Materials 2021, 14, 4880. https://doi.org/10.3390/ma14174880 www.mdpi.com/journal/materials
Supplementary Information
M(II)Al 4 Type Layered Double Hydroxides—Preparation Using Mechanochemical Route, Structural Characterization and
Catalytic Application
Márton Szabados
1,2, Adél Anna Ádám
2, Zsolt Kása
2, Kornélia Baán
3, Róbert Mucsi
3, András Sápi
3, Zoltán Kónya
3,4, Ákos Kukovecz
3and Pál Sipos
2,5,*
1 Department of Organic Chemistry, University of Szeged, Dóm tér 8, H-6720 Szeged, Hungary;
szabados.marton@chem.u-szeged.hu
2 Material and Solution Structure Research Group, Institute of Chemistry, University of Szeged, Aradi Vértanúk tere 1, H-6720 Szeged, Hungary; adeladam@chem.u-szeged.hu (A.A.Á.);
kasa.zsolt@chem.u-szeged.hu (Z.K.)
3 Department of Applied and Environmental Chemistry, University of Szeged, Rerrich B. tér 1, H-6720 Szeged, Hungary; kornelia.baan@chem.u-szeged.hu (K.B.); muule93@gmail.com (R.M.);
sapia@chem.u-szeged.hu (A.S.); konya@chem.u-szeged.hu (Z.K.); kakos@chem.u-szeged.hu (Á.K.)
4 MTA-SZTE Reaction Kinetics and Surface Chemistry Research Group, Rerrich B tér 1, H-6720 Szeged, Hungary
5 Department of Inorganic and Analytical Chemistry, University of Szeged, Dóm tér 7, H-6720 Szeged, Hungary
* Correspondence: sipos@chem.u-szeged.hu
10 20 30 40 50 60
γ-Al(OH)3
1:3.5 CuCl2:Al(OH)3 Cu2(OH)3Cl
{7.5Å} - LDH
1:4 CuCl2:Al(OH)3
1:3 CuCl2:Al(OH)3
1:2 CuCl2:Al(OH)3 1:1 CuCl2:Al(OH)3
2 θ ( ° )
In tensity (a. u.)
Figure S1. XRD patterns of the CuAl4–Cl−–LDH solids obtained using different initial Cu:Al molar ratios (reaction conditions: 96 h stirring, 90 °C, 6 h pre-milling). The basal spacing is shown in curly brackets.
10 20 30 40 50 60 {7.7Å} - LDH Al3O4Br
1:3.5 CuBr2:Al(OH)3 Cu2(OH)3Br
1:4 CuBr2:Al(OH)3
1:1 CuBr2:Al(OH)3 1:2 CuBr2:Al(OH)3 1:3 CuBr2:Al(OH)3
Intensit y (a. u. )
2 θ ( ° )
Figure S2. XRD patterns of the CuAl4–Br−–LDH solids obtained using different initial Cu:Al molar ratios (reaction conditions: 96 h stirring, 90 °C, 6 h pre-milling). The basal spacing is shown in curly brackets.
4000 3500 3000 2500 2000 1500 1000
Absorbance (a.u.)
Wavenumber (cm
−1)
LDH-ClO4−LDH-NO3−
1630 3465
3575
930 700
1085
620
1350
1050 820
Figure S3. Infrared spectra of the CuAl4–LDHs, prepared with nitrate and perchlorate interlayer anions.
10 20 30 40 50 60 {8.5Å} - LTH
0.125:1:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3
1:1:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3 0.25:1:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3 0.2:1:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3 0.17:1:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3
0.5:1:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3 (3 nm)
(3 nm) (4 nm)
(6 nm) (8 nm) (12 nm)
Intensit y (a. u. )
2 θ ( ° )
Figure S4. X-ray diffraction traces of the NiCuAl4–NO3−–LTH solids, with various initial Ni:Cu mo- lar ratios (gradual decrease of the added Ni(II) amount, crystallite thicknesses shown in nm, reaction conditions: 96 h stirring period, 90 °C, 6 h pre-milling). The basal spacing is shown in curly brackets.
10 20 30 40 50 60
Intensit y (a. u. )
{8.6Å} - LTH
1:0:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3 1:8:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3
2 θ ( ° )
1:2:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3 1:4:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3 1:5:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3 1:6:1 Ni(NO3)2:Cu(NO3)2:Al(OH)3 Cu2(OH)3NO3
(12 nm) (9 nm) (7 nm) (12 nm) (13 nm) (11 nm)
Figure S5. XRD patterns of the NiCuAl4–NO3−–LTH materials, with different initial Ni:Cu molar ratios (gradual increase of the added Cu(II) concentration,crystallite thicknesses denoted in nm, reaction conditions: 96 h stirring period, 90 °C, 6 h pre-milling). The basal spacing is shown in curly brackets.
5 10 15 20 25 30 35 40 {8.7Å} - LDH
In tensit y (a.u.)
1:1 Co(NO3)2:Al(OH)3 AlO(OH)
1:1 Zn(NO3)2:Al(OH)3 4:1 Co(NO3)2:Al(OH)3
2 θ ( ° )
4:1 Zn(NO3)2:Al(OH)3 α-Al(OH)3
Figure S6. XRD curves of the CoAl4– and ZnAl4–NO3–LDHs, with different initial Co/Zn:Al molar ratios (reaction conditions: 96 h stirring period, 90 °C, 6 h pre-milling). The basal spacing is shown in curly brackets.
10 20 30 40
32:1 Mg(NO3)2:Al(OH)3 8 h premilling
32:1 Mg(NO3)2:Al(OH)3 6 h premilling
16:1 Mg(NO3)2:Al(OH)3 6 h premilling {8.5Å} - LDH
Intensit y (a. u. )
2 θ ( ° )
γ-Al(OH)3
32:1 Mg(NO3)2:Al(OH)3 12 h premilling
Figure S7. XRD patterns of the MgAl4–NO3−–LDH solids, with various milling times and initial Mg:Al molar ratios (reaction conditions: 96 h stirring period, 90 °C). The basal spacing is shown in curly brackets.
Table S1. The molar ratios of the incorporated metal ions into the gibbsite structure; the initial and the measured values in the formed LTHs/LMHs are shown with perchlorate interlamellar anions.
Samples (LDH-ClO4) Initial Molar Ratios1 Measured Molar Ratios Mg Ni Co Cu Zn Mg Ni Co Cu Zn
NiCu-Al - 2 - 2 - - 4.32 - 1 -
- 1 - 2 - - 2.51 - 1 - - 1 - 4 - - 1.48 - 1 -
NiCo-Al - 2 2 - - - 20.70 1 - -
NiZn-Al - 2 - - 2 - 15.16 - - 1
CoCu-Al - - 2 2 - - - 1 7.27 -
CuZn-Al - - - 2 2 - - - 5.63 1
NiCoCu-Al - 2 2 2 - - 20.89 1 8.27 -
NiCoCuZn-Al - 2 2 2 2 - 15.20 1 4.81 1.15
MgNiCoZnCu-Al 2 2 2 2 2 0.04 16.83 1 4.83 1.22
1 The initial molar ratio of the aluminum was at 1 in every case.
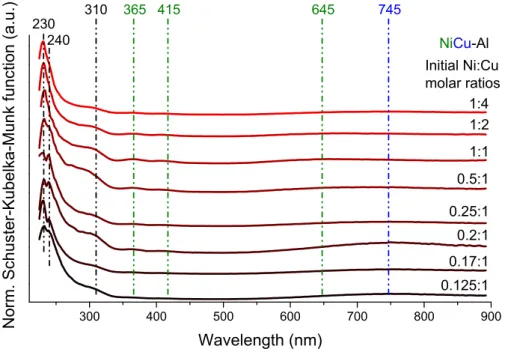
300 400 500 600 700 800 900
NiCu-Al Initial Ni:Cu molar ratios
0.17:1 0.2:1 0.25:1 0.5:1 1:1 1:2 1:4
0.125:1 240
Norm. Sch uster-Ku belka-Mun k function (a.u.)
Wavelength (nm)
645 745
415 365 230 310
Figure S8. UV-Vis diffuse reflection spectra of the NiCuAl–LTHs, prepared with various initial Ni:Cu molar ratios.
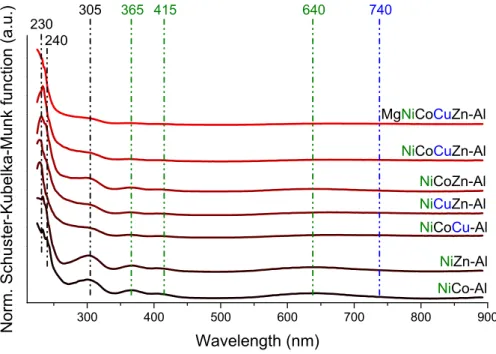
300 400 500 600 700 800 900
Norm. Schu ster-Kube lka-Munk function (a.u.)
NiCuZn-Al
Wavelength (nm)
NiCoCuZn-Al NiCoZn-Al NiCoCu-Al 240
640 415
230 305 365
MgNiCoCuZn-Al
NiZn-Al NiCo-Al 740
Figure S9. UV-Vis diffuse reflection spectra of nickel-containing LTHs/LMHs.
300 400 500 600 700 800 900
Norm. Schu ster-Kube lka-Munk function (a.u.)
255
MgCuZn-Al
Wavelength (nm)
MgCoCu-Al CoCuZn-Al CuZn-Al MgCu-Al
CoCu-Al Cu-Al 240
230 305 745
Figure S10. UV-Vis-DR spectra of copper-containing materials.
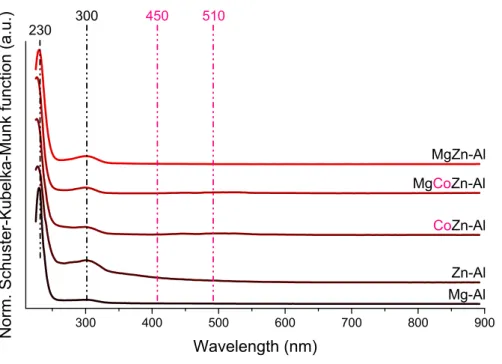
300 400 500 600 700 800 900
Norm. Schu ster-Kube lka-Munk function (a.u.)
Wavelength (nm)
Mg-Al Zn-Al CoZn-Al MgCoZn-Al MgZn-Al
230 300 450 510
Figure S11. UV-Vis diffuse reflection spectra of zinc-containing solids and the MgAl4–LDH.
300 400 500 600 700 800 900
Norm. Schuster-Kubelka-Munk function (a.u.)
*Unsuccesful LDH formation
Wavelength (nm)
Co-Al*
Co-Al MgCo-Al 255
240
230 305 450 510
625
Figure S12. UV-Vis-DR spectra of cobalt-containing samples; for the failed CoAl–LDH synthesis (marked with an asterisk), the reaction conditions were the following: 1:1 initial Co:Al molar ratio, 96 h stirring period, 90 °C, 6 h pre-milling.
Table S2. Optical properties of the prepared LDHs.
Samples Direct Band Gap (eV) Indirect Band Gap (eV)
6 h milled Al(OH)
35.30 5.11 NiAl
4–LDH 5.07 4.71 CuAl
4–LDH 4.79 3.93 CoAl
4–LDH 5.02 4.67 ZnAl
4–LDH 4.97 4.53 MgAl
4–LDH 5.17 4.89
Figure S13. SEM images of the ZnAl4– (A), MgAl4– (B) and CoAl4–LDH (C) prepared with nitrate interlamellar anions.
10 20 30 40 50 60 70 80
(511) (400)
(311) (440)
from NiCuAl8-LDH from ZnAl4-LDH
2 θ ( ° )
Inte nsity (a.u. )
from milled Al(OH)3 from MgAl4-LDH from CoAl4-LDH from NiAl4-LDH from CuAl4-LDH from NiCoAl8-LDH (220)
Figure S14. Powder X-ray diffraction patterns of the spent catalysts after long-term carbon monoxide oxidation at a 700 °C reaction temperature.
A B C
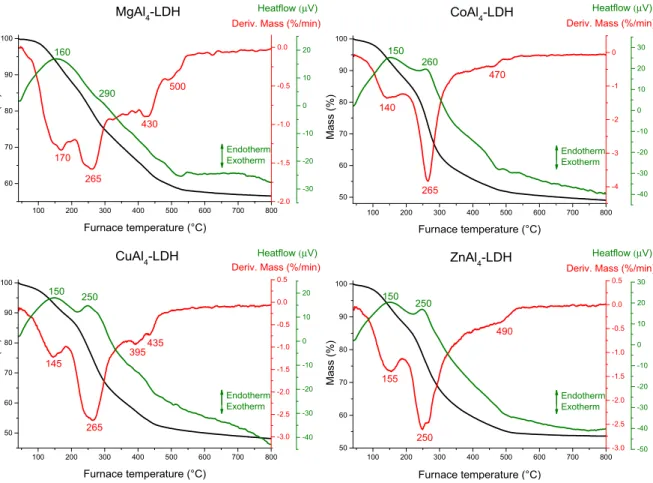
100 200 300 400 500 600 700 800 60
70 80 90 100
290
Mass (%)
Furnace temperature (°C)
Deriv. Mass (%/min) Heatflow (μV)
MgAl4-LDH
170 265
430 500 160
-2.0 -1.5 -1.0 -0.5 0.0
Endotherm Exotherm
-30 -20 -10 0 10 20
100 200 300 400 500 600 700 800
50 60 70 80 90 100
260 150
140
470
265
Furnace temperature (°C)
Heatflow (μV) Deriv. Mass (%/min)
CoAl4-LDH
Mass (%)
Endotherm Exotherm
-4 -3 -2 -1 0
-40 -30 -20 -10 0 10 20 30
100 200 300 400 500 600 700 800
50 60 70 80 90 100
Heatflow (μV) Deriv. Mass (%/min)
CuAl4-LDH
Furnace temperature (°C)
Mass (%)
Endotherm Exotherm
-3.0 -2.5 -2.0 -1.5 -1.0 -0.5 0.0 0.5
-40 -30 -20 -10 0 10 20
395 145
150 250
265
435
100 200 300 400 500 600 700 800
50 60 70 80 90 100
ZnAl4-LDH Heatflow (μV)
Deriv. Mass (%/min)
Mass (%)
Furnace temperature (°C)
Endotherm Exotherm 150 250
490
250 155
-3.0 -2.5 -2.0 -1.5 -1.0 -0.5 0.0 0.5
-50 -40 -30 -20 -10 0 10 20 30
Figure S15. Thermogravimetric, derivative thermogravimetric and differential thermal analysis curves of the magnesium-, cobalt-, copper- and zinc-containing LDHs prepared with nitrate interlamellar anions.
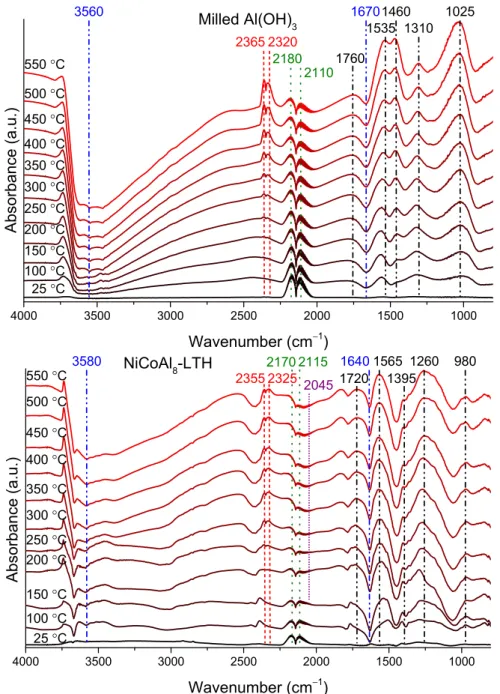
4000 3500 3000 2500 2000 1500 1000 2320
2365 21802110
1535 1310
500 °C 450 °C 400 °C 350 °C 300 °C 250 °C 200 °C 150 °C 100 °C 25 °C
Milled Al(OH)3
Absorbance (a.u.)
Wavenumber (cm−1) 550 °C
3560 1460 1025
1760 1670
4000 3500 3000 2500 2000 1500 1000
1565 1395
Absorbance (a.u.)
Wavenumber (cm−1) NiCoAl8-LTH
550 °C 500 °C 450 °C 400 °C 350 °C 300 °C 250 °C 200 °C 150 °C 100 °C 25 °C
2325 2045 2355
21702115
3580 1260 980
1720 1640
Figure S16. DRIFT spectra of the milled Al(OH)3 and NiCoAl8–LTH, heated up to 550 °C in the presence of a carbon monoxide–helium flow.