Nonprotein Nitrogenous Compounds
W . SIMIDU
Department of Fisheries, Kyoto University, Maizuru, Kyoto, Japan
I. Introduction 353 II. Dark-Fleshed Fish (Migratory Fish) 354
A. Types of Fish 354 B. Seasonal Changes 355
C. Histidine 5 3 5
D. Trimethylamine Oxide 360 III. White-Fleshed Fish 360
A. Free Amino Acids 360 B. Trimethylamine Oxide 363
IV. Elasmobranchs 365 A. Urea 365 B. Trimethylamine Oxide 368
C. Miscellaneous 368 V. Invertebrates 368
A. Crustaceans 369 B. Mollusks 372 C. Miscellaneous Invertebrates 374
References 375 I. Introduction
Nonprotein nitrogen (NPN) accounts for from 9.2 to 18.3% of the total nitrogen in teleost fishes and from 33 to 38.6% in elasmobranch fishes (Tarr, 1958). In the case of gadoids and flatfishes, NPN is in the range of 9 to 14%, and in the clupeids 16 to 1 8 % (Shewan, 1951). Indian marine fishes are comparatively low in NPN (Velankar and Govindan, 1958b), for reasons not yet explained, possibly species and diet. Elasmo
branchs show much higher values, up to 5 7 % (Joshi et al., 1953).
The NPN constitutes about one-third to one-half of the total water- soluble nitrogen of teleosts, approximately three-fourths in elasmobranchs.
In crustacean shellfish, this same relative figure is three-fifths, and in mollusks four-fifths.
During spawning migration, the nonprotein nitrogen of salmon drops in the same ratio as the weight declines while the percentage of the nitrogen in the muscle is constant (Duncan and Tarr, 1958). The creatine nitrogen remains practically unchanged (Greene, 1919).
353
The compounds occurring in this fraction may be grouped as follows:
(a) volatile bases (ammonia, mono-, di-, and trimethylamines), of minor significance in muscles of living fish but most important to fish handling, as they are found in the common pattern of spoilage; (b) trimethylam- monium bases (trimethylamine oxide and betaines); ( c ) guanidine deriv
atives (creatine and arginine); (d) imidazole or glyoxaline deriv
atives (histidine, carnosine, and anserine); (e) miscellaneous (urea, amino acids, and purine derivatives). The occurrence of these nitro
genous extractives in the muscles of fish has been the subject of extensive investigations.
White-fleshed fish, containing relatively less of nitrogenous extrac
tives, contrast sharply, in this respect, with dark-fleshed fish. The non
protein nitrogen content is very high in elasmobranchs and mollusks, chiefly due to the presence of relatively large amounts of urea and tri
methylamine oxide in the first group and certain free amino acids in the latter group.
A number of nonprotein nitrogenous compounds play a key role in the metabolic processes of marine animals, and also in their spoilage.
They contribute, besides, to the flavor of the sea food.
Ranke et al. (1955) have discovered a special peptide—designated PR—which is specific to all investigated marine fishes, crustaceans, and squid. It is lacking in mollusks and starfish. In fresh-water fish it has been encountered only in smelt and trout, which may both occasionally convert to a marine life. This peptide yields glutamic and aspartic acid, glycine or serine, α-alanine and traces of threonine on acid hydrolysis.
II. Dark-Fleshed Fish (Migratory Fish)
A. T Y P E S OF F I S H
Oceanic bonito, a dark-meat fish, contains more than 800 mg./lOO g.
flesh of nitrogenous extractives. Approximately 2 1 % of the total nitrogen in fresh yellowfin tuna meat consists of nonprotein nitrogen (Kochi and Era, 1959). Mackerel, yellowtail, and sardines, also largely dark-meat fish, show values of 500 to 600 mg./100 g. White-fleshed fish such as porgies, flatfish, and swellfish contain less of nitrogen extractives (about 300 mg./100 g.). Fish coming in a category in between are jack mackerel and sea bass, containing about 400 mg./100 g. The content of nitrogenous extractives, therefore, seems to be correlated with the amount of red muscle color or the degree of motility of the fish.
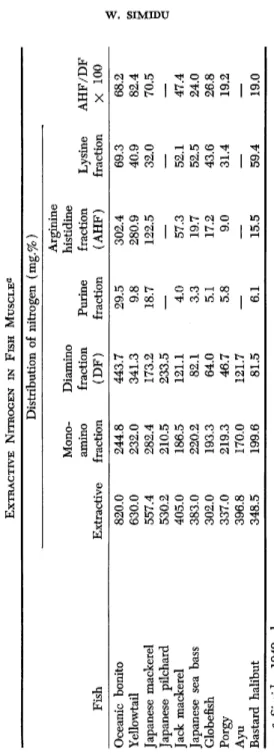
Differences in nitrogenous extractives are shown in Table I (Simidu, 1949a, b ) . Notable is the clear distinction between dark- and white- fleshed fishes as to the diamino fraction (precipitated by phosphotungstic acid). No corresponding difference prevails as to the monoamino fraction.
After fractionating, further distinctive features turn up as to the amount of arginine and histidine, while two other subtractions show no noticeable differences. The last column of Table I bears out the fact that the ratio of arginine and histidine to the diamino fraction nitrogen in dark-muscled fish contrasts sharply with the corresponding figures for white meat fish. Red-meat fish, in fact, is characterized by its predom
inant content of arginine and histidine, the latter being the main com
ponent.
B . SEASONAL CHANGES
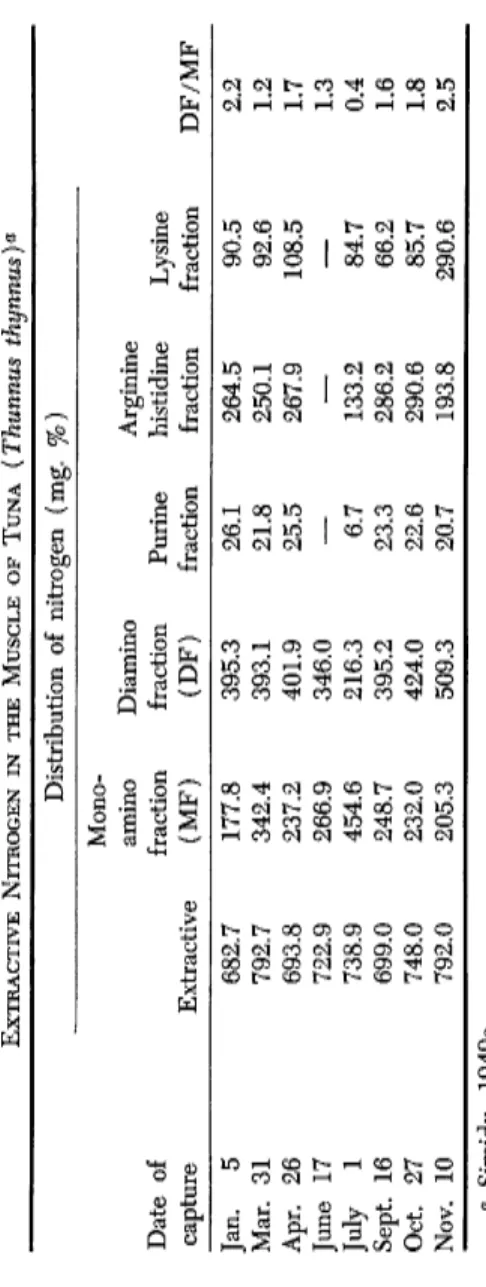
In bluefin tuna meat diamino nitrogen, or more clearly the ratio of this to monoamino nitrogen, increases in the winter and diminishes during the summer, although there is no significant change in the total amounts of nitrogen in the muscle. This implies that monoamino nitrogen must change seasonally in the reverse direction as compared to diamino nitro
gen (Table I I ) .
Summer albacore have a volatile base nitrogen content of 25 mg.%, as compared to winter specimens showing values of only 10 mg.% (Ka
wabata et al., 1952).
It is well recognized that the taste of bluefin tuna from the western Pacific Ocean shows a marked seasonal difference, the best flavor being encountered in the winter. In summer—in the season when spawning occurs—it becomes almost unpalatable. The fish tissue also contains more fat in the winter than in the summer (Simidu, 1947).
It can be assumed that the flavor of bluefin tuna is related to the amount of diamino or arginine and histidine nitrogen in the muscle, as well as to the amount of fat.
No correlation was found between sexual maturity and content of total or individual amino acids in herring muscle extracts (Hughes, 1959).
C . HISTIDINE
The occurrence of free histidine in fish tissue has been known for a long time (Suzuki and Yoshimura, 1909; Okuda, 1919). Quantitative re
sults have been reported by many authors (Spahr, 1937; Johnston and Johnston, 1939; Shewan, 1955), and show that the histidine content in
TABLE I EXTRACTIVE NITROGEN IN FISH MUSCLE« Distribution of nitrogen (mg.%) Ar ginine MonoDiamino histidine amine^ fraction Purine fraction Lysine AHF/DF Fish Extractive fraction (DF) fraction (AHF) fraction X 100 Oceanic bonito 820.0 244.8 443.7 29.5 302.4 69.3 68.2 Yellowtail 630.0 232.0 341.3 9.8 280.9 40.9 82.4 Japanese mackerel 557.4 282.4 173.2 18.7 122.5 32.0 70.5 Japanese pilchard 530.2 210.5 233.5
— — — —
Jack mackerei 405.0 186.5 121.1 4.0 57.3 52.1 47.4 Japanese sea bass 383.0 220.2 82.1 3.3 19.7 52.5 24.0 Globefish 302.0 193.3 64.0 5.1 17.2 43.6 26.8 Porgy 337.0 219.3 46.7 5.8 9.0 31.4 19.2 Ayu 396.8 170.0 121.7— — — —
Bastard halibut 348.5 199.6 81.5 6.1 15.5 59.4 19.0 α Simidu, 1949a, b.TABLE II EXTRACTIVE NITROGEN IN THE MUSCLE OF TUNA (Thunnus thynnus)a Distribution of nitrogen (mg. %) Mono- amino Diamino Arginine Date of fraction fraction Purine histidine Lysine capture Extractive (MF) (DF) fraction fraction fraction DF/MF Jan. 5 682.7 177.8 395.3 26.1 264.5 90.5 2.2 Mar. 31 792.7 342.4 393.1 21.8 250.1 92.6 1.2 Apr. 26 693.8 237.2 401.9 25.5 267.9 108.5 1.7 June 17 722.9 266.9 346.0 — —
—
1.3 July 1 738.9 454.6 216.3 6.7 133.2 84.7 0.4 Sept. 16 699.0 248.7 395.2 23.3 286.2 66.2 1.6 Oct. 27 748.0 232.0 424.0 22.6 290.6 85.7 1.8 Nov. 10 792.0 205.3 509.3 20.7 193.8 290.6 2.5 Simidu, 1949a.the dark-fleshed fish may occasionally reach high levels, sometimes more than 2,000 mg./100 g. muscle (Simidu et al, 1952; Hibiki and Simidu, 1959) (see Table I I I ) . The lighter the muscle color, the less histidine in the tissue.
Fish seem to be unique among meat-producing animals in containing
TABLE III
T H E HISTIDINE CONTENT IN TISSUE EXTRACTS OF MARINE ANIMALS (60% A L C O H O L )0
Species Histidine (mg.%) Dark-meat fish
Common dolphin 937
Frigate mackerel 1010
Japanese mackerel 802
Japanese mackerel 704
Japanese pilchard 606
Striped marlin 1320
Yellowtail 1035
Intermediate species
Barracuda 170
Common sea bass 6.9
Jack mackerel 368
Jack mackerel 164
White-meat fish
Filefish 4.6
Globefish 5.7
Opale-eye 5.5
Whip ray 4.8
Bastard halibut 4.7
Gurnard 7.4
"Kisu"*> 40.3
Porgy 47.7
Mollusks
Common octopus 25.9
Loligo edulis 29.5
Short-necked clam 8.9
Wreath shell0 4.6
Crustaceans
Blue crab 16.7
Northern shrimp 9.3
Penaeopsis joyneri 20.6
a Hibiki and Simidu, 1959.
δ Sälago sihama (Forskäl).
c Turbo cornutus Solander.
free histidine and 1-methylhistidine (Mishukova, 1955). This is the more remarkable as most protein analyses bear out the fact that fish proteins contain a far lower amount of histidine than the meat of mammals (see Volume II, Chapter 2, Section V ) . Muscles of mammals and poultry are characterized rather by other dipeptides than histidine, such as carnosine and anserine (Bate-Smith, 1958). Nevertheless, Lukton and Olcott (1958) reported that, besides histidine, relatively large amounts (10-40 μιηοΐββ per gram of muscle) of anserine were found in four tuna species. Salmon, swordfish, and sablefish contained only anserine, in the same amount.
Mackerel, sardines, and menhaden had insignificant quantities of anserine and were, like all fish, scanty in carnosine, but histidine was abundant (15-49 μπιο1θ8 per gram of flesh).
Little information is available as to the physiological role of free histidine in fish. With few exceptions, the motility of the fish increases with the color intensity of the red muscle. This might point to causal relationships. Within this dark-fleshed group, younger fish, which have less motility, also contain less histidine. Large amounts of histidine, as well as carnosine, stimulate the glycolitic oxido-reduction, but unlike carnosine, histidine has no effect on the formation of phosphoglyceric acid under aerobic conditions (Mishukova, 1955).
In sockeye salmon, the histidine content drops to one-fifth of its previous value, both in males and females during the early stage of migration (Wood, 1958). Other changes in nonprotein nitrogen and nitrogen constituents follow the weight changes.
No conclusive evidence has accumulated to prove that histidine con
tributes to the characteristic flavor of dark-fleshed fish. Some such fish acquire a better flavor only subsequent to a short period of "ripening"
of the meat after death. It might be significant that in this case the histidine content increases with the lapse of time after death (Simidu et al, 1952, 1953b). Yamamoto (1921) reported that palatable fish con
tain large amounts of free histidine, but no experimental proof was given.
The tasty yellowtail flounder is characterized by the ample presence of free histidine (Simidu, 1949a; Amano and Bito, 1951). Kodama (1913) attributed the flavor of "katuo-busi" (boiled and dried oceanic bonito used as a relish in Japan) to histidine inosinate. Most likely there is some kind of relationship between histidine and the flavor of dark- fleshed fish.
Histidine is liable to be converted into histamine by decarboxylation.
The pungent taste of stale fish has been ascribed to histamine formed in
the muscle even under aseptic conditions. Most of the histamine is pro
duced by bacterial action, even if minor quantities are formed in the autolytic process of fish muscle.
Histidine is decarboxylated chiefly through bacterial action forming histamine. In histidine-rich fish, this seems to be a common cause of poisoning. It has been suggested further that the freshness of some fish might be judged on the basis of histamine content. The entire histamine problem is discussed in great detail in Chapter 10, this volume. For further information and references in this area, see Section C. White- fleshed fish and mollusks contain only traces of histidine in their tissue;
hence, only a small amount of histamine is formed in these cases.
D . TRIMETHYLAMINE OXIDE
Trimethylamine oxide (TMAO) will be discussed chiefly in the fol
lowing section (see Section III, B ) . Mention will be made here only of the finding that large amounts of trimethylamine ( T M A ) are contained in the dark muscle of pelagic fish, such as albacore and frigate mackerel.
As this compound has been generally considered to be an indicator of bacterial reducing action, it is essential to know that the white flesh of the same fishes contained no TMA. Only the red-pigmented flesh had the ability to reduce TMAO in much the same way as the TMAO reductase of bacteria (Kawabata, 1953).
Ml. White-Fleshed Fish
A. F R E E AMINO ACIDS
As mentioned before, histidine and arginine occur in much smaller quantities in the tissue of white-fleshed fish than in dark-fleshed fish.
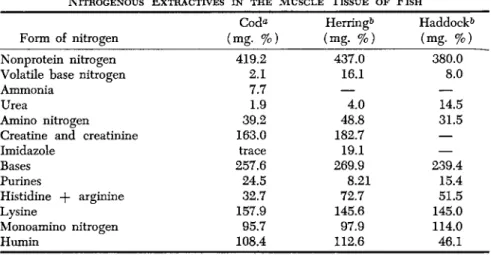
Wide variations were, however, established in carp (Duchäteau and Florkin, 1954a). This was confirmed by Shewan (1955), who compared flatfish with pelagic species. No conspicuous differences have been found as to various white-fleshed fish with respect to the distribution of NPN compounds. Consequently, there is, as yet, no clue to explain the flavor of white-fleshed fish in relation to these compounds. With few exceptions, most white-fleshed fish are characterized by a low percentage of imidazole compounds ( 7 - 0 . 1 % in the total amount of NPN), while dark-fleshed species show such percentage figures as 30 to 8 0 % (Lukton and Olcott, 1958). Taste has been attributed to such imidazole compounds (Simidu et al., 1952). The results of fractionating nitrogen extracts of cod, her-
ring (Campbell, 1934-35), and haddock (Komarov, 1934 Table IV.
TABLE IV
NITROGENOUS EXTRACTIVES IN THE MUSCLE TISSUE OF
) are listed in
FISH
Form of nitrogen
Cod«
(mg. % )
Herring0 (mg. % )
Haddock0 (mg. % )
Nonprotein nitrogen 419.2 437.0 380.0
Volatile base nitrogen 2.1 16.1 8.0
Ammonia 7.7
— —
Urea 1.9 4.0 14.5
Amino nitrogen 39.2 48.8 31.5
Creatine and creatinine 163.0 182.7
—
Imidazole trace 19.1
—
Bases 257.6 269.9 239.4
Purines 24.5 8.21 15.4
Histidine -|- arginine 32.7 72.7 51.5
Lysine 157.9 145.6 145.0
Monoamino nitrogen 95.7 97.9 114.0
Humin 108.4 112.6 46.1
« Campbell (1934-35).
» Komarov (1934).
Shewan et al. (1952) showed the presence of at least seventeen amino acids in haddock muscle extracts, as well as creatine, creatinine, tri
methylamine oxide, and a dipeptide, anserine. They pointed out that the presence of histidine, methylhistidine, ß-alanine, and anserine, but the absence of carnosine, might be of special significance; this condition may be interpreted, as it was by Yudaev (1950), to indicate that the dipep- tides carnosine and anserine are formed in the muscle through a con
densation of ß-alanine, histidine and methylalanine. Torry Research Station explains certain brown disoolorations in dried fish as due to transformations of 1-methylhistidine (Jones, 1956). In codling muscle, this compound is released after death by the activities of anserinase and, consequently, becomes available for further chemical changes (Reay, 1957).
Jones (1954, 1955b, 1959), fractionating the extracts of the codling muscles by chromatography, reported the presence of a large quantity of taurine and a small amount of ß-alanine, besides a number of other amino acids. These compounds showed seasonal variations in their values; taurine gave a peak of 350 to 450 mg./100 g. from May to July and a minimum value of 100 to 150 mg./100 g. in December to February;
lysine was found in amounts less than 0.5 mg./100 g. in all seasons
except the spawning time, when its value was increased to 12 to 15 mg./100 g. Budanova (1952) found that lysine in the protein of muscle from sturgeon was increased during its gonad maturation. Differences in free amino acids between winter and summer samples were also re
ported by Ranke and Bramstedt (1955).
Excess free cysteine characterizes such fresh-water fish as lake perch, pope, and perch-pike, and such marine species as the cat shark (Scyl- liorhinus canicuh L . ) and Ameiurus nebulosus ( L e Sueur). The latter, together with eel, also has a special surplus of lysine. Garfish is one of the richest sources of histidine (Ranke, 1959).
In fresh-water fish, with the exception of eel, neither carnosine nor anserine (Ackermann and Hoppe-Seyler, 1931) has been reported, but a considerable amount of histidine has been found (Yudaev, 1950). In well-fed carp, the free amino acids make up 5.8% of the muscle, ac
cording to Sorvachev (1959), but after the winter starvation, it drops to 4 % . In contrast to earlier findings Duchäteau and Florkin (1957) found histidine to dominate (55.8 mg. per 100 g. flesh) among 15 free amino acids in the muscle of lamprey. Glycine and glutamic acid came next.
By spring, the total level of amino acid nitrogen has declined by one- third. The major drop occurred in glycine, histidine, and glutamic acid.
In contrast, the arginine content increased 3-5 times. Lysine showed irregularities, with ups and downs. Glycine is more abundant than any other free amino acid. Besides those mentioned, the following were de
tected: cystine, aspartic acid, α-alanine, and saroosine (Sorvachev, 1959).
Histidine-oontaining fresh-water fish are generally devoid of ß-alanine.
Fish not containing histidine frequently contain carnosine. Histidine could not be found in the blood of the carp, although present in ample quantities in the muscle: 127-241 mg./100 g. of flesh (Yudaev, 1950).
Mainly glycocoll and histidine and to some extent lysine were found by Duchäteau and Florkin (1954a) to dominate the pool of free amino acids of the carp.
Anserinase is present in the muscle tissue of cod, hydrolyzing anserine to its constituent amino acids: 1-methylhistidine and β ^alanine. It is lack
ing in tuna flesh (Jones, 1955a). This might explain the abundance of this compound in such a dark-fleshed species.
Although this review is devoted primarily to fresh fish, mention should be made of the studies by Obata and his oo-workers [for further references, see Obata et al. (1951) ] on the breakdown products of some of the free amino acids, e.g., lysine, proline, and arginine. Several com-
pounds have been isolated and undoubtedly contribute to the char
acteristic flavor and odor of decomposing fish—in marine species fre
quently superimposed by TMA. In minor quantities these same sub
stances may constitute essential ingredients of the fresh-stage taste. Fur
thermore, the original free amino acids convey taste impulses of sweet
ness, occasionally bitterness, and other specific reactions. The major breakdown products of the above-mentioned amino acids have been tabulated below for the sake of comparison:
Possible ensuing breakdown products, Amino acid encountered in fish
Arginine Ornithine, putrescine, agmatine, δ-aminovaleric acid Leucine Ketoisocaproic acid, isovaleric acid
Lysine N-aminopiperidine, piperidine and pyridine Proline Pyrrolidine, δ-aminovaleric acid
B . TRIMETHYLAMINE OXIDE
The compound TMAO is encountered chiefly in marine fish. This compound was first isolated by Suwa (1909a, b ) in dogfish. Earlier in
vestigators considered it lacking in fresh-water fish (Kutscher and Acker
mann, 1933b). Present studies, however, have clearly shown that it may also occur in this group of fish (Reay, 1938; Lintzel et al., 1939), although in much smaller quantities than in marine types, with the one exception of landlocked salmon (Cook, 1931). Young king salmon in fresh water show TMAO values of 0-4.5 mg.% in the muscle tissue, whereas the same species at sea contain 50-60 mg.%. Significant amounts have, never
theless, been reported in some cases also in such fresh-water fish as the alewife, shad (185 m g . % ) , burbot (116 m g . % ) , trout and salmon (up to 83 mg.%) (Shewan, 1951).
The TMAO content may vary widely with species and location (see also Section IV, Β ) . The values for the teleosts have a wide amplitude. The clupeids contain about 40 mg. as TMAO in 100 g. wet weight, except for the Atlantic and Pacific herring, which average about 75 mg. The gadoids contain most, averaging 88 mg. for 6 species (Dyer, 1952). Pollack show about 70 mg.; cod, 95 mg.; haddock, 70 mg.; hake, 120 mg.; and cusk, 65 mg. Thus, cod contain about 2.7% TMAO calculated as per cent of dry weight; haddock contains about 1.9%, and hake is almost as high as the dogfish—about 3.7%. The salmonids are very low, below 2 5 % , as are also the smelt and the killifish. Dyer (1952) also discusses interesting examples of differences between Atlantic and Pacific specimens of the
same species. Feeding experiments indicate that this compound is wholly exogenous in origin. But, on the other hand, in some cases nearly 5 0 % of the total nitrogen is excreted in the form of TMA by marine fishes (Baldwin, 1957, p. 3 2 3 ) . In such a case it seems to originate in zoo- plankton rather than plant organisms. Norris and Benoit (1945) found no TMAO in marine algae or diatoms, but it was abundant in copepods.
A remarkable observation, for which no safe explanation is yet available, is the appreciable differences in the TMAO values between the east and the west coast of India. Whether they are due to diet or species is still an open question (Velankar and Govindan, 1958b).
The compound TMAO is present in small amounts in both amphibia (Wilson and Walff, 1938) and in mammals such as porpoises and whales (Shewan, 1951). Physiologically, this compound has some kind of os
motic regulatory function (Hoppe-Seyler, 1930; Krough, 1939; Cook, 1931). Barrenscheen and Pantlitschke (1949) advanced the idea that TMAO is a methyl donor in the general metabolism. A comprehensive review of this compound and its possible functions is available (Shewan, 1951).
Nevertheless, it appears difficult to resort to dietary effects as an ex
planation of the winter upsurge in the TMAO content of various United Kingdom herrings (Hughes, 1959). A similar finding is reported in Nor
wegian brisling and herring (Ronald and Jakobsen, 1947). In cod (Shew
an, 1951) and herring (Hughes, 1959), there is also a correlation be
tween age and TMAO content, the latter constantly increasing as the fish gets older.
Hashimoto and Okaichi (1958a, b ) and Okaichi et al. (1959), who fed marine fish as well as fresh-water fish on feed containing TMAO, have reported interesting observations on the accumulation of TMAO by certain fishes. Marine species accumulate TMAO which may amount to about 120 mg./100 g. in filefish (Monacanthus cirrhifer), and be retained for a long period in the tissue, while fresh-water species, such as the goldfish and the eel, accumulate this compound to a far lesser degree.
In this case it is excreted very rapidly and consequently, removed from the body and its tissues.
Miscellaneous. Choline, betaine, and γ-butyro-betaine have been identified in the lamprey (Strack et al, 1937).
IV. Elasmobranchs
A. UREA
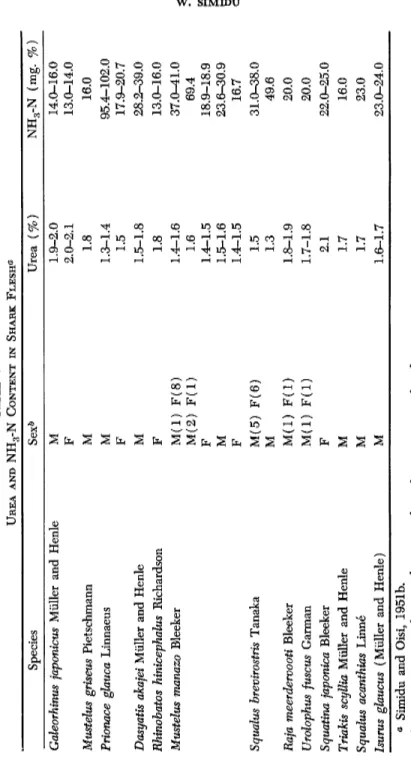
Since the discovery of urea by Städler and Fredricks in 1858 as a muscle constituent of a ray (Raja hatis) and a shark (Scyllium canicula), the high content of this substance in cartilaginous fish has been reported by many authors (Städler, 1859; Schröder, 1890; Baglioni, 1906; No- guchi, 1932). The amount varies with species. Thus, in the muscle of sharks caught in the sea of Japan, the content varied from 1.0 to 2 . 1 % , as shown in Table V (Simidu and Öisi, 1951b; Suyama and Tokuhiro, 1954a). Kisch (1930) established that urea in the blood also varies with species even when coming from similar environments, e.g., 2.3-2.5% for Scyllium and Raja, 1.6-1.7% for Torpedo, 1.4-2.3% for Trygor, and 1.7-1.8% for Mustelus. There is not, however, sufficient evidence to prove that these differences may not be due to individual variations. Dakin
(1922) observed that the freezing point of the blood of Raja was de
pressed correspondingly below that of the surrounding sea water. A similar phenomenon was registered for Scyllium (Hukuda, 1928). When the animal was transferred into diluted sea water, it took in almost the calculated amount of water required to regulate the osmotic pressure of the muscles.
As for land animals, urea is formed mainly in the liver but not in the muscle (Hoagland and Mansfield, 1917) and the biochemical mech
anisms of the formation of urea involving a number of reactions, which can be summarized as follow, were shown by Krebs (Baldwin, 1957):
( 1 ) C 02 + NH3 > Carbamylphosphate
( 2 ) Carbamylphosphate + Ornithine > Citrulline ( 3 ) Citrulline + Aspartate > Argininosuccinate ( 4 ) Argininosuccinate > Arginine
Arginase
( 5 ) Arginine > Ornithine + UREA
In elasmobranch fish, the presence of enzymes in liver tissue which catalyzed these reactions mentioned above other than a reaction forming carbamylphosphate were shown (Baldwin, 1958). As shown in Table VI (Simidu and Öisi, 1951b), appreciable quantities of urea are present in most organs. It is well known that arginase is encountered all over the body and the hepatectomized elasmobranch fish maintains its normal high level (Baldwin, 1957). From these facts, the synthesis may not be confined to the liver as it is in land animals. In a small experiment
TABLE V UREA AND NH3-N CONTENT IN SHARK FLESH« Species Sex* Urea (%) NH3-N (mg. %) Galeorhinus japonicus Müller and Henle Μ 1.9-2.0 14.0-16.0 Galeorhinus japonicus Müller and Henle F 2.0-2.1 13.0-14.0 Mustelus griseus Pietschmann Μ 1.8 16.0 Prionace ghuca Linnaeus Μ 1.3-1.4 95.4-102.0 Prionace ghuca Linnaeus F 1.5 17.9-20.7 Dasyatis akajei Müller and Henle Μ 1.5-1.8 28.2-39.0 Rhinohatos hinicephalus Richardson F 1.8 13.0-16.0 Mustelus manazo Bleeker M(l) F(8) 1.4-1.6 37.0-41.0 Μ(2) F(l) 1.6 69.4 F 1.4-1.5 18.9-18.9 Μ 1.5-1.6 23.6-30.9 F 1.4-1.5 16.7 Squalus brevirostris Tanaka M(5) F(6) 1.5 31.0-38.0 Squalus brevirostris Tanaka Μ 1.3 49.6 Raja meerdervooti Bleeker M(l) F(l) 1.8-1.9 20.0 Urolophus fuscus Garman M(l) F(l) 1.7-1.8 20.0 Squatina japonica Bleeker F 2.1 22.0-25.0 Triakis scyllia Müller and Henle Μ 1.7 16.0 Squalus acanthias Linne Μ 1.7 23.0 Isums glaucus (Müller and Henle) Μ 1.6-1.7 23.O-24.0 « Simidu and Oisi, 1951b. & Figures in parentheses indicate number of specimens analyzed.
(Simidu and Öisi, 1951b), a remarkable increase in urea content was seen during the storage of shark muscle held antiseptic through thymol and toluene.
The large amount of urea present in the flesh of elasmobranchs sug
gests that this substance may have a significant role in the metabolism of these fishes. Generally it has been accepted that urea is responsible for the osmotic regulation of the body fluids (Smith, 1936).
TABLE V I
UREA IN Mustelus manazo Bleeker (PERCENTAGE OF FRESH W E I G H T )A
Part of body %
Muscle 1.1-1.6
Skin 0.8
Blood 1.6
Heart 1.5
Liver 0.8-1.1
Stomach 1.2
Pancreas 2.6
Kidney 1.9
Ovary 2.3
Testes 1.8
Brain 1.6
Embryosac fluid 2.0
Spleen 1.8
a Simidu and Öisi, 1951b.
During the storage of the meat of elasmobranchs large amounts of ammonia are formed, clearly indicating urea as its precursor. For a time, the urease in the muscle itself was thought to be responsible for this decomposition (Mori and Kobayashi, 1949). But only traces of urease were found in fresh shark muscle, showing that urea is decomposed by bacterial action (Simidu and Öisi, 1951a, c ) .
During the breakdown of urea a parallel increase in the amount of ammonia takes place (Simidu and Öisi, 1951a). There is, however, a certain lag in the appearance of ammonia indicating that some inter
mediate compounds are formed (Suyama et ah, 1950). When shark muscle is stored at certain intermediate temperatures, a lag is also evi
dent in the decomposition of urea, causing a platform in the graph for ammonia formation. No such inactive period is observed when the muscle is stored at lower or higher temperatures (Simidu and Öisi, 1951b) or when it is minced (Simidu and Hibiki, 1953a). The conclusion was drawn from such discrepancies that the movement of intracellular
urea outside of the muscle cell was required for a continuous decom
position. Most of the urea-splitting bacteria are killed at about 5 5 ° C , indicating these to be of marine origin (Simidu and Hibiki, 1953b).
Certain species of soil origin are also known to split urea (Kimata and Hata, 1953).
B . TRIMETHYLAMINE OXIDE
The compound TMAO occurs in elasmobranchs in large amounts, no less than 2.5% of the dry weight (Shewan, 1951, 1953; Dyer, 1952). This seems to confirm the conception that this substance serves to regulate the osmotic condition in the muscle. Analyses by Suyama and Tokuhiro, (1954b) show that the urea concentration is almost equal in all parts of the body of a ray (Raja hoUandii). Excessive urea apparently is always excreted. On the other hand, the TMAO content varies considerably.
Muscles, heart, and pancreas show higher concentrations than other body tissues. TMAO has the function of eliminating waste ammonia which would otherwise become toxic (Baldwin, 1957). This is presumably ac
complished through transmethylation and oxidation in the liver and, in some species, in the dark muscle tissue. This causes an accumulation of TMAO in these tissues, if it is not excreted. Another important finding supporting the general contention that TMAO in elasmobranch fish has an essential metabolic function is the fact that the blood has a relatively high concentration of TMAO in this group of fishes, whereas teleostean blood is devoid of this substance (Benoit and Norris, 1945).
C. MISCELLANEOUS
Betaine and sarcosine are characteristic features of elasmobranch fish, in contrast to gadoid species, where anserine and methylhistidine chiefly are encountered (Shewan, 1953).
V. Invertebrates
The free α-amino acid nitrogen in the invertebrates accounts for over 4 0 % of the NPN, while, e.g., teleosts only contain about 6% (Velankar and Govindan, 1957, 1958a). While the urine of fishes contains only minor quantities of amino acid nitrogen, 1 4 % of the nitrogen excreted by invertebrates is represented by such compounds (Baldwin, 1957).
Baldwin has suggested that possibly the invertebrates are less efficient than the vertebrates in metabolizing amino acids.
Creatine is lacking among shellfish, both crustacean and molluscan, while arginine is prevalent (Roche et al., 1957). Considerable quantities
of glycine have repeatedly been found in various marine invertebrates (Chittenden, 1875).
Crustacean shellfish contain TMAO, while mollusks, with the excep
tion of cephalopods, scallops, and cockles, lack this compound (Norris and Benoit, 1945).
A. CRUSTACEANS
Few special studies are available on the soluble nitrogen compounds of crustaceans. Okuda (1919), summarizing analytical results obtained by Buglia and Costantino (1912) and Wilson (1914) in addition to his own data, pointed out that the muscles of crustaceans and mollusks contain higher amounts of nitrogenous extractives, soluble in phospho- tungstic acid, as contrasted to the flesh of fish or land animals. Öya and Fujikawa (1933) discussed these same data, together with results ob
tained by Albrecht (1921). Similar findings were made by Campbell (1934-35) in detailed studies of lobster muscles as compared to fish flesh. Simidu and Hujita (1954a, b ) carried out extensive investigations on nonprotein nitrogenous extractives in several Japanese shrimp species.
They discussed possible relationships between the flavor and such con
stituents. Shrimp contain large amounts of nitrogenous extractives similar to dark-fleshed fish, cuttlefish and shellfish, while their total nitrogen content is low. The monoamino is markedly abundant and in amounts generally higher than in molluscan shellfish, reaching 300 gm./100 g. or more. In the lobster, crab, and prawn, free amino acid nitrogen content accounted for 34 to 3 9 % of the water soluble NPN, while in fish muscle in most cases it constituted only about 6 % (Velankar and Govindan, 1957).
The free amino nitrogen content of the crustacean muscle is over 300 mg. N/100 g. of meat, whereas it is only about a tenth of this value in fish (Velankar and Govindan, 1958a). A great part of this free amino nitrogen is lost in storing the shrimp on ice, thus depreciating its quality
(Velankar and Govindan, 1958b).
Monoamino nitrogen is actually more abundant in palatable species (Simidu and Hujita, 1954a). When the species were listed according to their content of monoamino nitrogen, the resulting order corresponded unexpectedly well with the order of palatability. Both monoamino nitro
gen and glycine decrease in quantity during cold storage (Simidu and Hujita, 1954a, b ) . Diamino nitrogen and its subfractions show little con
sistency as to species.
Simidu and Hujita (1954b) found shrimp to contain a remarkably
high amount of glycine. Glycine nitrogen ranges from 80 to 160 mg./100 g. corresponding to 0.4 to 0.8% of glycine per fresh muscle. It is most noteworthy that glycine nitrogen forms the bulk of the monoamino nitrogen ( 5 0 - 6 0 % or more). Even in this case, the relative order as to glycine content corresponds reasonably well with the palatability of the shrimps. The substantial amounts of glycine present in shrimp muscle may seem to indicate that this amino acid plays some important physio
logical role, possibly in the osmotic regulation, but presumably also in other respects. De Almeida (1954), analyzing Brazilian shrimps, could confirm the abundance of glycine as a free amino acid, but in addition proline was predominant. No cystine or cysteine was found. In the spoiling of the shrimp, most free amino acids increase in quantity. Ex
ceptions are proline and serine, which decline appreciably. Camien et ah (1951) also found glycine and proline to be by far the leading free amino acids of lobster. These two, together with arginine, glutamic acid, and alanine, characterize most crustacean muscles. The total concentra
tion of free amino acids is far less in such lacustrine forms as Astacus fluviatilis, compared to marine crustaceans, or in euryhaline forms as Chinese crab adapted to fresh water (Duchäteau and Florkin, 1955b).
This is explained by the likely function of these amino acids as regulating the osmotic pressure. This is in accordance with the finding that most of these free amino acids are intracellular. An intriguing finding is re
ported by a Belgian research group (Duchäteau and Florkin, 1956;
Duchäteau et al, 1959), that crabs moving into brackish water exhibit a drastic drop ( 3 0 - 5 0 % ) in their total pool of fifteen free amino acids, particularly in proline, glutamic acid, and glyoocoll. This has been estab
lished for the Chinese crab and Carduus maenas. Arginine remains largely unchanged. The fresh-water crayfish exhibits no comparable regulation when moved into sahne water—only a minor increase in proline (Duchäteau and Florkin, 1956). At low temperatures (1-3°C.) no other change with the fifteen free amino acids studied takes place in the Chinese crab than a reduced proline concentration (Duchäteau and Florkin, 1955a).
Due to the abundance of extractive amino nitrogen in the flesh of most crustaceans, large amounts of volatile base nitrogen are produced rapidly during the storage of shrimp, for instance. Hence, the muscle shows a tendency to become alkaline rather rapidly. Part of the volatile base formed may be ascribed to TMA derived from the reduction of TMAO (Simidu et al, 1955).
The fact that several marine invertebrates, constituting food for various sea-water fish, contain TMAO may explain its prevalence among such fish (Dyer, 1952).
Recently, a complete analysis of extractive amino acids in prawn (Penaeus japonicus) muscle tissue was carried out by Könosu et al.
(1958b) through microbiological assays. Glycine was highest in amount, followed by arginine and proline.
Kermack et al. (1955) established by chromatographic analysis that more than 8 0 % of the NPN in a lobster muscle consists of about 4 2 % diverse α-amino acids, 1 2 % of TMAO, 1 2 % of glycine, 4 % of taurine, and 1 1 % of amino nitrogen other than α-amino acids. Robertson (1957) found 500 mg. of amino nitrogen and 100 mg. of TMAO per kilogram in Nephrops muscles. Free amino acids, taurine, and TMAO account for no less than 6 0 % of the osmotic pressure of the crab Carcinus maenas—
also being reduced when it moves into brackish water (Shaw, 1958;
Duchateau et al., 1959). Ackermann and Kutscher (1907) found betaine, cragitine ( a compound recently identified as analogous to betaine), neosin, crangonin, and a plant alkaloid, pyridil-ammonium hydroxide in the muscle extract of lobster. Homarine was discovered in lobster by Hoppe-Seyler (1933) and later encountered in many shellfish. The crab Crangon vulgaris also contains this pyridine compound (Ackermann and List, 1957). The isomer to homarine—trigonelline—was also found, to
gether with TMAO, by the same research group. A new unidentified base, C4H9O2N, was also isolated.
Japanese research workers encountered some aldehyde-like sub
stances in canned crab meat (Paralithodes camtschaticus, Chionectes opilis, etc.), later identified through crystallization as urotropine by Kawaguchi (unpublished data quoted by Kimura, 1932). Kimura (un
published, 1932) has been able to establish that there is no definite rela
tionship between the aldehyde content of the flesh and the quality grade of the canned product, nor does this factor measure the aging. The amount of aldehydes does not reflect the habitat of the crab nor the freshness of the meat when packed.
The presence of free amino acids in significant quantities in the crustacean muscle probably renders it more susceptible to bacterial in
vasion. This might partly explain the lower potential keeping quality of the crustaceans as compared with teleost fishes. See further Chapter 15, this volume. Shewan (1951) extends this statement to refer to the entire group of nitrogenous extractives, their total amount accounting primarily for the readiness of both fish and shellfish to succumb to microbial attack.
Β . MOLLUSKS
1. In General
Together with arginine, the guanidine derivative octopine has been found in numerous molluscan shellfish. For references to such earlier investigation, see Thoai and Robin (1959). Arginine, in turn, is the natural biochemical precursor to octopine. The chemical constitution of this latter compound was established by Akasi (1937a, b ) and later con
firmed by Irwin and Wilson (1937).
Amino acid nitrogen accounted for 52 to 6 3 % of the extractive nitrogen in these investigated clam species. The free amino acids oc
curring in the largest quantities were alanine, glycine, arginine, and glutamic acid, in the order given here. In subsequent spoiling, arginine declined most (Itö, 1959).
Molluscan muscle tissue is distinguished from that of fish by its pro
portionally larger content of free arginine, and aspartic and glutamic acids. The compound TMAO has not been found in oysters, mussels, or clams (Norris and Benoit, 1945). Marine mussels have a many times higher concentration of free amino acids than fresh-water species (Du
chäteau et al, 1952; Potts, 1958).
2. Squid
Squids are an important source of food in Japan and are eaten as
"sasimi" (slices of raw fish), dried or otherwise processed. Dried squid is exported to the Far Eastern countries. The taste of squid is very sweet and varies with species and season.
Squids are rich in extractive nitrogen or amino nitrogen (Simidu and Takeda, 1952; Endo et al, 1954; Velankar and Govindan, 1957). The amount of amino nitrogen, especially of monoamino nitrogen, is larger in squid having a superior flavor as compared to those with an inferior taste. Glycine is also more abundant.
Recently, various amino acids and bases have been determined quali
tatively or quantitatively by techniques of bioassay, chromatography, or others (Amano and Bito, 1951; Yoshimura and Shibata, 1953; Yoshimura and Kubo, 1953; Itö, 1957; Kojima and Kusakabe, 1956a, b ) . Another such study by Könosu et al. (1958a) showed that the common squid also contained appreciable quantities of proline besides TMAO and TMA. In addition, octopine, TMAO, betaine, etc., were found in squid extractives by Kojima and Kusakabe (1955a, b, 1956a, b ) . The large
amount of betaine is claimed to contribute to a sweet flavor (Takahashi, 1915b).
3. Octopus
Muscle extractives of octopus contain hypoxanthine (Henze, 1905), taurine, carnosine, and betaine (Takahashi, 1915a). Morizawa (1927) isolated guanine, adenine, xanthine, hypoxanthine, and octopine. This latter compound was found by him for the first time, as well as cytosine, carnitine, betaine, taurine, lactic acid, creatine, creatinine, and alanine.
The findings of Morizawa are partly contradicted by those of Kutscher and Ackermann (1933b), who concluded that creatine is restricted to vertebrates. Invertebrates have no creatine, but do contain arginine (Kutscher and Ackermann, 1933b). The absence of arginine, as found by Morizawa, may be explained by the postmortem conversion of ar
ginine to octopine. From this point of view, it is interesting that Humoto (1954) encountered octopine only in the epidermis. In addition to the above-mentioned compounds, TMA, adenine, adenylpyrophosphoric acid, agmatine, argininephosphoric acid, choline, creatinephosphoric acid, glutathione, glucose and ribose, hypoxanthine, methylagmatine, urea, etc., have all been identified in the muscle of various octopi (Acker
mann and Möhr, 1937; Asano, 1957).
The compound TMAO is common in all cephalopods (Norris and Benoit, 1945). It was particularly studied as to its distribution in the body and total amount by Asano and Sato (1954).
A remarkably rapid formation of ammonia, together with a rise in pH values, takes place in the spoilage of octopus. These processes seem to be responsible for the destruction of nitrogenous extractives, so abun
dant in their muscle tissue.
4. Scallops
Arginine constitutes 9 0 % of the total guanidine bases of scallops.
After death, arginine breaks down and a corresponding increase in oc
topine is noticed (Irvin and Wilson, 1939). It appears to be a regular constituent of the abductor muscle (Mayeda, 1936; Moore and Wilson, 1937a, b ) . It was originally thought to be a postmortem product and a combination of arginine with propionic acid. Irvin and Wilson (1939), however, showed that arginine condenses with lactic acid or pyruvic acid to form octopine. This compound has later been investigated and analyzed as to its quantity in relation to total monosubstituted guanidine derivatives. Both octopine and total guanidine show constant values in
fresh mucles and are more abundant in abductor than mantle muscles, but increase after death (Thoai and Robin, 1959).
Taurine and glycocoll have both been identified in significant quan
tities in scallop muscles (Chittenden, 1875; Kelly, 1904).
Both scallops and cockles contain TMAO, according to Norris and Benoit (1945).
5. Other Shellfish
It has been assumed that creatine, so characteristic of vertebrate muscles, is always replaced by arginine in the invertebrates, with a few exceptions presented by Albrecht (1921). He demonstrated the presence of creatine and creatinine in some shellfish. These guanidine bases, com
bining with phosphoric acid to form phosphagens, seem to play im
portant roles in muscular contraction. So far, there is no evidence from shellfish of glucosamine being methylated to form creatine in the pres
ence of methionine as a methyl donor as is the case in mammalian livers (Davenport et al, 1938; Borsook and Dubnoff, 1940).
Betaine is also present in some shellfish (Kutscher and Ackermann, 1933a; Deutsch et al, 1938). Hoppe-Seyler (1933) isolated homarine, which was identified as a-pyridine-carbonic acid-methyl betaine. Stachy- drin, γ-butyrobetaine, trigonelline, carnitine, arcaine (tetramethylene guanidine), etc., were also encountered. Taurine was isolated from abalone by Mendel (1904).
The presence of TMAO, 61.4 mg./100 g. in ear shell and 20 mg./100 g. in wreath shell, was proved by Simidu et al (1953a), while Dyer (1952) failed to find any in oysters. Nevertheless, TMA is a good in
dicator of spoilage (Lartigue et al., 1960). Its biochemical origin is obscure.
C. MISCELLANEOUS INVERTEBRATES
Some other marine invertebrates, such as jellyfish, sea urchins, sea squirts, and sea cucumbers, are commonly utilized as food in Southeast Asia.
The biochemical features of jellyfish were first studied by Haurowitz and Waelsch (1926). It lacks urea, uric acid, and creatine derivatives, thus showing a defective oxidative metabolism. On the other hand, it cannot feed on inorganic nutrition because of the lack of chlorophyll.
The jellyfish has a remarkable resemblance to fungi, not only nutri
tionally, but also in its biochemical outfit.
Nitrogenous extractives in the sea cucumber (Stichopus faponicus)
are also minor; thus, the taste is more tied to texture than chemical com
position, as is the case with jellyfish (Tanikawa et al., 1955),
Sea urchin eggs made into a salted paste are cherished as a delectable aquatic food in Japan. No systematic studies on nitrogenous extractives of this organism are available. Sea urchins contain a basic substance, presumably trigonelline (Holtz et at, 1924). This is, in effect, a plant alkaloid discovered by Jahns in 1885 in the seeds of fenugreek. Chem
ically, it is a methylbetaine of nicotinic acid. This makes it related to the substance homarine, which is a methylbetaine of picolinic acid (Hoppe-Seyler, 1933). Homarine has been isolated from the egg and embryo of a number of sea urchin species and is considered to have some significant metabolic function in the morphogenesis, due to its part in methylation reactions (Hultin et al., 1953).
REFERENCES
Ackermann, D., and Hoppe-Seyler, F . A. (1931). Über das Vorkommen von An- serin und Carnosin bei Selachiern und Teleostiern. Z. physiol. Chem. 197, 135- 140.
Ackermann, D., and Kutscher, F. (1907). Über Krabben-Extrakt. IV. Ζ. Unter
such. Nahr.- u. Genussm. 14, 687-091.
Ackermann, D., and Mohr, N. (1937). Über das Vorkommen von Octopin, Ag- matin und Ar ginin in der Octopodenart Eledone moschata. Z. physiol. Chem.
250, 249-252.
Ackermann, D., and List, P. H. (1957). Über das Vorkommen von Trimethyl- aminoxyd, Homarin, Trigonellin und einer Base C4H902N in der Krabbe (Cran- gon vulgaris). Z. physiol. Chem. 306, 260-264.
Akasi, S. (1937a). Studien über die Konstitution des Octopins, eines stickstoffhal
tigen Körpers in den octopoden Muskeln. I. Eigenschaften und Abbau des Oc
topins. /. Biochem. (Japan) 25, 261-280.
Akasi, S. (1937b). Studien über die Konstitution des Octopins, eines stickstoff
haltigen Körpers in den octopoden Muskeln. II. Synthetische Versuche. J. Bio
chem. (Japan) 25, 281-290.
Albrecht, P. G. (1921). Chemical study of several marine mollusks of the Pacific Coast. /. Biol. Chem. 45, 395-405.
Amano, K., and Bito, M. (1951). Consequence of free amino acid generated from decomposing fish muscle (In Japanese.) Bull. Japan. Soc. Sei. Fisheries 16, 526-532.
Asano, M. (1957). Biochemistry of octopus (In Japanese.) Kagaku no Ryoiki 11, 685-692.
Asano, M., and Sato, H. (1954). Biochemical studies on octopus. I. Trimethyl
amine and trimethylamine oxide contents of octopus. Töhoku J. Agr. Research 5 ( 3 ) , 191-195.
Baglioni, S. (1906). Einige Daten über die mengenmässige Zusammensetzung von verschiedenen Körperflüssigkeiten von Seetieren (Fischen und Inverte- braten) Beitr. chem. Physiol. Pathol. 9, 50-66.
Baldwin, E. (1957). "Dynamic Aspects of Biochemistry," 3rd ed. Cambridge Univ. Press, London and New York.
Baldwin, E. (1958). Ureogenesis in elasmobranchs. Nature 181, 1591-1592.
Barrenscheen, Η. K., and Pantlitschke, M. (1949). Die Methylierung durch pflan
zliche und tierische Gewebe. 7. Mitteilung. Zum Mechanismus der Methylüber
tragung des Cholins. Z. physiol Chem. 284, 250-256.
Bate-Smith, E. C. (1958). Carnosine and anserine in meat—a biochemical co
nundrum. Food Preserv. Quart. 1 8 ( 3 ) , 42-45.
Benoit, G. J., and Norris, E. R. (1945). Studies on trimethylamine oxide. II. The origin of trimethylamine oxide in young salmon. /. Biol. Chem. 158, 439-442.
Borsook, H., and Dubnoff, J. W. (1940). The formation of creatine from glyco- cyamine in the liver. /. Biol. Chem. 132, 559-574.
Budanova, A. M. (1952). Some amino acids in muscle proteins of sturgeons and trimethylamine oxide content of their blood during spawning migration. Biok- himiya 17, 1-6.
Buglia, G., and Costantino, A. (1912). Beiträge zur Muskelchemie IV. Mitteilung.
Der Extraktivstickstoff und der freie durch Formoltitrierbare Aminostickstoff in der Muskulatur verschiedener Tierarten. Z. physiol. Chem. 82, 439-462.
Camien, M. N., Sarlet, H., Duchäteau, G., and Florkin, M. (1951). Nonprotein amino acids in muscle and blood of marine and fresh water Crustacea. J. Biol Chem. 193, 881-885.
Campbell, J. (1934-35). The nonprotein nitrogenous constituents of fish and lobster muscle. /. Biol. Board Can. 1, 179-189.
Chittenden, Ν. H. (1875). Über Glykogen und Glykokoll in dem Muskelgewebe des Pecten irradians. Ann. Chem. Pharm. 178, 266-274.
Cook, A. S. (1931). Α study of the occurrence of trimethylamine in marine ani
mals. Can. Chem. Met. 15, 22-23.
Dakin, H. R. (1922). Quoted from Kawamoto, Ν. (1935). "Gyorui no Sein,"
p. 13. Yokendo, Tokyo.
Davenport, H. W., Fisher, R. B., and Wilhelm, A. E. (1938). The metabolism of creatine. III. The role of glycocyamine in creatine synthesis. Biochem. J. 32, 262-270.
De Almeida, M. E. W. (1954). Aminoacidos livros con camaroes variagoes decor- ridas durante a decomposigao. Rev. inst. Adolfo Lutz 15, 158-167.
Deutsch, Α., Eggleton, Μ. G., and Eggleton, P. (1938). The use of sodium sul
phate for the preparation of concentrated protein-free tissue extracts. Biochem.
J. 32, 203-207.
Duchäteau, Gh., and Florkin, M. (1954). La composition quantitative d'acides amines, non-proteiques des muscles de la carpe et la teneur de peptides. Compt.
rend. soc. biol. 148, 1287-1289.
Duchäteau, Gh., and Florkin, M. (1955a). Influence de la temperature sur l'etat stationnaire du pool des acides amines non-proteiques des muscles d'Eriocheir sinensis Milne Edwards. Arch, intern, physiol. et biochim. 63, 213-221.
Duchäteau, Gh., and Florkin, M. (1955b). Concentration du milieu exterieur et etat stationnaire du pool des acides amines non-proteiques des muscles d'Eno- cheir sinensis, Milne Edwards. Arch, intern, physiol. et biochim. 63, 249-251.
Duchäteau, Gh., and Florkin, M. (1956). Systemes intracellulaires d'acides amines libres et osmoregulation des crustaces. /. physiol. (Paris) 48, 220.
Duchäteau, Gh., and Florkin, M. (1957). Type de composition du pool d'acides amines, non-proteiques des muscles de la lamproie. Arch, intern, physiol. et bio
chim. 65, 378.
Duchäteau, Gh., Sarlet, Η., Camien, Μ. Ν., and Florkin, Μ. (1952). Acides ami
nes non proteiniques des tissus chez les mollusques lamellibranches et chez les vers. Comparaison des formes marines et des formes dulcicoles. Arch, intern, physiol. et biochim. 60, 124-125.
Duchäteau, Gh., Florkin, Μ., and Jeuniaux, Ch. (1959). Composante aminoacide des tissus chez les crustaces. I. Composante amino-acide des muscles de Carcinus maenas L. Lors du passage de l'eau de mer ä l'eau saumatre et au cours de la mue. Arch, intern, physiol. et biochim. 67, 489-500.
Duncan, D. W., and Tarr, H. L. A. (1958). Biochemical studies on Sockeye salmon during spawning migration III. Changes in the protein and nonprotein nitrogen fractions in muscles of migrating Sockeye salmon. Can. J. Biochem.
Physiol. 36, 799-803.
Dyer, W. J. (1952). Amines in fish muscle. VI. Trimethylamine oxide content of fish and marine invertebrates. /. Fisheries Research Board Can. 8, 314-324.
Endo, Κ., Hujita, Μ., and Simidu, W. (1954). Studies on muscle of aquatic ani
mals. XXII. On distribution of extractive nitrogens and free glycine content in squids. Bull. Japan. Soc. Sei. Fisheries 20, 723-725.
Greene, C. H. (1919). Changes in nitrogenous extractives in the muscular tissue of the king salmon during the fast of spawning migration. J. Biol. Chem. 39, 457-477.
Hashimoto, Y., and Okaichi, T. (1958a). Trimethylamine oxide in fish muscle. I.
The origin of trimethylamine oxide in goldfish muscle. (In Japanese with English summary.) Bull. Japan. Soc. Sei. Fisheries 24, 640-644.
Hashimoto, Y., and Okaichi, T. (1958b). Trimethylamine oxide in fish muscle.
II. Trimethylamine oxide in the muscle of eels kept in fresh and brackish water.
(In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 24, 645- 647.
Haurowitz, F., and Waeisch, H. (1926). Über die chemische Zusammensetzung der Qualle Velella spirans. Z. physiol. Chem. 162, 300-317.
Henze, M. (1905). Beiträge zur Muskelchemie der Oktopoden. Z. physiol. Chem.
43, 477-492.
Hibiki, S., and Simidu, W. (1959). Studies on putrefaction of aquatic products.
27. Inhibition of histamine formation in spoiling of cooked fish and histidine con
tent in various fishes. (In Japanese with English summary.) Bull. Japan. Soc.
Sei. Fisheries 24, 916-919.
Hoagland, R., and Mansfield, C. M. (1917). The function of muscular tissue in urea formation. J. Biol. Chem. 31, 487-499.
Holtz, F., Kutscher, F., and Thielmann, F. (1924). Über das Vorkommen des Pflanzenalkaloids Trigonellin in der Tierwelt. Z. Biol. 81, 57-60.
Hoppe-Seyler, F. A. (1930). Die Bedingungen und die Bedeutung biologischer Methylierungsprozesse. Z. Biol. 90, 433-466.
Hoppe-Seyler, F. A. (1933). Über das Homarin, eine bisher unbekannte tierische Base. Z. physiol. Chem. 222, 105-115.
Hughes, R. B. (1959). Chemical studies on the herring (Clupea harengus) I.
Trimethylamine oxide and volatile amines in fresh, spoiling and cooked herring flesh. /. Set. Food Agr. 10, 431-436; II. The free amino-acids of herring flesh and their behaviour during post mortem spoilage. /. Set. Food Agr. 10, 558-564.
Hukuda, K. (1928). Brit. J. Exptl. Biol. 9, 61; quoted from Kawamoto, N. (1935).
"Gyorui no Seiri" p. 13. Yokendo, Tokyo.
Hultin, T., Lindvall, S., and Gustafsson, K. (1953). On the occurrence of picolinic acid betaine in sea urchin embryos. Arkiv Kemi 6, 477-480.
Humoto, Y. (1954). Darstellung von Octopin aus Sepiahaut. Ζ. physiol. Chem.
297, 47-48.
Irvin, J. L., and Wilson, D. W. (1937). Synthesis of octopine (pectenine). Proc.
Soc. Exptl. Biol. Med. 36, 398-399.
Irvin, J. L., and Wilson, D. W. (1939). Studies on octopine. II. The precursor of octopine in autolyzing scallop muscle. /. Biol. Chem. 127, 575-579.
Itö, Κ. (1957). Amino acid composition of the muscle extracts of aquatic animals.
(In Japanese with English summary.) Bull. Japan. Soc. Sei. Fisheries 23, 497- 500.
Itö, Κ. (1959). Amino acid composition of the muscle extracts of aquatic animals.
II. The amounts of free amino acids in the muscle of shellfishes and their varia
tion during spoilage. (In Japanese with English summary.) Bull. Japan. Soc.
Sei. Fisheries 25(10-12), 658-660.
Johnston, W. W., and Johnston, Μ. L. (1939). An additional compound of histidine. J. Fisheries Research Board Can. 4, 363-366.
Jones, N. R. (1954). Factors affecting the free amino acid composition of fresh and iced skeletal muscle of North Sea codling (Gadus callarias). Biochem. J.
58, vii, xlvii-xlviii.
Jones, N. R. (1955a). The free amino acids of fish. I. Methylhistidine and ß-ala- nine liberation by skeletal muscle anserinase of codling (Gadus callarias). Bio
chem. J. 60, 81-87.
Jones, N. R. (1955b). The free amino acids of fish. II. Taurine in the skeletal muscle of codling (Gadus callarias). J. Sei. Food Agr. 6, 3-9.
Jones, N. R. (1956). Discoloration of muscle preparations from codling. Gadus callarias, by degradation products of 1-methyl-histidine. Nature 1 1 1 , 748-749.
Jones, N. R. (1959). The free amino acids of fish. III. Fresh skeletal muscle from lemon sole (Pleuronectes microcephalus). J. Set. Food Agr. 10, 282-286.
Joshi, S., Master, F., and Magar, N. G. (1953). Nutritive value of some Bombay fish. I. Distribution of nonprotein nitrogen extractives, thiamine, riboflavine, and niacin. Indian J. Med. Research 41, 431-439.
Kawabata, T. (1953). Studies on the trimethylamine oxide reductase I. Reduction of trimethylamine oxide in the dark muscle of pelagic migrating fish under aseptic conditions. (In Japanese with English summary.) Bull. Japan. Soc. Sei.
Fisheries 1 9 ( 4 ) , 505-512.
Kawabata, T., Fujimaki, M., Amano, K., and Tomiya, F. (1952). Studies on pH of fish muscle. Variation in pH of fresh albacore muscle on the locality ex
amined. (Studies on the tunny meat—I) (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 18(3), 124-132.
Kelly, A. (1904). Beobachtungen über das Vorkommen von Ätherschwefelsäuren,
von Taurin und Glycin bei niederen Tieren. Beitr. chem. Physiol. Pathol. 5, 377- 383.
Kermack, W. O., Lees, H., and Wood, J. D. (1955). Some nonprotein constitu
ents of the tissues of the lobster. Biochem. J. 60, 424-428.
Kimata, M., and Hata, Y. (1953). On the urea-splitting bacteria causing the spoilage of fresh fish. (In Japanese with English summary.) Mem. Research Inst. Food Sei. Kyoto Univ. 5, 55-64.
Kimura, K. (1932). "Suisan-Seizo-Gaku" (Technology of marine products), 282 pp. Koseikaku, Tokyo.
Kisch, B. (1930). Harnstoffuntersuchungen bei Selachiern. Biochem. Z. 225, 197- 207.
Kochi, M., and Era, S. (1959). Studies on the chemical composition, particularly nitrogen fraction of a fresh yellowfin tuna meat. (In Japanese with English sum
mary.) /. Shimonoseki Coll. Fisheries 8 ( 1 ) , 67-71.
Kodama, S. (1913). On the isolation of inosinic acid. (In Japanese.) Tokyo Ka- gaku Kaishi 34, 751-753.
Kojima, Y., and Kusakabe, H. (1955a). Isolation of natural substances by ion exchange resins. III. A new simple method for the isolation of octopine and taurine from squid muscles and the estimation of their amounts. /. Sei. Research Inst. (Tokyo) 49, 132-136.
Kojima, Y., and Kusakabe, H. (1955b). Isolation of natural substances by ion- exchange resins. IV. A note on the betaine content of dried squid. J. Sei. Re
search Inst. (Tokyo) 49, 262-266.
Kojima, Y., and Kusakabe, H. (1956a). Isolation of natural substances by ion exchange resins. V. Quantitative determination of trimethylamine oxide and betaine in squid muscles. /. Sei. Research Inst. (Tokyo) 50, 193-198.
Kojima, Y., and Kusakabe, H. (1956b). Studies on the isolation of nitrogenous substances by ion exchange resins. I. Isolation of amine fraction from extractives of squid muscles. (In Japanese.) Kagaku Kenkyusho Hökoku 32, 221-223.
Kömarov, S. A. (1934). The partition of non-protein nitrogen in haddock's muscle. Contribs. Can. Biol. and Fisheries 8, 125-130.
Könosu, S., Akiyama, T., and Mori, T. (1958a). Muscle extracts of aquatic ani
mals. I. Amino acids, trimethylamine and trimethylamine oxide in the muscle extracts of a squid, Ommastrephes sloani pacificus. (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 23, 561-564.
Könosu, S., Akiyama, T., and Mori, T. (1958b). Muscle extracts of aquatic ani
mals. II. Amino acids in the muscle extracts of a prawn, Penaeus japonicus. (In Japanese with English summary.) Bull. Japan. Soc. Set. Fisheries 23, 565-567.
Krough, A. (1939). "Osmotic Regulation in Aquatic Animals." Cambridge Univ.
Press, London and New York.
Kutscher, F., and Ackermann, D. (1933a). Über das Vorkommen von Betainen in der Archenmuschel. Ζ. physiol. Chem. 221, 33-39.
Kutscher, F., and Ackermann, D. (1933b). The comparative biochemistry of vertebrates and invertebrates. Ann. Rev. Biochem. 2, 255-376.
Lartigue, D., Novak, A. F., and Fieger, E . A. (1960). An evaluation of the indole and trimethylamine tests for oyster quality. Food Technol. 14 ( 2 ) , 109-112.
Lintzel, W., Pfeiffer, Η., and Zippel, I. (1939). Untersuchungen über Trimethyl-
ammoniumbasen. IV. Mitteilung: Über das Vorkommen von Trimethylaminoxyd in der Muskulatur von Süsswasserfischen. Biochem. Z. 301, 29-36.
Lukton, Α., and Olcott, H. S. (1958). Content of free imidazole compounds in the muscle tissue of aquatic animals. Food Research 23(6), 611-618.
Mayeda, H. (1936). Über die Extraktivstoffe aus den Schliessmuskeln von Pecten (Patinopecten) yessoensis Jay. Acta Schol. Med. Univ. Imp. Kioto 18, 218-225.
Mendel, L. B. (1904). Über das Vorkommen von Taurin in den Muskeln von Weichtieren. Beitr. chem. Physiol. Pathol. 5, 582.
Mishukova, G. A. (1955). Metabolie properties of skeletal muscles free from carnosine and anserine. (In Russian.) Biokhimiya 20, 307-316.
Moore, E., and Wilson, D. W. (1937a). Nitrogenous extractives of scallop muscle.
1. The isolation and a study of the structure of octopine. /. Biol. Chem. 119, 573-584.
Moore, E., and Wilson, D. W. (1937b). Nitrogenous extractives of scallop muscle.
2. Isolations from and quantitative analyses of muscles from freshly killed scal
lops. /. Biol. Chem. 119, 585-588,
Mori, T., and Kobayashi, T. (1949). Occurring of urease in muscle from shark.
(In Japanese.) Bull. Japan. Soc. Sei. Fisheries 15, 51-52.
Morizawa, K. (1927). Über die Extraktivstoffe von Octopus octopodia. Acta Schol.
Med. Univ. Imp. Kioto 9, 285-288.
Noguchi, E . (1932). On the urea content of muscle of marine animals. (In Japa
nese.) Bull. Japan. Soc. Set. Fisheries 1, 121-123.
Norris, E. R., and Benoit, G. J. (1945). Studies on trimethylamine oxide. I. Oc
currence of trimethylamine oxide in marine organisms. /. Biol. Chem. 158, 435- 438.
Obata, Y., Yamanishi, T., and Ishida, M. (1951). Chemical studies on the sub
stance of fish smell. II. Piperidine group compounds as the substances concerned with fishy smell. (In Japanese with English summary.) Dept. Fisheries Fac.
Agr., Hokkaido Univ. Contrib. No. 103.
Okaichi, T., Manabe, M., and Hashimoto, Y. (1959). Trimethylamine oxide in fish muscle. III. The origin of trimethylamine oxide in marine fish muscle. Bull.
Japan. Soc. Set. Fisheries 25, 136-140.
Okuda, Y. (1919). Studies on the muscle constituents of aquatic animals. (In Japanese.) Nogaku Kaihö 200, 279-416.
Oya, T., and Fujikawa, K. (1933). "Nantai Dobutsu no Kagaku," pp. 31-60. (In Japanese.) Koseikaku, Tokyo.
Potts, W. T. W. (1958). The inorganic and amino acid composition of some lamellibranch muscles. /. Exptl. Biol. 35, 749-764.
Ranke, Β. (1959). Über die nicht-eiweissgebundenen Aminosäurebestände von Fischen, Mollusken und Krebsen. Arch. Fischereiwiss. 1 0 ( 1 / 2 ) , 117-159.
Ranke, E., and Bramstedt, F. (1955). Der Einfluss der Lagerungsdauer des Fisch
fleisches auf den Gehalt an freien Aminosäuren. Arch. Fischereiwiss. 6(3-4), 193-198.
Ranke, E., Ranke, B., and Bramstedt (1955). Über ein bei frischen Seefischen vor
kommendes Peptid. Arch. Fischereiwiss. 6 ( 5 / 6 ) , 343-345.