OBTAINING MONONUCLEAR PHAGOCYTES FROM GRANULOMAS
Robert J. Bonney
I. INTRODUCTION
Granulomas are "focal chronic inflammatory reactions characterized by the accumulation and proliferation of leuko- cytes, principally of the mononuclear type" (1)· Granuloma- tous inflammatory lesions can be divided into (a) nonimmuno- logical or foreign body responses and (b) immune-based re- sponses resulting from delayed hypersensitivity reactions.
Although both types of granulomas contain macrophages as the predominant cell type, lymphocytes play a key role in the for- mation of the immune-based granuloma (2). The immune-based granulomas can be induced by slowly degradable antigens that normally elicit delayed hypersensitivity reactions in the host (3). Examples of such antigens are those of Schistosoma mansoni eggs (4), Bacillus - Calmette Guerin (5), Bordetella pertussis (6), spores of fungi such as Micropolyspora and Thermoactinomyces (7)f and Mycobacterium tuberculosis (8).
The granuloma lesion induced by Schistosoma eggs (4) or
METHODS FOR STUDYING Copyright © 1981 by Academic Press, Inc.
MONONUCLEAR PHAGOCYTES 1 1 1 All rights of reproduction in any form reserved.
ISBN 0-12-044220-5
Toxocara consists primarily of eosinophils initially, which are recruited by the acute inflammatory infiltrate (9). The eosinophils degranulate, and the resulting debris is taken up by macrophages that become the predominant cell type. After 10 - 12 weeks the granulomas are fibrotic encapsulated epi- thelioid lesions (9). In contrast, nonimmunological granulo- mas are induced by relatively inert nondigestible or poorly digestible particles, such as carrageenans (10), streptococcal cell walls (11), cotton pellets (12), polyvinyl sponges (13), and nylon and acrylic fibers (14). These lesions are composed of neutrophils, macrophages, and fibroblasts and do not have a prominent lymphoid cell component. Macrophages that comprise these lesions are long-lived and usually contain the undigested or partly digested inducing agent (10). For example, when car- rageenan is injected subcutaneously into an air pouch in rodents, an acute edematous reaction occurs in a few hours.
This is followed by accumulation of newly recruited bone marrow-derived macrophages after a few days (15). Up to 100%
of the macrophages can be shown to contain carrageenan 2 - 3 weeks after the injection (10), and the lesion is slow to re- solve, taking up to 3 months.
In order to study the functional capacity of the cells that comprise these various lesions, it is advantageous to de- vise methods for their removal from the lesions and for their purification and cultivation. Therefore, by utilizing tech- niques that have been used successfully to disperse parenchymal cells from adult liver (16), synovial cells from human rheuma- toid joints (17), and neoplastic cells (18), a method for pre- paring macrophages from granulomas has been devised (19). The starting tissue is a 7-day granuloma induced in mice by one subcutaneous injection of carrageenan that can be shown histo- logically to be comprised predominantly of macrophages (19).
The principle underlying this method is a gentle enzymatic dis- persion of the dissected granuloma followed by purification by adherence and finally cultivation in serum-free medium (to eliminate fibroblast proliferation). Thus, it is likely that other foreign- body- as well as immune-based granulomas could also be studied by these techniques.
II. REAGENTS
Carrageenan is a polysaccharide extracted from Irish moss Chondrus crispus composed of a mixture of a a and (B isomers of sulfated D-polygalactose. Sodium carrageenan, Viscarin brand, was used exclusively in this study and was a gift from Marine Colloids, Inc., Springfield, New Jersey. Collagenase (CLS II),
175 U/mg, was from Worthington Biochemical Corporation, Free- hold, New Jersey. Dulbecco's modified Eagles medium, lactal- bumin hydrolysate, fetal calf serum, and penicillin/strepto- mycin were from Grand Island Biological Co., Grand Island, New York. The fetal calf serum was inactivated by heating to
56°C for 30 min (HIFCS)· Nonclon tissue culture dishes were from Vangard International, Inc., Neptune, New Jersey.
Male SW-ICR mice were purchased from Hilltop Lab Animals, Inc., Scottdale, Pennsylvania and were fed a standard pellet diet and water ad libitum.
III. PROCEDURES
A. Induction of Granulomas
A solution of carrageenan (5 mg/ml) was prepared by slowly adding carrageenan to sterile distilled H2O, which was being stirred with a magnetic bar. The solution was then warmed to 37°C and 0.5 ml injected subcutaneously into the abdominal area using a 1-ml syringe fitted with a 25-gauge needle. The mice weighed 20 - 25 gm and were 4 - 6 months old. After 7 days the lesion was comprised mainly of mature macrophages with small numbers of immature macrophages, fibroblasts, and few neutro- phils (19).
B. Isolation of Granuloma Cells
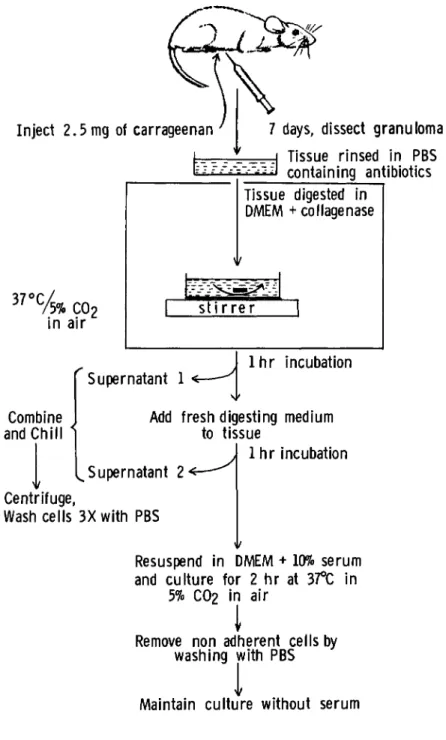
The 7-day-old granulomatous nodules from 30 mice were dis- sected with scissors under aseptic conditions from the subcu- taneous tissue underlying the skin and scraped with a scalpel into a bacterial petri dish containing sterile Dulbecco's phosphate-buffered saline (PBS) supplemented with 100 U/ml of penicillin and streptomycin. As outlined in Fig. 1, the tis- sue was sliced further into small pieces with scalpels and added to a 100-mm bacterial petri dish (Falcon) containing 20 ml of 0.2% collagenase in Dulbecco's modified Eagles medium
(DMEM). The dish is placed on a stirrer inside a 37°C incu- bator equilibrated with 5% CO2 in air and stirred slowly with a small magnetic stirrer for 45 - 60 min. The supernatant fluid containing released cells were removed into a sterile 50-ml conical centrifuge tube and chilled on ice. The diges- tion step is repeated for 1 hr with 20 ml of fresh digesting medium and the two supernatant fluids are combined. The cells are pelleted by centrifugation at 800 g for 10 min at 4°C and washed three times with ice-cold PBS. In three different ex-
Inject 2.5 mg of carrageenan
37 °C/5% C05 2
in air
7 days, dissect granuloma Tissue rinsed in PBS containing antibiotics I ]__ i Tissue rinsed in PBS
Tissue digested in DMEM + collagenase
I s t i r r e r I
Combine and Chill 1
Supernatant 1 I h r incubation Add fresh digesting medium
to tissue
I h r incubation
^Supernatant 2 Centrifuge,
Wash cells 3Xwith PBS
Resuspend in DMEM + 10% serum and culture for 2 hr at 37°C in
5% CO2 in air
Remove non adherent cells by v washing with PBS
Maintain culture without serum
I
Fig. 1. Schematic of procedures for isolating mononuclear phagocytes from carrageenan-induced granulomas in mice [repro- duced from Bonney et al. (19)].
periments, the yield was 4 x 10" - 5 x 10" cells per granuloma with 75 - 95% of cells excluding trypan blue. The cells were resuspended in DMEM containing 10% HIFCS and added to 35 or 60 mm Nunclon tissue culture dishes or to 15-mm glass cover- slips and incubated for 2 hr. The nonadherent cells were re- moved by washing the cell sheet four times with PBS, and the adherent cells were incubated overnight in fresh DMEM contain- ing 10% HIFCS. After 24 hr in the presence of serum, the cells were washed again and maintained in DMEM + 0.2% lactalbumin hydrolyzate.
IV. CHARACTERIZATION OF CULTURED CELLS
Cells isolated from carrageenan-induced granulomas in mice by the procedures described above exhibit the characteristics of mononuclear phagocytes (19). Table I summarizes several of the activities exhibited by these cells in comparison with
TABLE I. Activities Exhibited by Cultured Cells
Characteristic Granuloma Macrophage Fibroblast
Phagocytosisa + +
Lysozyme secretion** + + Binding of 125I-labeled IgGc + + Secretion of LAF^ + +
Trypsin detachmente - +
Elastase secretion^ + + High specific activities + +
of lysosomal hydrolases
aIngestion of several particles of 0,81 ]im latex beads in serum-free medium in 2 hr.
^Net increase in lysozyme activity in the culture medium over 72 hr.
cBinding of 125I-labeled IgG at 0°C and displacement by unlabeled IgG.
αSecretion of lymphocyte-activating factor (LAF) induced by lipopolysaccharide.
eResistant to detachment by trypsin-EDTA for 24 hr; fibro- blasts were completely removed in 10 min.
fSecreted an activity capable of hydrolyzing tritium- labeled insoluble elastin.
^specific activities of three lysosomal acid hydrolases were four to eight times greater than that found in fibroblasts.
mouse peritoneal macrophages and a mouse fibroblast line (CC-1, NCTC clone 929). It has been recently reported that the ac- tivities of two membrane enzymes, 5'-nucleotidase (20) and leucine aminopeptidase (21) are vastly different in resident populations of macrophages compared to populations elicited by the injection into the peritoneal cavity of certain inflam- matory stimuli. These enzymes were assayed in homogenates ob- tained from cultured macrophages isolated from the carrageenan granulomas, and it can be concluded that these cells resemble elicited population of macrophages (Table II).
The presence of 10% serum in the first 24 hr was essential for attachment and stability of the cells, perhaps due to re- sidual proteolytic enzymes from the collagenase digestion medium. However, after 24 hr the cells can be maintained for up to 3 weeks without serum. Medium should be changed three times a week and should contain penicillin - streptomicin or gentamicin.
V. CRITICAL COMMENTS
In the past macrophages have been isolated from granulo- matous lesions by mechanical treatment rather than by enzy- matic dispersion (22). These workers dissected the granuloma
induced by Bordetella pertussis and rubbed it against the ribbed internal surface of dissecting forcepts in heparinized M199 medium. The resulting suspension was found to adhere to coverslips (22). Yields, viabilities, and contamination by
TABLE II. Membrane Enzyme Activities of Granuloma Macro- phages Compared to Resident and Elicited Mouse Peritoneal Macrophages3
Enzyme-specific/Activity (U/mg protein) Macrophages 5r-Nucleotidase Aminopeptidase
Resident 13.28 ± 1.33 9.41 ± 4.50 Elicited 0.21 ± 0.15 41.67 ± 1.61 Granuloma 0.31 ± 0.12 36.67 ± 2.81
Cells were cultured for 24 hr, washed, and harvested in Triton-xlOO (0.1%) and saline. The enzymes were assayed as described (19). The elicited cells were obtained from mice injected 4 days previously with thioglycol late broth. Repro- duced from Bonney et al. (19).
other cell types were not discussed. It is most likely that a more gentle dispersion such as that achieved by proteolytic action would yield a population of cells with higher viability that could be further purified by other techniques and subse- quently cultivated. The methodology for enzymatic dispersion of granulomas described here is similar to that described by Russell et al. (18) for dispersing neoplasms and confirms the usefulness of their method.
The mononuclear phagocytes isolated from carrageenan- induced granulomas in mice by the technique described herein exhibit many of the characteristics of elicited populations of mouse peritoneal macrophages. The cultures are nearly homo- geneous as determined by the percentage of cells capable of phagocytosing latex beads in serum-free medium (>95%). This method most likely would be applicable to granulomas induced by other agents (foreign body or hypersensitivity induced).
Times of incubations with the proteolytic enzymes as well as the concentration of enzymes should be varied to obtain opti- mal conditions for each type of granuloma. In the case of
carrageenan granulomas, it is most crucial to determine a non- toxic but effective dose of carrageenan to be injected into the mice. Macrophages isolated from lesions induced by 5 mg of carrageenan did not survive in culture, and an injection of less than 1 mg of carrageenan did not elicit a sizable lesion.
This would be expected to vary with type of carrageenan and strain of mice.
REFERENCES
1. K. S. Warren. A functional classification of granuloma- tous inflammation. Ann. NY Acad. Sei. 278: 7-18, 1976.
2. W. G. Spector. Chronic inflammation. In "The Inflam- matory Process" (B. Zweifach, L. Grant, and R. T. McClus- key, eds.), p. 277. Academic Press, New York, 1974.
3. D. L. Boros. Granulomatous inflammations. Progr. Allergy 24: k83-267, 1978.
4. D. L. Boros and K. S. Warren. Delayed hypersensitivity granuloma formation and dermal reaction induced by a soluble factor isolated from Schistosoma mansoni eggs.
J. Exp. Med. 132: 488-507, 1970.
5. A. M. Danneberg, M. Ando, and K. Shima. Macrophage ac- cumulation, division, maturation, and digestive and mi- crobicidal capacities in tuberculous lesions. III. The turnover of macrophages and its relation to their acti- vation and antimicrobial immunity in primary BCG lesions and those of reinfection. J. Immunol. 109: 1109-1121, 1972.
W. G. Spector and A. W. J. Lykke. The cellular evolution of inflammatory granulomata. J. Pathol. Bacteriol. 92:
163-177, 1966.
R. P. McCombs. Diseases due to immunological reactions in the lungs (two parts). N. Engl. J. Med. 286: 1186- 1194 and 1245-1252, 1972.
M. B. Lurie. "Resistance to Tuberculosis: Experimental Studies in Native and Acquired Defensive Mechanisms,"
p. 6. Harvard University Press, Cambridge, Massachusetts, 1964.
S. G. Kayes and J. A. Oaks. Development of the granulo- matous response in murine Toxocariasis. Am. J. Pathol.
93: 277-286, 1978.
W. G. Spector and G. B. Ryan. New evidence for the existance of long lived macrophages. Nature 221: 860, 1969.
I. Ginsburg. Mechanisms of cell and tissue injury induced by group A streptococci. Relation to post streptococcal sequence. J. Infect. Dis. 126: 294-340, 1972.
C. A. Winter, E. A. Risley, and G. W. Nuss. Antiinflam- matory and antipyretic activities of indomethacin. J.
Pharmacol. Exp. Ther. 141: 369-376, 1963.
E. Kulonen and M. Potila. Effect of administration of antirheumatic drugs and experimental granuloma in rat.
Biochem. Pharmacol. 24: 219-225, 1975.
J. Cortez Pimentel. Sarcoid granulomas of the skin pro- duced by acrylic and nylon fibers. Br. J. Dermatol. 96:
673-677, 1977.
B. Morris, T. Weinberg, and G. J. Spector. The carragee- nan granuloma in the rat. A model for the study of the structure and function of macrophages. Br. J. Exp.
Pathol. 49: 302-311, 1968.
R. J. Bonney, P. R. Walker, and V. R. Potter. Isoenzyme patterns in parenchymal and nonparenchymal cells from re- generating and regenerated liver. Biochem. J. 136: 947- 954, 1973.
J. M. Dayer, S. M. Krane, R. G. G. Rüssel, and D. R. Ro- binson. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial ce-ls. Proc. Nat.
Acad. Sei. USA 73: 945-949, 1976.
S. W. Russell, W. F. Doe, R. G. Hoskins, and C. G.
Cochrane. Inflammatory cells in solid murine neoplasms.
I. Tumor disaggregation and identification of constituent inflammatory cells. Int. J. Cancer 18: 322-330, 1976.
R. J. Bonney, I. Gery, T. Y. Lin, M. F. Meyenhofer, W. Acevedo, and P. Davies. Mononuclear phagocytes from carrageenan-induced granulomas: Isolation cultivation and characterization. J. Exp. Med. 148: 261-275, 1978.
20. P. J. Edelson and Z. A. Cohn. S'-Nucleotidase activity of mouse peritoneal macrophages. I. Synthesis and degra- dation in resident and inflammatory populations. J.
Exp. Med. 144: 1581-1595, 1976.
21. E. D. Wachsmuth. Aminopeptidase as a marker for macro- phage differentiation. Exp. Cell Res. 96: 409-412, 1975.
22. G. B. Ryan and W. G. Spector. Natural selection of long- lived macrophages in experimental granulomata. J.
Pathol. 99: 139-151, 1967.