ContentslistsavailableatScienceDirect
Journal of Pharmaceutical and Biomedical Analysis
jou rn al h om e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / j p b a
Heart-cutting two-dimensional liquid chromatography coupled to quadrupole-orbitrap high resolution mass spectrometry for
determination of N,N-dimethyltryptamine in rat plasma and brain;
Method development and application
Tímea Körmöczi
a,b, Írisz Szabó
c, Eszter Farkas
c, Botond Penke
b, Tamás Janáky
b, István Ilisz
a, Róbert Berkecz
a,b,∗aInstituteofPharmaceuticalAnalysis,FacultyofPharmacy,UniversityofSzeged,SomogyiUtca4,H-6720,Szeged,Hungary
bDepartmentofMedicalChemistry,FacultyofMedicine,UniversityofSzeged,Dómtér8,H-6720,Szeged,Hungary
cDepartmentofMedicalPhysicsandInformatics,FacultyofMedicine,UniversityofSzeged,Korányifasor9,H-6720,Szeged,Hungary
a r t i c l e i n f o
Articlehistory:
Received21June2020
Receivedinrevisedform30August2020 Accepted2September2020
Availableonline9September2020
Keywords:
N,N-dimethyltryptamine
␣-methyltryptamine Ratplasma Ratbrain
Heart-cutting2D-LC-HRMS/MS
a b s t r a c t
Theorthogonalheart-cuttingliquidchromatography(LC)modescoupledtohigh-resolutiontandemmass spectrometry(HRMS/MS)provideanumberofpossibilitiestoenhanceselectivityandsensitivityforthe determinationoftargetedcompoundsincomplexbiologicalmatricies.Herewereportthedevelopmentof anewfast2D-LC-(HRMS/MS)methodanditssuccessfulapplicationforquantitativedeterminationofthe levelofplasmaandbrainN,N-dimethyltriptamine(DMT)using␣-methyltryptamine(AMT)asinternal standardinanexperimentalmodelofcerebralischemia/reperfusionusingDMTadministration.The2D- LCseparationwascarriedoutbyacombinationofhydrophilicinteractionliquidchromatography(HILIC) inthefirstdimensionfollowedbysecond-dimensionalreversed-phase(RP)chromatographywithina totalruntimeof10min.TheenrichmentofHILICeffluentofinterestcontainingDMTwasperformed usingaC18trappingcolumn.Duringmethoddevelopmentseveralparametersofsamplepreparation procedures,chromatographicseparationandmassspectrometricdetectionwereoptimisedtoachieve highDMTrecovery(plasma:90%,brain:88%)andsensitivity(plasma:0.108ng/mLofLOD,brain:0.212 ng/gofLOD)applyingtargetedanalyticalmethodwithstrictLCandHRMSMSconfirmatorycriteria.
Concerningratplasmasample,theconcentrationofDMTbeforehypoxia(49.3–114.3ng/mLplasma) wasgenerallyhigherthanthatafterhypoxia(10.6–96.1ng/mLplasma).Aftertreatment,theconcentra- tionofDMTinbrainwaselevateduptotherangeof2–6.1ng/g.
Overall,ouranalyticalapproachissuitabletodetectandconfirmthepresenceofDMTadministeredto experimentalanimalswiththerapeuticpurposeinareliablemanner.
©2020ElsevierB.V.Allrightsreserved.
1. Introduction
DMTis an endogenous hallucinogen and traceamine found invarioustissues suchasthebrain,pineal glandandlung, and bodyfluidssuchasurine,cerebrospinalfluidandbloodplasmain mammals[1–4].DMTisproducedbysomaticcellsunderphysi- ologicalconditions[1,3],anditsconcentrationhasbeenfoundto increaseinresponsetopathophysiologicalhomeostaticchallenges (e.g.hypoxiaoroxidativestress)[1,3].Concurrently,DMTisbest
∗Correspondingauthor.
E-mailaddress:berkecz.robert@pharm.u-szeged.hu(R.Berkecz).
knownasanindolealkaloidtypicallysynthesised byplants like PsychotriaviridisandDiplopteryscabrerana[5].
AlthoughDMTwastracedwithgas-chromatographyinbody fluidsofpsychiatricpatientsandcontrolsubjects[3,4],itsphysi- ologicalroleisstilltobeunderstood,inparticular,becauseDMT undergoesrapidenzymaticdegradationbymonoamineoxidases (MAO)[3].Ontheotherhand,DMThasbeenimplicatedinneuro- protectionathighconcentration,eithersynthesisedinthenervous tissueinresponsetohypoxiaoroxidativestress,ororiginatingfrom thelungand reachingthebrainviathebloodstream[1,6].The putativeneuroprotectiveactionofDMThasbeenutilisedinexper- imentalmodelsofcerebralorrenalischemia.DMTadministeredat supraphysiologicalconcentrationtoexperimentalrodentsresulted inasmallercerebralinfarctsize[7]andpromotedcellsurvivalina
https://doi.org/10.1016/j.jpba.2020.113615 0731-7085/©2020ElsevierB.V.Allrightsreserved.
cellcultureofhumancerebrocorticalneuronsexposedtohypoxia [8].
EarlyresearchesofDMTfocusedprimarilyonthepsychological andpharmacologicaleffectsofconsumingayahuasca[5].During the last 60 years, many studies reported finding DMT and its metabolitesin animal tissueand humanurine,blood and cere- brospinalfluidusingliquidchromatography(LC).Nowadaysliquid chromatography–tandemmassspectrometry(LC–MS/MS)isone ofthemostwidelyusedbioanalyticalmethodsforquantificationof smallmoleculesinbiologicalsamples.Itisduetoitshighsensitivity andhighselectivity,thusbeingabletoprovidemorereliabledata thantheLCtechniqueitself,whichisalsotrueoftheanalysisofDMT [9],onlyafewstudiesreportedthepresenceofDMTinmammalian tissue;forinstance,inpinealglandmicrodialysateofrat[2],ratkid- ney,lung,liver,brain,humanlungandrabbitliver[10].According tocertainviewpoints,detectionofendogenousDMTisnotrele- vantwhenit isexogenouslyadministereddue tothedissimilar concentrationrange[11].
The ultimatepurpose of ourupcoming study is toevaluate theneuroprotectiveactionofDMTinanexperimentalmodelof cerebralischemia/reperfusion,aggravatedbytransientanoxiaand recurrentspreadingdepolarisations.Here,wesetouttoestablish anovel,highlysensitiveanalyticaltooltodeterminetheconcen- tration of endogenous DMT in our model and to measure the accumulationof intravenouslyadministered exogenousDMT in bloodplasmaandbraintissue.
In modern pharmaceutical analysis, quantitation of analyte or analytes of interest in complex biological matrices requires highselectivityand sensitivity.Nowadays,two-dimensionalliq- uidchromatography(2D-LC)isoneofthemostpowerfulanalytical toolstoimprovethequalityofobtaineddata,especiallyincom- bination withHRMS/MS. The comprehensiveand heart-cutting analysesareknowntobethemaintechniquesof2D-LC.Theheart- cuttingmodeisatargeted2Dtechnique,becauseonlyaselected eluentfraction(singleheart-cutting)orfractions(multipleheart- cutting)arecollected,trappedforsecond-dimensionalseparation [12–14].
Nowadays, the typical methodutilised for determination of administeredDMTinplasmaandbrainsampleisreversedphase chromatographicseparationcoupledtotandemmassspectrome- tryusingelectrosprayoratmosphericpressurechemicalionization inpositivemode[15–18].However,thehighresolutionaccurate HRMS/MSmethod,incombinationwithorthogonal2D-LCsepara- tionincomparisonwithonedimensionalLC-HRMS/MSmethods, hastheabilitytoprovidemorereliabledata.Furthermore,itgives theopportunitytouseloweramountsofsampleswithsimilaror betterlimitof detectionfor DMT.The requiredamountof bio- logicalsample isa critical issue,especiallyin pharmacokinetics assaysorwhentheavailablesampleis limitedforinstanceata repeatedsamplingofbloodfromratandmouse.Anotherkeyfac- torofmethoddevelopmentisruntime,whichshouldbekeptas lowaspossible.FordeterminationofDMTincomplexbiological samplessuchasbrainandplasma,theapplicationofthegradient elutionisessential,thereforethetotalruntimeofanalysisincreases bythetimeofcolumnwashandequilibration.Intheliterature,the totalruntimeofgradientLC–MS/MSanalysisofDMTcanbefound intherangeof11.5−25mininbrainandplasmasamples[15–18].
Thus,themaingoalsofthisstudywerethedevelopmentofafast heart-cutting2D-LC-HRMS/MSmethodandoptimisationofsam- plepreparationproceduresforthequantitativeanalysisofDMTin ratbrainandplasmausingarelativelylowamountofbiological samples.Themethodthusdevelopedwasappliedforthedeter- minationofDMTlevel inratplasmaand brainofexperimental model of cerebral ischemia/reperfusionusing DMT administra- tion.
2. Materialsandmethods 2.1. Chemicalsandstandards
N,N-Dimethyltryptamine (DMT) standard with purity >98.5
% was obtain from Lipomed AG (Arlesheim, Switzerland). ␣- Methyltryptamine(AMT)withpurity>99%waskindlyprovided bytheDepartmentofForensicMedicine,FacultyofMedicine,Uni- versity of Szeged, Hungary, and used as internal standard (IS).
Acetonitrile,ammoniumformate,formicacid,methanol,water,(all LC–MSgrade),35%ammoniasolution,ethylacetate(HPLCgrade) and reagentgrade perchloric acid(70 %) werepurchased from VWR(Radnor,PA,USA).Isoflurane(MedicusPartnerLtd.,Biator- bágy,Hungary)forinvivopreparations,theanaestheticisoflurane wasproducedbyCP-Pharma(HandelsgesellschaftmbH,Germany);
atropineandlidocainewerepurchasedfromEgisPharmaceuticals Ltd.(Budapest,Hungary);EDTAandpargilinfromSigma-Aldrich, (StLouis,MO, USA);chloralhydrate fromMolarChemicals Ltd.
(Budapest,Hungary),andsterilephysiologicalsalinefromB.Braun AG,(Melsungen,Germany).
2.2. Animalsandtissuesamples
TheexperimentalprocedureswereapprovedbytheNational Food Chain Safety and Animal Health Directorate of Csongrád County,Hungary(licenseNo.XXXII./2015).Theprocedureswere performedaccordingtotheguidelinesoftheScientificCommittee ofAnimalExperimentationoftheHungarianAcademyofSciences (updatedLawandRegulationsonAnimalProtection:40/2013.(II.
14.)Gov.ofHungary),followingtheEUDirective2010/63/EUon theprotection.
Young3monthsold,maleSprague-Dawleyrats(CharlesRiver Laboratories,328±18g)wereusedinthisstudy.Standardrodent chowandtapwaterweresuppliedadlibitum.Theanimalswere housedunderconstanttemperature,humidityandlightingcondi- tions(23◦C,12:12hlight/darkcycle,lightsonat7a.m.).
Wesetouttodeterminethebloodplasmaandbrainconcentra- tionofDMTsynthesisedbytissuesinresponsetoischemia/hypoxia (i.e.endogenousDMT),andDMTcontentofbloodplasmaandbrain tissueafterDMTadministeredtotheanimalsbyintravenous(i.v.) infusion(i.e.exogenousDMT),withtheultimatepurposetoachieve neuroprotectionagainstcerebralischemia/hypoxia.
2.3. Surgicalproceduresandcollectionofsamples
For endogenous DMT measurements, rats were injected intraperitoneally(i.p.)withpargilin(50mg/bwkg,Sigma-Aldrich, USA),aneffectivemonoamine-oxidase(MAO)inhibitor,adayprior toandonthedayoftheexperiments.Forthesurgicalprocedures, animalswereanaesthetisedwith1.5–2%isofluraneinN2O:O2(3:2) andwereallowedtobreathespontaneouslythroughaheadcone duringtheexperiment.Bodytemperaturewaskeptat37.2◦Cby afeedback-controlledheatingpad(HarvardApparatus,Holliston, MA,USA).Atropine(0.1%,0.05mL;i.m.)wasadministeredaspre- medicationinordertoavoidtheproductionofairwaymucus.The leftfemoralarterywascannulatedfor blood sampling,andthe adjacentfemoralveinwascannulatedfordrugadministration.For thelaterinitiationofincompleteglobalforebrainischemia(n= 4),a midlineincisionwasmadeintheneck,andeach common carotidarterywascarefullyloopedaroundwithasurgicalthread.
Lidocaine(1%)wasusedforlocalanaesthesia.Controlratswere sham-operated(n=3).Allratswerefixedbytheirheadinaprone positioninastereotaxicapparatus.
After a baseline period (5–10 min), cerebral ischemia was inducedbytheocclusionofbothcommoncarotidarteries.Inhalf
anhourafterischemiaonset,theinsultwasaggravatedbyhypoxia achievedbythecompletewithdrawal (5min,endogenousDMT measurement)orcontrolledwithdrawal(1min,exogenousDMT measurement)ofO2fromtheanaestheticgasmixture.Thecom- plete withdrawal of O2 terminated the experiments,while the controlledwithdrawalofO2wasfollowedbyreoxygenationandthe promptreleaseofthecarotidarteriestoallowcerebralreperfusion foranotherhour.
InratspreparedforthemeasurementofexogenousDMT,the administrationofDMT(1mg/bwkg/hinphysiologicalsaline)or its vehicle (physiological saline) started upon ischemia induc- tionthrough thefemoralvein, withtheaidof amicroinjection syringepump(CMA/100MicroInjectionPump,CarnegieMedicine, Stockholm,Sweden).Thei.v.infusionofDMTwascontinuousand maintaineduntiltheendoftheexperiment.
ForthedeterminationoftheendogenousDMTcontentofblood plasmaandbraintissue,5mLarterialbloodwascollectedimme- diately after the 5-min withdrawal of O2. The full blood was centrifugedpromptlyinanEppendorftube(5000g,5min,4◦C, HeraeusFresco17Microcentrifuge,ThermoScientific,Waltham, MA,USA)coatedwithEDTA(ethylenediaminetetraaceticacid),and supernatantplasma(1mL)wasseparatedintoanotherEppendorf tube.Thebrainswereremovedandthen,aftersnap-frozeninliq- uidnitrogen,werestoredtogetherwiththeplasmaat−80◦Cuntil furtherprocessing.
Forthemeasurementofbloodplasmaandbraintissueconcen- trationofexogenousDMT,bloodsampleswerecollectedthrough thefemoralarteryatbaseline(i.e.priortoischemiaonset),under ischemia,afterhypoxiaandunderreperfusion.Theanimalswere transcardiallyperfused withice-cold physiological salineunder deepchloralhydrate anaesthesia(5%,i.p.,500mg/bwkg)atthe endoftheexperimentalprotocoltoeliminateanybloodfromthe brain.Thebrainswereremovedandsnapfrozeninliquidnitrogen, thenstoredat−80◦Cuntilfurtherprocessing.Bloodsampleswere processedasdescribedaboveandyielded0.2mLplasma.
2.4. Samplepreparationofratplasmasamples
Priortoextractionofplasma,5LIS(200ng/mLinmethanol) and5Lmethanolwasaddedto50Lofplasmasample.Aftervor- tex,50Lof5.3v/v%ammoniasolutionand300Lethylacetate wereaddedfollowedby30svortexandshakingfor10minatroom temperature(250rpm,orbital,Dual-ActionShakerKL2,Edmund Bühler,Bodelshausen,Germany).Thenthemixturewasrestedfor 5minonice.Thesamplewascentrifugedat4◦Cfor12minat 21,000g(Universal320R,Hettich,Tuttlingen,Germany).250L ofthe upperphase wascollectedand thelowerphase wasre- extractedwith300Lofethylacetate.Aftercentrifugation,300L oftheupperphasewascombinedwiththefirstportionofaliquot andwasdriedundernitrogenatroomtemperature.For2D-LC- HRMS/MSmeasurements,thedriedextractsweredissolvedin100
Lmethanol.
2.5. Samplepreparationofratbrainsamples
Rat brain tissue was homogenized based on the work by Kärkkäinen[10].Theweighedbrainsample(1gtissue,7mLice- cold0.1MHClO4)wasaddedinto12mLtubes.Thesamplewas sonicatedonicewithBiologicsModel150VTultrasonichomog- enizer(BioLogics,Inc.,Manassas, VA,USA) for3 × 1minusing mediumpowersettingonthemicro-tipprobeandan80%pulse.
Aftercentrifugationat9000gfor12minat4◦C(Universal320R, Hettich,Tuttlingen,Germany),5LIS(200ng/mLinmethanol), 5Lmethanolandfinally200Lof8%ammoniasolutionwere addedto400Lbrain homogenate.Aftervortex,500L ethyl acetatewasadded,followedby30svortexandshakingfor10min
atroomtemperature(250RPM,orbital,Dual-ActionShakerKL2, EdmundBühler,Bodelshausen,Germany).Thenthemixturewas restedfor5minonice.Thesamplewascentrifugedat4 ◦Cfor 12minat21,000g(Universal320R,Hettich,Tuttlingen,Germany).
350Loftheupperphasewascollectedandthelowerphasewas re-extractedwith500Lofethylacetate.Aftercentrifugation,500
Loftheupperphasewascombinedwiththefirstportionofthe aliquotfollowedbydryingundernitrogenatroomtemperature.
Forthe2D-LC-HRMS/MSmeasurements,thedriedextractswere dissolvedin100Lmethanol.
2.6. Preparationofsamplesforcalibrationandvalidation
1.00mg/mL stocksolutionsofDMTand AMTwereprepared by dissolving approximately1 mg aliquots of each component inmethanol.Thestockswereusedforthepreparation ofwork- ingcalibrationstandards.EightDMTworkingcalibrationstandard solutionswerepreparedinmethanol.Forexternalcalibration,the sample preparation wassimilar tothat of thetreated samples, exceptthatthesamplewasspikedwith5Lofthegivenconcen- trationofDMTinsteadof5Lmethanol.Thus,thefinalvolumes werethesameinthecalibrationsamplesandthetreatedsamples.
Externalcalibrationcurvesusinginternalstandardwereusedwith thefollowingcalibrationpointsofDMT(0,0.2,2,10,20,40,100 and200ng/mLplasma)inplasmaand(0,0.175,1.75,8.75,17.5,35, 87.5,175and350ng/g)inbrain.Eachcalibrationpointwasinjected inreplicateandrepeatabilitywasevaluatedfortheretentiontimes ofDMT.
Thelimitofdetection(LOD)andlimitofquantification(LOQ) werecalculated,respectively,onthebasisonthestandarddevia- tion(SD)andtheslope(S)ofthecalibrationcurve.
LOD= 3.3×SD S
LOQ= 10×SD S
Accuracyandprecisionwasdeterminedbyanalysingsamples at“Low”(10ng/mLinplasmaand17.5ng/ginbrain),“Mid”(40 ng/mLinplasmaand35ng/ginbrain)and“High”(200ng/mLin plasmaand350ng/ginbrain)concentrationsinthreeanalytical runs.
Carryoverwasassessedbyinjectingablanksampleafterinject- ingahighconcentrationofanalyte(plasma:200ng/mL,brain:350 ng/g).
ToevaluatethestabilityofDMTinplasmaandbrainsamplesin theautosampler(16◦C),calibrationsolutions(20ng/mLinplasma and35ng/ginbrain)werere-injected24hafterthefirstinjec- tionandcomparedtotheoriginalconcentrations.Theresultsofthe secondrunswereexpressedasthepercentageoftheirrespective valuesinthefirstrun.
2.7. 2D-LC-HRMS/MSconditions
The 2D-LC-HRMS/MS analysis was performed with Thermo Scientific Q Exactive Plus hybrid quadrupole-Orbitrap (Thermo FisherScientific,Waltham,MA,USA)massspectrometercoupled to a Waters Acquity I-Class UPLCTM (Waters, Manchester, UK) apparatus.TheUHPLCsystemequippedwithtwobinarysolvent managers,anauto-samplerandacolumnmanagerwithtwosix- port,two-positionautomaticswitchvalves.Theeluentofthetrap columnwasdilutedusingaKnauerHPLC-pump64(Knauer,Berlin, Germany)andahigh-pressurestainlesssteeltee(IDEX,OakHarbor, WA,USA).Thefirst-andsecond-dimensionalanalyticalcolumns werethermostattedinthecolumnmanageroftheUHPLCsystem,
whilethetrapcolumnwasinsertedintoanl-7350LaChromcolumn oven(Merck,Darmstadt,Germany).
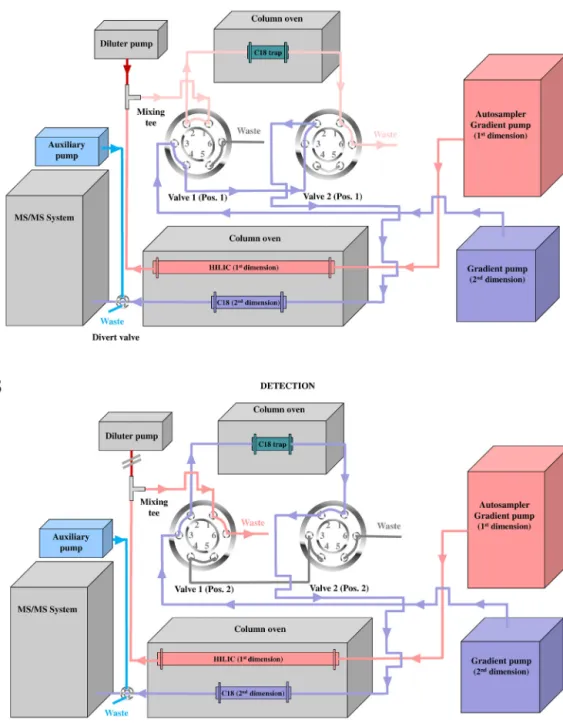
In the final 2D-LC-HRMS/MS analysis, the first-dimensional chromatographicseparation wascarried outonaKinetexHILIC column(150×2.1mm,2.6m,100Å,Phenomenex)protected byaHILICguardcolumn(4×3mm,2.6m,100Å,Phenomenex), whilethesecond-dimensionalseparationwasperformedonaLuna Omega Polar C18 column (50 × 2.1 mm, 1.6 m, 100 Å, Phe- nomenex).For theenrichmentof theanalytes,aLunaC18trap column(20×2mm,5m,100Å,Phenomenex)wasappliedand connectedthroughtwosix-portvalves(Fig.1).
Duringmethoddevelopment,thefollowing chromatographic columnsweretestedin thefirstdimension:LunaHILIC(150 × 3 mm,3 m, 200Å, Phenomenex), LunaOmega Sugar (100 × 2.1 mm,3 m, 100 Å,Phenomenex), LunaNH2 (150 × 2 mm, 3m, 100Å,Phenomenex)andKinetexC18(50× 2.1mm,2.6
m,100Å,Phenomenex).Columnstriedintheseconddimension wereLunaOmega PSC18 (50 × 2.1 mm,1.6 m, 100Å, Phe- nomenex),AcquityUPLCBEHC18(50×2.1mm,1.7m,130Å, Waters)andCORTECSUPLCC18+(50×2.1 mm,1.6m,90 Å, Waters).Bythe scheduledswitching ofthesix-portvalves,the dilutemobilephasefromtheHILICcolumninfirstdimensionwas trappedonLunaC18enrichmentcolumn(Fig.1).Forthepreven- tionoftheelutionoftargetedcompoundsfromtheenrichment columnsduringthetrappingprocess,thediluterpumpdelivered aqueousammoniasolution(pH10.2)at3mL/minflowrateinto thestaticmixingtee,whichwasconnectedtoaHILICcolumnand asix-portvalve.Thetemperatureoftrapcolumnswasmaintained at50◦C(Fig.1).
Inthefirstdimension,a10Lsamplewasinjectedwiththe Partial LoopWith Needle Overfill injection modeon a Kinetex HILICcolumnusingtheprogrammedgradientofeluentA(50mM ammoniumformatesolution)andeluentB(0.1%formicacidin acetonitrile).Thecolumntemperaturewassetat50◦Cduringthe analysis.Inthesecond dimension,eluent AandB,respectively, were0.1%formicacidsolutionand0.1%formicacidinacetoni- trile.ThemobilephasewashedthesubstancestrappedonaLuna C18enrichmentcolumnontoaLunaOmegaPolarC18column.The second-dimensionalcolumnwasmaintainedat50◦C.Thegradient programsofbothdimensionalseparationswiththetwosix-port valvepositionsaredetailedinTable1.
The mass spectrometer was operated in positive-ion mode usinga heatedelectrosprayionisation(ESI)sourcewiththefol- lowingconditions:capillarytemperature250◦C,S-LensRFlevel 50,sprayvoltage3.5kV,sheathgasflow45,sweepgasflow2and auxiliarygasflow10 inarbitraryunit.Inparallelreactionmon- itoring(PRM)withresolutionof17,500(FWHM),theautomatic gaincontrol (AGC)settingwasdefined as1 × 106 charges and themaximuminjectiontimewassetto50msThewidthofthe isolation windowof precursor ion was2 Da. Collisionenergies (CE)wereoptimisedforrespectivetransitionsofDMTandAMT (Fig.2).TheadditionalmainHRMS/MSparametersaresummarized inTable2.
During2D-LC-HRMS/MSmeasurements,theeluatewaspassed intotheESIsourceonlyinthetimerangeof4.5–5.5mininorder todecreasethecontaminationoftheMS.Fortheremainingtime, theESIsourcewasrinsedwithacetonitrile/watersolution(90/10, v/v)ataflowrateof0.1mL/minbyanauxiliary pump(Knauer HPLC-pump64,Knauer,Berlin,Germany).
TheLCsystemwascontrolledbyMassLynx4.1SCN901(Waters, Milford,MA,USA).ThecontroloftheMSsystemandMSdataacqui- sitionwasconductedbyXcaliburTM4.1software(ThermoFisher Scientific,Waltham, MA).GraphPad Prism5statistical software (GraphPadSoftware,SanDiego,CA,USA)wasusedfordrawingcol- umndiagrams,boxplotsandpointgraphs,andthecalculationof pairedt-test.
3. Resultsanddiscussion
3.1. Developmentofheart-cutting2D-LC-HRMS/MS
During analytical method development several parameters weretestedinbothdimensionsandtrappingproceduresuchas mobilephasecomposition,columntemperature,gradientsteep- ness, flow rate, type and length of column. For ESI-HRMS/MS detectionofDMTandAMT,theappropriatetransitions(quantifiers, qualifiersions)wereselectedandtherelatedcollisionenergiesand ESIparameterswereoptimised(Fig.2).
ThemainaimofoptimisationofHILICseparationofDMTand AMT(internalstandard)wastoachieveproperretainand peak shapeofDMTandAMT,whichisfeasibleinvolvingequilibration timewithinmaximum10minasaplannedcompleteruntimeof online2Dmeasurements.FortheoptimisationofHILIC,narrow- borecolumns(3and2.1mm)withlengthof100mmand150mm, packedwithtotallyporousorcore-shellparticleswerecompared withdifferentsolidphases.Thepresenceofwaterataminimum quantityof3%intheinitialmobilephasecompositionisessential inHILIC.Byvaryingitsconcentration,theretentionmechanismand separationcanbeinfluencedinbothisocraticandgradientelutions [19].
TheMS-compatibleammoniumformateasmobilephasemod- ifierwasselectedat50mMconcentrationapplyingacetonitrileas theorganiccontentofthemobilephase.Theflowrateisalsoacrit- icalparameterinthefirstdimension,sinceitmustbekeptaslow aspossible,thusassistingthetrappingoftheinvestigatedanalytes.
Unfortunately,theundesiredeffectoflowflowrateispeakbroad- ening.Inourcase,aflowrateof0.3mL/minprovidedanacceptable compromise.Forappropriatecolumnselection,fourHILICcolumns weretested(LunaHILIC,KinetexHILIC,LunaOmegaSugar,Luna NH2)usinga50mMammoniumformateaqueoussolution/0.1% formicacidinacetonitrile(90/10,v/v)mobilephasecomposition for6min.AcomparisonoftheseparationperformanceofAMTand DMTonthefourHILICcolumnsrevealedthatapplicationofLuna Omegawasdisadvantageousforbothretentionandpeakshapes ofDMT(1.35min)andAMT(1.40min).Similarly,whenLunaNH2
wasused,AMTgaveanextremepeakbroadening,whereasDMT (1.23min)hadarelativelylowretention.InthecaseofLunaHILIC, significantlyhigherretentiontimeswerefoundforbothDMT(3.89 min)andAMT(4.84min)withapeakwidthofapproximately1 min.Interestingly,theelutionorderchangedusingKinetexHILIC column,andtheretentiontimesofbothanalyteswerehigherthan 6 min.Therefore, theeluent strength wasincreased to50 mM ammoniumformateaqueoussolution/0.1%formicacidinacetoni- trile(85/15,v/v)resultingin3.09minand2.72minretentiontime forDMTand AMTand a peakwidthof approximately0.5min.
Consequently,thiscompositionwasselectedinthefinalmethod.
Interestingly,theelutionorderchangedusingtheKintexHILICcol- umnincomparisonwiththoseoftheotherthreecolumns.
Inthecaseofoptimisationofseconddimension,theprimary aimwastoobtainsharp,symmetricchromatographicpeaks(high sensitivity)withcloseretentionsforbothcompoundsusinggra- dientelutionandkeeping 10mintotalruntimeof2Danalysis.
Initially,fiveC18columnswerecompared,usinggradientelution (0–1min10%B,1–4min40%B,whereeluentsAandB,were0.1
%formicacidaqueoussolutionand0.1%formicacidinMeOH), respectively.UsingCORTECSC18,OmegaPSC18,andKinetexC18, relativelyhighpeakwidths(∼0.5and0.3minforDMTandAMT) wereobserved.TheapplicationofBEHC18andOmegaC18pro- videdhigherretentiontimesandlowerpeakwidthscomparedwith thoseoverotherC18columns.Finally,anOmegaC18microbore columnwithaparticlesizeof1.6mwasselectedforfurtheropti- misationprocedures.Thenatureoforganicsolventinfluencedboth peakwidthandretention.ForDMTandAMT,itcouldbeconcluded
Fig.1.Flowschemeoftheestablished2D-LC/MSsysteminbothvalvepositions:Position1(A)andPosition2(B).
Fig.2. ThestructureofDMT(A)andAMT(B)withassumedfragmentationcleavages,theexactmassofquantifierandqualifierionsandtheiroptimisedcollisionenergy.
thatACNinsteadofMeOHgavelowerretention,betterpeakshape andlowerpeakwidth.Forobtainingproperretentionwithin10- minruntimeincludingwashingandre-equilibrationofthecolumn
connectedtotrappingcolumnintheseconddimension,thetem- peratureofthecolumn,theflowrateandthegradientsteepness wereadjustedandsynchronizedwiththeHILICmethod.
Table1
Thegradientprogramsoffirstandseconddimensionwiththetwosix-portvalvepositionsandanalysismode.
Gradientprogramof1stdimension Gradientprogramof2nddimension Timetableofvalves Mode Time(min) B% Flowrate(mL/min) Time(min) B% Flowrate(mL/min) Time(min) Valve1 Valve2
0.00 85 0.3 0.00 2 0.5 0.00 Position2 Position2
1.80 Position1 Position1
trapping
3.00 2 0.5
3.10 2 0.3
3.50 2 0.3 3.50 Position2 Position2
4.00 85 0.3
analysis
4.50 50 1.0
5.50 100 0.3
6.00 100 0.3
6.10 100 0.5
6.50 50 1.0
7.00 85 1.2
8.00 Position1 Position1 8.01 Position2 Position2
9.00 100 0.5
9.50 2 0.5 9.50 Position1 Position1
9.51 Position2 Position2
9.90 85 1.2
10.00 85 0.3 10.00 2 0.5
Table2
MainHRMS/MSparametersforDMTandAMT(internalstandard)andretentiontimes.
Analyte Chemical formula
[M+H]+ Production1 (quantifierion)m/z
Collisionenergy (eV)
Production2(qualifier ion)m/z
Collisionenergy (eV)
Retentiontime (min)
DMT C12H16N2 189.1386 58.0651 10 144.0808 21 4.99
AMT C11H14N2 175.1230 158.0964 10 143.0730 30 4.99
Theonlinetrappingof DMTand AMTfromtheeluateofthe first-dimensionalHILICcolumnina2D-LCsystemwasperformed byusingC18trapcolumn.Thishighlyorthogonalseparation(HILIC
×RP-LC)requiredtodecreasethesolventstrengthoftheeffluentof thefirst-dimensionalcolumninordertoretainbothanalytesonthe enrichmentcolumn.Fig.1.demonstratestheexperimentalconfig- urationofour2D-LC-HRMS/MSsystem.The2Dinterfacebetween HILICandRPcolumnsconsistsofC18trapcolumnandtwoauto- maticallyoperatedtwo-positionsix-portvalves.Thedilutionofthe first-dimensionalHILICeluatewasperformedbyapplyingadiluter pumpconnectedtothecolumnthroughastaticmixingtee(Fig.1).
Theselectionofanappropriatedilutersolventandflowratewas crucialtoenrichDMTandAMTonthetrappingcolumnwithhigh efficiency.Itcanbeexplainedbythehighelutingpowerofeluate ofHILICseparationinRPmodeand,consequently,itneededtobe dilutedwithaweakRPsolvent.Initially,0.1%formicacidaque- oussolutionwasusedwithaflowrateof1mL/min.However,it resultedinpoorretentionforbothanalytesonthetrappingcolumn (Fig.1).Therefore,theeffectoftheflowrateofthedilutingeluenton retentionwasinvestigatedintherangeof1–3mL/min.Fig.3shows that,byincreasingtheflowrateupto3mL/min,trappingefficien- cieswereonlyslightlyimproved.Thesenegligiblechangescanbe explainedbythepKavaluesofDMT(8.68)[20]andAMT(9.96)[21].
AcomparisonrevealedthatatlowretentionintheRPmodeusing of0.1%formicacidaqueousdilutersolutionwithapHof2.7,the protonatedformsofDMTandAMTwerethedominantspecies.It seemedobviousthattheretentionofAMTandDMTonthetrapping columncanbeenhancedbysuppressingthepolarcationicforms byincreasingthepHofthedilutersolvent.TheincreaseofpHupto 10.2inaqueousammoniasolutionwasanacceptablecompromise consideringthesufficienttrappingofthetwoanalytesandthepH stabilityofboththetrappingandtheOmegaC18columns.Fig.3 demonstratesthedramaticimprovementoftrappingefficiencyof DMTandAMTbytheincreaseofpHofthedilutersolvent.ForDMT, higherenhancementwasfound,whichcouldbeexplainedbyits lowerpKavalueby∼1.3unitscomparedwiththatofAMT.
Fig.3.TheeffectoftheflowrateofdilutingeluenttotrappingefficiencyofDMT andAMTduringtrappingoftheheart-cut1Deluateintherangeof1–3mL/min (0.1%FA),and3mL/min(ammoniasolutionatpH10.2).Thepercentageincreases comparedtoconsecutiveexperimentsareindicatedbycurvedarrows.
Fig. 1 (A) displays the schematic diagram of our 2D-LC- HRMS/MSsystemin ¨Position1status,wherethe ¨C18Trap¨column wasusedtotrapDMTandAMTfromthediluteeffluentoftheHILIC column.Duringthetrappingperiod,thegradientprogramofHILIC methodwasinisocraticsection(Table1).Thesystemwaskept intrappingconfigurationfrom1.8minto3.49min.At3.50min theswitchingvalveswereturnedinto ¨Position2andthus ¨C18 trap¨columnwasconnectedtotheRPanalyticalcolumnasshown inFig.1(B).Theparticulardesignoftheeluentgradientprogram wasappliedtowashouttrappedanalytesfrom ¨C18trap¨column ontoC18analyticalcolumn,wherethechromatographicseparation occurredfollowedbyHRMS/MSdetectionofDMTandAMT.Table1 summarizesthefinalsetupofeventsandgradienttimeprograms inbothdimensions.Tokeeptheshort10min2Druntime,theHILIC columnwaswashedandequilibratedfrom3.5to10min,whilethe
Fig.4. ExtractedionchromatogramsofquantifieriontransitionofDMTandAMTdetectedbypositivemodewith1D-HILICmethod(A)and2D-LC-HRMS/MSmethod(B)in standardsolution.
trapandC18columnswereintherangesof8–10minandupto3.5 mininthenextrunusingeluentandtheflowrategradientpro- graminbothdimensions.Theobtained10mintotalruntimewas fasterthanvariouspublishedonedimensionalLC–MS/MSmethods forDMTanalysisinbrainandplasma[15–18].Theelutionprofiles ofDMTandAMTinthefinal1Dand2Dchromatogramsofstandard solutionsareshowninFig.4(A,B).
3.2. Determinationofextractionrecovery,matrixeffect,process efficiencyandmainvalidationparameters
Hence,thechallengeofthisworkwastofindarelativelysim- plesamplepreparationprocedure,whichissuitableforenrichment ofDMTfromratplasmaandbrainsamples.Intheliterature,sev- eral sample preparation methods related to DMT are reported [10,15–18,22,23].Unfortunately,inmanycasesthemainparam- eters,suchasextractionrecovery(RE),matrixeffect(ME),process efficiency(PE),characteristicsoftheefficiencyofagivenmethod, werenotpresented.Labour-intensiveandexpensivesolid-phase extraction procedures are widely employed nowadays for the enrichmentofDMTfrommammaliantissues[10,15,16].Theappli- cationof2D-LChasanabilitytodecreasethenegativematrixeffect underanalysistherebyprovidinganopportunitytosimplifythe samplepreparationprocedure.Therefore,theliquid–liquidextrac- tionprocedureseemedtobeanobviouschoiceforthedevelopment ofanewmethod.Withoutdetailingtheprocessoptimisationofthe samplepreparationprocedure,theuseofethylacetateasorganic solventandabasicpHinsamplesolutionhelpedtoenrichDMT properlyinthecaseofplasmaandbrainsamples(Fig.5(A,B)).
TherelevantRE,MEandPEofthenewmethodweredeterminedin accordancewiththeprocedureofMatuszewskietal.andCappiello etal.[24,25]
Fig.5(A,B)illustratesthemainindicators(RE,MEandPE)of thenewanalyticalmethodforDMTandAMTinratplasma(20 ng/mL)andbrain(17.5ng/mL).ForDMT,theobtained90%ofRE ofournewsample preparationprocedureinplasmawasbetter withapproximately20–30%thandatainearlystudies[17,18].The obtainedhighmeanvaluesofREwithreallygoodrepeatability(∼
Table3
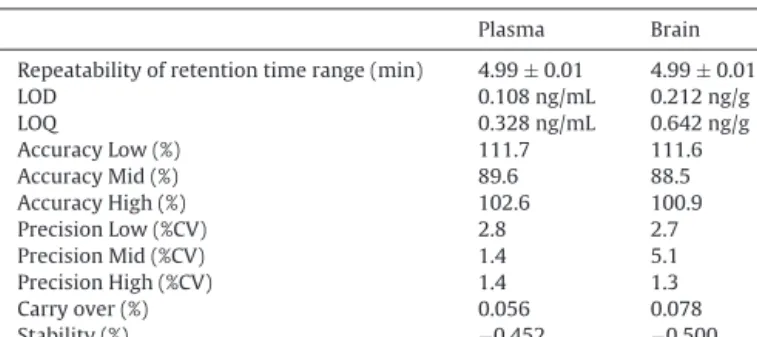
Mainvalidationparametersof2D-LC-HRMS/MSmethod.
Plasma Brain
Repeatabilityofretentiontimerange(min) 4.99±0.01 4.99±0.01
LOD 0.108ng/mL 0.212ng/g
LOQ 0.328ng/mL 0.642ng/g
AccuracyLow(%) 111.7 111.6
AccuracyMid(%) 89.6 88.5
AccuracyHigh(%) 102.6 100.9
PrecisionLow(%CV) 2.8 2.7
PrecisionMid(%CV) 1.4 5.1
PrecisionHigh(%CV) 1.4 1.3
Carryover(%) 0.056 0.078
Stability(%) −0.452 −0.500
Limitofdetection(LOD),limitofquantification(LOQ)andpercentcoefficientof variation(%CV).
10%SEM)forDMTandAMTstandardrevealthatournewextrac- tionmethodsarewell-suitedforbothplasmaandbrainsamples.
ThepresenceofstrongionisationenhancementduringHRMS/MS detectionresultedinahighpositiveMEvalueofDMTfromplasma (161%)andbrain(174%).TheobtainedhighMEinplasmawas much favourablethan reportedbyMeyers (46%) in 2014[17].
However,byinvestigatingMEofAMT,moderateionsuppression occurredresultinginavalueof87%inbrain(Fig.5(B)).Overall,the obtainedfavourableMEvaluesforbothcompoundsfromacomplex biologicalmatrixclearlyindicatedthebenefitofapplicationofthis 2D-LC-HRMS/MSmethod.InthecaseofDMT,the145%(plasma) and153%(brain)ofPE,asresultantsofvaluesofREandPE,lend evidencetotheadequacyofthenewmethod(Fig.5(A,B)).
Forbrainandplasmasamples,themainvalidationparameters ofDMTaresummarizedinTable3.Afterfulloptimizationofthe2D method,goodrepeatabilityoftheretentiontimeofDMTwithinthe rangeof4.98–5.00minwasachieved.Duringmethoddevelopment, themainissuewastokeeptherequiredvolumeofplasmaaslow aspossibleinordertodecreasethenegativeeffectofbloodcollec- tionduringsurgicalprocedures[26].ForDMT,our2D-LC-HRMS/MS methodprovided0.108ng/mLofLODusing50Lofplasma,which issignificantlybetterthanthosereportedinearlierstudies,suchas
Fig.5.Extractionrecovery,matrixeffectandprocessefficienciesforDMT(plasma:
20ng/mL,brain:17.5ng/mL)andAMT(plasma:20ng/mL,brain:17.5ng/mL)stan- dardsfromratplasma(A)andratbrainhomogenate(B).Theextractionrecoveryand matrixeffectpercentagewerecalculatedasfollows:Extractionrecovery(%)=C/B
×100,Matrixeffect(%)=B/A×100andProcessefficiency(%)=C/A×100,whereA representstheaveragepeakareaofthestandardsolution,Brepresentstheaverage peakareaoftheplasmaorbrainextractspikedatthesameconcentrationofthe standardandCrepresentstheaveragepeakareaofaplasmaorbrainpre-spikedat thesameconcentrationofthestandard.
100ng/mLusing500Lplasma[17]and0.45ng/mLfrom200L plasma[18].Interestingly,Oliveiraetal.reported0.1ng/mLofLOD forDMTinplasma[16].However,thesolidphaseextractionpro- cedureoftheiranalyticalmethodrequired1000Lofplasma.In brainsampletheachievedLODofDMTinourcasewasalsobetter thanthatreportedbyBarkeretal.(0.5ng/g)[15].
Thecalibrationcurves(Figs.S13–S24)forDMTwerelinear(R2
>99%)intherangeof0.2–200ng/mLplasmaand1.75–350ng/g brain.Accuracyofournewmethodinthreedifferentconcentration levelsweredeterminedwithinanacceptableintervalof12%in plasmaandbrainsamples.Theprecisionswerefoundtobe≤2.8% coefficientofvariation(CV)incaseofplasmaand≤5.1%CV(brain).
TheobtainedcarryoverofDMTwasnegligible(<0.08%)inplasma andbrain.
3.3. QuantificationofDMTinratplasmaandbrain
Inthefirstdimensionofourorthogonal2Dmethod,HILICmode was appliedfor theseparation of DMT and AMT from plasma andbrainmatrix-derivedendogenouscomponents.Inthemethod developmentprocess,animportantconsiderationwastocutthe peaksofinterest from1Dto2Din anarrowtime window(1.8 minfor 3.5min), thus tokeeptheamountof undesirable exo- andendogenousmatrixcompoundsinalowlevelinthesecond dimension.Itisworthtobenotedthatthetrappingprocessatbasic pHalsodecreasedtheamountofcompoundswithacidiccharac- terwithachromatographicbehavioursimilartothoseofDMTand AMTinHILICcondition.Forthesereasons,arelativelyfastgradient methodwasappliedintheseconddimension.TheobtainedMEval- uesconfirmedthevalidityofthesteepRPgradientprogramapplied.
AnotheradvantagewasthatAMTasinternalstandardallottedwith DMTastargetedcompoundatsameretentiontime,butnotindead time,whichisthechromatographicexpectationofthestandard calibrationmethodtowardsaninternalstandard(Figs.S1–S4).
ThedevelopedmethodwasappliedtothequantificationofDMT inratplasmaandbraintissue.Themainaimsoftheapplication ofthisnew2D-LC-HRMS/MSmethodweretofollowtheplasma DMTlevelbeforeischemiaonset, underischemia,afterhypoxia andunderreperfusion,anddeterminationofDMTconcentration inrelatedbraintissueafterreperfusion.Therefore,theplasmaand brainsamplesofpreliminaryexperimentswereusedforoptimi- sationofthesamplepreparationprocedureanddeterminationof theconcentrationrangeofexternalcalibrationusinginternalstan- dards.Awidecalibrationconcentrationrangewasattemptedfor quantificationofendogenousandexogenousDMTlevelsinasingle run.Toourknowledge,thereexistsonlyasinglereportfrom2005 inwhichendogenousDMTwasquantifiedinbothratbrainand plasmausingisoctraticLC–MS/MSmethod[10].Unfortunately,we couldnotconfirmthepresenceofendogenousDMTinratbrainand plasma.Itisimportanttonote,however,thatour2D-LC-HRMS/MS methodprovidedmorereliabledatadueto2D-LCseparationand accuratehighresolutionmassspectrometrydetectionwithrelated strictLCandHRMSMSconfirmatorycriteria:specificretentiontime (4.99min)withtwocharacteristicprecursor–productiontransi- tionsusing50ppmmassaccuracy(quantifierionat58.0651m/z andqualifierionat144.0808m/z)andtheratioofthepeakarea ofthequantifierionandqualifierion(2.1±0.21)(Figs.S5–S12).
Despitethefactthatthequantifierionwasdetected,butonethe above-mentionedotherparameterswasnotcompleted,thecon- centrationofDMTwasfoundtobelowerthanthelimitofdetection.
Inthefinalmethod,thecalibrationcurvewasoptimisedtomea- sureexogenousDMTlevel,andDMTshowedagoodlinearityrange of0.2–200ng/mLplasmaand1.75–350ng/gbrainwithR2values higherthan0.99(Figs.S13,S14).
ThelevelofDMTinplasmasamplesoftreatedratsisillustrated inFig.6(A).Allplasmasamplescollectedbeforeadministrationof DMTshowedlessthanthedetectableamountsofDMT.Thehigh- estconcentrationofDMTintherangeof49.3–114.3ng/mLwas observedinplasmaforeachanimalbeforehypoxia.Foranimals1, 3and4,theconcentrationsofDMTdecreasedsignificantlyafter hypoxiainplasma.Afterhypoxiatotheendofthetreatment,the concentrationofDMTdecreasedtoameanvalueof3.8ng/mL,in linewiththeterminationofDMTapplication.
Another question that remains to be answered is whether DMTaccumulatestoadetectableconcentrationinthebrain.Thus, endogenousandexogenousDMTwereanalysedinbraintissuein boththecontrolandtreatedanimals.Similartoplasmasamples, theendogenousDMTwasnotdetectableinthebrainofcontrolani- mals.However,aftertreatment,theconcentrationofDMTchanged intherangeof2–6.1ng/gbrain(Fig.6(B)).
Fig.6. TheobtainedconcentrationofDMTinplasma(A)andbrain(B).Three repeatedmeasurementswereusedforstatisticalanalysis(*p≤0.05;**p≤0.01;***
p≤0.001).
Theconcentrationsof exogenousDMTmeasuredareconsid- eredtobeunderestimatedoftheactualconcentrations,because DMTmusthavebeenrapidlydegradedduringcollectingbiological samples[27],inparticular,whenharvestingbraintissue.
4. Conclusion
Thispaperdescribesthedevelopmentandapplicationofafast heart-cutting2D-LC-HRMS/MSmethodand relatedliquid-liquid extractionprocedurefortheanalysisofDMT.Byconnectingorthog- onalHILIC and RPchromatography through theuseof RP trap column and high resolution MS/MS detection, a sensitive and selectiveanalyticalmethodwassuccessfullyutilisedwithintotal run time of 10 min for determination of the concentration of DMTinratplasmaandbrainofexperimentalmodelofcerebral ischemia/reperfusion using DMT administration The developed andoptimisedplasmaandbrainmatrix-relatedsampleprepara- tionprotocolsprovided high extractionrecoveries(∼90 %) and highpositive matrix effect (plasma: +61 %, brain: ∼ +74 %) of DMT.During analysis,DMTwasidentifiedandconfirmedbyits specificretentiontime inthesecond dimension,twocharacter-
isticprecursor–productiontransitionsandtheratioofquantifier toqualifier masstransitions. External calibrationusinginternal standard wasappliedtoobtainaccurateconcentrationsof DMT inplasmaandbrainsamples.Thisheart-cutting2D-LC-HRMS/MS methodwithnewsamplepreparationproceduregaveanopportu- nitytoimprovetheLODwiththedecreaseoftherequiredamount ofbiologicalsamples.
ConcerningexogenousDMT,tothebestofourknowledge,thisis thefirstattempttoconfirm–withananalyticaltool–thepresence ofDMTadministeredwiththerapeuticalpurposeinmammalian tissues.Insummary,ouranalyticalapproachissuitabletodetect andconfirmthepresenceofDMTadministeredtoexperimental animalswiththerapeuticpurpose.Furtherrefinementofbiologi- calsampleharvestingwillprovidemoreaccuratedeterminationof actualDMTconcentrations.
DeclarationofCompetingInterest Authorsdeclarenoconflictofinterest.
Acknowledgement
This research was supported by the EU-funded Hungarian grantEFOP-3.6.1-16-2016-00008 and <GN1>G</GN1>INOP-2.3.2- 15-2016-00060,andtheMinistryofHumanCapacitiesofHungary grantNo.ÚNKP-19-3-SZTE-266.
WewishtothankÉvaSija(InstituteofForensicMedicine,Uni- versityofSzeged),whokindlyprovidedtheAMTstandard.
AppendixA. Supplementarydata
Supplementarymaterial related tothis articlecan befound, in theonline version, at doi:https://doi.org/10.1016/j.jpba.2020.
113615.
References
[1]J.G.Dean,T.Liu,S.Huff,B.Sheler,S.A.Barker,R.J.Strassman,M.M.Wang,J.
Borjigin,Biosynthesisandextracellularconcentrationsof
N,Ndimethyltryptamine(DMT)inmammalianbrain,Sci.Rep.9(1)(2019) 9333,http://dx.doi.org/10.1038/s41598-019-45812-w.
[2]S.A.Barker,J.Borjigin,I.Lomnicka,R.Strassman,LC/MS/MSanalysisofthe endogenousdimethyltryptaminehallucinogens,theirprecursors,andmajor metabolitesinratpinealglandmicrodialysate,Biomed.Chromatogr.27(12) (2013)1690–1700,http://dx.doi.org/10.1002/bmc.2981.
[3]L.Corbett,S.T.Christian,R.D.Morin,F.Benington,J.R.Smythies,
HallucinogenicN-methylatedindolealkylaminesinthecerebrospinalfluidof psychiatricandcontrolpopulations,Br.J.Psychiatry132(2)(1978)139–144, http://dx.doi.org/10.1192/bjp.132.2.139.
[4]F.R.Franzen,H.Gross,N.Tryptamine,N-dimethyltryptamine,
n,n-dimethyl-5-hydroxytryptamineand5-methoxytrytamineinhumanblood andurine,Nature206(1965)1052,http://dx.doi.org/10.1038/2061052a0.
[5]T.J.Wolff,TheTouristicUseofAyahuascainPeru.Sozialwissenschaftliche Gesundheitsforschung,SpringerVS,Wiesbaden,2020,ResearchaboutDMT andayahuasca.
[6]N.V.Cozzi,A.Gopalakrishnan,L.L.Anderson,J.T.Feih,A.T.Shulgin,P.F.Daley, A.E.Ruoho,Dimethyltryptamineandotherhallucinogenictryptamines exhibitsubstratebehaviorattheserotoninuptaketransporterandthevesicle monoaminetransporter,J.NeuralTransm.116(12)(2009)1591–1599,http://
dx.doi.org/10.1007/s00702-009-0308-8.
[7]S.Sato,T.Kawamata,T.Kobayashi,Y.Okada,Antidepressantfluvoxamine reducescerebralinfarctvolumeandamelioratessensorimotordysfunctionin experimentalstroke,NeuroReport25(10)(2014)731–736,http://dx.doi.org/
10.1097/WNR.0000000000000162.
[8]A.Szabo,A.Kovacs,J.Riba,S.Djurovic,E.Rajnavolgyi,E.Frecska,The endogenoushallucinogenandtraceamineN,Ndimethyltryptamine(DMT) displayspotentprotectiveeffectsagainsthypoxiaviasigma-1receptor activationinhumanprimaryiPSC-derivedcorticalneuronsandmicroglia-like immunecells,Front.Neurosci.10(2016)423,http://dx.doi.org/10.3389/fnins.
2016.00423.
[9]S.A.Barker,E.H.McIlhenny,R.Strassman,Acriticalreviewofreportsof endogenouspsychedelicN,N-dimethyltryptaminesinhumans:1955–2010, DrugTest.Anal.4(7–8)(2012)617–635,http://dx.doi.org/10.1002/dta.422.
[10]J.Kärkkäinen,T.Forsström,J.Tornaeus,K.Wähälä,P.Kiuru,A.Honkanen, U.-H.Turpeinen,A.Hesso,Potentiallyhallucinogenic5-hydroxytryptamine receptorligandsbufotenineanddimethyltryptamineinbloodandtissues, Scand.J.Clin.Lab.Invest.Suppl.65(3)(2005)189–199,http://dx.doi.org/10.
1080/00365510510013604.
[11]D.E.Nichols,N,N-dimethyltryptamineandthepinealgland:separatingfact frommyth,J.Psychopharmacol.32(1)(2018)30–36,http://dx.doi.org/10.
1177/0269881117736919.
[12]S.Bäurer,M.Ferri,A.Carotti,S.Neubauer,R.Sardella,M.Lämmerhofer, Mixed-modechromatographycharacteristicsofchiralpakZWIX(+)andZWIX (−)andelucidationoftheirchromatographicorthogonalityforLC×LC application,Anal.Chim.Acta1093(2020)168–179,http://dx.doi.org/10.
1016/j.aca.2019.09.068.
[13]R.Berkecz,F.Tömösi,T.Körmöczi,V.Szegedi,J.Horváth,T.Janáky, Comprehensivephospholipidandsphingomyelinprofilingofdifferentbrain regionsinmousemodelofanxietydisorderusingonlinetwo-dimensional (HILIC/RP)-LC/MSmethod,J.Pharmaceut.Biomed.149(2018)308–317, http://dx.doi.org/10.1016/j.jpba.2017.10.043.
[14]M.Kula,D.D.Głód,M.Krauze-Baranowska,Applicationofon-lineandoff-line heart-cuttingLCindeterminationofsecondarymetabolitesfromtheflowers ofLoniceracaeruleacultivarvarieties,J.Pharmaceut.Biomed.131(2016) 316–326,http://dx.doi.org/10.1016/j.jpba.2016.09.010.
[15]S.A.Barker,M.A.Littlefield-Chabaud,C.David,Distributionofthe hallucinogensN,N-dimethyltryptamineand5-methoxy-N,
N-dimethyltryptamineinratbrainfollowingintraperitonealinjection:
applicationofanewsolid-phaseextractionLC–APcI–MS–MS–isotopedilution method,J.Chromatogr.BBiomed.Sci.Appl.751(1)(2001)37–47,http://dx.
doi.org/10.1016/S0378-4347(00)00442-4.
[16]C.D.R.Oliveira,G.G.Okai,J.L.daCosta,R.M.deAlmeida,D.Oliveira-Silva,M.
Yonamine,Determinationofdimethyltryptamineand-carbolines (ayahuascaalkaloids)inplasmasamplesbyLC–MS/MS,Bioanalysis4(14) (2012)1731–1738,http://dx.doi.org/10.4155/bio.12.124.
[17]M.R.Meyer,A.Caspar,S.D.Brandt,H.H.Maurer,Aqualitative/quantitative approachforthedetectionof37tryptamine-deriveddesignerdrugs,5
-carbolines,ibogaine,andyohimbineinhumanurineandplasmausing standardurinescreeningandmulti-analyteapproaches,Anal.Bioanal.Chem.
406(1)(2014)225–237,http://dx.doi.org/10.1007/s00216-013-7425-9.
[18]E.H.McIlhenny,J.Riba,M.J.Barbanoj,R.Strassman,S.A.Barker,Methodology fordeterminingmajorconstituentsofayahuascaandtheirmetabolitesin blood,Biomed.Chromatogr.26(2012)301–313,http://dx.doi.org/10.1002/
bmc.1657.
[19]P.Hemström,K.Irgum,Hydrophilicinteractionchromatography,J.Sep.Sci.
29(12)(2006)1784–1821,http://dx.doi.org/10.1002/jssc.200600199.
[20]DimethyltryptamineBioinformaticsandCheminformaticsData,Drugbank, 2020(Accessed15June2020)https://www.drugbank.ca/drugs/DB01488.
[21]IndopanBioinformaticsandCheminformaticsData,Drugbank,2020 (Accessed15June2020)https://www.drugbank.ca/drugs/DB01446.
[22]R.W.Walker,H.S.Ahn,G.Albers-Schönberg,L.R.Mandel,W.J.A.
Vandenheuvel,Gaschromatographic-massspectrometricisotopedilution assayforN,N-dimethyltryptamineinhumanplasma,Biochem.Med.8(1) (1973)105–113,http://dx.doi.org/10.1016/0006-2944(73)90014-8.
[23]L.J.Riceberg,H.V.Vunakis,DeterminationofN,N-dimethylindolealkylamines inplasma,bloodandurineextractsbyradioimmunoassayandhighpressure liquidchromatography,J.Pharmacol.Exp.Ther.206(1)(1978)158–166.
[24]B.K.Matuszewski,M.L.Constanzer,C.M.Chavez-Eng,Strategiesforthe assessmentofmatrixeffectinquantitativebioanalyticalmethodsbasedon HPLC−MS/MS,Anal.Chem.75(2003)3019–3030,http://dx.doi.org/10.1021/
ac020361s.
[25]H.Trufelli,P.Palma,G.Famiglini,A.Cappiello,Anoverviewofmatrixeffects inliquidchromatography–massspectrometry,MassSpectrom.Rev.30(3) (2011)491–509,http://dx.doi.org/10.1002/mas.20298.
[26]K.H.Diehl,R.Hull,D.Morton,R.Pfister,Y.Rabemampianina,D.Smith,J.-M.
Vidal,C.V.D.Vorstenbosch,Agoodpracticeguidetotheadministrationof substancesandremovalofblood,includingroutesandvolumes,J.Appl.
Toxicol.21(1)(2001)15–23,http://dx.doi.org/10.1002/jat.727.
[27]S.A.Burchett,T.P.Hicks,Themysterioustraceamines:protean neuromodulatorsofsynaptictransmissioninmammalianbrain,Prog.
Neurobiol.79(5–6)(2006)223–246,http://dx.doi.org/10.1016/j.pneurobio.
2006.07.003.