Propionibacterium acnes Induces
Autophagy in Keratinocytes: Involvement of Multiple Mechanisms
Q5 Kla´ra Megyeri1,La´szlo´Orosz1,7,SzilviaBolla2,Lilla Erdei2,ZsoltRa´zga3,Gyo¨rgySepre´nyi4,8, EditUrba´n5,Korne´liaSzabo´6and Lajos Keme´ny2,6
Propionibacterium acnesis a dominant member of the cutaneous microbiota. Herein, we evaluate the effects of differentP. acnesstrains and propionic acid on autophagy in keratinocytes. Our results showed thatP. acnes strain 889 altered the architecture of the mitochondrial network; elevated the levels of light chain 3B-II, Beclin-1, and phospho-50-adenosine-monophosphate-activated protein kinase
a
; stimulated autophagic flux; facilitated intracellular redistribution of light chain 3B; increased average number of autophagosomes per cell; and enhanced development of acidic vesicular organelles in the HPV-KER cell line. Propionic acid increased the level of phospho-50-adenosine-monophosphate-activated protein kinasea
, enhanced lipidation of light chain 3B, stimulated autophagic flux, and facilitated translocation of light chain 3B into autophagosomes in HPV-KER cells. P. acnes strains 889 and 6609 and heat-killed strain 889 also stimulated autophagosome formation in primary keratinocytes to varying degrees. These results indicate that cell wall components and secreted pro- pionic acid metabolite ofP. acnesevoke mitochondrial damage successively, thereby triggering 50-adenosine- monophosphate-activated protein kinase-associated activation of autophagy, which in turn facilitates the removal of dysfunctional mitochondria and promotes survival of keratinocytes. Thus, we suggest that low-level colonization of hair follicles with noninvasive P. acnes strains, by triggering a local increase in autophagic activity, might exert a profound effect on several physiological processes responsible for the maintenance of skin tissue homeostasis.Journal of Investigative Dermatology(2017)-,-e-;doi:10.1016/j.jid.2017.11.018
INTRODUCTION
Propionibacterium acnes is a dominant member of the cutaneous microbiota that is composed of highly variable and topographically diverse microbial communities. The skin microbiota provides colonization resistance, and thereby hampers invasion of virulent microbes. The structural com- ponents, secreted products, and metabolites of normal flora members have the potential to decrease pH; to modulate
inflammation, cell viability, and differentiation; and to manipulate the virulence of pathogenic microbes. In contrast, an altered cutaneous microbiota may contribute to diseases, including acne vulgaris (Belkaid and Hand, 2014;
Bouslimani et al., 2015; Christensen and Bru¨ggemann, 2014; Oh et al., 2014; Schommer and Gallo, 2013; Szabo´
et al., 2017; Weyrich et al., 2015).
The skin commensal P. acnesis a Gram-positive, anaer- obic rod that predominates in the anoxic, lipid-rich envi- ronment of sebaceous glands.P. acnesproduces propionic acid, which protects the skin from virulent microbes.
P. acnes carries several pathogen-associated molecular patterns, which bind to Toll-like receptor 2 (TLR2) and TLR4, leading to the production of cytokines and b-defen- sins (Drott et al., 2010; Kim et al., 2002; Nagy et al., 2005;
Thiboutot et al., 2014). Some invasive strains interact with intracellular pathogen-associated molecular pattern sensors and trigger inflammasome assembly, stimulate a proin- flammatory response, and facilitate the establishment of persistent infection (Qin et al., 2014; Tanabe et al., 2006).
P. acnes expansion in the pilosebaceous unit can trigger tissue damage during the course of acne vulgaris (Weyrich et al., 2015). P. acnes has elaborated a strain-specific variability manifesting in the production of virulence determinants, cellular effects, invasiveness, and pathogenic potential. It is now widely accepted that truly commensal and pathogenic lineages of P. acnes exist, which can be useful members of skin microbiota and causative agents
1Department of Medical Microbiology and Immunobiology, University of Szeged, Szeged, Hungary;2Department of Dermatology and Allergology, University of Szeged, Szeged, Hungary;3Department of Pathology, University of Szeged, Szeged, Hungary;4Department of Medical Biology, University of Szeged, Szeged, Hungary;5Institute of Clinical Microbiology, University of Szeged, Szeged, Hungary; and6MTA-SZTE Dermatological Research Group, Szeged, Hungary
7Current address: Public Health and Food Chain Safety Service of Government Office for Csongra´d County, Laboratory Department, Derkovits fasor 7-11, Szeged, Hungary.
8Current address: Department of Anatomy, University of Szeged, Kossuth Lajos sgt. 40, Szeged, Hungary.
Correspondence: Lajos Keme´ny, Department of Dermatology and
Allergology, University of Szeged, Kora´nyi fasor 6, Szeged, Hungary. E-mail:
kemeny.lajos@med.u-szeged.hu
Abbreviations: AMPK, 50-adenosine-monophosphate-activated protein kinase; BFLA, bafilomycin A1; LC3B, light chain 3B; ROS, reactive oxygen species; TLR, Toll-like receptor
Received 13 August 2017; revised 4 November 2017; accepted 6 November 2017; accepted manuscript published online 27 November 2017; corrected proof published online XXX XXXX
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60
61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120
of acne vulgaris or systemic infections, respectively (Achermann et al., 2014; Beylot et al., 2014; McDowell et al., 2012).
Previous observations demonstrated that bacterial pathogen-associated molecular patterns, exotoxins, and some type 3 or type 4 secretion system effector proteins are powerful activators of autophagy. The autophagic process can function as an early antimicrobial defense pathway by targeting bacteria for autolysosomal destruction, a process known as xenophagy. Several intracellular bacteria have developed strategies with which to evade the degradative power of autophagy. These interesting studies have revealed the importance of autophagy in bacterial infections (Deretic et al., 2013; Deretic and Levine, 2009;
Mathieu, 2015).
A recent study has demonstrated that a cell-invasive P. acnes strain triggers the accumulation of autophago- somes in Raw 264.7 macrophages, mesenchymal cells, and the HeLa cell line (Nakamura et al., 2016). Further ob- servations have indicated that propionic acid is a powerful autophagy inducer in the HCT116 cell line. Autophagy, in response to propionic acid, was shown to develop by a succession of hierarchical steps involving mitochondrial dysfunction, reactive oxygen species (ROS) overproduction, and 50-adenosine-monophosphate-activated protein kinase (AMPK)-mediated inhibition of the mechanistic target of rapamycin. It has also been revealed that propionic-acid- associated autophagy helps to overcome energy crisis, and promotes cell survival by blocking apoptotic demise (Tang et al., 2011). However, investigations of the pro-autophagic effects of extracellular P. acnes strains have not yet been reported in keratinocytes.
In this study, therefore, we investigated the effects of differentP. acnesstrains on autophagy in keratinocytes, and, in parallel, measured the involvement of the AMPK- associated autophagic pathway.
RESULTS
P. acnesinduces autophagy in keratinocytes
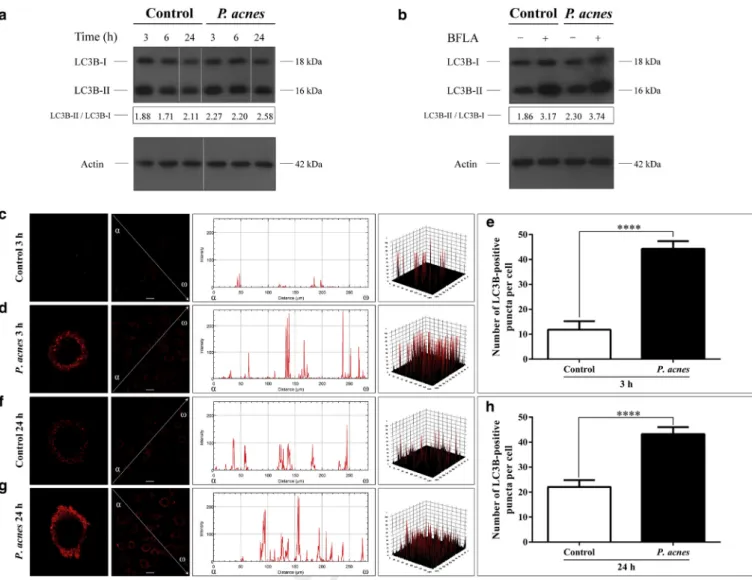
To elucidate how live P. acnes strains 889 and 6609 and heat-killed strain 889 (HK-889) affect the cellular autophagic cascade, we incubated keratinocytes with bacteria in vitro at a multiplicity of infection of 100 and measured (i) the levels of microtubule-associated protein 1 light chain 3B-I (LC3B-I) and LC3B-II, (ii) autophagic flux, (iii) subcellular localization of LC3B and Beclin-1, (iv) the ultrastructural features of autophagic vacuoles, and (v) cytoplasmic acidification.
To study the effects of P. acnes strain 889 on basal autophagy, the levels of LC3B-I and LC3B-II were deter- mined by western blot analysis in the HPV-KER cell line.
The control cells displayed endogenous expression of both the lipidated and the nonlipidated forms of LC3B.P. acnes- treated cells displayed elevated LC3B-II and decreased LC3B-I levels compared with controls at each time point (Figure 1a). Furthermore, P. acnes strains also increased LC3B-II/LC3B-I ratios in normal human keratinocytes, live P. acnes strain 889 being the most powerful trigger (Supplementary Figure 1a online).
To investigate autophagic flux in cells incubated with P. acnesstrains at 6 hpi, LC3B-II levels were measured under
conditions where autophagosome degradation was blocked by bafilomycin A1 (BFLA), a pharmacological inhibitor of autophagosome-lysosome fusion and lysosomal hydrolase activity. The cultures were treated with bacteria first, and incubated with BFLA for a 4-hour period just before the preparation of cell lysates. BFLA elevated the level of LC3B-II as compared with the untreated control cells, indicating that this drug efficiently blocked autophagic flux under the experimental conditions used. In the presence of BFLA, P. acnes triggered a higher increase in the LC3B-II/LC3B-I ratio than that observed in the corresponding drug control (Figure 1b andSupplementary Figure 1b).
Indirect immunofluorescence assay to determine the intracellular localization of LC3B revealed that the control cells displayed a faint cytoplasmic LC3B staining; the fluo- rescence intensity profiles consisted of a few peaks of low height. In contrast,P. acnes-treated cells exhibited very bright LC3B staining; the fluorescence intensity profiles consisted of numerous robust peaks (Figure 1c, d, f, g andSupplementary Figure 2aed online).
To investigate the effects ofP. acnesstrains on autophago- some formation, the abundances of LC3B-positive vesicles were determined. The average numbers of LC3B-positive vesicles per cell in P. acnes-treated cultures were significantly higher than that observed in the control cultures (Figure 1e, h andSupplementary Figure 2e, f). The liveP. acnes strain 889 was again more efficient than strain 6609 and HK-889 in promoting the autophagic process (Supplementary Figure 2e).
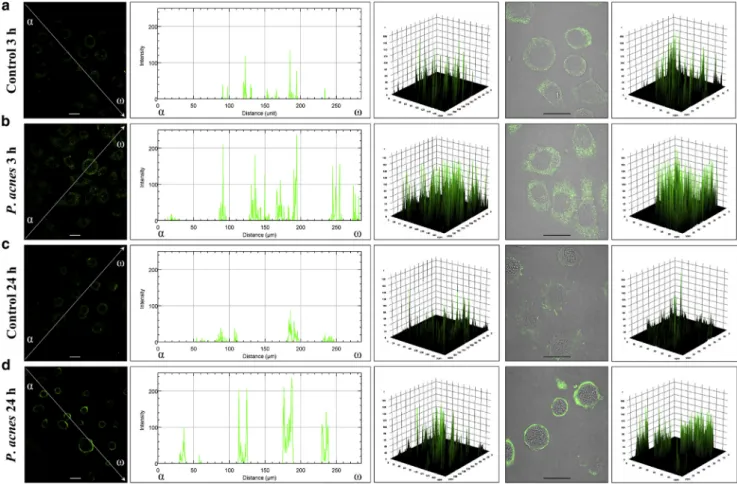
Indirect immunofluorescence assay to determine the intracellular localization of Beclin-1 revealed that the control HPV-KER cells displayed a faint cytoplasmic Beclin-1 stain- ing. In contrast, cells incubated with liveP. acnesstrain 889 exhibited very bright Beclin-1 staining; the fluorescence in- tensity profiles consisted of numerous robust peaks (Figure 2).
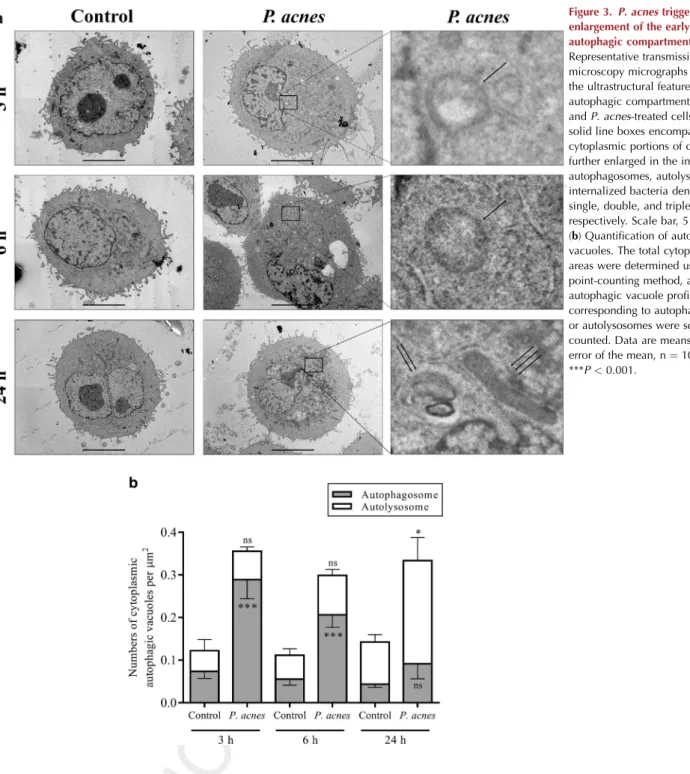
Transmission electron microscopy to investigate the ultra- structural features of autophagic compartments revealed that the control HPV-KER cells displayed a few autophagic vac- uoles. In contrast, cells incubated with live P. acnes strain 889 exhibited a significant rise in the number of autopha- gosomes as early as 3 hpi and an accumulation of autoly- sosome stage vacuoles at 24 hpi (Figure 3). Furthermore, this test also revealed the intracytoplasmic presence ofP. acnes partially surrounded by extensions bulging out of the endo- plasmic reticulum membrane (Figure 3a). However, bacterial invasion of HPV-KER cells seems to be a rare event occurring only in the late phase of incubation.
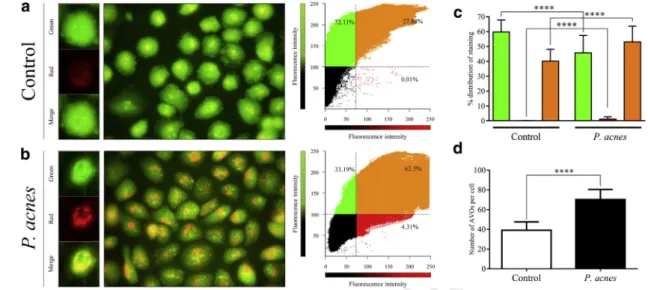
To determine the effects of liveP. acnesstrain 889 on the formation of acidic vesicular organelles, acridine orange staining was used. In the control HPV-KER cultures, the cytoplasm stained green. In P. acnes-treated cells, the cytoplasm exhibited bright-red staining with a marked punctate structure (Figure 4a and b). Analysis of the fluo- rescence intensities in green, red, and overlapping spectral regions revealed an enhancement in red and a reduction of green fluorescence in response to P. acnes treatment (Figure 4c). Moreover, the average numbers of acidic vesicular organelles per cell in P. acnes-treated cultures were significantly higher than that observed in control cultures (Figure 4d).
121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180
181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240
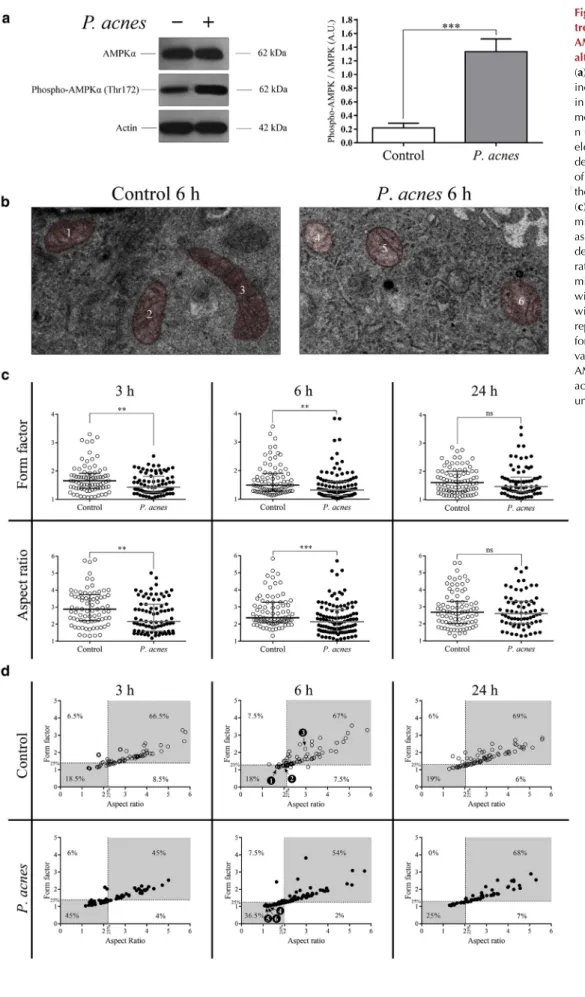
P. acnestriggers abnormal mitochondrial dynamics, entailing AMPK activation and induction of autophagy To gain some insight into the mechanism ofP. acnes-medi- ated induction of autophagy, we incubated HPV-KER cells with the live strain 889 in vitro at a multiplicity of infection of 100, and measured (i) the levels of AMPKa and phospho- AMPKa (Thr172) and (ii) the ultrastructural features of mitochondria.
Western blot analysis revealed that the control cells dis- played endogenous expression of both AMPKaand phospho- AMPKa (Thr172). Phosphorylation of AMPKa at Thr172 is known to be essential for the activation of AMPK (Stein et al., 2000).P. acnestriggered a pronounced increase in the level of phospho-AMPKa; the phospho-AMPKa/AMPKa ratio in P. acnes-treated cultures was considerably higher than that observed in controls (Figure 5a).
P. acnes induced spherical and swollen mitochondria displaying destructive changes of their cristae (Figure 5b). The median aspect ratio and form factor values inP.acnes-treated
cultures were significantly lower than that observed in con- trols at the 3- and 6-hour time points (Figure 5c). To investi- gate further the effect ofP. acneson mitochondria, dot plots of aspect ratios versus form factors were generated, and divided into four quadrants defined by the 25th percentiles of the corresponding controls. The analyses ofP.acnes-treated cells indicated a dramatic increase in the mitochondrial compartment with <25th percentile values for both aspect ratios and form factors at 3 and 6 hpi (Figure 5d). There were no significant differences in the morphological features of mitochondria between the control cells andP. acnes-treated cultures at 24 hpi.
Propionic acid induces autophagy in the HPV-KER cell line To investigate the effect of propionic acid on autophagy, we treated HPV-KER cells with 10 mM propionic acid, and measured (i) the levels of LC3B-I, LC3B-II, AMPKa, and phospho-AMPKa; (ii) autophagic flux; and (iii) subcellular localization of LC3B.
print&web4C=FPO
Figure 1. P. acnestreatment increases the LC3B-II/LC3B-I ratio, stimulates autophagic flux, and triggers autophagosome formation.(a) Western blot analysis showing the kinetics of LC3B-I and LC3B-II expression in control andP. acnes-treated cells. (b) Western blot analysis showing increased autophagic flux inP. acnes-treated cells. (c,d,f,g) Immunofluorescence assays showing the fluorescence intensities of LC3B-positive autophagic vacuoles. The line intensity scan graphs depict the intensity values along the arrows drawn across the images, whereas the 3D surface plots represent the intensity values of the whole image. (e, h) Immunofluorescence assays showing the average numbers of LC3B-positive autophagic vacuoles. Data are meansstandard error of the mean, n¼500. Scale bar, 10mm. ****P<0.0001. BFLA, bafilomycin A1; LC3B, light chain 3B.
241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300
301 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325 326 327 328 329 330 331 332 333 334 335 336 337 338 339 340 341 342 343 344 345 346 347 348 349 350 351 352 353 354 355 356 357 358 359 360
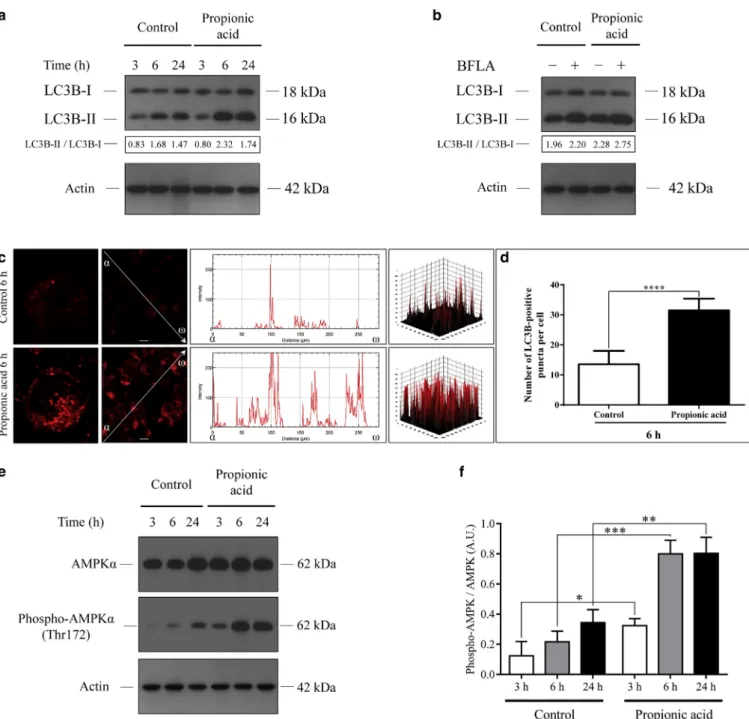
Western blot analysis revealed that propionic acid stimu- lated the lipidation of LC3B at the 6- and 24-hour time points, and increased autophagic flux (Figure 6a and b).
Indirect immunofluorescence assay to determine the intracellular localization of LC3B revealed that the control cells displayed a faint cytoplasmic LC3B staining at the 6-hour time point. In contrast, propionic-acid-treated cells exhibited very bright LC3B staining; the fluorescence in- tensity profile consisted of numerous robust peaks (Figure 6c). The average number of LC3B-positive vesicles per cell in propionic-acid-treated cultures at the 6-hour time point was significantly higher than that observed in the control cultures (Figure 6d).
To examine the involvement of AMPK in the propionic- acid-mediated induction of autophagy, the levels of AMPKa and phospho-AMPKa(Thr172) were determined by western blot analysis. Propionic acid induced moderate increases in AMPKalevels at the 3- and 6-hour time points, and triggered pronounced increases in the level of phospho-AMPKa at each time point. The phospho-AMPKa/AMPKa ratios in propionic-acid-treated cultures were considerably higher than that observed in the control cultures (Figure 6e and f).
DISCUSSION
Compelling evidence indicates that autophagy functions as an important cellular defense mechanism against the
invasion of pathogenic microorganisms (Benjamin et al., 2013). Commensal bacteria were shown to exert complex effects on the autophagic activities of tissues located at the entry sites of pathogens (Benjamin et al., 2013). However, we are just beginning to understand the protective role of the pro-autophagic effect exerted by the skin microbiota. Thus, in this study, we considered the question of whether different P. acnesstrains are able to stimulate the autophagic process in keratinocytes.
Initially, we evaluated five distinct criteria for increased autophagic activity in keratinocytes incubated withP. acnes.
As LC3B is a well-characterized marker of autophagy (Klionsky et al., 2016), we first measured the levels of LC3B-I and LC3B-II.P. acneselevated LC3B-II and decreased LC3B-I levels, indicating that the lipidation of LC3B is stimulated by live P. acnes strains and heat-killed bacteria. We also assessed autophagic flux inP. acnes-treated cultures. In the presence of BFLA, the LC3B-II level of cells incubated with P. acnesstrains was higher than that seen in the drug control, demonstrating that autophagic flux is increased by this bac- terium. Next, we performed confocal imaging to investigate the intracellular distributions of LC3B and Beclin-1. These experiments revealed thatP. acnesraises the intensity levels of LC3B and Beclin-1 staining and stimulates translocation of these proteins into autophagosomes. Interestingly, the live P. acnesstrain 889 was more efficient than strain 6609 and
print&web4C=FPO
Figure 2. P. acnestreatment alters the intracellular distribution of Beclin-1 protein.The samples were stained for the endogenous Beclin-1 protein, and images were obtained by confocal microscopy. The images were subjected to line scan fluorescence intensity analysis and 3D surface plotting using the Image J software (Schneider et al., 2012). The line intensity scan graphs depict the intensity values along the arrows drawn across the images, whereas the 3D surface plots represent the intensity values of the whole image. Scale bar, 10mm.
361 362 363 364 365 366 367 368 369 370 371 372 373 374 375 376 377 378 379 380 381 382 383 384 385 386 387 388 389 390 391 392 393 394 395 396 397 398 399 400 401 402 403 404 405 406 407 408 409 410 411 412 413 414 415 416 417 418 419 420
421 422 423 424 425 426 427 428 429 430 431 432 433 434 435 436 437 438 439 440 441 442 443 444 445 446 447 448 449 450 451 452 453 454 455 456 457 458 459 460 461 462 463 464 465 466 467 468 469 470 471 472 473 474 475 476 477 478 479 480
HK-889 in promoting autophagosome formation. In addition, we determined the effects of P. acnes strain 889 on acidic vesicular organelle formation. The results showed that these bacteria increase cytoplasmic acidification and enhance the development of acidic vesicular organelles. Finally, we studied the ultrastructural features of autophagic compart- ments by transmission electron microscopy. The data demonstrated that, at 3 and 6 hpi,P. acnesstrain 889 triggers the accumulation of autophagosome-stage vacuoles, which subsequently evolve into degradative autolysosomes, sug- gesting that these bacteria trigger a transient increase in autophagic activity at the early phase of incubation, which declines thereafter. It was earlier reported that an invasive P. acnes strain induces autophagy in Raw 264.7
macrophages, mesenchymal cells, and HeLa cell line (Nakamura et al., 2016). Overall, our experiments demon- strate thatP. acnescan stimulate autophagy in keratinocytes when it is present extracellularly, as the level of bacterial invasion was negligible at the low multiplicity of infection used. These results together suggest that P. acnes-induced autophagy might exhibit significant cell-type specificity and bacterial-strain dependency.
The diversity of bacterial structural components, secreted virulence factors, and metabolic products involved suggests a highly intricate mechanism inP. acnes-mediated induction of autophagy. It has already been revealed that TLR4 ligands and complex TLR2 agonists, engaging additional receptors, are strong autophagy inducers, whereas individual TLR2
Figure 3.P. acnestriggers
enlargement of the early and the late autophagic compartments.(a) Representative transmission electron microscopy micrographs depicting the ultrastructural features of autophagic compartments in control andP. acnes-treated cells. The solid line boxes encompassing cytoplasmic portions of cells were further enlarged in the insets to show autophagosomes, autolysosomes, and internalized bacteria denoted by single, double, and triple arrows, respectively. Scale bar, 5mm.
(b) Quantification of autophagic vacuoles. The total cytoplasmic areas were determined using the point-counting method, and the autophagic vacuole profiles corresponding to autophagosomes or autolysosomes were scored and counted. Data are meansstandard error of the mean, n¼10. *P<0.05;
***P<0.001.
481 482 483 484 485 486 487 488 489 490 491 492 493 494 495 496 497 498 499 500 501 502 503 504 505 506 507 508 509 510 511 512 513 514 515 516 517 518 519 520 521 522 523 524 525 526 527 528 529 530 531 532 533 534 535 536 537 538 539 540
541 542 543 544 545 546 547 548 549 550 551 552 553 554 555 556 557 558 559 560 561 562 563 564 565 566 567 568 569 570 571 572 573 574 575 576 577 578 579 580 581 582 583 584 585 586 587 588 589 590 591 592 593 594 595 596 597 598 599 600
ligands are unable to provoke autophagy (Delgado et al., 2008). Another interesting observation clearly demonstrated that the scavenger receptor CD36 is also implicated in autophagy induction (Sanjurjo et al., 2015). CD36 functions as a TLR coreceptor and participates in the formation of the CD36-CD14-TLR2/4-TLR6 signaling module, which is capable of evoking diverse biological responses, including the increased production of ROS (Di Gioia and Zanoni, 2015). CD36 stimulates ROS generation via the nicotin- amide adenine dinucleotide phosphate oxidase, whereas TLR2/4 trigger the TRAF6-ECSIT-NLRX1-dependent formation of mitochondrial ROS (Park et al., 2009; West et al., 2011).
We and others have previously shown thatP. acnesactivates the TLR2/4 signal transduction mechanisms in keratinocytes (Drott et al., 2010; Kim et al., 2002; Nagy et al., 2005).
Moreover, the cell wall lipoproteins of this bacterium were shown to trigger the production of ROS through the CD36 pathway (Grange et al., 2009). Excessive ROS levels might lead to mitochondrial dysfunction, which in turn evokes ATP depletion, activation of AMPK, and induction of the auto- phagic cascade (Inoki et al., 2003; Wang and Klionsky, 2011;
Wu et al., 2014; Zhang et al., 2016; Zhao and Klionsky, 2011). It is widely accepted that AMPK-dependent auto- phagy functions as an important adaptive mechanism during oxidative stress by facilitating the removal of damaged mitochondria (He and Klionsky, 2009; Kroemer et al., 2010).
In light of these interesting observations, we investigated how P. acnesaffects the levels of phospho-AMPKa (Thr172) and the architecture of the mitochondrial network. Our studies have shown that the level of phospho-AMPKa(Thr172) was strongly increased in HPV-KER cells incubated with live P. acnesstrain 889, indicating that extracellular bacteria are powerful activators of AMPK. The ultrastructural features of mitochondria were significantly altered at the early phase of P. acnes treatment, whereas at the late stage, the mitochon- drial configurations and shape heterogeneities were largely
restored. The time course of the increased autophagy level correlated well with the changes in mitochondrial morphology inP. acnes-treated cells. These results indicate thatP. acnestriggers mitochondrial dysfunction and, in par- allel, activates AMPK-dependent autophagy that can function as an antioxidant defense mechanism promoting the removal of damaged mitochondria.
SCFAs produced by bacterial fermentation act as signalQ2 molecules between microbiota and host cells and regulate several specialized functions of various tissues (Ganapathy et al., 2013). The importance of commensal-derived metab- olites in the regulation of autophagy is highlighted by the greatly increased autophagic activity in SCFA-treated cells (Adom and Nie, 2013; Jan et al., 2002; Tang et al., 2011).
Although the level of propionic acid in the skin has not yet been determined, the propionic acid quantity in the large intestine varies between 1.5 and 26.7 mmol/kg contents (Cummings et al., 1987). Propionic acid levels can reach high concentrations at sites of bacterial colonization and infec- tion, the subgingival concentration of this SCFA was 9.5 mM in patients with periodontal disease (Al-Lahham et al., 2010).
P. acneshas been reported to produce 13.85 mM propionic acid during in vitro culture (Douglas and Gunter, 1946).
Thus, we additionally considered the question of whether propionic acid at 10 mM concentration affects autophagy in HPV-KER cells. We found that propionic acid activated AMPK via phosphorylating AMPKaat Thr172, and stimulated the lipidation of LC3B, increased autophagic flux, as well as enhanced translocation of LC3B into autophagosomes. These data demonstrate that propionic acid enhances the auto- phagic activity of keratinocytes via AMPK activation. Strik- ingly, the time course of autophagic response was different in propionic-acid- andP. acnes-treated keratinocytes. Thus, we suggest that this SCFA metabolite might also be implicated in the autophagic response of keratinocytes, but only after a short delay followingP. acnesencounter.
print&web4C=FPO
Figure 4. Propionibacterium acnesstimulates AVO formation.(a,b) Representative fluorescence micrographs and correlation plots showing the fluorescence intensity and intracellular localization of AVOs. Fluorescence intensities were determined and analyzed using an “apoptosis correlator”
plugin (Mironova et al., 2007) operated in Image J. Thresholds indicated by dashed lines were chosen empirically so as to separate visible fluorescence from the dark pixels. (c) Distribution of fluorescence measured in green, red, and overlapping spectral regions. Fluorescence intensities were quantified, and the average distribution of fluorescence within the green, red, and overlapping regions was calculated. Data are meansstandard error of the mean, n¼50. (d) The average numbers of AVOs. Data are meansstandard error of the mean, n¼500. ****P<0.0001. AVO, acidic vesicular organelle.
601 602 603 604 605 606 607 608 609 610 611 612 613 614 615 616 617 618 619 620 621 622 623 624 625 626 627 628 629 630 631 632 633 634 635 636 637 638 639 640 641 642 643 644 645 646 647 648 649 650 651 652 653 654 655 656 657 658 659 660
661 662 663 664 665 666 667 668 669 670 671 672 673 674 675 676 677 678 679 680 681 682 683 684 685 686 687 688 689 690 691 692 693 694 695 696 697 698 699 700 701 702 703 704 705 706 707 708 709 710 711 712 713 714 715 716 717 718 719 720
Figure 5.Propionibacterium acnes treatment increases the level of AMPKaphosphorylated at Thr172and alters mitochondrial morphology.
(a) Western blot analysis showing increased levels of phospho-AMPKa inP. acnes-treated cells. Data are meansstandard error of the mean, n¼3. (b) Representative transmission electron microscopy micrographs depicting the ultrastructural features of mitochondria corresponding to the numbered data points in (d).
(c) Graphical representation of mitochondrial form factor and aspect ratio values. The dot plots depict the form factor and the aspect ratio values of each individual mitochondrion. The median values with interquartile ranges are shown within the graphs. (d) Graphical representation of mitochondrial form factor versus aspect ratio values. **P<0.01, ***P<0.001.
AMPK, 50-adenosine-monophosphate- activated protein kinase; AU, arbitrary unit.
print&web4C=FPO
721 722 723 724 725 726 727 728 729 730 731 732 733 734 735 736 737 738 739 740 741 742 743 744 745 746 747 748 749 750 751 752 753 754 755 756 757 758 759 760 761 762 763 764 765 766 767 768 769 770 771 772 773 774 775 776 777 778 779 780
781 782 783 784 785 786 787 788 789 790 791 792 793 794 795 796 797 798 799 800 801 802 803 804 805 806 807 808 809 810 811 812 813 814 815 816 817 818 819 820 821 822 823 824 825 826 827 828 829 830 831 832 833 834 835 836 837 838 839 840
On the basis of the present results, we propose that P. acnesinduces autophagy via its complex interactions with keratinocytes. We hypothesize that P. acnes stimulates the CD36-CD14-TLR2/4-TLR6 signaling module, triggers ROS generation through nicotinamide adenine dinucleotide phosphate oxidase and TRAF6-ECSIT-NLRX1 pathway, and evokes mitochondrial dysfunction. The P. acnes-derived propionic acid causes mitochondrial damage and aggravates oxidative stress. ROS, generated via multiple mechanisms, trigger AMPK-dependent activation of autophagy, which in
turn facilitates the removal of damaged mitochondria and promotes the survival of keratinocytes (see Supplementary Figure S3 online). Thus, P. acnes-induced autophagy may increase the adaptive potential of keratinocytes to cope with oxidative damage.
The human skin provides an extremely potent barrier against microbial invasion because its outermost layer is composed of dead cells formed as a result of the epidermal cornification process, whereas the hair follicles can function as convenient entry sites for pathogenic bacteria (Galluzzi
print&web4C=FPO
Figure 6. Propionic acid increases the LC3B-II/LC3B-I ratio, stimulates autophagic flux, and induces autophagosome formation.(a) Western blot analysis showing the kinetics of endogenous LC3B-I and LC3B-II expression in control and propionic-acid-treated cells. (b) Western blot analysis showing increased autophagic flux in propionic-acid-treated cells. (c) Immunofluorescence assays showing the fluorescence intensities of LC3B-positive autophagic vacuoles. (d) Immunofluorescence assays showing the average numbers of LC3B-positive autophagic vacuoles. Data are meansstandard error of the mean, n¼500. (e,f) Western blot analysis showing increased levels of phospho-AMPKain propionic-acid-treated cells. Data are meansstandard error of the mean, n¼3. Scale bar, 10mm. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. AMPK, 50-adenosine-monophosphate-activated protein kinase;
BFLA, bafilomycin A1; LC3B, light chain 3B.
841 842 843 844 845 846 847 848 849 850 851 852 853 854 855 856 857 858 859 860 861 862 863 864 865 866 867 868 869 870 871 872 873 874 875 876 877 878 879 880 881 882 883 884 885 886 887 888 889 890 891 892 893 894 895 896 897 898 899 900
901 902 903 904 905 906 907 908 909 910 911 912 913 914 915 916 917 918 919 920 921 922 923 924 925 926 927 928 929 930 931 932 933 934 935 936 937 938 939 940 941 942 943 944 945 946 947 948 949 950 951 952 953 954 955 956 957 958 959 960
et al., 2015; Schommer and Gallo, 2013; Szabo´ et al., 2017).
Thus, low-level colonization of hair follicles with noninvasive P. acnes strains might confer remarkable antimicrobial pro- tection by triggering a local increase in autophagic activity of keratinocytes. In addition to infectious agents, keratinocytes are also exposed to other harmful environmental stimuli, such as the UVR, chemicals, and temperature variations that lead to various pathological conditions via triggering exten- sive oxidative damage. Interestingly, P. acnes is endowed with the ability to decrease oxidative damage of bacteria and keratinocytes via the secretion of the RoxP (radical oxygenase of P. acnes) antioxidant enzyme (Allhorn et al., 2016). In view of the importance of autophagy in keratinocyte physi- ology (Li et al., 2016), the pro-autophagic effect of P. acnes might represent another indirect mechanism for how this commensal bacterium exerts a beneficial role in cutaneous homeostasis.
MATERIALS AND METHODS
An extended description of materials and methods is given in Supplemental Materials and Methodsonline.
Cell culture
The HPV-KER cell line was established and grown as previously described (Tax et al., 2016). Primary keratinocytes were obtained from healthy individuals who underwent plastic surgery after written informed consent according to the institutional review board pro- tocol. The Medical Research Council Ethics Committee of Hungary approved the use of skin samples (ETT-TUKEB 39361). Human epidermal keratinocytes were isolated and cultured as described previously (Nagy et al., 2005). For experimental purposes, kerati- nocytes were cultured in antibiotic-free medium for a 24-hour period beforeP. acnestreatment.
P. acnesstrain and growth conditions
P. acnes strain 889 was isolated and cultured as previously described, whereas the strain 6609 was obtained from ATCC (Tax et al., 2016). For experiments, keratinocytes were incubated with P. acnes strains at a multiplicity of infection of 100 CFU/cell. To prepare heat-killed suspensions of bacteria,P. acnesstrain 889 was killed by incubation at 60C for 30 minutes.
Indirect immunofluorescence assay
Cytospin cell preparations were fixed in methanol-acetone (1:1). The slides were incubated with rabbit polyclonal antibodies to LC3B or Beclin-1 (Sigma-Aldrich, St. Louis, MO). After washing, the samples were reacted with CF640R- or CF488A-conjugated anti-rabbit antibodies (Sigma-Aldrich). The cells were visualized by confocal microscopy using an Olympus FV1000 confocal laser scanning microscope. LC3B-positive vacuoles were quantified as previously described (Orosz et al., 2016; Pa´sztor et al., 2014). The fluorescence intensities were determined using the line scan analysis and surface plot functions of Image J
Q3 (Schneider et al., 2012).
Transmission electron microscopy
The samples were fixed, dehydrated, and embedded in Embed 812 (Electron Microscopy Sciences, Hatfield, PA). Ultrathin sections were stained with uranyl acetate and lead citrate, and examined in a JEOL JEM-1400Plus transmission electron microscope (JEOL USA, Peabody, MA). Autophagosomes and autolysosomes were scored according to their morphology. The results are presented as number
of organelles/cytoplasmic areastandard error of the mean. Mito- chondrial shape descriptors were determined using Image J.
Western blot assays
Protein samples were prepared for SDS-PAGE and western blot assay as previously described (Orosz et al., 2016; Pa´sztor et al., 2014). The membranes were developed using a chemiluminescence detection system, the autoradiographs were scanned with a GS-800 densi- tometer (Bio-Rad), and band intensities were quantified using the ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Acridine orange staining
Cytoplasmic acidification was assessed by the acridine orange staining procedure as previously described (Pa´sztor et al., 2014). The fluorescence intensities were analyzed by using an “apoptosis correlator” plugin operated in the Image J software.
Statistical analysis
Differences in autophagic vacuole numbers and protein levels between control and P. acnes-treated cells were evaluated with Student’s unpaired t-test, and the values are expressed as means standard error of the mean. Mitochondrial shape de- scriptors followed nonnormal distributions as determined by the Shapiro-Wilk normality test. Differences in aspect ratios and form factors therefore were evaluated by the Kolmogorov-Smirnov test, and the values are expressed as medians with interquartile ranges.
P-values of less than 0.05 were considered statistically significant.
CONFLICT OF INTEREST
The authors state no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by research grants OTKA 105369, GINOP-2.3.2-15- 2016-00015, GINOP-2.2.1-15-2016-00007, GINOP-2.3.3-15-2016-00007, GINOP-2.3.3-15-2016-00012, and EFOP-3.6.1-16-2016-00008.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper atwww.
jidonline.org, and athttps://doi.org/10.1016/j.jid.2017.11.018.
REFERENCES
Achermann Y, Goldstein EJC, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant path- ogen. Clin Microbiol Rev 2014;27:419e40.
Adom D, Nie D. Regulation of autophagy by short chain fatty acids in colon cancer cells. In: Bailly Y, editor. Autophagy—double-edged sword—cell
survival or death? InTech; 2013. Q4
Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Bio- logical effects of propionic acid in humans; metabolism, potential appli- cations and underlying mechanisms. Biochim Biophys Acta 2010;1801:
1175e83.
Allhorn M, Arve S, Bru¨ggemann H, Lood R. A novel enzyme with antioxidant capacity produced by the ubiquitous skin colonizer Propionibacterium acnes. Sci Rep 2016;6:36412.
Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation.
Cell 2014;157:121e41.
Benjamin JL, Sumpter R, Levine B, Hooper LV. Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe 2013;13:723e34.
Beylot C, Auffret N, Poli F, Claudel J-P, Leccia M-T, Del Giudice P, et al.
Propionibacterium acnes: an update on its role in the pathogenesis of acne.
J Eur Acad Dermatol Venereol 2014;28:271e8.
Bouslimani A, Porto C, Rath CM, Wang M, Guo Y, Gonzalez A, et al. Mo- lecular cartography of the human skin surface in 3D. Proc Natl Acad Sci USA 2015;112:E2120e9.
Christensen GJM, Bru¨ggemann H. Bacterial skin commensals and their role as host guardians. Benef Microbes 2014;5:201e15.
961 962 963 964 965 966 967 968 969 970 971 972 973 974 975 976 977 978 979 980 981 982 983 984 985 986 987 988 989 990 991 992 993 994 995 996 997 998 999 1000 1001 1002 1003 1004 1005 1006 1007 1008 1009 1010 1011 1012 1013 1014 1015 1016 1017 1018 1019 1020
1021 1022 1023 1024 1025 1026 1027 1028 1029 1030 1031 1032 1033 1034 1035 1036 1037 1038 1039 1040 1041 1042 1043 1044 1045 1046 1047 1048 1049 1050 1051 1052 1053 1054 1055 1056 1057 1058 1059 1060 1061 1062 1063 1064 1065 1066 1067 1068 1069 1070 1071 1072 1073 1074 1075 1076 1077 1078 1079 1080
Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987;28:1221e7.
Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J 2008;27:1110e21.
Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 2009;5:527e49.
Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol 2013;13:722e37.
Di Gioia M, Zanoni I. Toll-like receptor co-receptors as master regulators of the immune response. Mol Immunol 2015;63:143e52.
Douglas HC, Gunter SE. The taxonomic position ofCorynebacterium acnes.
J Bacteriol 1946;52:15e23.
Drott JB, Alexeyev O, Bergstro¨m P, Elgh F, Olsson J.Propionibacterium acnes infection induces upregulation of inflammatory genes and cytokine secretion in prostate epithelial cells. BMC Microbiol 2010;10:126.
Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ 2015;22:58e73.
Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Trans- porters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol 2013;13:
869e74.
Grange PA, Che´reau C, Raingeaud J, Nicco C, Weill B, Dupin N, et al. Pro- duction of superoxide anions by keratinocytes initiatesP. acnes-induced inflammation of the skin. PLoS Pathog 2009;5:e1000527.
He C, Klionsky DJ. Regulation mechanisms and signaling pathways of auto- phagy. Annu Rev Genet 2009;43:67e93.
Inoki K, Zhu T, Guan K-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003;115:577e90.
Jan G, Belzacq AS, Haouzi D, Rouault A, Metivier D, Kroemer G, et al.
Propionibacteria induce apoptosis of colorectal carcinoma cells via short- chain fatty acids acting on mitochondria. Cell Death Differ 2002;9:179.
Kim J, Ochoa M-T, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al.
Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol 2002;169:1535e41.
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016;12:1e222.
Kroemer G, Marin˜o G, Levine B. Autophagy and the integrated stress response. Mol Cell 2010;40:280e93.
Li L, Chen X, Gu H. The signaling involved in autophagy machinery in ker- atinocytes and therapeutic approaches for skin diseases. Oncotarget 2016;7:50682e97.
Mathieu J. Interactions between autophagy and bacterial toxins: targets for therapy? Toxins 2015;7:2918e58.
McDowell A, Barnard E, Nagy I, Gao A, Tomida S, Li H, et al. An expanded multilocus sequence typing scheme forPropionibacterium acnes: investi- gation of “pathogenic”, “commensal” and antibiotic resistant strains. PLoS One 2012;7, e41480.
Mironova EV, Evstratova AA, Antonov SM. A fluorescence vital assay for the recognition and quantification of excitotoxic cell death by necrosis and apoptosis using confocal microscopy on neurons in culture. J Neurosci Methods 2007;163:1e8.
Nagy I, Pivarcsi A, Koreck A, Sze´ll M, Urba´n E, Keme´ny L. Distinct strains of Propionibacterium acnes induce selective human b-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors.
J Invest Dermatol 2005;124:931e8.
Nakamura T, Furukawa A, Uchida K, Ogawa T, Tamura T, Sakonishi D, et al.
Autophagy induced by intracellular infection ofPropionibacterium acnes.
PLoS One 2016;11:e0156298.
Oh J, Byrd AL, Deming C, Conlan S, Barnabas B, Blakesley R, et al. Bioge- ography and individuality shape function in the human skin metagenome.
Nature 2014;514:59e64.
Orosz L, Papanicolaou EG, Sepre´nyi G, Megyeri K. IL-17A and IL-17F induce autophagy in RAW 264.7 macrophages. Biomed Pharmacother 2016;77:
129e34.
Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest 2009;119:
136e45.
Pa´sztor K, Orosz L, Sepre´nyi G, Megyeri K. Rubella virus perturbs autophagy.
Med Microbiol Immunol 2014;203:323e31.
Qin M, Pirouz A, Kim M-H, Krutzik SR, Garba´n HJ, Kim J.Propionibacterium acnes induces IL-1b secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol 2014;134:381e8.
Sanjurjo L, Ame´zaga N, Aran G, Naranjo-Go´mez M, Arias L, Armengol C, et al. The human CD5L/AIM-CD36 axis: a novel autophagy inducer in macrophages that modulates inflammatory responses. Autophagy 2015;11:
487e502.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671e5.
Schommer NN, Gallo RL. Structure and function of the human skin micro- biome. Trends Microbiol 2013;21:660e8.
Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 2000;345:
437e43.
Szabo´ K, Erdei L, Bolla BS, Tax G, Bı´ro´ T, Keme´ny L. Factors shaping the composition of the cutaneous microbiota. Br J Dermatol 2017;176:344e51.
Tanabe T, Ishige I, Suzuki Y, Aita Y, Furukawa A, Ishige Y, et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellularPropioni- bacterium acnes. Biochim Biophys Acta 2006;1762:794e801.
Tang Y, Chen Y, Jiang H, Nie D. Short-chain fatty acids induced autophagy serves as an adaptive strategy for retarding mitochondria-mediated apoptotic cell death. Cell Death Differ 2011;18:602e18.
Tax G, Urba´n E, Palota´s Z, Puska´s R, Ko´nya Z, Bı´ro´ T, et al. Propionic acid produced byPropionibacterium acnesstrains contributes to their patho- genicity. Acta Derm Venereol 2016;96:43es9.
Thiboutot DM, Layton AM, Anne Eady E. IL-17: A key player in theP. acnes inflammatory cascade? J Invest Dermatol 2014;134:307e10.
Wang K, Klionsky DJ. Mitochondria removal by autophagy. Autophagy 2011;7:297e300.
West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011;472:476e80.
Weyrich LS, Dixit S, Farrer AG, Cooper AJ, Cooper AJ. The skin microbiome:
associations between altered microbial communities and disease: the skin microbiome. Australas J Dermatol 2015;56:268e74.
Wu S-B, Wu Y-T, Wu T-P, Wei Y-H. Role of AMPK-mediated adaptive re- sponses in human cells with mitochondrial dysfunction to oxidative stress.
Biochim Biophys Acta 2014;1840:1331e44.
Zhang D, Wang W, Sun X, Xu D, Wang C, Zhang Q, et al. AMPK regulates autophagy by phosphorylating BECN1 at threonine 388. Autophagy 2016;12:1447e59.
Zhao M, Klionsky DJ. AMPK-dependent phosphorylation of ULK1 induces autophagy. Cell Metab 2011;13:119e20.
1081 1082 1083 1084 1085 1086 1087 1088 1089 1090 1091 1092 1093 1094 1095 1096 1097 1098 1099 1100 1101 1102 1103 1104 1105 1106 1107 1108 1109 1110 1111 1112 1113 1114 1115 1116 1117 1118 1119 1120 1121 1122 1123 1124 1125 1126 1127 1128 1129 1130 1131 1132 1133 1134 1135 1136 1137 1138 1139 1140
1141 1142 1143 1144 1145 1146 1147 1148 1149 1150 1151 1152 1153 1154 1155 1156 1157 1158 1159 1160 1161 1162 1163 1164 1165 1166 1167 1168 1169 1170 1171 1172 1173 1174 1175 1176 1177 1178 1179 1180 1181 1182 1183 1184 1185 1186 1187 1188 1189 1190 1191 1192 1193 1194 1195 1196 1197 1198 1199 1200