UNIVERSITY OF PANNONIA GEORGIKON FACULTY
DOCTOR OF PHILOSOPHY (PhD)
THESIS
RAHIM AHMADVAND
KESZTHELY, HUNGARY
2013
2
3
UNIVERSITY OF PANNONIA GEORGIKON FACULTY
DOCTORAL SCHOOL OF CROP PRODUCTION AND HORTICULTURAL SCIENCES PLANT BREEDING, GENETICS AND AGROBIOTECHNOLOGY PROGRAM
HEAD OF THE DOCTORAL SCHOOL PROF. DR. LÁSZLÓ KOCSIS, DSC
ANALYSIS OF RESISTANCE GENES IN POTATO WITH SPECIAL ATTENTION TO EXPRESSIONAL APPROACHES
DOCTOR OF PHILOSOPHY (PhD) THESIS WRITTEN BY
RAHIM AHMADVAND
SUPERVISORS DR. ZSOLT POLGÁR, PhD
AND
DR. JÁNOS TALLER, PhD
KESZTHELY, HUNGARY 2013
4
ANALYSIS OF RESISTANCE GENES IN POTATO WITH SPECIAL ATTENTION TO EXPRESSIONAL APPROACHES
Written By
RAHIM AHMADVAND
Written at the University of Pannonia, Doctoral School of Crop Production and Horticultural Sciences, Plant Breeding, Genetics and Agrobiotechnology Program
Supervisors: Dr. Zsolt Polgár
I propose for acceptance (yes /no)
Signature Dr. János Taller
I propose for acceptance (yes /no)
Signature The candidate has achieved………..% at the comprehensive exam,
I propose the thesis for acceptance as the reviewer:
Name of reviewer: ……….yes /no
Name of reviewer: ………..yes /no
The candidate has achieved………..% at the public discussion.
Veszprém/Keszthely,
….………..
Chairman of the Committee
Labeling of the PhD diploma ………..
………
President of the UCDH
5
Table of Contents
ABSTRACT ... 9
KIVONAT ... 10
ABSTRAKT ... 11
ABBREVIATIONS ... 12
1. INTRODUCTION ... 14
Research objectives ... 17
2. LITERATURE REVIEW ... 18
2.1. Origin and evolution of potato crop ... 18
2.2. Potato production in Hungary ... 19
2.3. History of potato research at Keszthely, Hungary ... 19
2.4. Potato viruses ... 20
2.4.1. Potato virus X ... 21
2.4.2. Potato virus Y ... 22
2.5. Genetics of resistance ... 24
2.5.1. Structure and function of R genes ... 25
2.5.2. Mechanism of virus resistance ... 28
2.5.3. Signaling mediated resistance ... 29
2.5.4. Gene silencing ... 31
2.5.5. Types of resistance to potato viruses ... 32
2.5.5.1. Hypersensitive reaction (HR) ... 32
2.5.5.2. Extreme resistance (ER) ... 33
2.5.5.3. Resistance to infection ... 35
2.5.5.4. Resistance to virus accumulation ... 36
2.5.5.5. Resistance to virus movement in plants ... 36
2.5.5.6. Mature plant resistance ... 37
2.5.5.7. Tolerance... 37
2.5.5.8. Resistance to virus vectors ... 38
6
2.6. The potato resistance genes Rx1 and Rx2 ... 39
2.7. Resistance breeding and molecular markers ... 40
2.8. Marker assisted selection ... 41
2.9. Intron-targeting... 42
2.10. Gene expression profiling in plant-virus interactions ... 43
2.10.1. Subtraction Suppressive Hybridization (SSH) ... 44
2.10.2. cDNA-AFLP ... 46
2.10.3. Serial Analysis of Gene Expression ... 46
2.10.4. Microarray ... 47
2.10.5. Transcriptome analysis ... 48
2.10.5.1 De novo transcriptome analysis ... 51
2.10.5.2. Transcriptome analysis of the sequenced genomes ... 51
3. MATERIALS AND METHODS ... 54
3.1. Plant materials ... 54
3.1.1. Plant materials used in PVX inoculation test ... 54
3.1.2. Evaluation of the validity of developed specific primers for Rx1 and Rx2 genes ... 54
3.1.3. Development of Intron targeting markers ... 56
3.2. DAS-ELISA test ... 57
3.3. PVX resistance tests ... 57
3.3.1. Mechanical inoculation ... 57
3.3.2. Graft inoculation ... 57
3.4. Genomic DNA isolation ... 58
3.4.1. Lysis of plant cells and protein denaturation ... 58
3.4.2. Purification ... 58
3.5. Identification of resistance gene to PVX... 59
3.5.1. Marker analysis... 59
3.5.2. Development of specific primers for Rx genes ... 59
3.6. Development of a multiplex PCR for the Rx genes ... 60
3.7. Transcriptome analysis ... 60
3.7.1. Inoculation with pathogens ... 61
7
3.7.1. 1. Inoculation with PVX and PVY ... 61
3.7.1.2. Inoculation with Ph. infestans... 61
3.7.2. mRNA isolation ... 61
3.7.3. mRNA analysis ... 62
3.7.4. mRNA preparation ... 63
3.7.5. Suppression subtractive hybridization (SSH) ... 63
3.7.5. 1. Construction of SSH library... 63
3.7.5.2. Cloning and PCR screening of the SSH library ... 64
3.7.5.3. Sequencing of cDNA clones and sequence analysis... 64
3.7.6. NGS transcriptome sequencing ... 65
3.8. Development of NGS derived intron-targeting markers ... 65
3.8.1. PCR amplification of intron targeting markers ... 66
3.8. 2. Data analysis ... 66
4. RESULTS ... 67
4.1. Development of functional markers and a multiple PCR for Rx1 and Rx2 resistance genes in potato ... 67
4.1. 1. PVX resistance tests ... 67
4.1.2. Identification of resistance gene to PVX... 67
4.1.2.1. Results of the published PVX resistance markers ... 67
4.1.2.2. Resistance gene analysis and development of gene-specific primers ... 69
4.1.3. Testing of the Rx2 specific primer pair in the F1 populations ... 73
4.1.4. Development of a multiplex PCR for the Rx genes ... 74
4.2. Isolation of important genes related to resistance against PVX, PVY and Ph. infestans ... 75
4.2.1. Construction of subtractive cDNA libraries in White Lady ... 75
4.2.1.1. Analysis of PVY induced subtracted cDNA library ... 75
4.2.1.2. Analysis of PVX induced subtracted cDNA library ... 76
4.2.2. NGS based transcriptome analysis ... 77
4.3. Development of Intron-targeting markers in potato ... 80
4.3.1. IT polymorphism in the potato genotypes ... 80
4.3.2. IT polymorphism in the wild Solanum species ... 82
8
4.3.3. Localization of the IT markers in the potato genome ... 84
5. DISCUSSION ... 85
5.1. Development of functional markers and a multiple PCR for Rx1 and Rx2 resistance genes in potato ... 85
5.2. Isolation of important genes related to resistance against PVX, PVY and Ph. infestans ... 87
5.2.1. Analysis of PVY induced subtracted cDNA library ... 87
5.2.2. Analysis of PVX induced subtracted cDNA library ... 90
5.2.3. NGS based transcriptome analysis ... 92
5.3. Development of intron-targeting markers in potato ... 94
LIST OF NEW FINDINGS ... 98
ÚJ TUDOMÁNYOS EREDMÉNYEK ... 99
ACKNOWLEDGEMENTS ... 100
PUBLICATION LIST ... 102
Referred articles related to thesis ... 102
Conference abstracts related to the thesis ... 102
Other publications ... 103
REFERENCES ... 105
Appendix 1 ... 124
Appendix 1 ... 125
Appendix 2 ... 126
Appendix 3 ... 128
Appendix 4 ... 130
Appendix 5 ... 131
Appendix 6 ... 152
Appendix 7 ... 156
Appendix 8 ... 169
Appendix 9 ... 172
Appendix 10 ... 175
Appendix 11 ... 176
Appendix 12 ... 178
9
ABSTRACT
Potato virus X (PVX) is one of the main viruses infecting potatoes worldwide. Two extreme resistant genes, Rx1 and Rx2, against PVX have a 98% sequence similarity at the nucleotide level that makes it complicated to identify these genes and to distinguish them from other highly similar genes, like the Gpa2 or from paralogous sequences by a single PCR. Here, we report the development of functional markers for the simple and rapid identification of the Rx1 as well as the Rx2 gene. Furthermore, a multiplex PCR reaction was developed for the simultaneous detection of both genes in a single reaction.
In further studies, we aimed to elucidate a better understanding of potato defense mechanisms against pathogens. To achieve this goal, two approaches, namely PCR-selected cDNA subtraction and RNA-Seq transcriptome were used to profile PVX, Potato virus Y (PVY) and Phytophthora infestans response genes in early stages of infection in the resistant potato cultivar, White Lady.
Firstly, two subtracted cDNA libraries of White Lady in response to PVX and PVYNTN were constructed. We could identify 28 and 35 resistance response EST-s to PVX and PVYNTN in White Lady, respectively. Out of these 17 and 12 could be annotated in the PVX as well as in the PVY pool, while five EST-s were found to be common between the two libraries. The remaining genes are genes with unknown function or novel sequences. Secondly, we generated a global transcriptome profile of early response of White Lady to PVX, PVY and Ph. infestans infection using next generation sequencing (NGS). Alignment of fragments with 39 031 protein-coding genes of the genome of S. tuberosum group Phureja DM1-3 5116R44 identified 38 675 genes in the pathogen treated sample. Seven hundred and forty eight transcripts were recognized in the treated sample but not in control indicating stress response specific genes. The data set contains 141 NBS-LRR encoding genes. From these, an NBS-encoding and an LRR-type gene were identified that were expressed only in the treated samples and would be interesting for future studies. The generated dataset has provided comprehensive information of transcriptome dynamics that can serve as a blueprint to elucidate the resistance mechanisms for biologists and potato breeders. In addition, NGS derived transcript sequences were applied for the development of intron-targeting (IT) markers. The efficiency of the developed IT primers was experimentally analyzed. The results revealed the efficiency of NGS derived IT marker development and indicate their utility in diverse molecular analyses including their applicability for cross-species studies.
10
KIVONAT
A burgonya X vírus (PVX – Potato virus X) világszerte elterjedt kórokozója a burgonyának. Két PVX extrém rezisztenciagén, a Rx1 és Rx2 ismert, melyek DNS szekvenciája 98%-os hasonlóságot mutat, ami megnehezíti e két gén azonosítását, illetve más, nagyon hasonló génektől, mint például a Gpa2 fonálféreg rezisztenciagéntől, vagy paralóg szekvenciáktól egyetlen PCR reakcióval történő elkülönítését. A jelen kutatási programban a Rx1 és a Rx2 gén egyszerű és gyors azonosítását lehetővé tevő funkcionális markereket fejlesztettünk ki. Továbbá, kifejlesztettünk egy multiplex PCR eljárást e két gén egyetlen reakcióban történő párhuzamos kimutatására.
Kutatásaink fő területe a burgonya kórokozókkal szembeni védekezési mechanizmusainak megismerése volt. E célból két megközelítést, nevezetesen a cDNS szubtraktálást, valamint a RNS- Szek transzkriptom analízist alkalmaztuk a PVX, PVY vírus és a Ph. infestans fertőzés korai stádiumaiban kifejeződő gének vizsgálatára. Modellnövényként az e három kórokozóval szemben rezisztens White Lady fajtát választottuk. Első lépésben két, PVX, illetve PVYNTN fertőzésre szubtraktált cDNS klóntárat készítettünk. A PVX fertőzéssel szemben 28, míg a PVY-nal szemben 35 EST-t azonosítottunk. Ezek közül 17-et, illetve 12-t tudtunk annotálni a PVX, illetve a PVY klóntárban, míg 5 EST azonosnak mutatkozott a két klóntár között. A többi EST vagy ismeretlen funkciójú gén vagy új, az adatbázisokban nem található szekvencia volt. A következő lépésben elkészítettük a White Lady PVX, PVY és Ph. infestans fertőzésre adott teljes transzkriptom profilját ún. következő generációs szekvenálással (NGS – next generation sequencing). A S. tuberosum Phureja csoport DM1-3 511R44 genom 39 031 ismert fehérjekódoló génje alapján 38 675 gént tudtunk azonosítani a kezelt mintában, melyek közül 748 nem fordult elő a kontrollban, ami arra enged következtetni, hogy e gének specifikusan a stressz-válaszban fejeződnek ki. A transzkriptom adatbázis 141 NBS-LRR gént tartalmaz, melyek közül egy NBS-kódoló és egy LRR- típusú gén csak a kezelt mintában fejeződött ki. E két gén funkcionális vizsgálata a közeli jövőben tervezett. A generált adatbázis átfogó információkat nyújtott a génkifejeződési változásokról, ezért úgy gondoljuk jó kutatási eszköz lehet a rezisztencia mechanizmusok feltárásában.
A fentiek mellett az NGS eljárással generált transzkriptomokat intron-targeting (IT) markerek fejlesztésére használtuk. A kifejlesztett IT primerek hatékonyságát kísérletesen elemeztük.
Eredményeink azt mutatják, hogy az NGS alapú IT marker fejlesztés a különböző molekuláris vizsgálatokban, - a fajok közötti tanulmányokat is beleértve, - hatékonyan alkalmazható eljárás.
11
ABSTRAKT
Potato virus X ( PVX ) ist weltweit einer der wichtigsten Kartoffeln Viren. Die zwei bekannte PVX extreme Resistenzgenen Rx1 und Rx2 haben 98% Sequenzähnlichkeit zu der Nukleotid- Sequenz. Das kompliziert die Identifizierung diese Genen und auch die Unterscheidung von anderen sehr ähnliche Gene, wie die Gpa2 oder paraloge Sequenzen durch einen einzigen PCR.
Hier berichten wir über die Entwicklung von funktionellen Markern für den einfachen und schnellen Identifizierung des Rx1 sowie die Rx2 Gen. Darüber hinaus wurde eine Multiplex-PCR- Reaktion zum gleichzeitigen Detektion beider Gene in einer einzigen Reaktion entwickelt.
In weiteren Untersuchungen sollen wir zu einem besseren Verständnis der Kartoffel Abwehrmechanismen gegen Krankheitserreger aufzuklären. Um dieses Ziel zu erreichen, wurden zwei Ansätze, nämlich PCR ausgewählte cDNA Subtraktion und RNA-Seq Transkriptom zum Expression-profil PVX, Potato virus Y ( PVY ) und Phytophthora infestans response-Genen in frühen Stadien der Infektion in der resistenten Kartoffelsorte, White Lady verwendet. Erstens wurden zwei subtrahierte cDNA-Sammlungen von White Lady in Reaktion auf PVX und PVYNTN konstruiert. Wir konnten 28 und 35 EST-s zu PVX und PVYNTN in White Lady identifizieren, beziehungsweise. Von diesen 17 und 12 könnten in den PVX sowie in der PVY Pool annotiert, während fünf EST-s wurde gemeinsam in beiden gemeinsamen cDNA-Sammlungen. Die übrigen sind Gene mit unbekannter Funktion oder neue Sequenzen. Zweitens wir erzeugten eine globale Transkriptom Profil der frühen Reaktion der White Lady zu PVX , PVY und Ph. infestans Infektion mit Next Generation Sequencing (NGS ). Angleichung der Fragmente mit 39 031 Protein-kodierenden Genen des Genoms von S. tuberosum Gruppe phureja DM1-3 5116R44 identifizierte 38 675 Gene des Erregers behandelten Probe. Siebenhundertachtundvierzig Transkripte wurden in der behandelten Probe erkannt, aber nicht in der Kontrolle, die als Stressantwort spezifischen Genen erkannt wurden. Der Datensatz enthält 141 NBS-LRR kodierenden Gene. Von diesen wurden ein NBS- und ein LRR-Codierende Gen identifiziert, die wurden nur in den behandelten Proben exprimiert und sollen für zukünftige Studien interessant sein. Der erzeugte Datensatz hat ausführliche Information über die Transkriptom Dynamik, und kann als Blaupause verwendet, in die Resistenzmechanism Untersuchungen für Biologen und Kartoffelzüchtern.
Darüber hinaus wurden NGS abgeleitet Transkript-Sequenzen für die Entwicklung von Intron- Targeting (IT) Marker aufgetragen. Die Effizienz der entwickelten IT Primere wurde experimentell untergesucht. Die Ergebnisse zeigten, die Effizienz der NGS abgeleitete IT Marker Entwicklung und zeigten ihren Nutzen in diverse molekulare Analysen einschließlich ihrer Anwendbarkeit für cross-species Studien.
12
ABBREVIATIONS
ACMV- African cassava mosaic virus AFLP - Amplified Fragment Length Polymorphism
AOX- alternative oxidase
APAF-1 - Apoptotic protease-activating factor 1 ARC - Apaf-1, R protein, CED-4
Avr - Avirulence gene
BCMV- Bean common mosaic virus BLAST - basic alignment search tool BLAT- The blast-like alignment tool BPB - Brome phenol blue
CAPS - Cleaved amplified polymorphic sequence
CC - Coiled coil domain
CDPK- Ca2+-dependent protein kinase
cDNA - Complementary deoxyribonucleic acid CIA - Chloroform isoamyl alcohol
CP - Coat protein
CTR1-constitutive triple response
DAS-ELISA – Double-antibody sandwich enzyme-linked immunosorbent assay DNA - Deoxyribonucleic acid
dsRNA - double-stranded RNA
EDTA - Ethylene diamine tetraacetic acid ER - Extreme Resistance
ERF- Ethylene-responsive transcription factor EST- Expressed sequence tag
ETI - Effector triggered immunity ET - Ethylene
FST- Wright’s fixation index
GMO - Genetically modified organism HC- heterozygosity
HC′- Shannon Wiener diversity index HT- Nei’s total genetic diversity
HSP - Heat shock proteins HR - Hypersensitive reaction IPM - Integrated pest management IT - Intron targeting
JA - Jasmonic acid
LRR - Leucine-rich repeat
MAPK - Mitogen-activated protein kinase MAS - Marker assisted selection
mRNA - Messenger ribonucleic acid NBS - Nucleotide-binding site
NCBI - National Center for Biotechnology Information
NGS - Next generation sequencing NO - Nitric oxide
ORFs- open reading frames
ORMV - Oilseed rape mosaic virus PAMPs - Pathogen-associated molecular patterns
PCL - Plant cell lysis PIs- Proteinase inhibitors
PCR - Polymerase chain reaction PLRV - Potato leaf roll virus
Potato-DM - Solanum tuberosum group Phureja DM1-3 5116R44
PMTV- Potato mop-top virus PR - Pathogenesis-related genes PRR - Pattern recognition receptors
PTNRD - Potato tuber necrotic ring spot disease PTI - PAMP triggered immunity
PVA - Potato virus A PVM - Potato virus M PVP - Polyvinylpyrrolidone PVS - Potato virus S
PVY - Potato virus Y
13 QTL - Qualitative trait loci
q-PCR - quantitative- PCR R-Avr- Resistance- avirulence RFLP - Restriction fragment length polymorphism
RNA - Ribonucleic acid ROS- Reactive oxygen species SA - Salicylic acid
SAGE - Serial analysis of gene expression SAR - systemic acquired resistance
SCAR - Sequence characterized amplified region
SCF- SKp1, Cullin, F-box protein SGN- SOL genomics network siRNA- small interfering RNA
SKP1- S phase kinase-associated protein 1 SNP - Single nucleotide polymorphism SOLiD - Sequencing by oligonucleotide ligation and detection
SSH - Suppressive subtraction hybridization SSR - Simple sequence repeat
ssRNA - Single-stranded RNA STS - Sequence tagged site
TBE - Tris-HCL, Boric acid, EDTA buffer TC- transcriptomes
TE - Tris-HCL, EDTA buffer TGB - Triple gene block
TIR - Toll interleukin-1 receptor domain TIGR -The institute for genomic research TMV- Tobacco mosaic virus
TNLs - TIR-NB–LRRs TuMV-Turnip mosaic virus TVCV- Turnip vein clearing virus TYLCV- Tomato yellow leaf curl virus UN - United Nations
VPg - viral protein linked to the genome VSRs - Viral suppressors of RNA silencing
14
1.
INTRODUCTION
Potato (Solanum tuberosum L.) is the third most important food crop after wheat and rice. According to FAOSTAT, potato production in 2009 reached 329.58 million tons produced from 18.65 million hectares at a mean fresh-weight yield of 17.6 t/ha. Potato is an excellent staple food crop and is grown as a vegetable for table use, or processed into French fries and crisps (chips), as well as it is used for dried products and starch production playing a main role in global food security (Kang and Priyadarshan, 2007).
The nutritional value of potato per unit of land is 2-3 times more than that of cereals.
Potatoes are a good source of carbohydrate and also provide a significant amount of protein, vitamins and minerals, such as calcium, potassium, phosphorus, magnesium, iron or zinc among others (Razdan and Mattoo, 2005; Storey, 2009). In addition, diverse non- food uses of potato are expanding, for example, potato as a source of starch for the production of biodegradable plastics (Doane 1994). In recognition of its important roles, the United Nations (UN) named 2008 as the International Year of the Potato. In recent years, there has been a shift in the end use of potatoes, with production for direct consumption being replaced by processing potatoes for the production of convenience foods such as French fries and potato chips (Kole, 2007).
Potatoes are grown in 149 countries (Hijmans and Spooner, 2001). Top five potato producing countries are China, India, the Russian Federation, Ukraine and the United States (FAO statistics, 2008). This crop is susceptible to a wide range of fungal, bacterial, and viral diseases as well as various nematodes and pests. From these, beside Phytophthora infestans viruses are the most dangerous parasites of the potato (Ross, 1986). Viruses are important pathogens that can substantially decrease yield and quality of this crop. Up to now over 40 viruses have been reported which infect cultivated potatoes in the field influencing, the distribution, agricultural practices and/or the varieties that can be grown in a given region. Most important viruses are Potato leaf roll virus (PLRV), Potato virus Y (PVY), Potato virus X (PVX), Potato virus A (PVA), Potato virus S (PVS) and Potato virus M (PVM) considering their distribution and effect on yield. Other viruses occur in potato only occasionally or locally (Salazar, 2003). These
15
viruses can cause serious yield and quality loss depending on the genotype or variety.
Chemical control of plant viruses is not commercially feasible; therefore, genetic resistance that decreases or prevents replication of the virus and the appearance of symptoms on the plant is the only solution to protect crops against viral infections.
Resistant varieties are considered to be the most cost-effective and reliable approach to control viruses and prevent yield and quality losses. Introgression of resistance genes to main potato viruses from wild species to cultivated potato has been successfully achieved in classical breeding programs. One of the most successful resistance breeding programs in terms of combination of cultural quality traits with complex virus resistance is operated by the breeders of Potato Research Centre, University of Pannonia, Keszthely.
During the last 50 years using considerable time, efforts and cost more than a dozen varieties with resistance to PVY, PVX, PVA and PLRV were developed in the program.
However over due to the loss of some pedigree data the origin of the resistance genes is uncertain in several cases.
In terms of genetic status, cultivated potato (Solanum tuberosum ssp. tuberosum) is an auto tetraploid (2n = 4x = 48) plant that displays tetrasomic inheritance. It is highly heterozygous due to inbreeding depression after repeated selfing. One to four different alleles are present per locus, resulting in one homozygous and four heterozygous genotypes (Gebhardt and Valkonen, 2001). Genetic knowledge of the potato has increased dramatically since the first molecular-marker map appeared in 1988 (Bonierbale et al., 1988). Molecular markers are considered as valuable tools for crop improvement, due to their usefulness in characterizing and manipulating genetic loci responsible for monogenic and polygenic traits. In addition, sequencing of potato genome has provided an important opportunity to accelerate isolation of important genes and identify their functions (PGSC, 2011).
One of the most important viral diseases of potato is PVX, against which two extreme resistance genes have been widely incorporated into different cultivars and breeding line. These genes, the Rx1 and Rx2, have already been cloned (Bendahmane et al., 1999; Bendahmane et al., 2000). They originate from different species and reside on different chromosomes. Nevertheless, these two genes have a 98% sequence similarity at the nucleotide level, respectively. The high level of sequence similarity makes it
16
complicated to identify these genes and to distinguish them from other highly similar genes, like the Gpa2 or from paralogous sequences by a single PCR.
In terms of the mechanism of virus resistance, the plants respond to the infection with general and virus-specific defense reaction, which also involves varieties of physiological changes. Physiological changes in the resistance response to viruses suggested that certain pathway(s) that confer a resistance response against the virus may be specifically activated in the resistant cultivar. In general, plant–virus interactions are the least studied among plant-pathogen interactions. Moreover, the very early response, which plays a main role in resistance to viruses, on the whole transcriptome level in these interactions is even less well understood (Baebler et al., 2009). Study on whole transcriptome response of resistant potato cultivars to viruses makes it essential to identify genes that play main roles in resistance. Several experimental approaches for investigation the changes in the transcriptional profiles induced by viral infection have been developed. Among them, suppression subtractive hybridization (SSH) and more recently next generation sequencing (NGS) technologies have considerable advantages over the other methods.
The former, is a powerful approach to identify and isolate cDNA-s of differentially expressed genes since SSH allows the isolation of differentially expressed cDNA-s without prior knowledge of their sequence. The latter has provided a capability to simultaneously sequence hundreds of thousands of DNA fragments, dramatically changing the landscape of genetics studies. RNA-Sequencing (RNA-Seq) is one of the new applications of NGS technologies for transcriptome studies which determine accurately the expression levels of specific genes, differential splicing, and allele-specific expression of transcripts. All these attributes are not readily achievable from previously widespread hybridization-based (microarray platforms) or tag sequence-based approaches (Costa et al., 2010).
An efficient method to generate gene-specific co-dominant markers for mapping in plants is the Intron Targeting (IT) method (Seres et al., 2007). On the other hand, high- throughput transcriptome sequencing has the advantage to generate large transcript sequence data sets for gene discovery and molecular marker development.
17 Research objectives
The research objectives of the present study are including:
1) Evaluation of the reaction of Hungarian potato cultivars to PVX and identification of the type of resistance gene using molecular approaches.
2) Development of specific molecular markers for the cloned PVX extreme resistance genes, Rx1and Rx2.
3) Development of a multiplex PCR to detect Rx1 and Rx2 in a single reaction for practical utilization in marker assisted selection.
4) Partial gene expression study of a resistant potato cultivar in response to PVX and PVYNTN in early stages of inoculation using PCR-selected subtractive approach.
5) Whole transcriptome analysis of a resistance cultivar in response to PVX, PVYNTN and Ph. infestans using next generation sequencing.
6) Development of NGS based intron-targeting markers in potato.
18
2. LITERATURE REVIEW
2.1. Origin and evolution of potato crop
Potato is a New World crop that was unknown to the rest of the world until the 1500's. The most obvious domestication originated in the Andes Mountains of South America. The potato of commerce today was first domesticated in present-day Peru and Bolivia, and played an important role in that society, as is witnessed by many representations of potato in ceramic artwork from the area (Bamberg and Del Rio, 2005).
The 219 wild tuber-bearing Solanum species recognized by Hawkes (1990) are distributed from the southwestern United States to central Argentina and adjacent Chile and cover a great ecogeographical range (Hawkes, 1990). They form a polyploidy series from diploid (2n = 2x = 24) to hexaploid (2n = 6x = 72). The result of domestication was the diploid species S. stenotomum from which six other cultivated species were derived, including S. tuberosum, which became the most widely grown species in South America (Bradshaw, 2008).
S. tuberosum is a tetraploid (2n = 4x = 48) species that displays tetrasomic inheritance. The short-day adapted landrace populations of the Andes and the long-day adapted ones of coastal Chile are genetically distinct groups that have been classified as separate subspecies (S. tuberosum subsp. andigena and subsp. tuberosum) and are also referred to as Andigena and Chilean Tuberosum potatoes (Raker and Spooner, 2002).
Potatoes (tetraploid S. tuberosum) were introduced into Europe in the 1570s and they were exported from Europe and cultivated in many other parts of the world (Hawkes and Francisco-Ortega, 1993). The today potatoes are grown in 149 countries (Hijmans and Spooner, 2001) and that potatoes are the fourth most important food crop after wheat and rice (Lang, 2001). Their distribution reflects the adaptation of S. tuberosum first to the short summer days of the highland tropics and subtropics, then to the long summer days of lowland temperate regions and finally to the short winter days of the low land subtropics and tropics.
19 2.2. Potato production in Hungary
The potato production area decreased during the last 15 years from 50.000 to 22.000 ha in Hungary. After Hungary joined the EU, the seed potato production area also significantly decreased from 1500 ha to 350 ha. The total production reached 600.000 Mts while 5000 Mts was only seed potato (FAO, 2010). The total production was only 1% of EU’s total potato production and could just cover the requests of local market. The national average yield is about 25-27 Mts/ha. Out of that less than 10% is consumed as processed food. The average consumption of potato is approximately 65 kg/year/capita in Hungary. According to FAO’s report, Hungary is in the 50th position in terms of production quantity. Twenty percent of the total production area is covered by Hungarian varieties; those were mainly bred at Keszthely. The leading varieties are named as: Red Scarlet (NL), Laura (D), Kondor (NL), Desiree (NL), Cleopatra (NL), Agria (D), as well as Balatoni Rózsa (HU), Hópehely (HU), Katica (HU), Démon (HU), Góliát (HU) and Rioja (HU).
2.3. History of potato research at Keszthely, Hungary
Based on 200 years long tradition in agricultural research University of Pannonia (UP), modern potato research and breeding activities exists since 1950 at the UP, Potato Research Centre, Keszthely. The Centre operates under a university system and is the only institution dedicated to potato research and breeding in Hungary. It is an appreciated center of basic and applied research, breeding, extension and education of experts for potato. One of its major duties is the breeding of profitable potato varieties those suitable for Central European agro-ecological conditions due to their resistance against major potato pests, pathogens and extreme weather conditions. The research fields of the Centre starting from basic to applied are all dedicated toward this goal and try to cover all important issues of the potato sector.
From the sixties till the middle of eighties of the previous century the Centre operated a consistent, large scale resistance-breeding program utilizing several wild species germplasm. There were years when 1.5 – 2 million of seedlings were produced and screened by artificial infection with major potato pathogens and pests (viruses, nematodes and late blight) to incorporate resistance genes into cultivated genetic background. In the
20
crossing program different accessions of S. stoloniferum, S. acaule, S. tub. ssp.
andigenum, S. vernei and S. hougasii were most intensively used directly or through species hybrids.
Due to the consistent resistance-breeding program utilizing wild species germplasm the Centre currently has 13 varieties on EU list (Arany Chipke, Démon, Balatoni rózsa, Katica, Lorett, Góliát, Rioja, Hópehely, White Lady, Vénusz Gold, Luca XL, Kánkán and Somogyi Kifli). These varieties except Somogyi Kifli due to their complex resistance, high yielding potential and outstanding consumption quality are unique of their type and some of them are especially advised for organic production.
All the varieties show extreme resistance to the economically most important potato virus PVY and PVA as well as high field resistance to PLRV. Out of thirteen, 9 is resistant to common scab, potato wart and golden cyst nematodes, while two of them to potato late blight which feature makes those especially advised for organic production.
Recently advanced parental line screening methods, somatic hybridization, genetic modification and markers assisted selection techniques are involved into the breeding methodology of the Centre. Current main research topics are: the development of marker assisted selection techniques for late blight and virus resistance genes; increasing the environmental and food safety of potato production by development of specialized production technologies integrated pest management (IPM), optimization of nitrogen use, etc.), screening and breeding for nitrogen utilization efficiency, breeding to combine multi-resistance with processing quality, and testing the virus resistance of genetically modified (GM) potato lines.
2.4. Potato viruses
Most potato-infecting viruses have a positive-sense, single-stranded RNA (ssRNA) genome that replicates in cytoplasm by the viral RNA-dependent RNA polymerase.
Virions are assembled from hundreds to thousands of viral coat protein (CP) molecules that encapsidate the viral RNA.
21 2.4.1. Potato virus X
Potato virus X (PVX) is one of the main potato viruses infecting potatoes worldwide.
PVX can cause yield loss in the range of 5-20% depending upon the virus strain, the potato genotype and the simultaneous infection with other viruses like PVY and PVA.
This virus belongs to the genus Potexvirus, a member of family Flexiviridae (Adams et al., 2004). Members of this family are characterized by flexuous, filamentous virions between 470 and 580 nm in length, built of subunits of a single coat protein (CP).
Potexviruses have monopartite, positive-strand RNA genomes encoding five open reading frames (ORFs). The 5′ end has a methylguanosine cap and the 3′ end has a poly (A) tail (Huisman et al., 1988; Huang et al., 2004). The genomic RNA encodes replicase for viral RNA synthesis, triple gene block (TGB) proteins for virus cell-to-cell movement and CP that functions in assembly, cell-to-cell movement and as an elicitor for Rx- mediated PVX resistance. The protein encoded by the first triple gene block gene (TGBp1) modifies the plasmodesmata to allow larger particles pass through and was also shown to be involved in inhibiting a post transcriptional gene-silencing pathway which is one of the plant’s defense mechanisms against virus replication (Howard et al., 2004;
Bayne et al., 2005). Replicase translated from the genome synthesizes minus- and plus- strand copies of the viral RNA and subgenomic RNA-s that are templates for translation of the TGB proteins and CP.
PVX can be transmitted in a mechanical way only. The natural host range of PVX has so far included plant species of the families Amaranthaceae, Brassicaceae and Solanaceae. PVX isolates generally produce mild symptoms in the potato crop, but sometimes they cause severe mosaic in infected plants. PVX strains have been classified into four groups according to their reactions with the genes for localized hypersensitivity (Nb and Nx), and extreme resistance (Rx).
Group 1 strains are sensitive to both the Nx and Nb genes. Group 2 strains are sensitive to Nx gene, whereas group 3 strains are sensitive to Nb genes. Group 4 strains overcome both Nx and Nb mediated resistance. Strain PVXHB, found in 7% of Bolivian clones of Solanum tuberosum subsp. andigena, resemble the normal group 4 strains in overcoming genes Nb and Nx but differs from these in its ability to overcome Rx genes
22
conferring extreme resistance to PVX (Cockerham, 1954; Moreira et al., 1980a; Querci et al., 1995). The most commonly occurring strains are in the group 3 strain.
2.4.2. Potato virus Y
Potato virus Y (PVY) nowadays is the most common and destructive virus of cultivated potato since the relative significance of Potato leaf roll virus (PLRV) has decreased all over the world. The yield loss caused by the virus depends on the interaction of specific virus strain and potato genotype varies between 30 and 80%. This virus is the type member of the genus Potyvirus in the family Potyviridae, the largest group of plant viruses (Hall et al., 1998). The viral genome consists of a single-stranded, positive-sense RNA molecule about 10 kb in length, with a VPg protein covalently attached to its 5′ end and a poly-A tail at its 3′ end. The viral RNA encodes a single large polypeptide, which is cleaved by three virus-encoded proteases into nine products (Dougherty and Carrington, 1988).
PVY can be transmitted mechanically and by aphids in a non-persistent manner.
Myzus persicae is probably the most efficient vector; and more than 40 other aphid species are known as vector belong to the Acyrtosyphon, Aphis, Aulacorthum, Brachycaudus, Capitophorus, Cavariella, Cerosipha, Dysaulacorthum, Hyadaphis, Idiopterus, Macrosiphoniella, Macrosiphum, Metopolophium, Myzus, Phorodon, Ropalosiphoninus, Ropalosiphum, Scisaphis genus and the number of known vectors has been increasing continuously. PVY generates a variety of symptoms with different intensity depending on the interaction of potato genotype and PVY strain. The most typical symptom is the ‘leaf-drop streak’ or necrosis along the veins of the underside of leaflets and leaf mosaic. In combination with Potato virus X it causes the disease called
‘rugose mosaic’. PVY has several genetic variants called strains. Strain groups are based on the host response and resistance gene interactions. Several distinct strain groups of PVY have been defined. These are: the common (ordinary) group (PVYO), the stipple streak group (PVYC), the tobacco veinal necrosis group (PVYN) and the tuber necrosis strain (PVYNTN) that induces potato tuber necrotic ring spot disease (PTNRD) (Beczner et al., 1984; Jones, 1990a; Blanco-Urgoiti et al., 1998a). Some strains can be distinguished by symptomatology in different indicator plants (Horvath, 1967), by
23
serological methods (monoclonal antibody), and via molecular genetic analysis. Two broad pathotypes of PVY are defined based on the symptoms induced in tobacco (DeBokx and Huttinga, 1981). Strains including PVYO and PVYC induce mosaic, vein clearing, and mild leaf mottling, while others including PVYN, PVYN-Wi, and PVYNTN induce mosaic and systemic vein necrosis. Some (mainly PVYNTN isolates) cause a tuber necrotic reaction in susceptible potato cultivars (Singh et al., 2008).
PVYO strain group: PVYO in general causes severe symptoms such as crinkling, rugosity or leaf-drop streaks and stunting although the type and severity of symptoms may depend on potato genotypes. This strain does not cause veinal necrosis in tobacco but induces symptoms ranging from typical mild mosaic to systemic mottle.
PVYC strain group: PVYC, the stipple streak group, was the first strain that was identified in the 1930s (Salaman, 1930). This strain causes mosaic patterns or stipple streak in susceptible potato. The PVYC isolates elicit a hypersensitive response (HR) in potato cultivars bearing the Nc resistance gene (Cockerham, 1970). Symptoms in tobacco are reported to be indistinguishable from those induced by PVYO isolates. Unlike the other strains of PVY, some PVYC strains are non-aphid transmissible (Horvath, 1966;
Blanco-Urgoiti et al., 1998b).
PVYN strain group: In the 1950s, a variant of PVY was detected in potatoes in many countries in Europe (Bode and Volk, 1957). It caused veinal necrosis in tobacco leaves and mild mottle symptoms in most potatoes and was referred to as ‘veinal necrosis virus’. Keller and Munster designated PVYN to describe the tobacco veinal necrosis strain (Keller and Münster, 1961). In Hungary PVYN was detected from tobacco by Szirmai, J.
in 1958. The PVYN isolates induce necrosis in tobacco but do not induce necrosis in the presence of the genes Nc or Ny in potato cultivars. The virus exists in the Andes in native cultivars; and thus it is believed to originate in the Andes and remained undetected in Europe for many years. The strain PVYNTN was first reported in Hungary (Beczner et al., 1984) and was characterized at molecular level (Thole et al., 1993). Since then outbreaks have been reported in many regions of the world. In Hungary more than 90% of PVY isolates collected from potato fields belong to the PVYNTN strain (Wolf and Horvath, 2000). The strain that is not an apparently uniform entity causes severe mosaic leaf symptoms and ring like tuber necrosis (PTNRD) on tubers of several susceptible cultivars
24
but not on all. PVYNTN isolates characterized at the molecular level were found to be recombinants of PVYO and PVYN in the CP-encoding region. The virus probably spread to Europe and North America in the last two decades due to inappropriate application of new technologies during seed production, such as the use of monoclonal antibodies for its detection in seed programs (Salazar, 2003). In addition other groups have been identified including the PVYZ (reacted serologically to PVYO- specific antibodies and did not cause veinal necrosis symptoms in tobacco) (Kerlan et al., 1999) and PVYN-Wi (reacted serologically to PVYO-specific antibodies and cause veinal necrosis symptoms in tobacco, (Chrzanowska, 1991) and in North America called PVYN:O (Nie and Singh, 2002). Some PVYN-Wi isolates may also cause tuber necrosis (Piche et al., 2004).
Researchers consider PVYN-Wi and PVYNTN as sub-strains of PVYN, and the identification of non-recombinant PVYNTN inducing PTNRD revealed that the recombinant structure of the genome is not a necessary prerequisite for PTNRD phenotype (Nie and Singh, 2003; Browning et al., 2004; Glais et al., 2005). Recently, Lorenzen et al. (2006) reported that strain variants of PVYN and PVYNTN from Europe were present in North America (Lorenzen et al., 2006). However, it is proposed that any newly found isolates should be described within the context of the original strain groups based on the original methods of distinguishing strains (Singh et al., 2008).
2.5. Genetics of resistance
Plants live in complex environments in which they intimately interact with a broad range of microbial pathogens with different lifestyles and infection strategies. In general, plants defend themselves against pathogens by a combination of active and passive defense. In passive defense, structural characteristics act as physical barriers and inhibit the pathogen from gaining entrance and spreading through the plant and in active defense, biochemical reactions that take place in the cells and tissues of the plant and produce substances that are either toxic to the pathogen or create conditions that inhibit growth of the pathogen in the plant (Agrios, 2005).
The evolutionary arms race between plants and their attackers provided plants with a highly sophisticated defense system that, like the animal innate immune system (active defense), recognizes pathogen molecules and responds by activating specific defenses
25
that are directed against the invader (Pieterse et al., 2009). Plants respond to infection using a two-branched innate immune system. The first branch recognizes and responds to molecules common to many classes of microbes, including non-pathogens. In this branch, the resistance is induced by the recognition of pathogen-associated molecular patterns (PAMPs) by plant cell surface pattern recognition receptors (PRR), which initiates PAMP triggered immunity, that usually prevent the infection of pathogens before invasion.
Defense responses activated by PAMPs are collectively termed PAMP triggered immunity (PTI) or basal resistance (Jones and Dangl, 2006).
In the majority of cases, PTI prevent pathogen growth at an early infection stage due to the induction of pathogen-responsive genes, production of reactive oxygen species, mitogen-activated protein kinase signaling and deposition of callose to reinforce the cell wall at sites of infection (Schwessinger and Zipfel, 2008). If a pathogen evades this line of defense, it must also overcome a second line of defense to become pathogenic. The second branch acts primarily inside the cell using disease resistance (R) proteins which recognize pathogen-delivered effectors or their effects on host proteins. R protein- mediated defenses are termed effector triggered immunity (ETI) or gene-for-gene resistance, in which the protein products of plant resistance (R) genes specifically recognize cognate pathogen avirulence (Avr) gene products and trigger a stronger resistance response. Direct or indirect recognition of effectors by R proteins initiates ETI, which is an amplified and accelerated PTI response resulting in disease resistance (Jones and Dangl, 2006). ETI usually induces a hypersensitive response (HR) with localized cell death and defense gene expression that suppresses the growth and spread of pathogens.
2.5.1. Structure and function of R genes
According to Sacco and Moffett (2009) over 70 R genes have already been cloned (Sacco and Moffett, 2009). However, the focus has been mainly on monogenic dominant resistance to fungal and bacterial pathogens. But, there is clear evidence that common mechanisms can be involved in virus resistance (Hammond-Kosack and Parker, 2003).
The majority of R genes belong to the nucleotide-binding leucine-rich repeat (NB-LRR) family. NB-LRRs contain a C-terminal LRR domain and a central NB domain (Sacco and Moffett, 2009). The NB is part of a larger domain that is called the NB-ARC (Apaf-1, R
26
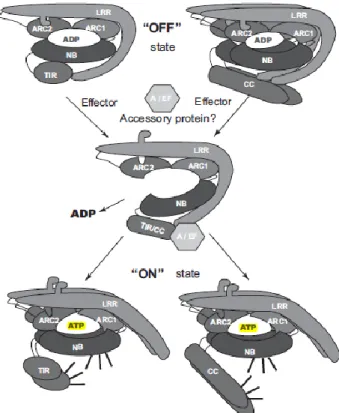
protein, CED-4) as it is shared between R proteins and the human apoptotic protease- activating factor 1 (APAF-1) and its Caenorhabditis elegans homolog CED- 4 (Takken et al., 2006). NB is proposed to act as a nucleotide dependent molecular switch regulating the conformation and signaling activity of these proteins. The LRR domain, positioned C- terminally to the NB-ARC, forms an arc-shaped conformation, forming a protein-protein interaction surface which provides recognition specificity (Fig 1.). Based on the identity of the N-terminal domain two main classes of NB-LRR, R proteins can be distinguished.
Some contains toll interleukin-1 receptor (TIR) domain and these R proteins are called TIR-NB-LRRs or TNLs (Burch-Smith and Dinesh-Kumar, 2007). Non-TIR-NB-LRR proteins contain predicted coiled coil (CC) motifs, this family is referred as CC-NB- LRRs (Lupas, 1997).
Direct interaction between the LRR and pathogen effectors has rarely been reported.
The guard-theory describes an alternative recognition mechanism by which the NB-LRR proteins known as the guardee are the target of the Avr protein. When the recognition mechanism detects interference with the guardee protein, it activates resistance (Van Der Biezen and Jones, 1998; de Wit, 2002). The guard hypothesis suggests that the host- pathogen interaction is more likely an interaction between the Avr protein and a host recognition complex. This complex must be able to recognize the pathogen and signal a defense response. Complex levels and activation of signaling must be tightly regulated and the recognition complex must be poised to perceive and respond to pathogens.
NBS-LRR encoded by the potato Rx gene is one of the best studied antiviral R proteins. Rx-mediated resistance to PVX and specificity of recognition depends on the presence of specific amino acid residues within the viral coat protein and properties of the Rx LRR domain (Farnham and Baulcombe, 2006). The NB domain of the Rx protein is found to be sufficient to induce defense responses (Rairdan et al., 2008). Whether this behavior extends to other CC-NB-LRR proteins remains to be determined.
Mutations in the CP of resistance breaking strains of PVX were responsible for their evasion of Rx1-mediated recognition. Initiation of defense signaling by interaction between Rx and the PVX coat protein appears to involve a conformational change in the Rx polypeptide, allowing its conversion from an inhibited to an active state. It is not clear
27
if this reorganization of the Rx protein requires direct interaction with the elicitor (Rairdan and Moffett, 2006).
More than 50 functional NB-LRR genes have been cloned from potato and related members of the Solanaceae (Hein et al., 2009). Recently, based on an amino acid motif based search of the annotated potato genome 438 NB-LRR type genes were identified among the ~39,000 potato gene models. Of the predicted genes, 77 contain an N-terminal toll/interleukin 1 receptor (TIR)-like domain, and 107 contain an N-terminal coiled-coil (CC) domain(Jupe et al., 2012).
All of the R proteins encoded resistance genes against plant viruses belong to the nucleotide binding site-leucine-rich repeat (NBS-LRR) class and are located intracellular.
However, there are no structural or other features of ‘‘antiviral’’ R proteins that sets them apart from the NBS-LRR proteins that confer resistance to bacteria, fungi, Oomycetes, or invertebrates (Jones and Dangl, 2006) and the signaling processes that they trigger are identical and lead to the activation of defenses against a broad spectrum of pathogens or pests, not only viruses (Murphy et al., 1999; Maule et al., 2007; Palukaitis and Carr, 2008; Moffett, 2009).
Twelve dominant R genes conferring resistance to viruses expressed either as HR or extreme resistance (ER) have been cloned and sequenced (Maule et al., 2007; Palukaitis and Carr, 2008). In potato, thirteen functional NB-LRR genes have been identified in which Rx1 and Rx2 confer resistant to PVX (Bakker et al., 2011). Dominant genes Rx1 and Rx2, against PVX in potato, have been identified by map-based cloning as well by Agrobacterium transient expression system, respectively (Bendahmane et al., 1999;
Bendahmane et al., 2000). Both genes belong to CC-NBS-LRR class. The potato gene Y- 1, an N gene homologue which confers HR to PVY, was also cloned and characterized (Vidal et al., 2002).
After a virus is recognized by the LRR, the function of an R protein complex must switch from recognition to signal transduction. Intramolecular interactions, activation of the NBS domain, and changes in signaling components that may associate with the CC or TIR domain and LRR domain have all been implicated during early signaling (Fig 1.) (Caplan and Dinesh-Kumar, 2006; Sessa, 2013).
28
Fig 1. A Model for NB-LRR protein activation. In the resting state, an NB-LRR protein is kept in a closed and auto-inhibited state in which the LRR and N-terminal domain (CC/TIR) fold back on the NB-ARC core. Effector recognition, often aided by an accessory protein, likely occurs by an interface formed by the C-terminal half of the LRR and the CC/TIR domain. Effector recognition results in a conformational change that is transduced via the N-terminal part of the LRR to the ARC2. This change allows exchange of ADP for ATP, triggering a second conformational change in the NB-ARC resulting in a more open structure in which interfaces on either the NB or the N-terminal domain (CC/TIR) become exposed and activate defense signaling (Sessa, 2013).
2.5.2. Mechanism of virus resistance
To complete their life cycles, viruses undergo a multistep process that includes entry into plant cells, uncoating of nucleic acid, translation of viral proteins, replication of viral nucleic acid, assembly of progeny virions, cell-to-cell movement, systemic movement, and plant-to-plant movement (Carrington et al., 1996). Plant viruses typically initiate infection by penetrating through the plant cell wall into a living cell through wounds caused by mechanical abrasion or by vectors such as insects and nematodes. When virus particles enter a susceptible plant cell, the genome is released from the capsid, typically in the plant cytoplasm. Once the genome becomes available, it can be translated from mRNA-s to give early viral products such as viral replicase and other virus-specific proteins. Hereafter the virus faces various constraints imposed by the host and also
29
requires the involvement of many host proteins, typically diverted for function in the viral infection cycle (Kang et al., 2005). Successful infection of a plant by a virus therefore requires a series of compatible interactions between the host and a limited number of viral gene products. Absence of necessary host factors for virus replication or movement or mutation to incompatibility has been postulated to account for recessively inherited disease resistance (passive resistance). Also, it could occur from the presence of physical barriers to inoculation including cell walls, cuticules or leaf hairs (Fraser, 1990, 1992). In contrast, dominant resistance has been shown to result from an active recognition event that occurs between host and viral factors, resulting in the induction of host defense responses. Basically, passive or active resistance can function at any stage of the virus life cycle, although most known viral resistance mechanisms appear to target virus replication or movement (Kang et al., 2005). In active resistance, plant R gene interacts with pathogen-encoded avirulence (Avr) gene (gene-for-gene hypothesis). The plant specific R protein can recognize the pathogen Avr protein and initiate signaling that leads to defense response. (Caplan and Dinesh-Kumar, 2006).
2.5.3. Signaling mediated resistance
The response of plant to virus could also be divided into two major categories such as cellular stress and developmental defects. Comparison of Arabidopsis and N.
benthamiana gene expression leads to the conclusion that virus infection causes characteristic changes in gene expression that is similar to stress and defense responses (Whitham et al., 2006). The stress responses are characterized by the induction of heat shock proteins (HSP) and defense responses by the induction of pathogenesis related (PR) genes and other genes associated with plant disease defense. The induction of HSP and PR genes represent cellular stress responses because of their non-specific nature and the lack of specific elicitors that induce them. Most viruses trigger these generic responses, which occur in the absence of typical gene-for-gene or resistance gene- avirulence gene interactions (Whitham et al., 2006). The expression of PR genes, mediated by salicylic acid (SA), is increased in many incompatible responses. In general, increasing of SA is required for the high accumulation of PR mRNA transcripts and
30
proteins that occur during resistance response to viruses but not in susceptible interactions (Malamy et al., 1990; Gaffney et al., 1993; Ryals et al., 1996).
The defense-related genes include numerous pathogenesis-related (PR) genes such as PR-1, PR-2 (β-1,3 glucanase), PR-3 (chitinase), PR-4, PR-5 (thaumatin like protein), genes associated with redox status such as superoxide dismutase and GST (glutathione S- transferases), resistance gene homologs. (Robatzek and Somssich, 2001, 2002).
Mitogen-activated protein kinase (MAPK) cascades play different roles in plant processes that include cytokinesis, phytohormone signaling, wound responses, osmotic stress, and pathogen resistance (Zhang and Klessig, 2001). Different transcription factors families such as TGA, MYB and WRKY have been implicated in disease resistance (Caplan and Dinesh-Kumar, 2006). In brief, some important signal transduction pathways involved in resistance to viruses are including:
Salicylic acid (SA): SA plays a main role in the signal transduction pathway that results in the induction of systemic acquired resistance (SAR) and it is required for localization of viral and other pathogens during the HR. SA is required for the expression of a group of proteins that collectively are referred to as pathogenesis-related (PR) proteins. SA can induce inhibition of virus replication, cell-to-cell movement, and systemic movement but the precise effects of SA-induced resistance on the life cycle of a virus can differ between hosts and between viruses (Murphy and Carr, 2002; Love et al., 2007; Wang et al., 2007). SA biosynthesis is induced most strongly during HR lesion development. SA may be necessary to regulate the timing and extent of the HR. During the HR, SA forms a gradient, with SA accumulating to high levels at the center of the HR lesions, moderate levels at the lesion borders, and low levels in healthy tissue (Enyedi et al., 1992).
Signaling mediated by Jasmonic acid and Ethylene: In spite of SA-dependent signaling pathways, the requirement of ethylene (ET) and jasmonic acid (JA) during R gene mediated resistance to viruses is more complex and variable. The crosstalk between ET-, JA- and SA-dependent signaling pathways can have synergistic or antagonistic effects on each other. ET and JA are secondary signaling molecules that function in microbial defense, wounding, and insect attack.
31
Signaling by reactive oxygen species, calcium, and nitric oxide: Reactive oxygen species (ROS) have been recognized as signals in defense, most notably during the oxidative burst or bursts that occur very early in the HR during a gene-for-gene response (Heath, 2000). ROS have several roles in the HR, but from the signaling point of view, perhaps two are the most important. Firstly, the oxidative burst activates Ca2+ ion influx across the plasma membrane via cyclic nucleotide-gated channels, in addition to mobilization of Ca2+ ions from intracellular stores (Torres and Dangl, 2005; Ma and Berkowitz, 2007). A second effect of changes in Ca2+- ion flux in the cytoplasm is triggering of the activity of calcium-dependent protein kinases, as well as highly complex MAPK cascades. ROS generated in the mitochondrion may also play roles in defensive signaling, particularly with respect to the induction by SA in resistance response to viruses. It has been proposed that alternative oxidase (AOX) is one of the factors that may influence this form, induction by SA, of defensive signaling (Ma and Berkowitz, 2007).
Nitric oxide (NO) is an important signal in plant defense. For example, the relative levels of NO and H2O2 regulate programmed cell death during an HR (Delledonne et al., 2001), and regulate defense gene expression both at the point of infection and in distal tissues, in part by inducing the biosynthesis of SA (Song and Goodman, 2001). NO also may stimulate changes in nuclear gene expression and defensive signaling indirectly through inhibition of cytochrome oxidase (Huang et al., 2002).
2.5.4. Gene silencing
Plants have also evolved mechanisms to actively target viruses. Viral RNA-s can be targeted for degradation by the RNA silencing machinery of the plant. This involves the processing of viral double-stranded RNA (dsRNA) by Dicer like enzymes, into small interfering RNA-s (siRNA-s), which are subsequently incorporated into protein complexes that target viral RNA-s for degradation, through an endonucleolytic process.
However, RNA silencing is generally insufficient to rapidly limit infections by viral pathogens, because most viruses encode viral suppressors of RNA silencing (VSRs) (Ding and Voinnet, 2007; Omarov et al., 2007). A rapid resistance response to viruses is afforded by gene-for-gene resistance. In addition to HR, many viral resistance genes
32
confer extreme resistance (ER), wherein cell death is not induced upon virus inoculation that occurs immediately after entry of the virus into plant cells and inhibits viral accumulation in the initially invaded cells (Ponz and Bruening, 1986; Kang et al., 2005).
2.5.5. Types of resistance to potato viruses
Resistant potato genotypes can react to the infection of viruses in the following ways.
2.5.5.1. Hypersensitive reaction (HR)
Hypersensitivity is expressed as a development of local and sometimes systemic necrosis following virus infection. It is generally virus strain specific and becomes non- effective, when another, non-necrosis inducing strain of the virus is introduced or selected in the plant (Jones, 1990b). The HR prevents spread of the virus throughout the plant. Plants with HR show either local necrotic lesions, which prevent the infection from spreading further, or systemic necrosis. Virus can be detected in affected leaves in most cases. The HR reaction can be affected by environmental conditions or by physiology of the host plant (Loebenstein and Carr, 2006).
Monogenic hypersensitive resistance to PVX, PVY and PVA has been reported in many potato cultivars and can confer useful field resistance to these viruses (Jones, 1990b). The hypersensitive resistance to the infection of PVX in potato is determined by two dominant genes, Nx and Nb (Cockerham,1955, 1970). Isolates of the PVX have been placed in strain groups according to their ability to elicit the hypersensitive response in potato cultivars carrying the dominant resistance genes Nb or Nx. The gene Nb confers resistance against PVX strains of the strain groups 1 and 2, whereas the gene Nx confers resistance against PVX strains of the strain groups 1 and 3 (Cockerham, 1954). The Nb gene has been localized to chromosome V to the same region that contains Rx2 (DeJong et al., 1997). HR to PVYO is expressed in genotypes containing the gene Nyadg but does not confer resistance to PVYN (Jones, 1990b; Valkonen et al., 1994). The gene Naadg confers HR to PVA in S. tuberosum subsp. andigena (Hamalainen et al., 1997;
Hamalainen et al., 1998; Valkonen et al., 2000).
33
The gene Ns derived from S. tuberosum ssp. andigena confers resistance to PVS in potato (Marczewski et al., 1998). Rm gene descends from S. megistacrolobum induces a hypersensitive response in potato plants to Potato virus M. The locus Rm was placed on the short arm of chromosome XI (Marczewski et al., 2006).
Hypersensitivity was not expressed in isolated protoplasts, suggesting that it is a tissue-related phenomenon and that cell to cell contact is required for the expression (Adams et al., 1986). The underlying mechanisms of HR resistance in potato are not yet clear, but some indications can be gathered from work done in tobacco. The N gene of tobacco mediates resistance to Tobacco mosaic virus (TMV) (Loebenstein and Carr, 2006). The N gene product is a cytoplasmically localized protein with a protein sequence motif known as ‘leucine-rich repeats’ (LRR) (Whitham et al., 1994). The N protein appears to recognize the presence of virus by binding, probably indirectly, to the TMV replicase protein. Subsequently, a series of steps is triggered that results in a hypersensitive response (HR) in the host plant. During the HR cells containing the virus die, resulting in a small necrotic lesion in the leaf.
2.5.5.2. Extreme resistance (ER)
Extreme resistance is expressed as a low incidence of infection in intact plants with an extremely low virus titer in infected plants; no symptoms develop, except for limited systemic necrosis following graft inoculation in some cultivars. It is active against a broad spectrum of virus strains and breakdown of resistance in the field has not been documented, except for a limited area of the Andean region in South America where a resistance-breaking isolate of PVX occurred (Moreira et al., 1980b; Jones, 1985;
Kavanagh et al., 1992). This type of resistance that functions at single cell level (Adams et al., 1986) is presumed as the best type of resistance to breed into potato. Rx gene upon recognition of PVX coat protein is highly effective in blocking of virus replication and also inhibit the translation of viral RNA-s by the host’s ribosomes (Bhattacharjee et al., 2009).
Plants with ER to a virus show no symptoms, or limited necrosis (pinpoint lesions, flecks, or localized stem necrosis) when inoculated with virus. Only extremely low amounts of virus, if any, can be detected by sensitive techniques. ER is often regarded as