University of Szeged Faculty of Pharmacy
Institute of Pharmaceutical Technology and Regulatory Affairs
Summary of the Ph.D. thesis
Investigation of passive and active permeation enhancement methods on skin permeation
Dr. Pharm. Nikolett Kis
Supervisors:
Dr. habil. Szilvia Berkó Ph.D.
Dr. habil. Anita Kovács Ph.D.
Szeged 2022
University of Szeged
Doctoral School of Pharmaceutical Sciences Head: Prof. Dr. Judit Hohmann D.Sc.
Educational Program: Pharmaceutical Technology Head: Prof. Dr. Ildikó Csóka
Institute of Pharmaceutical Technology and Regulatory Affairs Supervisors:
Dr. habil. Szilvia Berkó Ph.D.
Dr. habil. Anita Kovács Ph.D.
Nikolett Kis
Investigation of passive and active permeation enhancement methods on skin permeation
Complex examination committee:
Head: Prof. Dr. Piroska Szabó-Révész D.Sc., Institute of Pharmaceutical Technology and Regulatory Affairs, University of Szeged
Members: Prof. Dr. Ildikó Bácskay Ph.D., Department of Pharmaceutical Technology, University of Debrecen
Dr. habil. Zoltán Aigner Ph.D., Institute of Pharmaceutical Technology and Regulatory Affairs, University of Szeged
Reviewer committee:
Head: Prof. Dr. Zsolt Szakonyi, D.Sc., Institute of Pharmaceutical Chemistry, University of Szeged
Reviewers: Dr. Tamás Spaits, Ph.D., Egis Pharmaceuticals Plc.
Dr. Pálma Fehér, Ph.D., Department of Pharmaceutical Technology, University of Debrecen
Members: Dr. habil. Eszter Ducza, Ph.D., Institute of Pharmacodynamics and Biopharmacy, University of Szeged
Dr. habil Andrea Vasas, Ph.D., Department of Pharmacognosy, University of Szeged
SZEGED 2022
1. INTRODUCTION
The skin provides an attractive alternative of drug delivery to conventional drug administration. The preferred use of the dermal administration route stems from its advantages, e.g., the gastrointestinal tract and the first-pass metabolism of the liver can be avoided, patient adherence can be improved. However, the stratum corneum (SC), the outermost layer of the skin, sets an effective barrier against drug permeation. Thereby, getting through the SC is one of the greatest challenges for pharmaceutical technology.
Three permeation pathways can be distinguished, the intercellular, the transcellular, and the appendageal pathway, through which permeation can occur. For hydrophobic compounds, the diffusion pathway will mainly go intercellularly (through the extracellular lipids), while more hydrophilic compounds are expected to pass through a route through both corneocytes and extracellular lipids. The low permeability of SC can be related to its highly organized structure. However, there are different permeation enhancement techniques to overcome this skin barrier, using which the effectiveness of dermal drug administration can increase.
Permeation enhancement techniques can be categorized into passive and active methods.
This categorization is based on increasing skin permeability with or without providing the driving force of drugs. On the one hand, passive enhancement methods can increase the permeation with SC modification, for example, using chemical permeation enhancers, or with improved drug-vehicle attributes using specialized carriers (e.g., film-forming system (FFS)).
In contrast, active permeation enhancement techniques, such as electroporation, use external energy to reduce the skin barrier function, which allows a higher range of drugs to permeate.
The field of dermal permeation enhancement is emerging because they have an important role in developing effective dermal products in both the pharmaceutical and cosmetic industries.
These techniques are widely used to optimize skin permeation and the efficacy of dermal drug delivery; thus, their comprehensive knowledge is essential in ensuring the appropriate application during medical treatments.
2. EXPERIMENTAL AIMS
The aim of my Ph.D. work was to investigate passive and active dermal permeation enhancement techniques and use different methods alone and in combination to get an increased dermal permeation.
In the first part, dermally applicable semisolid in situ FFSs were formulated and investigated as a passive permeation enhancement method. The following aims were set:
• to identify and measure the critical parameters of FFSs using QbD approach;
• to form FFSs with or without silicones to examine the effects of silicones on the critical properties of FFS identified by QbD;
• to formulate an appropriate FFS as a drug delivery system for lidocaine-hydrochloride (LID-HCl), and investigate the effect of LID-HCl on the critical attributes of FFS;
• to investigate the permeation of LID-HCl into the skin from FFSs.
In the second part of my work, different glycols as CPEs were examined and applied in LID-HCl containing FFS. The aims were:
• to investigate how these molecules influence the mobility of SC lipids and protein;
• to relate these molecular effects to how these compounds may act as permeation enhancers when applied in a formulation with LID-HCl as a model drug;
• to apply glycol as a CPE in LID-HCl containing FFS, to improve the drug permeation from this drug delivery system.
In the third part, electroporation, as an active permeation enhancement technique was examined. The following aims were set:
• to examine the effect of EP on the skin barrier function under the applied circumstances;
• to develop a routine examination method to investigate the changes in permeation with fluorescence microscopy;
• to investigate the permeation enhancing effect of EP in combination with different dermal formulations on a model macromolecule;
• to investigate the molecular weight and the treatment time dependence of permeation enhancer effect of EP.
3. MATERIALS AND METHODS
3.1. Experiment part I – Investigation of film-forming systems 3.1.1. Quality by Design approach
Quality by Design (QbD) method begins with defining the targets and emphasizes product and process understanding and process control based on quality risk management. The QbD concept involves identifying the target product profile (TPP), the quality target product profile (QTPP), the critical material attributes (CMAs), the critical process parameters (CPPs), and the critical quality attributes (CQAs) of the product at the beginning of the development.
3.1.2. Preparation of film-forming systems
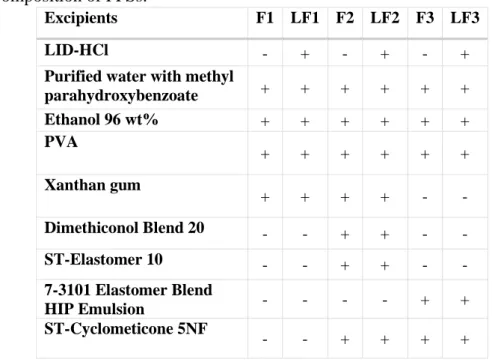
Lidocaine hydrochloride, poly(vinyl alcohol) (PVA), ethanol 96 wt%, xanthan gum, ST – Cyclomethicone 5-NF, Dimethiconol Blend 20, ST Elastomer 10, 7-3101 Elastomer Blend HIP Emulsion, and purified water with methyl parahydroxybenzoate were used.
During this research work, three different types of blank (F) and drug-containing (LF) formulations were prepared (Table 1). 5 wt% LID-HCl was dissolved in ethanol 96 wt%. PVA was dissolved separately in purified water at 80 °C under mixing. The two solutions were mixed at room temperature. Then, xanthan gum was added to formulations to ensure the required consistency. Finally, silicones were added slowly and mixed with a high shear mixer. The F2, LF2 contained 25 wt%, while the F3, LF3 contained 50 wt% silicone; however, the amount of volatile silicone was the same in these formulations.
Table 1. Composition of FFSs.
Excipients F1 LF1 F2 LF2 F3 LF3
LID-HCl - + - + - +
Purified water with methyl
parahydroxybenzoate + + + + + +
Ethanol 96 wt% + + + + + +
PVA + + + + + +
Xanthan gum
+ + + + - -
Dimethiconol Blend 20 - - + + - -
ST-Elastomer 10 - - + + - -
7-3101 Elastomer Blend
HIP Emulsion - - - - + +
ST-Cyclometicone 5NF
- - + + + +
3.1.3. Characterization of the critical parameters of the semisolid system 3.1.3.1. Investigation of drying time
PVA-based artificial skin was used as a model skin surface to detect the drying time of the preparations. A microscope slide was touched to the top of the film, and if no mark was left on the slide, the film could be considered dry. The elapsed time was measured with a timer.
3.1.4. Characterization of the critical parameters of the films 3.1.4.1. In vitro drug release and permeation studies
A Franz diffusion cell system (Logan Automated Dry heat sampling system) was applied during the experiments. The drug release was investigated through a cellulose acetate synthetic membrane, while the drug permeation was examined through heat-separated human epidermis (HSE). The drug concentration was measured by high-performance liquid chromatography (HPLC) (Shimadzu Nexera X2 UHPLC). To the further examination of drug permeation, Raman spectroscopy was applied on full-thickness human subcutaneous-fat-free abdominal skin. The exposition time was 30, 60, and 180 minutes. After the treatment, the films were
pulled down and the residues were wiped. The treated skins were frozen and sectioned (Leica CM1950 Cryostat), then Raman spectroscopic analysis was carried out with a Thermo Fisher DXR Dispersive Raman Spectrometer.
3.1.4.2. Investigation of the film mechanical properties
TA.XT plus Texture Analyzer was used to carry out the skin adhesion, film burst strength, and film flexibility studies. A 90-degree peel rig was used in the skin adhesion test, and a film support rig was applied in the film burst strength and film flexibility tests. Sticking plaster was used as a reference for skin adhesion and HSE for film burst strength and flexibility.
3.1.5. Statistical analyses
One-way ANOVA analysis of variance (Dunnett) with GraphPad Prism 8 for Windows software (GraphPad Software Inc., La Jolla, CA, USA) was applied. Four parallel measurements ± SD were carried out. The differences were significant if p< 0.0001****, p<
0.001***, p< 0.01**, p< 0.05* versus the control.
3.2. Experiment part II – Investigation of chemical permeation enhancer glycols 3.2.1. The effect of glycols on the molecular mobility of SC
Dipropylene glycol (diPG), propylene glycol (PG), and 1,3-butanediol (BG) were used.
Hydration of SC and corneocyte samples was performed by adding purified water. Polarization transfer solid-state NMR (PT ssNMR) (500 NMR spectrometer, Bruker NEO console, Bruker E-free 4 mm magic angle spinning probe) was applied to obtain qualitative information about the molecular mobility of SC components. The method consists of three experiments: DP (direct polarization), CP (cross polarization), and INEPT (insensitive nuclei enhanced by polarization transfer). The operating parameters were 500 and 125 MHz resonance frequencies of 1H and
13C, respectively. In-house Matlab code partially derived from matNMR was used for data processing. 20 mg of dry SC was mixed with 10 wt%, 20 wt%, and 30 wt% of different glycols, then 40 wt% water was added to the SC (the concentrations are based on the dry weight of SC).
The samples were placed in a 97% RH desiccator and incubated at 32 °C for 24 hours before the measurements to reach an equilibrated hydration condition.
3.2.2. The effect of glycols on skin permeation
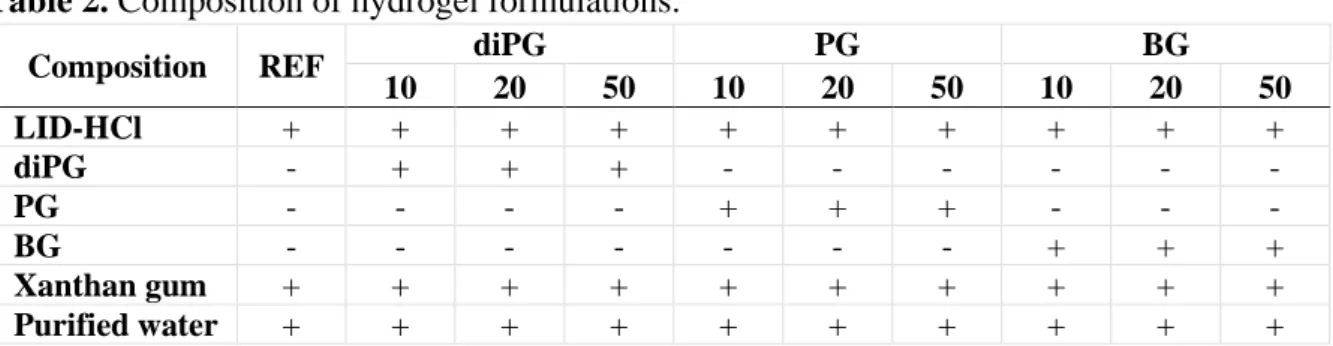
Four types of hydrogels were prepared (Table 2). 5 wt% LID-HCl was used as a model drug. The reference formulation (REF) did not contain any permeation enhancer. In the others, glycols – diPG, PG, and BG – were used as CPEs at concentrations of 10 wt%, 20 wt%, and 50 wt%. LID-HCl was dissolved in the glycol, while xanthan gum was dissolved in the purified water separately to ensure the appropriate consistency. Then, the two phases were mixed.
Table 2. Composition of hydrogel formulations.
Composition REF diPG PG BG
10 20 50 10 20 50 10 20 50
LID-HCl + + + + + + + + + +
diPG - + + + - - - - - -
PG - - - - + + + - - -
BG - - - - - - - + + +
Xanthan gum + + + + + + + + + +
Purified water + + + + + + + + + +
Raman measurement was carried out on full-thickness human skin samples to examine skin permeation as described in 3.1.4.1., except that the skin sections were placed in a thermostat at 60% RH and 32 °C for 1 hour before the treatment. Then, the skin samples were treated for 3 hours at 60% RH and 32 °C. During the Raman mapping, a 200x500 μm area of skin was captured. ImageJ software was used to evaluate the color intensity of skin maps. The relative intensity was calculated compared to the skin treated with REF formulation.
3.2.3. Preparation and investigation of FFSs containing glycols
Four different formulations were prepared (Table 3). All formulations contained 5 wt%
LID-HCl as a model drug. The reference formulation (FFS0) did not contain any permeation enhancer, while in the other formulations, diPG, PG, and BG were used at 20 wt%. LID-HCl was dissolved in the mixture of glycol and ethanol 96 wt%, while PVA was dissolved separately in purified water at 80 °C under mixing. Then, the two solutions were mixed at room temperature. Finally, xanthan gum was added to the formulation.
Table 3. Composition of FFS containing glycols.
Composition FFS0 FFS1 FFS2 FFS3
LID-HCl + + + +
PVA + + + +
Ethanol 96 wt% + + + +
Glycol - diPG PG BG
Xanthan gum + + + +
Purified water with methyl parahydroxybenzoate + + + +
During the examination, the in vitro drug release was studied through cellulose acetate membrane, and the drug permeation was measured through HSE with Franz diffusion cell system. The treatment protocol was the same as described in 3.1.4.1., except that LID-HCl was detected by UV spectroscopy at 262 nm (BIO-TEK Instruments). Furthermore, Raman spectroscopy was also applied to study the drug permeation. Full-thickness human abdominal skin was treated with formulations for 3 hours according to the protocol described in 3.1.4.1., except that the skin samples were thermostat at 60% RH and 32°C for 1 hour before treatments, then they were placed on a heater providing 32 °C during to ensure the drying of FFSs.
3.3. Experimental part III – Investigation of the effect of electroporation 3.3.1. Protocol of EP treatment
Non-invasive dermal EP treatment was performed with Mezoforte Duo Mez 120905-D instrument. Modulation involves 900 V with a duration of 5 ms, followed by a 20 ms break.
The EP treatment lasted for 2 minutes. Then, the treatment time was increased to 4 and 6 minutes to examine the time dependence of EP.
3.3.2. The effect of EP on skin barrier function in vivo
The dorsal surface of 3-4-month-old male SKH-1 hairless mice were treated with EP during the experiments. Three parallel measurements were performed on three different mice. The change in the skin barrier function induced by EP was followed by measuring transepidermal water loss (TEWL) (Tewameter TM 300). The results are shown in percentage compared to the TEWL value of untreated skin ± standard deviation (SD).
3.3.3. The effect of EP and dermal formulations on skin permeation ex vivo 3.3.3.1. Preparation of different dermal formulations
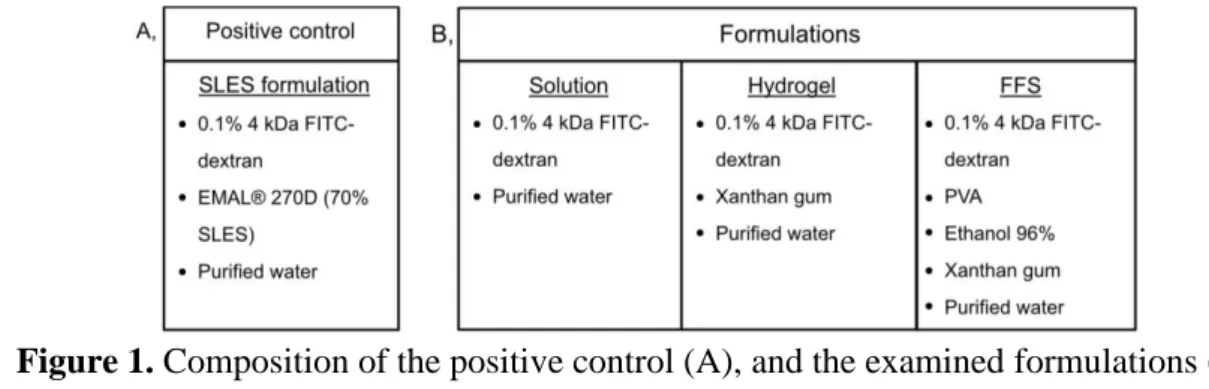
Fluorescein isothiocyanate-dextran (FITC-dextran) with an average MW of 4kDa, and EMAL® 270D (70 wt% sodium laureth sulfate (SLES)) were used during the formulation. In all compositions (Figure 1), 0.1 wt% FITC-dextran was used as a model macromolecule to detect its permeation under the fluorescence microscope. During the examination, sodium laureth sulfate (SLES) formulation was used to make a positive control. 0.1 wt% FITC-dextran was dissolved in 10 wt% purified water and dispersed in EMAL® 270D to 100 wt%.
Furthermore, solution, hydrogel, and FFS were made. In the case of the solution and the hydrogel, FITC-dextran was dissolved in purified water, and xanthan gum was added to the hydrogel. In the case of FFS, PVA was dissolved in water at 80 °C, and FITC-dextran and ethanol were added after it cooled down.
Figure 1. Composition of the positive control (A), and the examined formulations (B).
3.3.3.2. Examination of skin permeation
During the examination, full-thickness human abdominal skin was treated at room temperature. Firstly, the skin samples were treated only with the examined formulation with 5,
10, and 30 minutes of observation time, then, the combined effect of EP and the formulations were examined. EP was administered first on the skin surface for 2 minutes, then the examined formulation was applied on the treated skin for 5, 10, and 30 minutes of observation time. After the treatment, the skin samples were frozen and sectioned, then investigated with a fluorescence microscope (LEICA DM6 B) at room temperature under red fluorescence filter. Pictures about the untreated skin were also recorded to provide a negative control, and skin treated with SLES formulation was used as a positive control. ImageJ software was used to evaluate the color intensity of the pictures based on the shades. The relative intensity was calculated compared to the negative control (untreated skin). The relative intensity can be evaluated as a relevant change only if it is higher than 1.5 and the increase in intensity is also seen in the pictures.
3.3.4. Examination of molecular weight and treatment time dependence of EP
Texas Red conjugated dextran (TR-dextran), at average molecular weight of 3 kDa, 40 kDa, and 70 kDa were used to formulate 0.1 wt% solutions. The EP treatment was performed on human skin according to the protocol (3.3.1.). The skin samples were investigated with fluorescence microscopy, and the pictures were evaluated with ImageJ software (3.3.3.2.).
4. RESULTS AND DISCUSSION
4.1. Experimental part I – Investigation of film-forming systems
The aim of this experiment was to formulate and investigate semisolid, in situ FFSs containing LID-HCl using QbD approach.
4.1.1. Definition of QTPP, CQAs, CMAs, and CPPs for in situ FFSs
Firstly, the QTPP, the CQAs of both the semisolid composition and the film were considered, then the CMAs and CPPs were determined. Risk Estimate Matrix was used to evaluate the relationships between CQAs and QTPPs, then, Pareto chart was constructed to highlight the highly influenced parameters of product quality. Four highly influencing CQAs were identified: in vitro drug release, in vitro drug permeation, drying properties and film mechanical properties, which were investigated during the research. Furthermore, the following four material parameters of CPPs and CMAs had the highest influence on the target product:
permeation enhancing, drying, and film-forming excipients, and emollients.
4.1.2. Results of the critical parameters of the semisolid system 4.1.2.1. Drying time
During this study, the formulations were compared to each other, and to the blank formulations. Furthermore, the results were evaluated according to the requirements defined by QbD. The results of drying time showed that F1 needed approximately 15 minutes to dry, while
the drying time of F2 (10 minutes) and F3 (around 6 minutes) were lower, so the silicone content significantly decreased the drying time. Additionally, LID-HCl decreased the drying time of all compositions even more, but it was the most noticeable in the case of LF2.
4.1.3. Results of the critical parameters of the films 4.1.3.1. In vitro drug release
According to the target defined during the QbD method, 30% of the drug had to be released in 0.5 h. The results show that the diffused drug was lower than 30% in 0.5 hour, but more than 30% of LID-HCl was released in the case of LF2 and LF3 after 1 hour. After 3 hours, the diffused drug was more than 60% from all formulations.
4.1.3.2. In vitro drug permeation
The drug permeation rate (flux) was calculated from the Franz diffusion cell measurement, which showed that the LF1 had the lowest permeation rate (1.57 µg/cm2/h), however volatile silicone increased this property (LF2=4.29 µg/cm2/h, LF3=6.76 µg/cm2/h).
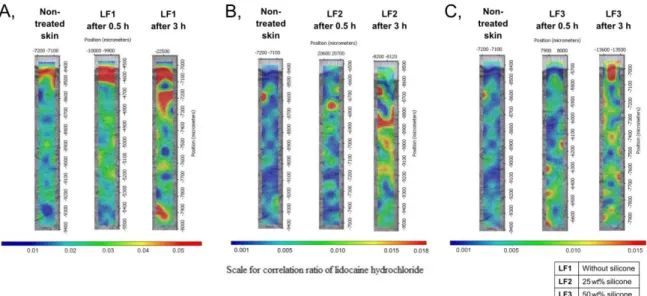
The in vitro skin permeation studies were also carried out with Raman spectroscopy. The correlation maps were established, which showed the distribution of LID-HCl containing FFSs.
A non-treated correlation map was used as a control during the evaluation. On the correlation maps (Figure 2), the warmer color indicates the higher amount of preparations in the skin layers.
Figure 2. Raman correlation maps of LF1 (A), LF2 (B), and LF3 (C).
The results showed that the preparations containing LID-HCl were detected in the different regions of the human skin. LF1 was found in the upper region of the skin, and LF2 permeated into the epidermis and the upper part of the dermis after 3 hours. LF3 could already be observed in the lower part of the dermis after 0.5 hour. After 3 h, this formulation was distributed in all skin layers. In conclusion, LF3 showed the fastest permeation rate, thereby the results of Raman mapping correlated with the measurements of the in vitro permeation study.
4.1.3.3. Mechanical properties of the films
The optimal mechanical properties ensure patient adherence and durable application.
During the evaluation, the formulations were compared to each other, and to the blank formulations. The results were also evaluated according to the requirements defined by QbD.
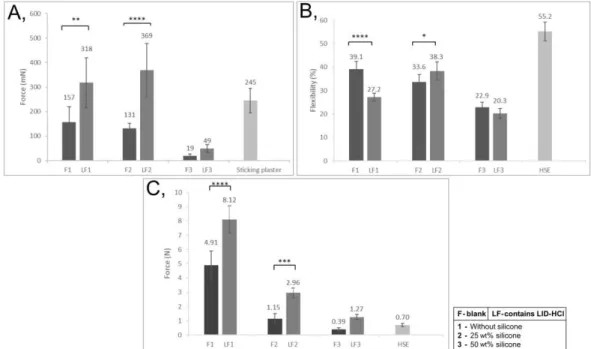
Figure 3. Mechanical properties of FFSs: Skin adhesion (A), Film flexibility (B), Film burst strength (C) (p < 0.05 *, p < 0.01 **, p < 0.001 ***, and p < 0.0001 ****).
The results showed that skin adhesion of LF1 and FL2 highly increased due to the LID- HCl content, so these parameters met our targets (100–500mN) (Figure 3A). In the case of LF1 and LF3, the drug decreased the flexibility of the films; however, the flexibility of LF2 increased (Figure 3B). The flexibility of LF1 and LF2 met the requirement (above 25%
compared to HSE), while LF3 showed lower flexibility. Film burst strength showed a significant increase in all cases when formulations contained LID-HCl, and in the case of LF1, this value was too high for our target (Figure 3C).
4.1.4. Summary of experiment I.
The LF3 (50% silicone content) showed the best permeation properties, while the LF2 (25% silicone content) had the best mechanical attributes. It can be concluded, that LID-HCl has a high effect on the formulation characteristics. The volatile silicone as a permeation enhancer improved the permeation rate of LID-HCl through the skin, while non-volatile silicones as emollients developed the mechanical properties of FFS.
4.2. Experimental part II – Investigation of permeation enhancer glycols
In this part of the research work, the effect of chemical permeation enhancer glycols was examined to detect how these molecules influence the molecular mobility and the structure of
SC lipid and protein components. Additionally, the connection between these molecular effects and the permeation enhancing properties of glycols were investigated.
4.2.1. Effect of glycols on molecular mobility
The PT ssNMR experiments provide information on the molecular mobility of lipids and proteins with close to atomic resolution. The DP spectrum (gray curve) shows the resonance from all carbons. The CP spectrum (blue curve) indicates rigid segments, while the INEPT spectrum (red curve) is seen for mobile segments of SC components and the added chemicals.
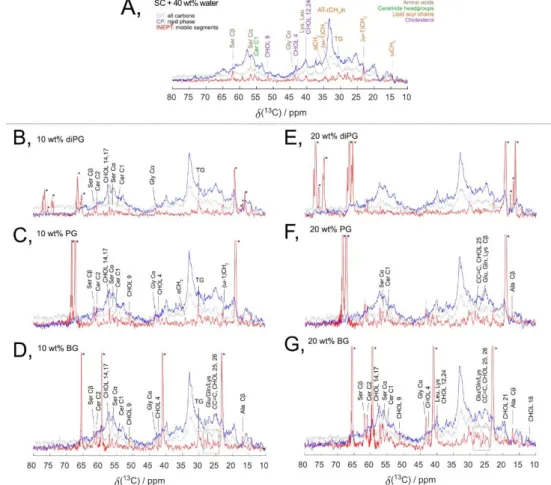
Figure 4. 13C MAS NMR spectra of intact SC hydrated at 97% RH (A) and treated with 10 wt% (B-D) or 20 wt% (E-G) of glycols and hydrated at 97% RH.
In all recorded spectra a broad high-intensity CP signal is present, which reveals that the major fractions of both SC lipid and protein components are rigid. The spectra for SC hydrated at 97% RH (Figure 4A) were used as a reference. The spectra in Figure 4B–D show that all the different glycols caused an increase in the mobility of SC components when added at 10 wt%.
However, PG and BG caused a strong increase in mobility of both the lipids as well as amino acids. DiPG shows a less strong effect in the lipids than the other glycols, with no observable effect on the amino acids. Then, the concentration of glycols was increased to 20 wt%. In the case of BG, the higher concentration (Figure 4G) led to an increase in the mobility of both lipid
and protein segments as compared to the BG at the lower concentration (Figure 4D). However, diPG and PG reached a saturation level below 20 wt% glycol and there was no observable difference in the SC mobility between the two doses investigated. To define the saturation limit of BG, an additional NMR measurement of SC treated with 30 wt% BG was performed, showing no additional mobility as compared to the sample with a lower concentration, thus suggesting that the saturation limit of BG in SC lies between 20 and 30 wt% in SC.
Previous studies have shown that the molecular mobility in keratin filaments is related to the presence of small polar compounds in NMF. To evaluate whether the observed mobility in the SC amino acids upon treatment with glycols stems from a similar mechanism as the effects observed after adding urea or lactate, we removed the water-soluble NMF components from SC through extraction in water. The NMF-depleted SC was thereafter treated with either diPG or PG. The results showed that the addition of diPG induces a small increase in the molecular mobility of the keratin filament, while PG has a much larger effect. This implies that the addition of glycols has an effect on the keratin filaments and not only on the free amino acids.
Similar trend was also observed for the lipids in the NMF-depleted SC, where the largest effect was shown for PG. The difference between diPG and the other two glycols is, that diPG has a bigger and more restricted structure, which may influence its partition into the SC segments.
4.2.2. Effect of glycols on skin permeation
In vitro drug permeation was examined using Raman spectroscopy. The relative intensity was calculated from the color shade of the correlation maps with ImageJ software, and the higher permeation is described by the higher relative intensity values. Conclusions are drawn only from relevant changes in the intensity, as this measurement is not sensitive enough to distinguish the small changes.
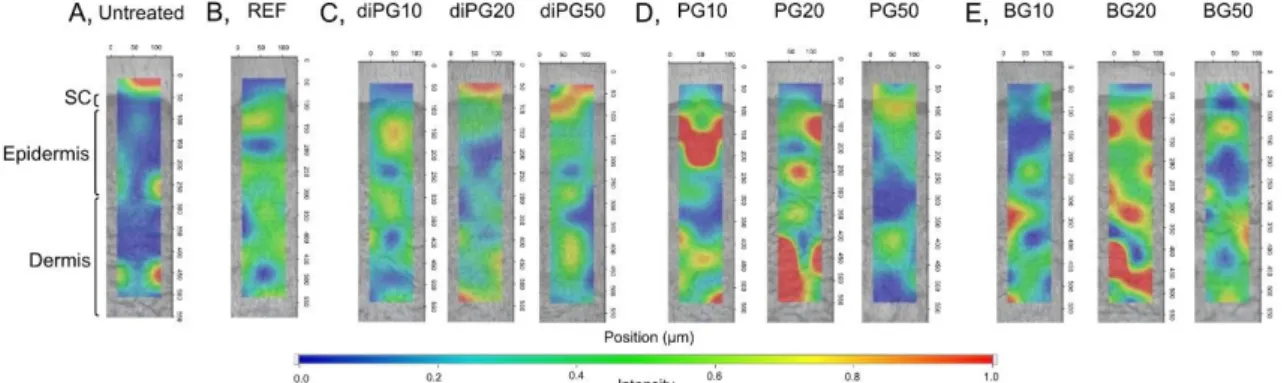
The correlation map showed that PG could increase the permeation already at 10 wt% in the upper part of the skin with 1.3 relative intensity (Figure 5D). At 20 wt%, the relative intensity was even higher (1.4), thereby indicating higher permeation of LID-HCl. The model drug can be noticed both in the epidermis and the examined dermal layers. For the higher glycol concentration, 20 wt% BG appeared as a more efficient permeation enhancer (Figure 5E) than PG, with 1.5 relative intensity. In this case, the correlation map showed a high LID-HCl permeation in all examined skin layers. Interestingly, the further addition of PG or BG (50 wt%) does not cause a further increase in the permeation, which can be explained by the substantial decrease in water activity. Finally, for diPG (Figure 5C), there are no distinguishable changes in the relative intensity at any concentrations compared to the REF.
Figure 5. Raman maps of untreated skin (A), and the skin treated with REF (B), diPG (C), PG (D), and BG (E) in 10, 20, and 50 wt%.
4.2.3. Combined effect of FFSs and CPEs on skin permeation
Glycols were used as CPE and emollient in the FFS at 20 wt% to examine their combined effect on the drug permeation. The in vitro drug release study showed that the drug release was slightly slower in the case of using PG (FFS2) and BG (FFS3) in the formulations at the initial times; however, the 100% of the drug released from all formulations in the examined period (24 h). The in vitro permeation study revealed that all glycols increased the permeation through HSE compared to the FFS without glycol (FFS0) significantly.
The in vitro drug permeation was also studied with Raman spectroscopy. On the correlation maps, a higher intensity in the lower skin layers can be observed in the case of FFS2 and FFS3 with 1.2 and 1.1 relative intensity, respectively. However, the map intensities and the relative intensity values of FFS1 were lower when diPG was used as a CPE. Based on these results, PG and BG have significant permeation enhancer effects in the FFSs.
4.2.4. Summary of experiment II
PG and BG have stronger effects on the SC molecular components (lipids, free amino acids, and keratin filaments), thus may be predicted a stronger permeation enhancer effect, where BG may be more efficient than PG in higher concentrations. The results of Raman spectroscopy correlated with the results of NMR. Furthermore, a non-monotonic response to glycol concentration could be observed, which may be explained by their effect on the SC components, and that the high concentration of glycols in formulations leads to a decrease in water activity.
So, the glycols cannot increase the drug permeation above this limit, rather the opposite.
Then the glycols were successfully applied in FFSs at 20 wt% concentration as CPEs, which were an efficient concentration to improve the permeation properties of FFSs.
4.3. Experiment part III – Investigation of the effect of electroporation
The aim of these experiments was to examine the effect of non-invasive dermal EP on the skin barrier function in vivo and investigate its permeation enhancing properties ex vivo.
4.3.1. Examination of the effect of dermal EP on the skin barrier function in vivo
We aimed to examine the changes in the skin barrier function caused by EP by measuring TEWL values and get information about how long it takes to get the ordered structure in the applied circumstances. The results showed that after the EP treatment, the TEWL values increased more than 500% abruptly. However, after 5 minutes, the TEWL was restored almost to the original condition.
4.3.2. The effect of EP and dermal formulations on the skin permeation ex vivo 4.3.2.1. Control experiments to fluorescence microscopy
The untreated skin was used as a negative control. In the case of positive control, pictures on the treatment with SLES solution containing FITC-dextran were taken without EP, where SLES provided the permeation certainly. The relative intensity (RI) was calculated from the color intensity of the pictures compared to the untreated skin, and the higher ratio predicts the higher permeation of the macromolecule. The results of the positive control studies showed a strongly increased light intensity after the SLES treatment (RI:1.8 in 5 min, 2.8 in 10 min).
4.3.2.2. The permeation enhancer effect of dermal formulations with EP
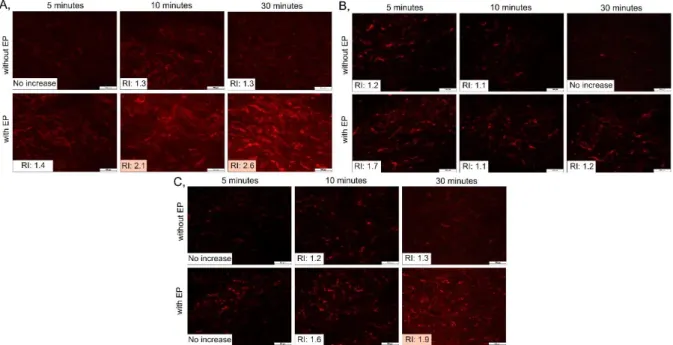
Firstly, the skin samples were treated only with the different dermal formulations (solution, hydrogel, and FFS) without EP; then another skin sample was treated with EP, and the formulation was put on the same skin surface to examine their combined effect. The pictures show the treatments with 5-, 10-, and 30-minutes observation time and the relative intensity compared to the untreated skin, which was calculated from the color intensity of pictures.
Significant increases are highlighted in orange (described in 3.3.3.2.).
There was no remarkable increase in the intensity during all treatments with formulations without EP; thus, significant permeation could not be reached passively, which can be explained by the high-molecular-weight dextran (Figure 6). Then, the combination of the EP and solution treatment was investigated (Figure 6A). With higher observation times, increased fluorescence intensity can be seen in the lower skin layers. After 10 minutes, the RI increased to 2.1, but the highest permeation was in 30 minutes of observation time (RI: 2.6). In the case of the EP and hydrogel treatment (Figure 6B), only the treatment with a 5-minutes observation time showed higher RI than 1.5; however, the picture did not show relevant increase in the intensity. These results can be explained by the composition of the formulation. The hydrogel contained xanthan gum to provide the gel consistency. However, xanthan gum is also a macromolecule, thereby it can interfere with the skin permeation of other macromolecules by retaining FITC-dextran in the formulation. In the applied concentration, the effect of xanthan gum was highly remarkable, so not even the EP treatment could ensure the permeation of FITC-dextran.
Finally, the combination of EP and FFS showed a significant increase in the fluorescence intensity after 30 minutes (Figure 6C), suggesting a higher presence of FITC-dextran in these skin layers. This formulation also contained xanthan gum, but its concentration was a third of the concentration in the hydrogel, so the composition allowed the release of FITC-dextran.
Figure 6. The skin treated with the solution (A), the hydrogel (B), and the FFS (C) containing FITC-dextran with or without EP and the relative intensity compared to the untreated skin.
4.3.3. Molecular weight and treatment time dependence of EP
Firstly, the skin was treated with just a solution containing 3, 40, or 70kDa TR-dextran with 30 minutes of observation time. Then, 2-minutes EP treatment was applied before the solution treatment. Just a slight or no permeation can be observed without EP; however, the intensity increased highly still in the case of the solution containing 70 kDa TR-dextran with EP. The intensity decreased with the increasing molecular weight; thus, the permeation rate may also be decreased. Then, the duration of EP treatment was changed (0, 2, 4, or 6 minutes).
The results showed that the maximum permeation rate could be assumed in the case of 4- minutes EP, and the further increase did not increase the intensity.
4.3.4. Summary of experiment III
The effect of EP on the skin barrier function was remarkable and reversible in vivo. The combined effect of different dermal formulations and EP showed just a slight skin permeation with passive diffusion. The EP could increase the permeation of a macromolecule remarkably;
however, the composition of the formulations highly influences the effect of EP. The solution and the FFS showed significant permeation in combination with EP, but the hydrogel inhibited the permeation due to the high amount of xanthan gum in the formulation. The molecular weight
dependence of EP showed a decreased permeation of the macromolecule with increasing molecular weight, but EP could increase the permeation of even the 70kDa-molecular-weight substance. The treatment time dependence of EP showed that with 4-minutes of EP, the permeation increased; however, with 6-minutes of EP, relevant increase could not be achieved.
THESIS FINDINGS
I. Investigation of FFS as a passive permeation enhancement method
Dermally applicable semisolid in situ FFSs were formulated and investigated by the QbD approach, which was applied for the first time in the development of FFSs. Texture Analyzer was applied to measure the mechanical properties of FFSs, which is a new measurement method for FFSs in the literature.
• The volatile silicone as a permeation enhancer improved the permeation rate of LID-HCl through the skin, while non-volatile silicones improved the mechanical properties of FFSs.
Furthermore, LID-HCl had a high influencing effect on the properties of these systems.
II. Investigation of chemical permeation enhancer glycols as a passive method
The effect of three well-known glycols, diPG, PG, and BG, was investigated on the SC lipids and proteins, and their permeation enhancing mechanism was defined.
• The examined glycols increased the mobility of SC lipids. PG and BG increased the mobility of amino acids both in the keratin filaments and the NMF free amino acids, thereby glycols appear to act similar manner as the NMF. Furthermore, a saturation level was detected for all glycols, after which the added glycols did not increase SC mobility.
• The permeation enhancer effect of glycols on a model drug was confirmed by Raman mapping, and these results correlated with the results of NMR. Additionally, a non- monotonic response to the concentration of glycol was observed.
• The glycols were applied in FFSs at 20 wt%, and they improved the drug permeation.
III. Investigation of the effect of non-invasive dermal EP as an active method
A routine examination method using fluorescent microscopy was developed to detect the permeation of a fluorescent material labeled model macromolecule.
• The effect of EP on the skin barrier was remarkable and reversible.
• The EP could increase the permeation of the model macromolecule from the dermal formulations, but the composition modified the permeation enhancer property of EP.
• The permeation of the macromolecule decreased with the increasing molecular weight;
however, EP could increase the permeation of the substances in all examined molecular weight. With the increasing treatment time of EP, the permeation of substances increased.
PUBLICATIONS RELATED TO THE SUBJECT OF THE THESIS
I. Nikolett Kis; Anita Kovács; Mária Budai-Szűcs; Attila Gácsi; Erzsébet Csányi; Ildikó Csóka; Szilvia Berkó: Investigation of Silicone-Containing Semisolid in Situ Film- Forming Systems Using QbD Tools
PHARMACEUTICS 11: 12 Paper: 660, 19 p. (2019) Q1, IF: 4.421
II. Anita Kovács; Nikolett Kis; Mária Budai-Szűcs; Attila Gácsi; Erzsébet Csányi; Ildikó Csóka; Szilvia Berkó: QbD-Based Investigation of Dermal Semisolid in situ Film- Forming Systems for Local Anaesthesia
DRUG DESIGN DEVELOPMENT AND THERAPY 2020: 14 Paper: 5059–5076, 18 p.
(2020) Q1, IF: 4.162
III. Nikolett Kis; Maria Gunnarson; Szilvia Berkó; Emma Sparr: The effect of glycols on molecular mobility, structure, and permeability in Stratum corneum
JOURNAL OF CONTROLLED RELEASE (2022) Q1, IF: 9.776, under publication IV. Nikolett Kis; Anita Kovács; Mária Budai-Szűcs; Gábor Erős; Erzsébet Csányi; Szilvia
Berkó: The effect of non-invasive dermal electroporation treatment on skin permeation JOURNAL OF DRUG DELIVERY SCIENCE AND TECHNOLOGY (2022), Q2, IF:
3.981, under publication
V. Kis Nikolett; Kovács Anita; Berkó Szilvia: In situ filmképző rendszerek, mint innovatív dermális gyógyszerforma,
GYÓGYSZERÉSZET (2022), IF: -, under publication
PUBLICATIONS NOT RELATED TO THE SUBJECT OF THE THESIS
I. Mónika Bakonyi; Szilvia Berkó; Anita Kovács; Mária Budai-Szűcs; Nikolett Kis;
Gábor Erős; Ildikó Csóka; Erzsébet Csányi: Application of Quality by Design principles in the development and evaluation of semisolid drug carrier systems for the transdermal delivery of lidocaine
JOURNAL OF DRUG DELIVERY SCIENCE AND TECHNOLOGY 44 Paper: 136–
145, 10 p. (2018) Q2, IF: 2.606
PRESENTATIONS RELATED TO THE SUBJECT OF THE THESIS
I. Nikolett Kis; Szilvia Berkó; Erzsébet Csányi: Examination of penetration through the skin by passive and active methods
I. Symposium of Young Researchers on Pharmaceutical Technology, Biotechnology and Regulatory Science, Szeged, 2019 (VP)
II. Kis Nikolett; Berkó Szilvia: Passzív és aktív penetrációfokozó módszerek hatása a bőr állapotára
II. Fiatal Technológusok Fóruma, Budapest, 2019 (VP)
III. Kis Nikolett; Csányi Erzsébet; Budai-Szűcs Mária; Kovács Anita; Gácsi Attila; Berkó Szilvia: A bőr fiziológiás paramétereinek változása passzív és aktív penetrációfokozó módszerek hatására
Gyógyszertechnológiai és Ipari Gyógyszerészeti Konferencia, Siófok, 2019 (PP) IV. Kis Nikolett; Kovács Anita; Csányi Erzsébet; Berkó Szilvia: In situ filmképző
rendszerek dermális alkalmazhatósága
Kozmetikai Szimpózium 2019, Budapest, 2019 (VP)
V. Nikolett Kis; Szilvia Berkó; Erzsébet Csányi: Investigation of semisolid in situ film- forming systems with QbD approach
II. Symposium of Young Researchers on Pharmaceutical Technology, Biotechnology and Regulatory Science, Szeged, 2020 (VP)
VI. Nikolett Kis; Szilvia Berkó; Erzsébet Csányi: Investigation of dermal semisolid in situ film-forming systems containing lidocaine hydrochloride with QbD approach
III. Symposium of Young Researchers on Pharmaceutical Technology, Biotechnology and Regulatory Science, Szeged, 2021 (VP)
VII. Nikolett Kis; Erzsébet Csányi; Mária Budai-Szűcs; Anita Kovács; Attila Gácsi; Szilvia Berkó: Investigation of physiological skin parameters influenced by penetration enhancer methods
12th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, Vienna, 2021 (PP)
VIII. Kis Nikolett, Kovács Anita, Berkó Szilvia: Dermális in situ filmképző rendszerek QbD alapú formulálása és vizsgálata
IV. Fiatal technológusok fóruma, 2021 (VP)
IX. Kis Nikolett: Glikolok, mint kémiai permeáció fokozó anyagok hatása a stratum corneum szerkezetre és permeabilitásra
XIV. Clauder Ottó Emlékverseny, 2021 (VP)
X. Nikolett Kis, Maria Gunnarsson, Emma Sparr, Szilvia Berkó: The effects of chemical permeation enhancer glycols on the skin
IV. Symposium of Young Researchers on Pharmaceutical Technology, Biotechnology and Regulatory Science, Szeged, 2022 (VP)
PRESENTATIONS NOT RELATED TO THE SUBJECT OF THE THESIS
I. Nikolett Kis: A bőr fiziológiás paramétereinek változása különböző hordozórendszerek és elektroporációs kezelés hatására
XXXIV. Országos Tudományos Diákköri Konferencia, Orvos- és Egészségtudományi Szekció, Debrecen, 2019 (VP)