ContentslistsavailableatSciVerseScienceDirect
Peptides
jo u r n al hom e p ag e :w w w . e l s e v i e r . c o m / l o c a t e / p e p t i d e s
Role of Pituitary Adenylate-Cyclase Activating Polypeptide and Tac1 gene derived tachykinins in sensory, motor and vascular functions under normal and
neuropathic conditions
Bálint Botz
a,c, András Imreh
a, Katalin Sándor
a, Krisztián Elekes
a, János Szolcsányi
a, Dóra Regl ˝odi
b, John P. Quinn
d, James Stewart
e, Andreas Zimmer
f, Hitoshi Hashimoto
g, Zsuzsanna Helyes
a,c,∗aDepartmentofPharmacologyandPharmacotherapy,UniversityofPécs,H-7624Pécs,Szigetiu.12,Hungary
bDepartmentofAnatomy,PTE-MPTE-MTALenduletPACAPResearchTeam,FacultyofMedicine,UniversityofPécs,H-7624Pécs,Szigetiu.12,Hungary
cJánosSzentágothaiResearchCenter,UniversityofPécs,H-7624Pécs,Ifjúságu.20,Hungary
dDepartmentofMolecularandClinicalPharmacology,InstituteofTranslationalMedicine,LiverpoolUniversity,Liverpool,UK
eSchoolofInfectionandHostDefense,UniversityofLiverpool,UK
fLaboratoryofMolecularNeurobiology,DepartmentofPsychiatry,UniversityofBonn,Bonn,Germany
gGraduateSchoolofPharmaceuticalSciences,OsakaUniversity,Japan
a r t i c l e i n f o
Articlehistory:
Received21January2013
Receivedinrevisedform4March2013 Accepted4March2013
Availableonline13March2013
Keywords:
Traumaticmononeuropathy Sciaticnerveligation Mechanicalhyperalgesia Neurogenicvasodilation SubstanceP
TachykininNK1receptor
a b s t r a c t
PituitaryAdenylate-CyclaseActivatingPolypeptide(PACAP)andTac1gene-encodedtachykinins(sub- stance P: SP, neurokinin A: NKA) are expressed in capsaicin-sensitive nerves, but their role in nociception,inflammationandvasoregulationisunclear.Therefore,weinvestigatedthefunctionofthese neuropeptidesandtheNK1tachykininreceptor(fromTacr1gene)inthepartialsciaticnerveligation- inducedtraumaticmononeuropathymodelusinggenedeficient(PACAP−/−,Tac1−/−,andTacr1−/−)mice.
Mechanonociceptivethresholdofthepawwasmeasuredwithdynamicplantaraesthesiometry,motor coordinationwithRota-RodandcutaneousmicrocirculationwithlaserDopplerimaging.Neurogenic vasodilationwasevokedbymustardoilstimulatingsensorynerves.Inwildtypemice30–40%mechani- calhyperalgesiadevelopedoneweekafternerveligation,whichwasnotalteredinTac1−/−andTacr1−/− mice,butwasabsentinPACAP−/−animals.MotorcoordinationofthePACAP−/−andTac1−/−groupswas significantlyworsebothbeforeandafternerveligationcomparedtotheirwildtypes,butitdidnotchange inTacr1−/−mice.BasalpostoperativemicrocirculationontheplantarskinofPACAP−/−micedidnotdif- ferfromthewildtypes,butwassignificantlylowerinTac1−/−andTacr1−/−ones.Incontrast,mustard oil-inducedneurogenicvasodilationwassignificantlysmallerinPACAP−/−mice,butnotinTacr1−/−and Tac1−/−animals.BothPACAPandSP/NKA,butnotNK1receptorsparticipateinnormalmotorcoordina- tion.Tachykininsmaintainbasalcutaneousmicrocirculation.PACAPisacrucialmediatorofneuropathic mechanicalhyperalgesiaandneurogenicvasodilation.Thereforeidentifyingitstargetanddeveloping selective,potentantagonists,mightopenpromisingnewperspectivesforthetreatmentofneuropathic painandvascularcomplications.
©2013ElsevierInc.Allrightsreserved.
1. Introduction
Painfulneuropathyisaveryimportantclinicalproblem,par- ticularlywiththegrowthofanelderlypopulation,thenumberof
Abbreviations:PACAP,PituitaryAdenylateCyclase-ActivatingPolypeptide;DRG, Dorsal,RootGanglion;SP,substanceP;CGRP,calcitoningene-relatedpeptide;Tac1- 4,tachykinin-encodinggenes;VIP,vasoactiveintestinalpeptide;NKA,neurokinin A;TRPA1,TransientReceptorPotentialAnkyrin1receptor.
∗ Correspondingauthorat:DepartmentofPharmacologyandPharmacotherapy, UniversityofPécs,Pécs7624,Szigetiu.12,Hungary.Tel.:+3672536001/5386/5591;
fax:+3672536218.
E-mailaddress:zsuzsanna.helyes@aok.pte.hu(Z.Helyes).
peopleaffectedbytheseconditionsisincreasing.Itstherapyisan emergingissue,becauseevenwiththelatestdevelopmentsonthe fieldofanalgesic-research,itisstillnotsatisfactory.
PituitaryAdenylateCyclase-ActivatingPolypeptide(PACAP)and tachykinins, such as substance P released from the capsaicin- sensitivepeptidergicsensorynerveswereshowntobeinvolved inacuteandchronicpainconditions[2,6,8,42,45,48,52],buttheir roleinchronicneuropathicpainsyndromeshasnotyetbeenclearly elucidated.
PACAP belongs to the vasoactive intestinal polypeptide (VIP)/secretin/glucagon family [31]. It is present in 27 and 38 aminoacid-containing forms, thelatter is more predominantin mammals.Sofarthreereceptorsofthispeptidehavebeencloned, 0196-9781/$–seefrontmatter©2013ElsevierInc.Allrightsreserved.
http://dx.doi.org/10.1016/j.peptides.2013.03.003
all of which belong to thefamily of G-protein coupledrecep- tors mediating their effect through the activation of adenylate cyclase and phospholipase C. PACAP is the main endogenous agonist on the PAC1 receptor, whereas PACAP and VIP have similaraffinities for theVPAC1 and VPAC2 receptors [22]. The PAC1receptoris expressedmainlyonneuraland smoothmus- clecells,whiletheVPAC1/VPAC2arelocalizedprincipallyonthe dorsalrootganglia(DRG),sensorynerveendings,andinflamma- torycells [6,9,50,55,64]. The fact, that PACAP shows increased expressionin thesuperficiallayerofthespinaldorsalhorn and incapsaicin-sensitiveprimarysensoryneurons,suggestedthatit hasarole innociception[33,34,39,61–63].However,theresults obtainedfromtheearlyinvivostudiesprovedtobecontradic- tory[41].IntrathecallyadministeredPACAP-38showedanalgesic effectintheearlyphase,subsequentlyfollowedbyalong-lasting algesia in the formalin test [48]. In several other models cen- trallyappliedPACAPshowedmarked pro-nociceptiveeffects: it decreasedthermalstimulation-evokedpawwithdrawallatencies andpotentiatednociceptivetransmissiontothedorsalhorn[37].
Itfacilitatedspinalnociceptiveflexorreflexes[41,60]andinduced hyperalgesia[35].WeprovidedevidencethatPACAP-38exertsan interestingdivergenteffectonpain-signaling.Peripherallyadmin- istrated PACAP induces anti-nociceptive, anti-hyperalgesic and anti-allodyniceffectsinbothacutesomaticandvisceralpainmod- els.However,it doesnot affectmechanical hyperalgesiain the traumaticmononeuropathy model, but induces sensitization of kneejointprimaryafferents[42].Furthermore,PACAPinhibitsthe releaseofseveralpro-nociceptiveandpro-inflammatorysensory neuropeptides (CGRP, SP and other tachykinins) from periph- eral terminals of capsaicin-sensitive nerves, and it diminishes acute neurogenic and non-neurogenic inflammatory processes [10,17,36].Wealsodescribed,thatPACAPhasadualroleinnocicep- tion.Thermalallodyniamediatedonlybyperipheralmechanisms is increased in PACAP gene-deficientanimals, but somatic and visceralnocifensivebehaviors,andneuropathicmechanicalhyper- algesiaarediminished.Thisdemonstrates thatPACAP canhave bothinhibitoryandexcitatoryrolesdependingonthesiteofaction andthepredominantmechanisminthepainmodel[44].
Tachykinins represent one of the largest families of neu- ropeptides released from the capsaicin-sensitive sensory nerve terminals.Allof thesepeptides arerelatively small,theiraver- agelengthis10–11aminoacids.Sofarthreetachykininencoding geneshave beencloned(Tac1,Tac3, andTac4),butthenumber ofthetachykininsismuchlargerduetopost-translationalmod- ifications[26,38].Thefirstidentified preprotachykinin-A(PPTA;
Tac1)geneencodessubstanceP(SP)andNeurokinin-A(NKA).This geneisexpressedpredominantlyinsensoryneurons,inflamma- toryandimmunecells[40].SPandNKAexertavarietyofeffects underbothnormalandpathophysiologicalconditions.Theyinduce neurogenicinflammation(vasodilation,plasmaproteinextravasa- tion,andstimulationofinflammatorycells),elicitsmoothmuscle contraction,regulatevasculartone,mucussecretionandimmune functions[26]. They inducethe release of histamine and sero- tonin throughmast cellactivation, which in turnincreasesthe neuropeptide-releasefromthesensory nerveterminalsthrough positivefeedbackmechanisms[18,51].
Threetachykininreceptorshavebeencloned,theNeurokinin1, 2,and3(NK1;NK2;NK3,respectively),whichareGs/Gq-protein coupled[26].Whilealltachykininscanactivateallreceptors,SP andNKAarethepreferredligandsfortheNK1andNK2receptors [15,23,27].TheinvolvementoftheTac1gene-encodedtachykinins inseveralmodelsofchronicinflammationandpainhasbeensug- gested[13].SPandNKAaresynthesizedinthedorsalrootganglia, andtheyareinvolved intheregulationofnociceptiveinforma- tion at the first sensory synapse in the spinal cord [7,56]. SP, NKA,andtheNK1receptorhavebeensuggestedtobeinvolvedin
inflammatoryandneuropathicpainsyndromes,suchastraumatic mononeuropathies[2,11,12],streptozotocin-induceddiabetes[5], andpaclitaxel-inducedperipheralneuropathy[52].However,in thesestudiesonlythealterationsofNK1receptorexpressionwas investigatedortheeffectofnon-peptideNK1receptorantagonists wereanalyzed,asTac1andNK1genedeficientanimalswereuntil recentlynotavailable.
Since PACAP and tachykinins are co-localized in capsaicin- sensitiveafferents,ouraimwastoelucidatetheirrolesinamodel oftraumaticmononeuropathywiththehelpofgene-deletedmice.
Furthermore,theinvolvementoftheNK1receptor,themaintar- getofSP, wasalsoinvestigated.In ordertohaveanintegrative approach, differentfunctional techniques toassess neuropathic mechanical hyperalgesia, motor coordination, and neurogenic vasodilationwereused.
2. Materialsandmethods 2.1. Animals
Experiments were performed with PACAP, Tac1 and Tacr1 gene-deficient(PACAP−/−,Tac1−/−and Tacr1−/−)miceand their respective,appropriatewildtypecounterparts.Thegenerationof PACAP−/− miceontheCD1backgroundattheOsakaUniversity hasbeenpreviously describedindetails [14].Theheterozygous mice(PACAP+/−)werebackcrossed for10 generations withthe CD1strain.Aftergenotypingtheoffspringsofthefirstgeneration ofthePACAP+/− breedingpairs,micewerebredonaswildtype (PACAP+/+)andhomozygousgene-deleted(PACAP−/−)linesinthe LaboratoryAnimalHouseoftheDepartmentofPharmacologyand PharmacotherapyoftheUniversityofPécs.Offspringswithinthe first threegenerations were usedfor the experimentsto mini- mizegeneticvariations.TheSPandNKAdeficient(Tac1−/−)and NK1receptorgene-deleted(Tacr1−/−)miceweregeneratedonthe C57Bl/6backgroundat theUniversity ofLiverpoolandtheUni- versity of Bonn,respectively, as previously described [16]. The homozygousgene-deletedanimalswerebackcrossedfor8-10gen- erationstotheC57Bl/6strain,therefore,thesemicewereusedas wildtype(WT)controls.Theiroriginalbreedingpairswerepur- chasedfromCharles-RiverLtd.(Hungary).Micewerebredandkept intheLaboratoryAnimalHouseoftheDepartmentofPharmacology andPharmacotherapyoftheUniversityofPécsat24–25◦Candpro- videdwithstandardmousechowandwateradlibitumunder12h lightand12hdarkcycles.Allexperimentalprocedureswerecarried outaccordingtothe1998/XXVIIIActoftheHungarianParliament onAnimalProtectionandConsiderationDecreeofScientificPro- ceduresofAnimalExperiments(243/1988)andcompliedwiththe recommendationsoftheInternationalAssociationfortheStudyof Pain[65]andtheHelsinkiDeclaration.Thestudieswereapproved bytheEthicsCommitteeonAnimalResearchoftheUniversityof PécsaccordingtotheEthicalCodex ofAnimalExperiments and licensewasgiven(LicenseNo.:BA02/2000-9-2011).
2.2. Thetraumaticmononeuropathymodel
Anesthesiawasperformedwitha combination of100mg/kg ketaminei.p.(RichterGedeonPlc.,Budapest,Hungary)and5mg/kg xylazinei.m.(LavetLtd.,Budapest,Hungary).Thentherightcom- monsciaticnervewasunilaterallyexposedhighinthethighandits 1/3wastightlyligatedusinganatraumaticsiliconizedsilksuture (Ethicone10-0)[28,47].Thereafterthewoundwasclosed,andthe animalswerenotexaminedforthenext7daysinordernottodis- turbthehealingprocess.Inaccordancewithourpreviousstudies [42,44],thisisareliablemodelofneuropathicmechanicalhyper- algesia,whichisnotinfluencedbyanimpairedweightdistribution.
2.3. Determinationofthemechanonociceptivethresholdby dynamicplantaraesthesiometry
The mechanonociceptive threshold of plantar surface of the hindpawwasmeasuredbydynamicplantaraesthesiometry(Ugo Basile37400,Comerio,Italy).Inthisdevicetheanimalscanfreely moveintheirseparatecompartmentsonametalmesh.Priorto each measurement there was 10–15min of acclimation period untilthecessationoftheanimalsexploratorybehavior.Thenthe stimulatorunitofthedevicewasplacedundertheanimal’spaw, usingan adjustable angled-mirrortoaidthe positioningofthe metalfilamentrightbelowtheplantarsurface.Thenanelectro- dynamicactuatorofproprietary designliftedthestraightmetal filament,whichtouchedtheplantar surfaceandbeganexerting anincreasing upwardforceat a presetrate (5s)of application untileither thenocifensivebehavior (removalof thepaw)was attained,oruntilthemaximal10gwasreached.Afteracondition- ingand3controlpreoperativemeasurements,thepawwithdrawal thresholdsweredeterminedondays7,10,14,19afterthenerve ligation.
2.4. AssessmentofthemotorcoordinationonaRota-Rod
MotorfunctionswerestudiedusinganacceleratingRota-Rod device(UgoBasile7750,Comerio,Italy).Thisinstrumentconsists ofaconstantlyfasterrotatingdrum,whichisdividedintofoursep- aratecompartments,eachforasingleanimal.Beforethebeginning oftheexperiment,weperformed3trainingmeasurementsonthree consecutivedays.Themotor performance wasindicatedbythe durationoftime(s)spentontherotatingdrum[43].Everymea- surementwasrepeated5timesforeachanimal,andtheirmeans wereusedforevaluation.
2.5. MeasurementofcutaneousmicrocirculationbylaserDoppler scanning
Microcirculationintheplantarskinofthehindpawwasmea- sured by laser doppler imaging (Perimed PIM II, Stockholm, Sweden).ThemechanismofthisdeviceisbasedontheDoppler- principle,asitemitsamonochromatic(wavelength:670nm)laser beam,whichisreflectedfromthemovingredbloodcells.Asthe reflected laserbeampasses throughDoppler-shift,detectingits wave-lengthallowsustomeasuretherateofbloodflowinthe superficialcapillaries.Becausemostofthelaserlightisabsorbed inthesuperficialtissues,thebloodflowofthelargerbloodves- selsdoesnotdisturbthemeasurement.Micewereanesthetized withurethane(2.4g/kgi.p.), theirbody temperaturewasmain- tainedat37◦Cwithacontrolledheatingpad.Atthebeginningofthe experiment3–4controlimagesoftheplantarsurfacesofboththe operatedandtheintactcontralateralhindpawsweretakensimul- taneouslytoobtainstablebaselineflowdata.Then30–30lof5%
mustard-oil(allyl-isothiocyanate)wasappliedtopicallyonboth plantarsurfacestoinduceneurogenicvasodilatation.Thischem- icalcompoundisaTransientReceptorPotentialAnkyrin1(TRPA1) receptoragonist,therefore,itinducesthereleaseofvasoactiveneu- ropeptidesfromtheintactsensorynerveendings,whichinturn leadstoanincreasedbloodflowin theinnervatedarea(neuro- genic vasodilation).Theplantar microcirculationwas measured for 60min aftermustard oil application.Altogether an average of 30 imageswererecorded for each animal including theini- tialcontrolmeasurements.5%mustard-oilwaspreparedfreshly beforethebeginning oftheexperiment fromthesameconcen- tratedsolution.Allknockoutandwildtypemiceweremeasured withinatimelimitof48htodecreasethepossibleenvironmental interferences.
2.6. Statisticalanalysis
Alldatawereexpressedasmeanswithstandarderrorsofmeans (s.e.m.).ForstatisticalevaluationrepeatedmeasuresANOVAfol- lowedbyBonferroni’smodifiedt-testwasusedfortheassessment ofmechanicalhyperalgesiaandmotorperformance,whereasthe resultsofthelaser dopplerimagingwereevaluated usingtwo- wayANOVAandBonferroni’smodifiedt-test.Whencomparingthe results*p<0.05wasconsideredstatisticallysignificant.
3. Results
3.1. Mechanicalhyperalgesiainresponsetopartialligationofthe sciaticnerve
TheinitialcontrolmechanonociceptivethresholdsofPACAP−/− miceweresimilartothewildtypes.Tightligationof1/3ofthesciatic nerveinducedasignificant,about30–40%decreaseofthethreshold oftheaffectedhindpawinwildtypeanimalsbetweenthe7thand 19thpostoperativedays.Incomparison,neuropathicmechanical hyperalgesiawasminimalinthePACAP−/−group,thenociceptive thresholdremainedsimilartotheinitialcontrolvaluesthroughout thetotaldurationoftheexperiment(Fig.1A).
The preoperative mechanonociceptive thresholdsof C57Bl/6 wildtypemicedidnotdiffersignificantlyfromeitherthePACAP+/+
wildtypesgeneratedontheCD1backgroundortheTac1−/−andthe Tacr1−/−ones.Furthermore,the35–45%mechanicalhyperalgesia detectedinresponsetothenerveligationwasalsoverysimilar inallthethreegroups,SP/NKAorNK1receptordeficiencydidnot influenceitsdevelopmentduringthewholestudy(Fig.1BandC).
3.2. Changesofthemotorfunctions
ThebasalmotorperformanceontheacceleratingRota-Rodwas significantlyworseinboththePACAP−/−andTac1−/−groupscom- pared to the respective wildtypes(Fig. 2A and B). In contrast, deletionoftheNK1tachykininreceptor(Tacr1−/−)didnotinfluence themotorcoordination(Fig.2C).Thepartialsciaticnerveligation didnotinfluencethemotorcoordinationinanyofthegroups.Dur- ingthecontrolpre-operativeandeventhepost-operativeperiodsa continuouslearningprocesswasobservedinthewildtypeandthe PACAPandTac1gene-deletedgroups,butnotinTacr1−/−animals.
However,thebasalimpairedmotorcoordinationinPACAP−/−and Tac1−/−micewasalsoobservedaftertheoperation(Fig.2AandB).
3.3. Changesofcutaneousbloodflowandneurogenicvasodilation
In both wildtype (PACAP+/+ and C57Bl/6) and all the three gene-deficient (PACAP−/−, Tac1−/−, Tacr1−/−) groups, the basal microcirculationintheplantarskinwasreducedontheoperated limb,butthedifferencewasnotstatisticallysignificant.Thebasal perfusionwassignificantlyhigherintheblackC57Bl/6,Tac1−/−,but notTacr1−/−miceascomparedtothewhitePACAP+/+andPACAP−/−
onesgeneratedontheCD1background.Meanwhile,topicalappli- cationof5%mustardoilactivatingthecapsaicin-sensitivesensory nerve terminals in the skinand inducing neuropeptide release evokedsignificantlysmallerneurogenicvasodilatorresponseinthe C57Bl/6-basedgroupscomparedtotheCD1-basedones(15–20%vs.
50–60%).Thisclearlyindicatesaremarkablestraindifferenceinthe regulationofmicrocirculation(Figs.3–5).
Basalperfusionin theplantarskinofboth theoperatedand intactlimbsofPACAP+/+andPACAP−/−micedidnotdiffersignif- icantly(3.4±0.32V,vs.3.97±1.04V).Inresponsetoactivationof thecapsaicin-sensitivesensorynerveterminalsbytopicalappli- cationof5%mustardoilmicrocirculationsimilarly increasedby
Fig.1. Changesofmechanonociceptivethresholdofthepawafterpartialsciatic nerveligationin(A)PACAPgene-deficient(PACAP−/−)micecomparedtotheir wildtypes(PACAP+/+)generatedontheCD1background,aswellasin(B)Tac1 gene-deletedanimals(Tac1−/−)lackingsubstancePandneurokininA,and(C)NK1 tachykininreceptordeficient(Tacr1−/−)miceincomparisonwiththeirC57Bl/6 wildtype(WT)counterparts.Datapointsrepresentmeansofn=12pergroupwith s.e.m.;**p<0.01,***p<0.001vs.respectivewildtypecontrol(repeatedmeasures ANOVA+Bonferroni’smodifiedt-test).
50–60%inbothgroupsonbothsidesafter5–10min.Thisneuro- genicvasodilationresponsepersistedinwildtypemiceduringthe next60minoftheexperiment,whereas inPACAP−/− animalsit startedtodecreaseafter10–15minSignificantlylowerperfusion
Fig.2. MotorperformanceontheacceleratingRota-Rodwheelbeforeandonthe7th and10thpostoperativedaysaftersciaticnerveligationin(A)PACAPgene-deficient (PACAP−/−)micecomparedtotheirwildtypes(PACAP+/+)generatedontheCD1back- ground,aswellasin(B)Tac1gene-deletedanimals(Tac1−/−)lackingsubstanceP andneurokininA,and(C)NK1tachykininreceptordeficient(Tacr1−/−)miceincom- parisonwiththeirC57Bl/6wildtype(WT)counterparts.Columnsrepresentmeans ofn=6–12pergroupwiths.e.m.;*p<0.05,**p<0.01,***p<0.001vs.respectivewild- typecontrol;#p<0.05vs.respectiveday1preoperativevalue(repeatedmeasures ANOVA+Bonferroni’smodifiedt-test).
wasdetectedinthePACAP−/−groupfromthe30thminuteofthe measurement(Figs.3and4AandB).
IntheTac1−/−andTacr1−/−groupsthebasalcutaneousmicro- circulationwassignificantlyloweronbothlimbscomparedtothe
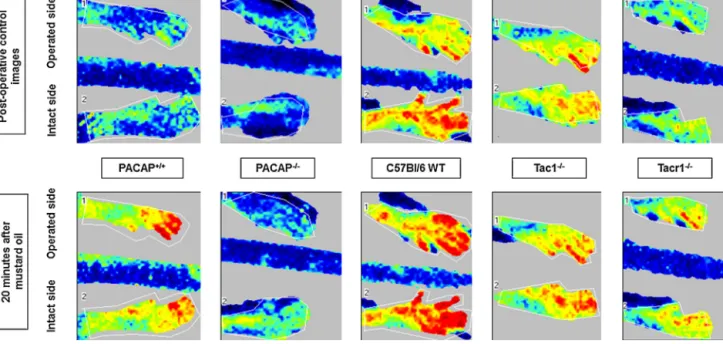
Fig.3.RepresentativelaserDopplerimagesoftheplantarskinoftheoperated(right)andtheintact(left)hindlimbsbeforeand20minaftertopicalapplicationof1%mustard oiltostimulatethepeptidergicsensorynerveterminals.PanelsshowdataofPACAPgene-deficient(PACAP−/−)micecomparedtotheirwildtypes(PACAP+/+)generatedon theCD1background,aswellasin(B)Tac1gene-deletedanimals(Tac1−/−)lackingsubstancePandneurokininA,and(C)NK1tachykininreceptordeficient(Tacr1−/−)mice incomparisonwiththeirC57Bl/6wildtype(WT)counterparts.Thebluecolorrepresentslowperfusionareas,greenandyellowrefertohigherperfusionandredshowsthe highestmicrocirculation.
C57Bl/6wildtypes(Fig.5).Inresponsetomustardoilsmearing theperfusionincreasedsteadilyby15–20%onbothlimbsofthe wildtypemice.Incontrast,incasesofSP/NKAandNK1receptor deficiencytherewasaninitialtransientdecreaseontheintactside, butafterthefirst10min,thebloodperfusiondidnotdiffersignifi- cantlyfromtheresultsoftheC57Bl/6wildtypeanimalsfortherest oftheexperiment(Fig.5).
4. Discussion
Theseresultsprovide clearevidence that:(1) Under normal conditionsPACAPandtachykininsplayimportantrolesinmotor coordination,buttheyarenotinvolvedinmechanonociception.(2) Tachykininsregulatethebasalcutaneousmicrocirculationthrough NK1 receptor activation, but PACAP is involved in neurogenic vasodilation.(3) Partial ligationof thesciaticnerve, which is a widely usedtraumatic mononeuropathy model, induces purely sensoryneuropathy(mechanicalhyperalgesia)withoutaffecting themotorandthevascularfunctions.(4)PACAPisacrucialmedi- atorofneuropathichyperalgesia.
Ourearlier datashowingthatPACAPhasa hyperalgesicand pro-nociceptiveroleinavarietyofpainmodelsareinagreement withthepresent findings [42].Others alsoshowed thatPACAP mightplayakeyroleinspinalsensitization,andtherefore,inthe developmentof neuropathicpain [8,25] throughPAC1 receptor activationinthedorsalrootganglia[6,20].Furthermore,PACAP islikelytohaveapivotalpro-nociceptivefunctioninanimalmod- elsofmigraineandalsoinhumanmigraneurs[29,46,54].Others suggested that theperipheralnociceptive responseselicited by intradermallyadministeredPACAParemainlymediatedthrough theVPACreceptors[45].Therefore, identificationofthe targets andthemechanismsofthehyperalgesicactionsofPACAPstillneed furtherinvestigations.
While the increased expression of the Tac1 gene-encoded tachykininsinthedorsalrootgangliaunderneuropathicconditions hasbeenestablished[57],ourresultsindicatethatSP/NKAandthe NK1receptordonotplayanimportantroleinthedevelopmentof neuropathichyperalgesia.Studyingtheanalgesicmechanismsof
theactionoftopicalcapsaicinapplicationinneuropathies,itwas foundthattheincreasedexpressionofSPhasonlymarginaleffect, andcapsaicinexertsitstherapeuticbenefitthroughotherpath- ways[1].OtherresultsalsoindicatethatSP/NKAup-regulationis notacrucialfactorinthedevelopmentofneuropathies:whileitwas foundtobeinvolvedinthedevelopmentofpaclitaxel-inducedneu- ropathy,oxaliplatintreatmentleadstoasimilarconditionwithout influencingtheSP/NKArelease[52].
ThemotorcoordinationofthePACAPandTac1genedeficient animalswassignificantlyworsethantheirwildtypecounterparts underbothnormalandneuropathicconditions,whilethemotor performanceoftheNK1 receptor deficientgroupwassimilarto thecontrolanimals.Theseresultssuggest,thatPACAPandSP/NKA mighthaveapotentialroleasmediatorsofnormalmotorcoordina- tion.However,thiseffectofthetachykininsisnotmediatedthrough theNK1 receptor, but theaction ofNKA at theNK2 tachykinin receptorcanbesuggested.Thesefindingsaresupportedbyear- lierdatashowingthatPACAPmaycontributetotheregulationof motorcoordination,thoughthemechanismandthesiteofaction hasnotbeendescribed[30].Currentlythereislessdataavailable aboutthe potentialrole of theTac1gene encoded tachykinins, butthefew earlierresultsobtainedinmarkedly differentmod- els(lesscomplexspecies)alsoindicatethattachykininsmightalso affectthemotorcoordination[21].SPhasbeensuggestedtohave aprotectiveroleinananimalmodelofamyotrophiclateralscle- rosis,whichisduetomotorneurondegeneration,supportingits roleinmotorcoordination[3].SPandtheNK1receptoractivation mayalsohaveapotentialroleinthedevelopmentofParkinson’s disease, in which the loss of motor coordination is the princi- palsymptom. IncreasedSPexpressioninthismodelaccelerated diseaseprogression,whereasNK1 receptorantagonisttreatment improvedthemotorperformance [53]. Thesignificantmechan- icalhyperalgesia,butnormalmotorperformanceandcutaneous microcirculationobservedinresponsetothesciaticnerveligation indicates,thatalthoughitcontainssensory,motorandautonomic fibers,thetraumaticmononeuropathyinducedbythisoperative processisexclusivelyofsensorynatureanditdoesnothindereither themotorperformanceorthevascularregulation.
Fig.4.Mustardoil-inducedvasodilationcomparedtotherespectiveinitialreferenceimagesintheplantarskinoftheoperatedandintactpawsofPACAPgene-deficient (PACAP−/−)micecomparedtotheirwildtypes(PACAP+/+)generatedontheCD1background(A–meanflux,B–percentage).n=5–6pergroupwiths.e.m.;#p<0.05,##p<0.01 PACAP+/+vs.PACAP−/−operatedside,*p<0.05,**p<0.01,***p<0.001PACAP+/+vs.PACAP−/−intactside(two-wayANOVA+Bonferroni’smodifiedt-test).
Besides neuropathic hyperalgesia, PACAP is involved in the development of neurogenic vasodilation, particularly in its long-termmaintenance.Asmustardoilactivatingthecapsaicin- sensitiveafferentspredominantlyviaTRPA1ionchannelactivation inducedaremarkableinitialvasodilationinPACAPdeficientmice, which very rapidlydecreased, it is suggested, that PACAPcon- tributes to the later, stable phase of neurogenic vasodilation.
Othersensoryneuropeptidesreleasedfromthestimulatedsensory nerves,suchasCGRParelikelytoberesponsiblefortheearlyphase ofthisresponse.
AsthebasalbloodflowwassignificantlylowerinbothTac1and Tacr1gene-deficientmice,SPthroughNK1 receptoractivationis likelytoplayanimportantroleinthemediationofthebasalvas- culartone,itdilatesthecutaneousvessels.However,theydonot haveanimportantfunctioninneurogenicvasodilation.Itshouldbe notedthatthewellestablishedeffectofSPinneurogenicinflam- mationisincreasingvenularpermeability,aphenomenwhichwas notmeasuredwiththelaserDopplerscanning[19].Inaddition, CGRPisconsideredasthemostpotentmediatorofvasodilation [24].Itwassuggested,thattheNK1 receptoractivationservesto
increaseindirectlytheproductionofnitricoxide(NO)andother localvasodilatorysubstances,howeveritisaccepted,thatthereis alotofinteractionsbetweentheneuropeptides,localmediators, andreceptorsinvolvedinthisprocess,andtheresponsiblepath- waysareredundant[59].Itmustbetakenintoaccount,thatSP isnotthesoleendogenousligandoftheNK1receptor,onwhich theotherknowntachykininsalsoactaspotentagonists,therefore probablybypassingtheroleofthelackingSP/NKA.Inadditionitis established,thatthoughtachykininsareinmostconditionspotent vasodilators,indifferentmodelsorspeciestheycaninducevaso- constriction,andthattheirneteffectisheavilyinfluencedbythe localdifferencesoftheendothelium[58].Thisassumptionalsocor- relateswiththefact,thatsofarnosubstancecouldbelabeledas theexclusivemediatorofcutaneousvasodilation[4,24].
WefoundremarkablestraindifferencesbetweentheC57Bl/6 andCD1mice(PACAP+/+)intheexaminedsensory,motorandvas- cularparameters.Itisnotsurprising,sincealargenumberofpapers describedsuchfindings,andreviewsfocusontheissueofstraindif- ference[32,49].Describingthesedifferencesisinfactvaluable,and highlightstheimportanceofusingtheappropriatewildtypeswhen
Fig.5.VasodilativeresponseinducedbymustardoilsmearintheplantarskinoftheoperatedandintactpawsoftheTac1gene-deletedanimals(Tac1−/−)lackingsubstance PandneurokininA,and(AandB)andNK1tachykininreceptordeficient(Tacr1−/−)mice(CandD)incomparisonwiththeirC57Bl/6wildtype(WT)counterparts.Data pointsrepresentmeansofn=5–6pergroupwiths.e.m.;*p<0.05,**p<0.01,***p<0.001,vs.intactsideofrespectivewildtypecontrol;#p<0.05,##p<0.01,###p<0.001vs.
respectiveoperatedpawofwildtypecontrol(two-wayANOVA+Bonferroni’smodifiedt-test).
interpreting dataof gene-deficientmice.In thepresent experi- mentalserieswedidnotaimtocomparethetachykininandNK1 deficientmicewiththePACAP knockouts,but onlytocompare themwiththeirrespectiveandappropriatewildtypesinorderto elucidate theirrole intheinvestigated biologicalfunctions.The conclusionsweredrawnfromtheresultsseparately.
It can be concluded that (1) PACAP has an overall pro- nociceptiveroleinperipheraltraumaticneuropathy.(2)PACAPand SP/NKAarebothinvolvedinmotorcoordination,whilethelackof theNK1receptordoesnotaffectthisfunction.(3)Tac1geneprod- uctsandNK1 receptorhaveapivotalroleinthemaintenanceof basalvasculartone,whereasPACAPisakeymediatorofneurogenic vasodilation,particularlyinvolvedinitslong-termmaintenance.
Acknowledgements
SROP-4.2.2.A-11/1/KONV-2012-0024, SROP-4.2.1.B-10/2/
KONV-2010-0002,SROP-4.2.2.B-10/1/2010-0029,OTKAK104984, ArimuraFoundation,PTE-MTA“Lendület”Program.
References
[1]AnandP,BleyK.Topicalcapsaicinforpainmanagement:therapeuticpotential andmechanismsofactionofthenewhigh-concentrationcapsaicin8%patch.
BrJAnaesth2011;107:490–502.
[2]CahillCM,CoderreTJ.Attenuationofhyperalgesiainaratmodelofneuropathic painafterintrathecalpre-orpost-treatmentwithaneurokinin-1antagonist.
Pain2002;95:277–85.
[3]CaioliS,CurcioL,PieriM,AntoniniA,MaroldaR,SeveriniC,etal.SubstanceP receptoractivationinducesdownregulationoftheAMPAreceptorfunctional- ityincorticalneuronsfromageneticmodelofAmyotrophicLateralSclerosis.
NeurobiolDis2011;44:92–101.
[4]CharkoudianN.Mechanismsandmodifiersofreflexinducedcutaneousvasodi- lationandvasoconstrictioninhumans.JApplPhysiol2010;109:1221–8.
[5]Coudoré-CivialeM,CourteixC,BoucherM,FialipJ,EschalierA.Evidenceforan involvementoftachykininsinallodyniainstreptozocin-induceddiabeticrats.
EurJPharmacol2000;401:47–53.
[6]Davis-TraberR,BakerS,LehtoSG,ZhongC,SurowyCS,FaltynekCR,etal.Central pituitaryadenylatecylase1receptorsmodulatenociceptivebehavioursinboth inflammationandneuropathicpainstates.JPain2008;9:449–56.
[7]DeKoninckY,HenryJL.SubstanceP-mediatedslowexcitatorypostsynaptic potentialelicitedindorsalhornneuronsinvivobynoxiousstimulation.Proc NatlAcadSciUSA1991;88:11344–8.
[8]DickinsonT,Fleetwood-WalkerSM.VIPandPACAP:veryimportantinpain?
TrendsPharmacolSci1999;20:324–9.
[9]EkbladE.Pharmacologicalevidence forboth neuronaland smoothmus- cularPAC1receptors andaVIP-specificreceptorinratcolon.RegulPept 1999;85:87–92.
[10]ElekesK,SandorK,MoriczA,KereskaiL,KemenyA,SzokeE,etal.Pitu- itaryadenylatecyclase-activatingpolypeptideplaysananti-inflammatoryrole inendotoxin-inducedairwayinflammation:invivostudywithgene-deleted mice.Peptides2011;32:1439–46.
[11]GoffJR,BurkeyAR,GoffDJ,JasminL.Reorganizationofthespinaldorsal horninmodelsofchronicpain:correlationwithbehaviour.Neuroscience 1998;82:559–74.
[12]GonzalezMI,FieldMJ,HughesJ,SinghL.EvaluationofselectiveNK1receptor antagonistCI-1021inanimalmodelsofinflammatoryandneuropathicpain.J PharmacolExpTher2000;294:444–50.
[13]HarrisonS,GeppettiP,SubstanceP.IntJBiochemCellBiol2001;33:555–76.
[14]HashimotoH,ShintaniN,TanakaK,MoriW,HiroseM,MatsudaT,etal.Altered psychomotorbehaviorsinmicelackingpituitaryadenylatecyclase-activating polypeptide(PACAP).ProcNatlAcadSciUSA2001;98:13355–60.
[15]HastrupH,SchwartzTW.SeptideandneurokininAarehigh-affinityligands ontheNK-1receptor:evidencefromhomologousversusheterologousbinding analysis.FEBSLett1996;399:264–6.
[16]HelyesZ,ElekesK,SandorK,SzitterI,KereskaiL,PinterE,etal.Involve- mentofpreprotachykininAgene-encodedpeptidesandtheneurokinin 1 receptorinendotoxin-inducedmurineairwayinflammation.Neuropeptides 2010;44:399–406.
[17]HelyesZs,PozsgaiG,BorzseiR,NemethJ,BagolyT,MarkL,etal.Inhibitoryeffect ofPACAP-38onacuteneurogenicandnon-neurogenicinflammatoryprocesses intherat.Peptides2007;28:1847–55.