Gap junctional coupling in the vertebrate retina: Variations on one theme?
Béla Völgyi
a,b,d,*,1, Tamás Kovács-Öller
b,d,1, Tamás Atlasz
a,c,d,1, Márta Wilhelm
c,1, Róbert Gábriel
b,d,1aDepartment of Ophthalmology, School of Medicine, New York University, 550 First Avenue, MSB 149, New York, NY 10016, USA
bDepartment of Experimental Zoology and Neurobiology, University of Pécs, Ifjúság Str. 6, Pécs 7624, Hungary
cInstitute of Physical Education and Sport Science, University of Pécs, Ifjúság Str. 6, Pécs 7624, Hungary
dJános Szentágothai Research Center, Ifjúság Str. 20, Pécs 7624, Hungary
a r t i c l e i n f o
Article history:
Available online 8 January 2013
Keywords:
Retina
Electrical synapse Conductance Connexin Phylogenesis
a b s t r a c t
Gap junctions connect cells in the bodies of all multicellular organisms, forming either homologous or heterologous (i.e. established between identical or different cell types, respectively) cell-to-cell contacts by utilizing identical (homotypic) or different (heterotypic) connexin protein subunits. Gap junctions in the nervous system serve electrical signaling between neurons, thus they are also called electrical synapses. Such electrical synapses are particularly abundant in the vertebrate retina where they are specialized to form links between neurons as well as glial cells. In this article, we summarize recent findings on retinal cell-to-cell coupling in different vertebrates and identify general features in the light of the evergrowing body of data. In particular, we describe and discuss tracer coupling patterns, connexin proteins, junctional conductances and modulatory processes. This multispecies comparison serves to point out that most features are remarkably conserved across the vertebrate classes, including (i) the cell types connected via electrical synapses; (ii) the connexin makeup and the conductance of each cell-to- cell contact; (iii) the probable function of each gap junction in retinal circuitry; (iv) the fact that gap junctions underlie both electrical and/or tracer coupling between glial cells. These pan-vertebrate fea- tures thus demonstrate that retinal gap junctions have changed little during the over 500 million years of vertebrate evolution. Therefore, the fundamental architecture of electrically coupled retinal circuits seems as old as the retina itself, indicating that gap junctions deeply incorporated in retinal wiring from the very beginning of the eye formation of vertebrates. In addition to hard wiring provided by fast synaptic transmitter-releasing neurons and soft wiring contributed by peptidergic, aminergic and purinergic systems, electrical coupling may serve as the ‘skeleton’ of lateral processing, enabling important functions such as signal averaging and synchronization.
Ó2013 Elsevier Ltd. All rights reserved.
Contents
1. Introduction . . . .2
2. Aims . . . .2
3. Gap junction protein families and retinal gap junction proteins in vertebrates . . . .2
3.1. Connexin families . . . 3
3.2. Gap junction proteins in the neural retina . . . 4
3.2.1. Conductance . . . 4
3.2.2. Modulation . . . 6
4. Cell-to-cell coupling patterns . . . .7
*Corresponding author. Department of Experimental Zoology and Neurobiology, University of Pécs, Ifjúság Str. 6, 7624 Pécs, Hungary. Tel.:þ36 72 503600/24614; fax:þ36 72 501 517.
E-mail addresses:volgyi01@gamma.ttk.pte.hu,Bela.Volgyi@nyumc.org(B. Völgyi),kovacstx@gmail.com(T. Kovács-Öller),attam33@gmail.com(T. Atlasz),mwilhelm@
gamma.ttk.pte.hu(M. Wilhelm),gabriel@ttk.pte.hu(R. Gábriel).
1 Percentage of work contributed by each author in the production of the manuscript is as follows: Béla Völgyi: 35%; Tamás Kovács-Öller: 20%; Tamás Atlasz: 10%; Márta Wilhelm: 10%; Róbert Gábriel: 25%.
Contents lists available atSciVerse ScienceDirect
Progress in Retinal and Eye Research
j o u r n a l h o me p a g e : w w w .e l se v i e r. co m/ lo ca t e / p r e r
1350-9462/$esee front matterÓ2013 Elsevier Ltd. All rights reserved.
http://dx.doi.org/10.1016/j.preteyeres.2012.12.002
4.1. Outer retinal junctions . . . 7
4.1.1. Photoreceptor-to-photoreceptor junctions . . . 7
4.1.2. Horizontal-to-horizontal cell junctions . . . 9
4.1.3. Bipolar-to-bipolar cell junctions . . . 10
4.2. Inner retinal junctions . . . 10
4.2.1. Amacrine-to-amacrine cell gap junctions . . . 10
4.2.2. Ganglion-to-ganglion cell gap junctions . . . 12
4.2.3. Ganglion-to-amacrine cell gap junctions . . . 12
4.2.4. Amacrine-to-bipolar cell gap junctions . . . 13
4.3. Junctions between glial cells . . . 13
5. Concluding remarks . . . .14
Acknowledgments . . . 14
References . . . 14
1. Introduction
Across the animal kingdom, two major forms of interneuronal communication are utilized. Chemical synaptic transmission is the numerically dominant mode of signal transmission. In this mode, the excited (pre-synaptic) neurons release chemical substances (transmitters, neuromodulators) that bind to receptor molecules on the plasma membrane of the targets (post-synaptic neurons). These receptors are highly selective for the appropriate transmitter, which in turn gate the excitation, inhibition or modulation of the post-synaptic neurons.
The other form of inter-neuronal signal transmission is medi- ated by so-called electrical synapses or gap junctions. Gap junctions are molecular bridges between cells that provide low resistance avenues for electrical and/or metabolic communication of coupled cells. Gap junctions were described by electron microscopy over 50 years ago in peripheral tissues (skin: Farquhar and Palade, 1965, muscle: Rosenbluth, 1965) and in the brain (Watanabe, 1958;
Brightman and Reese, 1969). Similar to chemical synapses, mem- brane surfaces of electrical synapse forming cells make close membrane appositions. However, the synaptic gap is some ten times narrower in the case of electrical synapses (1e2 nm), a characteristic feature that inspired Goodenough and Revel (1970) to dub them gap junctions. At the site of electrical syn- apses, plasma membranes are anchored to the cytoskeletal matrix via a zonula-occludens complex (Rash et al., 2004;Ciolofan et al., 2007;Li et al., 2004a,b,2008) and protein based structures span both cell membranes. These join to form continuous tunnels per- meable to several ions and small molecules (<1 kDa). The tunnel is created by aligned connexon halves (hemichannels), one inserted into each apposing membrane. Connexons are oligomerized from six connexin subunits that are members of a multigene family with relatively conservative molecular structure. All described con- nexins have four transmembrane domains, two extracellular and an intracellular loop with the C- and N- termini in the cytoplasm.
Various cells may express different connexin subunits to form gap junctions depending on the function served.
Although the existence of electrical synapses has long been known (Furshpan and Potter, 1957;Watanabe, 1958) they seemed less prevalent, thus thought to be inferior in the nervous system.
However, this dogmatic view has been challenged in the past two decades and it is now clear that electrical synapses are ubiquitous elements of the nervous system. The discovery of highly specific antibodies and the introduction of transgenic animal lines triggered a surge of experimental work that lead to the identification of the molecular building blocks of gap junctions, the connexin proteins.
Gap junctions have been recognized as significant components of the retinal circuitry almost 40 years ago (Raviola and Gilula, 1973). Most of them mediate lateral signaling by coupling similar
retinal neurons into extended, planar arrays and sometimes they also transmit signals vertically between different cell types (Cook and Becker, 1995). Retinal gap junctions are of a particular inter- est to neuroscientists, since there is no other area in the nervous system where gap junctions are found in such a great quantity and participate so extensively in signal processing. A number of recent reviews have surveyed the electrical and/or network properties, tracer coupling and cell-to-cell connections of retinal gap junctions (e.g.Bloomfield and Völgyi, 2009;Cook and Becker, 2009;Demb and Pugh, 2002;Roerig and Feller, 2000;Söhl et al., 2000;Vaney and Weiler, 2000;Weiler et al., 2000). However, the phylogenetic aspects and/or conservation of these properties have not been reviewed for over 15 years since the seminal work of Cook and Becker (1995). Yet a lot of new evidence has been presented since then, just to mention the characterization of gap junction forming connexin subunits in many retinal contacts. Given that the con- nexin amino acid sequences (and the coding DNA nucleotide se- quences, for that matter) have great potential to reveal new phylogenetic and functional aspects, we will provide a multiple species comparison of the vertebrate retinal gap junctions.
2. Aims
In this review, we blend old dogmas with recent advances in neurobiology with regard to retinal gap junctions in key neuronal circuits. To this end, we will provide a comprehensive overview on retinal gap junctions in vertebrate species and survey interspecies differences and similarities of: (i) the cell types connected with gap junctions, (ii) the connexin composition of individual junctions, (iii) the way gap junctional conductivity relates to function and (iv) the modulatory processes that influence the junctional properties.
Finally, we will use the acquired data to make conjectures both about the evolution of the retinal electrical wiring and the possible origin of connexin divergence.
3. Gap junction protein families and retinal gap junction proteins in vertebrates
Intercellular channels resembling gap junctions exist in many lower multicellular organisms, but connexins and their genes have only been identified in deuterostomes (Willecke et al., 2002). It has been suggested that the connexin gene family has developed through duplication of ancestral connexin gene(s) (Bennett et al., 1991). In the best-known mammalian genomes (mouse and hu- man), approximately 20 connexin genes were sequenced (Söhl and Willecke, 2003). However, there have been more connexin genes identified infish species than in mammals (Cruciani and Mikalsen, 2006), therefore it is sometimes difficult to categorize the numer- ous connexin orthologs (similar connexins of different species) and
paralogs (different connexins of one species). As for the origin of connexin diversity, one leading view claims that animals in each vertebrate class use their own unique pattern of connexins to build gap junctions (DeBoer & Van der Heyden, 2005). In contrast, other scientists (Bennett et al., 1991;Cruciani and Mikalsen, 2006,2007) argue that there are surprisingly few differences in the ortholog sequences and even the numerousfish connexin subtypes can be derived simply with well-accepted genome duplications in the early evolution offish (Jaillon et al., 2004;Dehal and Boore, 2005).
Moreover, only a few gene duplications and/or losses can explain all changes in vertebrate connexin gene sequences from agnathans (jawlessfish) to mammals (Saez et al., 2003;Cruciani and Mikalsen, 2006). Briefly, is the expression of each connexin related to a par- ticular subset of animals or invariably used by vertebrates to fulfill a certain retinal function? As mentioned above, this review will discuss this dispute in the light of the summarized data as well.
3.1. Connexin families
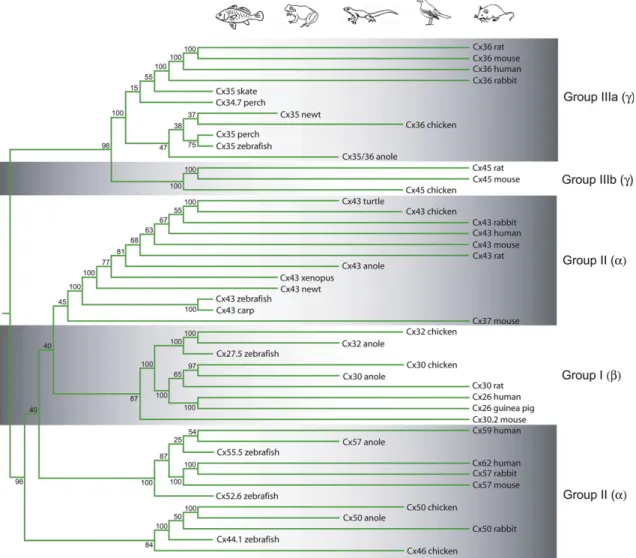
The literature follows a nomenclature in which the various types of connexin subunits are named after their molecular weights be- tween 21 and 70 kDa (Cx43, Cx40, Cx26 etc.) that tells us little about sequence homology and evolutionary relationships. Yet it has been shown that all major mammalian connexin genes display consid- erable sequence homology with ortholog counterparts (Fig. 1) of other vertebrates suggesting a rather conservative connexin evo- lution throughout the vertebrate phylogenesis (Bennett et al., 1991). Based on amino acid sequences, three main branches of the connexin phylogenetic tree can be separated (Cruciani and Mikalsen, 2006). Thea-connexins (Group II; Cx38, Cx40, Cx43, Cx45, Cx46, Cx50 etc.; Fig. 1) bear longer intracellular sequence, whileb-connexins (Group I; Cx26, Cx30.2, Cx31 and Cx32) have shorter intracellular C-termini (Cruciani and Mikalsen, 2006). The
Fig. 1.Connexin phylogenetic tree. This dendrogram was created for retinal connexin DNA sequences in the vertebrates. Following alignment, the most variable C-terminal tails and the N-terminals of connexin sequences were deleted. Subsequently, a neighbor-joining with bootstrap analysis was performed on the shortened homologous sequences. Pictograms on the top represent vertebrate classes offishes, amphibians, reptiles, birds and mammals in sequence. Paralogs and connexin sequences that belong to animals in the same phylogenetic class (marked by the pictograms) were grouped in the same column. Groups of connexin subtypes included:aor group II;bor group I;gor group IIIa and group IIIb (separated by alternating shadowed backgrounds) according to descriptions ofCruciani and Mikalsen (2006). Clearly, connexin paralogs are genetically far, whereas orthologs tend to group together. Species and DNA sequence IDs used for the analysis:Rattus norvegicusCx43-NM_012567, Cx30-NM_053388, Cx36-NM_019281, Cx45-NM_001085381;Mus musculusCx43-NM_010288, Cx36-NM_010290, Cx30.2-AJ414561, Cx57-NM_010289, Cx45-NM_008122, Cx37-NM_008120;Homo sapiensCx43-NM_000165, Cx26-BC017048, Cx36- BC148788, Cx62-NM_032602, Cx59-NM_030772;Oryctolagus cuniculusCx43-NM_001198948, Cx36-XM_002718001, Cx57-XM_002714475, Cx50-XM_002715651;Gallus gallus Cx43-NM_204586, Cx30-NM_204931, Cx32-NM_204371, Cx36-NM_204582, Cx45-NM_205503, Cx50-NM_204997;Anolis carolinensisCx43-XM_003215563, Cx30-XM_003219246, Cx32-XM_003228190, Cx35/36-XM_003214476, Cx57-XM_003215542, Cx50-XM_003219087;Cavia porcellusCx26-NM_001172823;Danio rerioCx27.5-BC056546, Cx35-BC162925, Cx55.5-NM_131812, Cx52.6-NM_212819, Cx43-AF035481, Cx44.1-BC095304;Morone americanaCx34.7-AF059184, Cx35-AF059183;Raja sp. Cx35-RSU43290;Cynops pyrrogaster Cx35-AB078502, Cx43-AB078503;Xenopus laevisCx43-NM_001085660;Trachemys dorbigniCx43-JQ356532;Cyprinus carpioCx43-AZ008286.
most recently identified group is theg(or Group III) that includes mammalian Cx36 (Figs. 1and3), skate, perch and zebrafish Cx35, perch Cx34.7. This latter g group has been further divided into subgroups IIIa and IIIb, which include the Cx36 and the Cx45 evolutionary lines, respectively (Cruciani and Mikalsen, 2006).
Similarly, the population ofa(Group II) connexins has been sug- gested to split up as well into two subgroups: one containing Cx43 and related sequences, and another including Cx57, Cx59 and Cx62.
The main lines (a,bandg) and the subdivisions of connexin se- quences are neatly separated in our own connexin family tree presented here (Fig. 1). In contrast to pores formed by eithera- orb- connexins,g-connexins have low voltage and pH sensitivity and are unable to form heterologous channels with members ofa- andb- connexin polypeptides. Interestingly enough, Revel et al. (1992) made a calculation using divergence times of 75 million years (myr) for the human/rodent, 275 myr for the mammal/bird and 350 myr for the mammal/amphibian splits and concluded that the two main connexin branches (a and b) diverged some 1.3e 1.9 billion year ago (Doolittle et al., 1989), way before multi- cellular organisms arose. It has been suggested that ancestors of connexin proteins served molecular exchange between unicellular organisms, similar to the plasmodesmata in plants (Yahalom et al., 1991;Revel et al., 1992).
3.2. Gap junction proteins in the neural retina
Retinal neurons are organized in cellular layers, including the distal-most outer nuclear layer (ONL), the inner nuclear layer (INL) in the middle, and the ganglion cell layer (GCL) in the vitreal (innermost) aspect of the retina (Fig. 2). The ONL contains the densely stacked cell bodies of the photoreceptors whose inner and
outer segments extrude distally toward the pigment epithelium, whereas axonalfibers reach proximally toward secondary neurons.
The somata of secondary bipolar and horizontal cells, as well as tertiary amacrine cells are located in the centrally positioned INL.
Bipolar cells relay information from photoreceptors to ganglion cells. In mammals, most bipolar cells contact either rods (rod bi- polar cells) or cones (cone bipolar cells) to establish separate streams of rod and cone pathways, whereas bipolar cells of other vertebrate species receive a mixture of rod and cone mediated signals. Horizontal cells and amacrine cells provide inhibitory (feed-back and feed-forward) contacts in the outer and the inner retina, respectively. The GCL is comprised of ganglion cells and displaced amacrine cells. The three cellular layers are separated by the outer and inner plexiform layers (OPL, IPL) that harbor pro- cesses and synapses of the above listed neurons. Finally, axonal fibers of ganglion cells are bundled together in the thin nervefiber layer (NFL) and then exit the retina through the optic disc. More than three dozen connexins have been identified in the retina in different vertebrates to date (Table 1and Fig. 1) with molecular weights ranging from 26 to 62 kDa. Most of these were first described in mouse (7 types), chicken (6 types) and human retina (5 types) but there are sporadic data available from homologous connexins in other mammalian species, reptiles, amphibians and fish as well.
3.2.1. Conductance
Gap junction pores, with a few exceptions, are non-selective for ions and for small molecules up to about 1 kD molecular mass. They rather select among molecules by their size. However, some are more permeable for cations (Veenstra et al., 1994a,b), whereas others for anions (Harris, 2001). Permeant molecules include
1 2 3
4 5
6 11 10
7 8
9 9
C
R
HC cone
BC rod
BC
AC
GC
AII AC
Fig. 2. Gap junctions in specific retinal circuits. The informationflow in the retina occurs vertically from rod (R) and cone (C) photoreceptors to rod and cone bipolar cells (BC) and ultimately to ganglion cells (GC). In this vertical axis synapses are excitatory glutamatergic, whereas feed back and feed forward inhibition is provided by horizontal (HC) and amacrine cells (AC) in the outer and the inner retina, respectively. The schematic shows gap junctions formed between cones (1), rods (2) and between rods and cones (3). In addition, the outer retina also contains gap junctions between horizontal cells (4) and dendritic processes of cone bipolar cells (5). In the inner retina, gap junctions are found between axon terminals of bipolar cells (6), between amacrine cell processes (7), between ganglion cell dendrites (8) and between amacrine and ganglion cells (9). AII amacrine cells (AII) represent a special group of amacrine cells with very specific connections; they receive their inputs from rod bipolar cells and provide glycinerg inhibition to OFF cone bipolar cells (not shown) and gap junction mediated excitation to ON center cone bipolar cells (10). Apart from this heterologous AII-to-cone bipolar cell contact they also form homologous AII-to-AII gap junctions (11).
intracellular messengers (e.g. cAMP, cGMP, IP3) and tracers (e.g.
Biocytin, Neurobiotin) that are used experimentally to image net- works of electrically coupled cells. The overall conductance of a particular gap junction connexon is dictated by the connexin subunit(s) that constitute its pores. Site-specific mutation studies have revealed that amino acid sequence of the N-terminal domain
determine conductance, permeability and ion selectivity (Beyer et al., 2012). Conductance characteristics are gathered inTable 2.
Single-channel conductance varies over a wide range (10e300 pS;
Harris, 2001) with some displaying moderate unitary conductance, e.g., Cx36 (10e15 pS;Srinivas et al., 1999b;Teubner et al., 2000), whereas others exhibit some 20 times greater conductance like zebrafish Cx44.1 (w280 pS;Dermietzel et al., 2000) or mammalian Fig. 3.Connexin expression in the mammalian retina.A. One-step PCR reactions were
performed in the adult rat retina with OneStep RT-PCR kit (Qiagen Inc., Valencia, USA) from RNA extracted with RNAÒRT (MRC, Inc.; Cincinnati, USA). Photopositive bands clearly show the presence of Cx36, Cx45 and Cx57 with amplicon sizes of 155 bp, 117 bp and 132 bp, respectively.B. Double label immunocytochemistry displays immunopositive Cx36 (magenta; monoclonal a-Cx35/36 Chemicon, Tremecula, USA) and Cx45 (green; polyclonal a-Cx45 Chemicon, Tremecula, USA) puncta in a whole- mount rabbit retina. The level of focus is set close to the GCL-IPL border thus voids mark the location of nearby somata in the GCL (asterisks). Besides the individual puncta some Cx36 and Cx45 labels seem to colocalize (arrow).C. Cross section of a mouse retina displays green punctuate Cx36 immunolabels (monoclonal a-Cx35/36 Chemicon, Tremecula, USA) in the IPL (arrows). Retinal cells are counter stained with propidium iodide (pseudocolored to magenta), nonspecific blood vessel staining is marked with open arrows in both the OPL and the IPL. Inset in the right upper corner highlights a selected IPL area for a better view of immunostained Cx36 puncta. PRLe photoreceptors layer, OPLeouter plexiform layer, INLeinner nuclear layer, IPLe inner plexiform layer, GCLeganglion cell layer. Scale bar: 20mm inB, 100mm inC.
Table 1
Connexin subtypes in the retina of vertebrate species.
Cx type Fishes Amphibians Reptiles Birds Mammals
Cx26 Turtlea Chickb
Cx27.5 Zebrafishc
Cx30 Ratd
Cx30.2 Mousee
Cx32 Chickb
Cx34.7 Bassf Cx35/36 Skaten
Zebrafisho Bassf
Salamanderp Chickq Mouseg,h,i,j,k,l,m Rabbitr
Rats Guine-pigt Humanu Monkeya,v
Cx37 Mousel
Cx43 Carp, zebrafishw
Newtx Turtlew Chickb,x Mousel,y Rabbitz,aa Ratd Humanab Cx44.1 Zebrafishc
Cx45 Chickx Mouseh,ac,l,y,ad
Ratd
Cx50 Chickx Rabbitae,af
Cx52.6 Zebrafishag,ah Cx55.5 Zebrafishc
Cx56 Chickx
Cx57 Mousel,ai,aj
Rabbitae
Cx59 Humanaa
Cx62 Humanaa
aPottek et al. (2003).
bBecker et al. (2002).
c Dermietzel et al. (2000).
d Zahs et al. (2003).
eMüller et al. (2010).
f O’Brien et al. (2004).
gDeans et al. (2002).
hHan and Massey (2005).
iHansen et al. (2005).
jLin et al. (2005).
kSchubert et al. (2005a).
lKihara et al. (2006a).
mPan et al. (2010).
nO’Brien et al. (1996).
oLiu et al. (2009).
p Zhang and Wu (2004).
qKihara et al. (2009).
rKothmann et al. (2009).
sHidaka et al. (2002).
tLee et al. (2003).
u Söhl et al (2010).
vO’Brien et al. (2012).
w Janssen-Bienhold et al. (1998).
xKihara et al. (2008).
yKihara et al. (2006b).
zJohansson et al. (1999).
aaZahs and Ceelen, 2006.
abKerr et al. (2010).
acSchubert et al. (2005b).
adHilgen et al. (2011).
aeHuang et al. (2005).
afO’Brien et al. (2006).
agZoidl et al. (2004).
ahShields et al. (2007).
aiJanssen-Bienhold et al. (2009).
ajPalacios-Prado et al. (2009).
Cx50 (w220 pS;Srinivas et al., 1999b). The amino acid sequence of connexins also determines the trans-junctional voltage (Vj) dependence, and thereby the kinetics of pore gating (Harris, 2001).
Except for Cx36 that forms largely voltage insensitive channels (Srinivas et al., 1999a;Gibson et al., 1999;Al-Ubaidi et al., 2000), most pores display maximum conductance when the membrane potentials of interconnected cells are equal (carp Cx43, zebrafish Cx27.5 and Cx44.1) and a few that show a minimum of conductivity atVj¼0 (Cx55.5;Dermietzel et al., 2000). The macroscopic con- ductance of a particular gap junction is also determined by two other factors:(i)the number of channels that aggregate in a plaque (see below conductance of small roderod plaques vs. large hori- zontal cell-horizontal cell plaques) and(ii)the opening status and the fraction of pores that are open at any given time, which can be as low as 4e9%, like Cx36 gap junctions in the brain (Lin and Faber, 1988; Teubner et al., 2000;Galarreta and Hestrin, 2002;Pereda et al., 2003). The diversity of gap junctions is further extended by different connexin subunits that pair up to form functional het- erotypic channels (Bennett and Verselis, 1992;Dedek et al., 2006) or form homotypic channels that aggregate in the same plaque as bihomotypic gap junctions (Li et al., 2008).
3.2.2. Modulation
It has now beenfirmly established that connexins are important to development, differentiation and growth control in all organs of the vertebrate body (Loewenstein, 1980; Lo, 1996; Cruciani and Mikalsen, 2006). Hence, their expression and operation must be strictly regulated via a variety of mechanisms. Indeed, a number of
signal-transduction pathways have connexin targets (Cruciani and Mikalsen, 2006). This indicates that gap junctions are not static bridges and the gating of individual channels is modified by vari- ous, mostly intracellular factors including pH, concentration of ions and second messengers. Although, the pH sensitivity of different connexins varies, the intracellular (but not extracellular) acid- ification closes most connexin pores (Spray et al., 1981;Liu et al., 1993), whereas intracellular alkalinization increases gap junction conductance (Chesler, 2003;Palacios-Prado et al., 2009). Accord- ingly, dye- and/or tracer-coupling of neurons in both the brain and the retina is reduced with acidification and increased with alka- linization (Church and Baimbridge, 1991; DeVries and Schwartz, 1989;Hampson et al., 1992;Jouhou et al., 2007). Direct evidence of gap junction regulation by pH came from work studyingin vitro expression systems, in which HeLa cells were transfected with connexins that paired up to form homotypic gap junctions. By using this technique both Cx36 and Cx57 gap junctions were shown to display detectable pH induced conductance decrease (Teubner et al., 2000; Palacios-Prado et al., 2009). Increase in the intra- cellular [Caþþ] also reduces gap junction conductance, or may even close gap junctions when [Caþþ] rises to pathologically high concentrations.
Apart from changes in [Hþ] and/or [Caþþ] levels, a variety of endogenous substances can modulate the gating of gap junctions.
Except forbconnexins whose carboxyl tails are truncated, most known connexins have intracellular phosphorylation sites, mainly on the C-terminal tail (Revel et al., 1992). Phosphorylation sites are accessible to protein kinase C and A (PKC and PKA) that are Table 2
Conductances of specific connexin formed gap junctions that are positioned in key retinal cell-to-cell contacts.
Cell-to-cell contact Species Connexin subunit Unitary conductance Transmembrane conductance
RodeRod Salamandera Cx36 e 500 pS
Guine pigb e e 386 pS
ConeeCone Ground squirrelc Cx36 e 20e150 pS (calculated)
220 pS (measured)
Monkeyd e e 650 pS
RodeCone e e e e
HorizontaleHorizontal In vitroexpression systeme Cx50 (mouse) 220 pS e
Rabbitf Cx50 e 2.29e5mS (calculated)
In vitroexpression systemg Cx57 e 0.2e2.5 nS
In vitroexpression systemh Cx57 (mouse) 57 pS 0.8e30 nS
Teleostfishesi,j,k,l Cx52.6 (zebrafish) 40e60 pS e
BipolareBipolar Teleostfishesm Cx35/36 (goldfish) e 800 pS
AmacrineBipolar Rat (AIIeON cone BC)n Cx36/Cx36 or Cx36/Cx45 e Type5 BC (106e646 pS)
Type6 BC (596e1379 pS) Type7 BC (1233 pS) Type8 BC (1414 pS)
AmacrineeAmacrine Rat (AIIeAII)o,p Cx36 e 320e700 ps
Fish (wide-field)q e e 2.4 nS
AmacrineeGanglion e e e e
GanglioneGanglion Rat (alpha GCs)r Cx36 e 1.35 nS
aZhang and Wu (2005).
bLi et al. (2012).
c Li and DeVries (2004).
d Hornstein et al. (2004).
eSrinivas et al. (1999a).
f O’Brien et al. (2006).
gManthey et al. (1999).
hPalacios-Prado et al. (2009).
iMcMahon et al. (1989).
jMcMahon (1994).
kLu and McMahon (1996).
lZoidl et al. (2004).
mArai et al. (2010).
nVeruki and Hartveit (2002b).
oVeruki and Hartveit (2002a).
p Veruki et al. (2010).
q Hidaka et al. (2005).
rHidaka et al. (2004).
integrators of many intracellular cascades. As a result, a number of surface receptor binding molecules that interfere with the same cascades can change conductance of gap junctions. Besides their influence on channel gating, kinases also regulate trafficking, as- sembly and turnover of connexins (Lampe and Lau, 2000). In many cases dopamine initiates changes by binding to D1 and/or D2re- ceptors (Weiler et al., 2000) particularly when its extracellular level rises during daylight. D1activation activates, whereas D2receptors inactivate adenylate cyclase, respectively. This results in a corre- sponding change in the intracellular cAMP concentration through which PKA activity and therefore PKA-mediated phosphorylation or phosphatase-mediated dephosphorylation of connexins occurs (McMahon et al., 1989). Interestingly, the leading cause of the curiously rapid turnover of junction proteins is the essentially irreversible phosphorylation of connexins (Vaughan and Lasater, 1990; Bennett et al., 1991; Cook and Becker, 1995). Besides dop- amine, several other active substances including nitric oxide (NO), hormones, arachidonic acid and retinoic acid can modify gap junction coupling or conductance between horizontal cells (Cobbett and Hatton, 1984;Yang and Hatton, 1987; DeVries and Schwartz, 1989;Miyachi et al., 1994;Li and Hatton, 1996;Weiler et al., 2000).
4. Cell-to-cell coupling patterns
In this section we survey the gap junctions between cell types, starting from photoreceptors and moving toward the inner retina.
Generally, similar to gap junctions elsewhere in the vertebrate body, there are homologous (junction between cells of one par- ticular type) and heterologous (interconnection of two morpho- logically and physiologically different cell types) forms of cell-to- cell coupling in the retina mediated by homotypic and heterotypic gap junctions.
4.1. Outer retinal junctions
In the outer retina, gap junctions couple neighboring photore- ceptors, or horizontal cells or bipolar cells (Fig. 2), but only rarely join different major cell types. Interestingly,Trexler et al. (2001) reported various ON and OFF bipolar cells that were occasionally found to be coupled to the horizontal cell network in the rabbit.
However, these connections were concluded to be rare.
4.1.1. Photoreceptor-to-photoreceptor junctions
In most vertebrate species, photoreceptors couple one another via homologous, rod-to-rod, cone-to-cone as well as heterologous rod-to-cone electrical synapses (Figs. 2and4A and B). While such coupling may reduce visual acuity, it is also beneficial for retinal signaling.
Observations using tracer coupling, electrophysiology and electron microscopy demonstrate that rod-to-rod coupling exists in fish (Owen, 1985), amphibians (Fain et al., 1976;Owen, 1985;Krizaj et al., 1998), reptiles (Schwartz, 1975a;Firsov and Green, 1998) and mammals (Tsukamoto et al., 2001;Hornstein et al., 2005). However, the cellular location of gap junctions differs slightly. For example, gap junctions are located quite distally between amphibian pho- toreceptors; they are found on radially extruding membranousfins of rod myoids in axolotls and toads (Fain et al., 1976;Owen, 1985), or even more distal inner segment regions in theXenopus(Krizaj et al., 1998). In contrast, rod-to-rod gap junctions in teleostfish, turtles and mammals are found rather proximally between telo- dendria, between the soma and the rod terminal or the rod ter- minal and the passing rod axon (Owen, 1985; Tsukamoto et al., 2001; O’Brien et al., 2012). While the possession of a single rod type is quite general across vertebrates, some anurans have two or
more rod populations with different spectral sensitivities whose coupling is restricted to the like spectral type (Gold and Dowling, 1979). The connexin subunit makeup of rod-to-rod gap junctions has only been identified in a few animals, including Cx43 in zebrafish and carp (Janssen-Bienhold et al., 1998) and Cx35/36 in the salamander (Zhang and Wu, 2004). These connexin subunits typically form channels with relatively low unitary channel con- ductances (Cx43,Bennett and Verselis, 1992; Cx36,Teubner et al., 2000), allowing for reciprocal, symmetrical but weak electrical coupling between rods (Gj: 500 pS;Zhang and Wu, 2005). Further, low conductance is ascertained by the relatively small size of focal gap junctions between photoreceptors. It is important to point out here, that the strength of coupling is not a function solely of the gap junction conductance, but also that of the cell. So for example, a 500 pS conductance would be trivially small in a horizontal cell with 10 MU input resistance, but it is a lot more significant in a photoreceptor (which has about a 1 GUinput resistance). Col- lectively, therefore, the gap junctions play a large role in the rod network. A possible corollary point is that evolution has matched the gap junction conductance to its cell type.
There is a paucity of data on the connexin makeup of reptile and mammalian rod-to-rod electrical synapses but the extent of tracer- coupling (Firsov and Green, 1998;Hornstein et al., 2005) as well as the relatively small gap junction plaques between rod receptors in ultrastructural studies (Tsukamoto et al., 2001) suggest low overall junctional conductances similar to those offish and salamander. In fact, guinea pig rod-to-rod electrical synapses have recently been presented displaying a low overall conductance of 386 pS (Li et al., 2012) similar to those observed in lower vertebrates. The low conductance of rod-to-rod electrical synapses has been thought to favor the signal passage with slow kinetics, which is a characteristic of single photon evoked rod responses (Zhang and Wu, 2005). In addition, rod homologous coupling increases the signal-to-noise ratio of rod photoreceptors. This is especially important in low light levels when rods absorb photons only sporadically, making light-evoked signals relatively weak considering the high level of dark-noise of the individual rods. It is also possible that, similar to a feedback loop of an amplifier, they moderate the high gain of the output synapse to make the best possible use of its dynamic range (Jacobs and Werblin, 1994). Hypothetically, rod-to-rod coupling fulfills yet another role in mammals, where rods and cones gen- erally form synapses with a separate set of rod and cone bipolar cells, respectively. In this scheme, rod-to-rod coupling pools signals to those scarce (<20%) rods that form‘irregular’chemical synapses with one type of OFF cone bipolar cell (Hack et al., 1999;Tsukamoto et al., 2001;Fyk-Kolodziej et al., 2003;Li et al., 2004a,b).
Cone coupling has been found in most examined vertebrates (turtle:Baylor et al., 1971; turtle, monkey and rabbit:Raviola and Gilula, 1973). The molecular structure of cone-to-cone gap junc- tions are only known in a few systems, where they are formed by Cx36 subunits (birds:Kihara et al., 2009; mammals:Deans et al., 2002;Feigenspan et al., 2004;Lee et al., 2003) that allow for low trans-receptor conductance. It appears that coupling is generally present between spectrally identical receptor subtypes in reptiles (turtle:Detwiler and Hodgkin, 1979) and mammals (ground squir- rel:Li and DeVries, 2004; monkey:O’Brien et al., 2012). However, spectrally indiscriminate coupling occurs in the trichromate primate retina between red and green cones, in which case coupled cones contain spectrally different but evolutionarily closely related pho- tosensitive molecules (Hornstein et al., 2004). Similar to roderod gap junctions, cone coupling averages asynchronous noise out and sums correlated light-evoked signals (DeVries et al., 2002). In con- clusion, rod-to-rod and cone-to-cone electrical coupling seems ubiquitous in all vertebrate classes, in which both are comprised by similar connexin building blocks and play very similar roles.
Regardless of the retinal pathway by which photoreceptor sig- nals reach the output ganglion cells, rod and cone mediated signals mix in most vertebrate species. In lower vertebrates this mixing of information is achieved by the convergence of rod and cone signals onto the same second order bipolar and horizontal cells. In contrast, mammals have two sets of bipolar cells specialized to receive in- formation from either rods or cones. However, the mixing of rod and cone signals still occurs in mammals through electrical syn- apses that connect rods to neighboring cones (Schwartz, 1975b;
Scholes, 1975;Copenhagen and Owen, 1976). An unusual feature of
mammalian horizontal cells is that its perikaryal dendrites connect only to cones, whereas its axon terminal contacts rods exclusively.
Moreover, these two cellular compartments are electrically iso- lated. Thus mixing of rod and cone signals in horizontal cells de- pends on gap junctional connections between rod and cone photoreceptors. Mixing signals at the photoreceptor level ensures that both the soma-dendritic region and the axon terminal of horizontal cells operate under both scotopic and photopic condi- tions. Most vertebrates also contain axonless horizontal cells as well that receive classical chemical synaptic inputs exclusively from Fig. 4.Tracer coupling patterns of outer retinal neurons.A. Neurobiotin injection into a single rod photoreceptor (asterisk) in theXenopusretina displays the coupling of a group of neighboring rods (arrows) and a single cone (open arrow).B. Rodecone coupling in cross-sections of theXenopusretina. A tracer-coupled cone (asterisk) adjacent to neurobiotin- injected and tracer-coupled rod outer segments. OS: outer segments, IS: inner segments, ONL: outer nuclear layer, OPL: outer plexiform layer, INL: inner nuclear layer, IPL: inner plexiform layer, GCL: ganglion cell layer.C. Lucifer yellow injected luminosity-type horizontal cells in the turtle retina. Courtesy of Dr. Paul Witkovsky. An extensive array of coupled axon terminals (open arrow) as well as several coupled cell bodies (arrows) are shown. The soma of the Lucifer yellow injected horizontal cell is marked with an asterisk.D.
Neurobiotin-injected luminosity-type horizontal cells in a section from resin-embeddedXenopusretina. The injected cell is labeled with an asterisk, arrows indicate cell bodies of coupled cells while the open arrow shows a dendritic bundle.E. Neurobiotin-injected chromatic horizontal cells (arrows) in a cross section of theXenopusretina. Retinal layers are as above.F: Photomicrograph showing an extensively coupled synthitium of axon-bearing horizontal cells in the mouse retina. Arrows point to somata, whereas open arrows mark the horizontal cell axon terminals. Scale bars: 40mm inA,B,D,E; 100mm inCand 200mm inF.
cones, whereas rod signal reaches them indirectly via rodecone electrical coupling (Nelson, 1977; Smith et al., 1986). It appears that low conductance Cx36 subunits makeup the cone hemi- channels of most studied mammals (guinea pig:Lee et al., 2003;
mouse: Güldenagel et al., 2001; Feigenspan et al., 2001; Deans et al., 2002). Interestingly, a mixture of Cx34.7 and Cx35 (thefish orthologs of mammalian Cx36) was detected in the bass retina (O’Brien et al., 2004). Contrary to the apparent dominance of Cx36, the identity of rod hemi-channels is uncertain in cones, as a num- ber of reports claim that rods do not express Cx36 (Lee et al., 2003;
O’Brien et al., 2004). A more recent study nonetheless suggests that Cx36 in fact forms the rod hemichannel in the monkey retina as well (O’Brien et al., 2012). There is an unfortunate lack of data on the connexin structure of amphibian, reptile and bird retina.
However, the similar connexin makeup of such phylogenetically distant animal groups such asfishes and mammals suggests that all vertebrate rod-to-cone gap junctions are formed by similar connexins. Of course, one can argue that convergent evolution lead independently to a very similar connexin makeup infish and mammalian rod-to-cone gap junctions to serve similar functions and intermediary lineages do not necessarily share the same fea- ture. Descriptions of various species agree that rod-to-cone elec- trical synapses are not controlled by the ambient light level (monkey: Schneeweis and Schnapf, 1999; mouse and fish:
Ribelayga et al., 2008) but they rather follow the retinal circadian rhythm via dopamine controlled mechanisms in both fish and mammals (Ribelayga et al., 2008). During the day, the level of secreted dopamine increases by which a D2/D4receptor initiated and cAMP/PKA driven intracellular cascade decrease the con- ductance of rod-to-cone gap junctions (Xenopus: Krizaj et al., 1998). This quite similar dopamine mediated control of rod-to- cone gap junction gating infish, amphibians and mammals rein- force the above-mentioned hypothesis concerning the similar connexin building blocks of rod-to-cone electrical synapses.
Therefore, it is highly likely that rod-to-cone gap junctions are not just ubiquitous but their molecular structure is conserved across vertebrates as well.
4.1.2. Horizontal-to-horizontal cell junctions
Horizontal cells are second order inhibitory interneurons whose dendrites and axons extend laterally to contact cone pedicles and rod spherules, respectively (Van Haesendonck and Missotten, 1979;
Wässle and Riemann, 1978). In these synapses, horizontal cells provide inhibition onto photoreceptors, which is a crucial element in forming the receptivefield center-surround mechanism for down- stream retinal neurons. Various vertebrate species display one or more types of horizontal cell in their retina according to the level of photoreceptor diversity in the corresponding species. In essence, each photoreceptor population has its own horizontal cell type that feeds one spectrally tuned photoreceptor with inhibition while others are active (Wu,1992;Stell and Lightfoot, 1975). Irrespective of the number of subtypes, each horizontal cell population appears to form type-specific, extensively coupled syncytia (Figs. 2 and 4CeF), a universal feature of all vertebrate horizontal cells (rabbit e Bloomfield et al., 1995; mouseeJanssen-Bienhold et al., 2009;Xin and Bloomfield, 1999; dogfishe Kaneko, 1971; turtleePerlman and Ammermüller, 1994; frogseMascetti and Ogden, 1989; sala- mandereLasansky, 1980; chickeCooper and McLaughlin, 1981).
Interestingly enough, axon-bearing horizontal cells form two inde- pendent coupled arrays, one connecting the soma/dendritic regions of neighbor horizontal cells and another, spatially somewhat more restricted, array of horizontal cell axon terminals. Homologous coupling of horizontal cells serves to average background luminance mediated signals across the electrically connected horizontal cell array. This averaged signal provides an equalized inhibition over
large retinal areas (the luminous background signal) against which the local photoreceptor signal is distinguished. This is the basic cir- cuit for contrast detection in the photoreceptor layer, and it is reprized in the bipolar cells. Such signal averaging mechanism re- quires high conductance electrical synapses among horizontal cells.
The relatively easy passage of current and tracers through horizontal cell gap junctions has been indicated by a cohort of findings including: (i) the extensive tracer coupling, (ii) the extended receptive field size (Bloomfield et al., 1995; Xin and Bloomfield, 1999) and(iii)the passage offluorescent dyes like Procion Yellow and Lucifer Yellow (Kaneko, 1971;Dacheux and Raviola, 1982). The molecular structure of the low resistance horizontal cell connections were found to be slightly variable across species as Cx57 mediates coupling in mouse (Janssen-Bienhold et al., 2009;Palacois-Prado et al., 2009), Cx50/Cx57 in rabbit (Huang et al., 2005;O’Brien et al., 2006;Cha et al., 2012), Cx35/36 in carp (Liu et al., 2009) and both Cx52.6 and Cx55.5 in zebrafish (Zoidl et al., 2004). However, analyses of DNA coding sequences revealed that most of these connexins (with the lone exception of the carp Cx35/36) display remarkably high sequence homology (see alsoFig. 1), and represent connexin orthologs rather than different species-specific connexins. Among all vertebrates, perhapsfish display the highest variety of connexin subunits utilized to form horizontal-to-horizontal cell gap junctions.
This diversity in connexin makeup likely corresponds to the large diversity of horizontal cell subtypes described in the retina of vari- ousfish species (Stell and Lightfoot, 1975;Connaughton and Nelson, 2010;Schieber et al., 2012). It is tempting to speculate that each horizontal cell type expresses different connexin subunits to form homologous junctions. Indeed,Dermietzel et al. (2000)suggested that Cx55.5 is expressed by only a subset of zebrafish horizontal cells, whereas the rest of the horizontal cells expressed other connexins.
Apart from Cx50, these previously mentioned connexin subtypes form channels with relatively moderate unitary conductance (McMahon, 1994;Lu and McMahon, 1996;Srinivas et al., 1999a,b;
Zoidl et al., 2004;Palacios-Prado et al., 2009). However, many of them aggregate in huge plaque-like structures (Witkovsky et al., 1983;Janssen-Bienhold et al., 2009), thus providing afirm basis for the peculiarly high overall trans-junctional conductance (Lu and McMahon, 1996) that can be as high as 30 nS inin vitroexpression systems (Palacios-Prado et al., 2009) or can, in theory, reach themS range (O’Brien et al., 2006). The extensive coupling of horizontal cells can be dynamically modified by light levels and by circadian rhythms (Kohler et al., 1990;Kurz-Isler et al., 1992). Accordingly, horizontal cell coupling is more extensive in dim light than in darkness or bright light (Baldridge and Ball, 1991; Xin and Bloomfield, 1999), hence a gradual increase of the background light first enhances trans-junctional conductance, but further brightening initiates an opposite, conductance-decreasing mecha- nism. These light induced changes in horizontal cell coupling are mediated by altered dopamine levels (Piccolino et al., 1987). Dop- amine, by binding to D1receptors on the horizontal cell membrane, evokes an increase in intracellular cAMP level (Lasater, 1987) fol- lowed by the activation of PKA, which in turn triggers phosphor- ylation of connexins and the reduction of gap junctional conductance (McMahon et al., 1989). The observed large number of phosphorylation sites present in the C-terminal of horizontal cell connexins (Zoidl et al., 2004) is plausibly the site of intracellular regulation through phosphorylation. Besides dopamine, nitric oxide (NO) also affects horizontal cell coupling, acting through the alter- ation of the intracellular cGMP level (DeVries and Schwartz, 1989;
McMahon, 1994). In contrast to dopamine, however, NO only re- duces the opening frequency, not the average open time (McMahon, 1994), and may also suppress endogenous dopamine release (Bugnon et al., 1994). In addition to dopamine and NO, retinoic acid (RA) appears to act as a light-dependent modulator (Weiler et al.,
1998,1999,2000). It acts through a dopamine-independent mech- anism (Weiler et al., 1999) but the retinal RA receptors and the intracellular cascade involved have not yet been identified.
4.1.3. Bipolar-to-bipolar cell junctions
Bipolar cells are vertically oriented glutamatergic interneurons (with very few exceptions in some non-mammalian species) that relay signals from photoreceptors to ganglion and amacrine cells in the inner retina. While there is mounting evidence that lower vertebrate bipolar cells maintain electrical coupling with one another (Van Haesendonck and Missotten, 1983;Marc et al., 1988a;
Arai et al., 2010), their mammalian functional counterparts are less studied due to their small size and hence relative inaccessibility to intracellular recording methods. The rationale for electrical cou- pling of bipolar cells is still unclear. It may decrease the dispersion of input signals permitting bipolar cells to respond uniformly to light (Umino et al., 1994) and ultimately leading to a better signal- to-noise ratio, similar to that seen between photoreceptors (Jacobs and Werblin, 1994). Nevertheless, data on bipolar-to-bipolar cell coupling is not sufficient to assign a definite function to them, thus a comparison between vertebrate groups is premature.
Yet there are a few consistent and conserved features across examined species including:(i)electrical coupling is not general but rather is specific to only certain bipolar cell types;(ii)similar to those of photoreceptors, gap junctions connecting bipolar cells are small and focal and(iii)bipolar-to-bipolar gap junctions can occur between bipolar cell dendrites and/or axon terminals (Fig. 2;fishe Marc et al., 1988a;Arai et al., 2010; mammalseRaviola and Gilula, 1975;Kolb, 1979;Cohen and Sterling, 1990;Vaney, 1994). Although these junctions can be heterologous (Mills, 1999), coupled bipolar cells seem to follow the segregation of ON/OFF signal streams in mammals (Raviola and Gilula, 1975; Kolb, 1979; Saito and Kujiraoka, 1988;Cohen and Sterling, 1990). The connexin makeup of these peculiar gap junctions has only been described in a few instances. For example, Cx36 has been localized to mouse OFF bi- polar cell dendrites (Feigenspan et al., 2004) and goldfish Mb1 bi- polar cell axon terminals (Arai et al., 2010). In addition, Cx45 has recently been detected in juxtaposition with dendritic crossings of similar type OFF bipolar cells, whereas Cx45 subunits seem to form heterologous gap junctions between axon terminals of OFF bipolar cells in the mouse retina (Hilgen et al., 2011). It is even less detailed how these gap junctions are regulated, albeit it seems that NO rather than dopamine modulates bipolar-to-bipolar electrical and tracer coupling (Sakai and Ball, 1994).
4.2. Inner retinal junctions
Bipolar cells are interneurons that relay signals from the outer to the inner retina. As mentioned above, some bipolar cells form gap junctions with each other but they also participate in gap junctions with other cell types, particularly with amacrine cells in the inner retina (Fig. 5A). However, the most conspicuous gap junctions of the inner retina are formed between amacrine cells, ganglion cells and certain amacrine and ganglion cell subtypes (Figs. 2and5).
4.2.1. Amacrine-to-amacrine cell gap junctions
The majority of amacrine cells are inhibitory interneurons expressing either GABA or glycine as a neurotransmitter. Amacrine cells provide inhibition to bipolar cell axon terminals, ganglion cell dendrites and other amacrine cell processes in the inner plexiform layer (IPL). They represent a remarkably diverse population regarding their morphology, biochemistry and electrophysiology.
Most amacrine cell somata are located in the INL, although all examined species possess a cohort of amacrine cell somata dis- placed into the GCL and some that are embedded in the IPL. The
spatial geometry of amacrine cell processes is remarkably variable, from small and diffuse to large and monostratified (Masland, 1988).
Polyaxonal amacrine cells possess dendrites as well as axon-like processes. Functionally, amacrine cells show ON, OFF or ONeOFF physiology and, in addition to their primary transmitter, may ex- press one from the long list of neuromodulator substances like dopamine, serotonin, substance P, enkephalin, somatostatin, etc. It has widely been thought that the population of amacrine cells consists of some 30 subtypes, each playing a specific role in visual signal processing (reviewed byWässle and Boycott, 1991). There is mounting evidence from various vertebrates that amacrine cells are coupled via gap junctions to form homotypic mosaics.Teranishi et al. (1987)identified at least three independent amacrine cell populations infish, each showing evidence of both electrical and dye coupling indicative of electrical synapses. Other vertebrates also possess amacrine-to-amacrine cell gap junctions, e.g., salamander (MacLeish and Townes-Anderson, 1988), chicken (Kihara et al., 2009) and various mammals (Dacheux and Raviola, 1986;
Bloomfield, 1992;Strettoi et al., 1992;Chun et al., 1993;Xin and Bloomfield, 1997;Völgyi et al., 2001;Li et al., 2002;Aboelela and Robinson, 2004; Vaney, 2004; Wright and Vaney, 2004;
Bloomfield and Völgyi, 2007). Mammalian AII amacrine cells rep- resent a specific case, as they are integrated into the so-called pri- mary rod pathway (Fig. 2), synapsing onto ON center bipolar cells (see below). In addition, they form homologous electrical synapses with neighboring AII amacrine cells in all examined mammalian species (Dacheux and Raviola, 1986;Strettoi et al., 1992;Chun et al., 1993;Mills and Massey, 1995). The AII cell network mediates spatial averaging of AII cell activity (Vardi and Smith, 1996), thus improving the signal-to-noise ratio of AII cells and increasing the sensitivity of the primary rod pathway (Smith and Vardi, 1995;Vardi and Smith, 1996;Bloomfield and Völgyi, 2004;Völgyi et al., 2004). The trans- membrane conductance of AII-to-AII gap junctions is moderate (320e390 pS;Veruki et al., 2010) due to the low-conductance Cx36 subunit building blocks (Feigenspan et al., 2001;Güldenagel et al., 2001;Mills et al., 2001;Deans et al., 2002). Interestingly, the cou- pling strength of AII amacrine cells is modulated by the ambient light level (Xin and Bloomfield, 1999). A gradual increase of lightfirst induces an increase and then a decrease in the diameter of the tracer-coupled AII cell network indicating corresponding changes in the conductance through AII-to-AII gap junctions (Bloomfield and Völgyi, 2004). These light initiated changes are mediated by an increased extracellular level of dopamine (Hampson et al., 1992) that acts through D1receptors in the AII amacrine cell membrane. D1 activation initiates an intracellular cascade, whereby an increase in the intracellular level of cAMP results in the activation of PKA (Urschel et al., 2006). Interestingly, activated PKA does not act directly on Cx36 subunits of these gap junctions, but rather phos- phorylates another enzyme, the protein phosphatase 2A. Protein phosphatase 2A then dephosphorylates Cx36 to reduce gap junc- tional conductance (Kothmann et al., 2009). It has recently been found that glutamate released from presynaptic ON bipolar cells induced activation of nonsynaptic NMDA receptors of AII cells and a subsequent calmodulin kinase II (CaMKII) mediated phosphor- ylation of gap junction-forming Cx36 subunits (Kothmann et al., 2012). This increase in Cx36 phosphorylation works in opposition to the above-mentioned dopamine-driven reduction of phosphor- ylation. These two opposing mechanisms provide a firm back- ground for the observed changes in AII coupling by background illumination by which a gradual augmentation of background illu- minancefirst increases, then decreases the size of the coupled AII field (Bloomfield and Völgyi, 2004).
Amacrine-to-amacrine cell junctions of non-AII amacrine cells may in fact serve to summate sustained components of amacrine cell light responses as suggested by experimental data in fish
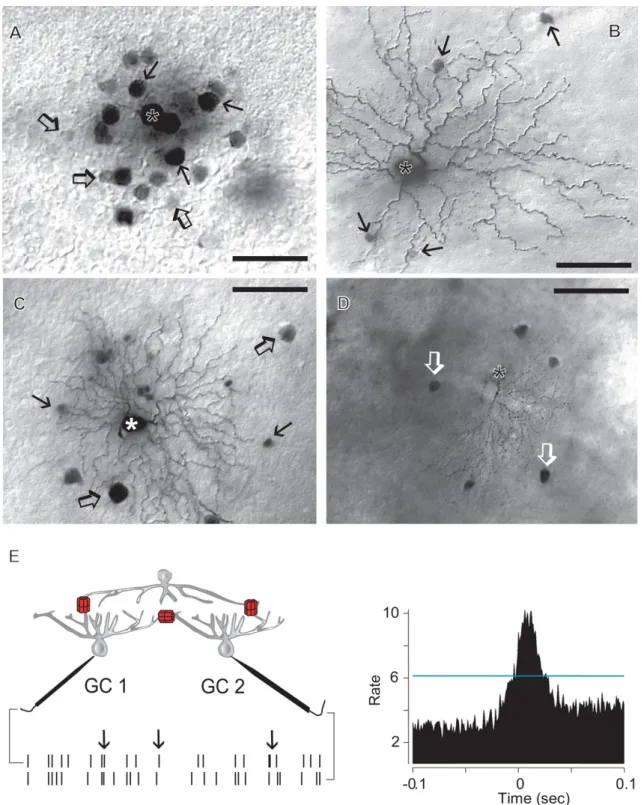
Fig. 5.Tracer-coupling patterns of mouse inner retinal neurons.A. Photomicrograph showing the tracer-coupling pattern of a Neurobiotin injected AII amacrine cell (asterisk). This AII amacrine cell displays coupling to an array of neighboring AII cells (arrows) and nearby bipolar cell bodies (open arrows).B. Neurobiotin injected retinal ganglion cell (asterisk) displays sporadic tracer-coupling to amacrine cell bodies (arrows) within thefield of the dendritic arbor.C. Neurobiotin injected ganglion cell (asterisk) displays coupling to both nearby ganglion (open arrows) and amacrine cell (arrows) somata.D. Neurobiotin injected ganglion cell (asterisk) displays tracer coupling to a local array of direct neighbor ganglion cells (open arrows).E. Drawing shows the recording paradigm when simultaneous spike recordings are performed from a pair of retinal ganglion cells (GC1 and GC2). Red symbols represent gap junctions located directly between the recorded ganglion cells and between ganglion cells and a common presynaptic amacrine cell. Time stamps on the bottom represent spike trains of the two recorded cells. In this recording spontaneous spikes of one cell tend to follow spikes of the other cell (arrows). Cross correlation function on the right shows a characteristic peak centered at 0 ms. Note, that the peak is above the 99% confidence interval (blue line) indicating that spikes of the two recorded neurons follow each other above chance. Such correlation peaks are generally found when ganglion cells share inputs form gap junction coupled amacrine cells (see left). Scale bars: 25mm inA, 50mm inBandCand 100mm inD.
(Hidaka et al., 1993). It has also been hypothesized that homologous amacrine cell gap junctions synchronize signals to provide spatially and temporally correlated inhibition, affecting the output signal of ganglion cells (Aboelela and Robinson, 2004). Such correlated amacrine cell inhibition has been shown to provide the basis for retinal ganglion cell oscillation in frogs that ultimately serve the basis for complex, visually guided motor responses like escape behavior (Ishikane et al., 2005). Descriptions of the molecular makeup of gap junctions are limited to Cx45 in some mammalian interplexiform cells (Dedek et al., 2009), Cx36 in bird amacrine cell gap junctions (Kihara et al., 2009) and to Cx43 infish (Janssen- Bienhold et al., 1998). However, based on the large variety of observed vertebrate amacrine cells, we assume that the number of known connexins serving amacrine-to-amacrine cell electrical synapses will increase in coming years. Even though it has only been studied in a few model systems, it is clear that amacrine-to- amacrine cell gap junction conductance is another feature that diversifies this neuron population: some ACs lack gap junctions while others have diverse gap junction subtypes. More specifically, macroscopic conductance of amacrine cell gap junctions can range from relatively low values, for instance, those of mammalian AII amacrine cells (320 pS;Veruki et al., 2010), to high ones; like those observed infish wide-field amacrine cells (2.4 nS;Hidaka et al., 2005). It has been shown that, similar to those of mammalian AII cells, transjunctional conductance of amacrine cell gap junctions in fish retina, are reduced by a D1 receptor-mediated increase of intracellular cAMP level (Mills et al., 2007; Hidaka, 2008 2012).
Nevertheless, the observed variety in molecular structure, con- ductance and intracellular modulation reflects the differences among examined amacrine cell types within each animal group rather than real evolutionary changes. On the other hand, AII amacrine cells are only found in mammals; therefore, vertebrate evolution did produce new gap junctions with new function(s), at least in those serving AII amacrine cells.
4.2.2. Ganglion-to-ganglion cell gap junctions
Ganglion cells are the only projection neurons, which means they provide the sole output from the vertebrate retina to visual centers of the brain. Retinal ganglion cells are feature detectors. As a result, the number of functionally different ganglion cell pop- ulations correlates well to the number of encoded visual features (luminosity, contrast, movement, direction of movement etc.) of the scene. The number of cells within each subtype determines the resolution by which a certain visual feature is interpreted toward the brain. Due to their central role in determining visual quality and acuity, ganglion cells have long been thought to act as independent encoders. Each ganglion cell perceives only a little facet of the image over its dendritic arbor, and signals are detected, analyzed and transmitted independent of the activity of nearby ganglion cells.
Over the past decades, however, this dogmatic picture has been challenged by both morphological and electrophysiological obser- vations showing the presence of gap junctions and tracer- and/or electrical coupling between ganglion cells (Mastronarde, 1983a,b,c;
Vaney, 1991,1994; Hitchcock, 1993; Xin and Bloomfield, 1997;
Brivanlou et al., 1998; DeVries, 1999; Hu and Bloomfield, 2003;
Schnitzer and Meister, 2003;Schubert et al., 2005a,b;Völgyi et al., 2005, 2009; Shlens et al., 2006; Hoshi et al., 2006; Trong and Rieke, 2008;Greschner et al., 2011). These observationsfirst sug- gested that ganglion cells signal laterally, consequently com- promising visual acuity of neuronal signals. It is now clear, however, that conductance of ganglion cell gap junctions is moderate, as seen in some ganglion cells of fish (1.35 nS; Hidaka et al., 2004).
Accordingly, electrical and tracer coupling is often confined to direct neighbors (Bloomfield, 1992;Xin and Bloomfield, 1997;Völgyi and Bloomfield, 2000). Some mammalian ganglion-to-ganglion cell
connections are comprised by Cx36 (Hidaka et al., 2002, 2004;
Schubert et al., 2005b) or Cx45 (Schubert et al., 2005b) in rats and mice, however, the identity of a number of homotypic ganglion cell gap junctions is yet to be determined. Moreover, the molecular makeup of ganglion cell gap junctions of other vertebrate species is largely unknown. The role of ganglion-to-ganglion cell electrical synapses has long been debated. The most recent view is that instead of signaling laterally, ganglion cells use electrical synapses to correlate their spiking activity with those of their direct neigh- bors. Based on computational models, it has been suggested that any possible information loss caused by spike averaging can be overcome by exploiting the information encoded by spike correla- tions (Kenyon et al., 2004). Such ganglion cell spike correlations have been detected in various vertebrate groups, including fish (Hidaka et al., 2002,2004), amphibian (salamander:Brivanlou et al., 1998), and various mammalian retinas (Mastronarde, 1983a,b,c;
DeVries, 1999; Hu and Bloomfield, 2003; Schnitzer and Meister, 2003;Schubert et al., 2005a,b; Völgyi et al., 2005,2009;Shlens et al., 2006;Hoshi et al., 2006;Trong and Rieke, 2008;Greschner et al., 2011). Ganglion cell spike synchronization has been sug- gested to encode information of the visual scene (Meister and Berry, 1999; Schwartz et al., 2007) to predict stimulus modulation (Schwartz et al., 2007;Schwartz and Berry, 2008) or to serve tem- poral binding of information or salient signaling along the visual axis (reviewed byUsrey et al., 1999). Mammalian ganglion cell gap junctions have been found modulated by dopamine through D2
receptors (Mills et al., 2007). Background illumination elevates the level of extracellular dopamine that, upon receptor binding, inhibits adenylate cyclase. Adenylate cyclase inactivation reduces the intracellular cAMP level and the PKA mediated Cx phosphorylation, thereby elevating the conductance of ganglion-to-ganglion cell electrical synapses in the alpha ganglion cell system (Mills et al., 2007). This light-induced conductance change was reflected by both a strengthening of ganglion-to-ganglion cell tracer coupling, and an enhanced spike correlation of neighboring alpha ganglion cells (Mills et al., 2007;Hu et al., 2010).
4.2.3. Ganglion-to-amacrine cell gap junctions
Besides the homologously coupled inner retinal neuronal net- works, amacrine and ganglion cells often form heterologous amacrine-to-ganglion cell electrical synapses, thereby interlacing between the two electrically connected homologous inner retinal arrays. Despite the fact that heterologous coupling of amacrine and ganglion cells has long been known in lower vertebrates like sal- amander (Brivanlou et al., 1998;Schnitzer and Meister, 2003) and various mammalian species (Vaney, 1991,1994;Xin and Bloomfield, 1997; Völgyi et al., 2005, 2009), the only complete survey for amacrine-to-ganglion cell coupling was performed in mouse retina (Völgyi et al., 2009). In this animal, 14 out of 22 morphologically identified ganglion cell subtypes were found tracer coupled to distinct subsets of amacrine cells. This strongly suggests that electrical coupling of amacrine and ganglion cells are integrated elements of most circuits that serve ganglion cell signaling.
The function of heterologous amacrine-to-ganglion cell con- nections is still elusive. It is possible that amacrine cells, by pro- viding common input to a number of neighbor ganglion cells, serve to correlate ganglion cell spikes. This process may seem similar to those proposed for amacrine-to-amacrine and ganglion-to- ganglion cell networks, still, it has been hypothesized that these three inner retinal electrical connections underlie different forms of ganglion cell spike correlation, likely serving three distinct visual functions (reviewed byBloomfield and Völgyi, 2009). Such gan- glion cell spike synchronization has been suggested to encode in- formation of the visual scene (Meister and Berry, 1999;Schwartz et al., 2007). In fact, ON directional selective ganglion cells have