Section //.β
Drug-Receptor Interaction:
Interaction of One or More Drugs with Different Receptor Systems*
Introduction . . . . . . . . . . . . 288
I I . B . l . Noncompetitive Interaction . . . . . . . . 290 1.1. Noncompetitive Antagonism . . . . 2 9 1 1.2. Noncompetitive Auto-Interaction . . . . . . 293 1.3. Noncompetitive Auto-Inhibition in Different Types of Drug
Combinations . . . . 2 9 7 1.4. Ε valuation of Noncompetitive Antagonists. . . . . 308 1.5. Dualism in Antagonism . . . . 3 1 0 1.6. Mutual Hindrance or Furtherance of Occupation of the Receptors
by Two Drugs 313 II.B.2. Uncompetitive Interaction. . . . 3 2 1
2.1. Uncompetitive Antagonism . . . . 3 2 1 2.2. Uncompetitive Auto-Inhibition. . . . . . . 323 II.B.3. Chemical Antagonism . . . . 3 2 4
3.1. Theory 325 3.2. Experiments 326
II.B.4. Functional Interaction . . . . . . . . . 329
4.1. Functional Synergism . . . . . . . . 329
4.2. A Comparison of Competitive and Functional Interactions . . 330 4.3. Functional Antagonism . . . . 3 3 3 4.4. Sequential Blockage . . . 337 II.B.5. Compounds with Multiple Actions . . . . . . . 340
5.1. Theory 340
5.2. Experiments . . . . . . . . . . 342
* B y E. J. Ariens and A. M. Simonis, in cooperation with J. M. van Rossum.
287
288 Ε. J. ARIENS, Α. Μ. SIMONIS, AND J . Μ. VAN ROSSUM
II.B.6. Specific and Nonspecific Drug Action . . . . . . . 345 6.1. Theories on the Action of Anesthetics . . . . . 345 6.2. Nonspecific or Specific Action? . . . . 3 4 7
6.3. Thermodynamic Activity . . . . . . . . 349
6.4. Binding to Specific Receptors or Accumulation in a Special Phase?. 351 II.B.7. Chemical and Physical Properties of Drugs with Local Anesthetic Action . 352
7.1. The Carbonyl Group in Local Anesthetics . . . . . 355 7.2. The Amino Group in Local Anesthetics . . . . . 3 6 1 7.3. Mechanism of Action of Local Anesthetics . . . . . 362
II.B.8. The pH and Drug Action 363 8.1. The pH and Drug Distribution 364
8.2. The pH and Local Anesthetic Action . . . . . 365 8.3. The pH and Drug-Receptor Interaction . . . . . 3 7 1 Concluding Remarks . . . . 3 8 5
References . . . . . . . . . . . . . 385
I N T R O D U C T I O N
The gradual change from agonists t o antagonists, via intermediate dualistic compounds, with a stepwise change in t h e chemical structure can, in m a n y cases, be ascribed t o a gradual decrease a n d loss of t h e intrinsic activity (see Section I I . A ) .
There are various series of homologous compounds known in which a change from agonistic t o antagonistic compounds, via intermediate compounds, takes place. The R N M e3 derivatives tested on t h e rectus abdominis muscle of t h e frog, mentioned in Table X of Section II.A, are an example. The heptyl com
pound (HeptNMe3) acts as a dualist or partial agonist, so possibly it has an intermediate intrinsic activity.
Combination of constant concentrations of this compound with increasing concentrations of a compound with a high intrinsic activity, for instance, succinylcholine, will provide t h e facts. If we are right, dose-response curves like those presented in Fig. 24, Section I I . A , will be found. If a high concen
tration of H e p t N M e3 is combined with increasing concentrations of succinyl
choline, it is expected t h a t , as a result of t h e competition between t h e two com
pounds, t h e latter will finally occupy all receptors. Then, t h e effect will reach a value equal t o t h a t obtained with high concentrations of succinylcholine alone. This does n o t happen as is demonstrated by t h e experiments represented in Fig. 1 . Here t h e theory is insufficient; t h e experimental curves do not even have t h e slightest similarity with t h e curves calculated from t h e theory given in Fig. 24 of Section I I . A .
A second experimental possibility is t h e reverse of t h e sequence of addition of both drugs—a combination of constant concentrations of an agonist, such as succinylcholine, with serial concentrations of t h e partial agonist, H e p t N M e3.
Ι , Π . Β . DIFFERENT RECEPTOR SYSTEMS 289
°/o contract.onjrect. abd (frog.) 10° "I Hept NMe3 0
°/o contraction !rect. abd (frog) - 256 '
100 80 60 40 20
S u C h M e3 O . l O ' ^ m M
L Γ
m Μ S u C h M e ^ 10" 10"
m M Hept Ν Me 3
FIG. 1. F I G . 2
FIGS. 1. and 2. Cumulative log concentration-response curves for the agonist SuChMe8
in the presence of various concentrations of the partial agonist HeptNMe8 (Fig. 1), and the converse (Fig. 2.). Note the differences between the curves obtained with this partial agonist and those obtained with, e.g., DecaMe2Et. Compare with Figs. 9 and 10, respectively.
°o contraction;rect.abd.(frog)
1 0 1 0 m ΜBuNMe-3
FIG. 3 F I G . 4
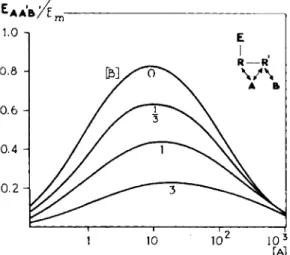
FIGS. 3 and 4. Noncompetitive antagonism. FIG. 3. Cumulative log concentration- response curves for the agonist BuNMe3 in the presence of various concentrations of the antagonist DecNMe3 (10, 12). Note the differences between the curves obtained with this antagonist and those obtained with a competitive antagonist, e.g., SuChEt3 (see Section I I . A, Figs. 12 and 13). FIG. 4. Theoretical log concentration-response curves for the agonist A combined with various concentrations of the noncompetitive antagonist Β (Eq. 1, KA = K'B = 1, α = 1, β' = -1 (10, 12). [A] and [B] in ikf- 1. Note the difference between this noncompetitive antagonism and the competitive antagonism. Compare with Fig. 11, Section I I . A.
290 Ε. J . ARIENS, Α. Μ. SIMONIS, A N D J . Μ. VAN ROSSUM
If t h e dualistic behavior of this compound has t o be a t t r i b u t e d t o a decrease in t h e intrinsic activity, dose-response curves as represented in Fig. 23, Section II.A, are t o be expected. Figure 2 gives t h e experimental results. Once more experiment a n d theory do n o t fit.
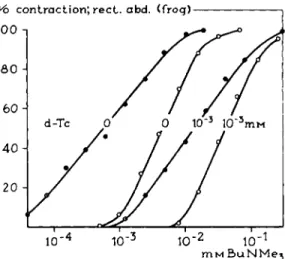
I n t h e series of R N M e3 substances studied t h e decyl compound (DecNMe3) acts as an antagonist. Is it a competitive antagonist? Combination of constant concentrations of this antagonist with increasing concentrations of an agonist, such as t h e butyl compound (BuNMe3), should give t h e answer. If t h e antagon
ism is of t h e competitive t y p e , dose-response curves like those in Fig. 11, Section II.A, can be expected. Figure 3 gives t h e experimental results; t h e decyl compound does not act as a competitive antagonist. The dose-response curves obtained resemble those expected in t h e case of a noncompetitive antagonism.
As mentioned before, t h e intrinsic activity, a, expresses t h e efficacy of drug A with respect t o t h e induction of a stimulus which sets in motion t h e chain of events or reactions leading from t h e occupation of t h e receptors R by A t o t h e effect, EA. A second drug, B , m a y interfere with this chain of events. Then Β interacts with t h e receptors R', which are different from those for A. The relation between A a n d Β is a noncompetitive one.
The contribution of drug A t o t h e effect is changed by B . The occupation of t h e receptors R ' by Β as such, does not result in any effect. Only if A is present, can Β change t h e response obtained with A. The degree to which this happens depends on t h e degree to which t h e receptors R ' are occupied by B . This rela
tion, too, can easily be p u t into an equation (10,12,14). The effect EAB> of t h e combined compounds A a n d Β as a fraction of Em becomes:
EAjEm represents t h e effect of A if applied without Β (Section II.A.2, E q . 1).
β' is t h e intrinsic activity of Β with respect t o t h e change induced in t h e effect of A. The occupation of R ' by Β results in a virtual change in t h e intrinsic activity of A. K'B is t h e dissociation constant of t h e drug-receptor complex R ' B . The interference of Β can result in an increase or a decrease of t h e response of t h e effector system. Then β' has t o be supplied with a positive or negative sign, respectively. This means t h a t there m a y be a noncompetitive sensitization or inhibition. Compound B , inactive as such, increases or de
creases t h e response of a biological object caused by a second compound A, in a noncompetitive way (10,12,15).
* "Noncompetitive" and "Uncompetitive" are to be distinguished as having different meanings.
M.B.1. N O N C O M P E T I T I V E I N T E R A C T I O N *
Ι , Π . Β . D I F F E R E N T RECEPTOR SYSTEMS 2 9 1
II.B.1.1. Noncompetitive Antagonism
I I . B . l . l . a . T H E O R Y
Depending on t h e value of t h e intrinsic activity, β', t h e antagonism m a y be complete or partial. If — 1 is substituted for β', for high doses of B , EAWjEm
becomes zero. Then there is complete antagonism. Figure 4 represents t h e theoretical dose-response curves calculated from E q . 1 b y substituting — 1 for β'. I n t h e case of a competitive antagonism the effect is determined b y the relation between t h e concentrations of agonist a n d antagonist; t h e antagon
ism is surmountable.
I n t h e case of a noncompetitive antagonism, t h e effect is determined b y t h e concentrations of t h e antagonist only. Independently of t h e dose of A, its effect is decreased b y a factor equal t o t h e second t e r m of E q . 1 . There is no shift in t h e curves b u t a gradual decline a n d a disappearance of t h e effect a t high concentrations of Β (see t h e theoretical dose-response curves in Fig. 4 ) . The noncompetitive antagonistic action is insurmountable. This is t r u e if high doses of t h e agonist or high doses of t h e antagonist are used. If lower doses of both t h e agonist a n d antagonist are involved, t h e decrease in t h e effect caused by t h e antagonist m a y be abolished t o a certain degree b y a n increase of t h e dose of t h e agonist. I n t h a t case t h e noncompetitive antagonism is surmount
able t o a certain degree. This m u s t be t a k e n into account if t h e surmountability is used as a n argument in t h e differentiation between competitive a n d non
competitive antagonism.
If a value between zero a n d — 1, let us say — 1 / 2 is substituted for intrinsic activity β', with high doses of Β t h e second t e r m of E q . 1 becomes 1/2 a n d t h e effect of A is reduced t o 5 0 % of its original value. Then there is a p a r t i a l noncompetitive antagonism.
I I . B . 1 . 1 . 6 . E X P E R I M E N T S
As is demonstrated in Fig. 3, t h e compound DecNMe3, if tested on t h e rectus muscle of t h e frog, acts as a noncompetitive antagonist of B u N M e3. Many other examples of a noncompetitive antagonistic action are known. While in t h e case of a competitive antagonism t h e relation between agonist a n d antagonist is highly specific, for t h e noncompetitive antagonism this is different. The com
pound papaverine antagonizes in a noncompetitive w a y t h e contractions of smooth muscle, as induced b y a great variety of drugs called spasmogens.
Examples are t h e contractions induced by acetylcholinomimetics in t h e rectus muscle of t h e frog a n d in t h e isolated gut, those caused b y histamine a n d b y B a C l2 in t h e isolated g u t , those induced b y sympathomimetics in t h e v a s deferens and in t h e isolated aorta strip, etc. Table I gives p^42
a n
d J>D\ values for combinations of various spasmogens a n d spasmolytics. T h e parasympa
tholytic atropine is most active against t h e parasympathomimetic H F M e3. *
* Dioxolane is designated F in the formulas for its derivatives.
292 Ε . J . A R I E N S , Α. Μ. SIMONIS, A N D J . Μ. VAN ROSSUM
Spasmogens
HFMe3 Histamine BaCl2
Spasmolytics pA2 pD\ P^ 2 P-^'2 pD'2
Atropine 8.1 6.5 3.2 Neobenodine 5.5 8.8 4.9 Diphenhydramine 6.6 7.5 5.3 Papaverine 5.3 4.9 5.0
concentrations of K+, which means t h a t t h e contractile elements are still able t o react after papaverine.
If t h e noncompetitive antagonist interferes with t h e sequence of events, which leads from t h e occupation of t h e specific receptors b y t h e agonist t o t h e effect, it m a y do so in various ways. This implies t h a t if one agonist, inducing its effect on a certain biological object, is antagonized by various noncompeti
tive antagonists, these do n o t necessarily interact with t h e same receptor system. Noncompetitive antagonists are often called nonspecific antagonists in contrast t o t h e competitive or specific antagonists.
A compound acting as a noncompetitive antagonist of a certain agonist on one object m a y act as a competitive antagonist on t h e other. DecNMe3, which behaves as a noncompetitive antagonist of t h e acetylcholinomimetics with respect t o t h e rectus muscle of t h e frog, behaves as a competitive antagonist with respect t o acetylcholinomimetics on t h e isolated g u t of t h e r a t . An ana
logous situation is found for atropine, which is a competitive antagonist for acetylcholinomimetics on t h e isolated gut, though on t h e frog rectus muscle in higher concentrations it behaves as a noncompetitive antagonist of ACh.
The antihistaminic neobenodine is similarly very active against histamine.
Benodine (diphenhydramine) takes some place in between. I n this respect, see Table X X V I I I , Section I I . A . Papaverine is practically equi-active against each of t h e three types of spasmogens (for t h e j)D'2 values, see Section I I . B . l .4).
Probably, papaverine interferes somewhere with t h e mechanism of muscle contraction. The compound is called therefore "musculotropic," e.g., in con
t r a s t t o acetylcholinomimetics a n d acetylcholinolytics which act on t h e neuro- effector junction a n d are called neurotropic. Papaverine does n o t , however, antagonize t h e contraction induced in t h e rectus muscle of t h e frog b y increased
T A B L E I
pA2 AND pD\ VALUES OF SUBSTANCES TESTED ON THE ISOLATED GUT OF THE GUINEA P I G
Ι , Ι Ι . Β . D I F F E R E N T RECEPTOR SYSTEMS 293 This points t o differences between t h e receptors for acetylcholinomimetics in t h e various tissues.
If we conclude on t h e basis of t h e t y p e of dose-response curves obtained (Fig. 3) t h a t there is a noncompetitive antagonism between B u N M e3 a n d DecNMe3, we m u s t be careful because it is possible t h a t t h e specific receptors for t h e agonist m a y be occupied in a n irreversible w a y b y t h e antagonist.
There m a y be a chemical binding between t h e drug a n d receptors on t h e basis of covalent bonds—this in contrast t o t h e binding forces supposed t o be operative in t h e case of a reversible receptor occupation. I n s t e a d of "irre
versible interaction," t h e t e r m "nonequilibrium i n t e r a c t i o n " is also used (141a).
Irreversibly blocking substances can definitely eliminate a n u m b e r of t h e specific receptors for t h e agonist. This n u m b e r increases with t h e concentration of t h e antagonist. I t will be clear t h a t this, t o o , gives a t y p e of antagonism, which results in dose-response curves r a t h e r similar t o those for t h e noncom
petitive antagonism (12, 13, 15, 16, 119, 156, 160). This will be discussed in Section I I I . If t h e antagonist can be easily washed away from t h e tissues, a n irreversible binding is highly improbable. I n t h e experimental examples given above, t h e drugs are easily washed out.
I n t h e following paragraphs t h e series of R N M e3 derivatives, which have caused trouble (see Figs. 1 a n d 2), will get closer attention. Now, we know t h a t in this series of compounds t h e change in t h e structure or t h e lengthening of t h e alkyl chain results in a change from agonists t o their noncompetitive antagonists (Fig. 3). Thus, with t h e change in t h e structure, a noncompetitive component is introduced into t h e action. T h e compound intermediate in structure m a y be expected t o behave as agonist a n d a t t h e same time as a noncompetitive antagonist for t h e spasmogenic action of B u N M e3 on t h e frog rectus muscle.
II.B.1.2. Noncompetitive Auto-Interaction
II.B.1.2.a. T H E O R Y
Ε I R—R'
A
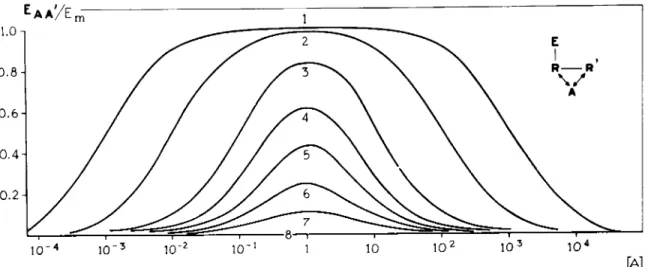
The situation just mentioned can easily be p u t into a n equation. W e have t o substitute in E q . 1 a' for β', K'A for K'B, A for Β a n d EAA,/Em for EAWfEm.
This means t h a t t h e compound A has a n affinity t o t h e receptor R, on which it induces a n effect, as well as t o t h e receptors R ' , on which t h e effect originally induced m a y be liquidated, a t least if 0 > α' ^ — 1.
If t h e affinity of A t o R is much greater t h a n t o R ' , or if KA <ξ.Κ'Α, A will occupy all receptors R first, producing t h e maximal effect, while a t still higher
294 Ε. J. ARIENS, Α. Μ. SIMONIS, AND J. Μ. VAN ROSSUM
FIG. 5. Theoretical log concentration-response curves for a series of compounds with an auto-inhibition (10, 12). In this series the values for KA increase, those for K\ decrease (a = 1 ; a' = — 1; [A] in Note the gradual change from active to "inactive" compounds, the latter being non-competitive antagonists for the other compounds.
Ι,Π.Β. DIFFERENT RECEPTOR SYSTEMS 295
FIGS. 6 .and 7. Cumulative log concentration-response curves for two series of homologous compounds, namely, RNMe3 derivatives (Fig. 6) (10,12) anddioxolanederivatives (Fig. 7) (156, 157). Note the gradual change from active to "inactive" compounds, the latter being noncompetitive antagonists of the active compounds. Compare with Figs. 3,5, and 8.
296 Ε . J . A R I E N S , Α. Μ. SIMONIS, AND J . Μ. VAN ROSSUM
concentrations, A will also occupy t h e receptors R ' , which results in a decrease of this effect. There is a n auto-inhibition.
If t h e affinity of A t o R ' is much greater t h a n t o R, or if KA > K'A) all R ' receptors will be occupied already before t h e receptors R are occupied, t h u s , before a n y effect is induced. The occupation of R b y A after all receptors R ' are occupied, will no longer result in a n y effect, a t least, if t h e intrinsic activity of A on R ' , α', is — 1.
If t h e difference between KA a n d Κ'Α is smaller, t h e auto-inhibition will already become manifest before all receptors R are occupied. Thus, t h e curves bend down again before t h e maximal effect for A on R is reached. Compound A t h e n behaves as a dualist in a double sense; it induces a partial response a t lower concentrations which is annihilated (antagonized) a t higher concentra
tions of t h e compound.
Figure 5 gives t h e theoretical dose-response curves for a series of compounds A, which interact with t h e receptors R a n d R ' . α equals 1 a n d a' equals — 1 a n d t h e quotient KAjK'A gradually increases in t h e series. The compounds exhibit a n auto-inhibition. A t t h e end of t h e series, " i n a c t i v e " compounds are found. These " i n a c t i v e " compounds a c t as noncompetitive antagonists of t h e active ones on t h e receptors R ' .
The auto-inhibition is analogous t o t h e so-called substrate inhibition in enzymology. Many of t h e types of drug-receptor interaction discussed here have their analogs in enzymology (60a,b,c} 142a, 160c, 171a).
If a' is greater t h a n zero, t h e occupation of R ' b y A results in a n increase of t h e effect; a so-called auto-sensitization takes place. Theoretical dose-response curves for this case can easily be calculated from E q . 1. If α' is equal t o —1/2, dose-response curves which represent a partial auto-inhibition are obtained.
II.B.1.2.6. E X P E R I M E N T S
The experimental results obtained with t h e series of R N M e3 derivatives, especially t h e noncompetitive antagonistic action of D e c N M e3 (Fig. 3), allow t h e supposition t h a t with a n increase of t h e length of t h e chain in t h a t series t h e affinity t o t h e receptors on which t h e effect is induced decreases, while t h e affinity t o receptors on which a noncompetitive auto-inhibition is induced, increases. This implies t h a t if we s t u d y dose-response curves over a broad dose-range, curves m a y be expected of t h e types given in Fig. 5. The experi
m e n t a l results are presented in Fig. 6. There is a remarkable similarity between t h e theoretical a n d t h e experimental set of curves. On this basis various other series of compounds were studied in which t h e change from agonist t o non
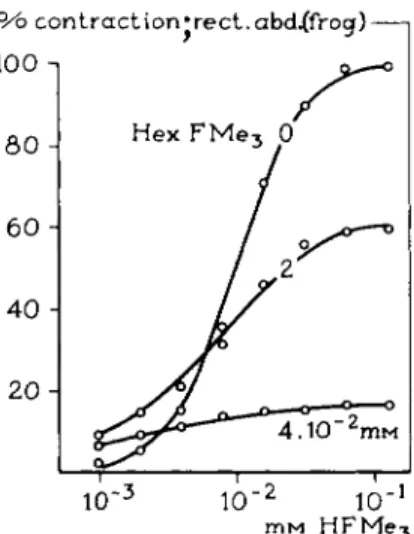
competitive antagonist occurs. Figure 7 demonstrates dose-response curves of a series of dioxolane derivatives R F M e3, in which t h e substituent was gradually lengthened, tested on t h e rectus muscle of t h e frog. As expected, t h e hexyl compound behaves as a noncompetitive antagonist for H F M e3 (Fig. 8).
If t h e interpretation of t h e log dose-response curves represented in Figs. 6
I , Π . Β . D I F F E R E N T RECEPTOR SYSTEMS 297 a n d 7 is correct, it will be possible t o predict t h e responses of combinations of compounds exhibiting a n auto-inhibition with various other drugs. W e can ask two questions:
1. W h a t will happen if constant concentrations of t h e dualistic compounds, e.g., H e p t N M e3, are combined with serial concentrations of an agonist, e.g., BuNMe3?
2. W h a t will be t h e result of a combination of constant concentrations of an agonist, e.g., B u N M e3 with serial concentrations of one of t h e dualistic compounds, e.g. H e p t N M e3?
°/o contractionjrect.abdXfrog) 1
—ι 1 1 — 1 0- 3 1 0- 2 1 0_ 1
m M H F M e 3
FIG. 8. Cumulative log concentration-response curves for the agonist HFMe8 in the presence of various concentrations of the antagonist HexFMe3 {156, 157). Note the con- competitive antagonism of HexFMe8, which itself is slightly active (see Fig. 7).
II.B.1.3. Noncompetitive Auto-Inhibition in Different Types of Drug Combinations
I I . B . 1 . 3 . a . T Y P E I T H E O R Y
Theoretical dose-response curves for t h e types of combination just men
tioned m a y be calculated (10,12,14). The case where a compound B , exhibiting an auto-inhibition is combined with a compound A, interfering with t h e action of Β on R , is represented b y E q . 2 :
Ε
+ 1.
= [ l + (1 + [ Β ] / ΖΒ) ΖΑ/ Α+1 + (1 + [ A ] / XA) XB/ [ B ] ] [* + (JTBtfB]) + 1 ] ( 2)
298 Ε. J. ARIENS j Α. Μ. SIMONIS, AND J. M. VAN ROSSUM
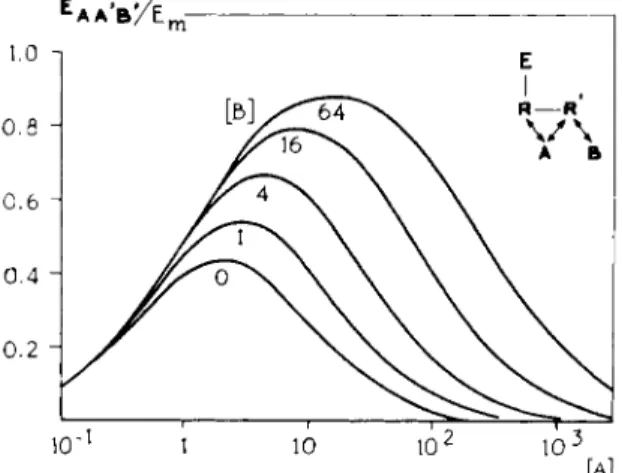
FIGS. 9 and 10. Theoretical log concentration-response curves for the agonist A combined with various concentrations of a partial agonist B (Fig. 9) and the converse (Fig. 10). [A] and [B] in M~x. Note the difference between the curves obtained with this type of partial agonists and those with an intermediate intrinsic activity. Compare with Figs. 24 and 23, respectively, from Section H.A. (a = ß = 1, ß' = — 1, KA = KB=l,K'B = 1 0 . )
Ι,Π.Β. DIFFERENT RECEPTOR SYSTEMS 299
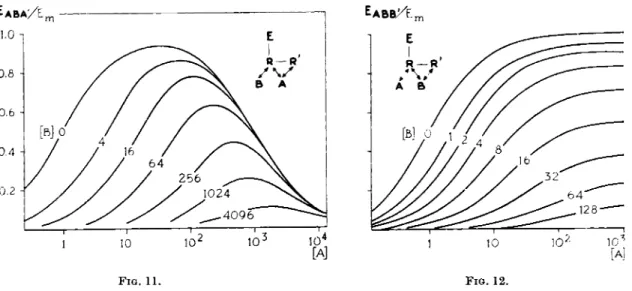
FIGS. 11 and 12. Theoretical log concentration-response curves. FIG. 11. Agonist A exhibiting an auto-inhibition, combined with various concentrations of a competitive antagonist Β (Eq. 2, KA = KB = 1, K\ = 10s, a = l , a ' = — 1, j8 = 0) (10, 12). [A] and [B] in M~x. Note the parallel shift in the ascending part of the curve only. FIG. 12. Agonist A combined with various concentrations of an antagonist Β with a dualism in antagonism, competitive and noncompetitive (Eq. 2, KA = KB = 1, K'B = 20, α = 1, β = 0, β' = — 1 (10, 12). [A] and [Β] in M~l. Note the parallel shift in the curves, as a result of the competitive antagonism on which is superimposed a decrease in the maximal height and in the slope of the curves as a result of the noncompetitive antagonism.
FIG. 11. FIG. 12.
3 0 0 Ε . J . A R I E N S , Α. Μ. SIMONIS, AND J . Μ. VAN ROSSUM
This equation is a combination of t h e E q . 6 , Section I I . A . 3 , a n d E q . 1 (Section I I . B . l ) . The first t e r m represents t h e competitive interaction of A a n d Β on t h e receptors R (see E q . 6 , Section I I . A . 3 ) . The second t e r m represents t h e noncompetitive interaction of Β on t h e receptors R ' (see E q . 1 ) . B y substitu
tion of suitable values for a, β, β', KA, KB, a n d K'B theoretical dose-response curves for t h e combination of various types of compounds can be calculated.
Suppose β is equal t o 1 a n d β' is equal t o — 1 ; Β is a dualistic compound. A is a n agonist with a high intrinsic a c t i v i t y : α = 1 . A cooperates in a competitive way with Β on R . F r o m E q . 2 , theoretical dose-response curves can be calcu
lated for t h e combination of constant concentrations of t h e dualistic compound B , with serial concentrations of A (Fig. 9 ) a n d t h e combination of constant con
centrations of A a n d serial combinations of Β (Fig. 1 0 ) . These cases correspond with t h e experiments as represented in Figs. 1 a n d 2 . Theory a n d experiment agree. This gives strong support t o t h e hypothesis used.
Ε I R—R' Β A
Now suppose t h e combination of a dualistic compound A, of which t h e in
trinsic activity α is equal t o 1 a n d a' is equal t o — 1, with a compound Β with an intrinsic activity β equal t o zero. Β acts as a competitive antagonist of A with respect t o t h e effect induced by A on R . Theoretical dose-response curves for combinations of constant concentrations of such a compound Β a n d serial concentrations of t h e dualistic compound A calculated from E q . 2 are repre
sented in Fig. 1 1 . T h e parallel shift in t h e ascending p a r t of t h e curves repre
sents t h e result of t h e competition between A a n d Β on R . The descending p a r t of t h e curves is n o t shifted; there is no interference of Β with t h e effect of A on R ' .
The case represented in Fig. 1 2 will be discussed in t h e section on dualism in antagonism (Section I I . B . l . 5 . a ) .
I I . B . l . 3 . 6 . T Y P E I E X P E R I M E N T S
Can t h e theoretical dose-response curves be realized experimentally? B y combining compounds chosen from those already discussed before, we can t r y t o predict t h e dose-response curves t o be obtained. F o r t h e theoretical dose- response curves presented in Figs. 9 a n d 1 0 t h e corresponding experimental ones have already been given in Figs. 1 a n d 2 . They are definitely in agreement.
Presumably, t h e change from agonist t o noncompetitive antagonist in t h e R N M e3 series is caused b y t h e introduction of a noncompetitive auto-inhibitive action into t h e compounds as a result of t h e change in t h e chemical structure.
How do m a t t e r s s t a n d with other results predicted b y t h e theory? I s it possible b y choosing t h e right compounds t o get dose-response curves like those in Fig. 1 1 ? This figure represents theoretical dose-response curves for a
Ι , Π . Β . D I F F E R E N T RECEPTOR SYSTEMS 301 combination of a dualistic compound of t h e t y p e of H e p t N M e3 a n d a competi
t i v e a n t a g o n i s t for t h i s compound for t h e receptors on which t h e effect is in
duced. The t r i e t h y l derivative of succinylcholine, a competitive a n t a g o n i s t of acetylcholinomimetics on t h e rectus muscle of t h e frog described earlier, m a y
°/o contraction;red. abd. (frog)—j
1 0 0 η
I
m Μ Hept Ν Me3
FIG. 13. Cumulative log concentration-response curves for HeptNMe3, exhibiting an auto-inhibition, in the presence of various concentrations of the competitive antagonist SuChEt3 (10). Note the parallel shift in the ascending part of the curves. Compare with Fig. 11.
m m / 1 2 hr g r o w t h 3 η
mg/1 IAA
FIG. 13. A. Log concentration-response curves for 3-indolylacetic acid (IAA)-induced growth in A vena coleoptile sections in the presence of various concentrations of the com
petitive antagonist 4-chlorophenoxyisobutyric acid (CPIA) (77a). Note the parallel shift in the ascending part of the curves. The descending part remains unchanged. Compare with Fig. 11. With higher concentrations of CPIA there is a decline in the ascending and in the descending part of the curves. Compare with Fig. 14.
302 Ε . J . A R I E N S , Α. Μ. SIMONIS, AND J . Μ. VAN ROSSUM
serve t h e purpose. Figure 13 represents experimental dose-response curves for combinations of constant concentrations of this triethyl derivative a n d serial concentrations of t h e compound H e p t N M e3. A comparison of Figs. 11 a n d 13 shows t h a t again there is a conformity between theory a n d experiment. Various sets of such experimental curves can be obtained if suitable compounds are chosen.
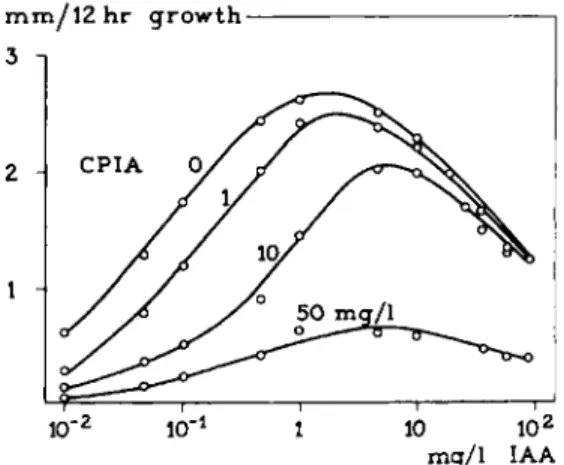
I n other fields of research, too, relations are reported of t h e t y p e represented in Fig. 11. Take, for instance, p l a n t hormones like t h e auxins. T h e growth promotor 3-indolylacetic acid (IAA) induces growth in Avena coleoptile sec
tions. T h e dose-response curves obtained demonstrate a clear auto-inhibition (Fig. 13A). Combination of I A A with its competitive inhibitor, 4-chloro- phenoxyisobutyric acid (CPIA), results in t h e dose-response curves presented in Fig. 13 A. There is a parallel shift in t h e ascending p a r t of t h e growth curves;
t h e descending p a r t remains unchanged (77a, 133a, 133b). I t appears t h a t a t higher concentrations, t h e inhibitor CPIA also h a s noncompetitive inhibiting properties (Section I I . B. l .5 ) . This is demonstrated b y t h e decline in t h e ascending p a r t of t h e curves for IAA in t h e presence of high concentrations of CPIA (see Fig. 13A).
II.B.1.3.C. T Y P E I I T H E O R Y
Another possibility is represented in E q . 3. Compare this equation with E q . 2.
Suppose A is a dualistic compound of which t h e intrinsic activity α is equal t o 1 and a' is equal t o — 1. I t is combined with a compound Β which interacts with R ' ; β' is equal t o — 1.
Ε
A Β
= α Γ / ^ £
" (ZA/[A]) + lL +l l + (l + [ B ] / Z 'B) Z 'A/ [ A ] + l + (l + [ A ] / Z 'A) ^
The first t e r m in E q . 3 represents t h e induction of t h e effect b y A on R , t h e second t e r m t h e noncompetitive inhibition of this effect on t h e basis of a competitive interaction of A a n d Β on R ' . Β behaves as a noncompetitive antagonist of A with respect t o t h e effect induced b y A on R a n d as a competi
tive synergist of A as far as t h e auto-inhibiting action of A on R ' is concerned (12, 14). Theoretical dose-response curves for this case calculated from E q . 3 are represented in Fig. 14. There is a decline in t h e ascending p a r t of t h e curves and a slight shift in t h e maxima of t h e curves, which points t o t h e competitive synergism of A a n d Β in this respect.
A situation closely related to t h e one just described is t h a t in which a dualistic compound A is combined with a compound B , which is a noncompetitive antagonist, interacting with receptors (R"), other t h a n those (R') on which t h e
Ι , Π . Β . D I F F E R E N T RECEPTOR SYSTEMS 303 auto-inhibition is induced b y compound A. As mentioned before, compounds noncompetitive with respect t o t h e action of a certain agonist m a y a c t on different receptor systems in t h e chain of reactions, leading from t h e induction of t h e stimulus b y t h e agonist t o t h e final effect. Β may, for instance, disable t h e effector system in such a w a y t h a t it can no longer respond. There is a cooperation between A a n d Β as far as t h e inhibitive action of these compounds
ι 1 1 Γ - i
ι 1 0 u r i o3
[A]
FIG. 1 4 . Theoretical log concentration-response curves for an agonist A, exhibiting an auto-inhibition combined with various concentrations of a noncompetitive antagonist Β (Eq. 3 , KA = K'B = 1, K \ = 1 0 0 , α = 1, α' =β' = - 1) (12). [A] and [Β] in M~K Note the shift in the maxima of the curves. This because common receptors are concerned with the auto-inhibitive action of A and the noncompetitive antagonistic action of B.
is concerned, b u t n o t a competitive one. Here, too, things can easily be p u t into a n equation (156).
R"—Ε
I R - R ' ^ A A - B - ^ g A A ^ £ 1 ) ( 4
\ S Em Em [ +1 + ( Z 'B/ [ B ] ) J 1 ] A
F o r t h e first p a r t of this equation see Section II.B.1.2.a. The last term repre
sents t h e noncompetitive inhibiting action of compound Β on t h e receptors R". Figure 15 represents theoretical dose-response curves calculated from this equation. F r o m these curves it m a y be seen t h a t there is now only a decline in t h e curves as a whole, while in t h e related situation, represented in Fig. 14, t h e competitive relation of A a n d Β on R ' is manifested by a slight shift in t h e maximal values for t h e different curves with increasing values of B .
II.B.1.3.d. T Y P E I I E X P E R I M E N T S
H o w can t h e case represented in t h e theoretical curves of Fig. 14 be realized?
I n order t o test this relation experimentally, we have t o combine serial
304 Ε. J . ARIENS, Α. Μ. SIMONIS, A N D J . Μ. VAN ROSSUM
concentrations of a compound with a n auto-inhibition, e.g., H e x N M e3, with constant concentrations of a compound exhibiting a competitive synergism with H e x N M e3 on t h e receptors on which t h e noncompetitive auto-inhibition of t h i s derivative is induced. T h e best t h i n g t o do is use t h e compound D e c N M e3.
Μ
FIG. 15. Theoretical log concentration-response curves for an agonist A, exhibiting an auto-inhibition combined with various concentrations of a noncompetitive antagonist Β (Eq. 4, KA = 1, K'A = 50, K \ = 1, α = 1, α' = - 1, β" = - 1) (156). Note the decline in the curves without a shift of the maximum. This is because different receptors are con
cerned with the auto-inhibitive action of A and the non-competitive antagonistic action of B. [A] and [B] in M~l. Compare with Fig. 14.
°/o c o n t r a c t i o n ; r e c t . a b d . ( f r o g ) . 1 0 0 - ,
m M H e x Ν M e3
FIG. 16. Cumulative log concentration-response curves for HexNMe3 exhibiting an auto-inhibition in the presence of various concentrations of the noncompetitive antagonist DecNMe3, tested on the rectus abdominis of the frog (12). Note the decline in the curves.
The shift in the maxima, expected because of the relation between HexNMe3 and DecNMe8, is not clearly manifested. Compare with Fig. 14.
Ι , Π . Β . D I F F E R E N T RECEPTOR SYSTEMS 305 According t o t h e experiments previously described, it has t h e required pro
perties. The experimental results are represented in Fig. 16. A comparison of these curves with t h e theoretical ones given in Fig. 14 shows t h a t again theory a n d experiment agree.
We have n o t y e t succeeded in demonstrating in experimental curves t h e slight differences between t h e theoretical curves of Figs. 14 a n d 15. The com
bination of H e p t N M e3 with papaverine was tried a n d resulted in curves very similar t o those represented in Fig. 15. The curves obtained with H e x N M e3
a n d D e c N M e3 (Fig. 16), however, did n o t clearly exhibit t h e slight shift in t h e m a x i m a of t h e curves as expected from Fig. 14.
I I . B . 1 . 3 . e . T Y P E I I I T H E O R Y Ε
I R—R'
A Β
An especially interesting case is t h a t in which a dualistic compound A, of which t h e intrinsic activity α is equal t o 1 a n d a is equal t o — 1, is combined with a compound Β which interacts with R ' , while i t s intrinsic activity /?' is equal t o 0 (Eq. 3). Now t h e compound Β behaves as a competitive antagonist of A o n R ' .
This implies t h a t t h e auto-inhibiting action of A is inhibited in a competitive w a y b y B . I n t h e curves represented in Fig. 11, A a n d Β are competitive an
tagonists on t h e receptor R , on which t h e effect is induced. This results in a parallel shift of t h e ascending p a r t of t h e curves. I n this case there is a com
petitive antagonism on t h e receptors R ' . This implies t h a t t h e descending p a r t of t h e curve is expected t o be shifted along t h e log-dose axis t o higher concen
t r a t i o n s of A. Theoretical dose-response curves for this case are represented in Fig. 17.
I I . B . 1 . 3 . / . T Y P E I I I E X P E R I M E N T S
A compound suitable t o produce experimental curves analogous t o t h e theoretical ones presented in Fig. 17 is n o t y e t known in pharmacology. I n enzymology, however, there are indications for t h e experimental reality of this case. N a y a r et al. (141) report t h a t t h e descending p a r t of t h e curve for t h e hydrolysis of phenolphthalein glucuronide b y t h e enzyme glucuronidase (the substrate inhibition) is shifted t o higher concentrations of t h e substrate in t h e presence of ethylene glycol. The set of curves obtained by t h e m resembles t h a t represented in Fig. 17 (see also 83a). I n t h e growth curves for 2-amino-5- carboxypyridine used as a growth factor for t h e p-aminobenzoic acid-deficient strain E. coli 273, a n auto-inhibition is observed, which is antagonized b y nicotinic acid amide (see Section I.B.5.1).
Various other sets of theoretical dose-response curves based on E q s . 2 a n d 3
306 Ε . J. ARIENS, Α. Μ. SIMONIS, AND J. Μ. VAN ROSSUM
a n d their experimental realizations are available (12,13). One can conclude t h a t based on t h e concepts "affinity" a n d "intrinsic a c t i v i t y " introduced into receptor theory, a large number of types of interaction between various drugs can be brought within a simple rational system.
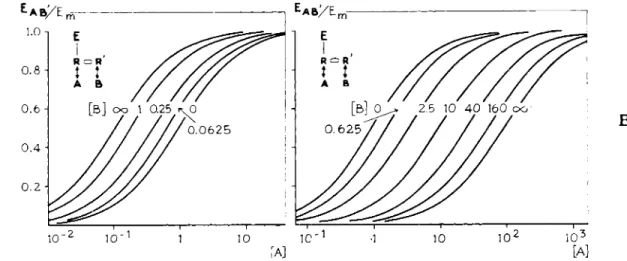
I n t h e section on mimetics a n d lytics (Section II.A.3.3) it was emphasized t h a t , if a compound is called a competitive antagonist, it is necessary t o mention t h e agonist or t y p e of agonist concerned and t h e tissues for which t h e relation holds t r u e . A comparison of Fig. 14 in Section II.A.3.1.6 and Fig. 8 (in this section) demonstrates t h a t t h e compound H e x F M e3 acts as a competitive antagonist for acetylcholinomimetics if tested on t h e isolated g u t of t h e r a t b u t
FIG. 17. Theoretical log concentration-response curves for a compound A, exhibiting an auto-inhibition, combined with a competitive antagonist B, which interacts with the receptors, on which the auto-inhibition is induced (Eq. 3, KA = 1, K'A = 5, K'B = 1, α = 1, α' = — 1, β' = 0. [A] and [Β] in Μ- 1. Note the parallel shift in the descending part of the curves. Compare with Fig. 11.
as a noncompetitive antagonist for acetylcholinomimetics if tested on t h e rectus muscle of t h e frog. The same is t r u e for t h e compound D e c N M e3 (156,157,158) (see Table X I X , Section II.A, and Fig. 3 in this section).
The existence of competitive as well as noncompetitive antagonists for t h e acetylcholinomimetics has its consequences. One m a y expect t h a t besides curare, a muscle relaxant which acts as a competitive antagonist of acetyl
choline, a n d decamethonium, a muscle relaxant with an acetylcholinomimetic depolarizing action, a third t y p e exists, viz., noncompetitive muscle relaxants.
The compound D e c N M e3 acts as such a noncompetitive curariform drug for t h e striated muscle (62a). Various other drugs with a noncompetitive t y p e of curariform action are known (122, 155, 157, a n d Section III.6.2.1).
The existence of competitive a n d noncompetitive antagonists for one agonist is not exceptional. The effects of acetylcholine, histamine, and arterenol
% contraction;guinea pig jejunum;
100 Ί
Nicotine
% contraction;guinea pig jejunum
10"" Μ
N i c o t i n e
10 Μ 10"6 1 0 "3 1 0 ' * 1 0 " - Μ
Nicotine Nicotine FIG. 1 8 . A , Β , and C. Log concentration-response curves for nicotine in the presence of
various concentrations of ganglionic blocking agents (160a, b). A . Pentamethonium and hexamethonium act as competitive antagonists of nicotine. There is a parallel shift in the curves. B. Presidal and Ecolid act as noncompetitive antagonists of nicotine. There is a decline in the curves. C. Mecamylamine and pempedine have a dual mode of action. There is a combination of a shift and a decline in the curves.
% contraction; g u i n e a p i g jejunum-
308 Ε. J . ARIENS, Α. Μ. SIMONIS, AND J. Μ. VAN ROSSUM
>C*Me510"6M • C6Me3 3.2xl0- Μ • QMe, 10~5 Μ
are antagonized in a competitive way by acetylcholinolytics, antihistaminics and sympatholytics, respectively, and, in a noncompetitive way b y papaverine.
The same holds true for ganglionic actions of acetylcholinomimetics and lytics. The ganglionic blocking agents can be distinguished as competitive and noncompetitive antagonists of t h e agonist nicotine (160a, 160b, 197a). Figure
18A and Β represent dose-response curves for nicotine in t h e presence of t h e respective types of antagonists. The ganglionic blocking agents mecamylamine and pempedine have a mixed t y p e of action: competitive a n d noncompetitive (see Fig. 18C). There is a dualism in action (see Section I I . B . l . 5 ) . Figure 18D a n d Ε represent registrograms of t h e t y p e of experiments given in Fig. 18A and B. I t is not possible t o obtain cumulative dose-response curves with nicotine because of t h e strong fade in t h e contraction. The dose-response curves represent t h e effects of sequential single doses (160a).
The effects of hexamethonium and mecamylamine on neurotransmission in the superior cervical ganglion of t h e r a t a t t e s t to a competitive inhibitive action of these compounds with respect to cholinergic transmission there (133c).
I n Section I I . B . l . 5 compounds will be discussed t h a t act simultaneously as competitive and noncompetitive antagonists for one agonist.
II.B.1.4. Evaluation of Noncompetitive Antagonists
For noncompetitive antagonists a procedure analogous t o t h a t used for an agonist m a y be followed. I n case of t h e agonist, pDx values are used (Section I I . A.3.2). The pZ)2 value corresponds t o t h e negative logarithm of t h a t dose of t h e drug for which EAJEA reaches a value 2. F r o m E q . 1 it follows t h a t if t h e effect EA of an agonist A is reduced, by a noncompetitive antagonist B , of which the intrinsic activity β' is equal to — 1, to 5 0 % of its original value, there
fore, if EJEAB, = x = 2, then [B] is equal to K'B. This implies t h a t the pD'2
value, which is —log [B] for χ = 2, becomes equal then to —log K'B. A criterion for the noncompetitive inhibition is t h a t the pD'x value of the
Ι , Π . Β . D I F F E R E N T RECEPTOR SYSTEMS 309
Nicotine 1 3.2 10 32 100 1 52 10 3 2 100 1 3.2 10 3 2 tX) i 3.2 10 32 100 1 32 10 3 2 100 1 3.2 10 3 2 100 1 3.2 X) 32 100
X10-6M I 1 I — I I Η
. e c o l i d 3.2 IK)"7M • ecolid XT* Μ • ecolid 3.2 χ KT*M
FIG. 18. D and E. Registrograms of the contractions induced in the isolated jejunum of the guinea pig. D. Various doses of nicotine and the influence of various doses of the com
petitive antagonist hexamethonium on them (160ayb). Ε. Various doses of nicotine and the influence of the noncompetitive antagonist Ecolid on them.
antagonist is independent of t h e original effect EA. I n t h e case of a competitive antagonist, t h e j)D'x value decreases with EA (14). F o r noncompetitive antagonists acting by t h e same pharmacological mechanism (thus, on common receptors), t h e difference in \}D'X values is constant for varying values of x,
TABLE I I
NONCOMPETITIVE ANTAGONISTS TESTED ON THE ISOLATED RECTUS ABDOMINIS MUSCLE OF THE FROG AGAINST CHOLINOMIMETICS0
Intrinsic activity
OctNMe3 —1 5.1
DecNMe3 —1 5.9
DodecNMe3 —1 6.1
H e p t N E t3 —1 4.7
a From Ariens and van Rossum (14).
which means t h a t t h e dose-response curves for t h e noncompetitive antagonists run parallel (14).
Schild (169) independently introduced a different nomenclature, t h e ρ^4Λ
value, which is a n empirical (experimental) magnitude. The \>D'X value links t h e experiments t o t h e receptor theory. Table I I gives j>D'2 values for a n u m b e r of noncompetitive antagonists.
310 Ε . J . A R I E N S; Α. Μ. SIMONIS, AND J . Μ. VAN ROSSUM
The relations are more complicated for compounds with a noncompetitive auto-inhibition (Section I I . B . l .2). Then, a j)Dx as well as a pZ>'x value is in t h e picture. If t h e auto-inhibition cuts in so early t h a t a bell-shaped log-dose- response curve results, a special procedure is necessary t o determine t h e j)Dx
a n d p D' s value (14).
II.B.1.5. Dualism in Antagonism
Ι Ι . Β . Ι . δ . α . T H E O R Y
Ε I R—R A Β
The drug-receptor interaction described in E q . 2 leaves still another interesting possibility open. Suppose a case in which t h e intrinsic activity of A on R is high, α is equal t o 1, while t h e intrinsic activity of Β on R is very low, β is equal t o 0 a n d t h e intrinsic activity of Β on R ' , β' is equal t o — 1. A is a n agonist and Β behaves as a competitive as well as a noncompetitive antagonist of A.
Depending on t h e affinities of Β t o R a n d R ' or t h e value of KB/K'B, t h e non
competitive inhibition will be superimposed on t h e competitive inhibiting action of Β a t lower or higher concentrations of B . Theoretical dose-response curves for this case, calculated from E q . 2, are represented in Fig. 12. If KB/K'B is large, compound Β behaves mainly as a noncompetitive antagonist;
if KBIK'B is small, Β behaves mainly as a competitive antagonist of A. If KBjK'B has a value of a b o u t 1, Β exhibits a clear dualism in antagonism with respect t o A.
I I . B . l . 5 . 6 . E X P E R I M E N T S
Consider t h e situation presented in t h e theoretical dose-response curves of Fig. 12. An agonistic compound has t o be combined with a compound which acts simultaneously as a competitive a n d as a noncompetitive antagonist. H o w can such a compound be obtained? The chemical structure of an agonist has t o be changed in such a way t h a t t h e intrinsic activity is reduced t o zero, while a noncompetitive component is introduced into t h e action.
I n t h e action of compound B u N M e3 a noncompetitive component is already available (see Fig. 6). The only thing t h a t m u s t be done is reduce t h e intrinsic activity. This m a y be done by gradual ethylation on t h e onium group.
The experimental results are given in Fig. 19A, which shows dose-response curves for t h e compounds obtained, a n d Fig. 19B, which shows t h e result of a combination of an agonist with t h e triethyl compound B u N E t3. This compound behaves practically as a purely noncompetitive antagonist. The effort failed;
t h e stepwise ethylation n o t only resulted in a decrease in t h e intrinsic activity, b u t also in a decrease of t h e affinity t o t h e receptors on which t h e effect is induced b y t h e butyl compound. I t is on these receptors t h a t t h e competitive
Ι , Π . Β . D I F F E R E N T RECEPTOR SYSTEMS 311 antagonistic action b y B u N E t3 h a d t o be induced. Thus, for B u N E t3, t h e affinity t o t h e receptors on which t h e competitive interaction h a d t o t a k e place is surpassed by t h e affinity t o t h e receptors on which t h e noncompetitive interaction is induced. The result is practically a pure noncompetitive antagonist.
Maybe another series of compounds has a better chance. I n t h e monoethyl derivative of decamethonium, t h e intrinsic activity is already reduced (see Fig. 3, Section II.A). If t h e length of t h e alkyl chain (the ethyl group) is increased, possibly a noncompetitive antagonistic component in t h e action m a y be introduced, analogous t o t h e results of a lengthening of t h e alkyl chain
9^6 contraction; re ct. abd. (fro g)
m M m M B u N M e 3 FIG. 1 9 . A . and B. Cumulative log concentration-response curves. A . A series of grad
ually ethylated BuNMe3 derivatives (14). Note the gradual change from active to "inac
tive '' compounds. Β. The agonist BuNMe3 in the presence of various concentrations of the antagonist BuNEt3. Note the noncompetitive character of the antagonist. Compare with Fig. 4 .
in t h e R N M e3 series (Fig. 6). Maybe t h e intrinsic activity is also further reduced, as t h e larger alkyl chain results in a stronger steric hindrance around t h e onium group.
If this speculation holds, in t h e series of alkyl decamethonium derivatives one can expect, successively:
1. compounds behaving mainly as competitive antagonists on which, if higher concentrations are used, a noncompetitive inhibiting action is super
imposed,
2. compounds exhibiting a competitive inhibition simultaneously with a noncompetitive one—a mixed t y p e of antagonism,
3. compounds behaving mainly as noncompetitive antagonists, because with longer alkyl chains t h e noncompetitive component becomes pre
dominating.
312 Ε . J. A R I E N S , Α. Μ. SIMONIS, AND J. Μ. VAN ROSSUM
m M m M BuNMe-}
FIG. 20 F I G . 21
°/o contraction',rect. abd. (frog) ·
m M B u N M e} m Μ BuN M e3
FIG. 22 F I G . 23
FIGS. 20-23. Cumulative log concentration-response curves for a homologous series of DecaMe2R derivatives (Fig. 20) (156, 157) and cumulative log concentration-response curves for the agonist BuNMe3 in the presence of various concentrations of antagonistic DecaMe2R derivatives (Fig. 21-23) (156, 157). Note the gradual change from active to
"inactive" compounds (Fig. 20) and the dualism in antagonism for DecaMe2Pr (Fig. 21) which changes with an increase of the alkyl chain to a purely noncompetitive antagonism for DecaMe2Hept (Fig. 23). Compare Figs. 21 and 23 with Figs. 12 and 4, respectively.
A gradual change from compounds mainly competitive t o compounds mainly noncompetitive, via compounds with a double t y p e of antagonism, is t o be expected. Figures 2 0 - 2 3 give experimental results. W h e n comparing these curves with t h e theoretical ones represented in Fig. 12 t h e conclusion m a y be
Ι , Π . Β . D I F F E R E N T RECEPTOR SYSTEMS 313 drawn t h a t t h e experimental curves accord well with t h e theoretical ones. The t y p e of compounds expected, were obtained. Examples of a dualism in antagonism are also known in t h e field of parasympatholytics. I n t h e clinic a n u m b e r of compounds a r e applied, in which, n e x t t o a parasympatholytic action, a spasmolytic action of a musculotropic type—e.g., a papaverine-like action as in adiphenine (13,18, 76b, 84)—is present.
As mentioned in Section II.A.5.3, in t h e series of arterenol derivatives obtained by substitution of alkyl or aralkyl groups on t h e Ν a t o m , t h e heavier substituted derivatives behave as sympatholytics, competitive antagonists of arterenol. The alkyl-substituted derivatives have a low affinity, t h e aralkyl substituted derivatives have again a much b e t t e r affinity (the ρA 2 values in Table X X V in Section II.A). Practically all higher substituted secondary amines have a noncompetitive spasmolytic activity. Papaverine itself is such a n amine.
The doses of t h e alkyl-substituted arterenols necessary t o get a shift in t h e dose-response curves for arterenol a r e very large, so large t h a t t h e non- competititive spasmolytic action also comes into t h e play (the p D '2 values in Table X X V , Section II.A). T h e consequence is t h a t t h e Aralkyl arterenol derivatives have, with respect t o arterenol, a competitive antagonistic action on which a noncompetitive antagonistic action is superimposed, t h u s again, an example of a double t y p e of antagonism. F o r some derivatives, e.g., N- butylarterenol t h e noncompetitive action predominates. Ganglionic blocking agents with a mixed t y p e of action are represented i n Fig. 18C.
The combination of a n antagonist Β with a dual mode of action a n d a n agonist A which produces a n auto-inhibition, gives another interesting set of dose-response curves for which α = 1, α' = — l, β = 0, β' = — 1 .
The theoretical dose-response curves for this case can easily be calculated. The first t e r m of E q . 2 m u s t be combined with t h e second t e r m of E q . 3. I n t h e curves t h e characteristics of those in Fig. 11 a n d Fig. 14 are combined. An experimental example of this t y p e of combination is represented in Fig. 13A.
W i t h t h e concentration of 50 mg/1 there is a decline in t h e descending p a r t of the curve (77a, 101a).
//.β.ί.6. Mutual Hindrance or Furtherance of Occupation of the Re
ceptors by Two Drugs
I I . B . l .β . α . T Y P E I : T w o D R U G S ON D I F F E R E N T R E C E P T O R S
II.B.l.6.α (1) Theory. The change in t h e affinity of a drug as a consequence of t h e change in t h e chemical structure is well-known. The blockade of t h e
R R'
A Β
314 Ε. J . ARIENS, Α. Μ. SIMONIS, A N D J . Μ. VAN ROSSUM
specific receptors by competitive antagonists causes a virtual change in t h e affinity, as demonstrated in E q . 6, Section I I . A . 3 . Competitive antagonism in t h e classical sense had an all-or-none character. The compounds were agonists, with a high intrinsic activity, or t h e y were competitive antagonists, with an intrinsic activity equal to zero. The receptor was blocked totally or not a t all.
The introduction of a gradual variation for t h e intrinsic activity led t o t h e conclusion t h a t t h e intermediates, compounds t h a t behave as agonists or as competitive antagonists a t t h e same concentration, were also possible (Section II.A.3.4). There is an intermediate t y p e of blockade in another sense, something like a partial blockade. I t is possible t h a t t h e affinity of an agonistic compound A t o its receptors R, is influenced by t h e occupation of receptors R ' by a compound B , which, if applied singly, is inactive. The receptors R and R ' have to be located close t o each other, because t h e occupation of one of t h e two influences t h e occupation of t h e other. They are called interdependent a n d represented by R C R'. The m u t u a l interference of A a n d Β on their respective receptors is n o t competitive in t h e usual sense.
The presence of Β on t h e receptors R ' m a y increase or decrease t h e affinity of A to R. Suppose t h a t A as well as Β is electrically positive or negative. This m a y result in a m u t u a l hindrance; t h e presence of Β on its receptors, then, decreases t h e affinity of A t o its receptors, a n d vice versa. If A a n d Β have opposite charges there m a y be a m u t u a l furtherance of receptor occupation.
The presence of Β on its receptors increases t h e affinity of A t o its receptors, and conversely.
The dissociation constants of t h e drug-receptor complexes A R C R ' and R C R ' B are KA and Κ'Β, respectively. W h e n both interdependent receptors are occupied a t t h e same time, i.e., if A R C R ' B is formed, t h e dissociation constants change to KAK'B (1-κΑΒ>), in which κΑ Β> represents t h e m u t u a l interference of t h e drug molecules on their respective receptors.
There m a y be an increase of t h e dissociation constants—a m u t u a l inhibition, as far as t h e occupation of t h e receptors is concerned; t h e n KA W is negative.
There m a y be a decrease of t h e dissociation constants—a m u t u a l furtherance;
t h e n κΑΒ> is positive. If this reasoning is p u t into an equation on t h e basis of t h e mass action law, we get (12, 156) :
\ ~ \ x : = ar T/ A B W [ A ] ) ( 5)
in which
f = 1 + [ B ] / Z 'B
1 + ( [ Β ] / * 'Β) [ 1 / ( 1 - κΑ Β' ) ] ' As m a y be seen from E q . 5, t h e addition of compound Β results in a parallel
shift in the dose-action curves for compound A. The dissociation constant of t h e drug-receptor complex R A is changed. Κ/ Α Β ' > 1> t h e curves are shifted t o