ContentslistsavailableatScienceDirect
Journal of Materials Science & Technology
jou rn a l h o m e p a g e :w w w . j ms t . o r g
Research Article
Preparation of sulfur hydrophobized plasmonic photocatalyst towards durable superhydrophobic coating material
Emese Lantos
a, László Mérai
a, Ágota Deák
a, Juan Gómez- Pérez
b, Dániel Seb ˝ok
a, Imre Dékány
a, Zoltán Kónya
b, László Janovák
a,∗aUniversityofSzeged,InterdisciplinaryExcellenceCentre,DepartmentofPhysicalChemistryandMaterialsScience,H-6720,RerrichBélatér1,Szeged, Hungary
bUniversityofSzeged,InterdisciplinaryExcellenceCentre,DepartmentofAppliedandEnvironmentalChemistry,H-6720,RerrichBélatér1,Szeged,Hungary
a r t i c l e i n f o
Articlehistory:
Received6February2019
Receivedinrevisedform23March2019 Accepted15April2019
Availableonline16November2019
Keywords:
Nanocomposite Coatingmaterials Wetting
Lotus-typestructure Visiblelightphotocatalyst
a b s t r a c t
Thewidelyusedphotocatalyticself-cleaningcoatingmaterialsareoftenmadeofpolymersandpolymer basedcomposites,wherethephotocatalystimmobilizationoccurswithmacromolecules.However,these organicpolymersareoftenunstableunderexposuretoUVirradiationandeasilydegradedbyreactive radicalsproducedinthephotocatalyticreaction.Inordertosolvethisproblem,inthispaper,wepresent thefacilepreparationofamultifunctionalcoatingwithdualsuperhydrophobicandphotocatalyticprop- erties,wherethefixationandthehydrophobizationoftheplasmonicAg-TiO2photocatalystparticles withvisiblelightactivitywasperformedwithnon-watersolublesulfur,whichisacheapandeasily availablematerial.Theresultednovelnanocompositewithroughandnano-tructuredsurfaceroughness (1.25–2.45nmdeterminedbysmall-angleX-rayscattering)hassufficientlowsurfaceenergy(3.3mJ/m2) forsuperhydrophobic(=151.1◦)properties.Moreover,incontrastoftheorganicandexpensivefluo- ropolymerbasedcomposites,thisnon-wettingnaturewasdurable,becausethemeasuredwashigher than150◦duringthelong-termLED(max=405nm)lightirradiation.
©2019PublishedbyElsevierLtdonbehalfofTheeditorialofficeofJournalofMaterialsScience&
Technology.
1. Introduction
Photocatalyst-basedfunctionalsurfacesareoneofthestate-of- the-artmaterialsthatarecommerciallyappliedinseveralfields, including wastewater and air treatment or even food-packing.
After the discovery of the photo-induced superhydrophilicity of photocatalyst (e.g.,TiO2) layers [1], commercially applicable photofunctionalmaterialswithself-cleaningpropertiesweresyn- thesizedusingTiO2 particlesandcompositethinfilms.Sincethe bandgapof TiO2 fallswithintheUV A range, it ispossibleto extendtheabsorptionspectrumofthesemiconductorphotocata- lystsbymodifyingthecatalystparticleswith,forexample,different metallicornon-metallicelements[2–4].Thefunctionalizedcata- lystscanbeexcitedbytheUV Aorvisible(VIS)lightduetothe surfaceplasmonresonanceeffect:themetalnanoparticlesabsorb electromagneticradiationatlowerenergywavelengths[4,5].
The incorporation of semiconductor photocatalyst particles in an appropriate polymer-ased support or binder material is expected to create antimicrobial and self-cleaning properties,
∗Correspondingauthor.
E-mailaddress:janovakl@chem.u-szeged.hu(L.Janovák).
whichwouldextenditsfield ofapplication.Thestate-of-the-art materialsattheareaofphotocatalyst/polymercompositelayers arethefunctionalsurfaces,whicharegainingattentionfordifferent practicalfieldsofapplication,suchasfoodpackingmaterialswith antibacterialbehaviours [6]and self-cleaning superhydrophobic coating[7,8].
Sincetheirdiscovery,superhydrophobicsurfaces(≥150◦,low contactanglehysteresis)areinfocusowingtotheirlowsurfacefree energyandbeneficialself-cleaningnature.Besidestheoriginally hydrophobicnatureofthematerial,asurfacehastobetextured inboththemicro-,andnano-scaletopossesssuperhydrophobic characteristics[9].Therearenumerousreportedwaystoproduce morphologicallyproper surfaces,including lithographic,chemi- caletching,templatesandevenspray-coatingmethods,whichis cheap,scaleable,andeasilyimplementable.Inthiscase,particulate material(e.g.,aphotocatalyst)getssprayedonasubstrate,forming thedesiredsurficialstructureswithadequatesurfaceroughness.
Ifhydrophobizedphotocatalystsareusedasroughnessenhancer particulate materials,it resultsin theformation ofbifunctional compositesurfaceswithdualphotocatalyticandextremewetting properties[10–13].
Whenpolymerimmobilizedphotoreactivehybridsurfacesare prepared,thephotodegradationofthematrixalsohastobetaken
https://doi.org/10.1016/j.jmst.2019.04.046
1005-0302/©2019PublishedbyElsevierLtdonbehalfofTheeditorialofficeofJournalofMaterialsScience&Technology.
160 E.Lantosetal./JournalofMaterialsScience&Technology41(2020)159–167
intoconsideration,sothereportedlyappliedhydrophobicorganic polymers(e.g.perfluorynatedpolyacrylates,orPTFE)maybeinap- propriateforlongtermuses.Becauseofthis,thedemandishighfor durablematrixmaterials.Tosatisfytheneedsforincreaseddura- bility,sulfuralsocanbeappliedasaninert,non-photodegradable andinsolubleinorganichydrophobizingagent.Therearevarious applicationofelementalsulfurandsulfurnanoparticlesnowadays suchasagrochemicalindustries[14]fungicidesinagriculturefields [15],modificationofcarbonnano-tubes[16],antibacterialmate- rial[17], andsynthesisofnanocompositesforlithium batteries [18].However,accordingtoourbestofknowledge,thisisthefirst demonstrationofutilizingsulfurnanoparticlesasinerthydropho- bizingagentforcreationofsuperhydrophobicandphotoreactive functionalsurface.
Thus, in this paper, we present a simple and cheap spray- coatingmethodtopreparenon-photodegradableVISlight-active andmicro/nanostructuredsuperhydrophobicsurfacesoninorganic sulfurbasis:theVISlight-activityowestothe10wt%plasmonicAg- TiO2 photocataystcontent,whilethedurablesuperhydrophobic natureisattributed tosulfur-nanoparticles,prepared bya sim- pleprecipitationmethodinaqueousmedium.Thesebifunctional inorganiccompositelayerswithlong-termdurabilityshowedcon- siderable photocatalytic efficiency duringEtOH (g) degradation testsatsolid/gasinterface.
2. Materialsandmethods
2.1. PreparationofthesulfurhydropobizedAg-TiO2layers
The VIS light-active plasmonic Ag-TiO2 photocatalyst with 0.5wt% surfacesilver nanoparticle contentwassynthesized via thedirectfunctionalizationofTiO2particles.Thesyntheticmethod andtheopticalcharacterizationofthecatalystaredetailedinour previouspublication[4,5].
Toobtainsulfurnanoparticles,0.03gofsulfurpowder(Sigma Aldrich) wasdissolved in 4ml of 10M aqueousNaOH-solution (pH=14.15)atatemperatureof80◦C.Afterwards,theliquidwas stirredthendilutedwith1mlof10MNaOHsolution,then4.65ml of2.5MH2SO4solutionwasaddeddropwiseuntilthedispersion becameturbid(pH=13.95atprecipitation)duetotheformedsulfur nanoparticles(c≈0.09g/100ml).
Theresulting precipitatedsulfur wascentrifuged (8000rpm, 5min),thoroughlywashedwithdistilledwateranddriedovernight in a drying cabinetat 60◦C. Themixing of theobtained sulfur nanoparticlesandthepreviouslysynthetizedAg-TiO2 photocata- lystoccurredinaqueousdispersion(c=0.01g/100ml).Thesulfur/
photocatalystratio(0,40,60,80,85,90and100wt%S-content)was systematicallychangedduringthepreparation.
To prepare 5cm×5cm photoreactive inorganic layers con- sisting of 10wt% Ag-TiO2 photocatalyst and 90wt% sulfur nanoparticles,theaqueousdispersions wereevenly sprayedon glasssubstrates,usinganR180-typeairbrushspraygunatanoper- atingpressureof3bar.
2.2. Methodsofsamplecharacterization
Thesizedistributionandmorphologyofthepreparedsulfur,and theAg-TiO2/Scompositenanoparticleswerecharacterizedduring highresolution transmissionelectronmicroscopy(HRTEM).The appliedFEITecnaiG220X-TWINmicroscope,isequippedwitha tungstencathode(200kVaccelerationvoltage).
SurfacemorphologyoftheAg-TiO2/Slayerswasexaminedwith ascanningelectronmicroscope(SEM,HitachiS-4700,secondary electrondetector,accelerationvoltage:10or20kV),whilethesur- ficialcompositionwasstudiedusingaRöntecEDSdetector.
OpticalcharacterizationbydiffusereflectanceUV–VISspectra wererecordedviausingaCHEM2000UV–VIS(USB2000+UV-VIS, OceanOpticsInc.,Dunedin,FL)spectrophotometer,equippedwith anintegratedsphere.
Thesurfacefractaldimensions(DS)andsurfaceroughness(SR) ofthesampleswereinvestigatedbysmall-angleX-rayscattering (SAXS)technique.SAXScurveswererecordedwithaslitcollimated Kratkycompactsmall-anglesystem(KCEC/3Anton-PaarKG,Graz, Austria)equipped witha position-sensitive detector(PSD 50M fromM.BraunAG.Munich,Germany)containing1024channels, 54minwidth.CuK˛radiationwasgeneratedbyusingaPhilips PW1830X-raygeneratoroperatingat40kVand30mA.TheKratky camerawascalibratedusingsilverbehenatewithawell-defined lamellarstructure(d=5.848nm).
Surface charge values of the photocatalyst and the sulfur suspensionsweremeasuredbymeansofaparticlechargedetec- tor (PCD-04 Particle Charge Detector; Mütek Analytic GmbH, Germany)withmanualtitration.Underatitrationprocessthepos- itivesurfacechargeoftheAg-TiO2photocatalystsparticles(in0.01
%aqueousmedium,pH=5.0setbyHCl)willbecompensatedwith negativelychargedsulfurnanoparticles(in0.09%aqueousmedium, pH=5.0 setbyHCl)withconcomitantstreamingpotentialmea- surements.
Composition-dependent crystalline properties were studied usingaPhilipspowderX-raydiffractometer(PW1830generator, PW1820goniometer,CuK˛:=0.1542nm,40–50kV,30–40mA, 2:2◦–70◦,25.0±0.5◦C).
TheRamanspectrawererecordedwith1.5cm−1interferometric resolutionapplyingaSenterra(Bruker)Ramanmicroscopeunder 50xmagnificationwith2.50mWoutputpowerofthe=532nm He–Nelaserlightsourceand3000msintegrationtime.Thepre- sentedspectraarethecoadditionsof5singlespectrainallcases, recordedatdifferentspotsofthesample.
Theapparentstaticcontact anglesonAg-TiO2/Slayerswere measuredaccordingtothesessiledroptechniqueat25.0±0.5◦C under atmospheric pressure, applying an EasyDrop drop shape analysissystem(KrüssGmbH,Hamburg,Germany)equippedwith DSA100software,aPeltiertemperaturechamberandasteelsyringe needleof0.5mmdiameter,andusingdistilledwaterasatestliquid.
Beside thestatic contact anglesthedynamic wetting ofthe compositeswasalsomeasuredbecauseaccordingtothetheoryof DrelichandChibowski[13,19]themeasuredadvancing(adv)and receding(rec)contactanglesaresuitablefortheestimationofthe totalapparentsurfacefreeenergy(stot)ofthelayer,knowingthe surfacetensionoftheprobeliquid,(l=72.1mN/minthecaseof distilledwaterat25◦C)anditscontactanglehysteresis,whichis definedasthedifferencebetweentheadvandrec,asshownby Eq.(1):
stot= 1
1+cosadv 22+cosrec+cosadv
(1)
TostudythephotocatalyticpropertiesoftheAg-TiO2/Slayers, ethanol(EtOH)degradationtestswererununderblueLEDlight (GeneralElectric’s,Veresegyház,Hungary,=405nm)illumination atsolid/gasinterface,whilegaschromatographicmeasurements werecarriedout(ShimadzuGC-14B)toquantifytherateofEtOH degradation.Duringthemeasurements,5lofEtOH(abs.,Molac Chemicals,Hungary)wasinjectedintothereactionchambercon- taining25cm2(25.0±0.5◦C)hybridlayers(5mg/cm2),fixedata 5cmdistancefromthelightsource.Thec/c0valuescalculatedfrom peakareasweredeterminedasthefunctionofilluminationtime, wherecistheconcentrationofEtOHattime(t)andc0istheinitial concentration(c0=0.36mM).Theapparentreactionrateconstants (k,min−1)werecalculatedforfirst-orderdecayasshownbythe equation:ln(c/c0)=−kt.
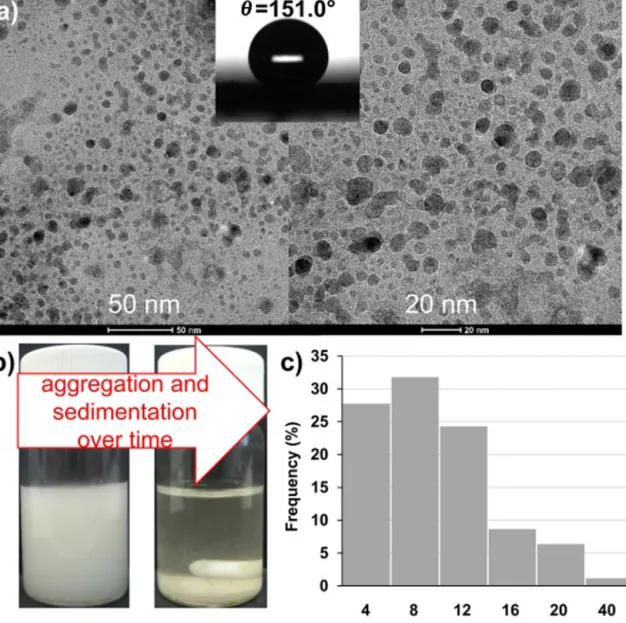
Fig.1.TEM-micrographsofprecipitatedsulfurnanoparticlesandwettingofspray-coatedS-nanoparticlelayerswithdeionizedwaterasatestliquid(a),aqueousdispersion ofpreparedsulfurnanoparticlesuponprecipitationand24hlater(b),size-distributionofprecipitatedsulfurnanoparticles(c).
During the stability tests, photoreactive composite layers of 5mg/cm2specificmasswereilluminatedfor14daysbythesame blueLED-light(=405nm)placedata5cmdistancefromthesam- ples, underambientatmosphere. Thechanges in water contact angleofthelayerswasmeasureddailyduringtheexperiment.Poly- merbasedcompositethinfilmswith60wt%incorporatedAg-TiO2
particleswereusedasreference.
Similartopreviouswork[4],theadhesivetapetestwasusedto investigatethemechanicalpropertiesofthepreparedcomposite films.Briefly,thedouble-sidedadhesivefoamtape(1.6mmthick) wasadheredtothefilmsfirstly,anda100gweightwasrolledover thetapetoensureconsistency.Thetapewasthenpeeledbackat anangleofapproximately45◦.
3. Resultsanddiscussion
3.1. Structuralcharacterizationofcompositenanoparticles
AccordingtotheWenzelandCassie-Baxtermodels[20],toreach thesuperhydrophobicextremewettingcharacter,theprovisionof hierarchicalroughnesstoanaturallyhydrophobicsurfaceiscrucial.
Toachievethisgoal,sulfurnanoparticleswithamaximumdiam- eterof40nmweresynthesizedbyapplyingasimpleprecipitation method.Hencetherearemanyreportedbottom-upwaystopre- paresulfurnanoparticles,includingthesulfurvapourdeposition oncoldwater[21]ortheWeimarn-[22],andRaffo-methods[23], onlythelastoneoffersparticleyieldshigherenough(∼600g/l)in aqueousmediatobeindustriallyprofitable[24].Ourversionofthe appliedRaffo-methodisbasedonthefollowingchemicalreactions [25]:
S+6NaOH =Na2S2O3+2Na2S+3H2O (2) Na2S2O3+H2SO4=H2S2O3+Na2SO4 (3) xH2S2O3=yH2O+zSO2+H2SmO6 (4) H2SmO6=H2Sm-nO6+Sn(n>5) (5) Upon the acidification of S2O32−-containing solutions with sulfuric acid, SO2 and colloidal S forms. We obtainedS2O32−- containing solution as a result of dissolving elemental S in pH=14.15NaOH-solution at 80◦C, then S-nanoparticles witha maximum meandiameterof 40nm formedupon thefollowing additionof 2.5MH2SO4 solution,which is thecorestepof the
162 E.Lantosetal./JournalofMaterialsScience&Technology41(2020)159–167
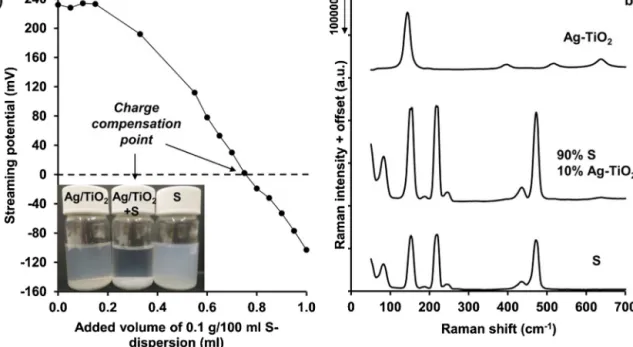
Fig.2. Measuredstreamingpotentialvaluesofthe0.01%Ag-TiO2photocatalystsuspensionduringtheadditionofthe0.09%suspensionofthesulfurnanoparticlesatapH of5.00(a)andtherecordedRamanspectraofAg-TiO2photocatalyst,sulfurnanoparticles,andAg-TiO2/Scompositesamplewithsuperhydrophobicwettingproperties(b).
classicalRaffo-method.AccordingtoEqs.(2–5),thedissolutionof sulfurin suchmediaisa disproportionationreaction,whilethe reverseprecipitationreactionissynproportionation.Asourprocess requiresbothelementalandprocessedS(H2SO4)andoffersgood yieldsitsindustrialapplicationcouldbebeneficialintheelimina- tionofresidualSasaby-productofthepetrolindustry.
Asshown in Fig. 1(a), the size distribution of theobtained sphericalsulfurnanoparticlesisrelativelypolydisperse.Themean diametersrange between1and 40nm,which is comparableto thesizeoftheappliedVISlight-activeplasmonicAg-TiO2photo- catalystparticles(dprim.∼25nm)[4,5].Becausethehydrophobic natureofthesulfurparticles,thestabilityoftheobtainedaqueous suspensionwasverylow.
Thesulfurnanoparticlesalsoshowsuperhydrophobiccharacter (=151.0◦)astheyareevenlydispersedonplainsurfaces(Fig.1(b)), because the formed sulfur layer consists of hydrophobic non- water-solublenanoparticleswithroughsurfaceinthenanometer scale.ThefixationoftheplasmonicAg-TiO2 photocatalystparti- clesonthesurfaceofthesulfurparticleswasexecutedthrough electrostaticinteractionbecausethepH-dependentsurfacecharge ofTiO2andAg-TiO2iswell-knownintheliteratureandithaspos- itivelychargedbelowpH=6(pointofzerocharge)[25],whilethe chargeofthesulfurnanoparticleswasslightlynegative(pointof zerochargeatpH=2[24]).Thus,thesurfacefixationwascarried outatpH=5becauseat thisvaluethecomponentshave oppo- sitecharge and theobtainedcharge titrationcurve achievedat thispHispresentedonFig.2(a).Itcanbeseenthatthepositive chargeoftheAg-TiO2 particleswascontinuouslydecreaseddur- ingtheadditionofthenegativelychargedsulfursuspension.The insertedphotosalsoshowthatatthechargecompensationpoint (streamingpotential=0)thestabilityoftheinitialAg-TiO2andsul- fursuspensionwasdrasticallydecreasedbecauseofthelost(i.e., compensated)chargeofthecomponentsandtheformationofthe heterocoagulatedS/Ag-TiO2system.
Fig.2(b)showstherecordedRamanspectraoforS,Ag-TiO2,and Ag-TiO2/Scompositenanoparticles.HencetheP25TiO2useddur- ingthesynthesisoftheplasmonicphotocatalystconsistof75wt%
anataseand25wt%rutile,onlythecharacteristicpeaksofanatase at144cm−1(Eg),397.5cm−1(B1g),517.5cm-1(B1g)and637.5cm-1
(Eg)could bedetectedupon =532nm laser-lightillumination [26].
Thepresence ofsilver isundetectableatthese experimental conditions,duetoitslowconcentration(0.5wt%).Inthecaseof sulfur samples,peaks(withthecorrespondingmolecularvibra- tionalmodes)at82.5cm−1(B2g),153cm−1(Eg),187.5cm−1(Eu), 219cm−1(A1g),244.5cm−1(A1g),435cm−1(B2g)and472.5cm−1 (A1g and Eg)are characteristics tothe vibrational bondsof S8- molecules[27,28], whilethepeak at51cm−1 belongs totheag
lattice-vibrational mode. Fig. 2(b) also shows, that if we form superhydrophobiccompositefromtheinitialsulfur,andAg-TiO2 nanoparticles, the intensity of sulfur peaks increases, while at 90wt% S-content, thecharacteristic peaks of rutile almostdis- appear, indicating the decreased surface concentration of the photocatalyst. However,no chemical interactionwas identified betweenthesulpurandtheAg-TiO2particlesevidencedbyRaman measurements.
Fig.3showsthepowderX-raydiffractogramsoftheresulting composites:asthesulfur-contentincreases,thecharacteristicpeak oftheanatasestructureofTiO2at2=25.32◦decreaseinintensity, whileotherpeaksappear,attributedtotheorthorhombic␣-sulfur (withhigherintensities:23.10◦,25.88◦,26.76◦,27.76◦,28.96◦)[29], makingtheidentificationofthephotocatalystmoredifficultdueto convolution.
Moreover,somepeaksarecharacteristicstomonoclinic-sulfur (27.74◦,31.08◦,37.84◦)[30].Theobservationsindicatethecrys- talline nature of theprecipitated sulfur-nanoparticles,since no broadbandofamorphousphaseweredetected.Ascanbeseenin theinsertedpicturesofFig.3,thetwocomponentspowdersamples havehighdispersibility inaqueousmediaunder 90wt% sulfur- content,butthis waterdispersibilitydrasticallydropsathigher sulfurcontents.Thisindicatesanoptimallyhydrophobicnatureand lowsurfacefreeenergytobeappliedinsurfacehydrophobization.
Besidetheabovepresented X-raydiffractogramsresults,the compositeswerealsocharacterizedbysmall-angleX-rayscatter- ing (SAXS) methodbecause thefractal plotof thesescattering curvesgivesreliableinformationaboutthequalityofthesurface roughness(SR)withaquantitativeinformationbasedonthesur- facefractal dimensions.The doublelogarithmic (fractal)plotof
Fig.3. PowderX-raydiffractogramsandaqueousdispersionsofAg-TiO2,SandAg- TiO2/S.
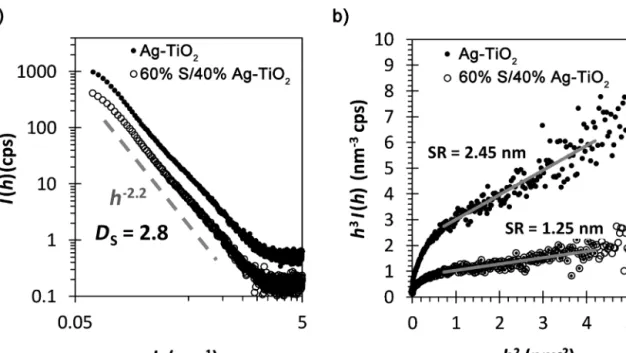
thescatteringcurvesofAg-TiO2 andAg-TiO2/Spowdersamples with60wt%S-contentshowsthatthesampleshavearatherrough surface,indicatedbytheDS=2.8(Fig.4(a)).
Furthermore,theh3I(h)vs.h2plotofthecurvesshowsapositive deviationfromtheasymptoticbehaviour(seeFig.4(a)),mainlyin thecaseoftheinitialAg-TiO2samplewhichindicatesasignificant interfacialelectrondensityinhomogeneity,i.e.surfaceroughness.
ThecalculatedvaluesareSR=2.45and1.25nminthecaseofAg- TiO2and40%Ag-TiO2/60%Ssamples,respectively(seeEq.E(6)in SupplementaryInformation):thesurfaceroughnessofthesam- plesdidnotdecreasedsignificantlyduringtheadditionofsulfur.
Becausethesurfaceroughnessisacrucialfactorofthesuperhy-
Fig.5.ApparentstaticwatercontactanglesonAg-TiO2/Scompositesurfacesasa functionofS-content.Thephotographillustratesthewater-repellentbehaviourof 90wt%S-containingcompositepowderfilmwithwaterdropcolouredwithmethy- lenebluedye.
drophobiccoatings,amoredetaileddescriptionofthecomposites structurecanbefoundinSupplementaryInformation(Figs.S1–S3).
3.2. Wettingandmorphologicalpropertiesandsurfacefree energydetermination
Theabovepresentedresultsindicatedthatthepresenceofthe sulfurnanoparticleschangedthewetting andstructuralproper- ties oftheAg-TiO2,whichwasalsoevidenced bycontactangle measurements(Fig.5).
As thesulfur content increases, thecomposite-covered sur- faces (spray-coated, with 1±0.5mg/cm2 specific layer mass) changedtheirwettingcharacterfromsuperhydrophilic(≈0◦)to superhydrophobic(>150◦).Thissulfurinducedsuperhydrophobic characterofthecompositelayeralsocanbeseenontheinserted photographinFig.5.Thewaterdropscolouredwithmethyleneblue
Fig.4. I(h)vs.h(a)andh3I(h)vs.h2(b)plotsofthescatteringcurvesofAg-TiO2andAg-TiO2/Spowdersampleswith60wt%S-content.
164 E.Lantosetal./JournalofMaterialsScience&Technology41(2020)159–167
Fig.6.Evolutionofadvancingandrecedingcontactanglesonspray-coated90wt%
sulfur-hydrophobizedphotoreactiveAg-TiO2layers,aswellasthedeterminedtotal apparentsurfacefreeenergy(␥stot)valuesbeforeandafter180minblueLEDlight illumination(=405nm).
dyewererolleddownfromthesurfaceofthesuperhydrophobic compositefilm.
Advancingandrecedingcontactangleswerealsomeasuredon thesuperhydrophobicfilmwith90wt%S-content,accordingtothe protocolofDrelich[13].ThedatawasanalysedaccordingtoEq.(1) ofChibowsky[19]toobtainthecorrespondingtotalsurfacefree energyvalues.
Fig.6showsalargecontactanglehysteresis(adv=146.8◦±3.0◦
→rec=30.1◦±0.6◦),indicatingtheWenzel-typeroughnesschar- acteristicsof the surface [19]. However, this superhydrophobic naturewithhighcontactangleandlowsurfaceenergywasdurable andresistanceforirradiation,becauseafterilluminatingthesam- plewithmax=405nmblueLED-lightfor180min,therecvalues showedamoderatedecrease(rec=19.4◦±2.0◦),whiletheadvval-
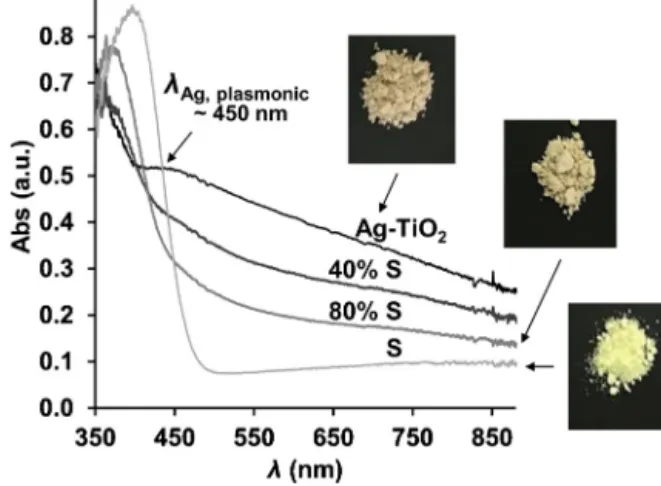
Fig.8.UV-VISdiffuse-reflectancespectraandphotographsofAg-TiO2,Snanopar- ticlesandAg-TiO2/ScompositeswiththecorrespondingS-content(giveninwt%).
uesremainedalmostthesame(148.2◦±2.5◦),andthecalculated total surfacefree-energyvalues of thesurfacesdecreased upon illuminationfrom3.3mJ/m2to2.3mJ/m2.
As evidenced by SEM-images and EDX-measurements, the surfaceofthespray-coated layersof90wt%sulfur-hydrophized Ag-TiO2photocatalysthascauliflower-likeaggregates(Fig.7)with relativelyhomogenousandevendistributionofthetwocompo- nents.The presence of both components onthe surface ofthe roughcompositefilmisadvantageousbecausethesulfurnanopar- ticlesareresponsibleforthesuperhydrophobicnature,whilethe presenceoffreephotocatalystparticlesensuresthephotocatalytic activity.Thismeansthatphotocatalyticreactionscouldtakeplace alloverthepreparedsurfaces.
3.3. Opticalandphotocatalyticproperties
Theopticalpropertiesofthehydrophobizedphotocatalystsam- pleswereslightlychangeduponsulfur-addition,whichcanbeseen intheUV-VISdiffuse-reflectancespectraonFig.8.Theabsorption
Fig.7.SEMmicrographsandEDX-mappingofAg-TiO2/Ssuperhydrophobicsurfaceswith90wt%S-content.
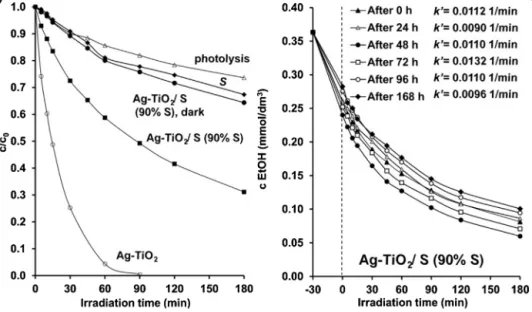
Fig.9.CharacterizationofEtOHdecomposition(c0=0.36mM)onpureAg-TiO2andsulphurlayers,andonAg-TiO2/Scompositefilm(90wt%S)underLEDlightillumination (=405nm)asthefunctionofilluminationtime.Thecompositelayerwasalsomeasuredunderdarkconditionforreference(a).EtOHconcentrationchangeonAg-TiO2/S filmafter0,24,48,72,96and168hcontinuousLEDlightirradiation(b).
wavelengthmaximagraduallyincreasefrom349to401nmasthe sulfurcontentisincreasedfrom0to100wt%,whiletheinitialpale browncolouroftheAg-TiO2graduallyturnedtoyellow.Theplas- monicabsorbancepeakataround450nmisduetothepresence ofAgNPsonTiO2photocatalystparticlesasdemonstratedinour previouspapers[4,5].Inthisplasmonicphotocatalyst,visiblelight isharvestedbecausethesurfaceplasmonresonance,attributedto theAgNPs,whilethemetal–semiconductorinterfacetakespartin theseparationofthegeneratedholesandelectrons[4].
ItwasalsopresentedearlierthattheusedblueLEDlightsource with405nmemissionmaximumissufficientforexcitationofthe Ag-TiO2catalyst[31,32].Inthisworkthephotocatalyticefficiency ofthesulfurhydrophobizedcompositephotocatalystlayerswere studiedusingthesameblueLEDlampandethanolasmodelvolatile organiccompound(Fig.9(a)).Asaresultof90minillumination,the pureAg-TiO2 photocatalystfilmdecomposedapproximatelythe wholeamount(c0=0.36mM)oftheEtOH,whilethephotocatalytic effectivenessofthesulfurcontainingsuperhydrophobiclayerwere lower,butitwasshownsignificantphotocatalyticactivitycom- paredtothereferencesample(photolysiswithoutphotocatalyst andS/Ag-TiO2 sampleindarkcondition).Thisdecreasedphoto- catalytic activityis becauseof thelower Ag-TiO2 photocatalyst content(only10%in90%non-photoreactivesulfur)butaccording tothewetting(Figs.5and6)andphotocatalyticresults(Fig.9), itcanbeclearly statedthatat acomposition of90%sulfur and 10wt%Ag-TiO2photocatalystthecompositelayerwasshownnot onlysuperhydrophobicbutalsophotocatalyticproperties.Inorder toprove thelong-term photoactivity of the composite sample, the EtOH concentration change was measured occasionally on S/Ag-TiO2 filmafter0,24,48,72, 96and 168hcontinuousLED lightirradiation.TheobtainedresultsinFig.9(b)representthat theinitial(c0=0.36mM)concentrationofEtOHwasdecreasedto 0.26±0.015mM after30minadsorption timeonthesurface of 25cm2compositefilmwith5mg/cm2specificsurfacemass.After thisadsorptionequilibriumtheEtOHconcentrationwascontinu- ouslydecreasedduringtheLEDlightirradiationandthecalculated k’values (0.0090–0.0132 1/min)indicatethealmostunchanged photocatalyticactivityofthecompositeduringthestudiedtime interval.
Fig.10.Effectoflong-termUV-A(blueLED)irradiationonthewettingproperties oftheorganicpolymer-basedandinorganicsulfur-containingsuperhydrophobic compositecoatings.
In ourprevious work it was reported that this dual super- hydrophobic and photocatalytic properties of the spray coated compositessurfacescouldbeveryusefulinvariousapplications, however,theundesiredself-photodegradationoftheearlierused organic(fluoro)polymerbindercouldhindertheapplicationofthis coating materials [4,33]. In this work,the hydrophobization of theAg-TiO2photocatalystparticleswasachievedbytheapplica- tionofinorganicsulfurinordertoavoiditsself-photodegradation.
Fig.10showsthatthemeasuredcontactanglevaluesofthesulfur hydrophobizedAgTiO2wasunchanged(∼150◦)duringthestudied timeinterval(14daysofcontinuousLEDlightirradiation).
Two polymer based composites were chosen as reference:
the Ag-TiO2/ polyacrylate hybrid layer contains poly(ethyl- acrylate-co-methyl methacrylate) copolymer [4], while the
166 E.Lantosetal./JournalofMaterialsScience&Technology41(2020)159–167
polymer component of the Ag-TiO2/fluoropolymer composite waspoly(perfluorodecylacrylate)fluoropolymer[31,32,34].Asa results,theinitialoftheAg-TiO2/polyacrylatehybridlayerwas 85.2◦±1.75◦ which wasdrasticallychanged to0◦ justafter two daysirradiation.The effectwasslowerinthecase of themore resistancefluoropolymerbasedbinderwithinitialsuperhydropho- bic(=151.1◦±5.4◦)properties.Thesedecreasingvaluesarethe resultsofthepreviouslyreportedpartialpolymerphotodegrada- tion:theilluminationchanges intheration ofcatalyst/polymer composition as the photocatalyst particles became uncoated [35]. This resulted in higher catalyst availability on the sur- face region. However, the sulfur hydrophobized photoreactive thin composite film shows durable hydrophobicity during the irradiation.
Finallythe mechanical properties ofthe preparedS/Ag-TiO2
film were also investigated and compared to the fluoropoly- mer based superhydrophobic thin film. The adhesive tape test was used to get information about theadhesion of the layers [4]. The tape was applied on the coating surface and subse- quently was removed by a quick pull. As indicate in Fig. S4, minoramountofsuperhydrophobic layercouldberemovedby the tape from both surfaces. However, this mechanical dam- agecaused thatboththefluoropolymerandsulfur basedlayers werelosttheirsuperhydrophobicity(seeinserted contactangle values) after the peeling. This vulnerability of superhydropho- bic surfaces is well known in the literature and it originated fromtheroughsurface andporousstructureof theselotus-like coatings[36,37]. However, in ourprevious paper we also pre- sentedthatthenatureinspiredself-healingabilityoftheartificial coatingscouldsolvethisproblem,ifwecanachievethatthedam- agedsurfaceprotrusionscanregenerateitself[32].Inthefuture, wetrytoimplementthisself-assembledmechanism(airhumid- itycatalysedsyneresisofthehydrophobicdodecyltrichlorosilane molecules) into the sulfur containing superhydrophobic layer in order to get thin film with quick superhydrophobic recov- ery.
4. Conclusions
To satisfy the needs for superhydrophobic coatings with increaseddurability,sulfuralsocanbeappliedasaninert,non- photodegradableand insolubleinorganichydrophobizingagent.
Inthispaperwe presentedasimple methodtoutilizesulfur in thearea of functional surfaces: bifunctional, superhydrophobic andphotocatalyticcoatingswerepreparedof90wt%(d≤40nm) sulfur-nanoparticles and 10wt% (d≈25nm) visible light-active Ag-TiO2 photocatalyst. The surface fixation of sulfur on Ag- TiO2 particles occurred through electrostatic interaction and althoughnochemical bondwasidentifiedbetweenthecompo- nents,theinitiallysuperhydrophylicphotocatalystnanoparticles (≈0.0◦)becamesuperhydrophobic(=150.3◦±1.0◦)uponform- ingcomposite particles.AsevidencedbyEDX-SEM,theextreme wettingpropertiesareattributedtothemultiscalesurfacerough- ness: the spray-coated layers have cauliflower-like aggregates with relatively homogenous distribution of the two compo- nents.
Thephotocatalyticactivity ofthesulfur containingsuperhy- drophobic layer waslower than that of the pureAg-TiO2 film (degraded 100% of c0=0.36M EtOH in 180min), still showing significant photoreactivity (photocatalytc effectiveness: 68.9%) comparedtothereferencesample(photolysiswithoutphotocat- alyst;effectiveness:26.4%).Thisdecreasedphotocatalyticactivity isbecausethelowerphotocatalystcontent(only10%)butaccord-
ingtothewettingandphotocatalyticresultsitcanbeclearlystated thatatoptimalcompositionthelayerwasshownnotonlysuper- hydrophobicbutalsophotocatalyticproperties.
As a main result, in contrast to the tested polymer-type hydrophobizingagents,sulfurnanoparticlesdidnotphotodegrade inthetimeintervalof14daysofcontinuousLED-lightillumina- tion(=405nm),sothehydrophobizedphotocatalystlayerskept theirwettingproperties(≈150◦).Inconclusion,astheexpensive fluoropolymerbinderscanbereplacedwithsulfurinself-cleaning superhydrophobicsurfaces.
Acknowledgements
ThisworkwasfinanciallysupportedbytheHungarianScien- tificResearchFund(OTKA)K116323,PD116224andtheprojectof GINOP-2.3.2-15-2016-00013,theUNKP-18-4NewNationalExcel- lence Programof theMinistryofHuman Capacitiesand bythe JánosBolyaiResearch ScholarshipoftheHungarianAcademyof Sciences and the Ministry of Human Capacities, Hungary (No.
20391-3/2018/FEKUSTRAT).
AppendixA. Supplementarydata
Supplementarymaterialrelatedtothisarticlecanbefound,in theonline version,at doi:https://doi.org/10.1016/j.jmst.2019.04.
046.
References
[1]A.Fujishima,T.N.Rao,D.A.Tryk,J.Photochem.Photobiol.CPhotochem.Rev.1 (2000)1–21.
[2]T.Boningari,S.N.R.Inturi,M.Suidan,P.G.Smirniotis,J.Mater.Sci.Technol.34 (2018)1494–1502.
[3]W.Liu,Y.Xu,W.Zhou,X.Zhang,X.Cheng,H.Zhao,S.Gao,L.Huo,J.Mater.Sci.
Technol.33(2017)39–46.
[4]Á.Veres,J.Ménesi,Á.Juhász,O.Berkesi,N.Ábrahám,G.Bohus,A.Oszkó,G.
Pótári,N.Buzás,L.Janovák,I.Dékány,ColloidPolym.Sci.292(2014)207–217.
[5]Á.Veres,T.Rica,L.Janovák,M.Dömök,N.Buzás,V.Zöllmer,T.Seemann,A.
Richardt,I.Dékány,Catal.Today181(2012)156–162.
[6]J.Xiong,M.Z.Ghori,B.Herkel,T.Strunskus,U.Schürmann,L.Kienle,F.Faupel, ActaMater.74(2014)1–8.
[7]Á.Deák,L.Janovák,E.Csapó,D.Ungor,I.Pálinkó,S.Puskás,T.Ördög,T.Ricza, I.Dékány,Appl.Surf.Sci.389(2016)294–302.
[8]F.Zhang,C.Zhang,L.Song,R.Zeng,S.Li,H.Cui,J.Mater.Sci.Technol.31 (2015)1139–1143.
[9]I.Dékány,L.G.Nagy,J.ColloidInterfaceSci.147(1991)119–128.
[10]M.Bleszynski,M.Kumosa,Compos.Sci.Technol.164(2018)74–81.
[11]K.Chen,W.Gou,XuLe,Y.Zhao,Compos.Sci.Technol.156(2018)177–185.
[12]Z.Caia,L.Shena,X.Wang,Q.Guo,Compos.Sci.Technol.164(2018)238–247.
[13]J.Drelich,Surf.Innov.1(2013)248–254.
[14]P.Devendar,G.F.Yang,Top.Curr.Chem.(Cham.)375(6)(2017)82–89.
[15]M.Ellis,D.Ferree,R.Funt,L.Madden,PlantDis.82(1998)428–439.
[16]J.Barkauskas,Res.Bull.42(2007)1732–1741.
[17]Sr.Choudhury,S.Roy,A.Goswami,S.Basu,J.Antimicrob,Chemother.67 (2012)1134–1137.
[18]W.Zheng,Y.W.Liu,X.G.Hu,C.F.Zhang,Electrochim.Acta51(2006) 1330–1340.
[19]E.Chibowski,Adv.ColloidInterfaceSci.103(2003)149–172.
[20]U.Eduok,O.Faye,J.Szpunar,Prog.Org.Coat.111(2017)124–163.
[21]A.Gutbier,Z.Anorg,AllergyChem.152(1926)163.
[22]P.P.vonWeimarn,Kolloidchem.Beihefte22(1926)38.
[23]M.Raffo,Kolloid-Z2(1908)358.
[24]R.Steudel,Top.Curr.Chem.230(2003)153–166.
[25]T.Preocanin,N.Kállay,Croat.Chem.Acta79(2006)95–106.
[26]U.Balachandran,N.G.Eror,J.SolidStateChem.42(1982)276–282.
[27]B.Meyer,Chem.Rev.76(1976)367–388.
[28]M.Ritz,L.Vaculíková,J.Kupková,E.Plevová,L.Bartonová,Vibrat.Spec.84 (2016)7–15.
[29]A.Caron,J.Donohue,J.Phys.Chem.64(1960)1767–1768.
[30]A.G.Pinkus,J.S.Kim,J.L.McAtee,C.B.Concilio,J.X-Ray,Am.Chem.Soc.81 (1959)2652–2654.
[31]L.Mérai,Á.Deák,D.Seb"ok,E.Csapó,T.S.Kolumbán,B.Hopp,I.Dékány,L.
Janovak,ExpressPolym.Lett.12(2018)1061–1071.
[32]L.Mérai,N.Varga,Á.Deák,D.Seb"ok,I.Szenti,Á.Kukovecz,Z.Kónya,I.
Dékány,L.Janovák,Catal.Today328(2019)85–90.
[33]L.Janovák,Á.Dernovics,L.Mérai,Á.Deák,D.Seb"ok,E.Csapó,A.Varga,I.
Dékány,C.Janáky,Chem.Commun.54(2018)650–653.
[34]Á.Deák,L.Janovák,E.Csapó,D.Ungor,I.Pálinkó,S.Puskás,T.Ördög,T.Ricza, I.Dékány,Appl.Surf.Sci.389(2016)294–302.
[35]L.Janovák,Á.Deák,Sz.P.Tallósy,D.Seb"ok,E.Csapó,K.Bohinc,A.Abram,I.
Pálinkó,I.Dékány,Surf.Coat.Technol.326(2017)316–326.
[36]H.Xu,C.R.Crick,R.J.Poole,J.Mater.Chem.A6(2018) 4458–4465.
[37]T.Verho,C.Bower,P.Andrew,S.Franssila,O.Ikkala,R.H.A.Ras,Adv.Mater.23 (2011)673–678.