60
NEUTRAL PROTEASES BY
3H-LABELED CASEIN
D. O. Adams
PRINCIPLE
Macrophages secrete a variety of proteases, which include enzymes such as collagenase that react only with specific sub- strates. It may on occasion, however, be of use to the inves- tigator to quantify either multiple proteases in one assay or proteases that have a wider range of reactivity. These goals can be achieved by quantifying lysis of casein, which is a broadly reactive substrate hydrolysed by a wide variety of proteases (1). Supernatants of macrophages have been shown to split azo dyes from casein (2). The development of a highly sensitive assay employing
3H-labeled casein as a substrate has made studies of caseinases quite easy (3). By use of the
3H- labeled casein substrate, lysates and supernatants of murine peritoneal macrophages can be shown to contain serine, métallo, and thiol proteases (4). Supernatants of macrophages contain at least five peaks of caseinolytic activity of greater than approximately 10,000 daltons when the supernatants are parti-
METHODS FOR STUDYING Copyright © 1981 by Academic Press, Inc.
MONONUCLEAR PHAGOCYTES 5 9 3 All rights of reproduction in any form reserved.
ISBN 0-12-044220-5
tioned by molecular gel filtration (5). These observations suggest the assay for caseinolytic activity, when applied to macrophages, determines the action of multiple proteases.
The assay quantifies the release of TCA-soluble, 3H-label- ed peptides cleaved from ^H-labeled casein by macrophage-de- rived proteases.
The secreted and intracellular proteases of macrophages are often labile, adherent to glass, and present in relatively low amounts (4,6). These factors dictate the strategy for preparation of supernatants. Medium is conditioned by several hours to several days of culture over purified populations of mononuclear phagocytes. Since serum contains inhibitors of hydrolases (particularly of neutral proteases), cultures are generally conducted in the absence of serum. The cultures, which are washed free of serum before the conditioning, are maintained in medium treated with acid to remove 0t_2-macroglo- bulin, in medium containing no serum and supplemented with lactalbumin hydrolysate, or in a designed serumless medium such as that of Neumann and Tytell (Grand Island Biological Co., Grand Island, New York.) (4,6). Siliconized glassware or plasticware and pipettes may be useful in harvesting and col- lecting supernatants, because many of the secreted proteases of macrophages adhere to glass (4). The conditioned medium is rapidly collected with Siliclad pipettes into previously chill- ed (4°C) Siliclad containers and freed of cells by centrifuga- tion (10,000 g for 10 min at 4°C). The conditioned medium is assayed immediately or stored at -20°C. If the desired product is present in low concentration, the conditioned medium can be concentrated by lyophilization or dialysis against an adsorbent
(i.e., Aquacide II, Calbiochem, San Diego, California). We have found concentration at 4°C in a stirred cell against an appropriately sized UM membrane (Amicon, Inc., Lexington, Massachusetts.) to be the most satisfactory method (4).
II. REAGENTS
(1). Labeled substrate. Tritiated casein is prepared by acetylation of casein with ^H by the method of Levine et al.
(3). Briefly, casein nach Hammersten (EM Chemical, Elmsford, New York) is dissolved in distilled water at a concentration of 50 mg/ml. To aid in dissolution, the pH is adjusted to 7.4 with 0.1 M NaOH. [3H]acetic anhydride (New England Nuclear, Boston, Massachusetts) is added with vigorous stirring and the solution is incubated at 4°C for 1 hr. After acetylation, the solution is exhaustively dialyzed against buffer (0.1 M sodium phosphate, pH 7.5, containing 0.14 M NaCl, 0.02 M KC1, and 0.05 M sodium acetate) until the activity in the dialysate for
VIII. SECRETION BY MONONUCLEAR PHAGOCYTES 595 24 hr is less than 10b cpm/ml of dialysate. Background re- lease of the dialyzed substrate should be less than 100 cpm in the final assay. The specific activity (i.e., radioacti- vity per microgram casein) of the labeled casein is deter- mined. Each newly prepared batch of substrate is checked at multiple times (5 min to 2 hr) toward multiple concentrations of trypsin to ensure linearity of release. Dilute the sub- strate in distilled water such that a 20 yl aliquot containing 5 yg of casein yields / 50,000 cpm (10,000 cpm/yg casein).
Store in small aliquots at -20°C.
(2). Unlabeled substrate. Unlabeled casein (EM Labs, Elmsford, New York, Catalog No. 2242) is dissolved in dis- tilled water, 30 mg/ml. To aid in dissolution, adjust the pH of the mixture to approximately 7.5 with NaOH. Store at -20°C.
(3). TCA: 6% trichloroacetic acid (TCA) in water. Store at 4°C.
(4). Buffer: 0.1 M phosphate buffer, pH 7.3.
(5). Stock Ά: 0.1 Ai KH2PO4, anhydrous monopotassium dihydrogen phosphate (FW 136) 27.2 gm/liter. Stock B: 0.1 M Na2HP04 anhydrous disodium hydrogen phosphate (FW 142)
28.4 gm/liter. Mix 23 parts of A to 77 parts of B. Check pH.
Adjust pH to 7.3 by addition of A or B.
(6). Standards: Use freshly prepared solution of trypsin (Worthington Biochemical). Make a stock solution containing 1.0 yg/ml. For each assay, standards of 1, 5, 10, and 20 ng of trypsin in 20 yl of stock are tested.
(7). Do not use HC1 to adjust the pH of any reagent, or premature precipitation of proteins may occur.
III. PROCEDURE
(1). Prepare samples, standards, and blanks in triplicates.
(2). In each 400 yl microfuge tube (No. 46/6P, Walter Sarstedt, Princeton, New Jersey), combine 20 yl of buffer, 20 yl of sample (or buffer for blank), and 20 yl of labeled casein.
(3). Flick all liquids down in the tube. Incubate at 37°C for li hr. Halt the reaction with 50 yl unlabeled casein.
Add 100 yl cold 6% TCA to precipitate proteins.
(4). Centrifuge for 10 min in a Beckman Microfuge B centri- fuge. Use of this centrifuge is facilitated by employing Beckman tube racks No. 338751 and 9-hole slides No. 338742 throughout the assay.
(5). Put an aliquot of 20 yl of the supernatant into a scintillation vial containing a suitable liquid scintillation cocktail (e.g., Aquasol II, New England Nuclear, Boston, Massachusetts). Place in a liquid scintillation counter and count after chilling in the dark overnight.
IV. CALCULATION OF DATA
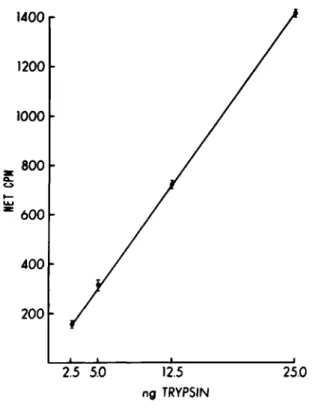
The assay performed as described is linear with respect to concentration of protease added or with time (Figs. 1 & 2).
It is independent of substrate concentration (3).
Subtract the background (release of label in the buffer blank) from each unknown to give net released counts. If the net released counts are less than 100, the standard errors of the sample and the buffer blank should be carefully compared to ensure differences are statistically meaningful. Multiply by 2/3 to give counts per minute released in one hour. Then, divide by 100 to give enzyme unit per sample.
One EU can be defined as 100 net released counts of TCA- soluble peptides over background per hour. By using the speci- fic activity of the substrate, an EU should be further defined in terms of microgram casein hydrolyzed per hour. One enzyme unit should be roughly equivalent to the activity of 1 to 2 ng of trypsin prepared and tested as described above. The assay is reported as EU secreted per unit number of macrophages or unit of macrophage protein per unit of time.
V. COMMENTS
It is worth reemphasizing that casein is a broadly reactive substrate. This breadth of reactivity, the ease of the assay, and the stability of the reagents make this assay useful in many circumstances. Supernatants of macrophages should be har- vested into siliconized tubes at 4°C and concentrated. The present assay may be more sensitive than the spectrophotometric assay for azocaseinases.
Acknowledgment
Supported in part by USPHS Grant CA-16784.
VIII. SECRETION BY MONONUCLEAR PHAGOCYTES 597
1400.
1200 1000 800 600 400 200
2.5 5.0
12.5 ng TRYPSIN25.0
Fig. 1. The net counts per minute released at 90 min by various amounts of t r y p s i n .
1400 1200 1000 800 600 400 200
30 60 90 TIME IN MINUTES
120
Fig. 2. The net counts per minute released by
50 ng of trypsin at various times.
REFERENCES
1. E. H. Reimerdes and H. Klostermyer. In "Advances in Enzymology," Vol. 45 (S. Colowick and M. Kaplan, eds.), pp. 26-28. Academic Press, New York, 1970.
2. S. Gordon and Z. Werb. Secretion of macrophage neutral proteinase is enhanced by colchicine. Proc. Natl. Acad.
Sei. USA 73:872-876, 1976.
3. N. Levine, V. B. Hatcher, and G. S. Lazarus. Proteinases of human epidermis: A possible mechanism for polymorpho- nuclear leukocyte chemotaxis. Biochem. Biophys. Acta 452:458-467, 1976.
4. D. 0. Adams. Effector mechanisms of cytolytically acti- vated macrophages. I. Secretion of neutral proteases
and effect of protease inhibitors. J. Immunol. 124:286, 1980.
5. D. 0. Adams, K. J. Kao, R. Farb, and S. V. Pizzo.
Effector mechanisms of cytolytically activated macrophages.
II. Secretion of a cytolytic factor by activated macro- phages and its relationship to secreted neutral proteases.
J. Immunol. 124:293, 1980.