QUANTITATIVE DETERMINATION BY A SPECIAL
"AMPULLA-DIVER" OF CHOLINESTERASE ACTIVITY IN INDIVIDUAL CELLS, W I T H NOTES O N O T H E R
USES OF THE METHOD
By J . ZAJICEK AND E. ZEUTHEN
Department of Cytology, Instituteof Badiopathology,Stockholm, Sweden, and Biological Institute of the Carlsberg Foundation, Copenhagen, Denmark
I . I n t r o d u c t i o n . . . 1 3 1 I I . M a t e r i a l a n d M e t h o d s . . . ·. . 1 3 2
A . S u b s t r a t e 1 3 2 B . P r e p a r a t i o n o f B o n e - m a r r o w C e l l S u s p e n s i o n s . . . . 1 3 2
C. I s o l a t i o n o f I n d i v i d u a l M e g a k a r y o c y t e s . . . . . 1 3 3 D . T h e M a n o m e t r i c M e a s u r e m e n t o f C 02 . · · · . 1 3 4
E . C a l c u l a t i o n o f C 02 E v o l u t i o n . . . . 1 3 6
F . D e t e r m i n a t i o n o f VF a n d VG . . . . 1 3 7
I I I . R e s u l t s 1 3 7 A . C o n t r o l E x p e r i m e n t s . . . · . 1 3 7
B . C h o l i n e s t e r a s e E x p e r i m e n t s . . . . 1 3 9
I V . C o m m e n t s 1 4 0 A . T h e I n i t i a l P e r i o d . . . . 1 4 0
B . L e a k a g e o f C O2 f r o m t h e D i v e r . . . 1 4 2
C. S e n s i t i v i t y a n d A c c u r a c y o f t h e M e t h o d . . . 1 4 3 V . S u m m a r y . . . · 1 4 4
V I . N o t e s 1 4 4 R e f e r e n c e s . . .' . . . 1 5 1
I. INTRODUCTION
The most sensitive method hitherto evolved for cholinesterase (ChE) determination is the Cartesian diver microgasometer of Linderstrom- Lang (1937) developed by him and Holter (Holter, this volume). With this apparatus analyses were made of serum samples (Linderstrom-Lang andGlick, 1938) and tissue fragments (Boell and Shen, 1950) with ChE ac
tivity corresponding to the liberation of about 0 · 01 to 0 · 1 μΐ C 0
2per hour.
The following paper describes a Cartesian diver method which permits quantitative determination of enzyme activity in single megakaryocytes of rat bone marrow. The method measures the evolution of Ι Ο
- 4μΐ C 0
2per hour with a sensitivity of ± 5%. I t is a modification of the ampulla- diver respirometer (Zeuthen, 1953) used in conjunction with the sensi
tive manometer for measuring the respiration of single protozoan or egg cells (Zeuthen, 1953, 1955).
131
1 3 2 J . ZAJICEK AND E. ZEUTHEN
S o l u t i o n % ( w / v ) m l
N a C l 0 - 8 10
N a H C 03 0 - 2 8 10
C a C l2. 6 H20 1 - 3 4 0 - 4
T o t a l 2 0 - 4
The quantity of AThCh dissolved to make the concentration 6 χ 1 0 ~
3M was 3 5 · 4 mg. The p H of the AThCh-bicarbonate buffer solution when saturated with a mixture of 5 % C 0
2and 9 5 % N
2is about 7 · 4 (Umbreit
et al, 1 9 4 9 ) .Β . PREPARATION OF BONE-MARROW CELL SUSPENSION
Bone-marrow cells from the femur of an 8-week-old albino rat were suspended in a solution of 5 % sodium citrate ( 1 part) and 0 - 8 % sodium chloride (4 parts). As the anticoagulant citrate is an inhibitor of ChE, the cells were washed free of citrate by centrifugation and were placed in
II. MATERIAL AND METHODS A. SUBSTRATE
The substrate found to meet the requirements for both stability and specificity was the thioanalogue of acetylcholine (ACh), acetylthiocholine (AThCh). This ester was first synthesized by Renshaw et al., ( 1 9 3 8 ) , who noted that its pharmacologic actions were similar to those of ACh, but were of briefer duration. Glick ( 1 9 3 9 ) reported that AThCh underwent more rapid hydrolysis, both enzymatically and non-enzymatically, than ACh. This finding was confirmed by Koelle and Friedenwald, who also introduced the thiocholine esters as substrates for the histochemical demonstration of ChE and acetylcholinesterase (AChE) ( 1 9 4 9 ) . With a modified thiocholine method it was demonstrated histochemically that the esterase found in the megakaryocytes was AChE (Zajicek, et al., 1 9 5 3 ) . This enzyme displays optimum activity at AThCh concentrations ranging from 4
χ1 0 ~
3to 6
χ1 0 ~
3M (Koelle, 1 9 5 0 ) . We therefore decided to measure the esterase activity in megakaryocytes with 6 χ 1 0 ~
3M AThCh as the substrate (Acetylthiocholine iodide, F . Hoffmann-La Roche, Basle).
T A B L E I
QUANTITATIVE DETERMINATION OF CHOLINESTERASE ACTIVITY 133
γ
Gas mixture
F I G. 1. P o l y m o r p h o n u c l e a r m e g a k a r y o c y t e i s o l a t e d f r o m r a t b o n e m a r r o w a n d p l a c e d i n d i v e r ( f r o m Z a j i c e k , 1 9 5 6 b ) .
0 · 9 % sodium chloride. Centrifugation at 1500 r.p.m. for about 45 sec then produced a fraction rich in megakaryocytes. A small drop of this fraction was resuspended in 0 · 5 ml of AThCh-bicarbonate buffer solu
tion.
C. ISOLATION OF INDIVIDUAL MEGAKARYOCYTES
A few drops of the bone-marrow cell suspension in AThCh-bicar- bonate buffer were placed on a hollow slide. Megakaryocytes, like plate
lets, display a strong tendency to adhere to glass. This makes the hand
ling of individual megakaryocytes very difficult, if not impossible. To
overcome this difficulty we first tried, without success, coating the slides
with paraffin or silicone. I t was finally found that agar gave the desired
effect. On an agar-coated hollow slide the megakaryocytes behave as
134 J . ZAJICEK AND E. ZEUTHEN
though freely suspended. The cell to be investigated can thus readily be sucked into a braking pipette and transferred to another agar-coated slide. When the accompanying erythropoietic or granulopoietic cells have been pipetted off, the megakaryocyte is sucked into the diver (Fig. 1), to the position indicated in Fig. 2, V. With a little practice all these operations can be performed under a standard dissection micro
scope, without the aid of a micromanipulator.
D . T H E MANOMETRIC MEASUREMENT OF C 0
2The method for determining ChE is best described graphically. Figure 2 and its extended legend give an impression of how the method func
tions. The reader is therefore advised at this point to consult Fig. 2.
F I G . 2,
Q U A N T I T A T I V E D E T E R M I N A T I O N O F C H O L I N E S T E R A S E A C T I V I T Y 135 F I G. 2 . ( I ) T h e b r a k i n g p i p e t t e . A c a p i l l a r y ( d i a m e t e r 0 · 5 m m ) is p u l l e d f r o m a t h i n - w a l l e d t e s t t u b e a n d is f i t t e d i n t o a g l a s s j a c k e t , u s i n g D e - K h o t i n s k y c e m e n t . T h e p r e s s u r e is r e g u l a t e d b y m o u t h , t h r o u g h r u b b e r t u b i n g . D e t a i l s i n s i d e t h e d o t t e d f r a m e a r e e n l a r g e d i n I I t o V .
( I I ) T h e l o w e r p o r t i o n o f t h e b r a k i n g p i p e t t e f o r m s a n " a m p u l l a " a p p r o x i m a t e l y 3 m m l o n g w i t h a " s h a f t " m e a s u r i n g a b o u t 2 0 m m a n d a " t i p " o f a b o u t 10 m m . T h e i n t e r n a l d i a m e t e r o f t h e s h a f t a n d t h e t i p is 4 0 t o 6 0 μ.
( I I I ) S u b s t r a t e p r e v i o u s l y b u b b l e d t h r o u g h w i t h 5 % C 02 a n d 9 5 % N2 is s u c k e d i n t o t h e a m p u l l a , s h a f t a n d a d j a c e n t p a r t s o f t h e p i p e t t e . T h i s i s f o l l o w e d b y a b u b b l e o f t h e s a m e g a s m i x t u r e , t a k e n f r o m t h e s l a n t e d t u b e t o t h e l e f t ( d u r i n g i n t e r r u p t i o n o f t h e g a s f l o w ) . F i n a l l y s u b s t r a t e is s u c k e d i n t o t h e t i p a n d t h e l o w e r p a r t o f t h e a m p u l l a , l e a v i n g g a s a n d f l u i d s i n s i d e t h e d i v e r i n t h e p o s i t i o n s i n d i c a t e d .
( I V ) T h e cell i s d e p o s i t e d i n a d r o p l e t o n a n a g a r - c o a t e d h o l l o w s l i d e . (V) T h e c e l l is s u c k e d u p t o t h e p o s i t i o n s h o w n . T h e t i p is s e a l e d w i t h a r e s i n - wra x m i x t u r e (1 p a r t r e s i n , 2 p a r t s w a x ) h e a t e d t o a b o u t 8 0 ° C . B r e a k i n g o f t h e s h a f t a t t h e s i t e i n d i c a t e d s e p a r a t e s t h e c h a r g e d a m p u l l a - d i v e r f r o m t h e p i p e t t e . T h e l a t t e r n o w h a s a s e a l e d t i p a n d w h a t w a s t h e s h a f t b e c o m e s t h e " t a i l " . T h e d i v e r is t r a n s f e r r e d t o a s p e c i a l v e s s e l ( V I ) c o n t a i n i n g C O2- N2- b u b b l e d s u b s t r a t e .
( V I ) I f t h e d i v e r f l o a t s t o t h e s u r f a c e t h e s t o p p e r is i n s e r t e d a n d t h e g a s s p a c e m a y b e r e d u c e d s t e p w i s e u n t i l b u o y a n c y is a t t a i n e d a t , o r i n t h e v i c i n i t y of, a t m o s p h e r i c p r e s s u r e . T h i s c a n b e a c c o m p l i s h e d b y s u c t i o n ( a r r o w ) , w i t h r e t u r n t o a t m o s p h e r i c p r e s s u r e w h e n e v e r a s m a l l b u b b l e is o b s e r v e d t o e s c a p e t h r o u g h t h e t a i l o f t h e d i v e r . [ B y r e p e a t e d s u c t i o n , h o w e v e r , t h e l i q u i d i n t h e d i v e r i s d e p l e t e d o f g a s e s a n d t h e t i m e r e q u i r e d f o r e q u i l i b r a t i o n m a y t h u s b e p r o l o n g e d . W e t h e r e f o r e l a t e r w o r k e d w i t h s l i g h t l y o v e r w e i g h t d i v e r s , i.e. d i v e r s w i t h e x c e s s i v e l y l o n g t a i l s (2 c m ) a n d r e g u l a t e d b u o y a n c y b y p r o g r e s s i v e l y s h o r t e n i n g t h e t a i l s ] . F r o m ( V I ) t h e d i v e r is t r a n s f e r r e d t o t h e f l o t a t i o n v e s s e l o f t h e " m a n o m e t e r " ( V I I ) , d i s c o n n e c t i n g a n d r e a s s e m b l i n g b e i n g c a r r i e d o u t a t (c).
T h e m a n o m e t e r is r e a l l y a b u r e t t e (d, e, f ), b y m e a n s o f w h i c h a c c u r a t e l y m e a s u r e d v o l u m e s o f f l u i d c a n b e w i t h d r a w n f r o m , o r i n j e c t e d i n t o , a c l o s e d a i r s p a c e ( b ) , t h e r e b y c r e a t i n g r e g u l a t e d p r e s s u r e c h a n g e s i n t h e s p a c e . I n o u r a p p a r a t u s t h e a p p r o x i m a t e c a l i b r a t i o n o f t h e b u r e t t e is 1 μΐ/mm. a n d t h a t o f t h e a i r s p a c e ( b ) w i t h a l l r a m i f i c a t i o n s 2 0 0 , 0 0 0 μ\. A 1 - m m s h i f t o f t h e m e n i s c u s (e) i n t h e b u r e t t e t h e r e f o r e c o r r e s p o n d s t o a p r e s s u r e c h a n g e o f 5 χ 1 0 ~6 a t m . o r 0 · 0 5 m m H20 . T h e d i v e r f l o a t s i n s u b s t r a t e i n a p o c k e t (a) w h i c h o p e n s t o ( b ) . T h e a s s e m b l e d m a n o m e t e r i s f i x e d a t t h e e d g e o f a s p e c i a l ( ± 0 · 0 0 2 ° ) w a t e r b a t h ( s u p p l i e d b y O . D i c h , A v e d o r e , C o p e n h a g e n ) , i n w h i c h ( a ) , ( b ) , (c) a n d (d) a r e s u b m e r g e d . T h e m a n o m e t e r is p e r f u s e d w i t h 5 % C 02 a n d 9 5 % N2 t h r o u g h n a r r o w p o l y e t h y l e n e t u b i n g , w h i c h is i n t r o d u c e d d e e p i n t o t h e p o c k e t (a) (cf. i n s e r t B ) . T h e t u b i n g p a s s e s t h e 3 - w a y s t o p c o c k s g a n d d a b o v e t h e f l o t a t i o n v e s s e l ( i n s e r t A ) . T h e f l o w i s c o n t i n u e d f o r 2 0 m i n a t a n a p p r o x i m a t e r a t e o f 5 0 m l / m i n . T h e g a s e s c a p e s a t ( h ) , (i). M e a n w h i l e t h e b u r e t t e ( d ) , (e) a n d t h e w a t e r m a n o m e t e r ( k ) , (1) a r e c l e a r e d o f a i r b y p u m p i n g , u s i n g s c r e w s (f) a n d (1). T h e f l o w o f g a s i s s t o p p e d b y d i s c o n n e c t i n g t h e p o l y e t h y l e n e t u b i n g ( i n s e r t A ) a n d c l o s i n g t h e s t o p c o c k s (g) a n d (h) t o t h e a t m o s p h e r e . W i t h t h e w a t e r l e v e l (e) i n a p o s i t i o n s u i t a b l e f o r t h e p r o p o s e d m e a s u r e m e n t s , t h e i n i t i a l n o t a t i o n p r e s s u r e of t h e d i v e r is a d j u s t e d a n d r e a d w i t h t h e a i d o f t h e m a n o m e t e r k , 1. F i n a l l y ( d ) i s c l o s e d t o t h e a t m o s p h e r e . D u r i n g t h e a c t u a l e x p e r i m e n t s — w h i c h m a y n o w b e b e g u n — g a s e x c h a n g e i n t h e d i v e r is r e a d a s t h e p r e s s u r e c h a n g e s ( m o v e m e n t s o f t h e m e n i s c u s (e)) n e c e s s a r y t o k e e p t h e d i v e r a f l o a t a t a p r e d e t e r m i n e d l e v e l . I f r e a d i n g s a r e d i s c o n t i n u o u s a s u i t a b l e e x c e s s p r e s s u r e m u s t b e a p p l i e d b e t w e e n t h e m e a s u r e m e n t s , s o t h a t t h e d i v e r r e m a i n s a t r e s t o n t h e b o t t o m o f t h e f l o t a t i o n v e s s e l ( f r o m Z a j i c e k a n d Z e u t h e n , 1 9 5 6 ) .
136 J . ZAJICEK AND E. ZEUTHEN
The method discussed is for a standard diver, gas volume 0-5 μ,Ι, liquid charge 0· 6 μ,Ι. I t is easy to charge divers with less than 0· 6 μΐ of liquid, and indeed in most of our experiments the charge was only about 0-2 μΐ. A large liquid charge, however, causes increase of the initial period (t
s, cf. p. 140) before gas formation or gas uptake can be registered manometrically. The standard diver then provides less favourable experi
mental conditions. Since the absorption coefficient for C 0
2in water at 22° C is 0-83, our standard diver compares with a 1 μ,Ι-diver without liquid charge.
The diver floats only if made of glass so thin that it readily cracks during manipulation with fingers. In all the steps of making and filling, however, the diver remains attached to the braking pipette capillary from which it is pulled, and need not be touched by hand. Our divers were made from thin-walled test tubes (Jena glass) pulled in several operations to the dimensions indicated in Fig. 2. I t is not essential that the cross- section of the capillary (diameter c. 0 · 5 mm) used for the braking pipette be circular. Most often, in fact, it is not. The "shaft" and " t i p " of the
"ampulla " (cf. legend, Fig. 2) are pulled in a microflame without rotation of the glass. The shaft becomes the "tail " of the diver. The glass is heated from one side when the shaft is pulled and from the other side when the tip is made. Thus the glass tapers excentrically into both tip and shaft, but the balance of the two is well adjusted and the diver will float up
right (Fig. 2, VI). Personal experience is the only true guide in the choice of glass and in the method of pulling it to form a diver which can be made to float with a reasonable charge, i.e. one not essentially different from that illustrated in Fig. 2.
E . CALCULATION OF C 0
2EVOLUTION
The formula reported in earlier papers (Zeuthen, 1953,1955) was used for calculating exchange of gases with low solubility, such as 0
2. When measuring the evolution of C 0
2the amount of this gas dissolved in the liquid contents of the diver must be taken into consideration. Some un
certainty remains concerning the extent to which the liquid in the narrow channels in both ends of the diver—far removed from the gas space—
serves as a solvent for the C 0
2, formed during the experiments. This liquid volume, however is very small. In the experiments presented in Fig. 4 it comprised less than 5% of the liquid in the diver and less than 3 % of the total volume of the diver. The formula employed in this study for calculating C 0
2evolution was
C 0
2_ ivx V
Gx(B + h-e)x213 ν χ V
Fχ aCQ
2χ (B + h-e)\
~T ~
x x\ f
x10300 χ (273 + f)
+~ 7 x 10300 )
QUANTITATIVE DETERMINATION OF CHOLINESTERASE ACTIVITY 137 in which χ is the change in scale reading (mm per accepted time unit), ν is the volume (/xl) per mm burette and V is the volume of the gas phase of the "manometer" (/xl); in our apparatus v/V was almost 1/200,000.
V
Gis the gas volume of the floating diver. Β is the barometric pressure in mm H
20 (760 mm H g ; 10,300 mm H
20 ) , h is the initial equilibrium pressure of the diver (mm H
20 ) read as the difference in height of the menisci on the double-branched manometer (le) (cf. Fig. 2, in which the initial pressure h is positive), β is the vapour tension of water at t° (mm H
20 ) , V
Fis the volume of water in the diver (/xl) and a C 0
2is the absorp
tion coefficient for C 0
2at the temperature during the experiment.
F . DETERMINATION OF V
FAND V
GThe volume of the liquid charge ( V
F) is computed by subtracting the weight of the diver after oven-drying from the weight of the diver as soon as the actual experiment ends—after washing in distilled water, removal of external liquid with a strip of filter paper and brief exposure to the air. The value thus arrived at is modified by the correction
V
G(h/B), to be added if the diver floats at negative pressure. The volume of gas ( V
G) in the floating diver is determined as follows. There is a con
stant ratio (/) between the weight of the glass used for the diver and the volume of air required to make the aggregate of glass and air buoyant in the medium. / is determined independently for the type of glass used.
V
Gis the dry weight of the diver multiplied b y / .
III. RESULTS
A . CONTROL EXPERIMENTS .
We are aware that our filling procedure (cf. stages I I I to V, Fig. 2) does not ensure initial saturation of the medium in the diver with 5% C 0
2and 95% N
2. I t is possible for the pretreated medium to lose to the atmosphere some of its C 0
2and N
2while being poured into the dish (Fig. 2, III) or on to the hollow slide (Fig. 2, IV) and to take up small amounts of 0
2. When the diver has been filled, sealed and balanced to buoyancy, there
fore, the gases will diffuse to equilibrium inside the diver. This process may last long when the diffusion distances in the system are considerable.
With the same concentration gradient the amount of any one gas dif
fusing must vary directly with the absorption coefficient. Reference is made to note 3.
Because of its high α the diffusion of C 0
2can be expected to dominate
over that of other gases. I t should be possible to define filling procedure
and diver dimensions so as to reduce to an acceptable minimum the time
necessary for initial equilibration of the control diver. Our search for
138
J . Z A J I C E K A N D E . Z E U T H E Nsuch conditions, however, led us to conclude that other sources of error play a dominant role. Glass, when suddenly exposed to C0
2-bicarbonate, seems to absorb C 0
2and to continue to do so for more than 24 hr. Figure 3 shows the series of control experiments from which this inference was drawn. Curve I in this figure shows typical scale readings for a freshly- pulled control diver charged with 5% C 0
2and 95% N
2, and with bubbled bicarbonate buffer medium as described on p. 135, but without AThCh.
The flotation medium was the same as that in the diver charge. The mano
meter was perfused with gas as shown in Fig. 2. Curve I indicates that the diver became heavier with high initial rates. The system did not approach equilibrium for about 24 hr. This is much longer than we anticipated,
(hours)
F I G . 3 . T h e s c a l e r e a d i n g s f o r f r e s h l y - p u l l e d c o n t r o l d i v e r s c h a r g e d w i t h b i c a r b o n a t e b u f f e r s o l u t i o n ( c u r v e I ) a n d w i t h a c e t y l t h i o c h o l i n e ( A T h C h ) - b i c a r b o n a t e s o l u t i o n ( c u r v e I I I ) . T h e p r e c o n d i t i o n i n g o f t h e d i v e r s b y p r o l o n g e d i n t e r n a l c o n t a c t w i t h b i c a r b o n a t e ( c u r v e I I ) o r w i t h A T h C h - b i c a r b o n a t e s o l u t i o n ( c u r v e I V ) c l e a r l y e l i m i n a t e d t h e i n i t i a l p h a s e i n w h i c h t h e d i v e r s r a p i d l y b e c o m e h e a v i e r ( f r o m Z a j i c e k a n d Z e u t h e n , 1 9 5 6 ) .
judging from calculations based on formulas published by Linderstrom- Lang (1943, 1946).
With a slight variation in procedure we obtained quite different results. We interrupted the diver fillings at stage I I I (Fig. 2) for 24 hr, during which time the filled ampulla was kept in air. We then blew the ampulla empty and refilled it, but this time concluding the procedure and setting the diver afloat as described above. Curve I I , Fig. 3, shows a typical result. Pre-conditioning of the glass by prolonged internal con
tact with C0
2-bicarbonate clearly eliminated the initial phase in which the diver rapidly becomes heavier.
When the diver, instead of being preconditioned, was filled as for
the experiment shown in curve I, but with 6 χ 10 ~
3M AThCh added to the
buffer (inside and around the diver), the result was curve I I I . In our
QUANTITATIVE DETERMINATION OF CHOLINESTERASE ACTIVITY 139
opinion the improvement as compared with curve I resulted from auto- hydrolysis of AThCh, with subsequent liberation of C 0
2wherever the C 0
2tension inside the diver was below that corresponding to 5% (in gas of atmospheric pressure), and the p H thus higher than the stability limit of the substrate. This implies that the non-conditioned diver filled with C0
2-bicarbonate and in contact with this medium becomes progressively heavier because C 0
2is lost from the diver's gas phase. Since this loss does not occur by diffusion (cf. curves I and II), we suggest that the glass continues to adsorb or absorb C 0
2for 24 hr or more after it is brought into contact with C0
2-bicarbonate.
With the diver preconditioned by 12 hr internal exposure to C0
2-bi- carbonate with added AThCh, and then refilled as described above with AThCh-bicarbonate solution, curve IV is obtained. In five experiments it was established that after an initial period of about 30 min such divers showed no further significant change in equilibrium pressure. Over 8 hr the scale readings were constant within ±10 mm, or within a pressure range of less than 1 mm H
20 . During the 30-min initial period, however, the equilibrium pressure in some divers showed variations up to ± 20 mm.
See also the following Section B.
B. CHOLINESTERASE EXPERIMENTS
In the ChE experiments the divers were first filled with AThCh-bi- carbonate solution plus gas, and the tips were sealed with wax. After
x l O "4
30
25
Ο
20u
10 5
0 I 2 3 4
Time (hr.)
F I G . 4 . C h o l i n e s t e r a s e a c t i v i t y of a m e g a k a r y o c y t e ( u p p e r c u r v e ) a n d o f a m e g a k a r y o b l a s t ( l o w e r c u r v e ) ( f r o m Z a j i c e k a n d Z e u t h e n , 1 9 5 6 ) .
about 12 hr the seals were broken off and the solution present in the tips
was expelled. Fresh substrate-bicarbonate solution was sucked into the
divers along with a cell, and stages V to VII of Fig. 2 were carried out.
140 J . ZAJICEK AND E. ZEUTHEN
ChE activity curves of megakaryocyte (diameter 48 μ) and of a megakaryoblast (diameter 24 μ)—both from rat—are shown in Pig. 4.
The experiments were conducted at 25° C. The megakaryocyte (upper curve) liberated from the bicarbonate buffer 100 χ 10~
5μ\ C 0
2per hour, and the megakaryoblast (lower curve) 1 9 · 2 χ 1 0 ~
5μ\ C 0
2per hour.
These values correspond to the hydrolysis of about 0-013 and 0 · 0024 μg, respectively, of AThCh per hour.
The constants for the two divers were: V
G= 0-8 μ\, V
F= 0-44 μ\
(upper curve), and V
G= 0-54 μ,Ι, V
F= 0-24 μΐ (lower curve).
After the completion of the experiments presented above, it was found that the observed absorption of carbon dioxide by the divers pulled from Jena glass did not take place in divers made from Phoenix glass. The use of Phoenix glass, therefore, eliminates the time-consuming pre-condi
tioning of the wall of the diver, and makes it possible to introduce the cells immediately after the divers have been charged with the gas bubble and the substrate. Phoenix glass has not recently been easily available.
It may be replaced by Pyrex or other heat-resistant glass. The user of the method is advised to perform proper control runs with the type of glass selected for use. The substrate used is 5 χ 10~
3M acetylcholine chloride.
The non-enzymatic hydrolysis of 0 · 1 μΐ of acetylcholine-bicarbonate solution (pH 7 · 4) was below the sensitivity of the present diver method.
IV. COMMENTS A. T H E INITIAL P E R I O D
When the control diver, filled and treated as described for the typical experiment of Fig. 3, curve IV, is set afloat, we have frequently observed considerable ( + 20 mm scale) changes of its equilibrium pressure. These initial changes, which usually involve less than the first half-hour of the experiment, probably reflect the diffusion to equilibrium of the gases inside the diver. I t is essential to point out that the time (t) required for diffusion to equilibrium of a finite amount of C 0
2, introduced at time 0 into a defined location in the diver, is expected to be much longer than the time (t
s) which must elapse before constant rate of gas formation or gas uptake is reflected in linear changes in equilibrium pressure with time (Linderstrom-Lang, 1943). In experiments with a biologic object, t
sand not t is of interest. The purpose of the following evaluation is to give some idea of the dependence of t
son the dimensions of the diver, a question of considerable practical importance.
According to Linderstrom-Lang (1943), t
svaries with b
2, b represent
ing the length of the diffusion path in the liquid medium in the diver. The
t
svalue for C 0
2is little different from that for 0
2. Linderstrom-Lang
Q U A N T I T A T I V E D E T E R M I N A T I O N O F C H O L I N E S T E R A S E A C T I V I T Y 141
L . - L . example 3.p.269 pp
ts= 6 min.
és = 25min.
' ts = 56min.
A Β
C D
10 mm
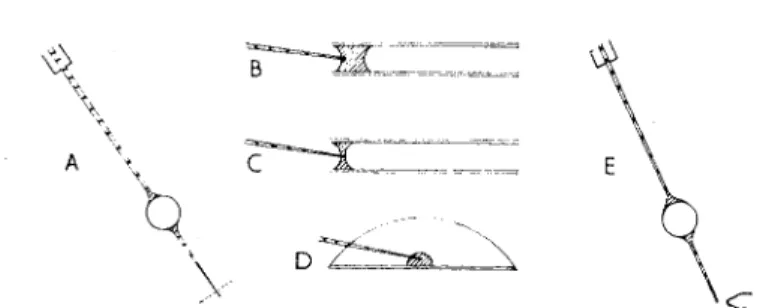
F I G . 5 . D i v e r s (B-D) c h a r g e d i n v a r i o u s w a y s . T h e d u r a t i o n (ts) o f t h e i n i t i a l p e r i o d i n w h i c h r e l i a b l e r e a d i n g s c a n n o t b e o b t a i n e d i s c a l c u l a t e d b y c o m p a r i s o n w i t h L i n d e r s t r o m - L a n g ' s ( 1 9 4 6 ) r e s u l t s ( p . 2 6 9 ) ( f r o m Z a j i c e k a n d Z e u t h e n , 1 9 5 6 ) .
b = 2 mm (Linderstr0m-Lang, 1946, p. 247, Table 4), accepting that t
svaries with b
2. The fact that the diver tapers off at both ends probably exaggerates the values given for t
s:
The situation, however, is somewhat complicated by the fact that the diver—in addition to the liquid between the lines in Fig. 5—carries small volumes of liquid in the long tail and tip. If the volume in these channels is not allowed to exceed 5% of the total volume (air plus fluid) of the diver, its influence on the measurements can be disregarded, provided we do not claim more than about 5% accuracy for the method. In any case it is probably difficult, for other reasons, to improve on this.
In our experiments the initial period required about 30 min, which is the time necessary for performing stages V, VI and VII of Fig. 2. The readings, however, were not commenced until 30 min after closing the stopcock d to the atmosphere (stage VII, Fig. 2), when the control divers were shown to have attained equilibrium.
(1946) applied calculations to a diver system resembling the sketch A in Fig. 5. The diver is charged with two columns of liquid, 3 and 2 mm long (total volume 16 μ\), separated by an air bubble (volume 2 μ\). The re
spiring layer is situated in the smaller water volume, either adjacent to the air bubble or close to the impermeable membrane which forms the floor of the diver chamber. When the respiring sheet is adjacent to the air bubble the value of b which determines t
swas accepted to be close to the average length of the two columns (Linderstrom-Lang, 1946, p. 269).
Our diver system closely resembles the system discussed by Linderstr0m-
Lang. The actual shape of the charged diver may be any of those shown
in Fig. 5. Lines have been drawn across the divers at distances of 0 · 5,1 · 0
or 1-5 mm from the gas bubble, and the calculated values for t
sare
reproduced. They are derived from the calculation that t
s= 100 min for
142 J . ZAJICEK AND E. ZEUTHEN B. LEAKAGE OF C 0
2FROM THE D I V E R
The equilibrium pressure of the control diver remained constant within 1 mm H
20 (20 mm scale) for about 8 hr. This did not necessarily indicate that the diver was impermeable to C 0
2. I t may merely have reflected the establishment of complete equilibrium between the gases inside and around the diver. Introduction of the cell entailed a C 0
2concentration difference which increased as C 0
2formed in the diver in the course of the experiment.
With our apparatus the capacity for pressure adjustment, without resetting of the instrument, is exhausted when the C 0
2tension in the diver exceeds the tension in the flotation medium by 35 mm H
20 (initial equilibrium is assumed; length of burette—700 mm—corresponds to 35 mm water pressure). It is therefore of interest to calculate the maxi
mum diffusion loss of C 0
2, i.e. when the gradient is 35 mm H
20 . The formula employed is :
AGQ
2_ 8C0
2xAx(p
1-p
2) h IxB
8C0
2is the "standard rate of passage " of C 0
2in water. I t is defined as μ\
passing through a column of 1 m m
2, 1 mm long, in 1 hr, when the gradient is 1 atmosphere. Using Hufner's values (Krogh, 1919, p. 407) we find that
§C0
2= 5-4; (ρχ— p
2) is 35 (mm H
20 ) ; for Β is introduced 10,300 (mm H
20 ) . The cross-sectional area (A) of the tail of the diver, through which pressure adjustment takes place, varies somewhat, but can easily be kept at less than 0-003 m m
2(diameter 60 μ). As a rule the tail is longer than 15 mm, but not at its narrowest part. Hence we have inserted (10 mm) for 1 in the above formula. For A = 0-003 m m
2we find J C O
2/ A = 5 x l 0 -
e/ i l .
In our standard diver a pressure change of 35 mm H
20 corresponds to the formation or consumption ο ί 3 · 5 χ 1 0 ~
3μ 1 C 0
2. If this amount of C 0
2were linearly produced over 1 hr {p\—p
2) would average 17-5 mm, and the C 0
2loss by diffusion through the diver tail would amount to 0-7% of the simultaneous production in a diver with a tail 60 μ in dia
meter. But we seldom measure enzyme activities high enough to liberate
C 0
2at that rate. With the production o f d 0
2constant at, say, 3 · 5 χ 10 ~
4μ\ per hour, the experiment can be run for 10 hr before the C 0
2tension in
the diver reaches the accepted maximum of 35 mm H
20 . The loss of
C0
2—in percentage of the simultaneous C 0
2production—evidently
increases linearly with time, and attains a maximum by the end of the
experiment. I n the last hour it will be 1 · 4% of the simultaneous
C 0
2production in the diver in question. It should also be pointed out that
QUANTITATIVE DETERMINATION OF CHOLINESTERASE ACTIVITY 143 for identical divers and for any fixed hour of the experiment the leakage of C 0
2represents a constant percentage error, irrespective of the rate of production.
We have not considered diffusion through the tip of the diver, assum
ing the solid wax to be impermeable to gases. Although there is some justification for this assumption (Zeuthen, 1943, Sect. X I I , p. 3), we may be mistaken. In that case C 0
2leaks from the diver about twice as fast as the calculated rate. I n other respects, however, we have assumed more adverse conditions, i.e. favourable for diffusion. We feel justified in stating that the diver used can be made impermeable to C 0
2in the sense that leakage is negligible as compared with other sources of error in the measurements. The tip and tail of the standard diver should not be wider than 60 μ and the length of the narrow parts should exceed 10 mm.
C. SENSITIVITY AND ACCURACY OF THE METHOD
The sensitivity of the method for absolute measurements is limited by the instability of the control diver. As reported in the foregoing, five runs with control divers showed that after an initial period of about 30 min the equilibrium pressure was stable within 1 mm H
20 for 8 hr.
Stability is not substantially better over 1 hr than over many, as the scale readings vary rapidly within the 0 · 5 mm H
20 limit, probably reflect
ing the offs and ons of the heater in the water bath. A pressure change of 10 mm H
20 (200 mm scale) accumulated in an experiment can thus be measured with 5% accuracy. Accordingly, if 8 hr is the time stipulated to be the maximum duration of an experiment and the standard diver is used, half of the C 0
2being dissolved in the liquid, 10 ~~
4μ\ C 0
2per hour will be measured with ± 5% accuracy. I t is advisable, however, either to select the biologic object or to reduce the dimensions of the diver below those of the standard diver, so as to obtain more pro
nounced pressure changes. This will probably enhance the accuracy of the method.
The accuracy thus claimed for our diver technique of ChE deter
mination is theoretical. We therefore checked the method by charging three divers with equal amounts of purified human plasma ChE. The error in charging the divers with this soluble plasma ChE was about 5%.
For diver 1 T F
G= 0-54 μ[, 7 ^ = 0 - 2 4 μΙ; for diver 2 : 7 ^ = 0-38 /xl, V
F= ο · 15 μ\ ; and for diver 3 : V
G= 0 · 4 μ\, V
F= 0 · 12 μΐ. The evolution of C 0
2in μ\ per hour was 22· 1 χ 1 0~
5i n diver 1,21 · 0 χ 1 0
- 5in diver 2 and 20-2 χ 1 0 ~
5in diver 3. The experimental evidence thus supports the accuracy claimed for the method on the basis of theoretical consider
ations.
144
J . Z A J I C E K A N D E . Z E U T H E NV. SUMMARY
A Cartesian diver method is described for the determination of cholinesterase activity in single somatic cells (megakaryoblast, mega
karyocyte). A megakaryoblast 24 μ in diameter was found to hydrolyse about 0-0024 μg, and a megakaryocyte measuring 48 μ, 0-013 /xg of acetylthiocholine per hour. The accuracy of the method is discussed.
VI. NOTES
11. The bath ( ± 0 · 002° C) and the double-branched water manometer is that shown in Fig. 2 of Holter's account in this volume. The sensitive manometer (Fig. 2, this paper) is hung over the edge of the bath in much the same way as a Warburg manometer. The air space b is submerged to between the stopcocks d and g, the burette and scale is fastened on the front side of the bath: Up to four manometers may be run simultaneously.
The photograph Fig. 6 shows the general set-up.
F I G . 6. G e n e r a l s e t - u p for w o r k w i t h t h e a m p u l l a d i v e r .
1 B y E . Z e u t h e n .
QUANTITATIVE DETERMINATION OF CHOLINESTERASE ACTIVITY 1 4 5 2. The original method (Fig. 7, with text) for respiration (Zeuthen, 1953) is simpler than the one here described for C 0
2. For respiration measurements the diver is charged with air, and the manometers are not gassed. Stopcock g in Fig. 2 may be omitted, and the water manometer is hooked directly to the free vertical glass tubing above d. In the equa
tion, p. 136, the second member in the parenthesis drops out. This method has been used also for determining succino-oxidase, succinic dehydrogenase and cytochrome oxidase in individual Deiter's nerve cells and in lumps of glia cells, together representing the same mass as a nerve cell (Hydén, et al.
9(1958) and Hydén and
P i g o n(unpublished, cf. Hydén
1 9 5 8 , 1959)).
Substrates for cytochrome oxidase (Slater, 1949a; Potter,
1 9 4 9 ) :(a) With sodium ascorbate as reducing agent: Phosphate buffer, p H 7 · 4, 0 · 0375 M ; cytochrome c (Sigma) 10~
4M ; Na-ascorbate 0 · 0 1 2 5 M; A1C1
35 x 1 0 ~
4M .
(b) With p-phenylenediamine as reducing agent : Phosphate buffer, p H 7-4, 0-043 M; cytochrome c 1-2 χ 10~
4M; ^-phenylene- diamine 0-4)43 M; A1C1
35-7 χ 10~
4M.
Substrate for succinic dehydrogenase (Slater, 1949b) :
Phosphate buffer, p H 7-4, 0-075 M; Na-succinate 0-025 M;
methylene blue 0 · 009 M ; KCN, neutralized, 0 · 009 M.
The flotation media used for the divers were of the same composition as the respective substrates, except that cytochrome G and methylene blue were omitted, and sodium ascorbate and j9-phenylenediamine were replaced by isotonic concentrations of sodium chloride. The fact that the flotation medium is, or may be, continuous with the substrate solution in the diver, makes the determination of enzymes by 0
2-uptake in certain respects simpler than respiration measurements in which the air bubble must constitute a safe separation between the alkaline flota
tion medium and the biologic reaction mixture (Zeuthen, 1953).
3. We have later (Brzin and Zeuthen, 1 9 6 1 ) introduced a funnel (Fig. 8, VII), in which the operations I I I , IV and V shown in Fig. 2 may be performed. 5% C 0
2, 95% N
2flows continuously through the funnel.
This ascertains that the substrate solution is saturated with the gas mix
ture with which first the diver and later the manometer is charged. The
" t i p " of the diver is sealed with a wax-resin mixture ( 2 : 1 ) which is
deposited on a wire to be electrically heated. When, upon heating, the
mixture just melts, the diver is sealed (wax-resin to water) in a quick
operation: touch-suck-withdraw. To simplify the illustration only the
sealing is shown to take place in the funnel. Steps V and VI are or may also
be performed in the flow of N
2, C 0
2.
146
J . Z A J I C E K A N D E . Z E U T H E NH I F I G. 7. ( I ) T h e t i p o f a g l a s s c a p i l l a r y is p u l l e d i n a m i c r o f l a m e t o f o r m w h a t is t o b e t h e d i v e r r e s p i r o m e t e r . T h e p o i n t e d t i p s h o u l d b e a b o u t 5 - 7 m m l o n g , t h e w i d e r p i e c e a b o u t 1 c m . , a n d t h e n a r r o w c h a n n e l a b o u t 1 c m . I n n e r d i m e n s i o n s o f t h e o r d e r 50μ,, 5 0 0 μ, a n d 5 0 μ, r e s p e c t i v e l y . T o s e l e c t t h e r i g h t c a p i l l a r y f r o m w h i c h t o m a k e t h e d i v e r o n e s h o u l d a i m a t a t u b e w h i c h w i l l f l o a t i n N / 1 0 N a O H w h e n filled w i t h a i r t o a b o u t h a l f i t s l e n g t h . A f t e r t r a n s f e r t o t h e v a c u u m b o t t l e s h o w n b e l o w i n t h e f i g u r e t h e d i v e r is m a d e b u o y a n t . S u c t i o n i s a p p l i e d s o t h a t f i r s t t h e n u t r i e n t m e d i u m filling t h e n a r r o w c h a n n e l , t h e n a i r , i s s u c k e d o u t o f t h e d i v e r . W h e n n o r m a l p r e s s u r e is r e - e s t a b l i s h e d , a i r h a s b e c o m e r e p l a c e d b y a l k a l i . T h i s is r e p e a t e d i n s t e p s s o t h a t b u o y a n c y is a p p r o x i m a t e d .
( I I ) T h e d i v e r f l o a t s i n N / 1 0 N a O H i n ( a ) , t h u s f r e e l y e x p o s e d t o t h e p r e s s u r e p r e v a i l i n g i n t h e a i r - s p a c e (b) w h i c h e x t e n d s t h r o u g h (c) a n d ( d ) t o t h e w a t e r s u r f a c e (e). A t t h e b e g i n n i n g o f t h e e x p e r i m e n t t h e p r e s s u r e i n ( b ) is a d j u s t e d b y m o u t h t h r o u g h ( n ) , ( m ) , (1), wTi t h ( d ) i n t h e p o s i t i o n s h o w n i n t h e figure.With(m) c l o s e d , t h e m a n o m e t e r ( k ) i s u s e f u l f o r t h e fine r e g u l a t i o n o f t h e i n i t i a l f l o t a t i o n p r e s s u r e i n ( b ) , ( n ) is a C 02- t r a p ; (1) is a n a i r - b r a k e .
D u r i n g a c t u a l m e a s u r e m e n t s (d) is c l o s e d t o (1), ( m ) , (n) a n d t o ( k ) , b u t o p e n t o (e).
T h e v o l u m e of t h e " b u r e t t e " f r o m 0 c m t o 7 0 c m is l e s s t h a n 2 % o f t h e v o l u m e of ( b ) , e x t e n d i n g t o (e). T h e r e f o r e l i n e a r m o v e m e n t s o f t h e i n d i c a t o r b u b b l e (g) i n t h e ( v e r y u n i f o r m ) " b u r e t t e " s e t s u p p r o p o r t i o n a l ( w i t h i n 2 % ) p r e s s u r e c h a n g e s i n ( b ) . T h e s y s t e m c a n b e r e s e t w h e n (g) h a s b e e n m o v e d a c r o s s t h e s c a l e . T h e g a s e x c h a n g e p e r u n i t t i m e (A02/At) i s c a l c u l a t e d f r o m t h e f o r m u l a A
J 02 xx vx gBxf(B + h — e) χ 2 7 3
~ΔΓ = F x 1 0 3 0 0 χ ( 2 7 3 +1 ° )
i n w h i c h χ is t h e m o v e m e n t (in m m p e r u n i t t i m e ) o f t h e b u b b l e (g) i n t h e " b u r e t t e " , t?vis t h e v o l u m e (μ\) p e r m m " b u r e t t e " , V is t h e v o l u m e o f (b) e x t e n d i n g t o (c) (c.
1 0 0 , 0 0 0 μ\; v/V = 2 . 1 0 ~5) , gD i s t h e d r y w e i g h t ( m g ) o f t h e d i v e r d e t e r m i n e d a f t e r
Q U A N T I T A T I V E D E T E R M I N A T I O N O F C H O L I N E S T E R A S E A C T I V I T Y 147
F I G . 8 . T e c h n i q u e f o r r e f i l l i n g d i v e r s . T h e h o l e i n t h e s t o p p e r is p i e r c e d w i t h a h y p o d e r m i c n e e d l e w h i c h i s t h e n w i t h d r a w n l e a v i n g t h e d i v e r ' s t a i l i n s e r t e d i n t h e s t o p p e r ( f r o m B r z i n a n d Z e u t h e n , 1 9 6 1 ) .
4. Refilling of divers. Adding reactants and inhibitors in the course of the experiment. The specificity of AChE is best defined by its reaction to a number of inhibitors. I n view of the fact that the activity for AChE varied considerably from one cell to another, Zajicek (1957) developed techniques which permitted a diver to be reused after full or partial refilling. A single cell could then be studied first in the absence, then in the presence of the inhibitor. In this procedure (Fig. 9) the diver is broken off
F I G . 7 . —c o n t .
t h e e x p e r i m e n t . / is a f a c t o r — d e t e r m i n e d i n d e p e n d e n t l y f o r t h e g l a s s f r o m w h i c h t h e d i v e r i s m a d e — i n d i c a t i n g t h e r a t i o b e t w e e n v o l u m e a i r (μ\) a n d d r y w e i g h t ( m g ) o f g l a s s w h e n t h e s y s t e m is b u o y a n t . * Β is t h e b a r o m e t r i c p r e s s u r e , m m H20 ( 7 6 0 m m H g ~ 1 0 3 0 0 m m H20 ) . h is t h e i n i t i a l e q u i l i b r i u m p r e s s u r e o f d i v e r r e a d o n ( k ) a s t h e d i f f e r e n c e i n h e i g h t o f t h e m e n i s c i ( m m ) , e is t h e v a p o u r t e n s i o n of w a t e r ( o r o f N/ 1 0 N a O H a n d o f 2 % p r o t e o s e - p e p t o n e ) a t t° ( m m H20 ) .
I n t h e p r e s e n t s t u d i e s h i n t h e e q u a t i o n h a s b e e n s m a l l c o m p a r e d t o B. Tt s h o u l d b e n o t i c e d , h o w e v e r , a s a n i n t e r e s t i n g a n d p r o m i s i n g f e a t u r e o f t h e p r e s e n t m e t h o d , t h a t χ v a r i e s i n v e r s e l y w i t h (B + h — e). T h e r e f o r e t h e s e n s i t i v i t y o f t h e s y s t e m s h o u l d i n c r e a s e g r e a t l y a t l o w p r e s s u r e s a n d s h o u l d a p p r o a c h i n f i n i t y w h e n h a p p r o a c h e s n e g a t i v e v a l u e s e q u a l t o B — e ( f r o m Z e u t h e n , 1 9 5 3 ) .
* ÇD'X f t h u s e q u a l s Vq in t h e e q u a t i o n p . 136.
148
J . Z A J I C E K A N D E . Z E U T H E Nfrom the tail (a) before it is charged and sealed. The tail is excessively long. A small amount of water is introduced via the tip by briefly touching a water surface. The diver is then quickly submerged so that the air in it is locked between two menisci, one in the tip and one in the tail. The diver is tested for buoyancy. At this stage the diver should be too heavy because of the long tail. Buoyancy is obtained by chopping small bits off the tail.
Divers which are buoyant with a minimum of liquid charge are used (Fig. 9 (c)).
To charge the diver with a cell, the tail is now inserted into a fine rubber tubing (inner diameter about 200 μ) which is fitted over a braking pipette containing the biological medium used (Fig. 9 (6)). By compress
ing the lumen of the rubber with a screw, the diver is held firmly in posi
tion. The filling procedure may now be continued according to the type
( α )
<Z> - < Ι Γ
Screw
(b) ο
[ — Σ Γ
Seal Cell (c) / \ Ε
F I G. 9. T h e d i v e r is p u l l e d f r o m a t h i n - w a l l e d c a p i l l a r y (a) b r o k e n off, a n d i t s t a i l f i r m l y f a s t e n e d i n t o r u b b e r t u b i n g p l a c e d o v e r a b r a k i n g p i p e t t e ( b ) . T h e d i v e r is c h a r g e d (c) w i t h b i c a r b o n a t e b u f f e r s o l u t i o n (in t h e t a i l ) , w i t h C 02- N2 g a s m i x t u r e (in a m p u l l a ) a n d w i t h a cell s u r r o u n d e d b y s u b s t r a t e - b i c a r b o n a t e b u f f e r s o l u t i o n . T h e t i p is s e a l e d wri t h w a x ( f r o m Z a j i c e k , 1 9 5 7 ) .
of experiment to be performed. The final sealing is with hot wax. After the enzymatic hydrolysis of the substrate has been determined, the diver is removed from the flotation vessel and fastened by the tail to the braking pipette as before. After removal, by cutting with a diamond, of the waxed part of the tip (unsignificant in terms of weight) the diver is refilled with the same cell, but this time with a substrate containing the inhibitor. This method may not work with all cells. Whether or not the cell adheres to the glass is important ; so is the degree of mechanical stability of the cell and of chemical stability of the enzyme. A further development is illustrated in Fig. 9,1-V (from Brzin and Zeuthen, 1961).
In the second filling the amount of inhibitor introduced may be con
trolled as follows : a small but well-defined volume of the inhibitor-sub
strate solution is placed under paraffin oil on a slide or in a capillary.
QUANTITATIVE DETERMINATION OF CHOLINESTERASE ACTIVITY 149
F I G . 1 0 . A . T h e t i p o f t h e d i v e r m i c r o p i p e t t e is b r o k e n a t i t s e n d u n d e r m i c r o s c o p e c o n t r o l . B . T h e o p e n e x t r e m i t y is i n t r o d u c e d i n a c a p i l l a r y h a v i n g a c o n s t a n t d i a m e t e r ( 1 0 - 2 0 μ) a n d tc o n t a i n i n g a s m a l l q u a n t i t y o f t h e i n h i b i t o r p r e v i o u s l y i n t r o d u c e d b y m e a n s o f a m i c r o p i p e t t e . T h e v o l u m e o f t h i s f l u i d i s c a l c u l a t e d b y t h e d i m e n s i o n s d e t e r m i n e d w i t h a m i c r o m e t e r e y e p i e c e . C. T h e l i q u i d is s l o w l y flowing i n t o t h e d i v e r . T h e v o l u m e o f t h e l i q u i d s u c k e d m a y b e c a l c u l a t e d b y t h e d i f f e r e n c e b e t w e e n t h e v o l u m e o f t h e l i q u i d p r e v i o u s l y i n t r o d u c e d a n d t h a t o f t h e l i q u i d l e f t i n t h e c a p i l l a r y a f t e r t h e o p e r a t i o n . D . T h e s o l u t i o n c o n t a i n e d i n t h e n e c k o f t h e d i v e r is m i x e d w i t h t h e i n h i b i t o r b y m e a n s o f s o m e b l o w i n g a n d s u c k i n g o f a p a r t o f i t i n a d r o p o f p a r a f f i n o i l . E . T h e m i c r o p i p e t t e i s c l o s e d w i t h t h e m i c r o f o r g e u n d e r m i c r o s c o p e c o n t r o l ( f r o m G i a c o b i n i , 1 9 5 7 ) .
Figure 11 (Giacobini, 1957) shows four curves which, listed from below, represent : a control, a 1 ΙΟ-μ,-long piece of the neurite from a sympathetic neurone from the frog, a sample of purified ChE, and the cell body (25 μ) of the sympathetic neurone mentioned. The interruptions of the three latter curves reflect the introduction of 5 x l 0
- 6M mipafox, which inhibits unspecific esterases.
5. The methods described have been applied without further changes in a number of haematogical and neurochemical studies in which it was desirable that single cells be studied. Zajicek (1956a) followed the in
creasing contents in AChE of rat megakaryoblasts (24 μ) maturating to megakaryocytes (45-55 μ). The measurements were in the range
^Without blowing anything out of the diver, this volume is quantitatively taken into the diver, and suction is continued until the oil-water inter
phase reaches the close vicinity of the megakaryocyte which always
attaches itself somewhere to the glass. Mixing is accomplished by pushing
the interphase back and forth a couple of times. The diver is then resealed
and set afloat. The inhibitor concentration is defined if also the volume in
which the cell was first introduced has been defined analogously to what
has just been described. An illustration of a slight modification of Zajicek's
procedure is shown in Fig. 10 (from Giacobini, 1959b).
150
J . Z A J I C E K A N D E . Z E U T H E N1 χ ΙΟ"
4to 15 x 10
4μ\ C 0
2per hour. He (1956b, 1957) confirmed his ^ histochemical results (1954) on the inverse distribution of AChE between erythrocytes on the one side and platelets on the other. The hypothesis was advanced that the megakaryocytic and the erythropoietic cells have a common origin in the body. It is at the level of this origin that the enzyme is partitioned, differently in different species of animals, between the two lines. Clearly, the platelets are in the megakaryocytic line.
Time (hr.)
F I G. 1 1 . A C h E a c t i v i t y c u r v e o f a c e l l b o d y ( 2 5 μ) i s o l a t e d f r o m a s y m p a t h e t i c g a n g l i o n o f t h e r a t ( O), t h a t o f 1 1 0 μ l o n g p a r t o f i t s n e u r i t e ( • ) , o f a s a m p l e o f p u r i f i e d C h E ( # ) , a n d o f a c o n t r o l b u f f e r - s u b s t r a t e s o l u t i o n ( χ ) ( f r o m G i a c o b i n i , 1 9 5 9 b ) .
The diver was used for single motor end-plates by Brzin and Zajicek (1958), by Giacobini (1959c) and by Brzin and Zeuthen (1961).
In the latter case it was accepted as the standard against which a gravi
metric method for AChE was checked (cf. the chapter on the diver balance in this volume).
Giacobini and Zajicek (1956) initiated a series of studies by Giacobini (1957,1959a, b, c (c is a review)), and by Giacobini and Holmstedt (1958) in which the AChE in single neurones and their constituent parts was determined. In single sympathetic ganglion cells from the frog and from the rat, the AChE varied from 2 to 30 χ 10~
4μ\ C 0
2per hour. No classi
fication into uniform groups was possible (Giacobini, 1957). The spinal
ganglion cells of the rat showed about the same content of AChE, and
the same variation per unit volume. Two separate groups of cells, one
Q U A N T I T A T I V E D E T E R M I N A T I O N O F C H O L I N E S T E R A S E A C T I V I T Y 151
REFERENCES
B o e l l , E . J . , a n d S h e n , S. ( 1 9 5 0 ) . J. Exptl. Zool. 1 1 3 , 5 8 3 . B r z i n , M . , a n d Z a j i e e k , J . ( 1 9 5 8 ) . Nature 1 8 1 , 6 2 6 .
B r z i n , M . , a n d Z e u t h e n , E . ( 1 9 6 1 ) . Compt. rend. trav. lab. Carlsberg. 3 2 ( 1 0 ) , 1 3 9 . G i a c o b i n i , E . ( 1 9 5 7 ) . J. Neurochem. 1, 2 3 4 .
G i a c o b i n i , E . ( 1 9 5 9 a ) . Acta Physiol. Scand. 4 5 , 2 3 8 . G i a c o b i n i , E . ( 1 9 5 9 b ) . Acta Physiol. Scand. 4 5 , 3 1 1 . G i a c o b i n i , E . ( 1 9 5 9 c ) . Acta Physiol. Scand. 4 5 , s u p p l . 1 5 6 .
G i a c o b i n i , E . , a n d H o l m s t e d t , B . ( 1 9 5 8 ) . Acta Physiol. Scand. 4 2 , 1 2 . G i a c o b i n i , E . , a n d Z a j i e e k , J . ( 1 9 5 6 ) . Nature 2 8 , 1 8 5 .
G l i c k , D . J . ( 1 9 3 9 ) . J. Biol. Chem. 1 3 0 , 5 2 7 .
H y d é n , H . (-1958). Proc. IVth Int. Cong. Biochem. 3 , 6 4 . H y d é n , H . ( 1 9 5 9 ) . Nature 1 8 4 , 4 3 3 .
H y d é n , H . , L o v t r u p , S., a n d P i g o n , A . ( 1 9 5 8 ) . J. Neurochem. 2 , 3 0 4 . K o e l l e , G . B . ( 1 9 5 0 ) . J. Pharmacol. 1 0 0 , 1 5 8 .
K o e l l e , G . B . , a n d F r i e d e n w a l d , J . S. ( 1 9 4 9 ) . Proc. Soc. Exptl. Biol. & Med. 7 0 , 6 1 7 . K r o g h , A . ( 1 9 1 9 ) . J. Physiol. 5 2 , 3 9 1 .
L i n d e r s t r o m - L a n g , K . ( 1 9 3 7 ) . Nature 1 4 0 , 1 0 8 .
L i n d e r s t r o m - L a n g , K . ( 1 9 4 3 ) . Compt. rend. trav. lab. Carlsberg, Sér. Chim. 2 4 , 3 3 3 . L i n d e r s t r o m - L a n g , K . ( 1 9 4 6 ) . Compt. rend. trav. lab. Carlsberg, Sér. Chim. 2 5 , 2 2 9 . L i n d e r s t r o m - L a n g , K . , a n d G l i c k , D . ( 1 9 3 8 ) . Compt. rend. trav. lab. Carlsberg, Sér.
Chim. 2 2 , 3 0 0 .
P o t t e r , V . R . ( 1 9 4 9 ) . " M a n o m e t r i c T e c h n i q u e s a n d T i s s u e M e t a b o l i s m " , p . 1 3 6 , B u r g e s s P u b l i s h i n g C o . , M i n n e a p o l i s .
with a high level of enzyme and one with a low level, could be dis
tinguished. The AChE is several times higher in the axon than in the cell body. AChE is much lower in the motor horn cell than in the spinal ganglion cell (Giacobini, 1959a, c).
Using his micro-edition (0-005 μ\) of this diver, Giacobini (1959b) sampled the neurone at various levels. The nucleus or μμΐ samples of cytoplasm, of nucleoplasm or of nucleoli were removed from the cell.
The equilibrium pressure of the controls was constant within 3 · 5-7 · 5
mm H
20 over 12 hr. For a 0-005 μί diver this would correspond to a
stability of 10 ~
7μ\ C 0
2per hour. The isolated nucleus of a large anterior
horn cell showed an activity of 5 χ 10~
6μ\ C 0
2per hour. A sample of
cytoplasmic material exhibited the same activity, and a sample of
nuclear sap evolved 4 χ 10~
7μ\ C 0
2per hour. (For more data, see Table
I I I in Giacobini, 1959c. ) I t would have been useful had the proper controls
been published. The experiments demonstrate at least the possibilities
of this diver method for the study of the intracellular distribution of
enzymes. I t is the inherent beauty of the method that the diver itself is a
pipette before it becomes a diver-gasometer. I t may therefore be used
for the removal of samples from inside individual living cells.
1 5 2 J . Z A J I C E K A N D E . Z E U T H E N
R e n s h a w , R . R . , D r e i s b a c h , P . F . , Ziff, M . , a n d G r e e n , D . ( 1 9 3 8 ) . J. Amer. Chem.
Soc. 6 0 , 1 7 6 5 . .
S l a t e r , E . C. ( 1 9 4 9 a ) . Biochem. J. 4 4 , 3 0 5 . S l a t e r , E . C. ( 1 9 4 9 b ) . Biochem. J. 4 5 , 1.
U m b r e i t , W . W . , B u r r i s , R . H . , a n d S t a u f f e r , J . F . ( 1 9 4 9 ) . " M a n o m e t r i c T e c h - n i q u e s a n d T i s s u e M e t a b o l i s m " , p . 2 7 . B u r g e s s P u b l i s h i n g C o . , M i n n e a p o l i s . Z a j i c e k , J . ( 1 9 5 4 ) . Acta Haematol. 1 2 , 2 3 8 .
Z a j i c e k , J . ( 1 9 5 6 a ) . Experientia X I I , 2 6 6 . Z a j i c e k , J . ( 1 9 5 6 b ) . Acta Haematol. 1 5 , 2 9 6 .
Z a j i c e k , J . ( 1 9 5 7 ) . Acta Physiol. Scand. 4 0 , s u p p l . 1 3 8 . Z a j i c e k , J . , a n d D a t t a , N . ( 1 9 5 3 ) . Acta Haematol. 9 , 1 1 5 .
Z a j i c e k , J . , S y l v é n , B . , a n d D a t t a , N . ( 1 9 5 3 ) . J. Histochem. Gytochem. 2 , 1 1 5 . Z a j i c e k , J . , a n d Z e u t h e n , E . ( 1 9 5 6 ) . Exptl. Cell Research 1 1 , 5 6 8 .
Z e u t h e n , E . ( 1 9 4 3 ) . Compt. rend. trav. lab. Carlsberg, Sér. Chim. 2 4 , 4 7 9 . Z e u t h e n , E . ( 1 9 5 3 ) . J. Embryol. Exptl. Morphol. 1 (3), 2 3 9 .
Z e u t h e n , E . ( 1 9 5 5 ) . Biol. Bull. 1 0 8 , 3 6 6 .