Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology 80:
181–187.https://doi.org/10.1212/WNL.0b013e31827ccf34 Petrarca M, Scipioni R, Di Giosia P, Giorgini P, Ferri C (2017) A case of
brain calcifications in postsurgical hypoparathyroidism. Intern Emerg Med 12:113–115.https://doi.org/10.1007/s11739-016- 1430-x
Ramos EM, Oliveira J, Sobrido MJ, Coppola G (2017) Primary familial brain calcification. In: Adam MP, Ardinger HH, Pagon RA, et al.
(eds) GeneReviews® [internet]. Seattle (WA): University of Washington, Seattle, 1993–2021. Available from:https://www.
ncbi.nlm.nih.gov/books/NBK1421/
Saade C, Najem E, Asmar K, Salman R, Achkar BE, Naffaa L (2019) Intracranial calcifications on CT: an updated review. J Radiol Case Rep 13:1–18.https://doi.org/10.3941/jrcr.v13i8.3633
Saleem S, Aslam HM, Anwar M, Anwar S, Saleem M, Saleem A, Rehmani MAK (2013) Fahr's syndrome: literature review of current evidence. Orphanet J Rare Dis 8:156.https://doi.org/10.1186/1750- 1172-8-156
Savino E, Soavi C, Capatti E, Borrelli M, Vigna GB, Passaro A, Zuliani G (2016) Bilateral strio-pallido-dentate calcinosis (Fahr's disease): re- port of seven cases and revision of literature. BMC Neurol 16:165.
https://doi.org/10.1186/s12883-016-0693-1
Taborda KNN, Wilches C, Manrique A (2017) A diagnostic algorithm for patients with intracranial calcifications. Rev Colomb Radiol 28:
4732–4739
Tadic V, Westenberger A, Domingo A, Alvarez-Fischer D, Klein C, Kasten M (2015) Primary familial brain calcification with known gene mutations: a systematic review and challenges of phenotypic characterization. JAMA Neurol 72:460–467.https://doi.org/10.
1001/jamaneurol.2014.3889
Wang C, Li Y, Shi L, Ren J, Patti M, Wang T, de Oliveira JRM, Sobrido MJ, Quintáns B, Baquero M, Cui X, Zhang XY, Wang L, Xu H, Wang J, Yao J, Dai X, Liu J, Zhang L, Ma H, Gao Y, Ma X, Feng S, Liu M, Wang QK, Forster IC, Zhang X, Liu JY (2012) Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat Genet 44:254–256.https://doi.org/10.
1038/ng.1077
Westenberger A, Balck A, Klein C (2019) Primary familial brain calcifi- cations: genetic and clinical update. Curr Opin Neurol 32:571–578.
https://doi.org/10.1097/WCO.0000000000000712
Yamada M, Asano T, Okamoto K, Hayashi Y, Kanematsu M, Hoshi H, Akaiwa Y, Shimohata T, Nishizawa M, Inuzuka T, Hozumi I (2013) High frequency of calcification in basal ganglia on brain computed tomography images in Japanese older adults. Geriatr Gerontol Int 13:706–710.https://doi.org/10.1111/ggi.12004
Publisher’s noteSpringer Nature remains neutral with regard to jurisdic- tional claims in published maps and institutional affiliations.