Differential Expression of Proteoglycans on the Surface of Human Melanoma Cells Characterized by Altered Experimental Metastatic Potential
Jozsef Timar,* Andrea Ladanyi,*
Karoly Lapis,* and Magdalene Moczart
FromtheFirstInstituteof Pathology andExperimental CancerResearch,*SemmelweisMedicalSchool, Budapest, Hungary,andthe Laboratoire de Biochimie du Tissue
Conjonctif,
Facultyof Medicine, UniversityofParis,FranceHeparan sulphate (HS) and chondroitin sulphate (CS) proteoglycans (PGs) frequently have opposite biologicfunctions incell-matrixadhesionaswellas inthe regulationof cell
proliferation.
Datarevealed thatsulphatedglycosaminoglycans (sGAGs) (sugar chains ofPGs) are differently expressed in tumor cellscharacterizedbydifferentmetastaticpotential;the more metastatic cells containahigherHS/CSra- tio. As theproliferative capacity oftumorcells is also frequently altered inparallel with their metastatic potentia4 itwas notclearwhetherobserved PG al- terations
reflect
changes in cellproliferationormet- astaticpotential The cell-associated PG expression andsGAGbiosynthesiswasstudied intumorcellsof human melanoma lines characterized by different experimentalmetastaticpotential to the mouse liver but similarinvitro/invivoproliferation rates. Using antibodies against PGswefound differentexpression of PG epitopes in melanoma lines, exceptfrom the melanomaantigen. Unlike the low CSPG(melCSPG) metastatic melanomacells;
the cell line with high metastaticcapacitycontainedahigherproportionof positivecellsforsurface-HSPGwithout the coexpres-sion ofcertain cartilage-type CSPGepitopes (recog- nizedby MAb HSFPG529)aswell as by an increased pericellularHS/CSratio duetointracellularaccumu- lation/retentionof CS. Immunocytochemistry of ad- herent cells revealed HSPGs at substrate-attached membraneareasonly in casesofhighly metastatic melanomacells Thesedatafurthersupport ourview that the absolute or relative dominance of HSPGs over CSPGsat the cellsurface of metastatic tumor
cells can be considereda markerofa more meta- staticphenotype. (Am JPathol 1992, 141:467-474)
Cell
membrane proteoglycans(PGs)
are known to be involved incell-cell andcell-matrix interactions.1Some
of themareextracellularcomponentslike thelargePGs
ag- grecan,perlecan, and versican,orthesmall PGs decorin andfibromodulin1'
belonging to the chondroitin sul- phate proteoglycan(CSPG),
aggrecan, versican, and decorin,ortheheparan sulphateproteoglycan (HSPG),
perlecan,family.
Interest has beenfocusedoncellmem- brane-type PGs. Important members of this subgroup aresyndecan and betaglycan; bothareglycosylated
by heparan sulphate (HS) and chondroitin sulphate(CS) chains,34 fibroblast-membrane HSPGs5
andCSPG
as- sociated withMHC-11.1
3PGs
were implicated in the regulationof
cellgrowth.68
It was demonstrated that the membraneHSPG,
betaglycan,serves as coreceptorfortransform- ing-growthfactor-p
(TGF,), providing and/orconcentrat- ingTGFP
for thesignaling-TGFO
receptor.9 Another membraneHSPG wasshowntoserveas acaptivede- viceforFGF through its HS side chains,alinkessentialfor FGFeffect.1"12
The sugarchains of HSPGs, HS and heparin, were also shown to have a direct role in the regulation of the proliferation of smoothmuscle, endothe- lial, andtumorcells.6,13
Transformed cellsarefrequently characterized by al- tered GAG pattern, decreased HS, and increased CS
content,1315
aswellasaccumulation ofHSPG and HSin theGolgi region.16
Well-characterizedhuman tumorPGsSupportedby theHungaranMinistry of Health and Social Services (K.
Lapis), by the BntishCouncil(J. Timar), and by the Association for Cancer Research(France) (M. Moczar).
Accepted for publication February 5, 1992.
Address reprint requests to Dr. Jozsef Timar, First Institute of Pathol- ogy andExperimental CancerResearch, Semmelweis Medical Univer- sity,Budapest,VIII.Ulloi u.26. H-1085, Hungary.
467
are themeICSPG,17'18 HSPG of colon carcinoma,19 and
glioma.'
Meanwhile, thefunction(s) oftumorcell PGs is notclear.The mostimportant consequenceof themalig-
nanttransformation is theability oftumorcellstoinvade host tissuesand formdistantcolonies, i.e., metastases.Datashow that rodent tumors with different metastatic capacity are characterized by altered sGAG pattern;
there isfrequentlyanincreasedHS/CS ratioin more met- astatic
variants.21
We have established and described human mela- noma xenograft lines,
HT168/HT168-Mi,
with different liver-colonizingpotentials.21'22
In thismodel there is no difference in cell proliferation characteristics orexpres- sionof melanoma markers.22 However, using this model this study describes increased HSPG and decreasedCSPG
epitopeexpressiononthe surface ofhighlymet- astatic cells. The alterations at the PG level were also reflected in theglycosylationpattern;dueto adecreased CScontent,theHS/CS
ratioincreased in thepericellular
compartmentof highly metastatic cells.Insitu immuno- chemistryshowed surface and substrate attachment site accumulationof HSPG epitopes onlyinthe caseofhighly metastatic human melanoma cells.Materials and Methods Human Melanoma Xenografts
HT168 human melanoma xenograft was established from human melanoma cell line
A205821
(provided by L.A. Liotta, NCI, Bethesda, MD).HT168-Mi
xenograft was derived from liver metastasis of HT168tumor.22 HT168-M1
cells provedtobe eight- totenfoldmore met- astatic than the HT168cells in liver-colonization assay after intrasplenic injection of106 cells.22
In Vitro Cultures
HT168 cell line was established from the sc tumor whereas
HT168-M1
was from a liver metastasis of the HT168tumor;both were cultured in RPMI 1640 medium(Gibco,
Grand Island,NY)
supplemented with10%fetal calfserum (FCS) (Boehringer, Mannheim, Germany)at370C
in5%C02.
The cellswereeitherscrapedfrom the plasticsurfacesby rubber policeman or digested at room temperature by 0.2% trypsin for5 minutes. The human and melanoma characterof thetumorcellswastested regularlyby chromosomal analysis as well as by measur- ingthe expressionof humanandmelanomaantigens.22
Biosynthetic Labeling of GAGs
Cells
instationarygrowthphase (1
C5 cells/cm2)werein- cubated with a mediumcontaining
10 ,uCi/ml 3H- glucosamine(8 mC/mmol, CEA, Saclay, France)
for 24 hoursat37°C. The mediumwasremoved, and the cells werewashed with3 x 5 mlphosphate-buffered
saline (PBS)anddigested by 0.05%trypsin/PBS
for 15 minutes atroomtemperature.Thetrypsinizationwasstopped by
adding20 ,ulFCS. Cells
werescraped
from theplastic,
sedimentedat 2000rpmfor 5 minutes at40C,
and the supernatant,containing thepericellular
proteins,was re- moved.Thecell pelletwaswashed with2x 1 mlPBS.Separation of GAGs
Thesugarchainswerereleased from the PG corepro- teins byreductive alkalinehydrolysisin0.05mol/lNaOH inthepresenceof 1 mol/l NaBH4 at
450C
for 24 hours.The hydrolysates were desalted on Biogel P2 column equilibrated with 10 mmol/l
Tris-HCI,
pH 8.2. The ex- cluded3H-macromolecules
eitherwereprecipitated by
cetylpyridinium chloride(CPC)
or weresubmittedtoion- exchange chromatographyusing DEAE-Trisacryl
col- umn(IBF, France),
equilibrated with 10 mmol/A Tris-HCIbuffer,
pH8.2. Elutionwasperformed
with linearNaCI
concentrationgradient.Characterization of GAGs
Analytical scale precipitation of
3H-GAGs
wascarriedout accordingtoWasteson.23
CSwasidentified by itssensi-
tivity to chondroitinaseAC
(Seikagaku-Kogyo, Japan), whereasHSwasidentified by deaminative cleavagewith nitrous acid. The specific degradation was assessed from the elution diagrams of the startingmaterials and the hydrolysates obtainedon a Biogel P6column using 0.2mol/l
aceticacid-pyridine, pH5.0.Anti-PG Antibodies
Anti-CSPGAntibodies
ME.31.3, an
IgGl
(provided by Dr. M. Herlyn, Wistar Institute, Philadelphia, PA), wasproduced against core protein epitope ofa250 kd melanoma associatedCSPGantigen.24
HFPG-529/4(IgM)and MK1 72(IgGl) were produced againsthumanfetalarticularcartilageCSPGrecognizing
core protein epitopes at the linkage region of the CS stub.25'26
Anti-HSPG Antibodies
FW16 antibodyproduced against pig skin fibroblast membraneHSPG (mw 60 kd)recognizinga coreprotein epitopewasprovided byM. F. Watt(ICRF, London, UK).
BN42antibody produced againstratliver basement membrane HSPG (J.V. Hascall, NIH, Bethesda, USA)
was agift of B. Nusgens (Liege University, Liege, Bel- gium).27
Flow Cytometry and Indirect Immunofluorescence
For flowcytometry,confluent phase melanoma cellswere
collected by scraping or by trypsinization, suspended (106 cells/sample), and labeled at 40C (native cells) or
after 10 minutes of fixation inmethanol(MetOH). For im- munofluorescence, cellsweregrownonglass coverslips atadensity of 105 cells/cm2.
Cells insuspensionor oncoverslipswerelabeled ei- therat40Corroomtemperature. First, 10% nonimmune goatserumwasapplied for 10 minutes,then thesamples
wereincubated with the primary antibody (1 :100 working dilution formonoclonals and 1:50 forpolyclonals) for 30 minutes. After washings in PBS (three times),anappro-
priate FITC-labeled secondary antibody (goat anti-
mouseIgG orgoatanti-rabbitIgG, Nordic)wasapplied (working dilution 1:20 in PBS) for another 30 minutes.
Afteranother washing in PBS (three times), the unfixed samples were fixed in 1% paraformaldehyde/PBS (10 min), while the fixedones werekept in PBSat40C. Neg- ativecontrols for flowcytometryorimmunofluorescence
were exposed tothe secondary FITC-labeled antibody alone. Experiments in which the background fluores-
cence washigher than the autofluorescence ofunstained cells wereexcluded from this study. Cells adherent to coverslipswereviewed and photographed in aVanox- Olympus epiluminescence microscopeorinaconfocal laserscanning microscope (BRL). Cell suspensionswere
tested in FACStar flow cytometer (Becton Dickinson,
Sunnyvale, CA) by measuring at least104 cells/sample (in triplicate) and the percent of positive cells (the fluores-
cenceof whichwas higherthanthe intensity of 90%of thenegativecontrol population)wasdetermined.
Results
Flow Cytometric Analysis and Localization of CSPG Epitopes
Thesurface of HT1 68 and HT1 68-Mi cellswereequally positive for the melanoma-CSPG antigen (-60%).One of thecartilage CSPG epitopes, detected by MAbMK172,
was equally represented, though ata lowlevel, onthe surface of the melanoma cells, but another cartilage, CSPG epitope, recognized by MAb HFPG529, was
present onlyon the native low metastatic HT1 68 cells (Table 1). After MetOH fixation, which allows the detec- tionof intracellular antigensaswell, similarCSPGantigen patternwasdetected inthetwocell populations (Table 1). The lowerproportion of positive cells in fixed samples comparedwiththe nativeonesisprobably due to the loss ofantigenicityuponfixation. In the adherent cells of both lines, the melanoma CSPG antigen was present atthe apical cell surface and in the cytoplasm (Figure 1). In permeabilized adherent cells, MAb HFPG529 bound dif- fusely to cytoplasmic domains of the low metastatic HT168 cells (Figure 2a) but it did notlabel the highmet- astatic variant(Figure 2b). Therewas adiffusefaint cy-
toplasmic positivity in asmall subpopulation of cells in bothtumorcell lines reacted withMAb MK1 72antibody (not shown).
Flow Cytometry and Localization of HSPG Epitopes
On the surface of native highly metastatic HT168-Mi cells, thetwoanti-HSPGantibodiesweredetectedatlow frequency(15%) but HT1 68cells remained negative (Ta- ble2). Themajority of the HSPG epitopesonHT168-M1 cells were masked according to the significantly in- creased surface labeling after trypsinization (Ta-
Table 1. Expression ofCSPGAntigensinHumanMelanoma Cells (Flow Cytomety)
Cell surface Cell surface + cytoplasm
Antibodies HT168 HT168-Mi HT168 HT168-Mi
ME.31.3 51.5 + 10.3 69.4 ± 15.4 41.8± 11.7 37.2± 7.6
HFPG529 47.8±9.2 0.5±0.4 40.6±8.3 0.0±0.0
MK172 20.6±5.2 28.5 ±6.3 23.6±4.2 22.2±3.3
Dataareexpressedin %of positive cells. Each point represents mean of three measurements(±SD). Cell surface; immunolabeling of native cellsat40C.Cellsurface + cytoplasm = immunolabeling ofMetOH fixed cells.
Figure 1. Immunofluorescent localization of melanoma-CSPG antigen in adherent HT168 cells. Methanol fixation/perme- abilization; primary Ab: ME.31. 1, secondary antibody:goatanti-
mouseIgG-FITC Note the heterogenous staining of the apical cell surfaces (arrows), x600.
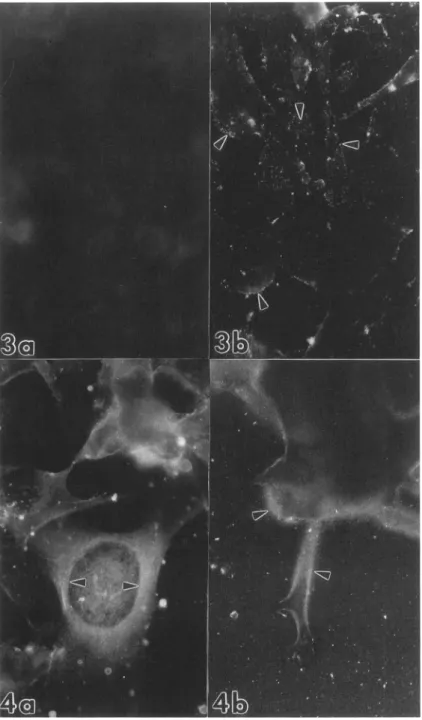
Figure 2. Immunofluorescent localization of cartilage-type CSPG antigen in adherent human melanoma cells. Methodologyasin Figure 1, but the primary antibodywasMAbHFPG529.a:Inthe HT168 line there isadiffuse cytoplasmic staining insomecells (arrows) butnegativecellsaremorefrequent, x600; b: HT168-Mi cellsareuniformly negative, x600.
ble 2). One of these antigens, recognized by the poly- clonalantibody PAb BN42,wasalso maskedonthesur-
face ofasmallproportion of HT1 68 cells (Table 2). After MetOH fixation, both cell lines exhibited cytoplasmicpos-
itivity. The PAb FW1 6-positive cells are dominant over
PAb BN42-positiveonesinthe low metastatic HT168 cell
population. In the highly metastatic HT168-Mi cells, the expression of epitopes recognized by PAb FW1 6was lower than in HT168 cells, while the HSPG epitope, rec- ognized by PAb BN42, was similarly represented (Ta- ble 2).
Inadherent cells, PAb FW1 6wascompletely absent from the apical surface of HT1 68 cells (Figure 3a) but
wasdiffusely presentonHT168-M1 cellsasdiscretedo- mains (Figure 3b). This difference disappearedafterper- meabilization because both cell types showed positivity with PAb FW16. In the cytoplasm of the adherent low metastatic HT1 68 cells, the distribution of PAb FW16- recognized antigen was diffuse (Figure 4a). While in HT168-M1 cells, beside the diffuse cytoplasmic localiza- tion, these epitopeswereconcentratedatthe focalcon- tactareasatlamellopodia and filopodia (Figure 4b). The difference between thetwocelltypes in thelabeling of the ventral membranes with PAbFW16wasfurthersup-
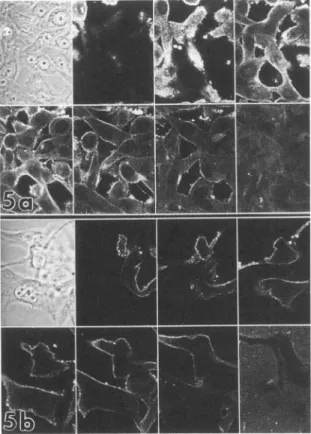
ported by confocal laser scanning microscopic analysis (Figure 5a, b). The staining pattern of PAbBN42atthe cell surface and in the cytoplasm was essentially the samethan in thecaseof PAbFW16(not shown).
Biochemistry of Cell-associated sGAGs
The incorporation of 3H-glcN into cell-associated sGAGs
was predominant in the pericellular (surface) compart- ment compared with the intracellularone, which repre-
sented only one-fifth and one-fourth of thetotal cellular incorporationin HT168 andHT168-M1 cells, respectively (Table 3). Therewasnodifference in theprecursorincor- poration into the pericellular sGAGs, buta50% increase
wasdetected in theincorporation into intracellular sGAGs in HT168-Mi cells compared withHT168(Table 1).
After ,8-elimination and ion-exchange chromatogra- phy, different sGAG composition wasfoundonthesur-
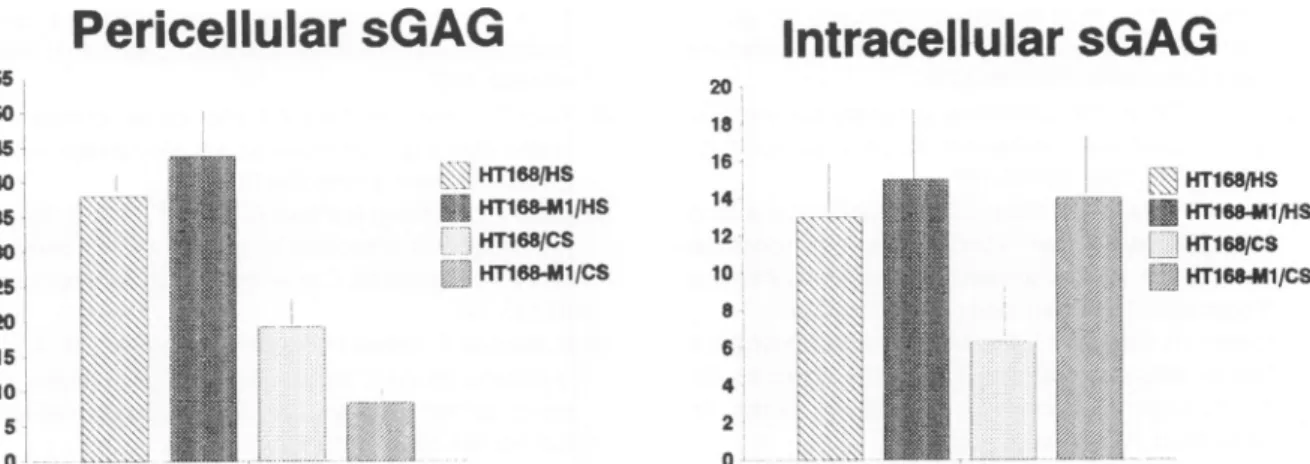
face of thetwomelanoma lines. Therewasnodifference intheprecursorincorporation into HS but duetothe lower level ofincorporation into CS in HT168-Mi cells, the HS/
CS ratiowasthree-foldhigheronthe surface of thehigh metastatic cells(6:1) thanonthelow metastaticcounter- part (2:1) (Figure 6). Intracellularly, there was nodiffer-
encebetween thetwocell lines intheprecursorincorpo- rationinto HS but duetothe increased incorporation into
Table2. ExpressionsofHSPG AntigensinHumanMelanoma Cells(FlowCytomety)
Cell surface Cell surface-masked Cell surface + cytoplasm
HT168 HT168-M1 HT168 HT168-M1 HT168 HT1 68-M1
FW16 0.0±0 13.6± 2.9 1.5± 0.8 30.3±8.2 73.7±9.2 47.5± 6.0
BN42 0.0±0 18.7± 5.6 21.5± 7.2 71.5±7.0 27.9±5.4 37.2± 4.4
Data is expressedin %of positive cells. Each point represents mean of three measurements (-SD). Cell surface = immunolabeling native cellsat40C. Cell surface-masked = immunolabeling of trypsinized cells at40C.Cell surface + cytoplasm = immunolabeling after MetOH fixation.
Figure 3. Immunofluorescent localization of membrane-typeHSPGepitopesonthesur-
face ofadherent human melanoma cells.
Methodology:
40C labeling with PAb FWJ6 andgoatanti-rabbitIgG-FITCG
a:TherezsnolabelingonthesurfaceofHT168 cells. X900.
b: Membrane-HSPG epitopes are localized into discrete surface domains (arrows) in HT168-Micells. X900.
Figure 4. Immunofluorescent localization of
memnbrane-type
HSPGepitopesinhumtan melanomacells.Methodology:methanolfix-ation/permeabilization,
immunlabelingasin caseofFigure3.a:Thereisadiffuse cytoplas-mic-mostlypermnuclear-staining
inHT168 cells(arrow), x1600. b. InHT168-Ml cells HSPGepitopes(recognized byPAbFWJ6)are concentratedatthoseplasma
membranear- eas, whichare in contactwith thesolid sub- strate(arrow), x1600.CS in the highly metastatic cells, the ratio between HS and CSwasmuch lower(around one) in HT1 68-M1 cells than in the HT168 (around two) (Figure 6). Based on
thesedata,weconcludedthat the differentsGAGcom-
position inthe two cell lines is not due to different HS biosynthesis, but rather due to the intracellularaccumu-
lationorretention of CS in the highly metastatic cells.
Discussion
Thebiosynthesis of PGs and their GAG chains is altered during malignant transformation, resultinginadecreased
HS sulphation and/or production and increased
CS
con- tentinthemajorityoftumortypes.13
TheGAG expression
is altered intumorcellsduringprogression
andmetasta- tization.21 Inseveral rodent and insomehumanmetasta- sis models the increased metastaticcapacity
isassoci- ated withanincreasedHS/CS
ratio insGAGs.21
We have studied thesephenotypic
changesinhuman melanoma xenografts, HT1 8/HT1 68,characterized
bydifferent
pro- liferationrate aswellasexperimental liver metastaticca-pacity.21
In thismodel, wefound an increasedHS/CS
ratio insGAGs
inthemoremetastatictumorbut the totalGAG
content was notaltered.21
Duetothefact
that be-Figure5. Confocal laserscanning microscopy ofadherentbhuman melanoma cells labeled for intracellular membrane-typeHSPG epitopes.Methodology:asinthecaseofFigure 4.Six serialoptical sections(1Fm)werephotographedincaseofanarea.Upperleft:
apical membrane; lower right: substrateattached site. a:HT168 cells.Notethediffusecytoplasmic staining (uppercenterandright) and the lack of labelingatattachmentmembranes(lowerright), X500. b:HT168-Mlcells.Notethe continuousmembranelabel- ingaswellas asignificantfluorescenceatthemembranes attached tosubstrate (lowerright), x500.
side the metastatic capacity, the
proliferation
rate was also different inthese melanomas, itwas notclear how the difference insGAG-composition
was relatedto thebiological
properties. To approach theproblem,wehave established human melanoma xenograft lines(HT168/
HT168-Mi)
with different liver metastatic capacity but similar in vivo/in vitro growth rate.22 In this model, we found again characteristic differencesincellular PGex- pression. In the cell-associated sGAG compartment, therewasonlyaslight difference betweenthe two tumor lines, unlikeintherelative ratio betweenHSandCSof theTable3. Incorporationof3H-glcNinto Cell-associated sGAGs inMelanoma Cells
sGAG fraction HT168 HT168-M1
Pericellular 76.83±8.2 81.13± 4.5
Intracellular 18.90± 1.8 28.00± 1.4 Data are expressed as cpm X103/105 cells and represent meansof three measurements ±SD. Cells were labeled with 3H- glcN,the peri- as well as the intracellular fractions were separated and sGAGs were isolated using p-eliminationand ion-exchange chromatography.
individualfractions(surfaceor
intracellular).
The intracel- lularsGAGwas theminorityof total cellular sGAGs and the highlymetastaticvariant, HT1 68-M1, contained 50%moreintracellularsGAG duetotheincreased CScontent than the lowmetastaticHT1 68.However, the intracellular HScontent wassimilarin the twocelltypes. Morepro- nounceddifferenceswerefound in thepericellular (sur- face)sGAG composition; theHS/CS ratio increased on highlymetastatic melanoma cells dueto adecrease in
CS
content.In harmony with these observations, flow
cytometric
measurements revealed characteristic differences be- tween the twomelanoma lines in the expression of PG epitopes. Acartilage-CSPG
recognizing antibody, MAb HFPG529, did not bind to the highly metastatic mela- noma cells unlike its low metastatic counterpart. MAb HFPG529 recognized Ser-Gly-CS-stub epitope on the high molecularweightcartilageCSPG,26
anepitope that iscommon in high molecularweightCSPGs.3 Previous biochemical studies indicated the presence of at least fourCSPG species in HT168 cellshavingapproximate
molecular weightsof 1000,600,500,and400kdbased ontheKav
valuesonSephadex
CL4B.28 However, only the 1000-kd species was characterized by hialuronic acid (HA) binding capacity.28 HA-binding characterizes severalCSPGs
including aggrecan, versican,PG-M,
aortic high molecular weightCSPG2,3,
as well asmeICSPG.1718
Furthermore, there areimmunologic sim- ilarities between thehigh molecular weightCSPGs.3 In- terestingly, melanomaCSPG
antigenwas detected not onlyin human melanomasbutinothertumorcelltypes as wellas in some normalcells includingchondrocytes.29
Decreased expression ofacommonCSPG
motif, Ser-Gly-CS-stub,
in the highly metastatic melanoma cells,HT168-Mi,
mayaffect theCS-biosynthesis as well. On the other hand, therewas nodifference between the lines inthe expression of melanoma specificCSPG
antigen, which supports those previous observations that the ex- pressionofthis antigen is not linked to theprogression.18
Thefact that in highly metastatic melanoma cells the high molecularweightCSPG disappeared28
when no alter- ation was found in meICSPG epitope expression sug- gests thatthehigh molecular weight HA-bindingCSPGin HT168 cells ismostprobablynotthemeICSPG.
Further studies have been undertaken tosolvethis question.Morecharacteristicdifferenceswere found in base- ment membrane-type and surface-type HSPG expres- sion in our human melanoma cells. There was an in- creasedsurfaceHSPG epitopeexpression in the HT1 68- Ml highly metastatic line; however, the majority of those surface HSPG antigenswasmaskedby trypsin-sensitive domains. Interestingly,there was nodifferenceinthe in- tracellularexpression of HSPGantigens between the two melanoma lines. Morphologicstudy showed that in the
Pericellular sGAG Intracellular eGAG
* ~~~~~~~~~~~~~~~20,
41
S,1
* ~~~~~~ 14
a [lifs/c 12'.W
*1 HTISSMI/cS 101l
HTISSMIlS15
lo. 41
5 2'1
Figure 6. Incorporationof3H-glcNintosGAGsofmelanomacells.Dataareexpressedincpm x1C3/105cells.Barsrepresentmeansof3 samples±SD.
highlymetastatic cells, thesurface-type HSPG-antigens recognized bythePAbFW1 6,waslocalizednotonlyon the apical plasma membrane but at the substrate at- tached membrane areas, suggesting that, at least this HSPG, mayplayarole in attachment. The meICSPGan- tigenwas not present atthoseareasin the HT1 68/HT1 68- Ml model,onthe contrary topreviousreportsinanother melanoma
line.30
The A2058 human melanoma cell line, theparentlineof the HT1 68tumorxenograft, containsat least two different HSPG species,3132 a hydrophobic transmembrane molecule with high affinitytothrombo- spondin and a basement membrane-type HSPG with high affinitytofibronectin.31 Infact, inHT168/HT168-Mi cellswehaveshown thepresenceoftwoHSPGspecies characterized by -200 and -400kd32 andinharmony with this, in the present work, we found binding ofan anti-basement membrane (PAb BN42) and anti-plasma membrane (PAb FW16) HSPG antibody to HT168/HT168-M1 cells. The lower level of expression of surface HSPG antigens in low metastaticHT168cells is dueto an intracellularaccumulationorretentionof these molecules asitwasprovenby biochemical
measurements.32
Thefunction(s) of PG molecules in humanmelanoma cells is still unknown.They mightplayanimportant role in matrix
adhesion1'
34 but accordingtorecentobservations they might be involvedintheautocrineregulationofthe cell proliferation as well.6 Functions ofHSPGs
andCSPGs
arefrequently
oppositeinadhesion and cellpro-liferation.1'
3,4, Ourpresent andpreviousresults21
sug- gest thatabsoluteorrelativedominance ofHSPGsoverCSPGs
atthesurfaceof human melanoma cells could be consideredas amarkerofamoremetastaticphenotype.Acknowledgment
The authors thank Mr. H. Paterson (Institute of Cancer Research, London, UK) for performing confocal laser microscopy.
References
1. Ruoslahti E: Structure and biology ofproteoglycans. Ann RevCell Biol 1988, 4:229-255
2. Barry F: Proteoglycans: structure and function. Biochem SocTrans 1990,18:197-200
3. Gallagher JT: The extended family of proteoglycans: social residentsof the pericellular zone.Curr OpinCellBiol 1989, 1:1201-1218
4. Kjellen L, Lindahl U:Proteoglycans:structuresandinterac- tions. AnnRevBiochem 1991, 60:443-475
5. Gallagher JT,TurnbullJE, LyonM: Heparan sulphate pro- teoglycans. BiochemSoc Trans 1990, 18:207-209 6. RuoslahtiE, Yamaguchi Y:Proteoglycansasmodulatorsof
growthfactor activities.Cell 1991, 64:867-869
7. Esko JD,RostandKS,WeinkeJL: Tumor formation depen- dent on proteoglycan biosynthesis. Science 1988, 241:
1092-1096
8. Yamaguchi Y, Ruoslahti E: Expression of humanproteogly- caninChinese hamster ovary cells inhibits cellproliferation.
Nature 1988, 336:244-246
9. Andres JL,Stanley K,CheifetzS, MassagueJ: Membrane- anchored andsolubleforms of betaglycan,apolymorphic proteoglycan that bindstransforming growthfactor-P.JCell Biol 1989,109:3137-3145
10.Yayon A, KlagsburnM, Esko JD, Leder P,Ornitz DM: Cell surfaceheparin-like molecules arerequiredforbinding of basic fibroblastgrowth factortoitshigh affinity receptor.Cell 1991, 64:841-848
11. D'Amore PA: Modes ofFGF release in vivo and in vitro.
Cancer Metast Rev 1990, 9:227-238
12. Flaumenhaft R, Moscatelli D, Rifkin DB: heparin and heparan sulphate increase the radius of diffusion and action of basic fibroblastic growth factor. J Cell Biol 1990, 111:
1651-1659
13. lozzoRV: Proteoglycans and neoplasia.Cancer Metast Rev 1988,7:39-50
14. UnderhillCB, Keller JM: Heparan sulphate of mouse cells.
Analysis of parent and transformed 3T3 cell lines. J Cell Physiol 1977,90:53-59
15. Winterburne DJ, More PT:Altered metabolismn of heparan sulphate in simian virus 40 transformed clonedmousecells.
JBiol Chem 1978, 253:5109-5120
16.Timar J, Paterson H: Localization andproduction of proteo- glycans by HT1 080 cell lines withaltered N-rasexpression.
Cancer Letts 1990, 53:145-150
17. Bumol TF, Walker LE, Reisfeld RA:Biosynthesis studies of proteoglycans in human melanomacells withamonoclonal antibody to a core glycoprotein of chondroitinsulphate pro- teoglycans. JBiolChem 1984, 259:12733-12741 18. Harper JR, Reisfeld RA: Cell-associated proteoglycansin
humanmalignant melanoma.Biology of Proteoglycans. Ed- ited by Wight TN, Mecham RP.Orlando, NY, London, To- ronto,Acad. Press, 1987, pp 345-366
19. lozzo RV: Biosynthesis of heparan sulphate proteoglycan by colon carcinoma cells and itslocalizationatthe cellsurface.
JCell Biol 1984, 99:403-417
20. Steck PA, Moser RP, Bruner JM, Liang L, Freidman AN, Hwang T-L, Yung WKA: Alteredexpression and distribution of heparan sulphate proteoglycansin humangliomas. Can- cerRes 1989, 49:2096-2103
21. Timar J, KovalszkyI, Paku S, Lapis K, Kopper L: Two human melanoma xenografts with differentmetastatic capacity and glycosaminoglycan pattern. JCancer Res Clin Oncol 1989, 115:554-557
22. Ladanyi A, Timar J, PakuS, Molnar G, Lapis K: Selection and characterization of human melanoma lines with different liver- colonizing capacity. Int JCancer 1990, 46:456-461 23. Wasteson A: Amethod for thedetermination of the molec-
ular weight distribution of chondroitin sulphate. J Chro- matogr 1971, 59:87-89
24. Ross AH, Herlyn M, ErnstCS,Guerry D, Bennicelli J, Christ BFD, Atkinson B, Koprowsky H: Immunoassay for mela-
noma associated proteoglycan in sera of patients using monoclonal and polyclonal antibodies. Cancer Res 1984, 44:4642-4647
25. Glant TT, Mikecz K, Poole AR: Monoclonal antibodies to protein relatedepitopesofhumanarticularcartilage proteo- glycans.Biochem J 1986,234:31-41
26. Kopper L,BankfalvyA, MihalikR,Glant TT, Timar J: Proteo- glycan-targeted antibodies as markers on non-Hodgkin lymphoma xenografts. Cancer Immunol Immunother 1990, 32:137-142
27. Matsunaga E, Shinkai H, Nusgens B, LapiereCM: Acidic glycosaminoglycans, isolation and structural analysisofa proteodermatan sulphate from dermatosparatic calf skin.
Coll Rel Res 1986, 6:467-479
28. Caux F, Timar J, Lapis K, Moczar M: Proteochondroitin sul- phate inhumanmelanoma cell cultures.Biochem Soc Trans 1990,18:293-294
29. Garin-Chesa P, Beresford HR,Carrato-Mena A, Oettgen HF, Old W, MelamedMR, Rettig WJ:Cell surface molecules of human melanoma. Immunohistochemical analysis of the gp57,GD3 andmel-CSPG antigenic systems. Am J Pathol 1989,134:295-303
30. De VriesJE, KeizerGD,TeVeldeAA,Voodouw A, RuiterD, Rumke P, Spits H, FigdorCG: Characterization of mela- noma-associatedsurface antigens involved in the adhesion and motilityof human melanoma cells. Int J Cancer 1986, 38:465-473
31. Roberts DD: Interactions of thrombospondin with sulphated glycolipids and proteoglycans of human melanoma cells.
Cancer Res 1988,48:6785-6793
32. Caux F, Timar J, Moczar E, Lapis K, Moczar M: Cell asso- ciated glycosaminoglycans in human melanoma cell cul- tures.Biochem Soc Trans 1990, 18:294-295