Microbial pathogens of cyanophycean blooms
W. D. P. STEWART and M. J. DAFT Department of Biological Sciences, University of Dundee, Scotland
1 Introduction . . . . 1 7 7 2 Viruses of blue-green algae . . . . 1 7 8 2.1 The source of algal lysing viruses . . . . 1 7 9 2.2 The isolation of cyanophages from natural populations . . . 1 7 9 2.3 Criteria used in the classification of cyanophages . . . . 182 2.4 The pattern of cyanophage multiplication . . . . . 1 8 7 2.5 Host physiology and virus replication . . . . 1 8 8 3 Bacteria that lyse blue-green algae . . . 194
3.1 The range of algal lysing bacteria. . . . 1 9 4
4 Fungal pathogens . . . . . . . . . . 205
5 Interactions within cyanophycean blooms . . . . . . 207 Acknowledgements . . . . 2 1 2 References . . . . 2 1 2
1 Introduction
References to algal blooms are found in the writings of the ancient Chinese and Romans, and in the Bible, but it is only within the last two decades, with the onset of man-made eutrophication, that algal blooms have been studied in detail on a world-wide scale. The prob- lems which these algae (mainly species of the blue-green algae Anabaena, Aphanizomenon, Coelosphaerium, Gloeotrichia, Microcystis and Oscillatorid) cause in drinking waters (Ridley, 1970), in amenity waters (Lund,
1972), in waters used for industrial processes such as steel making (Edwards, 1972) and in fishing waters where they may cause de- oxygenation are, perhaps, the main reasons why increased research effort is necessary.
177
To date the physical and chemical parameters such as light, tem- perature and nutrients, which may affect algal growth and primary production, have been studied in most detail. While advances have been made on the basis of such studies there are few lakes where one can predict the occurrence or composition of algal blooms. That is, physical and chemical parameters alone do not as yet give a complete understanding of the development and regulation of algal blooms and despite the faith of lake "modellers" in such parameters, it seems to us at least that attention will also have to be paid to the complex and as yet poorly understood biological interactions that occur within algal blooms (see e.g. Whitton, 1973). Amongst these are the effects of microbial pathogens such as viruses, bacteria and fungi on the blue- green algae. It is these pathogens, the ways in which they affect the blue-green algae, and how the blue-green algae in turn affect them that are the subject of this review.
2 Viruses of blue-green algae
In 1915 Twort, working in London, described a bacterial disease that caused "glassy transformation of micrococcal colonies". Two years later, in Paris, d'Hérelle (1917) showed that dysentery bacilli were lysed by an antimicrobial agent to which he gave the name "bacteriophage".
From these two papers has developed the study of viruses infecting prokaryotic cells. Wieringa and Wiebels (1936) first isolated a virus that infects actinomycetes (actinophage). A vast amount of information has now accumulated on viruses of prokaryotes but it is just over a decade since the first virus of blue-green alga-like organisms was described.
Then Lewin (1960) described a virus of Spirochaete rosea, an apochloro- tic, gliding, organism which was considered to be related to the blue- green alga Oscillatoria but which has since been reclassified as Saprospira grandis, a member of the Flexibacterales (Lewin, 1962, 1972). This study was followed by the isolation and partial purification of a virus of pigmented blue-green algae by Safferman and Morris (1963a). This virus which lyses species of Lyngbya, Plectonema and Phormidium was called LPP-1 after the genera that contain the susceptible species and is probably the most studied of all algal viruses. Since then viruses against unicellular, colonial, nonheterocystous filamentous, and heterocystous filamentous blue-green algae have all been described
(see below) and to these the general name phycoviruses or cyano-
PATHOGENS OF CYANOPHYCEAN BLOOMS 179
phages has been given. We prefer the latter term which not only de- scribes the host class (Cyanophyceae) but also the viral type (phage) ; it is also consistent with the terms bacteriophage and actinophage.
2.1 THE SOURCE OF ALGAL LYSING VIRUSES
Cyanophages have been reported from lakes (Adolph and Haselkorn, 1971), ponds (Padan et al, 1967; Koz'yakov et al, 1972; Singh, 1973), fishponds (Singh and Singh, 1967; Padan and Shilo, 1969), waste stabilization lagoons (Safferman and Morris, 1963a; Safferman and Morris, 1967; Safferman et al., 1969a, b ; Safferman et al., 1972), sewage works (Daft et al., 1970; Daft et al., 1975), rivers (Shane, 1971) and rice paddy soils (Singh, 1973). Viruses of the LPP type are the most common, having been recorded from the United States, Israel, the United Kingdom and India, and these are probably present in any habitat where their hosts occur. On the basis of the data so far available other types of cyanophage appear to be much less frequent.
2 . 2 THE ISOLATION OF CYANOPHAGES FROM NATURAL POPULATIONS
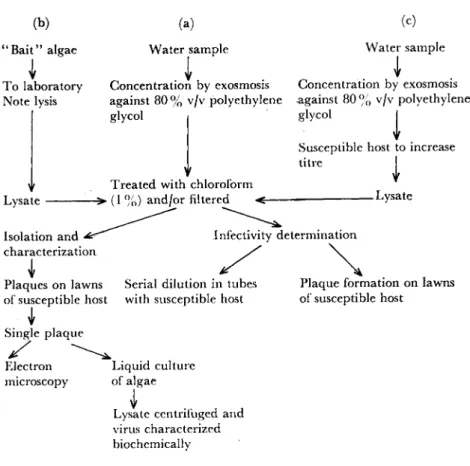
Several methods have been used to isolate cyanophages from natural populations of blue-green algae and the most successful of these are summarized in diagrammatic form in Fig. 1. In the first method (Fig. 1 (a)) filtered water samples are treated with chloroform either directly or after concentration by exosmosis to concentrate the virus particles (Padan et al., 1967). The chloroform treatment kills all lytic organisms other than viruses. The amount of virus in the water-soluble fraction is then determined, as will be described below. The main limitation of this approach is that large volumes of water have to be collected and concentrated if realistic quantitative data are to be ob- tained, because the viruses are often present only in low concentrations.
Even then it is questionable whether this method is sensitive enough for the isolation of any but the most common cyanophages in particular bodies of water.
A second method (Fig. 1 (b)) was used by Daft et al. (1970). They enclosed various blue-green algae in muslin attached to pieces of wood and floated these near the outflow of a sewage works. After 24-36 h, during which time large quantities of water flowed over the algae, these were returned to the laboratory and if lysis of the algae occurred these
(b) (a) (c)
" B a i t " algae Water sample Water sample To laboratory Concentration by exosmosis Concentration by exosmosis Note lysis against 80 % v/v polyethylene against 80 % v/v polyethylene
glycol | glycol I Susceptible host to increase t i n e I Treated with chloroform
Lysate - > ( 1 %) and/or filtered < LYs a t e
Isolation and ^ ^ ^ Infectivity determination characterization
Plaques on lawns Serial dilution in tubes Plaque formation on lawns
1
of susceptible host with susceptible host of susceptible host Single plaque
Electron Liquid culture microscopy of algae
\
Lysate centrifugea and virus characterized biochemically
Fig. 1. Outlines of three of the most commonly used techniques for the isolation of cyanophages (see also text).
were then placed in cultures of the same alga. If, after incubation, lysis again occurred, tests on the lysate for the presence of lytic viruses were carried out. Using this method, large quantities of water can be tested for the presence of lytic agents, but the method has the dis- advantage of being qualitative only.
The third method (Fig. 1(c)) involves adding the water sample without pretreatment (other than concentration) to actively growing algal cultures in order to increase the numbers of any host viruses present. After incubation the resultant lysate is passed through a bacteriological filter to remove contaminants and treated with chloro-
PATHOGENS OF CYANOPHYCEAN BLOOMS 181
Cd) (e)
Fig. 2. (a) Plaques of LPP virus D-l on a lawn of Plectonema boryanum after incubation for 3 days, (b) Plaques of LPP virus P-10 on a lawn of Plectonema boryanum after incuba- tion for 7 days, (c) Dilution end-point test on virus AS-2. Tubes, from left to right, show: (1) control, no virus added; (2-6) tubes containing 10-1, 10~2, 10~3, 10~4, and 10~5 dilutions of the virus suspension. These results show the presence of cyanophage in tubes 2-5 where the host has been lysed but not in tube 6 where no lysis has occurred.
(d) Flasks of Plectonema boryanum 54 h and 72 h after adding LPP isolate D-l. The centre flask is the control flask to which no virus was added (Daft et al., 1970). (e) The effect of cyanophage AS-2 on the unicellular Anacystis nidulans (Synechococcus sp.) 5 days after the addition of the virus (right-hand flask). The flask on the left had no virus added to it.
form as before (see above). This gives a preparation which can be used to infect other algal cultures and which is then checked critically for the presence of cyanophage.
To determine quantitatively the amount of infective virus present two common serial dilution techniques are available. In the first, a known dilution of the virus preparation is added to the algal host in test tubes and then incubated in the light for several days. The tube containing the highest dilution of the virus preparation that causes lysis of the host gives the dilution end-point of the preparation (Fig.
2(c)).
In the second test, double-layered plates are poured, the first layer containing agar plus nutrients and the second upper layer containing agar, alga and a dilution of the virus preparation. The number of plaques which develop is proportional to the number of infective virus particles. Typical plaques of LPP viruses D-l and P-10 on double- layered plates are shown in Fig. 2(a) and 2(b) respectively. These plaques may vary considerably in size (0-05-10 mm) depending on the viral strains present and on the time for which they are allowed to develop. All viral plaques have a characteristically clear edge.
Purification of particular viruses then starts by taking material from a single plaque and using this to infect fresh liquid cultures of the host (Fig. 2(d) and (e)). The cyanophages are then partially purified from the lysate by differential centrifugation and further purified using a sucrose gradient or by equilibrium centrifugation using caesium chlor- ide. Tris-magnesium-acetate buffer (0-01 M, p H 7-2) is commonly used during the purification, but some viruses such as AS-2 are un- stable in this mixture (Daft and Stewart, unpublished). Examination of cyanophages by electron microscopy is readily achieved by preparing algal lawns on glass-fibre filter paper and, on adding purified virus to these, plaques develop. One of these plaques is then gently touched with a carbon-coated grid which is then washed, dried and stained with uranyl acetate or uranyl formate and examined under the electron microscope.
2.3 CRITERIA USED IN THE CLASSIFICATION OF CYANOPHAGES
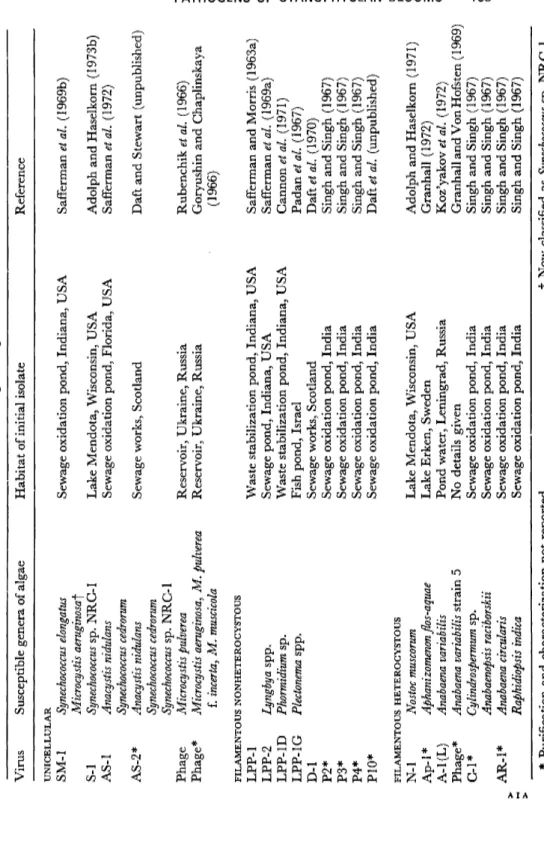
Unicellular, filamentous-nonheterocystous and heterocystous blue- green algae are all known to be susceptible to viruses and a list of some of the viruses is given in Table 1, together with their hosts and habitats
TABLE 1 Viruses of blue-green algae Virus Susceptible genera of algae Habitat of initial isolate Reference UNICELLULAR SM-1 S-l AS-1 AS-2* Phage Phage*
Synechococcus elongatus Microcystis aeruginosa^ Synechococcus sp. NRC-1 Anacystis nidulans Synechococcus cedrorum Anacystis nidulans Synechococcus cedrorum Synechococcus sp. NRC-1 Microcystis pulverea Microcystis aeruginosa, M. pulverea f. incerta, M. muscicola FILAMENTOUS NONHETEROCYSTOUS LPP-1 LPP-2 Lyngbya spp. LPP-1D Phormidium sp. LPP-1 G Plectonema spp. D-l P2* P3* P4* P10* FILAMENTOUS HETEROCYSTOUS N-1 Nostoc muscorum Ap-1 * Aphanizomenon flos-aquae A-l(L) Anabaena variabilis Phage* Anabaena variabilis strain 5 C-l* Cylindrospermum sp. Anabaenopsis raciborskii AR-1 * Anabaena circularis Raphidiopsis indica Sewage oxidation pond, Indiana, USA Lake Mendota, Wisconsin, USA Sewage oxidation pond, Florida, USA Sewage works, Scotland Reservoir, Ukraine, Russia Reservoir, Ukraine, Russia Waste stabilization pond, Indiana, USA Sewage pond, Indiana, USA Waste stabilization pond, Indiana, USA Fish pond, Israel Sewage works, Scotland Sewage oxidation pond, India Sewage oxidation pond, India Sewage oxidation pond, India Sewage oxidation pond, India Lake Mendota, Wisconsin, USA Lake Erken, Sweden Pond water, Leningrad, Russia No details given Sewage oxidation pond, India Sewage oxidation pond, India Sewage oxidation pond, India Sewage oxidation pond, India Safferman et al. (1969b) Adolph and Haselkorn (1973b) Safferman et al. (1972) Daft and Stewart (unpublished) Rubenchik et al. (1966) Goryushin and Chaplinskaya (1966) Safferman and Morris (1963a) Safferman et al. (1969a) Cannon et al. (1971) Padan^ö/. (1967) Daft et al. (1970) Singh and Singh (1967) Singh and Singh (1967) Singh and Singh (1967) Daft et al. (unpublished) Adolph and Haselkorn (1971) Granhall (1972) Koz'yakov et al. (1972) Granhall and Von Hofsten ( 1969) Singh and Singh (1967) Singh and Singh (1967) Singh and Singh (1967) Singh and Singh (1967) * Purification and characterization not reported. f Now classified as Synechococcus sp. NRC-1.
350
250
ε
Q.
<rt Q) CT
"S- I50
*o
a>
E
3
5 0
I 0- 6 I 0- 7 I0"8 I 0 "9 ΚΓ10 I 0 - " I0"12 Dilution
Fig. 3. Titration of LPP virus D-1 to show the relationship between dilution of the virus preparation and the number of plaques developing on a double-layered plate.
from which they were isolated initially. In this Table we have followed the convention used routinely in studies on algal viruses in that the viruses have been classified on the basis of the initial letters of the genera which they infect. Thus N-l infects Nostoc muscorum and LPP-1 infects species of Lyngbya, Plectonema and Phormidium etc. However, this ap- proach is open to criticism with the blue-green algae because of the taxonomic chaos which exists within the group (see, however, Stanier et al.y 1971). For example Stanier et al. (1971) have shown that the Microcystis reported to be infected with virus SM-1 (Synechococcus and Microcystis) should in fact be classified as a Synechococcus and they also consider that Synechococcus and Anacystis nidulans, which are infected by the quite distinct virus AS-1, are in a similar typological group.
In work on bacteriophages it is convenient to classify the viruses on the basis of particle morphology (Bradley, 1967). This can also be done with the viruses of blue-green algae and this we have done in Table 2.
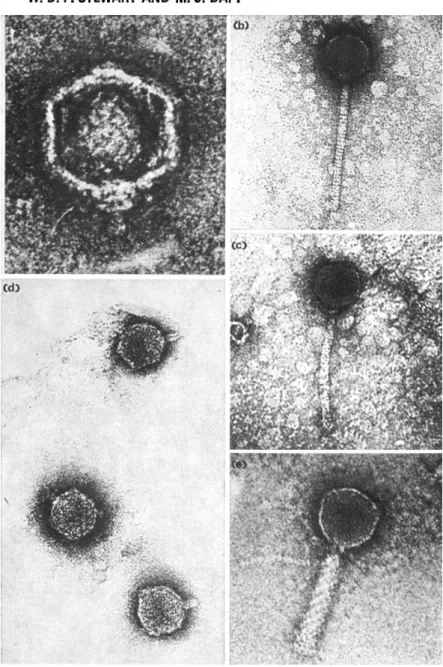
Viral tail length is probably the most characteristic of the features described and five viruses with varying tail lengths are shown in Fig. 4.
So far no filamentous cyanophages have been described.
PATHOGENS OF CYANOPHYCEAN BLOOMS 185
Group No distinct tail Short tailed
Long tailed, noncontractile Long tailed,
contractile
TABLE 2
Particle morphology of various cyanophages Virus
SM-1 LPP-1 LPP-2 LPP-1 G D-1 S-l AS-2 AS-1 N-l
Particle morphology
Polyhedral 67-0 nm ±1-3 nm with a collar 6-0 nm Polyhedral 58-6 n m ± 2-1 nm, tail 10-0-20-0 nm
57-3 n m ± 2-7 nm
Icosahedral 56-0-60-0 nm, tail 28-0-37-0 nm Icosahedral 58-6 nm, tail 20-0 nm
Hexagonal 50-0 nm, tail 140-0 nm 50-0 nm, tail 130-0 nm Polyhedral 90-0 nm, tail 243-5 nm
61-4 ± 2-8 nm, tail 100-0 ± 6-2 nm
Some of the physical and biochemical properties of the five most studied cyanophages are given in Table 3. SM-1 has the highest sedi- mentation coefficient and molecular weight of the DNA and S-l has the lowest. The guanine + cytosine ratios of cyanophages vary from 40 to 70 per cent and all contain double-stranded DNA. Stability of the particles to physicochemical parameters is of limited value in the classification of cyanophages at present.
Serology can be used to identify viruses specifically and to show relationships within a group. For example LPP-1, LPP-1 G, LPP-1D and D-1 are serologically identical, while the virulent LPP-1 and the temperate LPP-2 are unrelated although many of their properties are
TABLE 3
Physical and biochemical properties of some viruses
Virus SM-1 S-l N-l LPP-1 AS-1
Sedimentation coefficient (Svedberg
units) 820 353 539 555
—
Particle density (g cm~3)
1-48 1-501 1-498
— —
Type DNA DNA DNA DNA DNA
Nucle
Stranded- ness Double Double — Double Double
ic acid Molecular
weight (daltons
xlO6) 62-3 ±3-2 23 + 3 38 27
—
G + G ratio
(%) 66 70-74 37-41 53·7±2·3 53-54
—, Not recorded.
7-2
Fig. 4. Electron micrographs of five cyanophages. (a) SM-1 (by courtesy of R. Hasel- korn); (b) S-1 (from Adolph and Haselkorn, 1973b); (c) AS-2; (d) D-1 (from Daft et al., 1970); (e) N-1 (from Adolph and Haselkorn, 1973a). (See Table 2 for dimen- sions of these viruses.)
PATHOGENS OF CYANOPHYCEAN BLOOMS 187
similar. No serological relationship has been found between the two viruses that infect unicellular blue-green algae, SM-1 and AS-1.
The problems of nomenclature and grouping of higher plant viruses have been discussed by Gibbs et al. (1966). They propose that in addi- tion to the vernacular name, a cryptogram or code should be used, based on Adansonian principles of taxonomy. This uses biochemical and morphological properties of the virus particle together with details of host and vector as shown in Table 4. In sum, various ways of classifying cyanophages exist and all have advantages and disadvantages. The convention of naming the virus on the basis of the genera that it infects is simple, but will probably become more unsatisfactory as more viruses are recognized and it is possible that in the long term particle morphology and the use of cryptograms will prove increasingly useful.
TABLE 4
Cryptograms of cyanophages LPP-1, N-l and S-l
Virus LPP-1
N-l
S-l
Type of nucleic acid Strandedness
D 2 D 2 D
t
Molecular weight of nucleic acid*
% nucleic acid 27 40 38 23
t t
Outline of particle
Shape of Host nuclear capsid Vector
T X T X T X
Blue-green algae 0 Blue-green algae
0 Blue-green algae
0
* Daltonsx 106.
f No information available.
D, DNA; T, tailed; X, complex.
2 . 4 THE PATTERN OF GYANOPHAGE MULTIPLICATION
The pattern of cyanophage multiplication involves five main stages : (1) attachment of the cyanophages to the host; (2) introduction of the viral nucleic acid into the host; (3) the replication of viral nucleic acid and the synthesis of viral protein ; (4) assembly of the viral particles ;
(5) release of the particles from the host cell. The attachment or ad- sorption to the cell wall of the host alga is by means of the tail or by a modification of part of the virus head and the kinetics of attachment
have been determined for several systems. The rate constant for ad- sorption of LPP-1G onto Plectonema is 3-5 x 10~n cm3 s- 1 (Padan et al., 1970) and for N-l on Nostoc, 4-2 x lO"11 cm3 s"1 (Adolph and Hasel- korn, 1972). In these two systems and for AS-1 on Synechococcus the attachment is a first-order reaction and linear (Safferman et al., 1972).
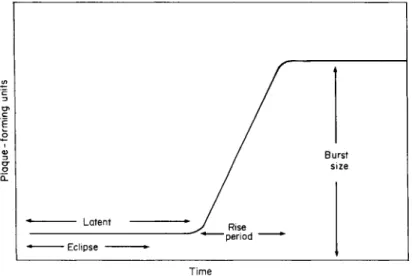
As with bacteriophage, once the nucleic acid in the head of the cyano- phage passes into the host cell via its tail or analogue it undergoes a fairly precise series of events, as shown by the use of the one-step growth experiment devised initially for bacteriophage synthesis by Ellis and Delbrück (1939). These various stages in virus multiplication within
Latent
Eclipse ··
I Γ
/
* ^/ Rise
" period *
J
Burst | s ze
r
Time
Fig. 5. Diagrammatic representation of one-step growth curve.
the host cell are shown diagrammatically in Fig. 5. During the eclipse phase no intracellular phage particles can be detected, while the latent period is the time taken from infection of the culture for the first phage particles to be released from the host. During the rise period the number of liberated phages increases and the difference between the initial and final phage titres represents the burst size.
2 . 5 HOST PHYSIOLOGY AND VIRUS REPLICATION
Viral replication within the host is regulated largely by the metabolism of the latter and viral infection in turn affects the physiological state of the alga.
PATHOGENS OF CYANOPHYCEAN BLOOMS 189
2.5.1 Effect of host cell physiology on viral infection and replication
ATP is necessary for viral replication and on the addition of the inhibitor of phosphorylation CCCP (carbonyl cyanide m-chlorophenyl hydrazone) to Plectonema boryanum there is an inhibition of viral syn- thesis (Padan et ai, 1970; Adolph and Haselkorn, 1972). In photo- autotrophic cultures this A T P comes mainly from photophosphoryla- tion with the virus yield being reduced substantially when oxidative phosphorylation and substrate level phosphorylation are the sources of ATP. For example the burst size of the LPP isolate G U I is reduced by 90 per cent of the light yield (Padan and Shilo, 1968) and the length of the infective cycle is much longer when the host, Plectonema boryanum, is incubated in the dark (Ginzburg et al., 1968). Also, as the rate of dark respiration is increased, for example by accumulating fixed carbon during the previous light period, the final burst size increases (Padan et al.,
1971). The time in the infection process during which photosynthesis provides the A T P is also critical. Padan et al. (1970) found for example that if the alga was illuminated during the eclipse phase a maximum burst size was obtained; if light was limiting during this period the burst size was reduced, and additional illumination after the eclipse phase had no effect on final burst yield. In Plectonema boryanum all the A T P can be supplied by cyclic photophosphorylation with D C M U (3'(3,4-dichlorophenyl) Ι,Γ-dimethyl urea) having no effect on viral synthesis (Padan et al., 1970). In Nostoc muscorum, however, D C M U reduces the burst size by 50-70 per cent (Sherman and Haselkorn, 1971 ; Adolph and Haselkorn, 1972) and in SM-1 there is also a marked dependency on photosystem II with incubation in the presence of D C M U (10~6 M) causing an irreversible loss of activity. For example when C 02 fixation is inhibited by 85 per cent there is a 96 per cent reduction in virus yield. These inhibitions by D C M U are probably due largely to a shortage of fixed carbon because in AS-1 the absence, in the light, of C 02 or the presence of D C M U rapidly reduces the number of infective centres that develop. Studies using algae growing photo- heterotrophically in the presence of D C M U would help to resolve the role of photosystem II in viral replication.
2.5.2 Effects of viral infection on the physiology of the host
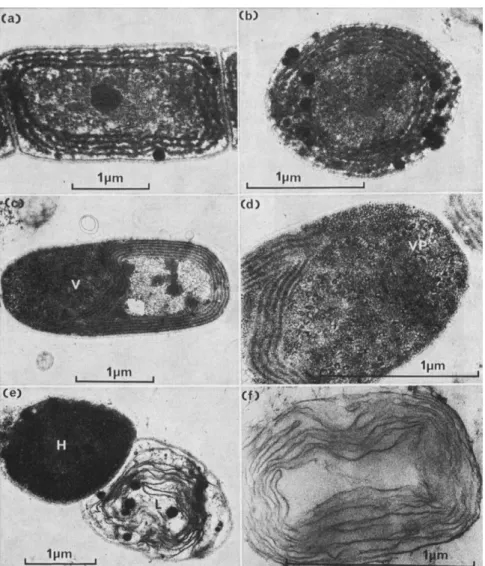
General cell metabolism is affected on infection of the alga with virulent cyanophage and although the effects on photosynthesis have been
Fig. 6. (a) and (b) Uninfected cells of Plectonema boryanum showing peripheral thyla- koids. (c) Infected cell of Plectonema boryanum showing invagination of the thylakoids due to the development of the virogenic stroma (V). (d) Virogenic stroma showing partially synthesized virus particles (VP). (e) Healthy (H) and lysed (L) cells of Plectonema boryanum, (f ) Membranous remains of a cell of Plectonema boryanum lysed by bacterium GP-1.
PATHOGENS IN CYANOPHYCEAN BLOOMS 191 studied most of all, data are available also on the effects on respiration and nitrogen metabolism.
a. Photosynthesis There are some differences in effects in the various algal-cyanophage systems investigated but in all the end-product is an inhibition of photosynthesis by the host. With Plectonema boryanum
1 4C 02 fixation is reduced within 2-5-3 h of infection with LPP-1G and is lost completely after 5 h, even although cell lysis does not occur until several hours later. It has been suggested that this rapid inhibition of C02 fixation might be due to the invagination of the photosynthetic lamellae which characteristically occurs on infection of Plectonema with the LPP virus but we question whether this is the complete answer because with the D-1 virus which also causes an invagination of the photosynthetic lamellae (Fig. 6(c)-(d)) there is no dramatic de- crease in 1 4C 02 fixation until just before cell lysis. Coupled with the cessation of 1 4C 02 fixation on infection with D-1 there is also the release
18
I 14 o o </>
o I 10 B a>
a: 0 6
, - X
-
, _ J
\
V ^
/ /
-J. . . .. . 1
\ \ \
1
\ \
\ \
^**""""*""**'*».* _
400 500 600 700 800
Wavelength (nm)
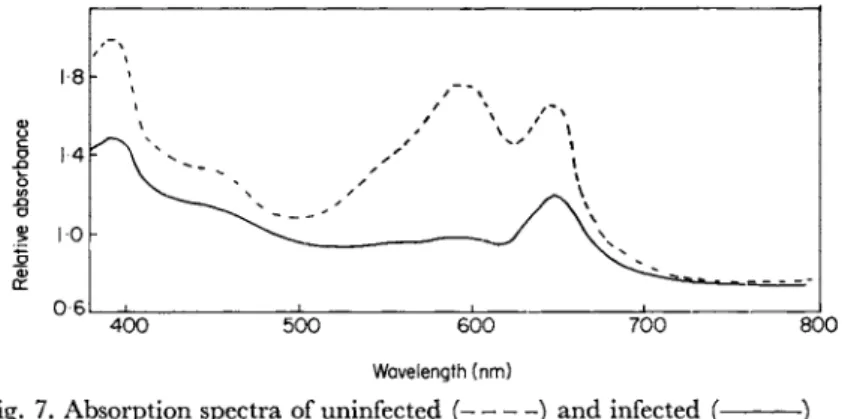
Fig. 7. Absorption spectra of uninfected ( ) and infected (- cultures of Plectonema boryanum after 36 h.
and breakdown of phycocyanin pigments (Fig. 7). The results obtained with D-1 on Plectonema are rather similar to those noted on infection of Synechococcus with SM-1 (MacKenzie and Haselkorn, 1972). They found that in this alga, where invagination of the thylakoids does not occur on infection, photofixation of C 02 was not seriously reduced during viral replication. In the case of Nostoc muscorum, infection with N-l reduced G 02 fixation significantly after 4 h and by 7 h inhibition was complete. This effect is slower than that noted for Plectonema boryanum by Ginzburg et al. (1968) but nevertheless the inhibition does precede cell lysis. There is no information available on whether or not
infection of Nostoc muscorum by N-l results in an invagination of the thylakoids.
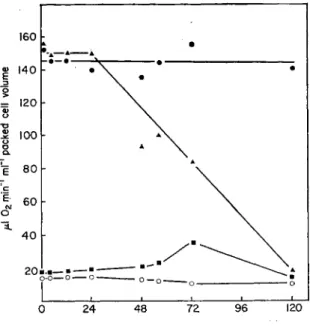
Gyanophage infection appears to have little effect on photophos- phorylation as evidenced by the fact that cell-free extracts of virus infected cells show the same rates of photophosphorylation as extracts from uninfected control filaments (Padan and Shilo, 1973). Similarly virus infection of Plectonema causes no change in the rate of photo- synthetic 02 evolution until lysis of the host occurs (Fig. 8) (Wu and Shugarman, 1967).
160
| 140
= 120
a>
o
I 100 υ
σ α.
E 80
\c Ε 60 O
40
20
0 24 48 72 96 120
Hours after infection
Fig. 8. Effect of cyanophage LPP-1 on photosynthesis and dark respiration by Plec- tonema boryanum. φ, Oxygen evolution by uninfected cells; A> oxygen evolution by infected cells; O, oxygen uptake by uninfected cells; ■ , oxygen uptake by infected cells. (Wu and Shugarman, 1967.)
b. Dark respiration Wu and Shugarman (1967) investigated the effect of viral infection on respiration by Plectonema boryanum and the results are shown in Fig. 8. It is seen that the respiratory rate did not change within 24 h of adding the virus but increased to about 150 per cent of the control after 48 h and to 300 per cent of the control after 72 h.
After 120 h the respiratory rate had dropped to the level of the control, but this could be due to a decrease in the number of intact cells rather
PATHOGENS OF CYANOPHYCEAN BLOOMS 193
than to a specific decrease in activity per intact cell. It appears there- fore that the respiratory mechanism can survive injury to the cells.
What is not clear is whether the increased respiratory rate noted is due simply to "wound damage" or whether it is due to biochemical pro- cesses associated with virus infection and virus synthesis. There is some preliminary evidence that it is not due entirely to wound damage.
c. Nitrogen metabolism Protein synthesis is essential for viral replication and thus a close interdependency between viral synthesis and nitrogen metabolism is to be expected. The effect of virus infection on protein syn- thesis and nucleic acid content of Plectonema boryanum cells infected with LPP-1 has been studied by Sherman and Haselkorn (1970a, b).
They found that within 3-7 h of infection with LPP-1 about 50 per cent of the host DNA was broken down to acid-soluble material and that this was subsequently reincorporated into viral DNA. This, however, had little effect on the synthesis of host DNA because when labelled nucleosides were supplied they were still incorporated into Plectonema DNA even although host DNA degradation was occurring.
Within about 5 h of infection, however, protein synthesis was inhibited completely (Sherman and Haselkorn, 1970b).
The effect of virus infection on nitrogenase activity of Plectonema boryanum has been studied in our laboratory and the data obtained are presented in Table 5. It is seen that on infection of this alga, which fixes nitrogen only under microaerobic conditions (Stewart and Lex, 1970),
TABLE 5
Multiplication of LPP virus D-l in Plectonema incubated in the light under micro- aerobic conditions and its effect on nitrogenase activity by the alga
The algae were grown initially in the presence of NaNOs, washed free of combined nitrogen and incubated under microaerobic conditions (N2IC02: 99-96/0-04 v/v). The virus was added to the cultures 9 h after C2H2 reduction was first detected. The light intensity was continuous at 3000 lux and the temperature 27 °C. Each value is the mean of triplicate determinations.
Time (h)
9 20 44 62
Virus multiplication
(PFU ml"1) Microaerobic
104 104 105 105
Rate of acetylene reduction G2H4 min- Control
2-1 3-2 3-6 3-9
(nmol -1 ml"1)
Infected 2-3 3-7 0-3 0-05
the rates of activity of the control and infected series remain similar for the first 20 h of adding the virus, but thereafter there is a rapid drop- off in the activity of the infected cultures. This drop-off precedes cell- lysis by several hours. While this could be due to a general inhibition of cell metabolism it could also be due to an 02 inactivation of nitrogen- ase on breakdown of the subcellular compartmentation of the 02
evolving photosynthetic system and the 02 sensitive nitrogenase. It may be noted that in these experiments viral replication occurred at the same rate under microaerobic conditions as it did under aerobic con- ditions in the light indicating that all the necessary A T P for viral syn- thesis could probably be supplied by photophosphorylation.
3 Bacteria that lyse blue-green algae
Söhngen (1927) was probably the first to report on the occurrence of lytic bacteria that were effective against other prokaryotes and since then bacteria that lyse fungi (Mitchell and Alexander, 1963), bacteria (see Dworkin, 1966) and blue-green algae have all been re- ported. In contrast to algal lysing viruses these lytic bacteria usually have a wide host range and can be cultured free from the host. They are widely distributed in freshwater habitats and it is surprising, per- haps, that it is only within the last few years that they have been recognized and studied in detail.
3.1 THE RANGE OF ALGAL LYSING BACTERIA
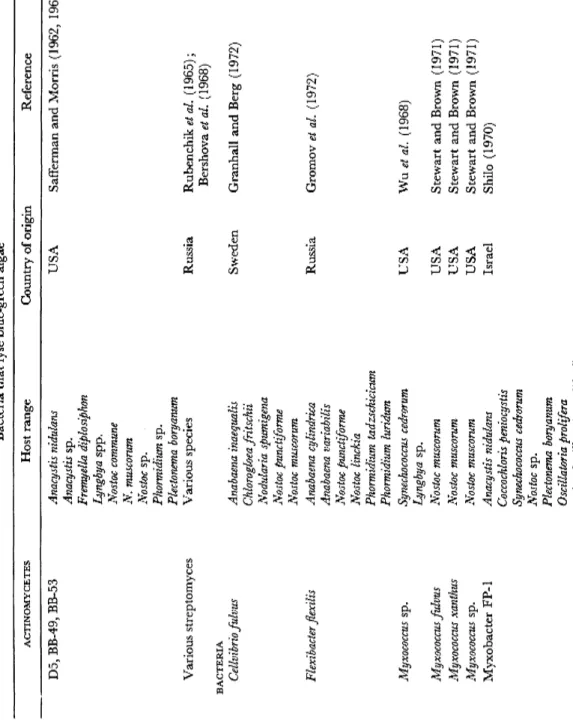
Table 6 lists the algal lysing bacteria reported to date and no doubt others remain to be discovered. The range includes:
3.1.1 Actinomycetes
Various actinomycetes have been reported to cause lysis of blue-green algae, but in general their mode of action has not been studied in any detail. Safferman and Morris (1962) noted, however, that many pro- duced extracellular products which were effective against blue-green algae, particularly Plectonema boryanum. Out of 403 actinomycetes that they isolated from soil, 24-6 per cent were effective against blue- green algae and 7-2 per cent were effective against both green algae and blue-green algae. Safferman and Morris (1963b) also reported briefly on actinomycete filtrates that affected the blue-green alga Anacystis
TABLE 6 Bacteria that lyse blue-green algae ACTINOMYCETES Host range Country of origin Reference D5, BB-49, BB-53 Various streptomyces BACTERIA Cellvibrio fulvus Flexibacter flexilis Myxococcus sp. Myxococcus fulvus Myxococcus xanthus Myxococcus sp. Myxobacter FP-1
Anacystis nidulans Anacystis sp. Fremyella diplosiphon Lyngbya spp. Nostoc commune N. muscorum Nostoc sp. Phormidium sp. Plectonema boryanum Various species Anabaena inaequalis Chlorogloea fritschii Nodularia spumigena Nostoc punctiforme Nostoc muscorum Anabaena cylindrica Anabaena variabilis Nostoc punctiforme Nostoc linckia Phormidium tadzschicicum Phormidium luridum Synechococcus cedrorum Lyngbya sp. Nostoc muscorum Nostoc muscorum Nostoc muscorum Anacystis nidulans Coccochloris peniocystis Synechococcus cedrorum Nostoc sp. Plectonema boryanum Oscillatoria proliféra ( = Oscillatoria prolifica?)
USA Russia Sweden Russia USA USA USA USA Israel
Safferman and Morris (1962, 1963b) Rubenchik et al. (1965); Bershova et al. (1968) Granhall and Berg (1972) Gromov et al. (1972) Wuetal. (1968) Stewart and Brown (1971) Stewart and Brown (1971) Stewart and Brown (1971) Shilo (1970)
$ I o o m Z C/) O m O -< > Z o no X ■< o m > O O CD CJ1
TABLE 6 {cont.) Bacteria that lyse blue-green algae ACTiNOMYCETES Host range Country of origin Reference Spirulina platensis Spirulina tenuis Myxobacter CP-type Anabaena ambigua Scotland Daft and Stewart (1971, 1973) Anabaena catenula Daft et al. (1973, 1975) Anabaena circinalis (4 strains) Anabaena cylindrica (2 strains) Anabaena flos-aquae (5 strains) Anabaena oscillarioides (2 strains) Anabaena spiroides Anabaena variabilis Anabaena sp. Anabaenopsis circularis Anacystis nidulans Aphanizomenon flos-aquae (3 strains) Aphanizomenon sp. (2 strains) Coelosphaerium sp. Cylindrospermum sp. Gomphosphaeria sp. Lyngbya sp. (2 strains) Microcystis aeruginosa Microcystis sp. (6 strains) Nostoc calcicola Nostoc ellipsosporum (2 strains) Nostoc muscorum (2 strains) Nostoc piscinale (2 strains) Oscillatoria redekei Oscillatoria sp. Phormidium luridum Phormidium uncinatum Phormidium sp. Plectonema boryanum (4 strains) Plectonema calothricoides Plectonema sp. (2 strains) Synechococcus cedrorum Myxobacter 44 Nostoc muscorum USA Stewart and Brown (1969, 1971 Plectonema boryanum
PATHOGENS OF CYANOPHYCEAN BLOOMS 197
nidulans and various green algae. Subsequently the Russian workers Rubenchik et al. (1965) found that out of 838 cultures that they tested 28 strains of Streptomyces and three strains of bacteria inhibited the growth of the bloom-forming alga Anabaena kassallii. The effect was most pronounced on solid medium. More recently Bershova et al.
(1968) reported on various actinomycetes that were effective against blue-green algae. It thus seems that although a variety of actinomycetes have been tested for lytic activity and although many appear to be active against blue-green algae, many more studies in depth on actino- mycete-cyanophyte interrelations are required before their importance in nature can be properly gauged.
3.1.2 Cellvibrio
Species of Cellvibrio occur in soil and in polluted waters. According to the Swedish workers Granhall and Berg (1972), who studied two strains of Cellvibrio, these produce an extracellular antibiotic that is effective against blue-green algae. This is a soluble, heat-resistant, low molecular weight compound that is produced in stationary phase culture only and is inhibitory to Anabaena inaequalis, Chlorogloea fritschii, Nodularia spumigena, Nostoc punctiforme, Nostoc muscorum and Spirulina platensis.
The effects vary depending on the host species but trichome breakage, the production of sphaeroplasts and cell lysis have all been noted. These effects are very similar to those of penicillin on blue-green algae
(Lamont, 1969; Fitz-James and Hancock, 1965) in that the mode of action appears to be to prevent cell wall synthesis. The bacteria also pro- duce cellulase and the vegetative cells are more susceptible to attack than either spores or akinetes. In contrast to myxobacterial attack (see below) the inhibition caused by the bacterium is reversible and the algal cells resume growth when the bacterial filtrate is removed; in addition certain resistant algal strains develop. Granhall and Berg
(1972) hypothesized that such Cellvibrio strains may be important in the degradation of algal species in the rivers where they occur, but no data on this aspect were presented.
3.1.3 Fruiting myxobacteria
As early as 1924 Geitler reported that the fruiting myxobacterium Polyangium parasiticum caused the death of green algae but he did not
test it against blue-green algae. More recently species of Myxococcus and Sorangium that lyse algae have been reported and the most ex- tensive studies have been those involving Myxococcus. Wu et al. (1968) published a preliminary account of the antimicrobial activity of an aerial contaminant, probably a Myxococcus, that lysed seven blue- green algae including Synechococcus cedrorum and a Lyngbya species, but activity was lost on subculturing. Cellular enzymes of the bacterium were studied in some detail and protease, enzymes causing lytic activity, ribonuclease, deoxyribonuclease, aryl sulphatase and acid phosphatase were all reported as being present. Cell-free filtrates with lytic activity were found only when growth of the bacterium was slow and clumpy, but protease activity was always found. Subsequently Stewart and Brown (1970) reported that four fruiting myxobacteria, Myxococcus xanthus, M.fulvus, Myxococcus sp. and Sorangium sp., lysed the blue-green alga Nostoc muscorum.
3.1.4 Nonfrutting myxobacteria with a low G + C ratio
Gromov et al. (1972) isolated an organism which they called Flexibacter flexilis (var. algavorum) from a naturally occurring Nostoc population in
Russia. This bacterium is a Gram-negative rod up to 200 μπι long that moves with a slow gliding movement. Microcysts, fruiting bodies, or flagella are never found. On agar plates the bacteria form a thin slime layer and show an orange pigmentation due to a carotenoid, probably saproxanthin, which absorbs at 450, 476 and 506 nm in hexane. The bacterium has little capacity to ferment, is highly sensitive to actino- mycin, does not hydrolyse cellulase or agar, but liquefies gelatine. It has a G + C ratio, based on melting point determinations of 35-9 per cent. The bacterium lyses species of Anabaena, Nostoc and Phormidium, but is ineffective against the cultures of Anacystis, Aphanothece, Plec- tonema and Lyngbya on which it has been tested. It does not lyse bacteria (species of Bacillus, Escherichia, Pseudomonas and Serratia).
Contact is required for lysis of the algae and the bacterium is most effective on algal lawns causing lysis there within 3-4 days; in liquid culture 6-7 days are required for lysis. The bacterium also inhibits nitrogen fixation by the algae. In many respects, apart from its low G + C ratio, which together with its other characters necessitates the placing of this organism in the genus Flexibacter (Lewin, 1969), the bacterium is very similar to the following group of organisms.
PATHOGENS OF CYANOPHYCEAN BLOOMS 199
3.1.5 Nonfruiting myxobacteria with a high G + C ratio
These organisms are all aflagellate, Gram-negative rods with rounded ends which move with a slow gliding movement in the direction of their longitudinal axis. None produce microcysts or other fruiting structures yet all have G + C ratios of 68-70 per cent. The rods increase in length with age and may become attached end-to-end to form chains. They are of widespread distribution in eutrophic freshwater ecosystems and are the most studied algal lysing bacteria. They have been isolated from Israel where Shilo (1970) studied one strain in detail; five strains from the United States have been investigated by Stewart and Brown (1969, 1971) and Daft et al. (1973, 1975) have isolated sixteen strains from freshwaters and soils in the United Kingdom. The most extensively studied have been FP-1 (Shilo, 1970), CP-1 (Daft and Stewart, 1971,
1973) and Myxobacter 44 (Stewart and Brown, 1969, 1971).
a. Isolation The technique here is to prepare algal lawns and look for plaques caused by a nonfilterable agent. The choice of test organism is not so critical because the bacteria usually have a wide host range but it is wise nevertheless to set up lawns of unicellular, nonheterocystous
and heterocystous filamentous species. We always include Anabaena flos-aquae and Nostoc muscorum in our tests as they produce very uniform lawns, grow rapidly and are susceptible to a wide range of bacterial isolates. When plaques develop, samples of these are taken, and if the agent is confirmed to be nonfilterable and if it can grow independently of the host, it can be assumed that it is not a virus. The characterization of the agent is then carried out using routine bacteriological techniques and if an axenic culture of the agent causes lysis when added to the alga it can be accepted as being an algal pathogen.
b. Characteristics of the organisms Table 7 lists the general characteristics of three myxobacteria with high G + C ratios, FP-1, CP-1 and Myxo- bacter 44 and compares these with Flexibacter flexilis, a. myxobacterium which has a low G + C ratio. CP-1 is typical of the group in mor- phology and ultrastructure and will be considered here in detail;
reference will be made to the others only where they differ substantially from CP-1.
CP-1, like other organisms in the same group, is a Gram-negative rod, about 2-4 x 10-6 μτη in size. In culture it produces a thin silky
TABLE 7
Characteristics of four bacterial isolates that lyse blue-green algae
Gram stain
Size (/mi) 3-0 Pigment
Maximum absorption of interacellular pigment (nm) G + C ratio (%) Temperature optimum
(°C) pH optimum Motility Mode of action
FP-11 Negative x 9-0x0-6-
Salmon 482*
70 26 7-2 Gliding Contact
442 Negative -1-0 0-4^0-6 x 1-9x3-7
Yellow 445|
69-71 30-35 8-7 Gliding Extracellular
excretions
CP-13 Negative 2-4x10-6 Yellow 435+
65-69 ± 1 37 Gliding 7-5 Contact
F. flexilis*
Negative 7-200 long
Orange 476*
36 25 Gliding 7-0 Contact
1 Shilo (1970) ; 2 Stewart and Brown (1971) ; 3 Daft and Stewart (1971) ;
4Gromovétfo/. (1972).
* In n-hexane; | in ethanol; ί in methanol.
growth over the surface of solid medium and in the young stage the colonies so produced are distinct, but later they become diffuse. A colony of CP-1, as seen under the scanning electron microscope, is shown in Fig. 9 (a) and the arrangement of the bacterial cells within such a colony is shown in Fig. 9(b). These colonies all have a yellow or pinkish pigmentation due to the production by the bacteria of intra- cellular carotenoids with absorption maxima within the range 410- 500 nm (see Fig. 10). These myxobacteria grow readily on artificial media and the compounds that they utilize are shown in Table 8.
These differ slightly from isolate to isolate although FP-1 and CP-1 are similar in many respects. In nature these organic compounds are no doubt provided by the organisms that they lyse. Healthy and lysing filaments of Anabaena flos-aquae are shown in Fig. 9 (c) and (d) respec- tively.
The appearance of CP-1 under the transmission electron miscoscope is shown in Fig. 11. The organism has a typical prokaryotic structure with a crenulated outer envelope (Fig. 11(a)) composed of three distinct layers : an outer unit membrane, an electron-dense layer, and an inner unit membrane (Fig. 11(c)). Slime material of a muco- polysaccharide nature (as evidenced by its staining with ruthenium red) is produced extracellularly as distinct vesicles, or as slime trails
Fig. 9. Scanning electron micrographs of bacterium CP-1 and host alga, Anabaena flos-aquae, (a) A bacterial colony growing on a membrane filter, (b) Bacteria within the colony. Note some very long cells, (c) Uninfected filaments of the alga Anabaena flos-aquae. (d) Infected filaments of Anabaena flos-aquae showing the presence of lysed
cells (arrows).
1-6 I j
l4l S~\
I-Oh / \
° \ / \
l
08| / \
0·4[ ^ ^ , 0 2l·
QI 1 1 1 1 1 350 400 450 500 550
Wavelength (nm)
Fig. 10. Absorption spectrum of the pigments extracted in methanol from bacterium CP-1 (Daft and Stewart, 1971).
(Fig. 11 (a) and (b)). The cell protoplasm has an outer layer rich in ribo- somes in which mesosomes (Fig. 11 (c)), and poly-/?-hydroxybutyrate granules can often be seen. The central nuclear area contains distinct DNA fibrils. The appearance of isolates FP-1 (Shilo, 1970) and Myxobacter 44 (Stewart and Brown, 1971) is very similar.
Both CP-1 and FP-1 require contact with the host for lysis to occur.
The bacteria attach themselves end-on to the algae, frequently, but not exclusively, close to the cross-septae in filamentous forms such as Oscillatoria redekei, attachment often taking place within 20 minutes of mixing the algae and bacteria together under a coverslip. The first change in appearance of an attacked cell, as seen under the light micro- scope, is that the protoplasm becomes paler and the gas vacuoles, if present, disappear. Subsequently the protoplasmic contents are re- leased from the cell into the surrounding medium leaving behind empty "ghosts" of cell wall and mucilage material, with the lytic bacteria often remaining attached to these remnants. The pattern of filament lysis is usually random except that heterocysts and akinetes, if present, are more resistant, and are often the only cells that remain apparently intact after attack. When lysis of heterocysts eventually occurs it is not certain whether this is due specifically to the bacteria or to the fact that the heterocysts have become detached from the vegetative cells.
The first stage in lysis to be seen under the electron microscope is the disappearance of the electron dense L2 layer of the wall, so that in its
TABLE 8 Fungi which occur parasitically on blue-green algae and the algal genera that have susceptible species (after Canter, 1972) Fungi Blastocladiella anabaenae Chytridium cornutum Chytridium microcystidis Phlyctidium globosum Phylctidium megastomum Rhizophydium deformans Rhizophydium megarrhizum Rhizophydium subangulosum Rhizophydium ubiquetum Rhizophydium oscillatoriae-rubescentis Rhizosiphon anabaenae Rhizosiphon akinetum Rhizosiphon crassum Scherjfeliomyces sp. Unknown Chytrid unnamed Unidentified sp.
Anabaena
+ + + + + + + +
Algal genera Aphanizomenon Gomphosphaeria Lyngbya
+ + + + + +
+ +
Microcystis + +
Oscillatoria
+ +
+ (possibly) Aphanizomenon) + +
and the presence of extensive slime vesicles (S) surrounding the cells, (b) Slime trails associated with bacterium CP-1. (c) Electron micrograph of part of bacterium CP-1 showing a developing mesosome (M). (d) Cultures of Anabaena flos-aquae 72 h after the addition of bacterium CP-1 to the right-hand flask only, (e) Plaques caused by bacterium CP-1 on a lawn of Nostoc ellipsosporum (Daft and Stewart, 1971).