Early occurrence of pseudocysts in acute pancreatitis e A multicenter international cohort analysis of 2275 cases
Lajos Szak o
a,b, No emi Gede
a,c, Alex V aradi
a,c, Benedek Tinusz
a,b,f, N ora V€ orhendi
a,b, D ora Mosztbacher
a,b,d,e, Aron Vincze
f, Tam as Tak acs
g, L aszl o Czak o
g, Ferenc Izb eki
h, L aszl o Gajd an
h, Veronika Dun as-Varga
h, J ozsef Hamvas
i, M aria Papp
j,
Krisztina Eszter Feh er
j, M arta Varga
k, Artautas Mickevicius
l, Imola T€ or€ ok
m, Klementina Ocskay
a,b, M ark F elix Juh asz
a,b, Szil ard V ancsa
a,b, N andor Faluhelyi
n, Orsolya Farkas
n, Attila Miseta
o, Andr as Vereczkei
p, Alexandra Mik o
a,b,
P eter Jen} o Hegyi
a,b, Andrea Szentesi
a,b,g, Andrea P arniczky
a,b,e,q, B alint Er} oss
a,b,1, P eter Hegyi
a,b,g,r,s,*,1aInstitute for Translational Medicine, Medical School, University of Pecs, Pecs, Hungary
bSzentagothai Research Center, Medical School, University of Pecs, Pecs, Hungary
cInstitute of Bioanalysis, Medical School, University of Pecs, Hungary
dFirst Department of Paediatrics, Faculty of Medicine, Semmelweis University, Budapest, Hungary
eDoctoral School of Theoretical Medicine, Faculty of Medicine, University of Szeged, Szeged, Hungary
fFirst Department of Medicine, Medical School, University of Pecs, Pecs, Hungary
gFirst Department of Medicine, Faculty of Medicine, University of Szeged, Szeged, Hungary
hSzent Gy€orgy Teaching Hospital of County Fejer, Szekesfehervar, Hungary
iBajcsy-Zsilinszky Hospital, Budapest, Hungary
jDepartment of Internal Medicine, Division of Gastroenterology, Faculty of Medicine, University of Debrecen, Debrecen, Hungary
kDepartment of Gastroenterology, Dr. Rethy Pal Hospital of County Bekes, Bekescsaba, Hungary
lVilnius University Hospital Santaros Clinics, Clinics of Abdominal Surgery, Nephro-urology and Gastroenterology, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
mCounty Emergency Clinical Hospital of T^argu Mures¸, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of T^argu Mureș, T^argu Mureș, Romania
nDepartment of Medical Imaging, Medical School, University of Pecs, Pecs, Hungary
oDepartment of Laboratory Medicine, Medical School, University of Pecs, Pecs, Hungary
pDepartment of Surgery, Medical School, University of Pecs, Pecs, Hungary
qHeim Pal National Pediatric Institute, Budapest, Hungary
rHungarian Academy of Sciences, University of Szeged, Szeged, Hungary
sMomentum Gastroenterology, Multidisciplinary Research Group, Szeged, Hungary
a r t i c l e i n f o
Article history:
Received 28 February 2021 Received in revised form 6 May 2021
Accepted 8 May 2021 Available online xxx
Keywords:
Pancreas Acute pancreatitis Pancreatic pseudocyst
a b s t r a c t
Background:Pseudocysts being the most frequent local complications of acute pancreatitis (AP) have substantial effect on the disease course, hospitalization and quality of life of the patient. Our study aimed to understand the effects of pre-existing (OLD-P) and newly developed (NEW-P) pseudocysts on AP.
Methods:Data were extracted from the Acute Pancreatitis Registry organized by the Hungarian Pancreatic Study Group (HPSG). 2275 of 2461 patients had uploaded information concerning pancreatic morphology assessed by imaging technique. Patients were divided into“no pseudocyst”(NO-P) group,
“old pseudocyst”(OLD-P) group, or“newly developed pseudocyst”(NEW-P) groups.
Results:The median time of new pseudocyst development was nine days from hospital admission and eleven days from the beginning of the abdominal pain. More NEW-P cases were severe (15.9% vs 4.7% in the NO-P group p<0.001), with longer length of hospitalization (LoH) (median: 14 days versus 8 days, p<0.001), and were associated with several changed laboratory parameters. OLD-P was associated with
*Corresponding author. Institute for Translational Medicine, University of Pecs, Medical School, Szigeti út 12, Pecs, 7624, Hungary.
E-mail address:hegyi2009@gmail.com(P. Hegyi).
1authors equally contributed.
Contents lists available atScienceDirect
Pancreatology
j o u r n a l h o m e p a g e :w w w .e l se v i e r. co m/ lo ca t e / p a n
https://doi.org/10.1016/j.pan.2021.05.007
1424-3903/©2021 The Authors. Published by Elsevier B.V. on behalf of IAP and EPC. This is an open access article under the CC BY-NC-ND license (http://creativecommons.
org/licenses/by-nc-nd/4.0/).
Pancreatology xxx (xxxx) xxx
Please cite this article as: L. Szako, N. Gede, A. Varadiet al., Early occurrence of pseudocysts in acute pancreatitiseA multicenter international cohort analysis of 2275 cases, Pancreatology, https://doi.org/10.1016/j.pan.2021.05.007
Pancreaticfluid collection Pancreatic local complication
male gender (72.2% vs. 56.1%, p¼0.0014), alcoholic etiology (35.2% vs. 19.8% in the NO-P group), longer hospitalization (median: 10 days, p<0.001), a previous episode of AP (p<0.001), pre-existing diagnosis of chronic pancreatitis (CP) (p<0.001), current smoking (p<0.001), and increased alcohol consumption (unit/week) (p¼0.014).
Conclusion: Most of the new pseudocysts develop within two weeks. Newly developing pseudocysts are associated with a more severe disease course and increased length of hospitalization. Pre-existing pseudocysts are associated with higher alcohol consumption and smoking. Because CP is more frequently associated with a pre-existing pseudocyst, these patients need closer attention after AP.
©2021 The Authors. Published by Elsevier B.V. on behalf of IAP and EPC. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Acute pancreatitis (AP) is one of the most common acute gastroenterological conditions, with a prevalence of 6e18.6% [2,3]
in acute pancreatitis and between 20 and 40% in chronic inflam- mation [4], which requires acute hospitalization [5]; however, several unanswered questions remain [6]. Although most AP cases are mild, local or systemic complications develop in 20e30% of cases, often necessitating interventions [7]. According to the revised Atlanta classification, the most common form of late local complication is the pancreatic pseudocyst, which usually occurs four weeks after the disease onset. It is described as a homogenous and capsulatedfluid collection on the pancreas without necrotic tissue [8].
Many risk factors have been associated with pseudocyst for- mation, but predictive factors remain underestimated. Akgül et al.
found that a lower serum calcium level may be a predictive factor for developing pancreatic pseudocysts after AP attack [9]. The prospective multicenter observational study of Cui et al., in 2013 concluded that lactate dehydrogenase (LDH) after 48 h of AP's onset seemed to be a risk factor of pseudocyst formation. Otherfindings were that age, CRP (48 h), and alcohol etiology appeared to be risk factors for pancreaticfluid collections [10]. An important limitation of cohort studies describing pancreatic pseudocysts that they do not investigate the differences between pre-existing (OLD-P) or newly developed (NEW-P) pseudocysts [11,12], causing difficulties in data interpretation. Furthermore, most of the cohort studies focus on the treatment of pseudocyst, but not on the risk factors of pseudocyst development, or the pseudocyst's impact on the dis- ease's clinical outcome.
Here we provide one of the most extensive and most detailed analysis of pseudocyst development, helping clinicians to under- stand the relevant risks factors and clinical characteristics of both pre-existing and newly developed pancreatic pseudocyst in AP.
Materials and Methods Data source
Data were collected from the multicenter international AP reg- istry run by the Hungarian Pancreatic Study Group (HPSG). At the time of data extraction (October 2019) our registry contained 2461 AP cases collected from 34 hospitals of 13 countries between 2012 and 2019. (Supplementary Table 1, Supplementary Figure 1).
Importantly, all patients were diagnosed with AP based on the 2/3 rules described in the guidelines [13,14], whereas the severity of AP was determined using the revised Atlanta classification [8].
Data quality
The accuracy of acquired data was ensured by a four-level quality monitoring system involving medical administrative
personnel and gastroenterologist specialists, as described in our earlier published cohort analyses [7,15e20]. 105381 of 125125 variables were uploaded, which resulted in 84% available data for analysis (Supplementary Table 2).
Identification of pseudocysts
Altogether 2275 AP cases contained valuable information on pancreatic morphology. Medically trained researchers reviewed the prospectively collected reports of abdominal imaging, but not the images themselves, for the presence of pancreatic pseudocysts.
These imaging modalities included abdominal ultrasonography, computer tomography (CT), endoscopic ultrasound (EUS), or mag- netic resonance cholangiopancreatography (MRCP). The identifi- cation of pseudocysts was based on the morphological recommendations of the revised Atlanta classification [8]. Gener- ally, thefluid and necrotic collections were categorized into four groups, namely acute pancreatic fluid collection (APFC), acute necroticfluid collection (ANFC), pancreatic pseudocyst, and walled- off necrosis (WON).Fig. 1describes the used morphological criteria for the diagnosis, along with examples of imaging examinations from our center (University of Pecs, Medical School) (Fig. 1). We included only pancreatic pseudocysts in our analysis.
Group formation and patients’characteristics
Patients where the latest imaging examinations of AP showed no pseudocyst formation were included in the “no pseudocyst” (NO-P) group. Patients with pseudocyst-positive imaging exami- nation during thefirst four days of hospital stay were assigned to the“old pseudocyst” (OLD-P) group. In contrast, patients with a negative imaging examination in thefirst four days of hospital stay and positive imaging during hospital stay were assigned to the
“new pseudocyst”(NEW-P) group.
Representativeness of the cohort
The analyzed cohort's representativeness was evaluated by comparing the following characteristics between the full cohort of 2461 patients and the cohort of 2245 patients, with data on pancreatic morphology included in the analysis. No significant differences were found between the two groups in terms of age, gender, severity, length of hospitalization, and pancreatitis etiology.
For the detailed analysis of representativity, see Supplementary Figure 2.
Statistical analysis
Statistical analysis was performed using the following statistical tests: for discrete and non-normally distributed continuous vari- ables Kruskal-Wallis rank-sum test with a significance level of 0.05, followed by Dunn's post hoc test with Holm-Sídak p-value
aradi et al. Pancreatology xxx (xxxx) xxx
correction, in which case a significance level of 0.025 was used.
Whereas for categorical variables, the incidence in each group was determined. In these cases, to analyze the relations between vari- ables, Chi-square test or Fisher's exact test was conducted. A p- value of less than 0.05 (0.05) was determined as statistically significant. All analyses were performed using R studio 1.3.1073 (R Foundation for Statistical Computing, Vienna, Austria) [21], and Dunn's tests were performed using the package dunn.test [22].
Ethical approval for the clinical study
The study was approved by the Scientific and Research Ethics Committee of the Medical Research Council (22254-1/2012/EKU).
All participants provided written, informed consent for the enrollment to the registry.
Results
From the 2275 patients, 2054 were included in the NO-P group, Fig. 1.Computer tomography (CT) images and description for morphological diagnosis.
L. Szako, N. Gede, A. Varadi et al. Pancreatology xxx (xxxx) xxx
while 108 patients had OLD-P;finally, 113 patients were included in the NEW-P group. 28.4% of patients had one, whereas 71.6% had at least two imaging examinations during the disease course. 33.33%
of the patients in the OLD-P group and 19.21% of the patients in the NEW-P group had only ultrasound as an imaging examination, while the rest was diagnosed with computer tomography.
OLD-P is associated with male gender and alcoholic etiology
Patients in the OLD-P group were predominantly male compared with the NO-P groups (72.2% vs. 56.1%, p ¼ 0.0014) (Fig. 2A). The average age was 56.1±17.1 years in the NO-P and 55.0 ± 13.7 years in the OLD-P (Fig. 2B), without a statistically significant difference (p¼0.1852). Alcoholic etiology was dominant in the OLD-P group (35.2% vs. 19.8%), while biliary etiology was less common (22.2% vs. 41.8%) compared to the NO-P group. Detailed results regarding the etiology are presented inFig. 2C.
NEW-P is not associated with gender or etiology
Gender did not differ significantly comparing NO-P and NEW- P (56.1% vs 64.6%, p¼0.0929). There was no significant difference regarding age between NO-P and NEW-P (56.1 ± 17.1 years vs 59.6±13.2 years respectively, p¼0.0300) (Fig. 2B). In the NO-P and NEW-P groups, the distribution of etiologic factors was not different from the entire cohort (Fig. 2C).
More than half of the NEW-P can be diagnosed in thefirst two weeks
In our cohort, the median of thefirst detection of NEW-P was on day 9 (IQR1-3: 7e15) calculated from admission, whereas 11 days (IQR 1e3: 7e16) from the beginning of the abdominal pain (Fig. 3A), although it should be highlighted that, the data quality regarding the duration of abdominal pain is 70%
NEW-P worsens the disease course and is associated with longer hospitalization
Since a NEW-P must be considered moderate AP, it is not sur- prising that no mild AP was detected in the NEW-P group. A significantly larger proportion of patients experienced moderate and severe course of AP compared to the NO-P group (p<0.001) (Fig. 3C). Patients were hospitalized for significantly longer the NEW-P (median: 14 days, IQR 1e3: 8e22, p < 0.001) group compared to the NO-P group (median: 8 days, IQR 1e3: 5e11 days) (Fig. 3D). Concerning mortality, we could not detect statistically significant differences between (p¼0.1486). Still, mortality was 5.2% in the NEW-P group versus 2.8% in the NO-P group (Fig. 3B).
OLD-P is also associated with undesirable outcomes regarding hospitalization and disease course
OLD-P was significantly associated with moderate severity compared to the NO-P group (56.5% and 19.33%, respectively, p < 0.001). (Fig. 3C). Patients were hospitalized for significantly longer in the OLD-P group (median: 10 days, IQR 1e3: 6.8e16.2 days, p<0.001) compared to the NO-P group (median: 8 days, IQR 1e3: 5e11 days) (Fig. 3D). Mortality did not differ significantly among the OLD and NO-P group (p¼0.7671)
Previous pancreatic inflammation, chronic pancreatitis, current smoking, and increased alcohol consumption are risk factors of OLD- P
A previous episode of AP (60% vs 25.3%, p<0.001), pre-existing diagnosis of CP (24.8% vs 4.9% p<0.001), current smoking (49.1% vs 30.5%, p < 0.001), and increased weekly alcohol consumption (800.9 g/week vs 495.9 g/week, p ¼ 0.014) were significantly associated with OLD-P compared to NO-P. At the same time, we could not detect significant differences comparing NO-P and OLD-P
Fig. 2. Demographic data A) Gender distribution B) Age C) Etiology. Statistically significant differences are indicated with*. AP: acute pancreatitis, NO-P: no pseudocyst group, OLD- P: old pseudocyst group, NEW-P: new pseudocyst, ERCP: endoscopic retrograde cholangiopancreatography, SD: standard deviation.
aradi et al. Pancreatology xxx (xxxx) xxx
Fig. 3.Outcomes A)first day of new cyst detection from admission and from the beginning of the abdominal pain, B) mortality, C) severity: percentages are indicated in the columns referring to each severity rank, D) length of hospitalization. Statistically significant differences are indicated with*. NO-P: no pseudocyst group, OLD-P: old pseudocyst group, NEW- P: new pseudocyst.
Fig. 4. Medical history. A) Previous acute pancreatitis (AP) episodes B) Previous chronic pancreatitis (CP) episodes C) Current smoking D) Former smoking E) Previous diabetes mellitus F) Alcohol consumption. Percentages are indicated, as well as the included number (n) of patients in the analysis. Statistically significant differences are indicated with*.
NO-P: no pseudocyst group, OLD-P: old pseudocyst group, NEW-P: new pseudocyst, AP: acute pancreatitis, CP: chronic pancreatitis.
L. Szako, N. Gede, A. Varadi et al. Pancreatology xxx (xxxx) xxx
in terms of diabetes mellitus (p ¼0.4204) and former smoking (p¼0.2257) (Fig. 4).
Former smoking is associated with newly developing pseudocyst
Former smoking was associated with NEW-P compared to NO-P.
(38.5% vs 25.4% p¼0.029). Differences were not found comparing NO-P and NEW-P regarding diabetes mellitus (p¼0.3359), alcohol consumption (p¼0.2806), current smoking (p¼0.7497), previous AP episodes (p¼0.5294) and previous CP episodes (p¼0.8908) (Fig. 4).
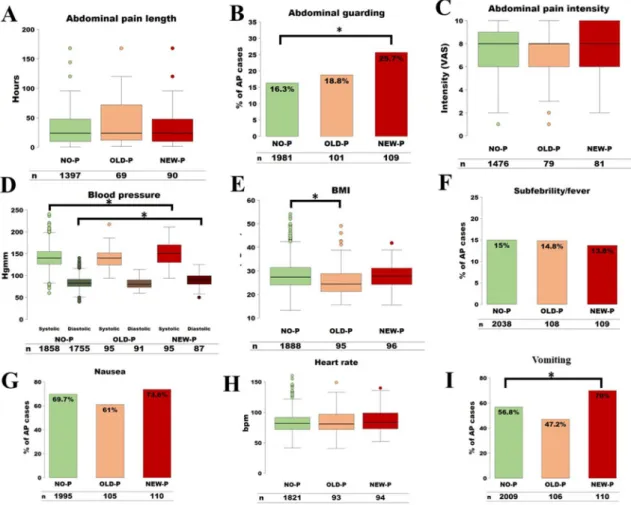
On admission abdominal guarding, vomiting, increased systolic and diastolic blood pressure are risk factors of NEW-P
Significant difference was found comparing NEW-P with NO-P considering abdominal guarding (25.7% vs 16.3%, p ¼ 0.0155), increased diastolic blood pressure (88.38 ± 15.06 mmHg vs 84.14±14.03 mmHg, p¼0.051), increased systolic blood pressure (150.89±27.28 mmHg vs 140.70±22.98 mmHg, p¼0.0035) and vomiting (70% vs 56.8%, p¼0.0088), while nausea (p¼0.4383), subfebrility/fever (p¼0.8261), abdominal tenderness (p¼0.2548), abdominal pain length (p ¼ 0.3788), abdominal pain intensity (p¼0.1917), heart rate (p¼0.2027) did not differ significantly.
On admission clinical parameters did not differ between NO-P and OLD-P
We did notfind statistical difference between NO-P and OLD-P regarding nausea (p ¼ 0.0752), vomiting (p ¼ 0.0633), sub- febrility/fever (p ¼1.0000), abdominal tenderness (p¼0.7814), abdominal guarding (p ¼ 0.5991), abdominal pain intensity (p ¼ 0.2209), abdominal pain length (p ¼ 0.0775), heart rate (p ¼0.3411), diastolic blood pressure (p¼0.1331), and systolic blood pressure (p<0.2844) (Fig. 5).
Lower levels of on-admission amylase and lipase were found in patients with OLD-P
Considering on admission laboratory parameters, amylase, and lipase levels were significantly lower in the OLD-P group than in the NO-P group (770.6±989.3 U/L vs. 1094±1129 U/L, p¼0.0003) (Fig. 6); however, this difference disappeared after excluding those patients who were suffering from CP or recurrent AP (Supplementary Figure 3).
Pancreatic enzyme levels were not associated with NEW-P
Amylase (p ¼0.4142) and lipase (p ¼0.4001) did not differ significantly between NO-P and NEW-P patients (Fig. 6).
Decreased red blood cell parameters and increased thrombocyte counts were found in the OLD-P
On admission red blood cell count (4.417 ± 0.6212 T/L vs.
4.699 ± 0.6231 T/L, p ¼ 0.0002) (Fig. 7D), hemoglobin (135.6±09.90 g/L vs. 142.9±18.9 g/L, p¼0.0017) (Fig. 7E), he- matocrit (39.88±5.363% vs. 41.65±5.079%, p¼0.0045) (Fig. 7B) levels were lower, while thrombocyte count (305 ±128.7G/L vs.
249.6±88.73G/L p¼0.0001) (Fig. 7A) was increased in the OLD-P group compared to NO-P group.
OLD-P is associated with lower BMI, cholesterol, and glucose levels Regarding clinical parameters on admission, patients in the
OLD-P group had significantly lower BMI (25.84 ± 6.63 kg/m2) compared to the NO-P group (28.09 ± 5.95 kg/m2, p < 0.001) (Fig. 5E). Glucose (7.436±2.433 mmol/L vs. 8.338±3.569 mmol/L, p ¼0.0178) (Fig. 7G) and cholesterol (4.593 ± 3.82 mmol/L vs.
5.465±4.02 mmol/L, p¼0.0081) (Fig. 7F) levels were significantly lower in the OLD-P group comparing it to NO-P.
On admission BMI and cholesterol levels were not associated with NEW-P
Significant differences were not found comparing NO-P and NEW-P in terms of on admission BMI (p¼0.3154) (Fig. 7E) and cholesterol (p¼0.3179) (Fig. 7F).
On admission calculated averages of lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, direct bilirubin, and gamma-glutamyl transferase (gamma GT) were lower whereas, the average of C reactive protein (CRP) levels was higher in the OLD-P group
Lactate-dehydrogenase (420.6±258.7 (U/L vs. 486.2±315.5 U/
L, p¼0.01), AST (68.69±117.7 U/L vs. 156.2±208.1 U/L, p<0.001), ALT (69.03±141.2 U/L vs. 154.7±199.6 U/L, p<0.001), total bili- rubin (27.83±50.67mmol/L vs. 35.81±39.28mmol/L, p¼0.0005), direct bilirubin (15.04 ± 27.3 mmol/L vs 27.79 ± 35.27 mmol/L, p ¼ 0.003) and GGT (245.2± 372.2 U/L vs. 362.9 ± 489.8 U/L, p < 0.001) were lower in the OLD-P as the result of etiological difference. CRP (78.48 ± 82.94 mg/L vs. 49.97 ± 75.32, mg/L p<0.001) levels were higher in the OLD-P group than the NO-P group (Figs. 7e8).
On admission liver function tests and C-reactive protein did not differ comparing NEW-P and NO-P
NEW-P did not differ from NO-P in terms of AST (p¼0.3423), ALT (p¼0.2928), gamma-GT (p¼0.2737) and CRP (p¼0.0826) (Fig. 8).
Increased inflammatory parameters, hemoglobin, hematocrit, glucose, blood urea nitrogen, LDH and creatinine levels are associated with NEW-P compared to NO-P
Patients in the NEW-P group had significantly higher on admission white blood cell (WBC) count (14.57 ± 4.411 G/L vs.
12.89±4.956 G/L, p¼0.0001), hemoglobin (150.1±20.38 g/L vs.
142.9 ± 18.9 g/L, p ¼ 0.0001), hematocrit (43.08 ± 5.445%, vs.
41.65± 5.079%, p¼0.0043), glucose (9.717± 3.633 mmol/L vs.
8.338±3.569 mmol/L, p<0.001), blood urea nitrogen (7.07±3.702 vs. 6.313±3.837, p¼0.0125), lactate dehydrogenase (592.2±319.5 vs. 486.2 ± 315.5, p ¼ 0.0002) and creatinine levels (89.021 ± 6.879 mmol/L vs. 75.426 ± 5.890 mmol/L, p< 0.0125) (Figs. 7e8), while also higher levels of maximum CRP (167±104.3 mg/L vs. 147.8 ±118.3 mg/L, p¼0.0141) and WBC (18.41±7.023 G/L vs. 14.38±6.651 G/L, p<0.001) levels compared to NO-P group (Suppl.Fig. 3). On admission thrombocyte count did not differ comparing NO-P and NEW-P (0.1877) (Fig. 7A).
On admission kidney function parameters and white blood cell count did not differ between NO-P and OLD-P groups
OLD-P did not differ from NO-P considering on admission WBC count (p ¼ 0.3184) (Fig. 7B), blood urea nitrogen (p ¼ 0.0322) (Fig. 7B), creatinine levels (p¼0.0322) (Fig. 7H) and maximum WBC count (p¼0.0429) (Suppl.Fig. 3).
aradi et al. Pancreatology xxx (xxxx) xxx
Systemic and local complications are more common in the cases of OLD-P
Concerning the complications, OLD-P was associated compared to NO-P with a higher incidence of the acute pancreatic fluid collection (APFC) (38.9% vs. 19% p<0.001), ascites (34.45% vs. 14.1%
p<0.001), systemic complication (13.15% vs. 7.8% p¼0.0113) and
respiratory failure (9.3% vs. 5.2% p¼0.0182), while pleural fluid (p¼0.7975), necrosis (p¼0.9062), heart failure (p¼0.6470), and renal failure (p¼0.4732) did not differ.
Systemic and local complications are associated with NEW-P too NEW-P was associated with higher rate of APFC (70.8% vs. 19%
Fig. 5. On admission clinical parameters A) Abdominal pain length B) Abdominal guarding C) Abdominal pain intensity D) Blood pressure E) Body-mass index (BMI) F) Subfebrility/
fever G) Nausea H) Heart rate (/min) I) Vomiting. Percentages are indicated, as well as the included number (n) of patients in the analysis. Statistically significant differences are indicated with*. AP: acute pancreatitis, NO-P: no pseudocyst group, OLD-P: old pseudocyst group, NEW-P: new pseudocyst.
Fig. 6. On admission laboratory parameters I.: A) on admission amylase, B) on admission lipase. Statistically significant differences are indicated with*. NO-P: no pseudocyst group, OLD-P: old pseudocyst group, NEW-P: new pseudocyst.
L. Szako, N. Gede, A. Varadi et al. Pancreatology xxx (xxxx) xxx
p<0.001), necrosis (38.1% vs. 7.8% p<0.001), systemic complica- tions (21.2 vs. 7.8% p¼0.0245) compared to NO-P, while there was no difference considering pleural fluid (p ¼ 0.7939), ascites (p ¼ 0.4995), respiratory failure (p ¼ 0.4995), heart failure (p¼0.0495) and renal failure (p¼0.0592) (Fig. 9).
One in four patients with OLD-P, whereas one in six patients with NEW-P required interventions
Interventions, such as endoscopic, percutaneous, and surgical drainage on pancreatic pseudocysts, were performed in 44 cases in our cohort. There were no significant differences comparing OLD-P and NEW-P regarding any intervention (p ¼ 0.3124), surgical intervention (p¼0.7058), percutaneous intervention (p¼1.0000), endoscopic intervention (p ¼0.7639) comparing the two groups (Fig. 10).
Further on admission laboratory parameters did not differ among NEW-P and NO-P
Differences were not found comparing NO-P and NEW-P considering on admission calcium (p ¼ 0.2945), potassium (p ¼0.4815), total protein (p¼ 0.4635), albumin (p¼0.4532), estimated glomerular filtration rate (p ¼ 0.1021), tryglyceride (p¼0.2392), direct bilirubin (p¼0.0713), alkaline phosphatase (p¼0.0489), procalcitonine (p¼0.6488), amylase (p¼0.4535) and lipase (p¼0.3506) including only AP cases.
Further on admission laboratory parameters did not differ among OLD-P and NO-P
OLD-P did not differ from NO-P regarding on admission calcium (p¼0.2201), potassium (p¼0.3090), total protein (p¼0.6143), albumin (p ¼ 0.0950), estimated glomerular filtration rate (p ¼ 0.0556), tryglyceride (p ¼ 0.1400), alkaline phosphatase (p¼0.0667), procalcitonine (p¼0.7398), amylase (p¼0.0989) and lipase (p¼0.0358) including only AP cases.
Discussion
The most intriguing finding of our results is that pancreatic pseudocysts can be diagnosed earlier than previously suggested.
The revised Atlanta classification of 2012 does not explicitly state that pseudocysts can only be diagnosed four weeks after the onset of pancreatitis; it only implies that it occurs most commonly after the mentioned time interval [8]). Our analysis showed that at least half of the pancreatic pseudocysts could be diagnosed in thefirst two weeks following the radiological morphology suggested by the same classification. Therefore, we recommend the nonmandatory use of this timeframe.
Delaying interventions based on the initially late diagnosis might be one reason why new pseudocysts were associated with a longer length of hospital stay. However, the association between LoH and OLD-P highlights the effect of pseudocyst itself on the severity, eventually resulting in longer hospitalization. While the Fig. 7.On admission laboratory parameters II.: Hematological parameters, cholesterol, glucose, kidney functions. Number (n) of patients included in the analysis are indicated.
Statistically significant differences are indicated with*. NO-P: no pseudocyst group, OLD-P: old pseudocyst group, NEW-P: new pseudocyst, Htc: hematocrit, WBC: white blood cell, RBC: red blood cell, HGB: hemoglobin, BUN: blood urea nitrogen.
aradi et al. Pancreatology xxx (xxxx) xxx
Fig. 8.On admission laboratory parameters III.: C-reactive protein (CRP) and liver functions. Number (n) of patients included in the analysis are indicated. Statistically significant differences are indicated with*. NO-P: no pseudocyst group, OLD-P: old pseudocyst group, NEW-P: new pseudocyst, CRP: C-reactive protein, GGT: gamma-glutamyl transferase, LDH: lactate dehydrogenase, ASAT/GOT: Aspartate aminotransferase/glutamic oxaloacetic transaminase, ALAT/GPT: alanine aminotransferase/glutamate pyruvate transaminase, TBIL: total bilirubin.
Fig. 9. Systemic and local complications. A) heart failure, B) respiratory failure, C) systemic organ failure, D) necrosis, E)acute pancreaticfluid collection (APFC), F) Ascites. Per- centages are indicated as well as the number (n) of patients included in the analysis. Statistically significant differences are indicated with*. AP: acute pancreatitis, NO-P: no pseudocyst group, OLD-P: old pseudocyst group, NEW-P: new pseudocyst.
L. Szako, N. Gede, A. Varadi et al. Pancreatology xxx (xxxx) xxx
analysis of mortality did not show a statistically significant differ- ence, an increased death rate was observed in the OLD-P and NEW- P groups compared to the NO-P group, which might imply type II statistical error due to the low number of cases. Furthermore, the higher rate of systemic organ failures in both groups compared to the NO-P patients can also lead to increased mortality.
The associations of newly developing pseudocyst, former smoking, and altered laboratory parameters are suggested as risk factors for cyst development by our analysis. They could be used as motives to perform additional imaging examinations to diagnose fluid collections.
The toxic effects of alcohol consumption, as well as smoking and their role in cyst formation, should be highlighted, which associa- tion is also presented in the larger proportion of alcoholic etiology in the OLD-P group, while former smoking also plays an important role in NEW-P. Alcoholic etiology was also highlighted by Cui et al.
regarding cyst formation. Furthermore, a meta-analysis from Sharanya et al. found that alcoholic etiology, male gender, and smoking are risk factors for chronic pancreatitis, which is the ground of pancreatic pseudocyst formation [23]. The association of alcoholic etiology and cyst formation was also found by an Indian prospective cohort study, where 65.6% of the patients had alcohol consumption in their medical history [24]. A multicenter and multinational (Germany, Italy, and the United States of America) study from Lankisch et al. also proved that alcohol consumption is associated with cyst formation (p¼0.0008) [25]. Thisfinding is also supported by a retrospective analysis from China, where alcoholic etiology (p¼0.031) and chronic pancreatitis (p¼0.006) were identified as risk factors for cyst formation. The more pseu- docysts associated with alcohol consumption are probably due to the direct toxic effects of these factors on acinar and ductal cells.
Both agents cause elevated intracellular calcium, adenosine
triphosphate (ATP) level, and membrane protein transition pore (MPTP) inhibition [26e28], leading the cell to necrosis in both exocrine cell types [29e31]. This can disrupt the epithelial barrier's integrity, causing leakage of ductal fluid to the peri-and intra- pancreatic space.
Former inflammatory episodes and chronic inflammations of the pancreas were associated with an increased risk of old pseu- docysts. Our result is in accordance with the South Korean cohort study, where the overall incidence of pseudocyst after acute or acute-on-chronic pancreatitis was 18.3% (n¼74/405) [32]. Pseu- docysts developed much more frequently in patients with under- lying chronic pancreatitis than in patients with acute pancreatitis (41.5% vs. 14.6%, p<0.001).
In our study, NEW-P was associated with abdominal guarding, vomiting and increased blood pressure, and more severe disease outcomes. Unfortunately, our cohort analysis did not allow us to investigate the cause-relationship in these associations.
We have earlier reported that the cluster of conditions called metabolic syndrome, most importantly diabetes mellitus, hyper- tension, and obesity have a detrimental effect on the disease course of pancreatitis [17]. On the other hand, we found that a lower BMI is a sensitive indicator of malnutrition in chronically affected pancreatic patients with old pseudocysts.
The malnourishment is also reflected by the on-admission lab- oratory parameters, meaning the decreased hemoglobin level, he- matocrit, and cholesterol. On the other hand, a study describing the African American population found that obesity is a risk factor of cyst formation (p ¼ 0.06) [33]. Another meta-analysis of our workgroup described fatty liver disease as a further risk factor of cyst development [34]. In the OLD-P group, biliary etiology is less common with lower levels of liver function tests, such as total bilirubin, GOT, GPT, and GGT. The exhaustion of exocrine functions Fig. 10. Management of the pseudocysts. A) Any intervention B)Percutaneous interventionC)Endoscopic interventionD)Surgical intervention. Percentages are indicated as well as the number (n) of patients included in the analysis. AP: acute pancreatitis, OLD-P: old pseudocyst group, NEW-P: new pseudocyst.
aradi et al. Pancreatology xxx (xxxx) xxx
of the pancreas in the OLD-P group is indicated in the significantly lower levels of on admission amylase and lipase, due to the decreased amount of acinus cells in the case of a chronic inflam- mation, which is also supported by the fact, that the difference disappears once the chronic pancreatic cases and recurrent acute pancreatic cases are excluded from the analysis. Thus, the shrinkage of the pancreas, thus the decreased amount of acinus cells in the cases of recurring or chronic inflammation, were formerly described by Steve et al. [35], supporting this idea. The fact that new pseudocysts are associated with increased white blood cell count and lactate dehydrogenase correlates with the effect of pseudocyst on the severity.
In contrast, increased hemoglobin, hematocrit, blood urea ni- trogen, and creatinine foreshadow the possibility of dehydration on admission. The significantly higher risk of systemic complications in NEW-P is partly driven by acute kidney injury, reflected by the significantly elevated blood urea nitrogen and creatinine levels.
More diabetogenic state of the NEW-P group is presented in the elevated level of on admission glucose. While Akgül et al. found that a lower serum calcium level may be a predictive factor for developing pancreatic pseudocysts after an AP attack [9], our analysis did notfind such association. On the other hand, we sup- port the findings of Cui et al. regarding increased LDH and CRP levels in the cases of the pancreatic pseudocyst. However, we found on admission laboratory parameters to be associated with the condition, not the 48-h value as the mentioned study did.
Interventions on the pancreatic pseudocysts should be carried out if it causes any symptom to the patient or in the case of the infected fluid collection according to the European Society of Gastrointestinal Endoscopy (ESGE) [36]. While interventions may vary, they should rely on local expertise and the location of thefluid collection. Although each treatment modality has its important undebatable role, minimally invasive techniques, including endo- scopic and percutaneous drainage, should be preferred. According to our recent meta-analysis, if multiple interventional modalities can be applied, endoscopic intervention should be preferred [37]. In our analysis, less than one-third of the patient required interven- tion. Still, this proportion was higher in the OLD-P group, where endoscopic interventions were carried out in more cases, compared to percutaneous or surgical drainage. Interestingly percutaneous drainage was more common in the NEW-P group, which might be due to the suspected benefit of this intervention according to the cyst's localization. However, our analysis did not investigate this question.
Limitations of our analysis
Our study has several limitations. Although all data were collected prospectively, clinical questions were post hoc defined.
Cases were included in the analysis with imaging reports from several centers from several radiologists. We aimed to minimize these limitations by uniform data collection forms. The imaging modalities included both ultrasound and computer tomography, which have different sensitivity and specificity for pancreatic pseudocysts. Furthermore, the criteria, we implied considering our cohort's grouping were based on empirical expert opinion since we believe that forming a new pseudocyst is unlikely to happen in the first four days.
Implications for research
While our analysis highlighted many aspects regarding pseu- docysts, a prospective observational study focusing on the risk factors of pseudocyst development and prognostic factors of the disease course of patients with diagnosed pseudocysts should be
proposed to achieve clearer insight into this complication of AP.
Implications for practice
Based on ourfindings, we encourage the nonmandatory use of the timeframes of the revised Atlanta classification for the diag- nosis of the pancreatic pseudocyst to allow earlier diagnosis and possible earlier treatment. Furthermore, considering the toxic and evoking effects of alcohol and smoking on cyst formation, cessation programs should be emphasized in medical therapy for AP patients.
Considering the severity and possible organ failures, newly diag- nosed pseudocysts require more attention as they indicate worse outcomes.
Conclusions
Newly developing pseudocysts are associated with a more se- vere disease course and increased length of hospitalization. Most of the new pseudocysts develop within two weeks. Pre-existing pseudocysts are associated with higher alcohol consumption and smoking. The fact that CP is more frequently associated with a pre- existing pseudocyst, these patients need closer attention after AP.
Funding
The research was supported by Project Grants (K131996 to PH, FK131864 to AM, FK124632 to BCN, K128222 to LC and K120335 to TT) of the National Research Development and Innovation Office, and Economic Development and Innovation Operative Programme Grant (GINOP 2.3.2-15-2016-00048, and GINOP-2.3.2-15-2016- 00015eI-KOM to PH), a Human Resources Development Opera- tional Programme Grant (EFOP-3.6.2-16-2017-00006 to PH), Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences.
Author contributions
Er}oss B and Hegyi P conceptualized and designed the study in cooperation with Szentesi A, VinczeA, P arniczky A, and Szako L;
Mosztbacher D, Vincze A, Takacs T, Czako L, Izbeki F, Gajdan L, Dunas-Varga V, Hamvas J, Papp M, Feher K E, Varga M, Mickevicius A, Miko A and T€or€ok I contributed to the data collection from their respective center; Tinusz B, V€orhendi N, and Szako L performed data extraction on patients from multiple centers; Tinusz B, Vancsa S, Juhasz MF, Ocskay K and Hegyi JP, conducted the quality assessment; Szako L and Er}oss B wrote the article; Miseta A, Ver- eczkei A, Miko A, Szentesi A, Parniczky A, and Hegyi P provided valuable feedback after critically reviewing thefirst drafts of the manuscript. Faluhelyi N and Farkas O assessed the imaging exam- inations. Gede N and Varadi A performed the statistical analysis. All the authors reviewed and approved thefinal manuscript for pub- lication, while providing critical input.
STROBE 2007 checklist statement
The authors have read the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) 2007 Checklist, and the manuscript was prepared and revised accordingly [1].
Declaration of competing interest
The authors declare no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at
L. Szako, N. Gede, A. Varadi et al. Pancreatology xxx (xxxx) xxx
https://doi.org/10.1016/j.pan.2021.05.007.
References
[1] von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ (Clinical research ed) 2007;335(7624):806e8.
[2] Imrie CW, Buist LJ, Shearer MG. Importance of cause in the outcome of pancreatic pseudocysts. Am J Surg 1988;156(3 Pt 1):159e62.
[3] Maringhini A, Uomo G, Patti R, Rabitti P, Termini A, Cavallera A, et al. Pseu- docysts in acute nonalcoholic pancreatitis: incidence and natural history. Dig Dis Sci 1999;44(8):1669e73.
[4] Barthet M, Bugallo M, Moreira LS, Bastid C, Sastre B, Sahel J. Management of cysts and pseudocysts complicating chronic pancreatitis. A retrospective study of 143 patients. Gastroenterol Clin Biol 1993;17(4):270e6.
[5] Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al.
Burden of gastrointestinal disease in the United States: 2012 update.
Gastroenterology 2012;143(5):1179e87. e3.
[6] Szentesi A, Toth E, Balint E, Fanczal J, Madacsy T, Laczko D, et al. Analysis of research activity in gastroenterology: pancreatitis is in real danger. PloS One 2016;11(10):e0165244.
[7] Parniczky A, Kui B, Szentesi A, Balazs A, Sz}ucsA, Mosztbacher D, et al. Pro- spective, multicentre, nationwide clinical data from 600 cases of acute pancreatitis. PloS One 2016;11(10):e0165309.
[8] Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al.
Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62(1):102e11.
[9] AkgülO, Ers€€ oz S¸, S¸enol K, Gündogdu SB, Çetinkaya E, Tez M. Calcium level may be a predictive factor for pseudocyst formation after acute pancreatitis. Acta Gastro-enterol Belgica 2015;78(2):219e22.
[10] Cui ML, Kim KH, Kim HG, Han J, Kim H, Cho KB, et al. Incidence, risk factors and clinical course of pancreaticfluid collections in acute pancreatitis. Dig Dis Sci 2014;59(5):1055e62.
[11] Pan G, Wan MH, Xie KL, Li W, Hu WM, Liu XB, et al. Classification and man- agement of pancreatic pseudocysts. Medicine 2015;94(24):e960.
[12] Hao L, Pan J, Wang D, Bi YW, Ji JT, Xin L, et al. Risk factors and nomogram for pancreatic pseudocysts in chronic pancreatitis: a cohort of 1998 patients.
J Gastroenterol Hepatol 2017;32(7):1403e11.
[13] IAP/APA evidence-based guidelines for the management of acute pancreatitis.
Pancreatology : Off J Int Assoc Pancreatol (IAP) [et al] 2013;13(4 Suppl 2):
e1e15.
[14] Hritz I, Czako L, Dubravcsik Z, Farkas G, Kelemen D, Lasztity N, et al. [Acute pancreatitis. Evidence-based practice guidelines, prepared by the Hungarian Pancreatic Study Group]. Orv Hetil 2015;156(7):244e61.
[15] Parniczky A, Lantos T, Toth EM, Szakacs Z, Godi S, Hagendorn R, et al. Anti- biotic therapy in acute pancreatitis: from global overuse to evidence based recommendations. Pancreatology : Off J Int Assoc Pancreatol (IAP) [et al]
2019;19(4):488e99.
[16] Mosztbacher D, Hanak L, Farkas N, Szentesi A, Miko A, Bajor J, et al. Hyper- triglyceridemia-induced acute pancreatitis: a prospective, multicenter, inter- national cohort analysis of 716 acute pancreatitis cases. Pancreatology : Off J Int Assoc Pancreatol (IAP) [et al] 2020;20(4):608e16.
[17] Szentesi A, Parniczky A, VinczeA, Bajor J, G odi S, Sarlos P, et al. Multiple hits in acute pancreatitis: components of metabolic syndrome synergize each other's deteriorating effects. Front Physiol 2019;10:1202.
[18] Farkas N, Hanak L, Miko A, Bajor J, Sarlos P, Czimmer J, et al. A multicenter, international cohort analysis of 1435 cases to support clinical trial design in acute pancreatitis. Front Physiol 2019;10:1092.
[19] Halasz A, Pecsi D, Farkas N, Izbeki F, Gajdan L, Fejes R, et al. Outcomes and timing of endoscopic retrograde cholangiopancreatography for acute biliary pancreatitis. Dig Liver Dis : Off J Italian Soc Gastroenterol Italian Assoc Stud Liver 2019;51(9):1281e6.
[20] Szakacs Z, Gede N, Pecsi D, Izbeki F, Papp M, Kovacs G, et al. Aging and comorbidities in acute pancreatitis II.: a cohort-analysis of 1203 prospectively collected cases. Front Physiol 2018;9:1776.
[21] RStudio Team. RStudio: integrated development environment for R. RStudio.
Boston, MA: PBC; 2020.http://www.rstudio.com/.
[22] Dinno Alexis. dunn.test: Dunn's test of multiple comparisons using rank sums.
R package version 1.3.5. https://CRAN.R-project.org/package¼dunn.test;
2017.
[23] Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta- analysis. Gastroenterology 2015;149(6). 1490-500.e1.
[24] Dr Sridhar Reddy P, Rajsiddharth Dr B, Karunakar Reddy Dr B. A study of clinical features and management of pseudocyst of pancreas - published at. Int J Sci Res Publ (IJSRP) September 2015;5(Issue 9). Edition.
[25] Lankisch PG, Weber-Dany B, Maisonneuve P, Lowenfels AB. Pancreatic pseu- docysts: prognostic factors for their development and their spontaneous resolution in the setting of acute pancreatitis. Pancreatology : Off J Int Assoc Pancreatol (IAP) [et al] 2012;12(2):85e90.
[26] Maleth J, Rakonczay Jr Z, Venglovecz V, Dolman NJ, Hegyi P. Central role of mitochondrial injury in the pathogenesis of acute pancreatitis. Acta Physiol 2013;207(2):226e35.
[27] Toth E, Maleth J, Zavogyan N, Fanczal J, Grassalkovich A, Erd}os R, et al. Novel mitochondrial transition pore inhibitor N-methyl-4-isoleucine cyclosporin is a new therapeutic option in acute pancreatitis. J Physiol 2019;597(24):
5879e98.
[28] Mukherjee R, Mareninova OA, Odinokova IV, Huang W, Murphy J, Chvanov M, et al. Mechanism of mitochondrial permeability transition pore induction and damage in the pancreas: inhibition prevents acute pancreatitis by protecting production of ATP. Gut 2016;65(8):1333e46.
[29] Luaces-Regueira M, Casti~neira-Alvari~no M, Castro-Manzanares M, Campos- Toimil M, Domínguez-Mu~noz JE. Pathophysiological events associated with pancreatitis in response to tobacco: an in vitro comparative study with ethanol in primary acinar cell culture. Pancreas 2018;47(10):1304e11.
[30] Sahin-Toth M, Hegyi P. Smoking and drinking synergize in pancreatitis:
multiple hits on multiple targets. Gastroenterology 2017;153(6):1479e81.
[31] Hegyi P, Petersen OH. The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev Physiol Biochem Pharmacol 2013;165:
1e30.
[32] Kim KO, Kim TN. Acute pancreatic pseudocyst: incidence, risk factors, and clinical outcomes. Pancreas 2012;41(4):577e81.
[33] Shahnazi A, Badurdeen D, Laiyemo AO, Nouraie M, Brim H, Wessly P, et al.
Obesity and pancreatic cysts in african American patients. Cureus 2018;10(8):
e3160.
[34] Vancsa S, Nemeth D, Hegyi P, Szakacs Z, Hegyi PJ, Pecsi D, et al. Fatty liver disease and non-alcoholic fatty liver disease worsen the outcome in acute pancreatitis: a systematic review and meta-analysis. J Clin Med 2020;9(9).
[35] DeSouza SV, Priya S, Cho J, Singh RG, Petrov MS. Pancreas shrinkage following recurrent acute pancreatitis: an MRI study. Eur Radiol 2019;29(7):3746e56.
[36] Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, et al.
Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy 2018;50(5):524e46.
[37] Szako L, Matrai P, Hegyi P, Pecsi D, Gy€ongyi Z, Csupor D, et al. Endoscopic and surgical drainage for pancreaticfluid collections are better than percutaneous drainage: meta-analysis. Pancreatology : Off J Int Assoc Pancreatol (IAP) [et al]
2020;20(1):132e41.
aradi et al. Pancreatology xxx (xxxx) xxx