CHAPTER 6
Microdetermination of Metals by the Ashing Technique
A number of metals* in organic compounds may be determined by a simple ashing procedure with or without the addition of sulfuric or nitric a c i d s .
1 7 , 1 8 , 9 0 , 9 1 , 1 7 1 - 1 7 3 , 1 7 6 , 1 8 4 , 1 9 5 - 1 9 8 , 2 2 3However, only one metal may be present in the com
pound, or this method is not applicable and determination must be accomplished by adapting one of the macromethods found throughout the l i t e r a t u r e .
8 2'
2 1 8 , 2 3 5Before attempting to determine any metal by this method, the reader should consult the l i t e r a t u r e
1 0 7 , 1 0 8 , 1 6 5to make certain in which state the metal would be present at the end, namely, the oxide, sulfate, or free metal, and if these would or would not be lost by volatilization at the temperature involved. The method is not applicable to compounds containing phosphorus, since this is not completely driven o f f .
1 0 5The substance is treated with sulfuric acidf and placed in a muffle in which the acid is driven off and the residue heated at red heat.
The reactions may be represented by the following, depending upon the metal involved.
—Na N a2S 04
— Κ , k2s o4
—Ca H0S 04 C AS° 4
— B a B a S 04
etc. etc.
- A g Ag
—Au > Au
—Pt Pt
—Cu CuO
—Fe ( H N 02) F e203 ( H2S 04)
etc. etc.
* Determination of arsenic is carried out according to the method given in Chapter 13.
f Sulfuric acid is added in cases where the sulfate is stable at red heat. For silver, gold and platinum, no acid need be added. Where the oxide will be formed at the end of the determination, nitric acid may be added, as for example with copper, iron, etc.
Silver may exist as sulfate or chloride in which case the factor would not be 1.000.
133
Reagents
DILUTED SULFURIC ACID
1 part cone. H
2S 0
4(sp. gr. 1.84) 5 parts distilled water.
DILUTED NITRIC ACID
1 part cone. H N 0
3(sp. gr. 1.42) 1 part distilled water.
Apparatus
PLATINUM BOAT
The platinum boat required is the standard i t e m
5'
2 2 3-
2 2 5described in Chapter 3, Fig. 24.
PLATINUM CYLINDER
The platinum cylinder
1-
6 1shown in Fig. 8 4 is used as a sleeve into which the platinum boat is placed. I f spattering occurs during the determination the
material is deposited on the inner surface of the cylinder, preventing loss. It is made of platinum foil approximately 0.04 mm. thick, is about 3 cm. long
η Λ ~Λ ~ * A i i and has a diameter of approximately 7.5 mm. T o
FIG. 84. The Coombs-Alber , , Γ ,
platinum cylinder (sleeve).
t hecylinder is attached a piece of thin platmum wire, the end of which is shaped into a hook.
This is used in pushing the cylinder into the micromuffle and pulling it out.
MICROMUFFLE
The micromuffle consists of a tube (Pyrex 1 7 2 0 glass,* Vycor,* or quartz) which is heated to 6 8 0 ° - 7 0 0 ° C. and through which passes a slow current of air. Although the original micromuffle of P r e g l
1 7'
1 8-
9 0'
9 1'
1 7 1-
1 7 3'
1 8 4-
1 9 5-
1 9 8was gas heated, the author prefers e l e c t r i c
1 7 2-
1 7 6-
1 9 2units as these provide more constant heat, with equal distribution. This helps to prevent creeping of the material during the early stages of the heating and insures more constant ashing conditions.
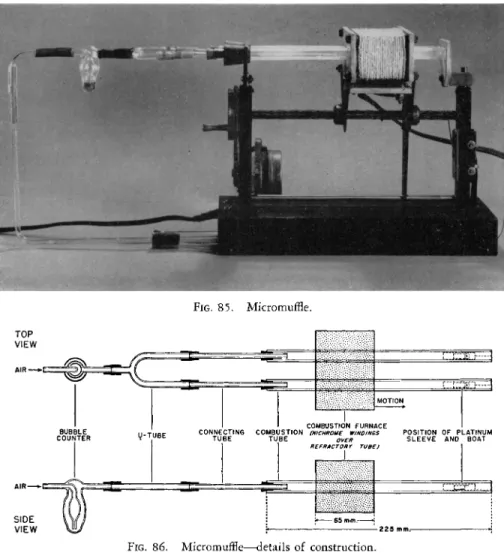
1 7 2The apparatus used by the author is shown in Figs. 85 and 86. It is a double unit which permits two determinations to be carried out simultane
ously. It consists of two sections of combustion tubing about 225 mm. in length, one tube for each determination. These are portions of standard combustion tubing, Pyrex 1720 glass,* Vycor,* or quartz, approximately 11 mm. O.D. and 8 mm. I.D. [ a section of the tube shown in Fig. 9 8 (Chapter 7 ) or Fig. 121
* Corning Glass Works, Corning, New York.
135 Apparatus
(Chapter 9 ) ] . The tubes are fastened in a support and pass through the two holes of the movable electric burner* or furnace. At one end, each tube is open and at the other end it is attached to a small bubble counterf which con
tains about 1 ml. of concentrated H
2S 0
4so that the rate of flow of air through
FIG. 8 5 . Micromuffle.
U - T U B E
COMBUSTION FURNACE COMBUSTION (NICHROME WINDINGS
T U B E OVER I REFRACTORY TUBE)
SIDE VIEW
FIG. 8 6 . Micromuffle—details of construction.
the tubes may be observed. The opposite end of the bubble counter is attached to a filter tube containing cotton, which in turn is connected to a compressed air supply.
The movable electric furnace is made from windings of small nichrome
* See footnote p. 154, Chapter 7.
f Portion of a broken bubble counter-U-tube (Chapter 9 ) may be used.
wire wound around two sections of alundum tubing approximately 1 3 - 1 4 mm. I.D. and about 65 mm. in length. The temperature is controlled to 6 8 0 ° - 7 0 0 ° C. by operating the furnace from a variable auto-transformer. The furnace is attached to a worm drive which is powered by a small electric clock motor,
2 2 2making the drive automatic with a forward speed of approximately 2.5 cm. in 10 minutes.
In many respects, the micromuffle is quite similar to that described by A. R.
Norton, G. L. Royer, and R. K o e g e l .
1 7 6Procedure
The boat and cylinder are cleaned by immersion in hot dilute nitric acid, washed with distilled water, strongly ignited in the flame of a gas burner, and set in a microdesiccator (Chapter 3, Figs. 4 3 - 4 6 ) to cool. The dismantled combination is then accurately weighed on the microchemical balance (taking into account the zero reading). About 5 mg. of sample is added to the boat and the combination reweighed. (Note: I f the sample is hygroscopic, the weighing pig is used to protect the sample as described in Chapter 3—see Figs. 29, 30, and 3 2 ) . Three drops of the diluted sulfuric acid* are care
fully added to the sample and the boat is then placed in the cylinder using a platinum-tipped forceps (Chapter 3, Fig. 4 1 ) to hold each piece during the manipulation. The combination is then placed in the open end of the combustion tubing and pushed far enough in so that all portions, including the wire hook, will be heated eventually. Care should be taken so that the boat is kept upright during the placing. The passage of air through the bubble counter is regulated so that bubbles pass through at the rate of about 1-2 per second. The furnace is placed back so that it is about at the one end of the cylinder opposite to the opening (about 5 - 6 cm. from the open end of the combustion tube). It is then switched on and the temperature brought up to 6 8 0 ° - 7 0 0 ° C. The mechanical drive is set into motion and the hot furnace is passed over the entire length of the cylinder and is allowed to remain in the end position (over the cylinder) for about 1 5 - 2 0 minutes. The combination is then carefully removed from the micromuffle and allowed to cool in a microdesiccator after which it is weighed to obtain the weight of the ash (taking into account the zero reading). It is good practice to reheat the combination in the micromuffle for 5 minutes and reweigh. This insures that constant weight has been attained.
Calculation:
Wt. ash X factor X 100
% Metal
W t . sample See f, Ρ- 133.
137 Additional Information for Chapter 6
Factors:*
Ash Element sought Factor
N a2S 04 Na 0 . 3 2 3 7
K2S O4 Κ 0 . 4 4 8 7
B a S 04 Ba 0 . 5 8 8 5
C a S 04 Ca 0 . 2 9 4 4
CuO Cu 0 . 7 9 8 8
F e2Os Fe 0 . 6 9 9 4
Pt Pt 1 . 0 0 0
Au Au 1 . 0 0 0
Ag Ag 1 . 0 0 0
Examples:
( a ) 5.632 mg. of ash ( N a2S 04) is obtained from a 7.631-mg. sample 5.632 X 0.3237 X 100
= 2 3 . 8 9 % Na 7.631
( b ) 2.610 mg. of ash ( C u O ) is obtained from a 8.001-mg. sample 2.610 X 0.7988 X 100
= 2 6 . 0 6 % Cu 8.001
( c ) 1.074 mg. of ash ( A g ) is obtained from a 7.016-mg. sample
1.074 χ 1 X 100 ^ A
Λ = " . 3 1 %
Ag
ADDITIONAL INFORMATION FOR CHAPTER 6
The micromuffle used by P r e g l
1 7-
1 8'
9 0-
9 1'
1 7 1-
1 7 3'
1 8 4-
1 9 5-
1 9 8is shown in Fig. 8 7 . It consists of a right angle tube and a straight one. The vertical portion of the right angle is heated to supply a hot air current.
Norton, Royer, and K o e g e l
1 7 6described an automatic electric microfurnace.
It provides for the ashing of two samples simultaneously. The two quartz com
bustion tubes are drawn through the furnace in contrast to that shown in Figs.
8 5 and 8 6 in which the tubes are stationary and the furnace moves along. The
above authors used a standard two-speed governor controlled phonograph motor geared so that the rate of travel is approximately either 1 or 2 inches
( 2 . 5 or 5 cm.) in 1 0 minutes. Platinum heating coils are used. The voltage
to these is controlled by means of a variable auto-transformer. The furnace is operated at a temperature of 8 0 0 ° C .
* Only a few typical factors are given here. For other cases, the reader is referred to the various handbooks containing gravimetric f a c t o r s .5 9-1 0 7'1 0 8 Also see Chapter 23.
Also see footnote on page 133.
FIG. 87. Pregl micromuffle.
T A B L E 16
ADDITIONAL INFORMATION ON R E F E R E N C E S * R E L A T E D TO C H A P T E R 6
Although this chapter deals with the determination of metals by the ashing technique, the author wishes to present additional information in the form of a table as in the preceding chapters. (See author's statement at top of Table 4 of Chapter 1.) The refer
ences listed in this table refer to the determination of metals by other means as well as the determination of several amphoteric elements not covered in detail in other chapters.
Books
Belcher and Godbert, 17, 18 Clark, E. P., 59
Clark, S. J . , 60
Fritz and Hammond, 81 Furman, 82
Grant, 90, 91
Milton and Waters, 167, 168 Niederl and Niederl, 171, 172 Niederl and Sozzi, 173 Pregl, 184
Roth, 195-198 Steyermark, 223
General, miscellaneous
Belcher, Gibbons, and Sykes, 16 Belcher, Macdonald, and West, 19 Cimerman and Selzer, 54, 55
General, miscellaneous (Conf.)
Duval, 69Martin, 158
Meyrowitz, and Massoni, 166 Norton, Royer, and Koegel, 176 Sykes, 231
Van Etten and Wiele, 239
Reviews
Belcher, Gibbons, and Sykes, 16 Sykes, 231
Apparatus
Norton, Royer, and Koegel, 176
Bombs
Kondo, 134 Kuck and Grim, 142
* The numbers which appear after each entry in this table refer to the literature citations in the reference list at the end of the chapter.
139 Table of References
T A B L E 16 (Continued) Perchloric acid
Smith, 217
Oxygen flask combustion Belcher, Macdonald, and West, 19 Corner, 62
Southworth, Hodecker, and Fleischer, 221 Kjeldahl flask digestion
Kreshkov, Syavtsillo, and Shemyaten- kova, 141
Simultaneous determinations Klimova and Bereznitskaya, 129, 130 Klimova, Korshun, and Bereznit-
skaya, 131, 132
Korshun and Chumachenko, 137 General volumetric methods
Belcher and Robinson, 20 Belcher and West, 21 Cimerman and Ariel, 4 8 - 5 0 Cimerman and Bogin, 51 Cimerman and Frenkel, 52 Cimerman and Selzer, 54 Deibner, 65
Gautier and Pellerin, 83
Gusev, Kumov, and Stroganova, 93 Kuck and Grim, 142
Schulitz, 210
Sloviter, McNabb, and Wagner, 215 Strahm and Hawthorne, 226 Sudo, Shimoe, and Miyahara, 230 Venkateswarlu, Ramanthan, and
Narayana Rao, 240 Zabrodina and Bagreeva, 248 Heterometric titration
Bobtelsky and Eisenstadter, 24 Bobtelsky and Graus, 25, 26 Bobtelsky and Hapern, 27 Bobtelsky and Jungreis, 2 8 - 3 2 Bobtelsky and Rafailoff, 33, 34 Bobtelsky and Welwart, 35-38 Complexometric, EDTA titration
Amin, 6
Ashby and Roberts, 10
Complexometric, EDTA titration (Conf.) Flaschka, 7 4 - 7 7
Flaschka and Amin, 78 Flaschka, Amin, and Zaki, 79 Harrison and Harrison, 96 Karrman and Borgstrom, 121 Kimbel, 122
Kinnunen and Merikanto, 125 Parry, 179
Socolar and Salach, 219 Southworth, Hodecker, and
Fleischer, 221
Flame photometric. X-ray spectropho- tometry, colorimetric, etc., methods
Almassy and Kavai, 4 Arnold and Pray, 9 Asperger and Murati, 11 Barrett, 14
Beauchene, Berneking, Schrenk, Mitchell, and Silker, 15
Buell, 41
Chatagnon and Chatagnon, 47 Gautier and Pellerin, 83 Gillam, 88
Grogan, Cahnmann, and Lethco, 92 Hegediis, Fukker, and Dvorszky, 99 Hunter, 112
Ikeda, 114
Ishibashi and Higashi, 115 Kingsley and Schafïert, 124 Kozawa, Tanaka and Sasaki, 139 Kreisky, 140
Lapin and Makarova, 148 Leonard, Sellers, and Swim, 149 Leroux, Mafïett, and Monkman, 150 Lewis, 151
Lindstrom, 153 Lipscomb, 154 Marier and Boulet, 157 Martin, 159, 160 Mehlig, 163
Natelson and Penniall, 170 Nonowa, 175
Nozaki, 177
Polley and Miller, 183 Sakuraba, 201
T A B L E 16 (Continued) Flame photometric, X-ray spectropho
tometry, colorimetric, etc., methods (Conf.)
Saltzman, 202 Schuhknecht, 206
Schuhknecht and Schinkel, 207 Schultz, 211
Solomon, 220 Teeri, 232
Toribara and Sherman, 234 Tunnicliffe, 236
Tutundzic and Mladenovic, 237 Umland and Weyer, 238 Zuehlke and Ballard, 250
Potentiometric, polarographic, etc., methods
Carruthers, 45 De Francesco, 64 Heyrovsky, 103 Jensen, 116
Kadowaki, Okamoto, and Nakajima, 118 Kuck and Grim, 142
Lambert and Walker, 145 Well, 243
Manometric methods See Chapter 18 Hoagland, 106
Gravimetric methods, precipitation, etc.
Barber and Kolthoff, 13 Cimerman and Selzer, 55 Fennel and Webb, 73
Gusev, Kumov, and Stroganova, 93 Kondo, 134
Walton and Smith, 242 Electrodeposition
Llacer, Sozzi and Benedetti-Pichler, 155 Chromatographic methods
Lacourt, 144 Lamm, 146
Radioactive methods Korenman, Sheyanova, and
Glazunova, 136
Antimony
Bahr, Bieling, and Thiele, 12 Gellhorn, Krahl, and Fertig, 85 Haight, 94
Jurecek and Jenik, 117 Schulek and Wolstadt, 209 Silvert and Kirner, 214 Arsenic
See Chapter 13 Barium
Pungor and Hegedus, 186 Pungor and Thege, 187 Beryllium
Toribara and Sherman, 234 Bismuth
Silvert and Kirner, 214 Boron
Allen and Tannenbaum, 3 Belcher, Macdonald, and West, 19 Buell, 41
Corner, 62
Gautier and Pignard, 84 Kuck and Grim, 142 Martin, 160
Pflaum and Wenske, 181 Roth, 194
Strahm and Hawthorne, 226 Cadmium
Cimerman and Selzer, 54 Furman, 82
Saltzman, 202 Steyermark, 223 Calcium
Andersch, 7
Ashby and Roberts, 10 Bobtelsky and Eisenstadter, 24 Gilbert, 86, 87
Harrison and Raymond, 95 Harrison and Harrison, 96 Herrmann, 102
Hunter, 112, 113
141 Table of References
T A B L E 16 (Continued)
Calcium (Conf.)
Ikeda, 114 Kimbel, 122
Kingsley and Schaffert, 123, 124 Kirk and Tompkins, 127 Lindner and Kirk, 152 Marier and Boulet, 157 McGregor, 161
Natelson and Penniall, 170 Nonowa, 175
Patel, 180
Pungor and Hegediis, 186 Pungor and Thege, 187 Rappaport and Rappaport, 189 Scholtis, 205
Schuhknecht, 206 Schultz, 211
Socolar and Salach, 219 Steyermark, 223 Teeri, 232 Wilkinson, 246
Chromium
Grogan, Cahnmann, and Lethco, 92 Kozawa, Tanaka, and Sasaki, 139 Lacourt, 144
Cobalt
Bobtelsky and Jungreis, 28 Cimerman and Selzer, 54 Ellis and Gibson, 70 Pohl and Demmel, 182 Sakuraba, 201 Saltzman, 203
Wenger, Cimerman, and Corbaz, 244
Copper
Amin, 6
Beauchene, Berneking, Schrenk, Mitchell, and Silker, 15
Benedetti-Pichler, 22 Bobtelsky and Graus, 26 Bobtelsky and Jungreis, 32 Bobtelsky and Welwart, 38 Carruthers, 45
Chatagnon and Chatagnon, 47 Dezsô and Fulôp, 66 Diehl and Smith, 67 Ellis and Gibson, 71
Copper (Conf.)
Emeléus and Haszeldine, 72 Furman, 82
Hecht and Reissner, 97 Hubbard and Spettelm, 111 Lapin and Makarova, 148
Llacer, Sozzi, and Benedetti-Pichler, 155 MacNevin and Bournique, 156 Mehlig, 163
Sakuraba, 201 Smith, 216 Steyermark, 223 Umland and Weyer, 238
Gold
Furman, 82
Konig, Crowell, and Benedetti- Pichler, 135
Onishi, 178 Steyermark, 223
Iron
Almassy and Kavai, 4 Belcher and West, 21 Bobtelsky and Jungreis, 29 Deibner, 65
Flaschka, 77 Furman, 82
Kirk and Bentley, 126 Knop and Kubelkova, 133 Nieuwenburg and Blumendal, 174 Rappaport and Hohenberg, 188 Steyermark, 223
Straub, 227
Tutundzic and Mladenovic, 237 Umland and Weyer, 238 Well, 243
Lead
Bobtelsky and Graus, 25 Bobtelsky and Rafailoff, 34 Brantner and Hecht, 40 Cimerman and Ariel, 4 8 - 5 0 Cimerman and Bogin, 51 De Francesco, 64 Furman, 82 Jensen, 116
Kuhn and Schretzmann, 143 Steyermark, 223
T A B L E 16 (Continued) Lithium
Hegedus and Dvorszky, 98 Nozaki, 177
Schuhknecht and Schinkel, 207 Steyermark, 223
Magnesium
Benedetti-Pichler and Schneider, 23 Bobtelsky and Welwart, 37 Butler, 43
Davidson, 63 Gillam, 88
Gusev, Kumov, and Stroganova, 93 Hoagland, 106
Hunter, 112, 113 Ikeda, 114
Karrman and Borgstrom, 121 Steyermark, 223
Strebinger and Reif, 229 Tunnicliffe, 236 Wilkinson, 246 Winkler, 247 Manganese
Flaschka, Amin, and Zaki, 79 Kozawa, Tanaka, and Sasaki, 139 Scott, 212
Mercury
Asperger and Murati, 11 Barrett, 14
Bobtelsky and Jungreis, 30, 31 Bobtelsky and Rafailoff, 33 Boëtius, 39
Cimerman and Frenkel, 52 Druzhinin and Kislitsin, 68 Emeléus and Haszeldine, 72 Furman, 82
Gautier and Pellerin, 83 Grant, 90, 91
Herd, 100 Hernler, 101
Hirai and Hayatsu, 105
Kadowaki, Okamoto, and Nakajima, 118 Korshun and Chumachenko, 137 Korshun and Lavrovskaya, 138 Leroux, Mafïett, and Monkman, 150 Lindstrom, 153
Mercury (Conf.) Lipscomb, 154
Meixner and Krocker, 164 Miura, 169
Parry, 179
Polley and Miller, 183 Rauscher, 190 Roth, F. J . , 193 Roth, H., 195-198 Rutgers, 199 Sachs, 200 Schulitz, 210
Shukis and Tallman, 213
Sloviter, McNabb, and Wagner, 215 Smith, 216
Southworth, Hodecker, and Fleischer, 221
Steyermark, 223
Sudo, Shimoe, and Miyahara, 230 Verdino, 241
Walton and Smith, 242 Zuehlke and Ballard, 250 Nickel
Benedetti-Pichler, 22
Bobtelsky and Welwart, 35, 36 Furman, 82
Llacer, Sozzi, and Benedetti-Pichler, 155 Steyermark, 223
Palladium
Kinnunen and Merikanto, 125 Konig, Crowell, and Benedetti-
Pichler, 135 Steyermark, 223 Platinum
Steyermark, 223 Potassium
Belcher and Robinson, 20 Bullock and Kirk, 42 Chapman, 46
Cimerman and Rzymowska, 5 3
Cimerman, Wenger, and Rzymowska, 58 Flaschka and Amin, 78
Hegedus and Dvorszky, 98 Herrmann, 102
143 Table of References
T A B L E 16 Potassium (Conf.)
Heyrovsky, 103
Kingsley and Schaffert, 123, 124 Klein and Jacobi, 128
Korenman, Sheyanova, and Glazu- nova, 136
Kreisky, 140 Lewis, 151
Robinson and Hauschildt, 191 Schuhknecht and Schinkel, 207 Steyermark, 223
Selenium
Alber and Harand, 2 Gould, 89
Kahane and Korach, 119 Kan, 120
Kondo, 134
Wernimont and Hopkinson, 245 Zabrodina and Bagreeva, 248 Silicon
Fennell and Webb, 73
Klimova and Bereznitskaya, 129, 130 Klimova, Korshun and Bereznitskaya,
131, 132
Kreshkov, Syavtsillo and Shemyaten- kova, 141
McHard, Servais and Clark, 162 Schoklitsch, 204
Thurnwald and Benedetti-Pichler, 233 Silver
Amin, 6
Emeléus and Haszeldine, 72 Flaschka, 75
Foulk and Bawden, 80 Furman, 82
Kuhn and Schretzmann, 143 Lambert and Walker, 145 Schulek, 208
Steyermark, 223 Sodium
Arnold and Pray, 9 Barber and Kolthoff, 13 Caley and Foulk, 44 Flaschka and Amin, 78
(Continued)
Sodium (Conf.)
Hegedus and Dvorszky, 98 Hegedus, Fukker, and Dvorszky, 99 Herrmann, 102
Holmes and Kirk, 109
Kingsley and Schaffert, 123, 124 Kreisky, 140
Schuhknecht and Schinkel, 207 Solomon, 220
Steyermark, 223 Strontium
Pungor and Hegedus, 186 Pungor and Thege, 187 Steyermark, 223
Strebinger and Mandl, 228 Zombory, 249
Thallium
Cimerman and Selzer, 55 Flaschka, 76
Thorium
Ishibashi and Higashi, 115
Venkateswarlu, Ramanthan, and Nara- yana Rao, 240
Tin
Furman, 82 Holtje, 110 Price, 185
Silvert and Kirner, 214 Uranium
Bobtelsky and Hapern, 27 Emeléus and Haszeldine, 72 Zinc
Anderson, 8
Cimerman and Selzer, 54 Cimerman and Wenger, 56, 57 Furman, 82
Hibbard, 104 Lamm, 146 Lang, 147 Martin, 159 Zirconium
Leonard, Sellers, and Swim, 149
REFERENCES 1. Alber, H. K., Mikrochemie, 18, 92 ( 1 9 3 5 ) .
2. Alber, H. K., and Harand, J . , / . Franklin Inst., 228, 243 ( 1 9 3 9 ) . 3. Allen, H., Jr., and Tannenbaum, S., Anal. Chem., 31, 265 ( 1 9 5 9 ) . 4. Almassy, G., and Kavai, M. Z., Magyar Kern. Folyoirat, 61, 246 ( 1 9 5 5 ) . 5. American Society for Testing Materials, AS Τ M Designations, E 1 2 4 - 5 7 T ( 1 9 5 7 ) . 6. Amin, A. M., Chemist Analyst, 44, 17 ( 1 9 5 5 ) .
7. Andersch, M. A , / . Lab. Clin. Med., 49, 486 ( 1 9 5 7 ) . 8. Anderson, C. W., Ind. Eng. Chem., Anal. Ed., 13, 367 ( 1 9 4 1 ) .
9. Arnold, Ε. Α., and Pray, A. R., Ind. Eng. Chem., Anal. Ed., 15, 294 ( 1 9 4 3 ) . 10. Ashby, R. O., and Roberts, M., / . Lab. Clin. Med., 49, 958 ( 1 9 5 7 )
11. Asperger, S., and Murati, I., Anal. Chem., 26, 543 ( 1 9 5 4 ) .
12. Bahr, G , Bieling, H , and Thiele, Κ. H., Z. anal. Chem., 145, 105 ( 1 9 5 5 ) .
13. Barber, H. H , and Kolthoff, I. M., / . Am. Chem. Soc, 50, 1625 ( 1 9 2 8 ) ; 51, 3233 ( 1 9 2 9 ) .
14. Barrett, F. R., Analyst, 81, 294 ( 1 9 5 6 ) .
15. Beauchene, R. E , Berneking, A. D., Schrenk, W . G., Mitchell, H. L., and Silker, R. E., / . Biol. Chem., 214, 731 ( 1 9 5 5 ) .
16. Belcher, R., Gibbons, D., and Sykes, Α., Mikrochemie ver. Mikrochim. Acta, 40, 76 ( 1 9 5 2 ) .
17. Belcher, R., and Godbert, A. L., "Semi-Micro Quantitative Organic Analysis,"
Longmans, Green, London, and New York, 1945.
18. Belcher, R., and Godbert, A. L., "Semi-Micro Quantitative Organic Analysis," 2nd ed., Longmans, Green, London, 1954.
19. Belcher, R., Macdonald, A. M. G., and West, T. S., Talanta, 1, 408 ( 1 9 5 8 ) . 20. Belcher, R., and Robinson, J . W., Mikrochim. Acta, p. 49 ( 1 9 5 4 ) .
21. Belcher, R., and West, T. S , Anal. Chim. Acta, 5, 472 ( 1 9 5 1 ) .
22. Benedetti-Pichler, Α. Α., "Introduction to the Microtechniques of Inorganic An
alyses," Wiley, New York, and Chapman & Hall, London, 1942.
23. Benedetti-Pichler, Α. Α., and Schneider, F., Mikrochemie, Emich Festschrift, pp. 1-17 ( 1 9 3 0 ) .
24. Bobtelsky, M., and Eisenstadter, J . , Anal. Chim. Acta, 14, 89 ( 1 9 5 6 ) . 25. Bobtelsky, M., and Graus, B . , Anal. Chim. Acta, 9, 163 ( 1 9 5 3 ) . 26. Bobtelsky, M., and Graus, B . , Anal. Chim. Acta, 11, 253 ( 1 9 5 4 ) . 27. Bobtelsky, M., and Hapern, M., Anal. Chim. Acta, 11, 84 ( 1 9 5 4 ) . 28. Bobtelsky, M., and Jungreis, E., Anal. Chim. Acta, 12, 248 ( 1 9 5 5 ) . 29. Bobtelsky, M., and Jungreis, E., Anal. Chim. Acta, 12, 351 ( 1 9 5 5 ) . 30. Bobtelsky, M., and Jungreis, E., Anal. Chim. Acta, 12, 562 ( 1 9 5 5 ) . 31. Bobtelsky, M., and Jungreis, E., Anal. Chim. Acta, 13, 72 ( 1 9 5 5 ) . 32. Bobtelsky, M., and Jungreis, E., Anal. Chim. Acta, 13, 449 ( 1 9 5 5 ) . 33. Bobtelsky, M., and Rafailoff, R., Anal. Chim. Acta, 14, 339 ( 1 9 5 6 ) . 34. Bobtelsky, M , and Rafailoff, R., Anal. Chim. Acta, 16, 321 ( 1 9 5 7 ) . 35. Bobtelsky, M., and Welwart, Y . , Anal. Chim. Acta, 9, 281 ( 1 9 5 3 ) . 36. Bobtelsky, M., and Welwart, Y . , Anal. Chim. Acta, 9, 374 ( 1 9 5 3 ) . 37. Bobtelsky, M., and Welwart, Y . , Anal. Chim. Acta, 10, 156 ( 1 9 5 4 ) . 38. Bobtelsky, M., and Welwart, Y . , Anal. Chim. Acta, 10, 459 ( 1 9 5 4 ) . 39. Boëtius, M., / . prakt. Chem., 151, 279 ( 1 9 3 8 ) .
40. Brantner, H., and Hecht, F., Mikrochemie, 14, 30 ( 1 9 3 4 ) . 4 1 . Buell, Β . E., Anal. Chem., 30, 1514 ( 1 9 5 8 ) .
145
References42. Bullock, Β . , and Kirk, P. L., Ind. Eng. Chem., Anal. Ed., 7, 178 ( 1 9 3 5 ) . 43. Butler, E. J . , Analyst, 81, 615 ( 1 9 5 6 ) .
44. Caley, E. R., and Foulk, C W., / . Am. Chem. Soc, 51, 1664 ( 1 9 2 9 ) . 45. Carruthers, C , Ind. Eng. Chem., Anal. Ed., 17, 398 ( 1 9 4 5 ) . 46. Chapman, G. W , / . Agr. Sci, 37, 29 ( 1 9 4 7 ) .
47. Chatagnon, C , and Chatagnon, P., Bull, soc chim. biol., 36, 911 ( 1 9 5 4 ) . 48. Cimerman, C , and Ariel, M., Anal. Chim. Acta, 12, 13 ( 1 9 5 5 ) . 49. Cimerman, C , and Ariel, M., Anal. Chim. Acta, 14, 48 ( 1 9 5 6 ) . 50. Cimerman, C , and Ariel, M., Anal. Chim. Acta^ld, 207 ( 1 9 5 6 ) . 51. Cimerman, C , and Bogin, D., Anal. Chim. Acta, 12, 218 ( 1 9 5 5 ) . 52. Cimerman, C , and Frenkel, S., Anal. Chim. Acta, 16, 305 ( 1 9 5 7 ) . 53. Cimerman, C , and Rzymowska, C. J . , Mikrochemie, 20, 129 ( 1 9 3 6 ) . 54. Cimerman, C , and Selzer, M., Anal. Chim. Acta, 9, 26 ( 1 9 5 3 ) . 55. Cimerman, C , and Selzer, G., Anal. Chim. Acta, 15, 213 ( 1 9 5 6 ) . 56. Cimerman, C , and Wenger, P., Mikrochemie, 2A, 148 ( 1 9 3 8 ) . 57. Cimerman, C , and Wenger, P., Mikrochemie, 24, 153 ( 1 9 3 8 ) .
58. Cimerman, C , Wenger, P., and Rzymowska, C. J . , Mikrochemie, 20, 1 ( 1 9 3 6 ) . 59. Clark, E. P., "Semimicro Quantitative Organic Analysis," Academic Press, New
York, 1943.
60. Clark, S. J . , "Quantitative Methods of Organic Microanalysis," Butterworths, Lon
don, 1956.
6 1 . Coombs, H. L, Biochem. ] . , 21, 404 ( 1 9 2 7 ) . 62. Corner, M., Analyst, 84, 41 ( 1 9 5 9 ) . 63. Davidson, J . , Analyst, 77, 263 ( 1 9 5 2 ) .
64. De Francesco, F., Boll. lab. chim. provinciali (Bologna), 6, 10 ( 1 9 5 5 ) . 65. Deibner, L., Mikrochemie ver. Mikrochim. Acta, 35, 488 ( 1 9 5 0 ) . 66. Dezsô, I., and Fiilôp, T., Mikrochim. Acta, p. 592 ( 1 9 5 9 ) .
67. Diehl, H., and Smith, G. F., "The Copper Reagents. Cuproine, Neocuproine, Bathocuproine," G. F. Smith Chemical Co., Columbus, Ohio, 1958.
68. Druzhinin, I. G., and Kislitsin, P. S., Trudy Inst. Khim. Akad. Nauk Kirgiz.
S.S.R., p. 21 ( 1 9 5 7 ) ; Referai. Zhur. Khim., Abstr. No. 57237 ( 1 9 5 8 ) ; Anal.
Abstr., 6, Abstr. No. 587 ( 1 9 5 9 ) . 69. Duval, C , Chim. anal., 34, 209 ( 1 9 5 2 ) .
70. Ellis, K. W . , and Gibson, Ν . Α., Anal. Chim. Acta, 9, 275 ( 1 9 5 3 ) . 71. Ellis, K. W., and Gibson, Ν . Α., Anal. Chim. Acta, 9, 368 ( 1 9 5 3 ) . 72. Emeléus, H. J . , and Haszeldine, R. N., / . Chem. Soc, p. 2948 ( 1 9 4 9 ) . 73. Fennell, T . R. F. W., and Webb, J . R , Talanta, 2, 389 ( 1 9 5 9 ) . 74. Flaschka, H., Mikrochemie ver. Mikrochim. Acta, 39, 38 ( 1 9 5 2 ) . 75. Flaschka, H., Mikrochemie ver. Mikrochim. Acta, 40, 21 ( 1 9 5 3 ) . 76. Flaschka, H., Mikrochemie ver. Mikrochim. Acta, 40, 42 ( 1 9 5 3 ) . 77. Flaschka, H., Mikrochim. Acta, p. 361 ( 1 9 5 4 ) .
78. Flaschka, H., and Amin, A. M., Chemist Analyst, 42, 78 ( 1 9 5 3 ) . 79. Flaschka, H., Amin, A. M , and Zaki, R., Chemist Analyst, 43, 67 ( 1 9 5 4 ) . 80. Foulk, C. W . , and Bawden, A. T., / . Am. Chem. Soc, 48, 2045 ( 1 9 2 6 ) .
81. Fritz, J . S., and Hammond, G. S., "Quantitative Organic Analysis," Wiley, New York, and Chapman & Hall, London, 1957.
82. Furman, Ν . H., ed., "Scott's Standard Methods of Chemical Analysis," 5th ed., Vol. II, Van Nostrand, New York, 1939.
83. Gautier, J . Α., and Pellerin, F., Prods, pharm., 13, 149 ( 1 9 5 8 ) ; Chem. Abstr., 52, 15338 ( 1 9 5 8 ) .
84. Gautier, J . Α., and Pignard, P., Mikrochemie ver. Mikrochim. Acta, 36/37, 793 ( 1 9 5 1 ) .
85. Gellhorn, Α., Krahl, M. E., and Fertig, J . W . , / . Pharmacol. Exptl. Therap. 87, 159 ( 1 9 4 6 ) .
86. Gilbert, A. B . , Nature, 183, 888 ( 1 9 5 9 ) . 87. Gilbert, A. B . , Nature, 183, 1754 ( 1 9 5 9 ) .
88. Gillam, W . S., Ind. Eng. Chem., Anal. Ed., 13, 499 ( 1 9 4 1 ) . 89. Gould, E. S., Anal. Chem., 23, 1502 ( 1 9 5 1 ) .
90. Grant, J . , "Quantitative Organic Microanalysis, Based on the Methods of Fritz Pregl," 4th ed., Blakiston, Philadelphia, Pennsylvania, 1946.
9 1 . Grant, J . , "Quantitative Organic Microanalysis," 5th ed., Blakiston, Philadelphia, Pennsylvania, 1951.
92. Grogan, C. H., Cahnmann, H. J . , and Lethco, E., Anal. Chem., 27, 983 ( 1 9 5 5 ) . 93. Gusev, S. I., Kumov, V . I., and Stroganova, A. M., Zhur. Anal. Khim.. 10, 349
( 1 9 5 5 ) ; Chem. Abstr., 50, 7654 ( 1 9 5 6 ) . 94. Haight, G. P , Anal. Chem., 26, 593 ( 1 9 5 4 ) .
95. Harrison, G. E., and Raymond, W . Η. Α., Analyst, 78, 528 ( 1 9 5 3 ) . 96. Harrison, Η. E., and Harrison, H. C , / . Lab. Clin. Med., 46, 662 ( 1 9 5 5 ) . 97. Hecht, F., and Reissner, R., Mikrochemie, 17, 127 ( 1 9 3 5 ) .
98. Hegedus, A. J . , and Dvorszky, M., Mikrochim. Acta, p. 160 ( 1 9 5 9 ) .
99. Hegedus, Α., Fukker, F. K., and Dvorszky, M., Magyar Kém. Folyoirat, 59, 334
• ( 1 9 5 3 ) ; Referai. Zhur. Khim., Abstr. No. 36,400 ( 1 9 5 4 ) . 100. Herd, R. L., / . Assoc. Offic. Agr. Chemists, 38, 645 ( 1 9 5 5 ) . 101. Hernler, F., Mikrochemie, Pregl Festschrift, p. 154 ( 1 9 2 9 ) . 102. Herrmann, R., Z. ges. exptl. Med., 122, 84 ( 1 9 5 3 ) . 103. Heyrovsky, Α., Chem. listy, 50, 69 ( 1 9 5 6 ) .
104. Hibbard, P. L., Ind. Eng. Chem-., Anal. Ed., 6, 423 ( 1 9 3 4 ) . 105. Hirai, M., and Hayatsu, R., Yakugaku Zasshi, 70, 670 ( 1 9 5 0 ) . 106. Hoagland, C. L., / . Biol. Chem., 136, 553 ( 1 9 4 0 ) .
107. Hodgman, C D., "Handbook of Chemistry and Physics," 28th ed., Chemical Rubber, Cleveland, Ohio, 1944.
108. Hodgman, C. D., and Lange, Ν. Α., "Handbook of Chemistry and Physics," 9th ed., Chemical Rubber, Cleveland, Ohio, 1922.
109. Holmes, B . , and Kirk, P. L., / . Biol. Chem., 116, 377 ( 1 9 3 6 ) . 110. Hôltje, R., Z. anorg. u. allgem. Chem., 198, 287 ( 1 9 3 1 ) .
111. Hubbard, D . M., and Spettelm, E. C , Anal. Chem., 25, 1245 ( 1 9 5 3 ) . 112. Hunter, G., Analyst, 83, 93 ( 1 9 5 8 ) .
113. Hunter, G., Analyst, 84, 24 ( 1 9 5 9 ) .
114. Ikeda, S., Nippon Kagaku Zasshi, 76, 783 ( 1 9 5 5 ) .
115. Ishibashi, M., and Higashi, S., Bunseki Kagaku, 4, 14 ( 1 9 5 5 ) . 116. Jensen, R., Chim. anal., 37, 53 ( 1 9 5 5 ) .
117. Jurecek, M., and Jenik, J . , Chem. listy, 51, 1316 ( 1 9 5 7 ) .
118. Kadowaki, H., Okamoto, J . , and Nakajima, M., Yakugaku Zasshi, 75, 485 ( 1 9 5 5 ) . 119. Kahane, E., and Korach, S., Mikrochemie ver. Mikrochim. Acta, 36/37, 781 ( 1 9 5 1 ) . 120. Kan, M., Takeda Kenkyusho Nempo, 11, 54 ( 1 9 5 2 ) .
121. Karrman, K. J . , and Borgstrom, S., Svensk Kern. Tidskr., 67, 18 ( 1 9 5 5 ) . 122. Kimbel, Κ. H., Z. physiol. Chem., Hoppe-Seyler's, 293, 272 ( 1 9 5 3 ) . 123. Kingsley, G. R., and Schafïert, R. R., Anal. Chem., 25, 1738 ( 1 9 5 3 ) . 124. Kingsley, G. R., and Schafïert, R. R., / . Biol. Chem., 206, 807 ( 1 9 5 4 ) . 125. Kinnunen, J . , and Merikanto, B . , Chemist Analyst, 47, 11 ( 1 9 5 8 ) .
147
References126. Kirk, P. L., and Bentley, G. T., Mikrochemie, 21, 250 ( 1 9 3 7 ) .
127. Kirk, P. L., and Tompkins, P. C , Ind. Eng. Chem., Anal. Ed., 13, 277 ( 1 9 4 1 ) . 128. Klein, B , and Jacobi, M., Ind. Eng. Chem., Anal. Ed., 12, 687 ( 1 9 4 0 ) . 129. Klimova, V . Α., and Bereznitskaya, E. G., Zhur. Anal. Khim., 11, 292 ( 1 9 5 6 ) . 130. Klimova, V . Α., and Bereznitskaya, E. G., Zhur. Anal. Khim., 12, 424 ( 1 9 5 7 ) ;
Chem. Abstr., 52, 1853 ( 1 9 5 8 ) .
131. Klimova, V . Α., Korshun, M. O., and Bereznitskaya, E. G., Doklady Akad. Nauk S.S.S.R., 96, 81 ( 1 9 5 4 ) .
132. Klimova, V . Α., Korshun, M. O., and Bereznitskaya, E. G., Zhur. Anal. Khim., 11, 223 ( 1 9 5 6 ) .
133. Knop, J . , and Kubelkova, Ο., Z. anal. Chem., 100, 161 ( 1 9 3 5 ) .
134. Kondo, Α., Bunseki Kagaku, 6, 583 ( 1 9 5 7 ) ; Chem. Abstr., 52, 15345 ( 1 9 5 8 ) .
135. Konig, O., Crowell, W . R., and Benedetti-Pichler, Α. Α., Mikrochemie ver.
Mikrochim. Acta, 33, 281 ( 1 9 4 8 ) .
136. Korenman, I. M., Sheyanova, F. R., and Glazunova, Ζ. I., Zavodskaya Lab., 21, 774 ( 1 9 5 5 ) .
137. Korshun, M. O., and Chumachenko, Ν . M., Doklady Akad. Nauk S.S.S.R., 99, 769 ( 1 9 5 4 ) .
138. Korshun, M. O., and Lavrovskaya, Ε . V., Zhur. Anal. Khim., 3, 322 ( 1 9 4 8 ) . 139. Kozawa, Α., Tanaka, M., and Sasaki, K., Bull. Chem. Soc. Japan, 27, 345 ( 1 9 5 4 ) . 140. Kreisky, F., Mikrochim. Acta, p. 242 ( 1 9 5 9 ) .
141. Kreshkov, A. P., Syavtsillo, S. V., and Shemyatenkova, V . T., Zavodskaya Lab., 22, 1425 ( 1 9 5 6 ) .
142. Kuck, J . Α., and Grim, E. C , Microchem. J . , 3, 35 ( 1 9 5 9 ) . 143. Kuhn, R., and Schretzmann, H., Chem. Ber., 90, 554 ( 1 9 5 7 ) . 144. Lacourt, Α., Mikrochim. Acta, p. 550 ( 1 9 5 4 ) .
145. Lambert, R. H., and Walker, R. D., Ind. Eng. Chem., Anal. Ed., 13, 846 ( 1 9 4 l ) . 146. Lamm, G. G., Acta Chem. Scand., 7, 1420 ( 1 9 5 3 ) .
147. Lang, R., Z. anal. Chem., 93, 21 ( 1 9 3 3 ) .
148. Lapin, L. N., and Makarova, V . P., Pochvovedenie, p. 82 ( 1 9 5 3 ) ; Referai. Zhur.
Khim., Abstr. No. 22,146 ( 1 9 5 4 ) .
149. Leonard, G. W . , Jr., Sellers, D . E., and Swim, L. E., Anal. Chem., 26, 1621 ( 1 9 5 4 ) .
150. Leroux, J . , Maffett, P. Α., and Monkman, J . L., Anal. Chem., 29, 1089 ( 1 9 5 7 ) . 151. Lewis, R. P., Analyst, 80, 768 ( 1 9 5 5 ) .
152. Lindner, R., and Kirk, P. L., Mikrochemie, 22, 291 ( 1 9 3 7 ) . 153. Lindstrom, O., Anal. Chem., 31, 461 ( 1 9 5 9 ) .
154. Lipscomb, W . N., Anal. Chem., 25, 737 ( 1 9 5 3 ) .
155. Llacer, A. J . , Sozzi, J . Α., and Benedetti-Pichler, Α. Α., Ind. Eng. Chem., Anal.
Ed., 13, 507 ( 1 9 4 1 ) .
156. MacNevin, W . M., and Bournique, R. Α., Ind. Eng. Chem., Anal. Ed., 12, 431 ( 1 9 4 0 ) .
157. Marier, J . R., and Boulet, Μ. Α., / . Agr. Food. Chem., 4, 720 ( 1 9 5 6 ) . 158. Martin, F., Mikrochemie ver. Mikrochim. Acta, 36/37, 660 ( 1 9 5 1 ) . 159. Martin, G., Bull. soc. chim. biol, 34, 1174 ( 1 9 5 2 ) .
160. Martin, G., Bull. soc. chim. biol., 36, 719 ( 1 9 5 4 ) . 161. McGregor, A. J . , Analyst, 75, 211 ( 1 9 5 0 ) .
162. McHard, J . Α., Servais, P. C , and Clark, Η. Α., Anal. Chem., 20, 325 ( 1 9 4 8 ) . 163. Mehlig, J . P., Ind. Eng. Chem., Anal. Ed., 13, 533 ( 1 9 4 l ) .
164. Meixner, Α., and Krôcker, F., Mikrochemie, 5, 131 ( 1 9 2 7 ) .
165. Mellor, J . W., "A Comprehensive Treatise on Inorganic and Theoretical Chemistry,"
Longmans, Green, New York, and London, 1922-1937.
166. Meyrowitz, R., and Massoni, C. J . , Anal. Chem., 27, 475 ( 1 9 5 5 ) .
167. Milton, R. F., and Waters, W . Α., "Methods of Quantitative Microanalysis."
Longmans, Green, New York, and Arnold, London, 1949.
168. Milton, R. F., and Waters, W . Α., "Methods of Quantitative Microanalysis,"
2nd ed., Arnold, London, 1955.
169. Miura, H., Kokumin Eisei, 25, 196 ( 1 9 5 6 ) ; Chem. Abstr., 52, 18083 ( 1 9 5 8 ) . 170. Natelson, S., and Penniall, R., Anal. Chem., 27, 434 ( 1 9 5 5 ) .
171. Niederl, J . B . , and Niederl, V., "Micromethods of Quantitative Organic Elementary Analysis," Wiley, New York, 1938.
172. Niederl, J . B . , and Niederl, V . , "Micromethods of Quantitative Organic Analysis."
2nd ed., Wiley, New York, 1942.
173. Niederl, J . B . , and Sozzi, J . Α., "Microanâlisis Elemental Orgânico," Calle Arcos, Buenos Aires, 1958.
174. Nieuwenburg, C. J . van, and Blumendal, H. B . , Mikrochemie, 18, 39 ( 1 9 3 5 ) . 175. Nonowa, D . C , Mikrochim. Acta, p. I l l ( 1 9 5 8 ) .
176. Norton, A. R., Royer, G. L., and Koegel, R., Ind. Eng. Chem., Anal. Ed., 12, 121 ( 1 9 4 0 ) .
177. Nozaki, T., Nippon Kagaku Zasshi, 76, 445 ( 1 9 5 5 ) . 178. Onishi, H., Mikrochim. Acta, p. 9 ( 1 9 5 9 ) .
179. Parry, E. P., Anal. Chem., 29, 546 ( 1 9 5 7 ) . 180. Patel, H. R., Drug Standards, 24, 159 ( 1 9 5 6 ) .
181. Pflaum, D . J . , and Wenske, H. H., Ind. Eng. Chem., Anal. Ed., 4, 392 ( 1 9 3 2 ) . 182. Pohl, Ε. Α., and Demmel, H., Anal. Chim. Acta, 10, 554 ( 1 9 5 4 ) .
183. Polley, D., and Miller, V . L., Anal. Chem., 27, 1162 ( 1 9 5 5 ) .
184. Pregl, F., "Quantitative Organic Microanalysis," ( E . Fyleman, trans., 2nd German ed.), p. 136, Churchill, London, 1924.
185. Price, J . W . , Paint Manuf., 28, 147 ( 1 9 5 8 ) ; Anal. Abstr., 6, No. 1384 (1959 ) . 186. Pungor, E., and Hegedus, A. J . , Mikrochim. Acta, p. 87 ( I 9 6 0 ) .
187. Pungor, E., and Thege, I. K., Mikrochim. Acta, p. 712 ( 1 9 5 9 ) . 188. Rappaport, F., and Hohenberg, E., Mikrochemie, 14, 119 ( 1 9 3 4 ) . 189. Rappaport, F., and Rappaport, D., Mikrochemie, 15, 107 ( 1 9 3 4 ) . 190. Rauscher, W . H., Ind. Eng. Chem., Anal. Ed., 10, 331 ( 1 9 3 8 ) .
191. Robinson, R. J . , and Hauschildt, J . D., Ind. Eng. Chem., Anal. Ed., 12, 676 ( 1 9 4 0 ) .
192. Rodden, C. J . , Mikrochemie, 18, 97 ( 1 9 3 5 ) .
193. Roth, F . J . , / . Assoc. Off. Agri. Chemists, 40, 302 ( 1 9 5 7 ) . 194. Roth, H., Angew. Chem., 50, 593 ( 1 9 3 7 ) .
195. Roth, H., "Die quantitative organische Mikroanalyse von Fritz Pregl," 4th ed., Springer, Berlin, 1935.
196. Roth, H., " F . Pregl quantitative organische Mikroanalyse," 5th ed., Springer, Wien, 1947.
197. Roth, H., "Pregl-Roth quantitative organische Mikroanalyse," 7th ed., Springer, Wien, 1958.
198. Roth, H., "Quantitative Organic Microanalysis of Fritz Pregl," 3rd ed. ( Ε . B, Daw, trans., 4th German ed.), Blakiston, Philadelphia, Pennsylvania, 1937.
199. Rutgers, J . J . , Compt. rend. acad. sci., 190, 746 ( 1 9 3 0 ) . 200. Sachs, G., Analyst, 78, 185 ( 1 9 5 3 ) .
201. Sakuraba, S., Bunseki Kagaku, 4, 496 ( 1 9 5 5 ) .
149
References202. Saltzman, Β . E., Anal. Chem., 25, 493 ( 1 9 5 3 ) . 203. Saltzman, Β . E., Anal. Chem., 27, 284 ( 1 9 5 5 ) . 204. Schoklitsch, K., Mikrochemie, 18, 144 ( 1 9 3 5 ) .
205. Scholtis, K., Mikrochemie ver. Mikrochim. Acta, 26, 150 ( 1 9 3 9 ) . 206. Schuhknecht, W., Z. anal. Chem., 157, 338 ( 1 9 5 7 ) .
207. Schuhknecht, W., and Schinkel, H., Z. anal. Chem., 143, 321 ( 1 9 5 4 ) . 208. Schulek, E., Mikrochemie, Emich Festschrift, p. 260 ( 1 9 3 0 ) . 209. Schulek, E., and Wolstadt, R., Z. anal. Chem., 108, 400 ( 1 9 3 7 ) . 210. Schulitz, P. H., Arch. Pharm., 286, 506 ( 1 9 5 3 ) .
211. Schultz, Y . O., Schweiz. med. Wochschr., 83, 452 ( 1 9 5 3 ) . 212. Scott, F. W., Chemist Analyst, 27 ( 1 9 3 8 ) .
213. Shukis, Α., Jr., and Tallman, R. C , Ind. Eng. Chem., Anal. Ed., 12, 123 ( 1 9 4 0 ) . 214. Silvert, F. C , and Kirner, W . R., Ind. Eng. Chem., Anal. Ed., 8, 353 ( 1 9 3 6 ) . 215. Sloviter, Η. Α., McNabb, W . M., and Wagner, E. C , Ind. Eng. Chem., Anal. Ed.,
13, 890 ( 1 9 4 1 ) .
216. Smith, G. F., Chemical Co., "The Trace Element Determination of Copper and Mercury in Pulp and Paper," Columbus, Ohio.
217. Smith, G. F., Chemical Co., "The W e t Ashing of Organic Matter Employing Hot Concentrated Perchloric Acid. The Liquid Fire Reaction," Columbus, Ohio.
218. Smith, G. McPhail, "A Course of Instruction in Quantitative Chemical Analysis for Beginning Students," rev. ed., Macmillan, New York, 1922.
219. Socolar, S. J . , and Salach, J . I., Anal. Chem., 31, 473 ( 1 9 5 9 ) . 220. Solomon, A. K., Anal. Chem., 27, 1849 ( 1 9 5 5 ) .
221. Southworth, B . C , Hodecker, J . H., and Fleischer, K. D., Anal. Chem., 30, 1152 ( 1 9 5 8 ) .
222. Steyermark, Al, Ind. Eng. Chem., Anal. Ed., 17, 523 ( 1 9 4 5 ) .
223. Steyermark, Al, "Quantitative Organic Microanalysis," Blakiston, Philadelphia, Pennsylvania, 1951.
224. Steyermark, Al, Alber, H. K., Aluise, V . A , Huffman, E. W . D., Jolley, E. L , Kuck, J . Α., Moran, J . J . , Ogg, C. L., and Willits, C. O., Anal. Chem., 26, 1186 ( 1 9 5 4 ) .
225. Steyermark, Al, Alber, Η. K., Aluise, V . Α., Huffman, E. W . D., Kuck, J . Α., Moran, J . J . , and Willits, C. O., Anal. Chem., 21, 1555 ( 1 9 4 9 ) .
226. Strahm, R. D., and Hawthorne, M. F., Anal. Chem., 32, 530 ( I 9 6 0 ) . 227. Straub, J . , Mikrochemie, 14, 251 ( 1 9 3 4 ) .
228. Strebinger, R., and Mandl, J . , Mikrochemie, 4, 168 ( 1 9 2 6 ) .
229. Strebinger, R., and Reif, W . , Mikrochemie, Pregl Festschrift, p. 319 ( 1 9 2 9 ) . 230. Sudo, T., Shimoe, D., and Miyahara, F., Bunseki Kagaku, 4, 88 ( 1 9 5 5 ) . 231. Sykes, Α., Mikrochim. Acta, p. 1155 ( 1 9 5 6 ) .
232. Teeri, A. E., Chemist Analyst, 43, 43 ( 1 9 5 4 ) .
233. Thurnwald, H., and Benedetti-Pichler, Α. Α., Mikrochemie, 11, 212 ( 1 9 3 2 ) . 234. Toribara, T. Y . , and Sherman, R. E., Anal. Chem., 25, 1594 ( 1 9 5 3 ) .
235. Treadwell, F. P., and Hall, W . T , "Analytical Chemistry," 6th ed., Vol. II, Wiley, New York, and Chapman & Hall, London, 1924.
236. Tunnicliffe, M. E., Trans. Inst. Rubber Ind., 31, T l 4 l ( 1 9 5 5 ) .
237. Tutundzic, P. S., and Mladenovic, S., Anal. Chim. Acta, 12, 390 ( 1 9 5 5 ) . 238. Umland, F., and Weyer, F. G., Klin. Wochschr., 33, 237 ( 1 9 5 5 ) . 239. Van Etten, C. H., and Wiele, M. B . , Anal. Chem., 25, 1109 ( 1 9 5 3 ) .
240. Venkateswarlu, P., Ramanthan, A. N., and Narayana Rao, D., Indian J . Med.
Research, 41, 253 ( 1 9 5 3 ) .
241. Verdino, Α., Mikrochemie, 6, 5 ( 1 9 2 8 ) .
242. Walton, H. F., and Smith, Α. Α., Anal. Chem., 28, 406 ( 1 9 5 6 ) . 243. Well, I. C , Anal. Chem., 23, 511 ( 1 9 5 1 ) .
244. Wenger, P., Cimerman, C , and Corbaz, Α., Mikrochim. Acta, 2, 314 ( 1 9 3 8 ) ; Mikrochemie ver. Mikrochim. Acta, 27, 85 ( 1 9 3 9 ) .
245. Wernimont, G., and Hopkinson, F. ] . , Ind. Eng. Chem., Anal. Ed., 12, 308 ( 1 9 4 0 ) . 246. Wilkinson, R. H., / . Clin. Pathol, 10, 126 ( 1 9 5 7 ) .
247. Winkler, L. W., Z. anal. Chem., % , 241 ( 1 9 3 4 ) .
248. Zabrodina, A. S., and Bagreeva, M. R., Vestnik. Moskov. Univ. Ser. Mat. Me khan., Astron.-Fiz. i Khim., 13, No. 4, 187 ( 1 9 5 8 ) ; Chem. Abstr., 53, 12946 ( 1 9 5 9 ) . 249. Zombory, L., Technika (Budapest), 10, 147 ( 1 9 2 9 ) .
250. Zuehlke, C. W., and Ballard, A. E., Anal. Chem., 22, 953 ( 1 9 5 0 ) .