MELANOGENESIS IN SOMATIC CELL HYBRIDS
FUNAN HU
Department of Cutaneous Biology
LINDA M. PASZTOR
Department of Pathology
Oregon Regional Primate Research Center Beaverton, Oregon
I. INTRODUCTION
Genetic control of a differentiated function such as melano- genesis has been extensively studied in the phenotype of hybrids which were produced by the fusion of pigmented cells and nonpig- mented fibroblasts derived from longterm cell lines (Davidson et al., 1966; Silagi, 1967). Like other "luxury" functions, melano- genesis was suppressed in cell hybrids which contained one genetic complement but was expressed in hybrids which had two genomes from the pigmented cell parent (Fougère et al., 1972; Davidson, 1974). We contend that some cells that have never functioned melanogenically and have been cultured in vitro for long periods
449
450 FUNAN HU AND LINDA M. PASZTOR
of time may be regulated by negative control mechanisms (Davidson et al., 1968), but that such factors as the ploidy and origin of the so-called undifferentiated cell parent are extremely critical in determining whether cell hybrids can express the differentiated function.
In earlier studies one of the parental cells in differentiated χ nondifferentiated hybrids was a mouse fibroblast line. Unlike melanoma cells which were differentiated, these cells were undif-
ferentiated in terms of melanogenesis. Strictly speaking, how- ever, the differentiated function of the fibroblast is collagen, not melanin, synthesis. Should fibroblasts that are capable of collagen synthesis be designated undifferentiated?
Melanoma cells, on the other hand, form melanin, but enzy- matically (Lerner and Fitzpatrick, 1953; Fitzpatrick and Kukita, 1956; Chang et al., 1959) as well as ultrastructurally (Mishima, 1965; Cesarini, 1971) do not behave like normal melanocytes in all respects. Conceivably, then, the control of differentiation in normal and neoplastic cells may differ somewhat.
In this report, we describe our observations of several cell hybrids in vitro derived from (1) diploid melanocytes χ pigmented and nonpigmented cells from long-term cell cultures and (2) melanoma χ nonpigmented cells from long-term cell cultures as well as in vivo hybrids produced by the fusion of melanoma cells from C57BL/6 mice with diploid cells from the host (Hu and Pasztor, 1975).
II. METHODS AND MATERIALS
A. Cells
Two types of melanogenic cells were used: (a) primary cul- tures of terminally differentiated, diploid melanocytes prepared from adult rhesus monkey choroid (Hu et al., in preparation) and
(2) a neoplastic melanocyte cell line (PAZG) (Pasztor et al.,
CO*
M · CO M CO
õ ì ο
Q) õ
Ν CO
Q) g, . q
^ +J -H 4J Ο £
Ο M « Q) - ^ Ü
3
" S.
• H m co
Q) g 0 • q II co co 0 0 * Ή 0 Q) + J
Ü q
õ tcj <D
• c: Q) H
• H A ï 0 lH Q) ><:
c; S Q) H I Q) - U . q Q)
ο > q 0 - U 4 J ο Q) «M 0
» q - U CO «β CO
Ü — s 0 • M q + J Ό * H t J
t J • H Q) Q) • Q)
q Q) Ü
••H g 0 0 0 (H 4 J co
CO 0
CO g 0 0 QJ • * q « H 0 co 0 co M
fcl Ή Q) <Ü Q) p Ü A i CO
• q Ο S CQ S CQ •
Û ; Q) Q) • ι—1 0
0 • u 4 J
È • • H
S QJ co t 3
D CD 0)
V 0) H + J <B
0 Ü 3 2h • H Q)
• H * H
452 FUNAN HU AND LINDA M. PASZTOR
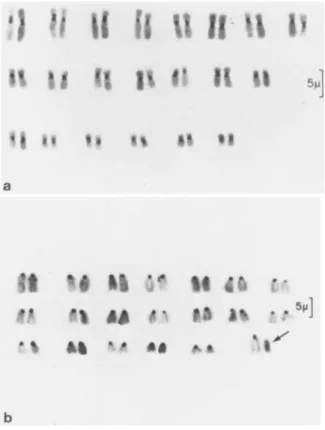
FIGURE 2 Chromosomes stained to show C-banding. Bar = 5μ.
(a) Karyotype of diploid rhesus cells. (b) Karyotype of male diploid C57BL/6 mouse cells. Arrow indicates the Y chromosome.
1974). The nonmelanogenic parental cells were murine renal adeno- carcinoma cells (RAG) (Klebe et al., 1970), mouse fibroblasts [LM (TK~)] (Kit et al., 1963), Chinese hamster peritoneal cells
(CH) (line B14 FAF 28-G3, obtained from American Type Culture Col- lection, CCL 14.1), and diploid cells from a male C57BL/6 mouse, most probably fibroblasts (Hu and Pasztor, , 1975). (Fig. la- d. Fig. 2a-b).
SOMATIC CELL HYBRIDS 453
B. Hybridizations
All cells were grown in Eagle's minimal essential medium (MEM) supplemented with 10% fetal calf serum. The medium for the rhesus choroidal melanocytes also contained 75 yg/ml cytosine arabino- side, which killed any cell contaminants such as proliferating fibroblasts or endothelial cells and ensured a pure melanocyte population.
The PAZG cells were grown in MEM containing 200 yg/ml 8- azaguanine (AZG); the RAG, in 3 yg/ml AZG; the CH, in 50 yg/ml 5- iododeoxyuridine; and the LM(TK~) in 30yg/ml 5-bromodeoxyuridine.
All fusions in vitro were done either in suspension or monolayer cultures with 3-propiolactone-inactivated Sendai virus.
A tumor produced when 10^ PAZG cells were injected into a normal C57BL/6 male mouse was excised, trypsinized, and grown in HATG medium to suppress the proliferation of the PAZG cells and to select for cell hybrids produced by the fusion of the host with the PAZG cells. Cell hybrids received the genetic informa- tion for HGPRT which was lacking in PAZG cells but was required for survival in HATG medium.
III. RESULTS
Rhesus choroid (RC) χ long-term cell line hybrids contained a reduced number of rhesus chromosomes (2n = 42). Most hybrid metaphases contained from 2 to 16 rhesus elements and two genomes contributed by the other parent. RC x LM(TK"), F96 (Fig. 3a) and RC χ RAG, F100 (Fig. 3b) hybrids were nonpigmented. Similarly, PAZG χ CH, F57 (Fig. 4a) and PAZG χ LM(TK~) , F34 (Fig. 4b) hy- brids were nonpigmented and contained a reduced number of PAZG chromosomes and of total chromosomes respectively (Hu et al., in press).
/ /
/ V
> Λ · / f » . · ÷. *
1 e
η; «
• M Ο 10 . q t J Ü
ι
U 0)
10 Ü Ό Ή
QJ CJ
*Η * Η
to CO
Q) Ο t0 ÎH
i s
tu q +J -H
«S! S
S in
D
g 3
to
CD A i Q)
SC 2 en ò
cd
CM
• QJ
$H +J Q) <U A i Ü
S ^
•H tsi to
Ο
- P
Ο EH
?H
— s a; ^
Ο °o
•m X 4 J
1 1 · s
' * . - . N X
(
f <0
A 3 to
l
to
tu qj
s,
to
Q)
to I
Ci. fΦ 4J QJ
t0 Ή QJ Ο ^ to
Ο ο
§ 3 Ü * õ ο
to er;
to
CO 4J tU v o
•M
•H
454
SOMATIC CELL HYBRIDS 455
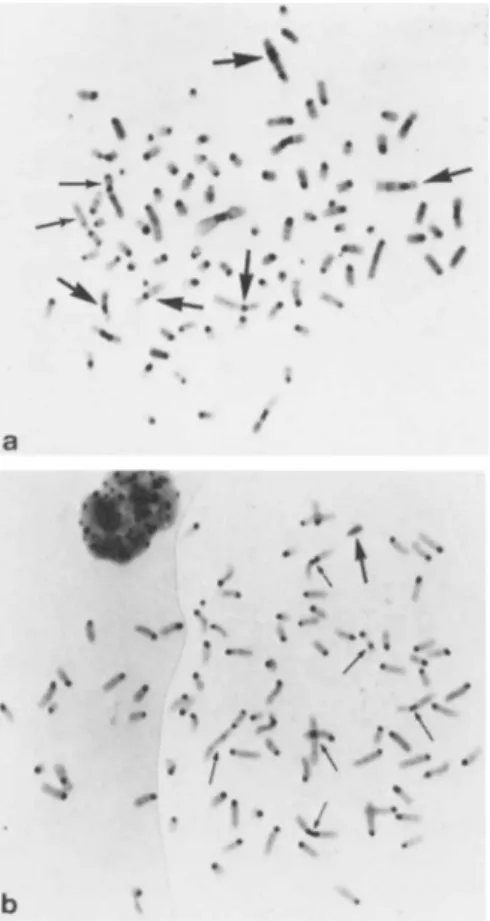
FIGURE 5 Metaphase spreads. C-bands. (a) PAZG ÷ RC (F 85).
Thin arrows indicate PAZG markers; thick arrows, rhesus chromo- somes, (b) PAZG x C57BL/6 diploid cells (MP), in vivo hybrids.
Thin arrows indicate PAZG markers; thick arrow, the Y chromosome.
RC χ PAZG, F85 (Fig. 5a) and PAZG x C57BL/6 host cell, MP hybrids (Fig. 5b) were pigmented. Indeed, these two hybrid lines were more pigmented than the parental PAZG cells (Fig. 6a- d).
456 FUN AN HU AND LINDA M. PASZTOR
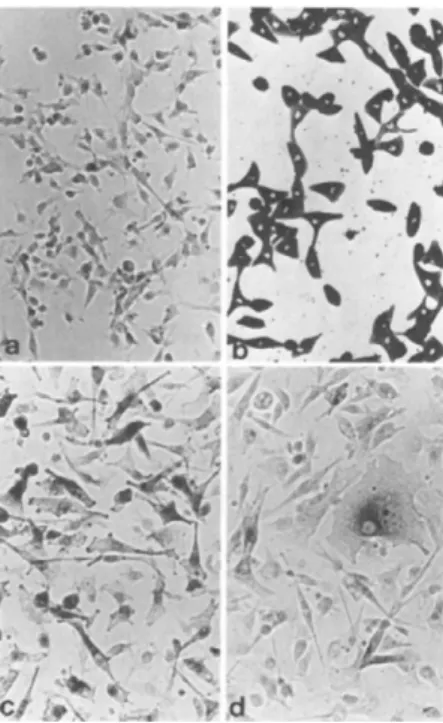
FIGURE 6 Monolayer cell cultures. (a) Mouse melanoma cells (PAZG), DOPA reaction. Note only a few scattered cells darkened by the dopa reagent. Mag. 256 X. (b) Rhesus monkey choroidal melanocytes (RC). Living culture, unstained. All cells filled with dark granules. Mag. 160 X. (c) PAZG ÷ diploid host cells (MP). DOPA reaction. Note larger and more dopa-reactive cells than (a). Mag. 256 X. (d) PAZG ÷ RC (F 85). DOPA reaction.
Note one large epithelial-like cell which contains dark dopa- reactive granules and several smaller dopa-reactive cells. Mag 256 X.
IV. DISCUSSION
Our experiments were designed to answer two questions: (1) Are hybrids derived from the fusion of two melanogenic parental cells (e. g., F85) different from hybrids derived from the fusion of one melanogenic with one nonmelanogenic cell? (2) Does the control of differentaition differ in neoplastic and normal melano- cytes?
SOMATIC CELL HYBRIDS 457
Our results indicate that when both parental cells were mel- anogenic, the hybrid was melanogenic and that when only one pa- rental cell was melanogenic, the hybrids were, with one exception, nonmelanogenic. Each of the nonmelanogenic hybrids was charac- terized by chromosome loss, however, and melanogenesis was not re- expressed in them. These results were the same whether the mel- anogenic parent was normal or neoplastic. The exceptional hybrid, MP, was initially characterized by a chromosome number which was consistent with its derivation from the fusion of one PAZG cell with one diploid host cell without chromosomal loss (Hu and Pasz- tor, 1975).
According to Harris (1972) and Wiener et al. (1971, 1974), malignancy develops when cells lose the gene (s) which control normal cell growth and can be corrected by the fusion of malignant with normal cells. Undoubtedly, the expression of melanogenesis depends upon whether the genetic information for this function is present. Davidson (1974) discussed a gene dosage effect in mel- anoma hybrids and suggested that a 2S genetic complement from the pigmented parental cell can overcome the negative control con- tributed by the nonpigmented parental cell. Since melanogenesis was expressed in our MP hybrid cells (an intraspecific fusion of melanoma and host cells from the same mouse strain), this require- ment was unnecessary.
We agree with Davidson's observation (1974) that to use dif- ferent species as parental cells complicates the interpretation of the results since some alterations in gene expression in the hybrids could be due to the combination of genomes of different species, not to the mechanisms of gene regulation. Silagi's hy- brids (1967) were intraspecific (C57BL/6 melanoma x C3H cells from a long-term cell line) but unlike our MP hybrids, the undif- ferentiated parental cell was not diploid and not derived from the same mouse strain.
What control mechanism accounts for the enhancement of mel- anogenesis in MP hybrids? If we could be absolutely sure that no
458 FUN AN HU AND LINDA M. PASZTOR
diploid pigment cell was involved in this fusion, we could postu- late that once the amelanotic cell is removed from its natural environment, it can be derepressed or activated to synthesize its own melanin. This would be similar to the induction of human al- bumin in mouse hepatoma χ human leukocyte hybrids (Darlington, 1974). This hypothesis is supported by Brumbaugh's (this Con- gress) finding of melanin formation in heterokaryons derived from amelanotic melanocytes χ erythrocyte nuclei from the pink-eyed mutant and wild-type fowl respectively.
ACKNOWLEDGMENTS
We are indebted to Dr. H. G. Coon of the National Cancer In- stitute, who generously supplied the ß-propiolactone inactivated Sendai virus used in our fusion experiments; to Coral Jean Cot- terell, Cathy Taylor and Dinah Teramura for expert technical as- sistance; and to Margaret Barss for careful editing of the manu- script.
Publication No. 816 of the Oregon Regional Primate Research Center supported in part by Grants CA 08499 of the National Can- cer Institute, RR 00163 and RR 05694 of the Animal Resources Branch, Division of Research Resources, AM 08445 of the National Institute of Arthritis and Metabolic Diseases, National Insti- tutes of Health; and by funds from the Cammack Trust, Portland, Oregon.
REFERENCES
Cesarini, J. P. (1971). Eur. J. Clin. Biol. Res. 16, 316-322.
Chang, J. P., Speece, A. J. and Russell, W. 0. (1959). In "Pigment Cell Biology" CM. Gordon, ed.), pp. 359-370, Academic Press, New York.
Darlington, G. (1974). In "Somatic Cell Hybridization" (R. L.
SOMATIC CELL HYBRIDS 459
Davidson and F. de la Cruz, eds.), pp. 159-162. Raven Press, New York.
Davidson, R. L. (1974). in "Somatic Cell Hybridization" (R. L.
Davidson and F. de la Cruz, eds.), pp. 131-146. Raven Press, New York.
Davidson, R. L., Ephrussi, Â., and Yamamoto, K. (1966). Proc.
Natl. Acad. Sei. 56, 1437-1440.
Davidson, R. L., Ephrussi, Â., and Yamamoto, K. (1968). J. Cell Physiol. 72, 115-128.
Fitzpatrick, T. B. and Kukita, A. (1956). J. Invest. Dermatol. 26, 173-183.
Fougère, C , Ruiz, F., and Ephrussi, B. (1972). Proc. Natl. Acad.
Sei. USA. 69, 330-334.
Harris, H. (1972). J. Natl. Cancer Inst. 48, 851-864.
Hu, F. and Pasztor, L. M. (1975). Differentiation 4, 93-97.
Hu, F., Pasztor, L. Ì., White, R., and Wilson, B. J. In "Proc. IX International Pigment Cell Conference." S. Karger, Basel. (In press).
Kit, S., Dubbs, D. R., Piekarski, L. J., and Hsu, T. C. (1963).
Exp. Cell Res. 31, 297-312.
Klebe, R. J., Chen, T., and Ruddle, F. (1970). J. Cell Biol. 45, 74-82.
Lerner, A. B. and Fitzpatrick, T. B. (1953). In "Pigment Cell Growth" (M. Gordon, ed.), pp. 319-333. Academic Press, New York.
Mishima, Y. (1965). Arch. Dermatol. 91, 519-557.
Pasztor, L. Ì., Hu, F., Stankova, L., and Bigley, R. (1974). J.
Natl. Cancer Inst. 52, 1143-1150.
Silagi, S. (1967). Cancer Res. 27, 1953-1960.
Wiener, F., Klein, G., and Harris, H. (1971). J. Cell Sei. 8, 681- 692.
Wiener, F., Klein, G., and Harris, H. (1974). J. Cell Sei. 15, 177-183.