Chemistry and Metabolism of the Adrenal Cortical Hormones1
B Y R . D . H . H E A R X ) CONTENTS
Page
I. Historical Introduction and Synopsis 550
II. Hormone Concentrates 551 III. The Steroids Isolated in Crystalline State from Adrenal Tissue 552
A. List of Cornpounds 552 B. Methods of Isolation 560 C. Structure and Properties 560
1. The Carbon Ring Skeleton. 561
2. T h e Side Chain 563 3. The Nuclear Substituents 567
a. Position 567 b. Stereoisomerism of the Hydroxyl Groups 570
IV. Artificial Preparation of the Active Adrenal Steroids 570
A. The 11-Desoxy Compounds 570 1. Partial Synthesis of 11-Desoxycorticosterone 570
2. Partial Synthesis of 17-Hydroxy-l 1-desoxycorticosterone 572
B. The 11-Oxygenated Compounds 574 1. Degradation of the Bile Acid Side Chain 575
2. Introduction of Oxygen at C - l l 578 3. Partial Synthesis of 11-Dehydrocorticosterone 586
4. Partial Synthesis of Corticosterone 587 5. Partial Synthesis of 17-Hydroxy-l 1-dehydrocorticostarone 589
V. The Amorphous Fraction 592 V I . Relationship between Chemical Structure and Physiological Action 594
A. Rings A and Β 594 Β. Ring C 597 C . The Side Chain 600 V I I . Metabolism of the Adrenocortical Hormones 602
A. Catabolism. Urinary Excretory Products as Indices of Adrenocortical
Function 602 1. 17-Ketosteroids 607
2. Cortin 609 3. Neutral Lipide-Soluble Reducing Substances 610
4. 17-Ketosteroids Generated on Periodic Acid Oxidation of
Neutral Non-ketonic Lipides 611
1 The author is much indebted to Drs. J. S. L. Browne, D . A. Prins and D . L.
Thomson for considered criticism of various aspects of this review and Miss J. Cohen for valuable help in the preparation of the manuscript.
549
550 R . D . H . H E A R D
Page 5. Formaldehyde Generated on Periodic Acid Oxidation of Neutral
Lipides 6 1 3 6. A8- A n d r o s t e n e - 3 ( j S ) , 1 7 ( a ) - d i o l 6 1 4
7. A * - A n d r o s t e n e - 3 ( / 3 ) , 1 6 , 1 7 - t r i o l 6 1 4 8 . A5- P r e g n e n e - 3( / 3) , 2 0 a - d i o l 6 1 5 9. P r e g n a n e - 3 ( a ) , 2 0 a - d i o l 6 1 5 1 0 . P r e g n a n e - 3 ( « ) , 1 7 , 2 0 - t r i o l 6 1 6 1 1 . P r e g n a n e - 3 ( a ) , 1 7 - d i o l - 2 0 - o n e 6 1 6 1 2 . Urinary Steroids Oxygenated at C - l l 6 1 7 1 3 . Urinary Steroids Probably of Cortical Origin 6 1 9
B . Anabolism 6 2 1 References 6 2 3
I. Historical Introduction and Synopsis
The first extracts of cortical tissue which would maintain life in the adrenalectomized animal were prepared in 1930 by Hartman and Brow- nell (81) and b y Swingle and Pfiffner (247). The active agent was designated "cortin" by Hartman and co-workers, a name which became ambiguous in meaning when subsequent investigations led to the isola- tion of at least six pure chemical compounds, two of which (the "desoxy "
group) are highly active with respect to life maintenance and the reten- tion of sodium and chloride ions but relatively impotent as regards carbohydrate metabolism, and four of which (the 11-oxygen group) show marked activity in the latter connection but are relatively ineffective in the survival test. Further, after the separation from cortical extract of all material crystallizable b y the application of the existing methods, there remains an "amorphous fraction" which retains of the order of
14-30% of the activity (based on life maintenance) of the original whole extract. The term "cortin" is used today in several senses, to denote (a) the active substances contained in whole adrenal extract, (b) any life maintenance factor of unestablished chemical nature (as in the amorphous fraction), and (c) substances, again of unrecognized constitution, which possess any kind of cortical activity (i.e., urinary "cortin," the active excretory products in urine, concentrates of which promote life main- tenance, sodium and chloride ion retention, and gluconeogenesis).
Intensive investigation aimed at the isolation and chemical charac- terization of the cortical hormones was undertaken from 1934 b y four investigators with their respective collaborators, E. C. Kendall, J. J.
Pfiffner, and 0 . Wintersteiner, in the United States, and T. Reichstein, in Switzerland. Several crystalline compounds were quickly obtained by each group, and designated, in the order of isolation, by the letters of the alphabet, but physiological activity was not clearly associated with a crystalline product until 1936, when Mason, Myers, and Kendall (167)
established the effectiveness of their Compound Ε (VIII, page 557) in the work performance test of Ingle (108). The same compound had also been isolated by Wintersteiner and Pfiffner—Compound F (268)—
and by Reichstein—Compound Fa (205)—who found it inactive at the dose levels employed for maintenance of life in the adrenalectomized dog and recovery of fatigued muscle (Everse-de Fremery test, 54). At the same time Reichstein (205) separated in crude state his Compound H, which in 1937 was obtained in pure form (206) and shown to be highly active (65, Everse-de Fremery test); the name corticosterone was assigned. There followed the elucidation of the structure of the com- pounds, and the isolation from cortical extract, chiefly by Reichstein and collaborators, of a series of 26 crystalline steroids, six of which possess marked cortical activity. The first of these to be prepared artificially was 11-desoxycorticosterone ( X X I I , page 558), which Steiger and Reichstein (243) obtained in 1937 in fair yield from cholesterol or stigmasterol as original starting material. The partial synthesis of the adrenal hormones bearing an oxygen atom in the 11 position presented serious chemical difficulties which were not overcome until 1943, when Lardon and Reichstein (127) first realized 11-dehydrocorticosterone (XVI, page 558) from desoxycholic acid in extremely minute yield.
II. Hormone Concentrates
In principle, the preparation of cortical extracts for experimental and clinical use involves the extraction of fresh whole adrenal glands with a neutral water-miscible organic solvent, such as acetone or alcohol, followed by the separation of the hormone mixture from much inert material and from adrenaline by various solvent partition procedures.
The final concentrate is then adjusted to contain an arbitrarily chosen number of biological units per unit volume, or the potency is stated in terms of the equivalent weight of whole adrenal tissue.
While many preparative methods are described, that of Kuizenga et al. (120,122) suffices to illustrate (Table I) a typical process, which yields, from 1000 lb. of beef adrenals, 9 g. of final product containing the biological equivalent of 2.5 g. of Kendall's Compound Ε (VIII), when compared by the work performance test of Ingle (111), or 8 rat units per mg. when assayed by the survival growth test of Cartland and Kuizenga (28).
Species variation in total hormone content of the gland is considerable.
Based on either the work test of Ingle (111) or the time survival test (28), extracts from hog adrenals show approximately double the potency of those from beef or sheep glands (122).
(See Table IV, page 560.)
552 R. D. H. HEARD T A B L E I
P R E P A R A T I O N OF A D R E N A L C O R T I C A L E X T R A C T
(Beef adrenals (1000 Ib.), ground into 300 gal. acetone, extracted 7 days, and filtered)
8 0 % Acetone extract (400 gal.)
Acetone removed; aqueous solution extracted with Skelly Solv. Β
Gland residue (discarded)
Aqueous solution
Extracted with ethylene dichloride
Skelly Solv. extract
Ethylene dichloride solution (30 g.)
Partition between (a) 70 % ethanol and Skelly Solv. Β (6) 30 % methanol and Skelly Solv. Β
3 0 % Methanol solution (13 g.)
Methanol removed; extracted with ethyl acetate
Ethyl acetate solution
Washed with 1 % N a2C 03 and 0.5 Ν HCl to remove acidic and basic material
i
Neutral ethyl acetate fraction
(9 g. = biological equivalent of 2.5 g. of V I I I ;
= 75,000 rat units)
Aqueous solution (adrenaline)
Skelly Solv. extract
Aqueous residue
III. The Steroids Isolated in Crystalline State from Adrenal Tissue A . LIST OF COMPOUNDS
Set out in Table II and formulated2 in Table III are the steroids isolated in crystalline state from adrenal extracts. The arrangement in Table II (according to carbon and oxygen content) and the recorded physical constants are taken from Reichstein and Shoppee (212). For the complete bibliography and a full description of the individual com- pounds, reference should be made to this and other review articles
(120,241).
The adrenal steroids lend themselves to several methods of classifica- tion, each of which possesses certain merits, both from the chemical and
2 The configurations at C - l l and C-17 are corrected and expressed in accord- ance with the findings recorded later (pages 564 and 574;. Some formulations there- fore deviate, in part, from those assigned in certain of the original communications.
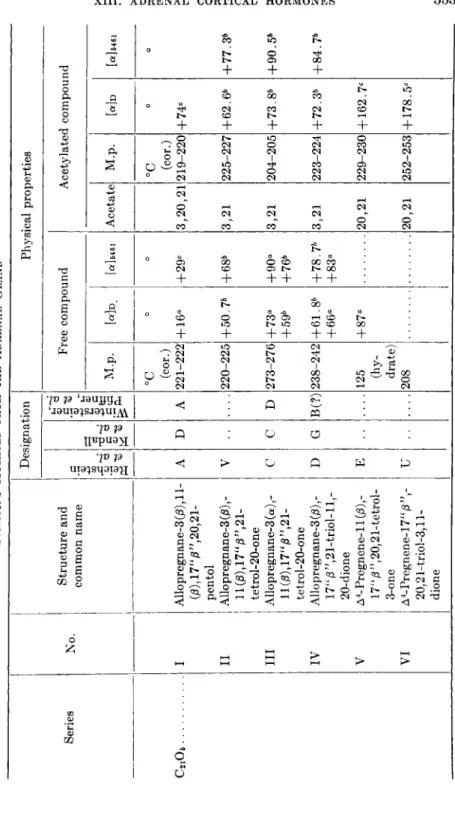
TABLE II STEROIDS ISOLATED FROM THE ADRENAL GLAND Series No. Structure and common name Designation Physical properties Series No. Structure and common name Reichstein et al.
Kendall et al.
Wintersteiner, Pfiffner, et al.
Free compound Acetylated compound Series No. Structure and common name
Reichstein et al.
Kendall et al.
Wintersteiner, Pfiffner, et al.
M.p. [α]ϋ [α]δ461 Acetate M.p. Mo [<x]646 °C ο ο °C ο ο (cor.) (cor.) C2i06 I Allopregnane-3 (β), 11-A D A 221-222 + 16« +29c 3,20,21 219-220 +74* G8),17"j8",20,21-3,20,21 pentol II Allopregnane-3 (β) ,-V 220-225 +50.7b +68* 3,21 225-227 +62.6b +77.3 llG8),17"j8",21- tetrol-20-one III Allopregnane-3 (a) ,-C C D 273-276 +73tt +90« 3,21 204-205 +73. Sb +90.5 \\{ß),\7uß",2l-+59b +76» tetrol-20-one IV Allopregnane-3 (β) ,-D G B(?) 238-242 +61. Sb +78.7b 3,21 223-224 +72.3b +84.7 17"/3",21-triol-ll,-+66« +83° 20-dione V A4-Pregnene-ll(/3),-Ε 125 +87« 20,21 229-230 + 162.7C l7"0",2O,21-tetrol-(hy- 3-one drate) VI A4-Pregnene-17"j8",-U 208 20,21 252-253 + 178.5C 20,21-triol-3,ll-U 208 20,21 252-253 + 178.5C dione
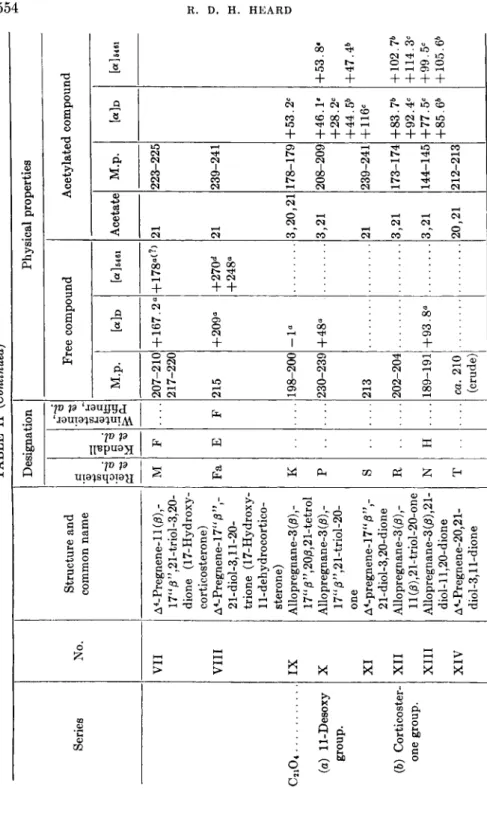
TABLE II (Continued) Series C2104 . (a) 11-Desoxy group. (b) Corticoster- one group.
No. VII VIII IX X XI XII XIII XIV
Structure and common name A4-Pregnene-ll(/S),- 17"0",21-triol-3,2O- dione (17-Hydroxy- corticosterone) A4-Pregnene-17"/3",- 21-diol-3,11-20- trione (17-Hydroxy- 11-dehy drocortico- sterone) Allopregnane-3 (β) ,- 17"/3",20/3,21-tetrol Allopregnane-3 (β),- 17"/3",21-triol-20- one A4-pregnene-17" β 21-diol-3,20-dione Allopregnane-3 (β) ,- ll(/3),21-triol-20-one Allopregnane-3 (β), 21- diol-ll,20-dione A4-Pregnene-20,21- diol-3,ll-dione
Designation M Fa Κ Ρ S R Ν Τ
σ3 . Η
α» 5*
Physical properties
Οι Οι Free compound M.p. 207-210 217-220 215 198-200 230-239 213 202-204 189-191 ca. 210 (crude) + 167.2° +209« -1° +48° +93.8°
[ot] 64 61 + 178α<?> +270d +248«
Acetylated compound Acetate 21 21 3,20,21 3,21 21 3,21 3,21 20,21
M.p. Md [α] 6461 223-225 239-241 178-179 +53.2C 208-209 +46.1* +53.8· +28.2C +44.5b +47.4b 239-241 + 116« 173-174 +83.7b + 102.7b +92.4C + 114.3C 144-145 +77.5C +99.5C +85.6* + 105.6b 212-213
σ a a > 50 σ
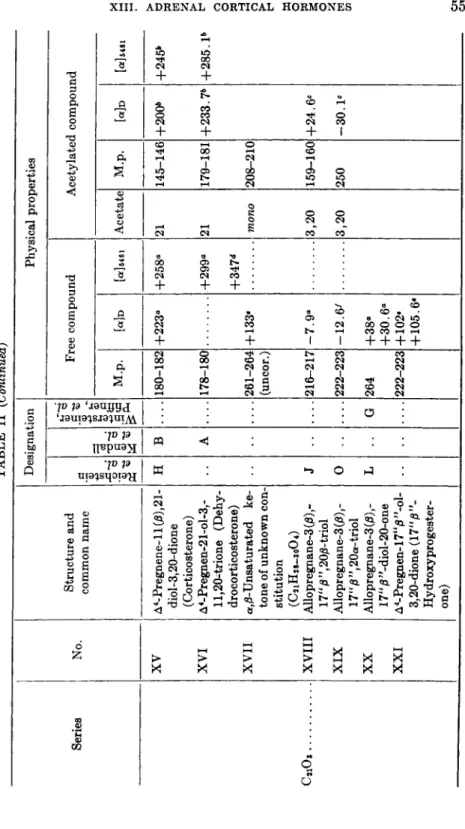
TABLE II (Continued) Series No. Structure and common name Designation Physical properties Series No. Structure and common name Reichstein et al.
Kendall 1 et al.
Wintersteiner, Pfiffner, et al.
Free compound Acetylated compound Series No. Structure and common name
Reichstein et al.
Kendall 1 et al.
Wintersteiner, Pfiffner, et al.
M.p. Wd [α]δ461 Acetate M.p. [<*] 546 XV A4-Pregnene-ll(/3),21-H Β 180-182 +223° +258* 21 145-146 +200* +245* diol-3,20-dione (Corticosterone) XVI A4-Pregnen-21-ol-3,-A 178-180 +299« 21 179-181 +233.7b +285. Ρ ll,2(Urione (Dehy- drocorticosterone) +347*" XVII α,/3-Unsaturated ke-261-264 + 133· mono 208-210 tone of unknown con-(uncor.) stitution (C21H28-30O4) C2iOî XVIII Allopregnane-3 (β) ,-J 216-217 -7.9* 3,20 159-160 +24.6« XVIII 17"j3",20/3-triol 216-217 -7.9* 3,20 159-160 +24.6« XIX Allopregnane-3 (β) ,-0 222-223 -12.6/ 3,20 250 -30. Ie 17"0",2Oa-triol 3,20 XX Allopregnane-3 (β), -L G 264 +38» 17"/3"-diol-20-one +30.6° XXI A4-Pregnen-17"0"-ol-222-223 +102· 3,20-dione (17" 0"-+105.6· Hydroxyprogester- one)
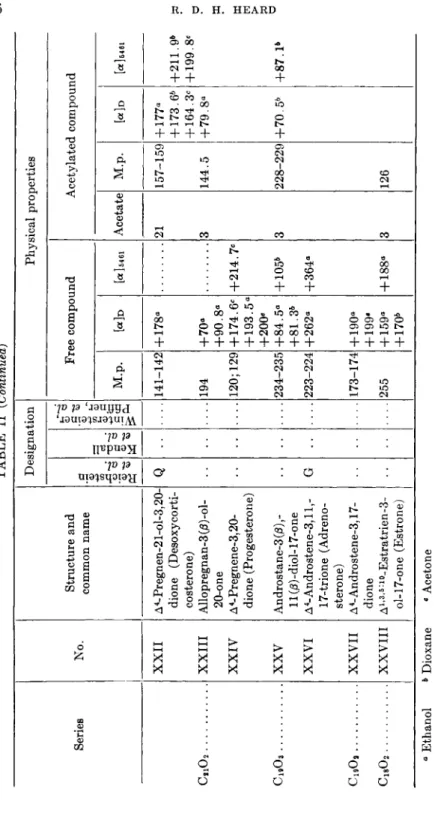
TABLE II (Continued) Designation Physical properties Series No. Structure and common name ein steiner, :r, et al.
Free compound Acetylated compound
Reichst et al.
Kendal et al.
Winten Pfiffne
M.p. [«]d [a] 54 6i Acetate M.p. [«]d M 64 61 XXII A4-Pregnen-21-ol-3,20- dione (Desoxycorti- costerone)
Q 141-142 + 178° 21 157-159 + 177° + 173.6* + 164.3C
XXII A4-Pregnen-21-ol-3,20- dione (Desoxycorti- costerone)
Q 141-142 + 178° 21 157-159 + 177° + 173.6* + 164.3C +211.9* + 199.8C CS102 XXIII Allopregnan-3 (ß)-o\- 20-one 194 +70° +90.8* 3 144.5 +79.8° XXIII Allopregnan-3 (ß)-o\- 20-one 194 +70° +90.8* 144.5 +79.8° XXIV A4-Pregnene-3,20- dione (Progesterone) 120;129 + 174.6C + 193.5° +200«
+214.7C C1903 XXV Androstane-3 (β) ll(/3)-diol-17-one 234-235 +84.5° +81.3* + 105* 3 228-229 +70.5* +87.1* XXV Androstane-3 (β) ll(/3)-diol-17-one 234-235 +84.5° +81.3* + 105* 228-229 +70.5* +87.1* XXVI Δ4-Androstene-3,11,- 17-trione (Adreno- sterone)
G 223-224 +262° +364° CjgOa XXVII Δ4-Androstene-3,17- dione 173-174 + 190° + 199« XXVII Δ4-Androstene-3,17- dione 173-174 + 190° + 199« C18O2 XXVIII Ai,3.5:io_Egtratrien-3-255 + 159° +170* + 188° 3 126 XXVIII ol-17-one (Estrone) 255 + 159° +170* + 188° 126 ° Ethanol * Dioxane c Acetone d Benzene « Chloroform f Methanol
physiological point of view, (A) With the exception of estrone ( X X V I I I ) , all contain 19 or 21 carbon atoms which admits of a C I9 series (Compounds X X V to X X V I I ) and a C2I series (Compounds I to X X I V ) . Further, the C19 and C21 compounds may be subgrouped in accordance with the num- ber of oxygen atoms inherent in the molecule (as listed in Table II). (6) In all instances, C-ll is either unsubstituted (the 11-desoxy series; Com- pounds I X - X I , X V I I I - X X I V , and X X V I I - X X V I I I ) , or bears a ketonic or alcoholic function (the 11-oxygenated series; Compounds I-VIII,
T A B L E I I I
S T E R O I D S I S O L A T E D FROM A D R E N A L G L A N D
C H2O H C H2O H C H 2 O H
I I I C H O H C O C O
I II III
C H2O H CH2OH CH2OH
ho C H O H < ^ Η Ο Η
VII VIII IX
558 R. D. H . H E A R D
HO
HO
X
O
XIII
o o
XVIHO
T A B L E ' I I I (Continued) C H2O H * C H2O H CO
-OH
O
C H2O H
I co
) < H
C H2O H
I co
'•••H
HO
C H3
I co
v - O H
O
XI
XIV
XVIII
co
-OHHO
C H2O H
I
CHOH
λ -Η
O
C H3
I
H Ç O H ' -OH
HO
C H3
I co
-OH
HO
C H2O H
I co
HXII
HO
C H2O H
I co
H
XV
C H3
I
HOCH OH
XIX
CH2OH
I co
J < - H
XX XXI XXII
C H ,
T A B L E III (Continued) C H3
C O H
H O
H H O Ο
11
ο
H OXXIII
ο
XXIV XXV
ο ο
ο •ν
H OXXVI XXVII XXVIII
X I I - X V I , and X X V - X X V I ) . Physiologically, the distinction is import- ant since high gluconeogenic activity is observed only in the latter group, while marked action with respect to salt and water metabolism is found only in the desoxy series, (c) Based on the substitution at C-17, the C21 compounds fall into a 17-hydroxylated group (Compounds I - X I and X V I I I - X X I ) and a group in which the fourth valency bond of C-17 is satisfied with hydrogen (Compounds X I I - X V I and X X I I - X X I V ) . Since the former give rise to the corresponding 17-ketosteroids by rela- tively mild oxidative procedures (Section III, C,2), the possible sig- nificance of the members of this group as precursors of the urinary 17-ketosteroids is of metabolic interest (see Section VII, A , l ) . (d) The compounds are divisible into an α,/5-unsaturated 3-ketosteroid group (Compounds V-VIII, X I , X I V - X V I , X X I - X X I I , X X I V , X X V I , and X X V I I ) and those in which ring A is saturated (Compounds I-IV, I X - X , X I I X - I I I , X V I I I - X X , X X I I I , and X X V ) . The first-mentioned resonant system is requisite to appreciable cortical activity, (e) The presence in the side chain of a primary hydroxyl group vicinal to a carbonyl oxygen atom (i.e., the primary α-ketol grouping) differentiates these compounds (II-IV, VII-VIII, X - X I I I , X V - X V I , and X X I I ) , which are strongly reducing, from the remainder, which are not (see Section III, C,2).
High cortical activity is confined to the first-mentioned class.
560 R. D. H. HEARD
There is species variation in the relative abundance of the various compounds in adrenal tissue. Kuizenga (120) has summarized (Table IV) the quantities of the four principal carbohydrate-active hormones which may be separated from beef, hog, and sheep glands.
T A B L E I V
S P E C I E S V A R I A T I O N IN Y I E L D S OF 1 1 - O X Y G E N A T E D A D R E N A L H O R M O N E S
Compound
Ox
Yield"
Hog Sheep
Corticosterone ( X V ) 2 0 0
Crude crystallizate6 7 0 0 1 5 0 0 6 8 0
Dehydrocorticosterone ( X V I ) 1 5 0
.. ·
ll-Dehydro-17-hydroxycorticosterone ( V I I I ) 2 0 0 2 2 0 4 0 0
Crude crystallizatec 1 7 0 0 8 5 0
17-Hydroxycorticosterone ( V I I ) 7 5 6 1 0 0
" M g . / l 0 0 0 lb. of whole adrenal gland (data of Kuizenga, 1 2 0 ) .
6 According to assays, consisting chiefly of corticosterone and dehydrocortico- sterone.
e According to assays, consisting chiefly of ll-dehydro-17-hydroxycorticosterone and 17-hydroxycorticosterone.
B . METHODS OF ISOLATION
A comprehensive description of the chemical procedures requisite to the isolation of the individual cortical steroids listed above would be superfluous. In general, advantage is taken of (a) the observations of Pfiffner and Vars (184) and of Mason, Myers, and Kendall (166) that certain of the more highly oxygenated C2I 05 and C2I 04 compounds tend to pass from ether or benzene to water on repeated extraction, (b) the elegant method of Girard and Sandulesco (75) of separating water- insoluble, lipide-soluble ketones as their water-soluble, lipide-insoluble trimethylaminoacethydrazone chlorides, which permits fractionation of a ketonic mixture according to the ease with which the carbonyl groups in various positions react to form these Girard complexes, and according to the hydrogen ion concentration required to hydrolyze the formed com- plexes, and (c) the application of the principles of chromatography, a method extensively employed by Reichstein, in which a mixture is adsorbed on a column of activated alumina and the components are eluted therefrom in order of their power of adsorption by the percolation through the column of appropriate organic solvent mixtures.
C . STRUCTURE AND PROPERTIES
In this section only the established structure and principal chemical properties of the adrenal steroids are dealt with. The many degradation
561 reactions, transformations from one series to another, and partial syn- theses of inactive compounds which led to the elucidation of the struc- ture of each individual are omitted; these are synopsized in the recent reviews on the chemistry of the adrenal steroids by Kuizenga (120), Reichstein and Shoppee (212), and Spring (241), in which references to earlier reviews and the original literature are listed in full.
The parent saturated hydrocarbons of the adrenal steroids are allo- pregnane and androstane, the stereochemical formulation and system of numeration of which are given below.
Consistent with the burden of evidence, the ring structures are formulated with the substituents of each pair of adjacent asymmetric carbon atoms (i.e., 5:10, 9:10, 8:9, 8:14, and 13:14) oriented in space in the opposite or trans relation to each other.
Thus the two angular methyl groups at C-10 and C-13 lie on the same side (arbitrarily the near side) of the flat plane of the molecule and serve as points of reference.
The spatial position of hydroxyl groups substituted in the nucleus is designated β or a. This nomenclature was originally introduced b y Fieser (56) to define the con- figuration of the 3-OH group; (β) indicates the orientation, with respect to the remainder of the molecule, that obtains in cholesterol and ß-cbolestanol, and ( « ) , the reverse stereochemical relationship, formulated, respectively, b y a solid and a dotted valency b o n d line. The convention has been universally adopted and extended to define the configuration of hydroxyl groups at centers of asymmetry other than C-3 (see also page 564). Except in the lumi series (page 555), (β) implies that the hydroxyl group is located on the same side of the flat plane of the molecule as the angular methyl groups at C-10 and C-13, and (a) indicates trans orientation with respect to these reference groups.
The observation which led to the establishment of the steroid ring skeleton of the adrenal compounds was provided by Reichstein (202),
1. The Carbon Ring Skeleton
2 1C H3
H2 H:
XXIX XXX
Androstane Allopregnane
562 R. D . H . H E A R D
who noted the high androgenic potency of Compound G of his series, adrenosterone ( X X V I ) , which immediately suggested a close chemical relationship to the male sex hormones. Also the biologically inactive
H O
T A B L E V
P R O O F OF T H E R I N G S K E L E T O N OF T H E A D R E N A L S T E R O I D S
C H2O H C H 2 O H
( ^ H O H I O
H O
HO
A
0 H H OC H2O H
^ O H
H O H O
III
C r 03
I CrOs 1 Ο Ο
IV
C r 03
Ο
Zn:HR HCl
XXXI XXX
Ο
CrOs
Ο
C H2O H
L -O H
XXVI VIII
compounds A, C, and D (I, III and IV), on treatment with chromic anhydride (Table V) yielded the saturated triketone X X X I with andro- genic activity (203), and identical with the product of reduction of the
double bond of adrenosterone ( X X V I ) . At about the same time, Mason, Myers, and Kendall (167) obtained, from the chromic acid oxidation of their cortically active Compound Ε (VIII), an androgenic product which proved to be adrenosterone ( X X V I ) . Unequivocal chemical proof of the carbon ring structure came with the Clemmensen reduction of X X X I to the known parent hydrocarbon, androstane ( X X X ) (Reichstein, 204).
Later Steiger and Reichstein (244) degraded corticosterone ( X V ) to allopregnane ( X X I X ) .
2. The Side Chain
Seven types of two-carbon side chains at C-17 are characteristic of the adrenal steroids:
CH2OH CH2OH CH3 CH3 CH2OH CH2OH CHS
A HOH io io A HOH io A HOH Ao
—A— OH —A— OH — A —OH —A— OH
— C - H-A»H —A-
ΗI I I I I I I (a) (b) (c) (d) (β) (/) (g)
The presence of a ketonic oxygen atom at C-20 adjacent to the primary hydroxyl group at C-21 (i.e., the a-ketol grouping; types b and e) is essential to cortical activity and imparts to the molecule reducing properties similar to tnose of fructose which contains the same a-ketol grouping. Only primary a-ketols of types b and e reduce alkaline silver diamine; also they reduce cupric ion in alkaline solution and phospho- molybdic acid, reactions which have been applied to the quantitative estimation of small quantities of reducing steroids of these types (Sect.
VII, A,3).
The behavior of the various types on oxidation with excess chromic or periodic acid is characteristic and has been extensively employed in the elucidation of the substituents at C-17, C-20 and C-21. With Cr03, the 17-hydroxylated compounds (types a to d) are oxidized to the corre- sponding 17-ketosteroids, | ®, and the C-17 unsubstituted types e
COOH
and / , to the corresponding etio acids, — • H. With periodic acid, the 17-hydroxylated a glycols (types a and d) give the corresponding 17-ketones, while, from the dihydroxyacetone type b, the 17-hydroxy
COOH
etio acids, —OH, are obtained, and from the C-20-C-21 glycols CHO
f, the 17-aldehydo compounds, H.
564 R. D. H . H E A R D
Owing to the asymmetry about C-17 in the allopregnane series, the hydrogen atom or hydroxyl group substituted at the junction of the side chain may be oriented in either the eis (β) or the trans (a) position with respect to the rest of the molecule; conversely the alkyl side chain must occupy the opposite spatial position. Since the sterols may be degraded (under conditions which tend to preclude inversion at C-17) to the naturally occurring bile acids and members of the C-21 hormone series not hydroxylated at C-17, it follows that the configuration of the side chain is the same in all three classes of steroids. Recent physical and chemical evidence (see page 574) makes it practically certain that the angular methyl group at C-13 and the side chain at C-17 are oriented on the same side of the flat plane of the molecule; accordingly the side chain is now assigned the 17(0) configuration, i.e., the opposite to that provisionally accepted prior to 1946.
17(β) and 17(a) here refer to the orientation of the side chain; the 17-hydrogen atom occupies conversely the a and β positions. As yet there is no universally accepted nomenclature to distinguish the natural from the artificial series of 17-hydro- gen steroids. In the 17-hydroxylated series, however, Reichstein and Gätzi (210) define the ^configuration about C-17 b y stating that of the 17-hydroxyl group, and in 1938 they arbitrarily assigned to the natural compounds the 17(jS)-OH configuration (see the text below). Selye (227), however, prefers a system of nomenclature in which the orientation and constitution of the alkyl side chain are specifically defined in relation to, and as a substituent of, the basic ring structure.
In an effort to reach a generally applicable and uniform system of nomenclature which permits convenient designation of the orientation about all centers of asymmetry in the steroid molecule, the author and D . A. Prins offer the following suggestions:
(1) That such a system should be based upon the accepted stereochemical struc- ture of the ring systems of androstane ( X X X ) and etiocholane on the one hand, and of allopregnane ( X X I X ) and pregnane on the other. Since the members of each pair differ only in the orientation of the hydrogen atom substituted at C-5, the long established use of the prefix alio is thus retained exclusively for the designation of the C-5 configuration of the parent hydrocarbons of all classes of steroids.
With respect to the inversion of a hydrogen atom, or a methyl group, about all other centers of asymmetry (i.e., carbon atoms 8, 9, 10, 13, 14, and 17 of the ring skeleton, and 20, 24, etc. of an aliphatic side chain), it is suggested that the prefix iso (not abbreviated) should be employed (together with the numbers of the carbon atoms concerned) to designate the configuration opposite to that which obtains in androstane (or etiocholane), allopregnane (or pregnane), allocholane (or cholane), cholestane (or coprostane), etc. This nomenclature merely represents an extension of the use of iso in the sense originally introduced b y Butenandt and adopted b y Reichstein and others; i (an abbreviation of iso) is thus retained for the designation of condensed five-ring systems containing a 3,5 bridge (i.e., members of the i-andro- stane, i-cholestane series, etc.).
(2) That the established configuration of functional groups substituted in the nucleus should be designated as (a) or (β) (see page 561), the indices being placed in parentheses (see Callow, 27, and Shoppee, 231). Where doubt exists concerning the stereochemical orientation, it is suggested that the indices be contained in inverted commas rather than brackets (see Petrow and Starling, 183), e.g., cholestane-3(/3),-
7 V - d i o l (151) and cholestane-3(/3),7"/3"-diol (267), in each of which the orientation of the 7-hydroxyl group is still in doubt (188). In instances of complete uncertainty of configuration, epi m a y serve to differentiate the members of a pair (see page 5 7 4 ) ; further, to avoid confusion, it would seem desirable to restrict the use of epi with reference to functional groups only.
As regards tertiary hydroxyl groups, Ruzicka and Muhr (217) have introduced a system of nomenclature, adopted b y others (189,193,211), in which the orientation of the 5-hydroxyl is defined in terms of the parent hydrocarbon, e.g., cholestan- 5-ol[5(a)-OH], and coprostan-5-ol[5(/3)-OH]. This system m a y well be extended to include all functional groups and to bear on all tertiary positions in the molecule;
thus, < <3/3,21-diacetoxy-14-oxy-20-keto-17-iso-5,14-diallo-pregnan,, (191) would be expressed 14-iSO-17-tso-allopregnane-3(/3),14,21-triol-20-one 3,21-diacetate.
The isomerism of hydroxyl and other functional groups substituted in an aliphatic side chain is of the diastereoisomeric t y p e ; consequently absolute configurations cannot b e assigned. The empirical use of a and β in this connection, as introduced b y Marker in the 17-hydrogen series, and the arbitrary assignments of Prins and Reichstein (195,196) in the 17-hydroxyl series, thus need to be retained until correlations are established. Omission of the brackets serves to distinguish diastereo- isomerism of this type from cis-trans isomerism (see Shoppee, 231), e.g., pregnane- 3(a),20a-diol.
The orientation of alkyl substituents of tertiary carbon atoms m a y be expressed in terms of the parent hydrocarbon; the configuration of those attached to nuclear methylene groups or in the side chain m a y be designated normal and iso.
In the main, the above proposals conform with general practise, but the terms alio, epi, and iso are not infrequently used indiscriminately with reference to a hydrogen atom, a hydroxyl group, or a methyl group. For example, to denote the reverse configuration of the 14-hydrogen atom, Hirschmann and Wintersteiner (101) employed epi (14-epî-equilenin), while Bachmann ei al. (2) describe the same c o m p o u n d as zsoequilenin; and, in the cardiac aglucone series, inversion of the 14-H atom is indicated b y 14-aZZo (Plattner etal., 191) and also b y 14-epi [Meyer (168)]. Similarly the prefix lumi has been used to designate inversion of both the methyl group at C-10 (i.e., lumisterol) and that at C-13—i.e., Zwmi-androsterone (21) and Zwww-estrone ( 2 0 ) , according to the nomenclature herewith suggested, these compounds would be more specifically defined respectively as 10-zso-ergosterol, 13-zso-androsterone and 13-zso- estrone. The two artificial C n isomers of etiodesoxycholic acid (formulas 3 and 4, page 575) are thus 3 (c0,12 (a )-dihydroxy-17-zso-etiocholanic acid and S(a),12(ß)- dihydroxy-17-tso-etiocholanic acid. Likewise reversal of the natural configuration about C-20 has been observed on several occasions—e.g., 3(A),12(A)-dihydroxy-20- iso-bisnorcholanic acid (238,239)—and instances of inversion about more than one center of asymmetry are not uncommon—e.g., pyrocalciferol (9-îso-lO-zso-ergosterol),
" 3/3oxy-17-*'so-5,14-dialloätiocholansäure" (191) [3(j8)hydroxy-14-zso-17-iso-etioallo- cholanic acid]. As in the designation of functional groups of uncertain orientation, inverted commas m a y profitably be employed when the site of inversion is not con- clusively established (e.g.,' ^ " - i s o - p r e g n a n e (urane), see page 618).
Several methyl ketones (type g) are known in both isomeric forms, which are reversibly interconvertible by the action of acid or alkali (17,18,23). The stable 17(0) isomer predominates in equilibrium mix- tures and is that which arises directly from the sterols on oxidation; the artificial 17(a) isomer is labile and physiologically inactive. In formula- tion, the side chain of the natural 17(0) series conventionally occupies
566 R. D. H. HEARD
the vertical position, and that of the artificial 17(a) series, the horizontal position, as illustrated below.
C H3
Ao H
•H x , A' C O — C H3
\ l \ l
/
Stable 17 (β) series
/
Labile 17(a) series
The stereochemical relationship of the side chain in those steroids hydroxylated at C-17 is difficult to establish since removal of the hydroxyl group destroys the asymmetry about C-17. However, strict chemical proof that all members of the ll-desoxy-17-hydroxy adrenal series (Compounds I X - X I and X V I I I - X X I ) ; types a to d, are simi- larly oriented at C-17 has been furnished by Reichstein and collabo- rators by interconversion and degradation reactions leading to the same 3(jS),17u/3,,-dihydroxyetioallocholanic acid ( X X X I I ) or 17"0"-hydroxy- 3-keto-A4-etiocholenic acid ( X X X I I I ) . Both C i7 epimers of X X X I I and
C O O H C O O H
- O H Λ , A- O H
H O Ο
XXXII XXXIII
of X X X I I I have been prepared artificially and are not identical with the breakdown products of the natural substances. In the case of the degradation of the ll-oxy-17-hydroxy compounds (I-VIII, types a tod), the corresponding 17-epimeric pairs of reference acids are not charac- terized, but the application of optical superimposition rules defining the contribution of the 11-hydroxyl or 11-ketone group to the molecular rotation leaves little doubt of the same configuration at C-17 in these instances. In 1938, when the side chain of the sterols and bile acids was believed to be trans oriented, Reichstein and Gätzi (210) arbitrarily assigned the β configuration to the 17-hydroxyl group of the natural adrenal series, and they are so described in the literature to date; this would orient the side chain of the 17-hydroxylated adrenal compounds as in the sterols and bile acids, which seems highly likely. With the
evidence now overwhelmingly in favor of the eis position of the side chain in the 17-hydrogen compounds (see page 574), reversal of the configura- tion previously assigned in the 17-hydroxy series is indicated. In their latest communications, Reichstein and collaborators (53,240) point to the probability that the so-called 17"0"-OH compounds are in reality 17(a). Because of this uncertainty and the fact that revision has not yet been effected, the original assignment of Reichstein and Gätzi (210) is retained throughout this review, with the use of inverted commas to indicate its arbitrary nature (see page 564) ; in formulation however, the natural series (probably 17(a)-OH) is indicated by a vertical side chain, and the artificial epimers (probably 17(0)-OH), by a horizontal side chain.
Isomerism about the center of asymmetry at C-20 is possible in types a, d, and / , and a naturally occurring pair is observed in Reichstein's Compounds J (XVIII) and Ο ( X I X ) , in which the 200 and 20a configura- tions, respectively, are arbitrarily assigned without commitment as to absolute spacial orientation (see Prins and Reichstein, 196, and page 564).
3. The Nuclear Substituants
a. Position. In the adrenal steroids not oxygenated at C-ll (the 11-desoxy series), the position and nature of the nuclear substituents were readily ascertained by degradation to known compounds. On oxida- tive cleavage of the side chain with chromic acid, the secondary alcohol groups are simultaneously oxidized to ketones, and the resulting product is one of (a) androstane-3,17-dione, (6) A4-androstene-3,17-dione, (c) 3-keto-etioallocholanic acid, or (d) 3-keto-A4-etiocholenic acid. With periodic acid, the nuclear hydroxyl groups remain unattacked, and, depending on the type of side chain (see Sect. I l l C,2), one or two carbon atoms are removed to give (a) androstan-3(0)-ol-17-one, (b) A4-andro- stene-3,17-dione, (c) 3(0), 17" 0 "-dihydroxyetioallocholanic acid ( X X - X I I ) , or (d) 17"0''-hydroxy-3-keto-A4-etiocholenk acid ( X X X I I I ) .
The substituent at C-ll in the remaining members of the series proved much more difficult to place, as at the time of the investigations no authentic 11-oxygenated steroid was available for comparison. As previously indicated (Table V, page 562), the unsaturated C21O5 com- pounds (V-VIII), give with chromic acid adrenosterone ( X X V I ) , which, on saturation of the double bond, yields the same androstane- trione ( X X X I ) as that formed on oxidative cleavage of the side chain of all saturated members of the C21O5 series (Compounds I-IV). The position of the nuclear substituents in Compounds I-VIII is thus the same in all cases. Dehydration (Table VI) of the 11-hydroxyl group
568 R. D. H . H E A R D T A B L E V I
P R O O F OF SUBSTITUTION A T C-3 A N D C-17 IN T H E C21O6 S E R I E S C H2O H
HO JHOH
- O H
ΗΙΟ4
HO
A c20
A c O XXV
OAc O H 1
HCl or KHSO4
Ο
! A c20
A c O A c O
J Η 2 Pt
A c O XXXIV
of the ketone X X V obtained on cleavage of the side chain of Reichstein's Compound A (I) and subsequent catalytic hydrogénation and acetyla- tion led to the known androstane-3(0)-17(AO-diol diacetate ( X X X I V ) , which established the presence of the 3 (β)-OH group in I and of carbonyl oxygen atoms at C-3 and C-17 in the triketone X X X I (229). The third ketone group of the latter exhibited unique chemical properties in that it failed to react in the usual manner to form an oxime, semicarbazone, etc. The hindered 11 position was assigned to this substituent by Steiger and Reichstein (242) in 1937 by a process of elimination. Posi- tions 1, 2, 4, 15, and 16 were excluded, as the products would behave as an a or β diketone, which was not in accordance with fact, and a carbonyl group in position 6, 7, or 12 would not be expected to be unduly unreac- tive. That the C21O4 corticosterone group (Compounds X I I - X V I ) also contain an 11-oxygen was shown by the elimination of the 17- hydroxyl group of the C21O5 compounds to products identical with or referable to the C21O4 substances (Table VII). On treatment of Ken- dall's compound Ε (VIII) with calcium hydroxide and oxidation with chromic acid, Mason (159) obtained small yields of 3,ll-diketo-A4-etio-
cholenic acid ( X X X V ) identical with the product of oxidation of corticosterone and dehydrocorticosterone ( X V and X V I ) . From the triacetate ( X X X V I ) of their Compound A (I), Shoppee and Reichstein
T A B L E V I I
C O R R E L A T I O N OF T H E C2I0 6 A N D C2I04 S E R I E S
Ο
Ο
VIII
H O
C H2O H
' — Ο Η
Ca(OH)2
C H2O A c (^HOAc
' - O H
Zn
C O O H (^HOH
• H
+
C O O H H O H
— O H
O
C 1 O 3
C O O H
I
H
O
H O
XXXV
H
k " C O C H2O A c
CrOs
A c O
XXXIX
(232) effected with zinc the elimination of acetic acid to a substance X X X V I I , isomeric at C-17 with Compound R (XII), which, on oxidation, yielded the diketone X X X V I I I ; rearrangement in acid of the labile side chain of the latter to the stable configuration gave the
570 R. D. H. HEARD
diacetate ( X X X I X ) , of Compound Ν (XIII). Full confirmation of the assignment at C-ll came with the artificial preparation (Sect. IV B) of 3,ll-diketo-A4-etiocholenic acid ( X X X V ) from desoxycholic acid (126).
b. Stereosomer ism of the Hydroxyl Groups. The hydroxyl group at C-3 is cis(ß) oriented in all of the natural adrenal steroids with the exception of Compound III (53); thus Compounds I, II, IV, IX, X , X I I , XIII, X V I I I - X X , X X I I I , and X X V form insoluble adducts with digitonin.
As regards the orientation of the 11-hydroxyl group in Compounds I, II, III, V, VII, X I I , X V , and X X V , the interconversion and correla- tion reactions formerly establish the same spatial relationship in all instances, but the assignment of the 11 (β) configuration has only recently received full confirmation through the synthetic approach from desoxy- cholic acid (LII) (see Sect. IV B, and reference 53). In the bile acid series, several pairs of 11-hydroxy epimers have been prepared artificially;
one epimer is esterifiable and resists dehydration on treatment with acid, while, in the opposite configuration, the alcohol is difficultly esterified, if at all, and loss of the elements of water proceeds easily in an acid medium to a mixture of the corresponding Δ9·1 1 and Δ1 1*1 2 derivatives (see Table VI, page 568). Reference to three dimensional models makes it appar- ent that the cis(ß)-oriented 11-hydroxyl group is subjected to consider- able steric hindrance from the angular methyl groups neighboring at C-10 and C-13 and lying on the same side of the flat plane of the molecule;
with the 11 (a)-hydroxyl group on the opposite side of the molecule to the angular methyl groups, interference is diminished and substitution may be expected. Accordingly the non-reactive, readily dehydratable 11- hydroxyl of the corticosteroids is assigned the β configuration.
IV. Artificial Preparation of the Active Adrenal Steroids A . T H E 11-DESOXY COMPOUNDS
1. Partial Synthesis of 11-Desoxycorticosterone
In 1937 Steiger and Reichstein (243) prepared desoxycorticosterone acetate (XLIV) from 3(ß)-acetoxy-A5-etiocholenic acid (XL), and showed that this hitherto unknown product was highly active in the Everse-de Fremery test. This conversion, while it marks the first preparation by artificial means of a naturally occurring biologically active adrenal steroid, antedates by approximately one year the isolation of desoxy- corticosterone from adrenal tissue (Reichstein and von Euw, 209). The process (Table VIII) is widely used in the commercial preparation of desoxycorticosterone. The immediate starting material in the partial
T A B L E V I I I
P A R T I A L S Y N T H E S I S OF 1 1 - D E S O X Y C O R T I C O S T E R O N E
A c O
XL
A c O AcO
XLI XLII
Κ Ο Η
I
i C H2N2
io
H
H O
XXII XLIV XLIII
synthesis,3 3(/3)-acetoxy-A5-etiocholenic acid (XL) is readily available as a by-product in the chromic acid oxidation of cholesterol to dehydro- isoandrosterone (XLV). Addition of diazomethane to the acetoxy acid
» In that the starting materials of all preparations are of natural origin (the sterols and bile acids), the term 1 'partial synthesis" is applied as distinct from "total syn- thesis," which implies that the products may be built up from the constituent elements.
572 R. D. H . H E A R D
chloride X L I gives the acetoxy diazo-ketone XLII, which is saponified and hydrolyzed with acetic acid to 21-acetoxypregnenolone (XLIII).
Desoxycorticosterone acetate (XLIV) is then furnished by the oxidation of the 3-hydroxyl group, either with aluminum alkoxide by the method of Oppenauer, or by chromium trioxide after previous saturation of the double bond with bromine and subsequent debromination; in the course of either oxidative procedure the 5,6 ethylenic linkage migrates to the 4,5 position in conjugation with carbonyl oxygen atom at C-3.
2. Partial Synthesis of 17-Hydroxy-ll-Desoxy corticosterone More difficulty attended the partial synthesis (Table I X ) of 17- hydroxy-ll-desoxycorticosterone ( X I ) , and the compound is not yet gen- erally available. From dehydroisoandrosterone (XLV), von Euw and Reichstein (51) prepared the tetrol XLVIII by the method of Butenandt and Peters (19) ; a Grignard reaction with allyl magnesium bromide and an Oppenauer oxidation lead to 17"<x"-allyltestosterone (XLVI), which, on dehydration of the tertiary alcohol, gives the triene XLVII, the two side chain double bonds of which are then hydroxylated with osmium
T A B L E I X
P A R T I A L S Y N T H E S I S OF 1 7 - H Y D R O X Y - 1 1 - D E S O X Y C O R T I C O S T E R O N E