ContentslistsavailableatSciVerseScienceDirect
Neuroscience Letters
j o ur na l ho me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / n e u l e t
Effect of PACAP on MAP kinases, Akt and cytokine expressions in rat retinal hypoperfusion
Aliz Szabo
a, Bese Danyadi
b, Eszter Bognar
a, Krisztina Szabadfi
c, Eszter Fabian
b, Peter Kiss
b, Laszlo Mester
a, Sridharan Manavalan
b,d, Tamas Atlasz
e, Robert Gabriel
c, Gabor Toth
f, Andrea Tamas
b, Dora Reglodi
b,∗,1, Krisztina Kovacs
a,1aDepartmentofBiochemistryandMedicalChemistry,UniversityofPecs,Pecs,Hungary
bDepartmentofAnatomy,PTE-MTA“Lendulet”PACAPResearchTeam,UniversityofPecs,Pecs,Hungary
cDepartmentofExperimentalZoologyandNeurobiology,UniversityofPecs,Pecs,Hungary
dDepartmentofBasicSciences,CollegeofProfessionalStudies,NationalUniversityofHealthSciences,St.Petersburg,FL,USA
eDepartmentofSportbiology,UniversityofPecs,Pecs,Hungary
fDepartmentofMedicalChemistry,UniversityofSzeged,Szeged,Hungary
h i g h l i g h t s
EffectsofintravitrealPACAPwastestedinhypoperfusion-inducedretinalinjury.
PACAPattenuatesinflammatorycytokineresponseuponischemiaintheretina.
PACAPinducesERK1/2andAktactivationinischemicretina.
PACAPinhibitsJNKandp38MAPKexpression.
a r t i c l e i n f o
Articlehistory:
Received5May2012
Receivedinrevisedform15June2012 Accepted17June2012
Keywords:
Retinalischemia PACAP Cytokinearray ERK JNK p38MAPK Akt
a b s t r a c t
Pituitaryadenylatecyclaseactivatingpolypeptide(PACAP)isknownforitspotentneuroprotectiveeffects, includingtheretinoprotectiveactionsinseveraltypesofretinalinjuries.Wehaveshownearlierthat PACAPtreatmentcausesactivationofprotectivepathwaysandinhibitionofpro-apoptoticsignalingin excitotoxicretinallesions.Theaimofthepresentstudywastogaininsightintotheinvivoprotec- tivemechanismofPACAPinretinalhypoperfusioninjuryinducedbybilateralcommoncarotidartery occlusion(BCCAO).RatsunderwentBCCAOandreceivedintravitrealPACAP(PACAP38)treatment.We investigatedtheactivationleveloftheprotectiveAktpathwayaswellasthedifferentmitogenactivated proteinkinases(MAPKs)byWesternblotanalysisandtheexpressionofcytokinesusingacytokinearray kit.WefoundthatPACAPtreatmentalonedidnotinfluencethephosphorylationofAktortheMAPKs, butdecreasedthehypoperfusion-inducedactivationofbothp38MAPKandJNKandincreasedtheacti- vationoftheprotectiveAktandERK1/2inhypoperfusedretinas.Thecytokineprofilewasdramatically changedafterBCCAO,withmostcytokinesandchemokinesshowinganincrease,whichwasattenuated byPACAP(suchasCINC,CNTF,fractalkine,sICAM,IL-1,LIX,Selectin,MIP-1,RANTESandTIMP-1).Inaddi- tion,PACAPincreasedtheexpressionofVEGFandthymuschemokine.Thepresentresultsprovidefurther insightintotheneuroprotectivemechanisminducedbyPACAPinischemicretinalinjuries,showingthat PACAPameliorateshypoperfusioninjuryinvolvingAkt,MAPKpathwaysandanti-inflammatoryactions.
© 2012 Elsevier Ireland Ltd. All rights reserved.
∗Correspondingauthorat:DepartmentofAnatomy,UniversityofPecs,7624Pecs, Szigetiu12,Hungary.Tel.:+367253600135398;fax:+367236393.
E-mailaddress:dora.reglodi@aok.pte.hu(D.Reglodi).
1 Theseauthorscontributedequallytothepresentwork.
1. Introduction
Pituitaryadenylatecyclaseactivatingpolypeptide(PACAP)isa neuropeptidewithmiscellaneousactionsinthenervoussystem andperipheralorgans[38,41,49].TheeffectsofPACAPintheeye arealsodiverse.Actionsonthepupillaryreflex,tearsecretionand retinohypothalamiccircadiansystem,aswellastrophiceffectsdur- ingretinaldevelopmenthavebeendescribed[7,17,20,24].Oneof thewell-studiedeffectsofPACAPintheeyeisitsretinoprotection 0304-3940/$–seefrontmatter© 2012 Elsevier Ireland Ltd. All rights reserved.
http://dx.doi.org/10.1016/j.neulet.2012.06.044
94 A.Szaboetal./NeuroscienceLetters523 (2012) 93–98
action [4]. PACAP protects against various harmful stimuli in theretina,suchasmonosodiumglutamate-,NMDA-orkainate- inducedexcitotoxicinjury,UVlight-inducedretinaldegeneration anddiabeticretinopathy[3,5,6,16,28,40,43,44].
Retinalhypoperfusionorischemiaactivatesinflammatoryreac- tions and induces apoptotic pathways that eventually lead to degenerationandvisualimpairment[33].Ischemiaisassociated withseveralretinalvasculardiseasesincludingdiabeticretinopa- thy, retinal artery occlusion and carotid artery stenosis [14].
Bilateralcommoncarotidarteryocclusion (BCCAO)causesmild cerebralhypoperfusion[18]and,dependingonratstrain,itleadsto retinaldegeneration.Wehavepreviouslystudieddifferentneuro- protectivestrategiesusingthisratmodel[42].Wehavealsoshown thatboth PACAPandVIP areretinoprotectiveinchronicretinal hypoperfusion[2,45].
The neuroprotective effects of PACAP seem to be mediated byinfluencing signaling pathwaysatdifferent levels.The main effectsinvolvedaretheanti-apoptotic,anti-inflammatoryandanti- oxidanteffects.AsafirststepininvestigatingthePACAP-induced retinoprotectivemechanism,wehavestudiedsignalingpathways in glutamate-induced excitotoxic degeneration.We have found thatPACAPinhibitspro-apoptoticpathways, whileitstimulates anti-apoptoticintracellular processes[35,36].However,itis not known,whichmechanismsareinvolvedintheprotectiveeffects ofPACAPinhypoperfusion-inducedinjury.Mitogenactivatedpro- teinkinases(MAPKs)andAktsignalingplayanimportantrolein theneuroprotectiveeffectsofPACAP.Also,PACAPisknowntoinflu- encelevelsofcytokinesandchemokinesafterinjuries,whichare importantmediatorsinischemicprocesses[8,11,13].Therefore,we investigatedtheactivationlevelofMAPKs,Aktandcytokinesinrat retinaexposedtoBCCAO.
2. Materialsandmethods
MaleWistarratsweighing250–300gweresubjectedtoperma- nentBCCAO.Experimentalprocedureswereperformedfollowing institutionalethicalguidelines(BA02/2000-24/2011).Underisoflu- raneanesthesia,bilateralcommoncarotidarterieswereexposed and ligated with a 3-0 filament. Immediately following the BCCAOoperation,PACAP38(100nmol/3lsaline)wasintravitre- allyinjectedintotherighteyewithaHamiltonsyringe.Theleft
eyereceivedthesame volumeofvehicletreatment.Agroupof animalsunderwentanesthesiaandallstepsofthesurgicalproce- dure,exceptligationofthecarotidarteries.Theseanimalsserved assham-operatedsaline-orPACAP-treatedanimals.
ForWesternblotexperiments,retinasfrom33 animalswere removedafter5,30 and 60minin ordertoinvestigatethesig- naling pathways activated within the first few minutes after an ischemic insult (n=6 animal/group in the ischemic groups and 5 animals/group in the sham-operated groups). Samples were processed for Western blot analysis as described earlier [35,36].Membraneswereprobedovernightat4◦Cwiththepri- maryantibodies:phospho-specificanti-Akt-1Ser473(1:1000;R&D Systems, Hungary), phospho-specific anti-ERK1/2 Thr202/Tyr204, phospho-specific anti-SAPK/JNK Thr183/Tyr185, phospho-specific anti-p38MAPK(1:1000;CellSignalingTechnology,USA),andanti- glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000;
Millipore,Hungary).Membraneswerewashedsixtimesfor5min inTrisbufferedsaline(pH=7.5)containing0.2%Tweenpriorto additionofgoatanti-rabbitoranti-mousehorseradishperoxidase- conjugatedsecondary antibody (1:3000; BioRad, Hungary).The antibody–antigen complexes were visualized by means of enhancedchemiluminescence.Afterscanning,resultswerequan- tifiedbymeansofScionImageBeta4.2program.Allexperiments were performedat least fourtimes. Data are expressed asthe mean±SEM.Statisticalanalysiswasperformedbyanalysisofvari- anceandunpairedStudent’st-test.Differenceswithpvaluesbelow 0.05wereconsideredtobesignificant.
Forsemiquantitativecytokinearray,retinas(n=18from9ani- mals/group) in the ischemic and sham-operated groups) were removedafter24hofischemiaandhomogenizedinPBSwithpro- teaseinhibitors. Sampleswere pooled in three replicates(n=3 per replicate). Triton X-100 was added to a final concentra- tion of 1%. The samples were stored at −80◦C prior to use.
CytokinearrayfromtissuehomogenateswasperformedusingRat Cytokine ArrayPanel A Array kit from R&D Systems (Biomed- ica,Budapest,Hungary).Afterblockingthearraymembranesfor 1handadding thereconstitutedDetectionAntibodyCoctailfor another 1h at room temperature, the membranes were incu- batedwith1.5mloftissue homogenatesat2–8◦Covernighton arockingplatform.Afterwashingwithbuffer3timesandaddi- tion of horseradishperoxidase-conjugated Streptavidin to each
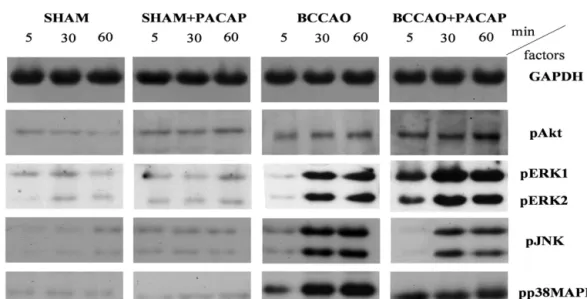
Fig.1. (A)RepresentativeWesternblotsshowingactivation(phosphorylation)ofAktandMAPkinasesinsham-operated,sham+PACAP-treated,ischemic(inducedbyBCCAO) andBCCAO+PACAP-treatedretinas.Samplesweretaken5,30and60minaftertreatments.GAPDHwasusedasinternalcontrol.(B)StatisticalanalysisofWesternblotresults.
Dataaregivenasmean±SEM.**p<0.01,***p<0.001vscontrol,##p<0.01;###p<0.001vsischemicretinas.
Fig.1. (A)(Continued).
membrane we exposed them to a chemiluminescent detection reagent(AmershamBiosciences,Hungary)thensideuptoanX-ray filmcassette[46].
3. Results
3.1. EffectofPACAPtreatmentonischemiainducedsignaling pathways
Insham-operatedgroups,nomarkeddifferencesinthephos- phorylationleveloftheexaminedfactorswerefounduponPACAP
treatment, only phosphorylation of Akt was slightly elevated byPACAPinsham-operatedretinas. Ischemiaitselfalsoslightly increasedthephosphorylationstateofAkt,butPACAPtreatment ledtoamarkedsignificantfurtherincreaseatalltime-pointsfol- lowingischemia(Fig.1AandB).ERK1/2phosphorylationwasclose tothedetectionlimitinshamoperatedsaline-treatedretinaswith littleeffectafterPACAPtreatment.Ischemiainducedastrongphos- phorylationofERK1/2comparedtosham-operation,andthiswas furtherincreasedbyPACAP.Wefoundtheactivationslightlymore intensiveafter30 than after60min,suggesting a fastresponse afteranischemicinsult.InPACAP-treatedretinas,thesignificant
96 A.Szaboetal./NeuroscienceLetters523 (2012) 93–98
Fig.2.Representativecytokinearraytodetecttheprotectiveeffectsof100nM PACAPinretinaexposedtoischemicinjurybybilateralcommoncarotidarteryocclu- sion(BCCAO).Thepanelsshowarraysfromsham-operatedcontrol(upperpanel), BCCAOischemic(middlepanel)andBCCAO+PACAP-treated(lowerpanel)retinas.
Thetableindicatestheexaminedfactorsineachbox,highlightingwitharrowsthe changesobservedafterPACAPtreatment.
activationofERK1/2appearedalready5minaftertheinductionof ischemia,furthersupportingthisrapidreaction.
Inthesham-operatedeyes,wecouldnotdetectmarkedphos- phorylationofSAPK/JNK.Ischemiainducedtheactivationofthis kinaseasitwasrevealedby itsslightlyincreasedphosphoryla- tionafter5minandstrongelevationafter30and60min.However, administrationofPACAPcausedsignificantdecreaseinthephos- phorylationstateofSAPK/JNKatalltime-pointsafterischemia.A phosphorylationpatternquitesimilartothatofJNKwasdetectedin caseofp38MAPK.Nophosphorylationofthiskinasewasobserved insham-operatedanimalsandwe foundnodifferencebetween salineandPACAP-treatedretinas.Ischemiainducedastrongphos- phorylationofp38MAPKthatwasattenuatedbyPACAPtreatment 30and60minafterBCCAO(Fig.1AandB).
3.2. Cytokinearray
The expression of several cytokines was increased after ischemia,includingchemoattractantproteins,thechemokinesof theCINC(cytokine-inducedneutrophilchemoattractant)andMIP (macrophageinflammatoryprotein)families:CINC-1,CINC-2␣/, CINC-3andMIP-1␣,MIP-3␣.Severalinterleukinswerealsoacti- vated:IL-1␣,IL-1andIL-1ra,whileotherinterleukinsremained unchanged,suchasIL-2,IL-3,IL-4,IL-6andIL-10.Theactivationof theciliaryneurotrophicfactor(CNTF),fractalkine,sICAM-1(inter- cellularadhesionmolecule),LIX(lipopolysaccharideinducedCXC chemokine),L-selectin,RANTES(regulatedonactivation,normalt cellexpressedandsecreted),thymuschemokineandTIMP-1(tis- sueinhibitor ofmetalloproteinase)wasincreasedin theretinas thatunderwentBCCAOcomparedwiththecontrolgroups(Fig.2).
PACAPtreatmentattenuatedactivationofalltheabove-mentioned cytokines,asmeasuredbyacytokinearraysystem.Exceptionswere VEGFandthymuschemokine,theactivationofwhichwasfurther increaseduponPACAPtreatment.Theexpressionofothercytokines analyzedbythearraydidnotshowanymarkedchanges(Fig.2).
4. Discussion
Thepresentstudyshowedthat PACAPcounteractedchanges in the phosphorylation of MAPKs and levelsof cytokinesafter ischemicinjuryintheratretina.PACAPhasbeenshowntoinflu- encecellsurvivalandinflammatorypathwaysinseveralischemic injuries,includingischemiaofthebrainanddifferentperipheral organs[19,34,38,46].TheMAPKfamilyseemstoplayanimportant rolein thePACAP-inducedcellularprotectioninseveralmodels [31,47].Incerebellargranulecells,thebalancebetweenERKand JNKMAPKsiscriticalforsurvival,andPACAPinducesERKphospho- rylationwhileitinhibitsJNKphosphorylation,therebyattenuating ceramide-inducedcelldeath[48].Ourpresentresultsarealsoin accordancewithpreviousobservationsinglutamate-inducedreti- nalinjury, where PACAP had opposingeffects on ERKand JNK phosphorylation[35,36].p38MAPK,thethirdmainmemberofthe MAPKfamily,istypicallyconsideredtobeapro-apoptoticMAPK, thephosphorylationofwhichisinducedbymanycellularinjuries.
PACAPhasbeenshowntoinhibitthephosphorylationofp38MAPK inseveralstudies[35,36].Aktsignalingisgenerallyassociatedwith increasedcellularprotection,includingretinalischemia[14].Akt phosphorylationwasalsoinducedbyPACAPinthepresentstudy, similarlytoourearlierobservationsinexcitotoxicretinalinjury and otherdescriptionsonPACAP-mediated neuronal protection [31,37].
Theanti-inflammatoryeffects of PACAPofferanother mech- anismagainstischemicinjury intheretina.Theinvolvementof PACAPininflammatoryreactionshaslongbeenknown,butthe exacteffectsofPACAPininflammationdependoncelltype,tissue, age,pathological conditionsandseveralotherfactors.However, PACAPisusuallyconsideredtobeananti-inflammatorypeptide [12].Inthepresentstudy,wealsofoundthatadditionofPACAP counteracted changes in several cytokines and chemokines in ischemicretinalinjury.Theoverallpictureofcytokineexpression showsthatischemiadramaticallyinducedseveralcytokinesand lessactivationwasfoundinmostcytokinesafterPACAPtreatment, exceptforVEGFandthymuschemokine,whichwereelevatedafter PACAPtreatment.Thestrongactivationofseveralcytokinesfound inourstudyisinagreementwithotherreportsshowinginduced cytokineexpressionafterretinalischemia[23,50].Thealterations inducedbyPACAPstronglysuggestananti-inflammatoryroleof thepeptideinischemicretinalinjury,keepinginmindthatthis wascheckedatonetime-point,namely24haftertheinductionof ischemia.
ThefamilyofCINC(CINC1-3)isapro-inflammatorychemokine family,involvedinseveralinflammatoryprocesses,usuallyduring theacuteinflammatoryresponsephase[21].Compromiseinblood flowhasbeenshowntoinduceexpressionofthecytokineCINCalso intheretina[52]andsubstancesdecreasingCINClevelsareusually associatedwithdecreasedtissuedamage[1,27].Thisis thefirst reportshowingthatPACAPattenuateselevatedCINClevelsafter ischemiainduction in theretina.Fractalkine, anotherchemoat- tractant,wasalso reducedafterPACAP treatment.The effectof PACAPoninterleukinswasvariable.Severalinterleukinswerenot affected,whiletheincreasedexpressionofotherswasattenuated byPACAP. Taken together,PACAPdecreased levelsof IL-1 sub- classes,thereduction ofwhichhasbeenshowntobebeneficial inretinalischemicinjuries[15,25].
RANTESalsoparticipatesinretinalinflammatoryreactions.The chemokineRANTEShasthepotentialtoinfluencethemigration ofmemoryTcells and monocytesacrosstheblood-retinalbar- rierduringinflammatoryeyedisease[10].Hereweshowedthat PACAPwasabletodownregulatetheischemia-inducedupregu- lationofRANTESintheretina.EarlierstudiesfoundthatPACAP inducedsecretionofRANTES,forexampleinastrocytes[8]andin corticalneurons[39].Inthesestudies,releaseofRANTEShasbeen
showntobeassociatedwithincreasedneuroprotection.Theroleof RANTESinretinalischemia,however,seemstobetheincreasein inflammatoryreactions,againstwhichPACAPmayprotectaccord- ingtoourfindings.Thisis,therefore,inaccordancewithfindings ofothersshowingthatPACAPandVIPinhibitchemokinereleasein microglialcells[11].
Severalother factors werefound tobe altered upon PACAP administration. Little is known about the effect of PACAP on these factors. Adhesion molecules have been demonstrated to be involved in several ophthalmic pathologies [26]. Similarly, theinvolvementofmatrixmetalloproteinasesinretinalischemic injuryhasbeenreportedbymanyauthors[30].MIP-1alphahas alsobeenfoundtobeelevatedafterretinalischemia[51].Inthe presentstudy,PACAPpreventedtheincreasedactivationofICAM, selectin,MIP-1alphaandTIMP-1.VEGF,ontheotherhand,was increasedafterPACAPtreatment.TheinducingeffectofPACAPon thelevelsofVEGFhasbeenreportedearlierinlungcancercells[32]
andinpituitaryfolliculostellatecells[22].VEGFisawell-studied angiogeneticfactorplayingaroleinseveralretinopathies,including retinopathyofprematurityanddiabeticretinopathy,butitalsohas aneuroprotectivefunction[53].Inretinalischemia,VEGFexpres- sionisaltereddependingonthetime,reperfusionandarterialor neuronallocalizationofVEGF[9,29].WhetherthisPACAP-induced increaseinVEGFhasaneuroprotectivefunction,aroleinreestab- lishingcirculationafterischemicretinainjury,oritplaysarolein inflammatoryprocesses,isnotknownatthemoment.Altogether, theexactquantificationofthechangesincytokineexpressionand activationisneededtobeperformedinfutureexperimentsfocus- ingonthecytokinesandchemokinesshowingthemostmarked changes.
Takentogether,thedramaticchangesinthecytokineprofileis counteractedbyPACAPtreatment.Thepresentresultsprovidefur- therinsightintotheneuroprotectivemechanisminducedbyPACAP inischemicinjuries,showingthatPACAPamelioratesischemicreti- nalinjuryinvolvingAkt,MAPKpathwaysandanti-inflammatory actions.
Acknowledgments
SupportbySROP4.1.2.B-10/2/KONV-20/0-0002,SROP-4.2.2/B- 10/1-2010-0029,OTKAK72592,104984,CNK78480,Momentum- ProgramoftheHungarianAcademyofSciences,BolyaiScholarship, Akira Arimura Foundation, National University of Health Sci- ences/Florida Campus Research Grant and Richter Gedeon CentenaryFoundationwasacknowledged.
References
[1]T.Asaga,M.Ueki,K.Chujo,S.Taie,JTE-607,aninflammatorycytokinesynthe- sisinhibitor,attenuatesischemia/reperfusion-inducedrenalinjurybyreducing neutrophilactivationinrats,JournalofBioscienceandBioengineering106 (2008)22–26.
[2]T.Atlasz,N.Babai,P.Kiss,D.Reglodi,A.Tamas,K.Szabadfi,G.Toth,O.Hegyi, A.Lubics,R.Gabriel,Pituitaryadenylatecyclaseactivatingpolypeptideispro- tectiveinbilateralcarotidocclusion-inducedretinallesioninrats,Generaland ComparativeEndocrinology153(2007)108–114.
[3]T.Atlasz,K.Szabadfi,P.Kiss,N.Babai,Z.Koszegi,A.Tamas,D.Reglodi,R.
Gabriel,PACAP-mediatedneuroprotectionofneurochemicallyidentifiedcell typesinMSG-inducedretinalregeneration,JournalofMolecularNeuroscience 36(2008)97–104.
[4]T.Atlasz,K.Szabadfi,P.Kiss,B.Racz,F.Gallyas,A.Tamas,V.Gaal,Zs.Marton, R.Gabriel,D.Reglodi,Pituitaryadenylatecyclaseactivatingpolypeptideinthe retina:focusontheretinoprotectiveeffects,AnnalsoftheNewYorkAcademy ofSciences1200(2010)128–139.
[5]T.Atlasz,K.Szabadfi,P.Kiss,A.Tamas,G.Toth,D.Reglodi,R.Gabriel,Evaluation oftheprotectiveeffectsofPACAPwithcell-specificmarkersinischemia- inducedretinaldegeneration,BrainResearchBulletin81(2010)497–504.
[6]T.Atlasz,K.Szabadfi,P.Kiss,Zs.Marton,M.Griecs,L.Hamza,V.Gaal,Zs.Biro,A.
Tamas,G.Hild,M.Nyitrai,G.Toth,D.Reglodi,R.Gabriel,EffectsofPACAPinUV- Aradiation-inducedretinaldegenerationmodelsinrats,JournalofMolecular Neuroscience43(2011)51–57.
[7]J.C.Borba,I.P.Henze,M.S.Silveira,R.C.Kubrusly,P.F.Gardino,M.C.deMello, J.N.Hokoc¸,F.G.deMello,Pituitaryadenylatecyclase-activatingpolypeptide (PACAP)canactasdeterminantofthetyrosinehydroxylasephenotypeof dopaminergiccellsduringretinadevelopment,DevelopmentalBrainResearch 156(2005)193–201.
[8]D.E.Brenneman,J.M.Hauser,C.Spong,T.M.Phillips,Chemokinereleaseisasso- ciatedwiththeprotectiveactionofPACAP-38againstHIVenvelopeprotein neurotoxicity,Neuropeptides36(2002)271–280.
[9]D.Cervia,E.Catalani,M.DalMonte,G.Casini,Vascularendothelialgrowth factorintheischemicretinaanditsregulationbysomatostatin,Journalof Neurochemistry120(2012)818–829.
[10] I.J.Crane,M.C.Kuppner,S.McKillop-Smith,R.M.Knott,J.V.Forrester,Cytokine regulationofRANTESproductionbyhumanretinalpigmentepithelialcells, CellularImmunology184(1998)37–44.
[11]M.Delgado,G.M.Jonakait,D.Ganea,Vasoactiveintestinalpeptideandpitu- itaryadenylatecyclase-activatingpolypeptideinhibitchemokineproduction inactivatedmicroglia,Glia39(2002)148–161.
[12] M.Delgado,C.Abad,C.Martinez,M.G.Juarranz,J.Leceta,D.Ganea,R.P.Gomariz, PACAPinimmunityandinflammation,AnnalsoftheNewYorkAcademyof Sciences992(2003)141–157,Review.
[13] M.Delgado,J.Leceta,D.Ganea,Vasoactiveintestinalpeptideandpituitary adenylatecyclase-activatingpolypeptideinhibittheproductionofinflamma- torymediatorsbyactivatedmicroglia,JournalofLeukocyteBiology73(2003) 155–164.
[14]J.C.Dreixler,J.W.Hemmert,S.K.Shenoy,Y.Shen,H.T.Lee,A.R.Shaikh,D.M.
Rosenbaum,S.Roth,TheroleofAkt/proteinkinaseBsubtypesinretinal ischemicpreconditioning,ExperimentalEyeResearch88(2009)512–521.
[15]G. Dvoriantchikova, D.J. Barakat, E. Hernandez, V.I. Shestopalov, D.
Ivanov, Liposome-delivered ATP effectively protects the retina against ischemia–reperfusioninjury,MolecularVision16(2010)2882–2890.
[16]K.Endo,T.Nakamachi,T.Seki,N.Kagami,Y.Wada,K.Nakamura,K.Kishimoto, M.Hori,D.Tsuchikawa,N.Shintani,H.Hashimoto,A.Baba,R.Koide,S.Shioda, NeuroprotectiveeffectofPACAPagainstNMDA-inducedretinaldamageinthe mouse,JournalofMolecularNeuroscience43(2011)22–29.
[17]A.Engelund,J.Fahrenkrug,A.Harrison,H.Luuk,J.Hannibal,Alteredpupillary lightreflexinPACAPreceptor1-deficientmice,BrainResearch1453(2012) 17–25.
[18]E.Farkas,P.G.Luiten,F.Bari,Permanent,bilateralcommoncarotidartery occlusionintherat:amodelforchroniccerebralhypoperfusion-relatedneu- rodegenerativediseases,BrainResearchReviews54(2007)162–180.
[19]A.Ferencz,P.Kiss,Gy.Weber,Zs.Helyes,N.Shintani,A.Baba,D.Reglodi,Com- parisonofintestinalwarmischemicinjuryinPACAPknock-outandwild-type mice,JournalofMolecularNeuroscience42(2010)435–442.
[20] V.Gaal,L.Mark,P.Kiss,I.Kustos,A.Tamas,B.Kocsis,A.Lubics,V.Nemeth,A.
Nemeth,L.Lujber,J.Pytel,G.Toth,D.Reglodi,Investigationoftheeffectsof PACAPonthecompositionoftearandendolymphproteins,JournalofMolec- ularNeuroscience36(2008)321–329.
[21] A.Ghaly,D.R.Marsh,Ischaemia–reperfusionmodulatesinflammationand fibrosisofskeletalmuscleaftercontusioninjury,InternationalJournalofExper- imentalPathology91(2010)244–255.
[22]J.Gloddek,U.Pagotto,M.PaezPereda,E.Arzt,G.K.Stalla,U.Renner,Pituitary adenylatecyclase-activatingpolypeptide,interleukin-6andglucocorticoids regulatethereleaseofvascularendothelialgrowthfactorinpituitaryfollicu- lostellatecells,JournalofEndocrinology160(1999)483–490.
[23]C.Gustavsson,C.D.Agardh,P.Hagert,E.Agardh,Inflammatorymarkersinnon- diabeticanddiabeticratretinasexposedtoischemiafollowedbyreperfusion, Retina28(2008)645–652.
[24]J.Hannibal,J.Fahrenkrug,TargetareasinnervatedbyPACAP-immunoreactive retinalganglioncells,CellandTissueResearch316(2004)99–113.
[25]Q.Ji,L.Zhang,R.Lv,H.Jia,J.Xu,Pentoxifyllinedecreasesup-regulatednuclear factorkappaBactivationandcytokineproductionintheratretinafollowing transientischemia,Ophthalmologica220(2006)217–224.
[26]J.B.Jonas,Y.Tao,M.Neumaier,P.Findeisen,Monocytechemoattractantprotein 1,intercellularadhesionmolecule1,andvascularcelladhesionmolecule1in exudativeage-relatedmaculardegeneration,ArchivesofOphthalmology128 (2010)1281–1286.
[27]S.P.Junnarkar,N.Tapuria,A.Mani,S.Dijk,B.Fuller,A.M.Seifalian,B.R.Davidson, Attenuationofwarmischemia–reperfusioninjuryintheliverbybucillamine throughdecreasedneutrophilactivationandBax/Bcl-2modulation,Journalof GastroenterologyandHepatology25(2010)1891–1899.
[28]P.Kiss,T.Atlasz,K.Szabadfi,G.Horvath,M.Griecs,J.Farkas,A.Matkovits,G.
Toth,A.Lubics,A.Tamas,R.Gabriel,D.Reglodi,ComparisonbetweenPACAP- andenrichedenvironment-inducedretinalprotectioninMSG-treatednewborn rats,NeuroscienceLetters487(2011)400–405.
[29]F.Lamoke,M.Labazi,A.Montemari,G.Parisi,M.Varano,M.Bartoli,Trans- chalconepreventsVEGF expressionandretinalneovascularization inthe ischemicretina,ExperimentalEyeResearch93(2011)350–354.
[30]N.Mathalone,N.Lahat,M.A.Rahat,K.Bahar-Shany,Y.Oron,O.Geyer,The involvementofmatrixmetalloproteinases2and9inratretinalischemia,Grae- fesArchiveforClinicalandExperimentalOphthalmology245(2007)725–
732.
[31]V.May,E.Lutz,C.MacKenzie,K.C.Schutz,K.Dozark,K.M.Braas,Pituitary adenylatecyclase-activatingpolypeptide(PACAP)/PAC1HOP1receptoracti- vationcoordinatesmultipleneurotrophicsignalingpathways:Aktactivation throughphosphatidylinositol3-kinasegammaandvesicleendocytosisforneu- ronalsurvival,JournalofBiologicalChemistry285(2010)9749–9761.
98 A.Szaboetal./NeuroscienceLetters523 (2012) 93–98
[32]T.W.Moody,J.Leyton,M.Casibang,J.Pisegna,R.T.Jensen,PACAP-27tyrosine phosphorylatesmitogenactivatedproteinkinaseandincreasesVEGFmRNAs inhumanlungcancercells,RegulatoryPeptides109(2002)135–140.
[33]I.Nenekidis,C.J.Pournaras,E.Tsironi,N.Tsilimingas,Visionimpairmentduring cardiacsurgeryandextracorporealcirculation:currentunderstandingandthe needforfurtherinvestigation,ActaOphthalmologica90(2012)168–172.
[34]H.Ohtaki,T.Nakamachi,K.Dohi,S.Shioda,RoleofPACAPinischemicneural death,JournalofMolecularNeuroscience36(2008)16–25.
[35]B.Racz,F.GallyasJr.,P.Kiss,G.Toth,O.Hegyi,B.Gasz,B.Borsiczky,A.Ferencz, E.Roth,A.Tamas,I.Lengvari,A.Lubics,D.Reglodi,Theneuroprotectiveeffects ofPACAPinmonosodiumglutamate-inducedretinallesioninvolvesinhibition ofproapoptoticsignalingpathways,RegulatoryPeptides137(2006)20–26.
[36]B.Racz,A.Tamas,P.Kiss,G.Toth,B.Gasz,B.Borsiczky,A.Ferencz,F.Gallyas Jr.,E.Roth,D.Reglodi,InvolvementofERKandCREBsignalingpathwaysinthe protectiveeffectofPACAPinmonosodiumglutamate-inducedretinallesion, AnnalsoftheNewYorkAcademyofSciences1070(2006)507–511.
[37]B.Racz,B.Gasz,F.GallyasJr.,P.Kiss,A.Tamas,Z.Szanto,A.Lubics,I.Leng- vari,G.Toth,O.Hegyi,E.Roth,D.Reglodi,PKA-Bad-14-3-3Akt-Bad-14-3-3 signalingpathwaysareinvolvedintheprotectiveeffectsofPACAPagainst ischemia/reperfusion-inducedcardiomyocyteapoptosis,RegulatoryPeptides 145(2008)105–115.
[38]D.Reglodi,P.Kiss,G.Horvath,A.Lubics,E.Laszlo,A.Tamas,B.Racz,P.Sza- kaly,Effectsofpituitaryadenylatecyclaseactivatingpolypeptideintheurinary system,withspecialemphasisonitsprotectiveeffectsinthekidney,Neuropep- tides46(2012)61–70.
[39]A.Sanchez,D.Tripathy,P.Grammas,RANTESreleasecontributestothepro- tectiveactionofPACAP38againstsodiumnitroprussideincorticalneurons, Neuropeptides43(2009)315–320.
[40]T.Seki,M.Nakatani,C.Taki,Y.Shinohara,M.Ozawa,S.Nishimura,H.Ito,S.
Shioda,NeuroprotectiveeffectofPACAPagainstkainicacid-inducedneurotox- icityinratretina,AnnalsoftheNewYorkAcademyofSciences1070(2006) 531–534.
[41]A.Somogyvari-Vigh,D.Reglodi,Pituitaryadenylatecyclaseactivatingpolypep- tide:apotentialneuroprotectivepeptide,CurrentPharmaceuticalDesign10 (2004)2861–2889.
[42] K.Szabadfi,L.Mester,D.Reglodi,P.Kiss,N.Babai,B.Racz,K.Kovacs,A.
Szabo,A.Tamas,R.Gabriel,T.Atlasz,Novelneuroprotectivestrategiesin ischemicretinallesions,InternationalJournalofMolecularSciences11(2010) 544–561.
[43] K.Szabadfi,T.Atlasz,P.Kiss,B.Danyadi,A.Tamas,Zs.Helyes,H.Hashimoto, N. Shintani, A. Baba, G. Toth, R. Gabriel, D. Reglodi, Mice deficient in
pituitaryadenylatecyclaseactivatingpolypeptide(PACAP)aremoresuscepti- bletoretinalischemicinjuryinvivo,NeurotoxicityResearch21(2012)41–48.
[44]K.Szabadfi,T.Atlasz,P.Kiss,D.Reglodi,A.Szabo,K.Kovacs,B.Szalontai,G.
SetaloJr.,E.Banki,K.Csanaky,A.Tamas,R.Gabriel,Protectiveeffectsoftheneu- ropeptidePACAPindiabeticretinopathy,CellandTissueResearch348(2012) 37–46.
[45]K.Szabadfi,B.Danyadi,P.Kiss,A.Tamas,E.Fabian,R.Gabriel,D.Reglodi, Protectiveeffectsofvasoactiveintestinalpeptide(VIP)inischemicretinal degeneration,JournalofMolecularNeuroscience,PMID:22544514,inpress.
[46] P.Szakaly,E.Laszlo,K.Kovacs,B.Racz,G.Horvath,A.Ferencz,A.Lubics,P.Kiss, A.Tamas,R.Brubel,B.Opper,A.Baba,H.Hashimoto,J.Farkas,A.Matkovits,T.
Magyarlaki,Z.Helyes,D.Reglodi,Micedeficientinpituitaryadenylatecyclase activatingpolypeptide(PACAP)showincreasedsusceptibilitytoinvivorenal ischemia/reperfusioninjury,Neuropeptides45(2011)113–121.
[47]D.Vaudry,T.F.Pamantung,M.Basille,C.Rousselle,A.Fournier,H.Vaudry, J.C. Beauvillain,B.J. Gonzalez, PACAPprotectscerebellar granule neurons againstoxidativestress-inducedapoptosis,EuropeanJournalofNeuroscience 15(2002)1451–1460.
[48]D.Vaudry,A.Falluel-Morel,M.Basille,T.F.Pamantung,M.Fontaine,A.Fournier, H.Vaudry,B.J.Gonzalez,Pituitaryadenylatecyclase-activatingpolypeptide preventsC2-ceramide-inducedapoptosisofcerebellargranulecells,Journal ofNeuroscienceResearch72(2003)303–316.
[49]D.Vaudry,A.Falluel-Morel,A.Bourgault,M.Basille,D.Burel,O.Wurtz,A.
Fournier,B.K.Chow,H.Hashimoto,L.Galas,H.Vaudry,Pituitaryadenylate cyclaseactivatingpolypeptideanditsreceptors:20yearsafterthediscovery, PharmacologicalReviews61(2009)283–357.
[50]S.Yoneda,H.Tanihara,N.Kido,Y.Honda,W.Goto,H.Hara,N.Miyawaki, Interleukin-1betamediatesischemicinjuryintheratretina,ExperimentalEye Research73(2001)661–667.
[51]S.Yoshida,A.Yoshida,T.Ishibashi,S.G.Elner,V.M.Elner,RoleofMCP-1and MIP-1alphainretinalneovascularizationduringpostischemicinflammationin amousemodelofretinalneovascularization,JournalofLeukocyteBiology73 (2003)137–144.
[52]A.Yoshida,S.Yoshida,Y.Hata,A.K.Khalil,T.Ishibashi,H.Inomata,Therole ofNF-kappaBinretinalneovascularizationintherat.Possibleinvolvement of cytokine-inducedneutrophilchemoattractant(CINC),amemberofthe interleukin-8family,JournalofHistochemistryandCytochemistry46(1998) 29–36.
[53]X.R.Zheng,S.S.Zhang,F.Yin,J.L.Tang,Y.J.Yang,X.Wang,L.Zhong,Neuropro- tectionofVEGF-expressionneuralstemcellsinneonatalcerebralpalsyrats, BehaviouralBrainResearch230(2012)108–115.