L . T . E V A N S
U.S. Department of Agriculture, Belts ville, Maryland
Work over the last 20 years, most notably that at Beltsville, has established that a photoreversible protein pigment, phytochrome, controls a wide spectrum of physiological displays in plants, including germination, stem lengthening, leaf expansion, anthocyanin synthesis and flowering. Phytochrome control of these displays is, to a large extent, saturated by brief exposures to red or far-red light at relatively low-energy levels.
However, prolonged exposures at high-energy levels, particularly to blue and far-red light, also have pronounced effects on most of these displays. The nature of the photoreceptor for this high-energy reaction is unknown. It is possible that some of the responses to high energy are mediated by phytochrome (HENDRICKS and BORTHWICK, 1959), by photosynthesis (DOWNS et al, 1964), or perhaps even by the phototropic pigment.
The relation between the high-energy reaction and phytochrome action is also uncertain. MOHR'S (1959) model suggests that they act independently. But the effects of the high-energy reaction on antho- cyanin synthesis, on stem elongation and on twining in dodder, depend entirely on the form in which phytochrome is left following the high- energy exposure (DOWNS and SIEGELMAN, 1963; EVANS et al, 1965;
LANE and KASPERBAUER, 1965). This suggests that the phytochrome- controlled step follows that of the high-energy reaction in one synthetic sequence. The results given in some of the papers presented below are interpreted in this light.
D E - A E T I O L A T I O N I N C I T R U S S E E D L I N G S
Dark-grown, aetiolated seedlings are characterized by long hypocotyls and small leaves. Another characteristic of some dark-grown seedlings is the presence of an epicotyl or hypocotyl hook. In some plants, such as many legumes, shortening of the hypocotyls, increase in leaf size and hook opening (i.e. de-aetiolation) appears to be largely mediated
187
ι88 P H O T O E N V I R O N M E N T
by phytochrome (PARKER et al, 1949; D O W N S , 1955; LIVERMAN et al,
1955). In other plants, such as lettuce (MÖHR and WEHRUNG, i960;
M O H R and NOBLE, i960), the high-energy reaction has a greater effect on de-aetiolation, while in Petunia, for example, the participation of both photoreactions is evident (EVANS et al, 1955).
The results presented by S.P.MONSELISE and A . K A D M A N - Z A H A V I in their paper' Morphogenetic responses of epicotyl hook, primary leaves
166' far red
Fi g . ι . Epicotyl hook angle and leaf length in citrus seedlings following
various light treatments (Mo n s e l i s e and Ka d m a n- Za h a v i ) .
and first internode of citrus seedlings to irradiance \ suggest that citrus may belong to this last category. Figure 1 presents the results of one of their experiments, in which the seedlings were exposed to various treatments for 26 h, and then returned to darkness for 6 days before measurement.
Neither a single exposure to red light for 3 min nor exposure to a long period of intermittent (3 min every 30 min) far-red light increased leaf length or opened the epicotyl hooks to any marked extent. How- ever, intermittent far red for 26 h followed by 3 min of red light increased leaf length and hook opening almost to the same extent as continuous white light. Intermittent red light was less effective, and when terminated by 3 min of far-red light the effect of the intermittent red light was largely lost.
Two other experiments confirmed these conclusions, except that intermittent red light greatly increased leaf length but opened the epicotyl hooks only slightly in one experiment. Far-red light alone, either continuously for 3 h, or 4 min every 30 min for 22 h, had little effect on either leaf length or hook opening.
The interpretation I would make of these results is that both leaf lengthening and hook opening in citrus are promoted by phytochrome Pf r action on the product of a high-energy reaction. The intermittent far-red irradiation for 26 h partially satisfies the high-energy reaction, and the terminal exposure to red light then allows Pf r action, giving a response similar to that in continuous white light, whereas prolonged far red alone, or a single exposure to red light, had no effect.
D E - A E T I O L A T I O N I N G H E R K I N S E E D L I N G S
In the experiments reported by G . Me i j e r and G . En g e l s m a in ' T h e synergistic influence of a pre-irradiation on the photo-inhibition of gherkin seedlings', the seedlings were grown in darkness at 2 50C for 66 h, then given an 8-h pre-irradiation treatment, after which they were moved to a second, continuous irradiation, growth rate being measured between the 16th and 40th hours of this.
The first irradiations were with blue, red or far-red light of high intensity (usually 200-2000 fiw/cm2), and alone they had little or no effect on increase in hypocotyl length during the measuring period.
The second irradiations were usually with red light of rather lower intensity. This reduced hypocotyl growth, even at intensities as low as 7 /xw/cm2, but the inhibitory effect of the red light increased with increase in intensity up to 660 jLtw/cm2 (see Fig. 2).
The low-energy action of red light suggests a phytochrome mediated de-aetiolation, but there is no action spectrum or evidence of reversi- bility to substantiate this suggestion. The exposures to red light are long enough that the red light could also be participating in a high- energy reaction.
The inhibitory effect of the red light was considerably enhanced by pre-illumination with blue light (see Fig. 2). Data from three separate but comparable experiments are combined in Fig. 3 to indicate the dependence of this pre-illumination effect on intensity and wave- length. At intensities of 200-400 f t w / c m2 far-red light potentiated the inhibitory effect of the subsequent red light more strongly than did blue light, while red light was still less effective. However, the effect of red light increased with intensity until it equalled the greatest effect of
IQO P H O T O E N V I R O N M E N T
far red. The marked dependence of these effects on intensity of pre- illumination, the fact that pre-illumination with far red had an effect
50
en c φ
^ \ ° \
•D
•8B
\
8D-RX\
\
8B-RX
Intensity red light in uW/cm32 2 (Rx
57
Fi g . 2. Effect of an 8-h pre-irradiation with blue light (8B —345
p / c m2) on the inhibitory effect on gherkin hypocotyl elongation of subsequent irradiation with red light (Rx) of various intensities
( Me i j e r and En g e l s m a ) .
only when exposures were longer than 2 h, and the greater response to far-red and blue light suggest that the pre-illumination effect is due to the high-energy reaction. Since red light apparently also participates
r30 mm
X
I ι I i i - ι ι ι ι ι ι
40 60 80 100 200 400 1000 2000 Intensity of pre irradiation μνν/cm2
FIG. 3. Effect of intensity and colour of pre-irradiation for 8 h on the inhibitory effect of subsequent red light on gherkin hypocotyl elonga- tion (MEIJER and ENGELSMA).
energy reaction since the inhibitory effect of pre-irradiation is apparent only when it is followed by red light and not, for example, when far red is followed by blue light.
A N T H O C Y A N I N S Y N T H E S I S I N T U R N I P S E E D L I N G S
Light may act at several points in the synthesis of anthocyanins, in the formation of both A ring (SIEGELMAN and HENDRICKS, 1957; GRIESE- BACH, 1958), and Β ring precursors (SCHMIDT, 1962), and possibly also in the high-energy reaction in gherkin, a clear separation of the high- energy reaction and phytochrome effects is not possible. However, the evidence suggests that a phytochrome mediated step follows the high-
I Q 2 P H O T O E N V I R O N M E N T
0-7
0-6
0-5
.0-4
Ε
f0
c
- Ω Ο
0-2
0-1
S 0
A Intact seedlings
cotyledons and hypocotyl separated
C Roots removed
iS) c
" Ό ο
>»
Ο Ο
< C
0-1
0·2·
ο··Η
ο
C L Χ
Dark Blue Far-red
F i g . 4. Effect of cotyledon excision and root removal on the amount of anthocyanin in cotyledons and hypocotyls of turnip in darkness, blue light (616 /xw/cm2) or far-red light (351 p / c m2) (G r i l l and Vi n c e ) .
in late steps in the flavonoid transformations (BOPP and MATTHISS, Ι961). The diversity of action spectra for anthocyanin synthesis is, therefore, not unexpected. In their paper 'Anthocyanin formation in turnip seedlings (Brassica rapa L.) : evidence for two light steps in the
biosynthetic pathway', R.GRILL and D . V I N C E present evidence for spatial separation of two light-dependent reactions required for antho- cyanin synthesis in the turnip hypocotyl, the first occurring in the cotyledons, the second in the hypocotyl itself.
Seedlings were grown in darkness at 25 0 C for 24-72 h, then exposed for up to 48 h to either blue or far-red light of high intensity (616 or 351 jaw/cm2, respectively), and the anthocyanin estimations made 48 h after the beginning of irradiation.
The results in Fig. 4 show that little anthocyanin was formed in darkness compared with that formed after 48 h in blue or far-red light.
Removal of roots from the seedlings (presumably reducing the drain on stored materials from the cotyledons) greatly increased anthocyanin synthesis in the cotyledons, which suggests that anthocyanin synthesis depends on materials stored in the cotyledons. This conclusion is supported by their finding that the amount of anthocyanin formed per seedling decreased progressively with increase in seedling age at the time illumination began, probably reflecting a decline in stored materials remaining in the cotyledons. Also, cotyledons excised after 24 h and then kept in darkness for a further 24 or 48 h before irradiation produced far more anthocyanin than those excised at 48 or 72 h and irradiated immediately.
The first light-dependent step in the cotyledons occurred under blue and far-red light. The yield of anthocyanin increased with duration of illumination up to 48 h, suggesting participation of the blue, far-red high-energy reaction.
Excision of the cotyledons at the beginning of the light treatments increased the synthesis of anthocyanin in them but prevented any being formed in the hypocotyls (Figs. 4 and 5). Covering the cotyle- dons, also, largely prevented anthocyanin synthesis in the hypocotyls (Fig. 5E ) .
Three explanations of these results are possible: (1) The antho- cyanins are wholly synthesized in the cotyledons and then translocated to the hypocotyl. This is unlikely in view of the results in Fig. 5 (C and D ) , where virtually no anthocyanin appeared in covered sections of the hypocotyl. (2) Translocation of a stored precursor from the cotyledons is dependent on light. This does not appear to be so since anthocyanin synthesis in the hypocotyl of intact seedlings increased with the duration of growth in darkness before illumination, which shows that the materials for anthocyanin synthesis are translocated from the cotyledons in darkness. (3) A precursor of anthocyanin synthesis is
13
194 P H O T O E N V I R O N M E N T
0-AH
0-3H
0-2
3, E 0 1 LT)
C aJ
l_ Ο ω rö 04
*c c >·, Ο rö
^ 0-2-ο
<
0·3·
ο·/η
0-5H
ΙΛ C Ό ο
φ
"δ ο
ο ο ο Q.
>.
Χ
Α = Intact
Β = Cotyledons excised C= Upper hypocotyl covered D = Whole hypocotyl covered Ε = Cotyledons covered F = Dark
Amount in covered part
Fi g . 5. Anthocyanin synthesis in turnip seedlings exposed to blue light for 48 h following cotyledon excision or covering of cotyledons or hypocotyl. The stippled areas show anthocyanin present in the parts of the hypocotyl above the cover (Gr i l l and Vi n c e ) .
formed in the cotyledons in high-energy light and is then translocated to the hypocotyls. The results in Fig. 5 (C and D) suggest that a further light reaction is then needed for anthocyanin synthesis to occur in the hypocotyls. All that can be said of this at the moment is that it can proceed in prolonged blue or far-red light. Action by phytochrome is not excluded, although SIEGELMAN and HENDRICKS (1957) found no evidence of its participation in anthocyanin synthesis by turnip seedlings. This second photoreaction could be a direct conversion of leucoanthocyanin to anthocyanin.
L A T E R A L R O O T I N I T I A T I O N I N P E A S
Another system in which both the high-energy reaction and phyto- chrome may have a role is in the initiation of lateral branches on isolated pea roots. In an earlier paper TORREY (1952) found that a single brief exposure to red light inhibited the formation of lateral roots otherwise induced by decapitation of cultured, isolated roots, whereas with intact plants repeated exposures were necessary. In the paper ' T h e reversible inhibition by red and far-red light of auxin-induced lateral root initiation in isolated pea roots', M.FURUYA and J.G.TORREY have found that single exposures of up to 32 min to low-intensity red light had no effect on lateral root initiation on segments from intact seedling roots, although phytochrome was readily detectable in them by differential spectrophotometry. When the root tips of 3-day-old seedlings grown in darkness were excised aseptically and grown for 7 days in darkness at 25 ° C on a modified Bonner medium, phyto- chrome was no longer detectable spectrophotometrically. However, the inhibition of auxin-induced lateral root development by brief exposures to red light was pronounced. Six-millimeter-long segments taken 6 - 1 2 mm behind the cultured root tips and treated with 5 χ i o ~5M I A A initiated about five lateral roots in darkness during the week following excision, whereas those exposed to 10 or more k-ergs c m2 of red light initiated about two. Segments taken further back from the root tip needed more red light to saturate the in- hibitory effect. Those taken 24-30 mm behind the tip needed about 120 k-ergs/cm2 for full effect. Thus, there may be an interaction between phytochrome action and auxin status in this system.
The dosage range of 10-100 k-ergs/cm2 for saturation of the in- hibition of lateral root formation by red light is comparable to that for other phytochrome-dependent phenomena in pea plants. The inhibition by red light was shown to be fully reversible by a brief
196 P H O T O E N V I R O N M E N T
subsequent exposure to far-red light, and to be repeatedly reversible.
Thus, the inhibition of lateral root initiation in these isolated root segments is clearly mediated by phytochrome even though the pigment could not be detected spectrophotometrically.
Although brief exposures (up to 8 min) to blue light had no effect on lateral root initiation, exposure to 21 k-ergs/cm2/min continuously for 7 days had a marked effect. With segments taken 24-30 mm behind the tip, about six laterals per segment were formed in darkness, while about three laterals were formed after saturating doses of red light. In continuous blue light the number was even less, about two roots per segment, which suggests that the high-energy reaction may also participate in this system.
F R O N D G R O W T H I N Lemna minor
The light requirement for the growth of Lemna minor can be met by brief exposures to red light, subsequent far red eliminating the light response (HILLMAN, 1957). In the paper ' T h e influence of periodic short illuminations on the growth, the mitotic activity and the phyto- chrome content of Lemna minor under heterotrophic conditions', J.ROMBACH has examined the relation between the growth rate and the extent of conversion of phytochrome Pr to Pf r, or of Pf r to Pr. Cultures of Lemna, otherwise grown in darkness on a medium containing sucrose, casein hydrolysate, tryptophane and kinetin, were given daily exposures of various lengths to red or far-red light, following a saturating exposure to light of the other colour. The method of calculation used by HENDRICKS et al (1956) was followed in order to estimate the proportion of phytochrome present as Pf r. The results in Fig. 6 show an approximately linear relation between the proportion of Pf r and the frond multiplication rate. Thus, frond growth in Lemna is apparently Pf r dependent as is leaf growth in many higher plants.
Because the Pf r form of phytochrome also absorbs in the red region, the maximum proportion of Pf r that can be achieved is about 80 per cent (BUTLER, 1964) and not 95 per cent as shown in the figure, but the approximately linear relation between the physiological display and the proportion of phytochrome as Pf r would still hold after correction.
Such a relation is similar to that estimated by HENDRICKS et al (1956) for lettuce germination, and unlike the relation for Lepidium germina- tion and bean internode length, where conversion of only a small proportion to the Pf r form had a relatively great effect. The linear
197 relation in Lemna suggests that the total phytochrome content is limiting the response.
Aetiolated tissues often contain relatively high amounts of phyto- chrome, much of which is destroyed following conversion to the Pf r
form by light (BUTLER et al, 1963; D E L I N T and SPRUIT, 1963). How- ever, although the phytochrome remaining may be difficult to detect by differential spectrophotometry, it is active in determining physio-
I Multiplication rate
p730
Fi g . 6. Relation between frond multiplication rate in Lemna and the estimated proportion of phytochrome as Pfr (Rombach). + with far red following saturating red ; Ο with red following saturating far red.
logical responses, as we saw in the preceding paper. Diurnal changes in the content of phytochrome in Lemna, as measured by differential spectrophotometry, are shown in Fig. 7 .
Destruction of phytochrome in the 4 h following each daily 8-min period of illumination is evident. The content of phytochrome then rose rather irregularly to the original level in the next 20 h. Rombach wondered whether this rise in phytochrome content could be connec- ted with the formation of new cells, and determined the mitotic
198 P H O T O E N V I R O N M E N T
0-3
0-2
0-1
Rel. phytochrome concentration
J I Ι L
0 8 12 16
Hours after illumination
20 24
FIG. 7. Diurnal changes in the phytochrome content of Lemna fronds, estimated by differential spectrophotometry. Results of several experiments (ROMBACH).
P H Y T O C H R O M E A C T I O N A N D N U C L E I C A C I D M E T A B O L I S M The biochemical processes controlled by the high-energy reaction and by phytochrome remain unknown. The extremely wide range of their physiological displays argues for their action at some basic metabolic control point. HENDRICKS (1963) has suggested that phytochrome may act in the region of pyruvate utilization, while GORDON (1964) suggests that it controls oxidative phosphorylation.
Even if the primary control by phytochrome is exerted in these areas of metabolism, removal of the bottleneck to further development by treatments with red light could very quickly yield many bio- chemical changes in no way indicative of the primary effect.
This is the problem that faces us with data of the kind presented by D.P.HOLDGATE and T . W . G O O D W I N in their paper 4T h e effect of red activity between illuminations by the colchicine method. Fluctuations in mitotic activity in the fronds were evident with minima at times when the rise in phytochrome content slowed. However, the data for phytochrome content were rather variable, and the evidence for a relation between mitotic cycle and phytochrome synthesis is not compelling.
and far-red light on nucleic acid metabolism in rye seedling shoots'.
Rye seedlings were grown in darkness at about 250 C for 5 days, were then given a 10-min exposure to either red, far red, or red followed by far-red light, returned to darkness, and analysed for total D N A and RNA every 12 h during the subsequent 2 days.
Compared with darkness, red light had no effect on either dry weight or R N A content throughout the 2-day period, but considerably increased D N A content, particularly in the first day after the red exposure.
Far-red light, and far red following red light, reduced growth and RNA content during the first day, but increased it during the second, relative to the dark controls. Far red, initially, had little effect on D N A content, but subsequently decreased it.
A possible interpretation of these results is that red light stimulates cell division, and therefore D N A synthesis, in the early stages of growth (cf. POWELL and GRIFFITH, i960; M Ö H R and PETERS, i960), while later growth by cell extension is increased by far red, and is in some way coupled to R N A content.
A curous feature of their results is that red light and darkness gave similar dry weight and R N A trends, while far-red light gave quite different ones. HOLDGATE and GOODWIN suggest that this may be due to action by phytochrome PR—the form usually considered inactive—
but it could also be due to the presence of some PF R in dark-grown seedlings, from imbibition or de novo synthesis, or to a fortuitous cancelling out of the many effects of red light.
T H E I N D U C T I O N O F F L O W E R I N G
Action by phytochrome in the induction of both long- and short-day plants is well established, and it has been widely inferred that the difference between them is that while long-day plants require con- tinued PF R action, short-day plants require the absence of PF R, by dark reversion, for at least part of each day. The papers to be dealt with now emphasize the similarities rather than the differences between long- and short-day plants, since they indicate that both require action by PF R, and that flowering may be reduced in both if PF R action is too prolonged or at too high a level. In both the short-day plant Cheno- podium rubrum and the long-day plant Lolium temulentum there appear to be rhythmic changes in the optimum PF R level for induction.
The role of the high-energy reaction in flowering is unclear. Since phytochrome PF R apparently acts on a product of the high-energy
200 P H O T O E N V I R O N M E N T
reaction in de-aetiolation and in anthocyanin synthesis, participation by the high-energy reaction in the induction of flowering is to be expected. Results obtained by Me i j e r (1959) suggested that the high- energy reaction must precede phytochrome action in both long- and short-day plants, but subsequent work by Me i j e r and v a n d e r Ve e n
(1961) makes the role of the high-energy reaction in flowering less clear.
f l o w e r i n g i n s h o r t - d a y p l a n t s : Pharbitis nil
Ta k i m o t o and Na i t o (1962) found that the flowering response of Pharbitis nil to one 16-h dark period increased with increase in the intensity of light in which the seedlings were grown for the preceding 2 days. White and red light were most effective in inducing flowering whereas blue and far-red light were ineffective. They were effective, however, when followed by a brief exposure to red light. These results suggest that a high-energy reaction—which can proceed in blue or far-red light, and also in red—must precede some phytochrome Pf r action for flowering to occur in Pharbitis. Fr e d e r i c q (1965) has also shown that induction in this short-day plant requires Pf r action follow- ing short exposures to high-energy light.
In her paper ' The effects of the duration of light and darkness on flowering of Pharbitis nil\ A . Ka d m a n- Za h a v i presents results which suggest that, at least when the seedlings are supplied with sucrose, participation by the high-energy reaction is not required for floral induction. In the experiments considered here the seeds were ger- minated and the seedlings grown in darkness for 5 - 6 days on nutrient agar with sucrose. In the experiments of Ta k i m o t o and Na i t o (1962) and Fr e d e r i c q (1965) they were in continuous illumination, but without sucrose, from germination. By starting the seedlings in darkness, Ka d m a n- Za h a v i is able to examine the dependence of flowering on the light conditions prior to a dark period. The amount of flowering increased with increase in the length of the light period from 36 to 72 h, with no evidence of a rhythmic dependence. Some flowering was obtained in the 'Violet' variety with a single 12-h light period, but single exposures to red light for 2 or 6 h gave no flowering. How- ever, 2· 16 min of red light given every 30 min for 24 h, for a total duration of red light exposures of slightly less than 2 h, resulted in 54 per cent flowering. Similarly, with variety 'Kidachi', 5-min illumination every 30 min for 48 h resulted in the same degree of flowering as continuous illumination for the same period. The
R A P P O R T E U R ' S R E P O R T
intensity of the red exposures is not given, but is likely to have been low, so the effectiveness of these intermittent red illuminations rather suggests that participation of the high-energy reaction is not essential when the plants are supplied with sucrose.
In several experiments there was no marked dependence of the flowering response on the length of the dark period between 14 and 40 h, and KADMAN-ZAHAVI concludes that endogenous rhythms do not play a primary role in the induction of Pharbitis nil. But, again, it should be emphasized that these experiments were with plants supplied with sucrose, because W . D . M I T C H E L L (private communication) has found no rhythmic response to the length of the dark period in Xanthium plants with two adjuvant leaves in continuous light, but a marked rhythm when no adjuvant leaves are present.
F L O W E R I N G I N S H O R T - D A Y P L A N T S : Chenopodium rubrum Experiments with Xanthium in which far-red light given at the begin- ning of near critical dark periods reduced the critical night length (BORTHWICK et al, 1952) led to the view that in short-day plants phyto- chrome Pf r must be removed, by dark reversion, before the dark reactions leading to induction could take place. On the other hand, the work of K Ö N I T Z (1958), CUMMING (1963) and FREDERICQ (1965) has indicated that Pf r must be present for at least part of each day for induction of short-day plants to occur, and CUMMING (1963) suggested that induction in Chenopodium rubrum may occur only within a narrow range of phytochrome-Pfr levels.
B . G . C U M M I N G , H . A . B O R T H W I C K and S.B.HENDRICKS, in their paper
* Flowering of Chenopodium rubrum in relation to phytochrome and an endogenous circadian rhythm', suggest that a considerable proportion of phytochrome is present as Pf r during most of very long dark periods, and is, in fact, required for induction to occur in this short-day plant.
Plants of selection 374 were germinated and grown in Petri dishes in continuous light for 4 days. They were then given a single dark period of various lengths or an inductive dark period of standard length, during which they were exposed briefly to red or far-red light, returned to continuous light, and examined for the presence of flower primordia several days later. Some of the results are presented in Fig. 8. The upper part of the figure shows a rhythmic dependence of flowering on the length of a single dark period. Dark periods of less than 8, of about 30, and of about 60 h length are clearly ineffective for
2 0 2 P H O T O E N V I R O N M E N T
100 η 9 0: 8 0 - 70 : 6 0 - 5 0 - 4 0 - 3 0 - cn 20 :
10 •
* > — 3 5 0 0 f t - C Y fluorescent S" preceding
period
0 4 8 12 16 20 24 28 32 36 40 4 4 48 52 56 60 64 68 72 Hours of single dark period (darkness followed by 1000ft-C continuous incandescent) 8100-
Incandescent dark control
0 4 8 12 16 20 24 28 32 36 4 0 4 4 48 52 5 6 60 6 4 6 8 Hour at which single irradiation of 4 minutes red was inserted within 72-hour dark period
(darkness followed by 1000ft,-C continuous incandescent) ,
° 100-,
I 80-
60·
40·
20·
ο >»
..- CT - c 100-Ο φ
c α>
8-D 80 Y 60- tô 40-
C T3
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 Hour at which single irradiation of 10 seconds far-red was inserted within 72-hour dark period
(darkness preceded by 3500ft-C fluorescent followed by 1000ft-C incandescent)
72-hours dark (Null point)
0 100 0 100 0 100 0 100 0 ' 100 0 100 0 Per cent R 100 0 100 0 100 0 100 0 100 0 100 0 100
- Per cent FR
17 t 23 29 35
100 0 100 0 100 0 100 0 100 0 100 0 100 0 100 0 100 0
· et^R/FR ratio Null point R/FR ratio ^v^resulting in at which flowering \ m a x i m u m
• ^ equivalent to dark S\lowering control + - /
41 \ \
47 53 \
59 + 65
Single 1-minute irradiation with light of specified red/far-red ratio inserted once within 72-hour dark period
Fi g . 8. Flowering of Chenopodium rubrum—374 at 200 C in response
to a single dark period with or without a single, brief red, far-red interruption. See text for details. Upper three sets of curves are moving averages (Cu m m i n g, Bo r t h w i c k and He n d r i c k s ) .
induction, while peak effectiveness is reached with about 16 and 45 h of darkness following fluorescent light, and with 12 and 40 h darkness following incandescent light.
The second part of Fig. 8 indicates the effects of single brief exposures to red light at various times during a 72-h dark period. A marked rhythmicity, parallel to that in the upper part of the figure, is evident, particularly following incandescent light. After fluorescent light the periods during which red light is inhibitory are more sharply confined, and exposure to red light is actually stimulatory during much of the long dark period.
The third part of Fig. 8 shows that at no time during the 72-h dark period is far-red light stimulatory, and that it is almost completely inhibitory throughout the first 50 h or so. When the far-red exposures were only sec, rather than 10 sec, induction was not inhibited as much. These results are to some extent comparable with those obtained by CARPENTER and HAMNER (1963) with soybeans.
The effects of red and far-red light were reversible by subsequent far red or red, respectively, indicating that they are mediated by phytochrome.
While exposure to far-red light for periods of 60-96 h prevented induction, exposure for such periods in light from BCJ lamps—in which about 20 per cent of the energy is red—led to some induction.
The simplest interpretation of these results is that some phyto- chrome Pf r must be present throughout the dark period for induction to occur, and that dark reversion of Pf r to Pr is slow.
Both the actual and the optimal proportion of phytochrome present as Pf r at various times during the dark period can be indicated by exposing plants at various times to a single 1 -min irradiation with light of a wide range of ratios of red to far-red energy, the total intensity being about 470 juw/cm2. The results of such an experiment are shown in the fourth part of Fig. 8.
Taking the responses to interruptions at the 23rd hour, it is clear that a high proportion of red light enhances induction compared with the dark controls, and that flowering response falls with increase in the proportion of far-red energy. A null response is achieved when about 50 per cent of the energy is red. The changes in the proportion of red energy in the light break which gives a null response are shown at the bottom of Fig. 8. These values suggest a fall in the proportion of phytochrome as Pf r from the 17th to the 29th hour, to perhaps about 40 per cent, a subsequent rise which could be due to de novo synthesis,
204 P H O T O E N V I R O N M E N T
and then another fall to a low level at the 53rd-5çth hours, and then another rise.
The greatest flowering response was obtained with high proportions of red energy in light breaks at all times except the 35th and 65th hours.
Thus it appears that not only is a considerable proportion of PF R present throughout the first 40 h of darkness, but also that a high proportion is favourable to induction. The inhibitory effect of red light at the 35th hour is seen to be due to the optimum proportion of red light, and therefore presumably of PF R being comparatively low at that time.
F L O W E R I N G I N L O N G - D A Y P L A N T S : Lolium temuletitutn Flowering in a number of long-day plants can be induced by exposure to a light break near the middle of long nights. Red light is most effective as the light break (BORTHWICK et al, 1948 ; PARKER et al, 1950) and this has led to the assumption that flowering in long-day plants is induced by maintaining a high phytochrome PF R level. However, BORTHWICK and PARKER (1952) drew attention to the fact that sugar- beet would flower well when extensions to the photoperiod were made with incandescent light, but would not flower in fluorescent light, and this observation has been extended to many long-day plants.
One possible explanation of this affect is that, as in Chenopodium rubrum, there is a rhythmic change in the optimum PF R level such that the intermediate PF R level maintained under incandescent light satisfies all phases of the rhythm better than the higher PF R level maintained under fluorescent or red light. Evidence in favour of this idea is presented by D . V I N C E in her paper ' T h e promoting effect of far-red light on flowering in the long-day plant Lolium temulentum ( B A 3081) \ The plants were grown in short days until the 3rd leaf stage, and were then exposed to various red and far-red light treatments in the 8 h following each 8 h of daylight. The plants were dissected 4-5 weeks after the first long day. The red and far-red energy in the various photo- period extensions totalled about 84 /xw/cm2.
Early experiments showed that an equal mixture of red and far-red energy was highly effective as a photoperiod extension, but red or far red alone were not. Inclusion of a small proportion of far red in the red, or of red in the far-red sources made them effective.
A most striking finding with this strain of L. temulentum was that while an 8-h extension with red light had virtually no inductive effect, exposure to 7 h of darkness followed by 1 h of red light gave a marked
R A P P O R T E U R ' S R E P O R T 205 flowering response, exceeded only by treatment with 7 h of far red followed by ι h of red. These results suggest that for optimal induction the phytochrome-Pfr level must be lowered following the daylight period, either by dark reversion or by exposure to far-red light, and must then be raised again after about 7 h, since 8 h of far red alone had no effect.
Figure 9 depicts the results of an experiment in which 2 h of far-red light was interpolated at various times during an 8-h photoperiod extension, the rest of the extension being in red light. The promotive effect of far-red light clearly reaches a peak 3 - 5 h after the daylight period. Other experiments with far-red interpolations of various lengths also showed peak effectiveness of the far red in the middle of the 8-h extension, and showed that the promotive effect of the far red increased with the length of the interpolation up to 7 h.
The results of a similar experiment in which 2 h of red light was interpolated at various times during an extension with 7 h of far red followed by ι h of red yield a complementary picture (see Fig. 9), red light being most inhibitory 3 - 5 h after the daylight period.
In a further experiment it was shown that whereas red light in the 8 h following the daylight period is inhibitory to induction, red light in the 8 h preceding the daylight is highly promotive. The evidence is clear that for this strain of L. temulentum the optimum Pf r level for the induction of flowering falls for several hours following the daylight period, and then rises again to a fairly high level for the rest of the diurnal period.
The results presented by L . T . E V A N S , H . A . B O R T H W I C K and S . B . HENDRICKS in their paper ' The spectral dependence of flowering in Lolium temulentum* suggest that, while there is a rhythmic change in the optimum Y\x level for induction, this does not wholly account for the superiority for L. temulentum of photoperiod extensions enriched with far-red light.
In these experiments the plants were grown for about 6 weeks in short days, reduced to one or a few leaves, and exposed to various light treatments for 1 day, being then returned to short days and dissected 3 weeks later.
As with the strain used by VINCE, extension of the photoperiod with sources having about equal red and far-red energy gave the greatest flowering response, and this decreased progressively as the proportion of red or far red increased. However, extension with either red or far-red light alone for one 16-h period led to a small flowering response
2-0 5-3
1-5
10A 2-2
1-2
1-0 1-0
0-2
0-2 L_
10 11p.m.
1-3 2-2
12
1-2 1-5
10 Β vol
0-4
0·95Γ 0-2
8 10 11p.m.
Fi g . 9. The effect of time of exposure to far red or red light on flowering in Lolium temulentum.
In A the basic treatment consisted of 7 h far red from 4- 1 1 p.m. with ι h of red from 11 p.m. to midnight: plants were transferred to red light for 2 h at the various times indicated by the positions of the bars.
In β plants were transferred to far red light for 2 h at the various times indicated by the positions of the bars ; otherwise plants remained in red light from 4 p.m. to midnight.
Α ι plants were in darkness from midnight to 8 a.m. and in daylight from 8 a.m. to 4 p.m. Plants were given 10 long days and were dissected about 4 weeks from the beginning of the long day treatment. The numbers above the bars refer to the mean floral stage reached in each treatment: the first recognizable stage of flowering, 'double redges', was designated stage 1 on this scale.
R A P P O R T E U R ' S R E P O R T 207
100 r
80h
60r
ο Ζ 40I-
ç 1
Έ 20h
16 hr
Darkness 16 32 64
Number of interruptions during 16hr dark period
Continuous 64 interruptions red at half intensity
Fi g . 10. The effect of number and intensity of red-light interruptions during a 16-h night on the rate of stomatal opening in Xanthium pensylvanicum ( Ma n s f i e l d ) .
with low-intensity red, far red or incandescent light, it was found that red light was slightly inhibitory at the beginning of the extension and promotive in the middle, while far-red light was promotive at the beginning and inhibitory in the middle. It would appear, then, as VINCE suggests, that the optimum Pf r level is lower during the early hours of the extension than it is later. However, at no time did inter- polation of red or far-red light significantly improve the response to an in all plants, whereas a brief red light break in the middle of a 16-h dark period caused no flowering whatever. In these respects our strain of L. temulentum differs sharply from that used by VINCE.
In three experiments in which 4-h periods of red or far-red light were interpolated at various times during a 16-h photoperiod extension
2θ8 P H O T O E N V I R O N M E N T
incandescent-light extension, which suggests that the optimum Pf r level is at all times fairly close to that maintained by incandescent light. Even 8 h of far red followed by 8 h of red light was less effective than continuous incandescent light.
Cyclic lighting treatments with only io per cent of each cycle (e.g.
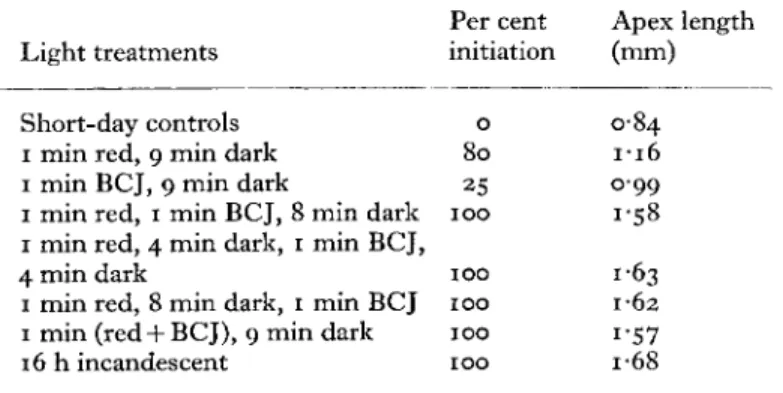
ι min every io min) illuminated by red or far red yielded only a minimal flowering response. It was thought that such treatments could be used to establish photoreversibility, by giving a brief far-red exposure after the red exposure in each cycle. Eight such experiments have been carried out, six with io-min cycles and two with 30-min cycles, and data from one are given in Table 1. In this experiment BCJ lamps, which emit a small proportion of red light, were used instead of far red, but individual treatments with a far-red source containing no red light support the conclusions to be drawn.
TABLE I. The effect of various cyclic light treatments, given every 10 min of a 16-h photoperiod extension, following 8 h of high-intensity fluorescent and incandescent light, on flowering
in L. temulentum.
Per cent Apex length Light treatments initiation (mm)
Short-day controls 0 0-84
ι min red, 9 min dark 80 1 1 6
ι min BCJ, 9 min dark 25 099
ι min red, 1 min BCJ, 8 min dark 100 1-58 ι min red, 4 min dark, 1 min BCJ,
4 min dark 100 1-63
ι min red, 8 min dark, 1 min BCJ 100 1-62 ι min (red + BCJ), 9 min dark 100 1*57
16 h incandescent 100 1-68
Although the cyclic red or far-red treatments alone produced a minimal flowering response, treatments in which both red and BCJ light were given, regardless of whether the BCJ light was given before, after, or simultaneously with the red light, gave a flowering response almost equal to that in continuous incandescent light. One minute of BCJ light following each red exposure might have been expected to reverse its effect, but has markedly enhanced it. Treatments with 2 min of red or far-red light per io-min cycle were only slightly more
effective than the i-min treatments, so the effects are not to be ascribed to the increased duration of illumination. Nor is any Emerson effect enhancement likely to be involved since interpolation of 4 min of darkness (or 12 min in the 30-min cycles) between the red and the far- red treatments had no effect on the enhancement. We conclude, then, that far-red energy in some way drives phytochrome Pf r action, and that this phenomenon may partly account for the superiority for many long-day plants of daylength extensions containing a propor- tion of far-red energy.
A L O W - E N E R G Y R E D L I G H T R E A C T I O N B Y S T O M A T A
Light of high intensity has a direct action in causing stomata to open, and the action spectrum obtained by KUIPER (1964) with Senecio odoris clearly implicates photosynthesis in the reaction. Another high- intensity blue reaction may also contribute to stomatal opening (MOURAVIEFF, 1958; KARVE, 1961).
Besides these photoreactions there is a low-energy reaction dis- covered by MANSFIELD and HEATH (1961) in Xanthium pensylvanicum, the after-effect of which is to reduce the rate of opening in subsequent high-intensity light. They found that the longer the dark period the more rapid was stomatal opening the following morning, and that very low light intensities could produce the short-night effect. MANSFIELD (1964) subsequently showed that this reaction was mediated by light at the red end of the spectrum.
In his paper ' Stomatal sensitivity in Xanthium pensylvanicum Wall, to interruptions of the dark period', T.A.MANSFIELD analyses this low-energy red-light effect further. Following an 8-h period of high- intensity light Xanthium plants were exposed to 16 h of either darkness or low-energy (14-4 μΛν/cm2) red light, and the rate of stomatal opening when they were returned to high-intensity light was then recorded. Of the plants placed in darkness, some received sym- metrically placed red-light breaks in one of the following regimes : one of 2 h 8 min length, three of 43 min, sixteen of 8 min, thirty-two of 4 min, sixty-four of 2 min length. The intensity of the red-light breaks was such that the total red energy received was the same as that for the plants in red light throughout.
One or three red interruptions had no effect on the subsequent rate of opening, while sixteen hourly interruptions had an almost significant effect, and thirty-two half-hourly interruptions a highly significant one. However, an effect equal to that of continuous light was obtained
14
2IO P H O T O E N V I R O N M E N T
only with sixty-four interruptions at 15-min intervals (see Fig. 10).
The effect of these sixty-four interruptions was dependent on the intensity of red light and was not reversed by following each red exposure with far-red light.
MANSFIELD suggests that the need for repeated red interruptions, the lack of far-red reversal, and the fact that he previously found greater action at 703 nm than at 660 nm, and some action at 730 nm, imply that phytochrome is not involved in this reaction. However, the peak action at 703 nm might merely reflect marked absorption of red light by the green tissue above the stomata, while the lack of rever- sibility could be due to rapid action by phytochrome Pf r, as found by FREDERICQ (1965). The need for repeated light breaks is not uncommon in systems controlled by phytochrome. Thus, phytochrome partici- pation in this timing reaction is not excluded, but neither is it clearly indicated.
C O N C L U S I O N
In these papers we have seen evidence for the participation of the high- energy reaction and of phytochrome in de-aetiolation of citrus and gherkin seedlings, and even in suppressing the branching of excised pea roots. T w o light reactions, which are spatially separable, also contribute to anthocyanin synthesis in turnip seedlings, but there is no evidence that phytochrome mediates either of them. Thus, we may have a third photoreaction to consider, and some of the results presented suggest there may be more than one component of the blue, far-red high-energy reaction.
In the growth of the Lemna frond, and in the flowering of Cheno- podium rubrum following light breaks at certain times, there was an
approximately linear relation between the proportion of phytochrome in the Pf r form and the physiological display. Yet in the pea root there was no clear relation between the amount of phytochrome present and the response to red light. Thus, the biologically active phytochrome may be a very small proportion of that detectable by differential spectrophotometry, or may not even be detectable by these means.
As to phytochrome action in the induction of flowering, it now seems easier to see what long- and short-day plants have in common than where they differ. Both apparently require some Pf r action, but in both a sustained high Pf r level may be inhibitory to induction. Both show rhythmic changes in the optimum Pf r level. The difference between them may be in the timing of these rhythmic changes relative to the
high-intensity light period, or in their rates of dark reversion of phyto- chrome Pf r to Pr, or it may lie in the effect that far-red light has on Pf r action. If small changes in the amount of Pf r are as important as they appear to be in Chenopodium rubrum and Lolium temulentum, we will have to consider not only the proportion of phytochrome present as Pf r but also changes in the total amount of phytochrome as governed by its rate of de novo synthesis on the one hand, and of destruction following conversion to Pf r on the other.
A marked rhythmic response to phytochrome-Pfr level was evident in the experiments with Chenopodium rubrum and with one strain of Lolium temulentum, but not with the other, nor with Pharbitis nil. It would be interesting to know just what experimental conditions reveal these rhythmic responses, and also what the relation is between phytochrome and the endogenous rhythms. The Chenopodium results suggest that an endogenous rhythm determines the range in Pf r level that the flowering processes can tolerate at any time. However, as B Ü N N I N G and L ö r c h e r (1957) suggest, this does not exclude phyto- chrome from itself determining the phase of the rhythm.
r e f e r e n c e s
Bopp M . and Ma t t h i s s B . (1961) Z. Naturf. 1 7 b , 811-18.
Bo r t h w i c k H . A . and Pa r k e r M . W . (1952) Rept. 13th Int. Hort. Congr., London, pp. 801-10.
Bo r t h w i c k H . A . , He n d r i c k s S . B . and Pa r k e r M . W . (1948) Bot. Gaz. n o , 103-18.
Bo r t h w i c k H . A . , He n d r i c k s S . B . and Pa r k e r M . W . (1952) Proc. nat. Acad.
Sei., Wash. 3 8 , 929-34.
Bü n n i n g Ε . and Lö r c h e r L. (1957) Naturwissenschaften 4 4 , 472.
Bu t l e r W. L . , La n e H . C . and Si e g e l m a n H . W . (1963) Plant Physiol. 3 8 , 514-19.
Bu t l e r W.L. (1964) Quart. Rev. Biol. 3 9 , 6-10.
Ca r p e n t e r B.H. and Ha m n e r K . C . (1963) Plant Physiol. 3 8 , 698-703.
Cu m m i n g B . G . (1963) Canad.J. Bot. 4 1 , 901-26.
DeLi n t P J . A. L . and Sp r u i t C J . P . (1963) Meded. LandbHoogesch., Wagenin- gen 6 3 (14), 1-7.
Do w n s R J . (1955) Plant Physiol. 3 0 , 468-73.
Do w n s R J . and Si e g e l m a n H.W. (1963) Plant Physiol. 3 8 , 25-30.
Do w n s R J . , Si e g e l m a n H . W . , Bu t l e r W. L . and He n d r i c k s S . B . (1964) Nature, Lond. (in press).
Ev a n s L. T . , He n d r i c k s S . B . and Bo r t h w i c k H . A . (1965). In preparation.
Fr e d e r i c q H . (1965) Plant Physiol, (in press).
Go r d o n S.A. (1964) Quart. Rev. Biol. 3 9 , i9~34-
Gr i e s b a c h H . (1958) Proc. Int. Congr. Biochem. 4th Congr., Vienna, pp.
56-69.
2 1 2 P H O T O E N V I R O N M E N T He n d r i c k s S.B. (1963) Science 1 4 1 , 21-7.
He n d r i c k s S . B . and Bo r t h w i c k H.A. (1959) Proc. nat. Acad. Sei., Wash.
45, 344-9.
He n d r i c k s S.B., Bo r t h w i c k H.A. and Do w n s R J . (1956) Proc. nat. Acad.
Sei.} Wash. 4 2 , 19-25.
Hi l l m a n W . S . (1957) Science 1 2 6 , 165-6.
Ka r v e A. (1961) Z. Bot. 4 9 , 47-72.
Kö n i t z W . (1958) Planta 5 1 , 1-29.
Ku i p e r P . J . C . (1964) Plant Physiol, (in press).
La n e H . C. and Ka s p e r b a u e r M J . (1965) Plant Physiol, (in press).
Li v e r m a n J.L., Jo h n s o n M . P . and St a r r L . (1955) Science 1 2 1 , 440-1.
Ma n s f i e l d T. A . (1964) Nature, Lond. 2 0 1 , 470-4.
Ma n s f i e l d T. A . and He a t h O . V . S . (1961) Nature, Lond. 1 9 1 , 974~5- Me i j e r G . (1958) Acta Bot. Neerl. 7 , 614-20.
Me i j e r G . (1959) Acta Bot. Neerl. 8 , 189-246.
Me i j e r G . and v a n d e r Ve e n R. (1961) Proc. 3rd Intl. Congr. Photobiol., pp. 387-8.
Mo h r Η . (1959) Planta 5 3 , 219-45.
Mo h r Η . and No b l e A. (i960) Planta 55, 327-42.
Mo h r Η . and Pe t e r s E . (i960) Planta 55, 637-46.
Mo h r Η . and We h r u n g M . (i960) Planta 55, 438-50.
Mo u r a v i e f f I. (1958) Bull. Soc. bot. Fr. 1 0 5 , 467-75.
Pa r k e r M . W . , He n d r i c k s S . B . , Bo r t h w i c k H. A . and We n t F . W . (1949) Amer. J. Bot. 3 6 , 194-204.
Pa r k e r M . W . , He n d r i c k s S . B . and Bo r t h w i c k H.A. (1950) Bot. Gaz. i n , 242-52.
Po w e l l R . D . and Gr i f f i t h M . M . (i960) Plant Physiol. 3 5 , 273.
Sc h m i d t Η . (1962) Biol. ZU. 8 1 , 213-26.
Si e g e l m a n H . W . and He n d r i c k s S.B. (1957) Plant Physiol. 3 2 , 393-8.
Ta k i m o t o A. and Na i t o Y. (1962) Bot. Mag., Tokyo 7 5 , 255-63.
To r r e y J . G . (1952) Plant Physiol. 2 7 , 591-602.