Excessive fuel availability amplifies the FTO-mediated obesity
risk: results from the TUEF and Whitehall II studies
Róbert Wagner 1,2,3, Ádám G. Tabák4,5, Ellen Fehlert1,2,3, Louise Fritsche1,2,3, Benjamin A.
Jaghutriz1,2,3, Róbert J. Bánhegyi6, Sebastian M. Schmid3,7, Harald Staiger2,3,8,9,10, Fausto Machicao10, Andreas Peter1,2,3, Hans-Ulrich Häring1,2,3, Andreas Fritsche1,2,3 & Martin Heni 1,2,3 Variation in FTO is the most important common genetic determinant of body weight. Altered energy metabolism could underlie this association. We hypothesized that higher circulating glucose or triglycerides can amplify the FTO impact on BMI. In 2671 subjects of the TUEF study, we investigated the interaction effect of fasting glucose and triglyceride levels with rs9939609 in FTO on BMI. We analysed the same interaction effect by longitudinally utilizing mixed effect models in the prospective Whitehall II study. In TUEF, we detected an interaction effect between fasting glucose and fasting triglycerides with rs9939609 on BMI (p = 0.0005 and p = 5 × 10−7, respectively). The effect size of one risk allele was 1.4 ± 0.3 vs. 2.2 ± 0.44 kg/m² in persons with fasting glucose levels below and above the median, respectively. Fasting triglycerides above the median increased the per-allele effect from 1.4 ± 0.3 to 1.7 ± 0.4 kg/m2. In the Whitehall II study, body weight increased by 2.96 ± 6.5 kg during a follow-up of 13.5 ± 4.6 yrs. Baseline fasting glucose and rs9939609 interacted on weight change (p = 0.009). Higher fasting glucose levels may amplify obesity-risk in FTO carriers and lead to an exaggerated weight gain over time. Since weight gain perpetuates metabolic alterations, this interplay may trigger a vicious circle that leads to obesity and diabetes.
Due to its increasing global prevalence, obesity has become a major health problem worldwide. Since it is strongly linked to insulin resistance, obesity increases the disease burden of type 2 diabetes. The pathogenesis of obesity comprises an intricate interplay of genetic and environmental factors. For the genetic contribution, around a hundred single nucleotide polymorphisms (SNPs) are reported to be associated with BMI1. However, the aggre- gate influence of these known variants explains the heritability of obesity to a small extent (~2.7%) only1. Genetic effects can show striking variation, depending on the metabolic environment which, in turn, reflects lifestyle or other genetic factors. Interactions between environmental factors and genetic loci might therefore account for some of the variability2. The impact of the common genetic variant in FTO, the polymorphism most strongly and consistently associated with obesity, is modulated by such factors. Physical activity, aerobic fitness, and diet interact with the FTO variant on obesity or the change of body weight over time3–6. For other diabetes-related genetic variants, such as TCF7L2, glycemia is an important interacting factor modulating the SNP’s effect7. We
1Department of Internal Medicine IV, Division of Endocrinology, Diabetology, Nephrology, Vascular Disease and Clinical Chemistry, University Hospital of Tübingen, Tübingen, Germany. 2Institute for Diabetes Research and Metabolic Diseases of the Helmholtz Centre Munich at the University of Tübingen (IDM), Tübingen, Germany.
3German Center for Diabetes Research (DZD), Neuherberg, Germany. 4Department of Epidemiology and Public Health, University College London, London, UK. 51st Department of Medicine, Semmelweis University Faculty of Medicine, Budapest, Hungary. 6Pándy Kálmán Hospital, Gyula, Hungary. 7Department of Internal Medicine I, Division of Endocrinology, Diabetology, and Metabolism, University Hospital of Lübeck, Lübeck, Germany. 8Interfaculty Centre for Pharmacogenomics and Pharma Research at the Eberhard Karls University Tübingen, Tübingen, Germany.
9Institute of Pharmaceutical Sciences, Department of Pharmacy and Biochemistry, Eberhard Karls University Tübingen, Tübingen, Germany. 10Institute of Experimental Genetics, Helmholtz Centre Munich, German Research Center for Environmental Health, Neuherberg, Germany. Correspondence and requests for materials should be addressed to M.H. (email: martin.heni@med.uni-tuebingen.de)
Received: 5 July 2017 Accepted: 31 October 2017 Published: xx xx xxxx
OPEN
have already demonstrated that the risk allele of FTO is associated with higher food intake8, altered processing of food signals in the brain9 and with insulin resistance of the human brain10. Recent data suggest that the variation in FTO also has an impact on the transcription factors that regulate adipocyte development11. Indeed, adipocytes from FTO risk allele carriers have an altered energy homeostasis: they store energy more efficiently and pro- duce less excess heat11. We thus hypothesized that higher plasma levels of energy substrates, namely glucose and triglycerides, could intensify this increased lipid storage, thereby causing excessive weight gain, particularly in subjects carrying the risk allele in FTO.
Methods
All methods were performed in accordance with the relevant guidelines and regulations.
Subjects. TUEFF. The Tübingen Family Study for type 2 diabetes (TUEF) is designed to establish a thor- oughly phenotyped cohort of a population at increased risk of type 2 diabetes. To this end, subjects with known prediabetes (without known diabetes), a family history of diabetes, or obesity are continuously recruited to eval- uate metabolic status with respect to glucose and lipid metabolism. The Ethics Committee of the Medical Faculty of the University of Tübingen approved the study protocol. Written informed consent was obtained from all participating individuals. Further details on the study have already been published12.
Whitehall-II. Data from the occupational Whitehall II cohort were accessed by a data sharing agreement.
Details of the study have already been published13. The study was approved by the Joint UCL/UCLH Committees on the Ethics of Human Research (Committee Alpha). All participants provided written informed consent. The Whitehall II study was initiated in 1985 and recruited 10,308 participants (3,413 women) aged 35–55 years with a response rate of 73% from 20 London based Civil Service departments. The initial visit (phase 1) included a clinical examination and a self-administered questionnaire in 1985–88. During follow-up, 5-yearly clinical exam- inations were performed (Phase 3: 1991–94, Phase 5: 1997–99, Phase 7: 2002–04, and Phase 9: 2007–09) and additional postal questionnaire only phases were conducted (Phase 2:1988–90, Phase 4: 1995–96, Phase 6: 2001, and Phase 8: 2006). As 75 g oral glucose tolerance tests (OGTT) were first performed in phase 3, this provides the baseline for the current analysis. Disclosed data was available for 5067 white participants for phases 3, 5, 7 and 9, amounting to a total of 24,029 person-examinations. After removing missing or non-fasted glucose measure- ments14, missing weight or genotyping information and participants with known diabetes, we gained a full dataset comprising 16,307 person-examinations.
Oral glucose tolerance test (OGTT) and laboratory analyses. In the TUEF study, all participants received a 75-g glucose solution (Accu-Check Dextro, Roche) at 8 a.m. following an overnight fast. Venous blood was obtained through an indwelling venous catheter before and 30, 60, 90 and 120 minutes after glucose ingestion. The proce- dure of OGTT in the Whitehall II study was already described in detail14.
Glucose was analysed in both the TUEF and the Whitehall II study using an YSI glucose analyser (Yellow Springs Instruments).
Fasting triglyceride concentrations were measured by a standard colorimetric method on a Bayer analyser (Bayer HealthCare) in TUEF, and by a Cobas Fara centrifugal analyser (Roche Diagnostics System) in Whitehall II15. Genotyping. Following appropriate cell lysis, protein precipitation and washing in the TUEF study, the SNP rs9939609 was genotyped from blood samples using the MassARRAY platform (Sequenom). Genotyping of Whitehall II participants was described earlier16.
Statistical analysis. Data are presented as median and interquartile range or mean ± SD. To determine whether glycemia or triglycerides modulate the effect of the SNP on body weight and BMI, linear regression modelling was performed with the least squares method, including the interaction term SNP × environmental factor. Age and sex were included as covariates in all linear regression models. From the linear regression models, the effect sizes are given as effect estimates (estimate). Records with missing data were excluded. The FTO SNP rs9939609 genotype was modelled according to the additive inheritance model; the SNP variable indicating the number of risk alleles. Longitudinal data in the Whitehall II cohort was investigated by testing fold-change BMI between adjacent visits. Linear mixed models were fitted, adjusting for the random effects subject and time since the beginning of the study. Fixed effect variables comprised sex, BMI on the previous visit, age, age-squared, elapsed time between the observations, fasting glucose on the previous visit, and the genotype, with all interactions of the latter three variables. The analyses were performed with R 3.3.2 (R Core Team), using the lme4 package for mixed models.
Data availability statement. The dataset of the TUEF study is not publicly available due to ethical reasons concerning the participants’ informed consents, but is available from the corresponding author on appropriate request. The availability of the Whitehall-II data is subject to an individual data-sharing agreement.
Results
TUEF Study. Participants of the cross-sectional TUEF study (N = 2671) had a median BMI above 28 kg/m2, and the interquartile ranges indicated a high proportion of subjects with higher BMI (see Table 1). As anticipated, genetic variation in FTO was strongly associated with BMI (p = 1.7·10−10), even after adjusting for sex and age (p = 2.9·10−10). The effect size on BMI amounted to 1.7 ± 0.3 kg/m2 per risk allele. We fitted linear regression models to assess the impact of both glucose levels and plasma triglycerides on the association of the genetic variant in FTO with BMI. We tested the interaction terms between FTO × fasting glucose, FTO × post-chal- lenge glucose and FTO × fasting plasma triglycerides on BMI. In all models, age and sex were included as
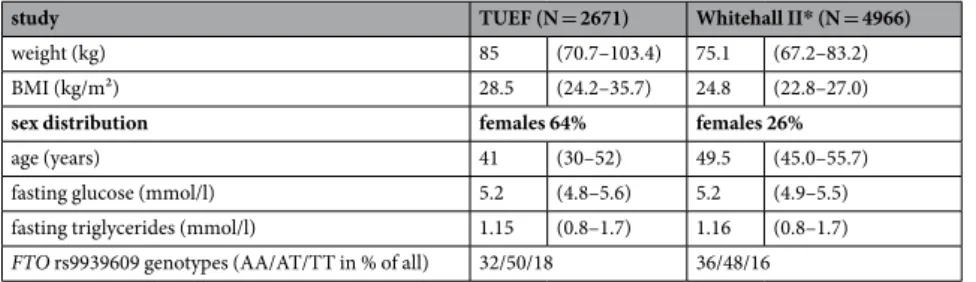
additional covariates. Genetic variation in FTO revealed significant interaction with fasting glucose in models with (p = 0.0005) and without (p = 0.003) additional adjustment for fasting plasma triglycerides. This translated to a 1.78 ± 0.73 kg/m2 higher BMI associated with each 1 mmol/l higher glucose value in the presence of the AA risk genotype than in the TT genotype. This interaction remained significant even after additional adjustment for insulin sensitivity (p = 0.003). In similar models, fasting plasma triglycerides showed interaction effects with FTO on BMI with (p = 5·10−7) and without (p = 0.0002) additional adjustment for fasting glucose. The presence of the AA risk genotype rather than the TT genotype implicated a 2.11 ± 0.51 kg/m2 higher BMI associated with each 1 mmol/l higher triglyceride value. However, no interaction of FTO with post-challenge glucose was observed (p = 0.3) (see Fig. 1).
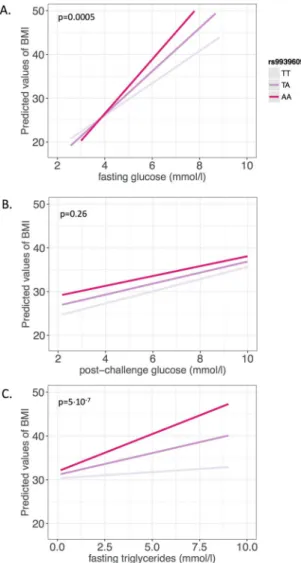
We next stratified the study population at the median of fasting glucose levels into low-glucose (<5.17 mmol/l) and high-glucose (≥5.17 mmol/l) groups. Genetic variation in FTO exhibited a lower effect size in the low-glucose group (estimate = 1.4 ± 0.3 kg/m2, p = 6·10−6) than in the high-glucose group (estimate = 2.2 ± 0.44 kg/m2, p = 6·10−7). A similar stratification was performed using low-triglyceride (<1.15 mmol/l) and high-triglyceride (≥1.15 mmol/l) groups. The impact of FTO on BMI was also lower in the low-triglyceride group (esti- mate = 1.4 ± 0.3 kg/m2, p = 2·10−5) than in the high-triglyceride group (estimate = 1.7 ± 0.4 kg/m2, p = 2·10−5).
All models were adjusted for sex, age, plasma triglycerides, and fasting glucose, respectively. The data are also shown in Fig. 2.
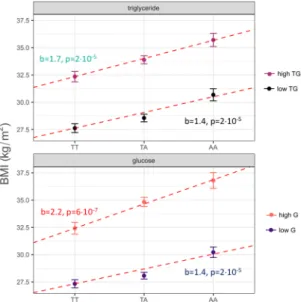
Whitehall II Study. In this longitudinal cohort, we tested whether fasting glucose and triglyceride concen- trations modulate the impact of the FTO SNP on BMI over time. During the observation period of 13.5 ± 4.6 years, the 4966 participants involved displayed a mean weight gain of 2.96 ± 6.5 kg, translating to a BMI change of 1.42 ± 2.2 kg/m2, i.e. a 5.7 ± 8.6% relative increase in BMI. BMI and diabetes incidence at the last observation, stratified by FTO genotypes, is provided in Supplementary Table 1. Using a linear mixed model, we modelled the BMI change between consecutive visits as a function of the FTO SNP and the fasting glucose levels assessed during the previous visit. The models also comprised the covariates sex, age, age-squared, and elapsed time since the last observation. Fasting glucose during the previous observation significantly interacted with FTO on BMI change (b = 0.002 ± 0.0008, p = 0.04, Supplementary Figure 1). This translates to a 0.2 percentage-points higher BMI increase per risk-allele for 1 mmol/l higher fasting glucose. We also ascertained an inversely directed sig- nificant interaction between elapsed time since the last observation, fasting glucose in the last observation, and FTO (b = −0.0019 ± 0.0008, p = 0.02), suggesting that the interaction effect diminishes over time. No comparable interaction was evident for fasting plasma triglycerides (p = 0.6). Moreover, post-challenge glucose levels did not modulate the impact of genetic FTO variation on weight gain (p = 0.67). To allow for robust visualization of the data, we computed individual average fasting glucose levels over all available time points and stratified this vari- able into quartiles. A comparison of the lowest and the highest mean fasting glucose quartiles showed that mean fasting glucose modulated the impact of the FTO genotype over the total observation period (p = 0.04). This is indicative of a progressively higher weight gain in subjects who have higher average fasting glucose levels and who are carriers of the FTO risk allele (see Fig. 3).
Discussion
In this study, we investigated whether plasma glucose or triglyceride levels modulate the effect of the BMI-associated genetic variant in FTO. In two independent study populations, we demonstrated that fasting glucose and FTO interact to increase BMI and weight change over time. This interaction effect was also observed in the cross-sectional TUEF population with a broad range of BMI. However, post-challenge glucose does not interact with FTO genotype to increase obesity.
Gene × environment interactions are proposed to contribute to substantial parts of the hitherto genetically unexplained heritability of metabolic traits such as obesity or diabetes17. A recent family-based study showed the presence of significant gene × environment interactions for BMI involving environmental factors such as sex, age, alcohol intake and other dietary habits18. While glycemia is also an outcome of genetic and environmental factors, it is nevertheless an important attribute of the metabolic environment which, in turn, modulates multiple cellular processes. We have already shown that glucose levels interact with what is to date the most important diabetes-associated common SNP in TCF7L2 to affect insulin secretion19.
Numerous biological mechanisms may underlie the interaction between glucose and FTO detected in this study. Recent research indicates that FTO variation has a profound effect on adipocyte energy handling via the transcription factors IRX3 and IRX511,20. Increased expression of these transcription factors leads to
study TUEF (N = 2671) Whitehall II* (N = 4966)
weight (kg) 85 (70.7–103.4) 75.1 (67.2–83.2)
BMI (kg/m²) 28.5 (24.2–35.7) 24.8 (22.8–27.0)
sex distribution females 64% females 26%
age (years) 41 (30–52) 49.5 (45.0–55.7)
fasting glucose (mmol/l) 5.2 (4.8–5.6) 5.2 (4.9–5.5)
fasting triglycerides (mmol/l) 1.15 (0.8–1.7) 1.16 (0.8–1.7)
FTO rs9939609 genotypes (AA/AT/TT in % of all) 32/50/18 36/48/16
Table 1. Baseline data from the TUEF and Whitehall II* studies. Given are medians and interquartile ranges or percentage. *Whitehall II data are presented for participants in the first available phase.
cell-autonomous changes during adipocyte development, causing a phenotype that is associated with increased lipid storage and reduced energy loss via thermogenesis11. Both circulating glucose and triglycerides are impor- tant substrate resources for lipid storage in adipocytes. Adipocytes synthesize triglycerides from glucose through de novo lipogenesis21, while circulating triglycerides are hydrolysed by lipoprotein-lipase and then re-esterified to triglycerides within the adipocytes22.
Abundance of energy carriers might thus augment the impact of FTO genotype by increasing substrate supply.
One might therefore expect basal energy expenditure to be lower in FTO risk allele carriers. Despite several stud- ies on this topic, however, there is still no convincing evidence that FTO has any impact on energy expenditure in humans23. However, we and others have shown that FTO risk allele carriers have an increased caloric intake8. In line with this, there is growing evidence that variation in FTO, the gene with the highest expression in the central nervous system, has an impact on central nervous appetite regulation9,24,25 and brain insulin sensitivity10. These processes in the human brain may involve the central dopaminergic system since genetically determined dopa- mine receptor density modulates FTO effects even further26,27. We speculate that insulin resistance and appetite dysregulation associated with the risk allele of FTO could also contribute to the observed shift in the glucose-BMI regression line, as shown in Fig. 1A, thus explaining this interaction. Elevated glucose levels are known to sup- press appetite, induce satiety and reduce food intake28,29. Since the association of FTO variation with brain activity is more pronounced in the context of elevated glucose9, higher glucose levels may enhance the impact of FTO on body weight also via the brain.
Limitations of this work include the different study populations, different measurement methods and differ- ences in OGTT conditions which could potentially reduce the comparability of the two studies. A longitudinal analysis was available in Whitehall II only. Furthermore, circulating glucose and triglycerides does not necessarily indicate cellular energy availability to a full extent.
Figure 1. Linear regression models plotting the predicted values of BMI against fasting glucose (A), post- challenge glucose (B), and fasting plasma triglycerides (C) in interaction with the FTO SNP rs9939609 in the TUEF study. Colors indicate genotypes of rs9939609. The p-values are given for the respective interaction terms in models adjusted for sex, age, and either fasting triglycerides in panel A or fasting glucose in panel C.
By demonstrating that glycemia and possibly also triglyceride levels interact with FTO to modulate body weight, our work provides additional evidence for the importance of gene × environment interactions in the pathogenesis of obesity. Although the underlying biological mechanism of this interaction cannot be eluci- dated by our data, our findings fit well into the known biological context. Our data suggest that individuals with increased fasting glucose levels have an even higher risk for weight gain if the strongest common genetic variant in the FTO gene is present. Since weight gain is known to further accelerate metabolic deterioration, this interplay may result in a vicious circle and thus lead to a more pronounced obese state and promote diabetes risk. Effective pharmacological and non-pharmacological glucose-lowering strategies may therefore be required to boost weight loss, particularly in FTO risk-allele carriers suffering from prediabetes or diabetes.
Figure 2. Relationship of FTO rs9939609 genotypes and BMI in subgroups stratified at the medians of fasting triglycerides (low TG, high TG) and fasting glucose (low G, high G). BMI per genotype is shown in each stratum as least squares mean with standard error (adjusted for sex, age, and either fasting triglycerides (in the glucose strata) or fasting glucose (in the triglyceride strata). The linear regression estimate, slope (b) with corresponding p-value (p), is shown by dashed lines.
Figure 3. Interaction effect of glycemia and rs9939609 in FTO on change of body weight in the longitudinal Whitehall-II study (p = 0.04). Panels show participants carrying different FTO rs9939609 genotypes, colours indicate quartiles of average fasting glucose per subject over the full observation period (red: bottom quartile, fasting glucose 4.07–4.9 mmol/l, blue: top quartile, fasting glucose 5.44–16 mmol/l). Lines indicate linear fits per glucose quartile with 95% confidence intervals in the respective colours.
References
1. Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206 (2015).
2. Wagner, R. et al. Untangling the interplay of genetic and metabolic influences on beta-cell function: Examples of potential therapeutic implications involving TCF7L2 and FFAR1. Mol Metab 3, 261–267 (2014).
3. Andreasen, C. H. et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation.
Diabetes 57, 95–101 (2008).
4. Sailer, C. et al. FTO Genotype Interacts with Improvement in Aerobic Fitness on Body Weight Loss During Lifestyle Intervention.
Obes Facts 9, 174–181 (2016).
5. Kilpeläinen, T. O. et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 8, e1001116 (2011).
6. Qi, Q. et al. Dietary intake, FTO genetic variants and adiposity: a combined analysis of over 16,000 children and adolescents.
Diabetes https://doi.org/10.2337/db14-1629 (2015).
7. Heni, M. et al. Glycemia determines the effect of type 2 diabetes risk genes on insulin secretion. Diabetes 59, 3247–3252 (2010).
8. Haupt, A. et al. Variation in the FTO gene influences food intake but not energy expenditure. Exp. Clin. Endocrinol. Diabetes 117, 194–197 (2009).
9. Heni, M. et al. Variation in the obesity risk gene FTO determines the postprandial cerebral processing of food stimuli in the prefrontal cortex. Mol Metab 3, 109–113 (2014).
10. Tschritter, O. et al. Variation in the FTO gene locus is associated with cerebrocortical insulin resistance in humans. Diabetologia 50, 2602–2603 (2007).
11. Claussnitzer, M. et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N. Engl. J. Med. https://doi.org/10.1056/
NEJMoa1502214 (2015).
12. Stumvoll, M. et al. Association of the T-G polymorphism in adiponectin (exon 2) with obesity and insulin sensitivity: interaction with family history of type 2 diabetes. Diabetes 51, 37–41 (2002).
13. Marmot, M. & Brunner, E. Cohort Profile: the Whitehall II study. Int J Epidemiol 34, 251–256 (2005).
14. Brunner, E. J. et al. Social inequality in coronary risk: Central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia 40, 1341–1349 (1997).
15. Shah, S. et al. Influence of common genetic variation on blood lipid levels, cardiovascular risk, and coronary events in two British prospective cohort studies. European Heart Journal 34, 972–981 (2013).
16. Talmud, P. J. et al. Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am. J.
Hum. Genet. 85, 628–642 (2009).
17. Marigorta, U. M. & Gibson, G. A simulation study of gene-by-environment interactions in GWAS implies ample hidden effects.
Front Genet 5, (2014).
18. Poveda, A. et al. The heritable basis of gene–environment interactions in cardiometabolic traits. Diabetologia 1–11 https://doi.
org/10.1007/s00125-016-4184-0 (2016).
19. Heni, M., Herzberg-Schafer, S., Machicao, F., Haring, H.-U. & Fritsche, A. Dietary Fiber Intake Modulates the Association Between Variants in TCF7L2 and Weight Loss During a Lifestyle Intervention. Diabetes Care 35, e24 (2012).
20. Smemo, S. et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507, 371–375 (2014).
21. Aarsland, A., Chinkes, D. & Wolfe, R. R. Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Am J Clin Nutr 65, 1774–1782 (1997).
22. Frayn, K. N., Karpe, F., Fielding, B. A., Macdonald, I. A. & Coppack, S. W. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord 27, 875–888 (2003).
23. Speakman, J. R. The ‘Fat Mass and Obesity Related’ (FTO) gene: Mechanisms of Impact on Obesity and Energy Balance. Curr Obes Rep 4, 73–91 (2015).
24. Loos, R. J. F. & Yeo, G. S. H. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol 10, 51–61 (2014).
25. Kühn, A. B. et al. FTO gene variant modulates the neural correlates of visual food perception. Neuroimage 128, 21–31 (2016).
26. Sevgi, M. et al. An Obesity-Predisposing Variant of the FTO Gene Regulates D2R-Dependent Reward Learning. J. Neurosci. 35, 12584–12592 (2015).
27. Heni, M. et al. Interaction between the obesity-risk gene FTO and the dopamine D2 receptor gene ANKK1/TaqIA on insulin sensitivity. Diabetologia 59, 2622–2631 (2016).
28. Chapman, I. M., Goble, E. A., Wittert, G. A., Morley, J. E. & Horowitz, M. Effect of intravenous glucose and euglycemic insulin infusions on short-term appetite and food intake. Am. J. Physiol. 274, R596–603 (1998).
29. Gielkens, H. A., Verkijk, M., Lam, W. F., Lamers, C. B. & Masclee, A. A. Effects of hyperglycemia and hyperinsulinemia on satiety in humans. Metab. Clin. Exp. 47, 321–324 (1998).
Acknowledgements
We gratefully acknowledge excellent technical assistance from Anja Dessecker, Ellen Kollmar, Andreas Vosseler, and Roman Werner, all from the Department of Internal Medicine, Division of Endocrinology, Diabetology, Nephrology, Vascular Disease and Clinical Chemistry, University Hospital, Eberhard Karls University, Tübingen.
We thank all the women and men who participate in the TUEF and Whitehall II Studies, as well as all Whitehall II research scientists, study and data managers and clinical and administrative staff who made this study possible.
The authors thank Shirley Würth (Eberhard Karls University Tübingen) for editorial assistance. This study was supported in part by a grant (01GI0925) from the Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.). The UK Medical Research Council, British Heart Foundation, and the US National Institutes of Health (R01HL36310, R01AG013196) have supported collection of data in the Whitehall II Study.
Author Contributions
R.W. and M.H. analysed data and wrote the manuscript. A.G.T., E.F., L.F., A.J., R.B., S.M.S. contributed to the interpretation of data and edited the manuscript. A.P. contributed to data analysis and edited the manuscript.
H.S., F.M., H.-U.H., A.F. contributed to the study design and interpretation of data and reviewed the manuscript.
Additional Information
Supplementary information accompanies this paper at https://doi.org/10.1038/s41598-017-15744-4.
Competing Interests: The authors declare that they have no competing interests.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Cre- ative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not per- mitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
© The Author(s) 2017